Abstract
Objective
A coagulation factor called fibrinogen is produced by the liver and is proteolyzed by thrombin to become fibrin. The latest studies have revealed that fibrin(ogen) palys an essential role in the regulation of cardiovascular disease. Understanding the relationship and mechanism between fibrin(ogen) and cardiovascular disease is of great significance for maintaining overall health. The objective of this review is to discuss the specific involvement and underlying mechanisms of fibrin(ogen) in cardiovascular disease.
Methods
A review was conducted using the PubMed database to identify and analyze the emerging role of fibrinogen in cardiovascular disease.
Results
The literature review revealed that fibrin(ogen) plays a pivotal role in maintaining cardiovascular disease and are involved in the pathogenesis of cardiovascular disease. Fibrin(ogen) mainly influence various pathophysiological processes, such as participating in thrombosis formation, stimulating the inflammatory response, and other molecular pathways.
Conclusion
This review focuses on the involvement of fibrin(ogen) in cardiovascular disease, with a particular emphasis on the main functions and underlying mechanisms by which fibrin(ogen) influence the pathogenesis and progression of these conditions. This review underscores the potential of fibrin(ogen) as therapeutic targets in managing cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver produces fibrinogen, a protein that is widely distributed and found in human plasma at 2–4 g/L. Fibrinogen forms fibrin through thrombin-mediated fragmentation [1]. Many studies have shown a close relationship between fibrin(ogen) and cardiovascular disease [2,3,4,5]. Recent studies have shown that cardiovascular diseases, including ischemic heart disease, stroke, heart failure, peripheral and aortic arterial disease, arrhythmias, and valvular diseases constitute the leading cause of global mortality and a major contributor to health loss worldwide [6, 7]. As the processes behind fibrin(ogen)’s activity in tissues are more understood, fibrin(ogen) ‘s role is evolving from that of a coagulation marker to that of a complex signaling molecule for cardiovascular disease. Additionally, further investigation into novel molecular pathways connecting coagulation to inflammation is opening up new targets for prospective treatments for a variety of systemic disorders, including the coagulation cascade factors [8]. However, the specific involvement and underlying mechanisms of fibrin(ogen) in cardiovascular disease have not been systematically discussed.
Fibrinogen and fibrin structures
The most prevalent coagulation factor is fibrinogen, often known as coagulation factor I. It is a big 340 kDa oligoglycoprotein that has a plasma concentration of 2–4 g/L [1]. The fibrinogen molecule is made up of three pairs of distinct polypeptide chains, namely Aα-、Bβ- and γ-chains. These chains are the result of three independent genes: FGA, FGB, and FGG, clustered within a 65-kilobase region on human chromosome 4 (4q23-q32) [9]. The expression of fibrinogen increases with age and is associated with inflammatory status. Corticosteroids can reduce circulating fibrinogen, and induction of acute phase response mediated by interleukin-6 can increase circulating fibrinogen by more than twice [9]. The heritability of circulating fibrinogen levels is estimated to be between 25% and 50%, but only a portion of gene regulatory factors have been identified. Using a gene silencing-based approach in HepG2 hepatoblastoma cells, Dobson et al. screened 28 genes and identified 7 novel potential genetic regulators of fibrinogen synthesis [10]. In an epigenome-wide associate study, Hahn et al. identified 208 and 87 significant CpG sites associated with fibrinogen levels from the 450 K (p < 1.03 × 10− 7) and EPIC arrays (p < 5.78 × 10− 8). The examples of genes located near these CpG sites were SOCS3 and AIM2, which are involved in inflammatory pathways [11].
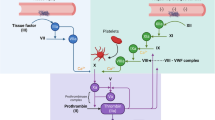
Each fibrinogen monomer has two external D structural domains connected to the central E structural domain by Aα-, Bβ-, and γ-chains. The N-terminal fibrinopeptide A (fibrinopepide-A, FpA) sequence is present in each fibrinogen Aα-chain, while the N-terminal fibrinopeptide B (fibrinopepide-B, FpB) sequence is present in the Bβ-chain. The C-terminal region of the α-chain begins at residue 220 of the D-structural domain, not far from where it emerges from the D-structural domain, and ends at Aα terminates at 610 (Fig. 1A) [12]. Fibrinogen is the primary source of fibrin, the main protein found in blood clots. The last step in the coagulation cascade is the transformation of fibrinogen into fibrin and the subsequent fibrin clot. This happens as a result of the thrombin-catalyzed cleavage of fibrin peptides, which causes molecules to aggregate and fibrin to form. Following FpA cleavage by thrombin from the N-terminus of the Aα-chain, fibrinogen monomers spontaneously assemble into half-interleaved double-stranded protofibrils via D-E-D interactions (Fig. 1B). Following FpB breakage, the protofibrils aggregate laterally to form the fibrin scaffold of the clot, and the C-terminal part of the Aα-chain separates from the central section (Fig. 1C) [13]. Extravascular deposition of fibrinogen or fibrin, collectively fibrin(ogen), is a powerful proinflammatory signal [4].
Post-translational modifications of fibrinogen can significantly affect its functionality. A systematic review described 11 different modifications of fibrinogen and showed that post-translational modifications of fibrinogen affect clot formation, clot characteristics, and susceptibility to fibrinolysis [14]. Therefore, post-translational modifications of fibrinogen may play an important role in the physiology and pathophysiology of cardiovascular disease.
Fibrinogen and fibrin structures (a)The two external D structural domains of fibrinogen monomers are linked to the core E structural domain by Aα-, Bβ-, and γ-chains, respectively. The C-terminal section of the fibrinogen α-chain begins at residue 220 of the D structural domain, not far from where it emerges from the D structural domain, and ends at Aα610. The fibrinogen Aα-chains each include a FpA sequence, while the Bβ-chains carry a FpB sequence. (b) Through D-E-D interactions, thrombin cleaves FpA from the Aα-chain’s N-terminus, causing a half-interleaved double-stranded protofibril to spontaneously form. (c) The fibrin scaffold of the clot is formed by the protofibrils aggregating laterally after FpB cleaves the α-chain, releasing the C-terminal portion from the central area
Fibrin(ogen) and cardiovascular disease
Globally, coronary heart disease (CHD), heart failure, stroke, and hypertension are the most common causes of death and hospital admission. Cardiovascular diseases account for the majority of these cases. The National Health and Nutrition Examination Survey (NHANES) reports that, with the exception of hypertension, the prevalence of cardiovascular disease in Americans aged 20 and older is 49.2% and 9.3%, respectively [15].
Numerous investigations have demonstrated an association between fibrin(ogen) and cardiovascular disorders (Table 1). Lowe et al. initially showed that D-dimer, the fibrin splitting product, possesses some prognostic significance for coronary risk [16]. Through follow-up, Rudnicka et al. discovered a distinct correlation between fibrinogen and coronary heart disease risk [17]. In addition, individuals with acute coronary syndrome (ACS) were found to have poorer outcomes when fibrin clots that are resistant to lysis were present [18]. Lee et al. also discovered that the severity of platelet fibrin clots was higher in individuals who had experienced an acute myocardial infarction (AMI) [19]. Fibrinogen, D-dimer, and C-reactive protein (CRP) levels were positively and significantly correlated with those of individuals with stable angina. Fibrinogen is particularly important in the process of coronary thrombosis [20,21,22]. It was also discovered that the fibrinogen to albumin ratio is an independent risk predictive factor for length of hospital stay and all-cause death after 90 days and a year in heart failure patients [23]. A high fibrinogen to albumin ratio (> 11.44) raised the risk of short- and long-term unfavorable functional outcomes, including disability and all-cause mortality, in patients with acute ischemic stroke, according to a follow-up research that included 8984 individuals [24]. Furthermore, there was a negative correlation found between the volume of intraplaque hemorrhage and the plaque macrolipid necrosis core volume and fibrinogen [25]. Besides, a high fibrinogen-to-albumin ratio level was associated with an increased risk of stroke recurrence, poor functional outcome, and dependence in patients with acute large artery atherosclerosis stroke [26]. Moreover, a systematic review investigating the association between fibrinogen and post-stroke vascular recurrence showed that, fibrinogen was independently associated with recurrence after stroke, and fibrinogen measurements might be useful to identify patients who are more likely to derive benefit from anti-inflammatory therapies after stroke [27]. Circulating fibrinogen levels were considerably greater in hypertensive stroke patients than in non-stroke patients, according to a follow-up research involving 115 hypertension patients (70 non-stroke patients and 45 stroke patients) [28].
Numerous investigations have confirmed the link between fibrin(ogen) and cardiovascular disease [29]. A small number of investigations, meanwhile, likewise reveal no connection between fibrin(ogen) and cardiovascular illness [30, 31]. A genome-wide association research including more than 100,000 participants found 23 loci linked to fibrinogen and refuted the idea that events related to cardiovascular disease are caused by plasma fibrinogen [32]. In the meanwhile, Keavney et al. concluded that fibrinogen is not related with CHD after finding that genotypes causing lifetime variations in fibrinogen concentrations do not significantly alter the incidence of coronary heart disease [33].
Potential mechanisms of fibrin(ogen) in cardiovascular disease
The association between fibrinogen and cardiovascular disease mainly comes from its role in thrombosis and inflammation.
The role of fibrin(ogen) in thrombosis
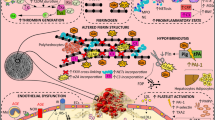
Most cardiovascular illnesses are caused by atherosclerosis [34]. Fibrinogen can influence cardiovascular disease by encouraging the development of atherosclerotic plaque in the following ways: (1) To generate an inflammatory response, induce proinflammatory cytokines (tumor necrosis factor) on monocytes, such as monocytes-α, interleukin-1β, and chemokines on endothelial and fibroblast cells, such as interleukin-8 and monocyte chemotactic protein-1 (MCP-1) [35, 36]; (2) Activating platelets via glycoprotein IIb/IIIa receptor stimulation amplifies inflammation, and activated platelets generate pro-inflammatory cytokines such as interleukin-1β and CD40 ligand [37]; (3) Intercellular adhesion molecule-1 (ICAM-1) binding on endothelial cells increases the expression of ICAM-1, which also binds to white blood cells, macrophages, and platelets [38]; (4) Fibrinogen binds to the endothelium and causes the secretion of vasoactive substances, enhancing endothelial permeability [39]; (5) Fibrinogen deposition can adsorb low-density lipoprotein cholesterol [40, 41]; (6) The buildup of fibrinogen on the vascular wall promotes macrophage infiltration, which is a precursor to foam cells [41]; (7) By mediating the adhesion of neutrophils to activated platelets on the damaged arterial wall [42].
Fibrin(ogen) stimulates the inflammatory response
Leukocyte transendothelial migration (TEM) is mediated by fibrin(ogen)
The term “TEM” describes the biological process by which leukocytes move to tissue organs and sites of inflammation from the vascular endothelium’s endothelial cell spaces. Leukocytes can quickly reach the site of infection or tissue injury thanks to this crucial immune response and inflammatory reaction mechanism. A substantial amount of evidence indicates that fibrinogen can mediate transferrin (TEM) via a number of pathways, potentially contributing to a range of physiopathological processes, including inflammation: (1) Fibrinogen can interact to promote TEM with ICAM-1 [43]. (2) The immobilized fibrinogen interacts with very low density lipoprotein receptors (VLDLR) with high affinity. However, fibrinogen in solution exhibits virtually no interaction with the VLDLR, indicating that the VLDLR-binding site is hidden within fibrinogen and can only be utilised when fibrinogen aggregates, adsorbs to the surface, or is converted to fibrin. Fibrinogen interacts with VLDLR through a pair of fibrin βN structural domains composed of β chains to promote TEM [43]. Notably, Yakovlev S et al. have proved their hypothesis about the dual role of the fibrin βN-domains in leukocyte transmigration and thereby inflammation, but when one of these two functions may prevail remains to be addressed [44]. Additionally, soluble fibrin and fibrin polymers anchored to endothelial cells, as well as the D-D: E1 complex, the main soluble fibrin degradation product released from cross-linked fibrin, and high molecular weight fibrin degradation products, also promote TEM through a VLDLR-dependent pathway [43].
Interactions between leukocyte integrin αMβ2 and fibrin(ogen)
It has been demonstrated that αMβ2 (Mac-1, CD11b/CD18, and CR3) are fibrin(ogen) receptors [45]. Fibrinogen binds to αMβ2 and causes leukocyte adherence to endothelial cells. It also causes peripheral blood mononuclear cells to produce pro-inflammatory cytokines, which in turn regulates the inflammatory response [46]. It has been demonstrated that fibrin(ogen) signaling via αMβ2 activates pro-inflammatory pathways such nuclear factor kappa-B (NF-κB), which causes the local production of inflammatory cytokines like tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) [47,48,49]. Hur et al. discovered that fibrin(ogen) devoid of the αMβ2 binding function significantly protected against obesity and associated conditions like hypercholesterolemia, diabetes mellitus, and non-alcoholic fatty liver disease, as well as decreased the incidence of these conditions [50]. In adenomas, fibrin(ogen) through a unique mechanism depending on the αMβ2 binding motif is engaged in altered inflammatory cell function within adenoma tissues, hence encouraging adenoma formation. Furthermore, it has been demonstrated that the early inflammatory process of adenoma development is promoted by local fibrin-leukocyte contacts mediated through αMβ2 [51]. An article in Science reported that in mice lacking fibrinogen, extravascular fibrin accumulation, gingival inflammation, and loss of periodontal bone were observed; on the other hand, in fibrinogen-deficient mice with concurrent αMβ2 recognition site defects in fibrinogen, periodontal inflammation and bone resorption could be recovered. Both in vitro and in vivo studies demonstrated that local periodontal microbes could stimulate fibrin deposition. Neutrophils gathered around fibrinogen through the action of surface integrin receptors αMβ2, which resulted in inflammatory effects like damage from reactive oxygen species and extracellular neutrophil trapping, ultimately causing periodontal inflammation [52]. Fibrinogen-αMβ2 interaction induces signaling pathways that support microglia activation, including fibronectin-dependent activation of protein kinase B (Akt) and Rho signalling, which increases cytoskeletal rearrangement and phagocytosis and determines the area of demyelination in multiple sclerosis [53]. Dean T et al. showed that cerebral fibrinogen deposits were associated with activated innate immune cells in both human and murine traumatic brain injury (TBI). Genetic elimination of fibrin-CD11b interaction reduced peripheral monocyte recruitment and the activation of inflammatory and reactive oxygen species (ROS) gene pathways in microglia and macrophages after TBI. Blockade of the fibrin-CD11b interaction was also protective from oxidative stress damage and cortical loss after TBI [54].
Interactions between fibrin(ogen) and other receptors
By stimulating various leukocyte subtypes through toll-like receptor 4, natural fibrinogen and fibrinogen derivative fractions create an extremely effective signaling complex that controls both the onset of allergic airway disorders and antifungal responses [55]. Furthermore, fibrinogen stimulates platelets via glycoprotein IIb/IIIa receptors to produce pro-inflammatory cytokines IL-1β and CD40 ligands to enhance inflammation and damage endothelial cells, resulting in the entry of LDL cholesterol and inflammatory cells into the subendothelium, leading to foam cell formation and AS. Fibrinogen also induces pro-inflammatory cytokines such as TNF-α, IL-1β, and chemokines on endothelial cells and fibroblasts such as interleukin-8 (IL-8) and Active Monocyte Chemotactic Protein 1 are secreted to promote inflammatory responses [37]. Other studies have found that the binding of retinogen to its astrocyte receptors induces the release of pro-inflammatory cytokines, leading to oxidative stress and ultimately neuronal death. This may be a mechanism of neurodegeneration and reduced short-term memory during TBI [56].
Other molecular pathways
It has been discovered that fibrinogen, through binding to proteins, may play a role in the development and progression of tumors. For instance, the Aα chain in the C-terminal region of fibrinogen is bound by plasma fibronectin, and the major site of covalent cross-linking between fibronectin Gln 3 and the Aα chain of fibronectin C-terminal region is fibronectin [57]. Through this interaction, fibrin(ogen) molecules are more easily incorporated into the extracellular matrix, where they act as a reservoir, control the use of growth factors, and have an impact on angiogenesis, angiogenic enzymes, cancer cell proliferation, and the inhibition of apoptosis. Furthermore, fibrin(ogen) and other adhesive glycoproteins are deposited into the extracellular matrix, where they serve as scaffolds to facilitate the binding of growth factors to fibrin(ogen), as well as to encourage angiogenesis and cellular responses to adhesion, proliferation, and migration during the growth of tumor cells [58]. Moreover, Sahni et al. found that fibrinogen can promote the growth of lung cancer and prostate cancer cells by interacting with fibroblast growth factor-2 [59]. Lastly, fibrinogen helps shield tumor cells from naturally occurring tumor cytotoxicity by interacting with platelets through β3-integrin, which enables them to elude host immune monitoring [60]. A research team has found that fibrin(ogen) enhances vascular permeability and mediates the occurrence and development of inflammation by inducing Akt activation, promoting microfilament depolymerization [61]. In addition, fibrin(ogen) promotes dysregulation related to colitis and the expression of dioxygenase 2, leading to the production of reactive oxygen species, which contributes to the pathogenesis of colitis and possible tumorigenesis [62]. Petersen MA et al. showed that the fibrinogen activates the bone morphogenetic protein (BMP) signaling pathway in oligodendrocyte progenitor cells and suppresses remyelination, thereby affecting the recovery of various diseases, such as multiple sclerosis, neonatal white matter injury and stroke [63]. The latest research indicates that fibrinogen inhibited neuronal differentiation in subventricular zone and hippocampal neural stem/progenitor cells while promoting astrogenesis via activation of the BMP receptor signaling pathway [64].
Factors affecting fibrin(ogen)
Genetics, age, gender, drugs, alcohol usage, smoking, diet, ozone, and mood can have an impact on fibrin(ogen) (Fig. 2). Fibrinogen levels and their reactivity to environmental stimuli are determined by genetic factors [65, 66]. Pankow et al. demonstrated that the twin approach uses genetics to determine 30–50% of plasma fibrinogen levels [67]. The mean fibrinogen concentration rose with age and was higher in females than in males at all ages, according to a study on the distribution of plasma fibrinogen concentrations in the population [68].
Numerous medications have been demonstrated to impact fibrin(ogen); for instance, fibrates that lower lipid levels can lower fibrinogen levels [69]. It has been demonstrated that oral melatonin lowers fibrinogen levels and inhibits fibrin production in the short term [70, 71]. The pretreatment with acetonitrocoumarol also inhibits the production of soluble fibrin and the fibrin splitting product D-dimer [72]. Oral contraceptives stimulate fibrinolysis [73]. Dexamethasone used for a brief period of time can raise fibrinogen levels [74].
The fibrin clot structure is improved by quitting smoking for at least a year; this results in fewer fibrin fibers in each region of the fibrin structure, wider branching angles, and less dense fibrin fibers [75]. Moderate alcohol intake dramatically and transiently reduces fibrinolysis [76]. The findings of two cross-sectional studies—one with 55 men and the other with 4434 participants aged 25–74 showed that heavy alcohol use raised fibrinogen levels whereas moderate alcohol consumption decreased them [68, 77]. Wang et al. gave rats food and water containing 5% ethanol for four weeks, during which time they monitored the levels of circulating fibrinogen by quantitative immunoassay every week. The findings demonstrated that daily use of modest doses of alcohol resulted in a drop in circulating fibrinogen levels of 18–20% [78]. In three groups of healthy non-alcoholic volunteers, Volpi et al. examined the effects of 500 ml of water (control study), 300 ml (28.4 g ethanol), or 750 ml (71 g ethanol) of table wine on hepatic protein metabolism. They discovered that moderate alcohol consumption decreased fibrinogen levels [79].
Numerous other factors can also impact fibrin(ogen). For instance, ozone can enhance the fibrinolysis process at high temperatures (32.5 °C) while activating it at moderate temperatures (22 °C) [80]. Butyrate-rich diets, like those high in soybeans and oats, alter the blood’s redox state and encourage the breakdown of fibrin [81]. It has also been discovered that there is an independent relationship between the induction of fibrin production and anxiety symptoms [82].
Conclusions
The exploration and advancement of the connection between fibrin(ogen) and cardiovascular disease has been facilitated by the advancement of diverse biotechnological methodologies. It is now acknowledged that fibrin(ogen) is linked to inflammation and plays an important role in many diseases. Furthermore, a wealth of evidence suggests that coagulation and inflammation are closely related processes, and that fibrin(ogen) may play a crucial role in tying together inflammation across the body [4, 8]. Nevertheless, there is a general dearth of medications that specifically target fibrinogen, and it is unclear how certain disorders are linked to fibrin(ogen). New therapeutic targets and medicines targeting fibrin(ogen) may become feasible with a clearer comprehension of the molecular mechanisms behind inflammation and coagulation. Further studies are needed to explore the complex interactions between fibrin(ogen), inflammation-related signaling pathways, and other immune cells in cardiovascular disease.
Data availability
No datasets were generated or analysed during the current study.
References
Pieters M, Wolberg AS. Fibrinogen and fibrin: an illustrated review. Res Pract Thromb Haemost. 2019;3:161–72.
Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020;105:284–96.
Surma S, Banach M. Fibrinogen and atherosclerotic Cardiovascular diseases-review of the literature and clinical studies. Int J Mol Sci 2021; 23.
Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133:511–20.
Tousoulis D, Papageorgiou N, Androulakis E, Briasoulis A, Antoniades C, Stefanadis C. Fibrinogen and cardiovascular disease: genetics and biomarkers. Blood Rev. 2011;25:239–45.
Mensah GA, Fuster V, Murray CJL, Roth GA. Global Burden of Cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. 2023;82:2350–473.
Mensah GA, Fuster V, Roth GA. A heart-healthy and stroke-free world: using data to inform global action. J Am Coll Cardiol. 2023;82:2343–9.
Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62.
Wolberg AS. Fibrinogen and fibrin: synthesis, structure, and function in health and disease. J Thromb Haemost. 2023;21:3005–15.
Dobson DA, Holle LA, Lin FC, Huffman JE, Luyendyk JP, Flick MJ, et al. Novel genetic regulators of fibrinogen synthesis identified by an in vitro experimental platform. J Thromb Haemost. 2023;21:522–33.
Hahn J, Bressler J, Domingo-Relloso A, Chen MH, McCartney DL, Teumer A, et al. DNA methylation analysis is used to identify novel genetic loci associated with circulating fibrinogen levels in blood. J Thromb Haemost. 2023;21:1135–47.
Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904.
Standeven KF, Ariëns RA, Grant PJ. The molecular physiology and pathology of fibrin structure/function. Blood Rev. 2005;19:275–88.
de Vries JJ, Snoek CJM, Rijken DC, de Maat MPM. Effects of post-translational modifications of fibrinogen on clot formation, clot structure, and Fibrinolysis: a systematic review. Arterioscler Thromb Vasc Biol. 2020;40:554–69.
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 update: a Report from the American Heart Association. Circulation. 2022;145:e153–639.
Lowe GD, Rumley A, McMahon AD, Ford I, O’Reilly DS, Packard CJ. Interleukin-6, fibrin D-dimer, and coagulation factors VII and XIIa in prediction of coronary heart disease. Arterioscler Thromb Vasc Biol. 2004;24:1529–34.
Rudnicka AR, Mt-Isa S, Meade TW. Associations of plasma fibrinogen and factor VII clotting activity with coronary heart disease and stroke: prospective cohort study from the screening phase of the thrombosis Prevention Trial. J Thromb Haemost. 2006;4:2405–10.
Sumaya W, Wallentin L, James SK, Siegbahn A, Gabrysch K, Bertilsson M, et al. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: a PLATO substudy. Eur Heart J. 2018;39:1078–85.
Lee SH, Kim HK, Ahn JH, Kang MG, Kim KH, Bae JS, et al. Prognostic impact of hypercoagulability and impaired fibrinolysis in acute myocardial infarction. Eur Heart J. 2023;44:1718–28.
Sagastagoitia JD, Sáez Y, Vacas M, Narváez I, Sáez de Lafuente JP, Molinero E, et al. Association between inflammation, lipid and hemostatic factors in patients with stable angina. Thromb Res. 2007;120:53–9.
Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European concerted action on thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–6.
Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring trends and determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42.
Xu Q, Zhu C, Zhang Q, Hu Z, Ji K, Qian L. Association between fibrinogen-to-albumin ratio and prognosis of patients with heart failure. Eur J Clin Invest. 2023;53:e14049.
Yilong L, Pan Y, Zhang R, Yilong M, Qin H, Meng L, et al. Association between Fibrinogen-to-albumin ratio and adverse stroke outcomes among patients with Acute ischemic stroke. Cerebrovasc Dis; 2023.
van Dijk AC, Donkel SJ, Zadi T, Sonneveld MAH, Schreuder F, Chohan MF, et al. Association between fibrinogen and fibrinogen γ’ and atherosclerotic plaque morphology and composition in symptomatic carotid artery stenosis: Plaque-At-RISK study. Thromb Res. 2019;177:130–5.
Wang Y, Bai L, Li X, Yi F, Hou H. Fibrinogen-to-albumin ratio and clinical outcomes in patients with large artery atherosclerosis stroke. J Am Heart Assoc. 2023;12:e030837.
McCabe JJ, Walsh C, Gorey S, Harris K, Hervella P, Iglesias-Rey R et al. Plasma fibrinogen and risk of vascular recurrence after ischaemic stroke: an individual participant and summary-level data meta-analysis of 11 prospective studies. Eur Stroke J 2024:23969873241246489.
Chekol Abebe E, Mengstie MA, Seid MA, Gebeyehu NA, Adella GA, Kassie GA, et al. Comparison of circulating lipid profiles, D-dimer and fibrinogen levels between hypertensive patients with and without stroke. Metabol Open. 2023;19:100252.
Ten Cate H, Meade T. The Northwick Park Heart Study: evidence from the laboratory. J Thromb Haemost. 2014;12:587–92.
Wang J, Jia L, Li X, Jin S, Li X, Liu F et al. New Insights into the Association between Fibrinogen and Coronary Atherosclerotic Plaque Vulnerability: An Intravascular Optical Coherence Tomography Study. Cardiovasc Ther. 2019; 2019:8563717.
Ward-Caviness CK, de Vries PS, Wiggins KL, Huffman JE, Yanek LR, Bielak LF, et al. Mendelian randomization evaluation of causal effects of fibrinogen on incident coronary heart disease. PLoS ONE. 2019;14:e0216222.
Sabater-Lleal M, Huang J, Chasman D, Naitza S, Dehghan A, Johnson AD, et al. Multiethnic meta-analysis of genome-wide association studies in > 100 000 subjects identifies 23 fibrinogen-associated loci but no strong evidence of a causal association between circulating fibrinogen and cardiovascular disease. Circulation. 2013;128:1310–24.
Keavney B, Danesh J, Parish S, Palmer A, Clark S, Youngman L, et al. Fibrinogen and coronary heart disease: test of causality by ‘Mendelian randomization’. Int J Epidemiol. 2006;35:935–43.
Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, aging, and Cardiovascular Disease: JACC Review topic of the Week. J Am Coll Cardiol. 2022;79:837–47.
Woods A, Brull DJ, Humphries SE, Montgomery HE. Genetics of inflammation and risk of coronary artery disease: the central role of interleukin-6. Eur Heart J. 2000;21:1574–83.
Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–94.
Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–704.
Tsakadze NL, Zhao Z, D’Souza SE. Interactions of intercellular adhesion molecule-1 with fibrinogen. Trends Cardiovasc Med. 2002;12:101–8.
Hicks RC, Golledge J, Mir-Hasseine R, Powell JT. Vasoactive effects of fibrinogen on saphenous vein. Nature. 1996;379:818–20.
Retzinger GS, DeAnglis AP, Patuto SJ. Adsorption of fibrinogen to droplets of liquid hydrophobic phases. Functionality of the bound protein and biological implications. Arterioscler Thromb Vasc Biol. 1998;18:1948–57.
Reinhart WH. Fibrinogen–marker or mediator of vascular disease? Vasc Med. 2003;8:211–6.
Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest. 1997;100:2085–93.
Yakovlev S, Medved L. Effect of fibrinogen, fibrin, and fibrin degradation products on transendothelial migration of leukocytes. Thromb Res. 2018;162:93–100.
Yakovlev S, Medved L. Dual functions of the fibrin βN-domains in the VLDL receptor-dependent pathway of transendothelial migration of leukocytes. Thromb Res. 2022;214:1–7.
Ugarova TP, Yakubenko VP. Recognition of fibrinogen by leukocyte integrins. Ann N Y Acad Sci. 2001;936:368–85.
Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med. 2011;17:568–73.
Fan ST, Edgington TS. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J Immunol. 1993;150:2972–80.
Perez RL, Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154:1879–87.
Perez RL, Ritzenthaler JD, Roman J. Transcriptional regulation of the interleukin-1beta promoter via fibrinogen engagement of the CD18 integrin receptor. Am J Respir Cell Mol Biol. 1999;20:1059–66.
Hur WS, King KC, Patel YN, Nguyen YV, Wei Z, Yang Y, et al. Elimination of fibrin polymer formation or crosslinking, but not fibrinogen deficiency, is protective against diet-induced obesity and associated pathologies. J Thromb Haemost. 2022;20:2873–86.
Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, Harmel-Laws E, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–43.
Silva LM, Doyle AD, Greenwell-Wild T, Dutzan N, Tran CL, Abusleme L, et al. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science. 2021;374:eabl5450.
Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–82.
Dean T, Mendiola AS, Yan Z, Meza-Acevedo R, Cabriga B, Akassoglou K, et al. Fibrin promotes oxidative stress and neuronal loss in traumatic brain injury via innate immune activation. J Neuroinflammation. 2024;21:94.
Landers CT, Tung HY, Knight JM, Madison MC, Wu Y, Zeng Z, et al. Selective cleavage of fibrinogen by diverse proteinases initiates innate allergic and antifungal immunity through CD11b. J Biol Chem. 2019;294:8834–47.
Sulimai N, Brown J, Lominadze D. Fibrinogen Interaction with Astrocyte ICAM-1 and PrP(C) results in the generation of ROS and neuronal death. Int J Mol Sci 2021; 22.
Stathakis NE, Mosesson MW. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977;60:855–65.
Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–25.
Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost. 2008;6:176–83.
Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859–65.
Zhang C, Chen H, He Q, Luo Y, He A, Tao A, et al. Fibrinogen/AKT/Microfilament Axis promotes colitis by enhancing vascular permeability. Cell Mol Gastroenterol Hepatol. 2021;11:683–96.
Sharma B, Kudira R, Rosenfeldt LA, Gourley BE, Cantrell R, Watanabe M et al. Fibrin(ogen) promotes Immune Cell Infiltration, Dysbiosis and ROS production in experimental colitis. Blood 2021; 138.
Petersen MA, Ryu JK, Chang KJ, Etxeberria A, Bardehle S, Mendiola AS, et al. Fibrinogen activates BMP Signaling in Oligodendrocyte Progenitor cells and inhibits remyelination after vascular damage. Neuron. 2017;96:1003–e10127.
Pous L, Deshpande SS, Nath S, Mezey S, Malik SC, Schildge S, et al. Fibrinogen induces neural stem cell differentiation into astrocytes in the subventricular zone via BMP signaling. Nat Commun. 2020;11:630.
Humphries SE, Cook M, Dubowitz M, Stirling Y, Meade TW. Role of genetic variation at the fibrinogen locus in determination of plasma fibrinogen concentrations. Lancet. 1987;1:1452–5.
Hamsten A, Iselius L, de Faire U, Blombäck M. Genetic and cultural inheritance of plasma fibrinogen concentration. Lancet. 1987;2:988–91.
Pankow JS, Folsom AR, Province MA, Rao DC, Williams RR, Eckfeldt J, et al. Segregation analysis of plasminogen activator inhibitor-1 and fibrinogen levels in the NHLBI family heart study. Arterioscler Thromb Vasc Biol. 1998;18:1559–67.
Krobot K, Hense HW, Cremer P, Eberle E, Keil U. Determinants of plasma fibrinogen: relation to body weight, waist-to-hip ratio, smoking, alcohol, age, and sex. Results from the second MONICA Augsburg survey 1989–1990. Arterioscler Thromb. 1992;12:780–8.
de Maat MP. Effects of diet, drugs, and genes on plasma fibrinogen levels. Ann N Y Acad Sci. 2001;936:509–21.
Wirtz PH, Bärtschi C, Spillmann M, Ehlert U, von Känel R. Effect of oral melatonin on the procoagulant response to acute psychosocial stress in healthy men: a randomized placebo-controlled study. J Pineal Res. 2008;44:358–65.
Wirtz PH, Spillmann M, Bärtschi C, Ehlert U, von Känel R. Oral melatonin reduces blood coagulation activity: a placebo-controlled study in healthy young men. J Pineal Res. 2008;44:127–33.
Hollenstein U, Homoncik M, Knöbl P, Pernerstorfer T, Graggaber J, Eichler HG, et al. Acenocoumarol decreases tissue factor-dependent coagulation during systemic inflammation in humans. Clin Pharmacol Ther. 2002;71:368–74.
Sidelmann JJ, Kluft C, Krug AH, Winkler U, Jespersen J, Gram JB. Fibrin clot structure - pro-fibrinolytic effect of oral contraceptives in apparently healthy women. Thromb Haemost. 2017;117:700–5.
Brotman DJ, Girod JP, Posch A, Jani JT, Patel JV, Gupta M, et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res. 2006;118:247–52.
Stępień E, Miszalski-Jamka T, Kapusta P, Tylko G, Pasowicz M. Beneficial effect of cigarette smoking cessation on fibrin clot properties. J Thromb Thrombolysis. 2011;32:177–82.
Pieters M, Vorster HH, Jerling JC, Venter CS, Kotze RC, Bornman E, et al. The effect of ethanol and its metabolism on fibrinolysis. Thromb Haemost. 2010;104:724–33.
Dimmitt SB, Rakic V, Puddey IB, Baker R, Oostryck R, Adams MJ, et al. The effects of alcohol on coagulation and fibrinolytic factors: a controlled trial. Blood Coagul Fibrinolysis. 1998;9:39–45.
Wang Z, Barker TH, Fuller GM. Alcohol at moderate levels decreases fibrinogen expression in vivo and in vitro. Alcohol Clin Exp Res. 1999;23:1927–32.
Volpi E, Lucidi P, Cruciani G, Monacchia F, Santoni S, Reboldi G, et al. Moderate and large doses of ethanol differentially affect hepatic protein metabolism in humans. J Nutr. 1998;128:198–203.
Kahle JJ, Neas LM, Devlin RB, Case MW, Schmitt MT, Madden MC, et al. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: a crossover study of healthy young volunteers. Environ Health Perspect. 2015;123:310–6.
Emmi G, Bettiol A, Niccolai E, Ramazzotti M, Amedei A, Pagliai G, et al. Butyrate-Rich diets improve redox status and fibrin lysis in Behçet’s syndrome. Circ Res. 2021;128:278–80.
von Känel R, Dimsdale JE, Adler KA, Patterson TL, Mills PJ, Grant I. Effects of depressive symptoms and anxiety on hemostatic responses to acute mental stress and recovery in the elderly. Psychiatry Res. 2004;126:253–64.
Acknowledgements
This work was sponsored by National Natural Science Foundation of China (No. NSFC 81700978), Natural Science Foundation of Shanghai (No. 22S11902800, 22ZR1467300), Shanghai Municipal Health Commission’s Clinical Research Project (202340064), Fundamental Research Funds for the Central Universities(22120240308).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HL and SZ wrote the manuscript; YX helped perform constructive discussions; XYan reviewed and edited the manuscript. All authors gave their approval for the final manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by John Di Battista.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, H., Zhao, S., Xiong, Y. et al. The emerging role of fibrin(ogen) in cardiovascular disease. Inflamm. Res. 73, 1435–1444 (2024). https://doi.org/10.1007/s00011-024-01916-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01916-2