Abstract
Background
The role of estrogen receptor β (ERβ) in the pathogenesis and development of breast cancer (BC) is controversial, and it is currently considered to play contradictory roles in different phenotypes. ERβ2 is thought to promote the BC process, but its role in triple-negative breast cancer (TNBC) has not been reported.
Methods
In this study, we collected tumor tissues from 15 patients with TNBC and obtained a variety of TNBC cell lines as research objects. The plasmid vectors and RNA interference techniques were used to change the level of target genes in cells, quantitative PCR and Western Blots were used to detect gene expression levels, CCK-8 and EdU assay were used to detect cell growth, and Transwell was used to detect cell migration and invasion. Dual-luciferase gene reports and RNA immunoprecipitation (RIP) were used to verify gene targeting relationships.
Results
ERβ2 was up-regulated in TNBC tissues and promoted the growth, migration, and invasion of TNBC cells. ERβ2 regulated hsa_circ_0000732 expression by binding to SCARF1 promoter. Knockdown of hsa_circ_0000732 inhibited TNBC cell proliferation, migration, and invasion by upregulating miR-1184.
Conclusion
Our present study found that ERβ2 is upregulated in some TNBC cells and promotes TNBC cell growth, migration and invasion by regulating hsa_circ_0000732 targeting miR-1184. The special role of ERβ2 in TNBC may be the breakthrough of a targeted treatment strategy for TNBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the most common cancers in women and a serious global health problem. It can be considered a heterogeneous group of tumors, including various diseases in clinical morphology and molecular aspects [1]. A unique subtype of BC tumor accounts for approximately 15–20% of all BCs and more than 50% of breast cancer mortality [2, 3]. It is triple-negative breast cancer (TNBC). TNBC lacks the expression of three important receptors: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2). Compared with other types of breast cancer, TNBC has a more aggressive clinical course and is usually diagnosed at a higher grade, often involving lymph nodes [4, 5]. Because TNBC patients do not benefit from targeted therapies for ER (such as tamoxifen) or HER-2 (such as trastuzumab), they have a poor prognosis and a higher rate of distal recurrence than women with other BC subtypes [4]. Therefore, it is a major clinical challenge to attempt to find new potential therapeutic targets and further research to improve the prognosis of this subtype.
Estrogen receptor α (ERα) is a major driver of breast cancer, and its role and the role of target genes have been widely studied. ERα and ERα regulatory genes are the main therapeutic targets for controlling hormone-responsive breast cancer [6]. The function and expression pattern of ERβ is different from that of ERα. ERβ is widely expressed in normal and neoplastic human breast tissues [7]. The exact role of ERβ in breast cancer is controversial ERβ. On one hand, studies have shown that ERβ exists in some patients with TNBC, and its expression is associated with improved prognosis. The fact that ERβ plays a tumor-suppressive role in TNBC cell models in vitro supports this view [8]. Transient transfection of ERβ in the MDA-MB 453 TNBC cell line significantly inhibited cell proliferation and metastasis potential and induced cell apoptosis [9]. On the other hand, we suggest that ERβ is associated with reduced TNBC relapse-free survival, and activation of ERβ may increase the migration and invasion capacity of TNBC cells [10].
In addition to full-length ERβ (ERβ1), there are four other spliced ERβ isoforms: ERβ2/cx, ERβ3, ERβ4, and ERβ5. Some scholars believe that the contradictory role of ERβ in BC is due to the different roles of different subtypes of ERβ [11]. In BC, after ERβ1, the most well-studied subtype is ERβ2, which mediates proteasome-dependent degradation of ERα [12], whose expression is associated with aggressive characteristics and malignant phenotypes [13]. In TNBC patients, high levels of ERβ2 are associated with early tumor recurrence [14]. Similar behavior of this subtype has been observed in TNBC cell lines, where ERβ2 and ERβ4-5 subtypes enhance hypoxia signaling that is associated with tumor aggressiveness [15].
In this study, we focused on verifying the role of ERβ2 in the pathogenesis and development of TNBC and exploring the internal mechanism of its influence on the growth and metastasis of TNBC. Studies have shown that ERβ can bind to the gene symbol promoter of circular RNA to regulate the expression level of circRNA, thus regulating the role of ERβ in transparent renal cell carcinoma [16]. Therefore, this study also started with circRNA, which ERβ2 may act on, to explore its influence on TNBC and its molecular mechanism.
Materials and methods
TNBC tissues collection
Human TNBC tissue samples (n = 15) and non-tumor tissues (n = 15) were collected from Beijing Anzhen Hospital, Capital Medical University after obtaining approval from the Institutional Human Ethics Committee. The samples were collected from patients who underwent primary surgery without prior chemotherapy or radiotherapy. Informed consent was taken from all the patients’ prior surgery/excision of tissue.
Cell culture
TNBC cells (BT-549, SUM-159, MDA-MB-231, MDA-MB-453, and MDA-MB-468) and normal breast cells (MCF-10A) were purchased from the Cell Culture Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were all cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific). Cells were cultured with 5% CO2 at 37 °C.
qRT-PCR
Tissues and cells were lysed via Trizol reagent (Invitrogen), and then total RNA was extracted and reverse transcribed into cDNA via a Reverse Transcription Kit (Invitrogen). Then, RT-qPCR was conducted through SYBR Green RT-PCR Kit (Invitrogen) on an Applied Biosystems 7300 (Thermo Fisher Scientific). The cycling conditions were a denaturation step of 94 °C for 10 min, 40 cycles of 94 °C for 45 s, 55 °C for 60 s, and 72 °C for 45 s, and a final extension step of 72 °C for 10 min. Results were calculated via the 2−ΔΔCt method. GAPDH and U6 level were measured as the internal control. The primer sequences used are shown in Table 1.
Cell transfection
The si-ERβ2 and si-circ_0000732, together with the negative control siRNA (si-NC), were obtained from Genepharma (Shanghai, China) and transfected into TNBC cells as per the protocol of Lipofectamine 2000 (Invitrogen, USA). pcDNA3.1 plasmids were inserted with the sequences of ERβ2. The oligonucleotide against miR-1184 (miR-1184 inhibitor) and control oligonucleotide (NC inhibitor) were designed and synthesized at Genepharma (Shanghai, China). After cells were grown to 50–80%, cells were transfected with the aforementioned plasmids or their controls, respectively.
CCK-8 assay
CCK-8 assay was performed to evaluate cell viability. TNBC cell lines were seeded onto 96-well plates at a density of 2 × 103 cells/well, cultured for 1, 2, 3, and 4 days, respectively. At the time points indicated above, 90 μL DMEM and 10 μL CCK-8 were added to each well and incubated for an additional 50 min. Then, the media supernatant was removed and the absorbance at 450 nm was measured using a microplate reader (Thermo Fisher Scientific, USA). Wells containing 10% CCK-8 (90 μL DMEM and 10 μL CCK-8) were regarded as blanks. The experiment was repeated three times, with four repeated measures of each experimental value.
EdU assay
EdU labeling medium was added to cell culture with EdU labeling kit (RiboBio, Guangzhou, China). TNBC cells after transfection were incubated for 2 h and then fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA). Cells were washed at room temperature by PBS (Sigma-Aldrich) dyed with an anti-EdU (Abcam, Cambridge, USA) working solution (Invitrogen). Under the same conditions, cells were again washed with Triton X-100 (Solarbio, Shanghai, China) in PBS (Sigma-Aldrich). Cells were observed via fluorescent microscopy (Leica, Wetzlar, Germany).
Transwell assay
The re-suspended cells in a serum-free medium were seeded into the upper chamber, and the basolateral chamber was filled with 10% PBS. Transwell chambers pre-coated with or without Matrigel (Millipore, Massachusetts, USA) were employed to carry out the invasion or migration assay. An inverted microscope (Carl Zeiss, Jena, Germany) was applied to observe the migrated or invaded cells.
Luciferase assay
Partial DNA sequences of SCARF1 promoter containing wild-type (WT) or mutant (MUT) circ_0000732 binding site 1/2 were amplified by PCR and then cloned into a luciferase plasmid (Promega, Madison, WI, USA) to produce site1-WT, site1-MUT, site2-WT, and site2-MUT reporter plasmids, which were separately co-transfected with pcDNA-ERβ2 or si-ERβ2 into TNBC cells. Finally, luciferase activities were evaluated through the dual-luciferase reporter assay system (Promega). Moreover, the binding of circ_0000732 and miR-1184 was also detected using a luciferase reporter assay. The sequences of circ_0000732 containing WT or MUT miR-1184 binding site were amplified by PCR and then cloned into the luciferase plasmids, which were separately co-transfected with miR-1184 mimic into TNBC cells.
RNA immunoprecipitation (RIP) assay
The Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA) was employed to implement RIP assay in TNBC cells. Cultured cells from RIP lysis buffer were collected and cultivated with beads and antibodies of interest with rotation for 30 min. Normal Rabbit IgG was seen as a control. After digestion, precipitated RNAs were isolated and tested by qRT-PCR.
Western blot
An equal amount of proteins were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked in 5% bovine serum albumin (Amresco, USA) with shaking for 1 h at room temperature, and then washed in 1X Tris-buffered saline-Tween-20 (TBST) buffer thrice for 5 min each. Membranes were incubated overnight at 4 °C with primary antibodies. The following antibodies purchased from Abcam and Bio-Rad were used: anti-ERβ2 (MCA4676, Bio-Rad), anti-MMP-2(ab92536), anti-MMP-9(ab76003), anti-TIMP-1(ab211926), anti-TIMP-2(ab230511) and anti-β-actin(ab8226). Then, membranes were washed thrice for 5 min each with 1X TBST and incubated with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG secondary antibodies (Cell Signaling Technologies, Danvers, MA, USA) for 1 h at room temperature. Membranes were washed thrice for 5 min each with 1X TBST, and antigen–antibody complexes were visualized with a chemiluminescent ECL detection system (Pierce, Rockford, IL, USA). Blots were scanned and analyzed via Image J software.
Statistical analysis
Statistical analysis was performed using the SPSS v. 21. A Student’s t test was used for continuous variables using GraphPad Prism v. 6.0. All results are presented as means ± standard deviation of the means. The correlation of gene expression level was analyzed by Pearson. A P < 0.05 was considered significant for all statistical tests.
Results
ERβ2 was up-regulated in TNBC and promoted the growth, migration, and invasion of TNBC cells
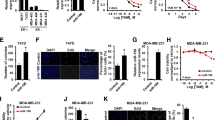
We collected the TNBC tissues and non-tumor control tissues of 15 patients, detected the mRNA expression level of ERβ2 in the tissues by qPCR, and found that the level of ERβ2 was significantly increased in TNBC tissues (P < 0.001, Fig. 1A). Detection of ERβ2 protein levels also showed the same results, and ERβ2 protein levels were significantly increased in TNBC tissues (P < 0.001, Fig. 1B). We also detected the expression levels of ERβ2 in various TNBC cells and found that the mRNA and protein levels of ERβ2 in MDA-MB-231, MDA-MB-453, and MDA-MB-468 cells were significantly higher than those in normal mammary gland cells MCF-10A (P < 0.001, Fig. 1C, D). However, the mRNA and protein levels of ERβ2 in BT-549 and SUM-159 cells were not significantly different from those in MCF-10A cells (P > 0.05, Fig. 1C, D).
ERβ2 was up-regulated in TNBC and promoted the growth, migration, and invasion of TNBC cells. A ERβ2 mRNA expression was elevated in TNBC tissues; B ERβ2 protein expression was elevated in TNBC tissues; C ERβ2 mRNA expression was significantly increased in TNBC cell line MDA-MB-231, MDA-MB-453 and MDA-MB-468; D ERβ2 protein expression was significantly increased in TNBC cell line MDA-MB-231, MDA-MB-453 and MDA-MB-468; E SiRNA was transfected into MDA-MB-231 cells to effectively knockdown ERβ2 expression; F knockdown of ERβ2 significantly inhibited the activity of TNBC cells; G Knockdown of ERβ2 significantly inhibited TNBC cell proliferation; H knockdown of ERβ2 significantly inhibited the mRNA expression of proliferation-related factors, CyclinD1 and PCNA, in TNBC cells; I knockdown of ERβ2 significantly inhibited TNBC cell migration and invasion; J knockdown of ERβ2 significantly inhibited the mRNA expression of MMP-2 and MMP-9, and promoted the mRNA expression of TIMP-1 and TIMP-2 in TNBC cells. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with non-tumor in A and B; compared with MCF-10A in C and D; compared with si-NC in E–K
Afterward, we tried to use si-ERβ2 to knock down the expression level of ERβ2. As shown in Fig. 1E, si-ERβ2-1 effectively knocked down the expression of ERβ2 in MDA-MB-231 cells, and si-ERβ2-1 was used for subsequent experiments. Using CCK-8 to detect the activity of MDA-MB-231 and MDA-MB-468 cells, it was found that knockdown of ERβ2 significantly inhibited the viability of the two TNBC cells (P < 0.001, Fig. 1F). EdU was used to detect the cell proliferation and it was found that knockdown of ERβ2 significantly inhibited the proliferation of both MDA-MB-231 and MDA-MB-468 cells (P < 0.001, Fig. 1G). We also detected the mRNA expression levels of proliferation-related factors CyclinD1 and proliferating cell nuclear antigen (PCNA) and found that knock down of ERβ2 significantly inhibited the expression levels of CyclinD1 and PCNA in both MDA-MB-231 and MDA-MB-468 cells (P < 0.001, Fig. 1H). These results indicated that knockdown of ERβ2 significantly inhibited the growth of TNBC cells, suggesting that ERβ2 promoted the growth of TNBC.
In addition, Transwell was used to detect the effects of ERβ2 knockdown on TNBC cell migration and invasion. In both MDA-MB-231 and MDA-MB-468 cells, knockdown of ERβ2 significantly inhibited cell migration and invasion epithelial-mesenchymal transformation (EMT) is the basis of cell migration and invasion (P < 0.001, Fig. 1I, J). In that case, we detected the expression of EMT-related genes in TNBC cells. In both MDA-MB-231 and MDA-MB-468 cells, ERβ2 knockdown significantly reduced the expression of MMP-2 and MMP-9 and significantly increased the expression of TIMP-1 and TIMP-2 (P < 0.001, Fig. 1K). These results suggest that ERβ2 can promote the metastasis of TNBC.
ERβ2 promoted TNBC cell proliferation, migration, and invasion by upregulation of hsa_circ_0000732
Previous studies have shown that ERβ plays a role in the tumor by regulating circRNAs. We further explored the mechanism of ERβ2 in TNBC. The circRNAs which expression up-regulated in breast cancer were screened out on circAltas website (http://circatlas.biols.ac.cn/): hsa_circ_0008717, hsa_circ_0004214, hsa_circ_0005239, hsa_circ_0001821, hsa_circ_0001175, hsa_circ_0001667, hsa_circ_0000732, hsa_circ_0001313, hsa_circ_0086241, hsa_circ_0001875, hsa_circ_0002113 and hsa_circ_0002874. After ERβ2 was knocked down in MDA-MB-231 and MDA-MB-468 cells, qPCR was used to detect the expression of the above circRNAs. It was found that hsa_circ_0000732 was significantly down-regulated in both MDA-MB-231 (P < 0.001, Fig. 2A) and MDA-MB-468 cells (P < 0.01, Fig. 2B). Subsequently, we detected the expression level of hsa_circ_0000732 in TNBC tissues and found that the expression level of hsa_circ_0000732 in TNBC tumor tissues was significantly up-regulated compared with non-tumor tissues (P < 0.001, Fig. 2C). It is noteworthy that in TNBC tissues, the expression level of ERβ2 is positively correlated with hsa_circ_0000732 (P < 0.01, Fig. 2D).
ERβ2 promoted TNBC cell activity and proliferation by upregulation of hsa_circ_0000732. A When ERβ2 was knocked down in MDA-MB-231 cells, hsa_circ_0000732 and hsa_circ_0002874 expressions were significantly down-regulated; B when ERβ2 was knocked down in MDA-MB-468 cells, hsa_circ_0001667, hsa_circ_0000732 and hsa_circ_0002874 expressions were significantly down-regulated; C the expression of hsa_circ_0000732 was elevated in TNBC tissues; D ERβ2 was positively associated with hsa_circ_0000732 expression in TNBC tissues; E ERβ2 overexpressed plasmid can effectively increase the mRNA expression level of ERβ2 in BT-549 and SUM-159 cells; F the expression level of hsa_circ_0000732 in BT-549 and SUM-159 cells was not significantly different from that in MCF-10A cells; G Si-circ transfection effectively knocked down the expression level of hsa_circ_0000732 in BT-549 and SUM-159 cells; H Hsa_circ_0000732 expression was significantly increased in BT-549 and SUM-159 cells overexpressing ERβ2, and hsa_circ_0000732 expression was cancelled by si-circ transfection; I ERβ2 promoted TNBC cell viability by up-regulating hsa_circ_0000732; J ERβ2 promoted TNBC cell proliferation by up-regulating hsa_circ_0000732; K ERβ2 promoted the expression of proliferation-related factors, CyclinD1 and PCNA, by up-regulating hsa_circ_0000732. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with si-NC in A and B; compared with non-tumor in C; compared with pcDNA3.1 in E, compared with MCF-10A in F, compared with Ctrl in G–K; ##P < 0.01 and ###P < 0.001 compared with ERβ2 + si-NC in G–K
The expression level of hsa_circ_0000732 was detected in TNBC cell lines and hsa_circ_0000732 was significantly increased in MDA-MB-231 and MDA-MB-468 cells (P < 0.001, Fig. 2E). However, the expression of ERβ2 and hsa_circ_0000732 did not change significantly in BT-549 and SUM-159 cells (P > 0.05, Figs. 1C and 2E), so these two kinds of cells were used in the gain-of-function experiment. As shown in Fig. 2F, G, ERβ2 overexpression plasmid and si-circ were used to effectively overexpress ERβ2 and knockdown hsa_circ_0000732 in BT-549 and SUM-159 cells (P < 0.001). We were pleasantly surprised to find that overexpression of ERβ2 significantly increased the expression level of hsa_circ_0000732 in both BT-549 and SUM-159 cells (P < 0.001, Fig. 2H), suggesting positive regulation of ERβ2 on hsa_circ_0000732.
By detecting the viability and proliferation of BT-549 and SUM-159 cells, it was found that knockdown of hsa_circ_0000732 could significantly inhibit the cell viability and proliferation promoted by overexpression of ERβ2 (P < 0.001, Fig. 2I, J). Knockdown of hsa_circ_0000732 also significantly reduced the expression levels of CyclinD1 and PCNA, which increased by overexpressed ERβ2, in both BT-549 and SUM-159 cells (P < 0.001, Fig. 2K). The results suggest that ERβ2 promotes TNBC cell growth by regulating hsa_circ_0000732.
Transwell was used to detect the migration and invasion of BT-549 and SUM-159 cells. It was found that knocking down hsa_circ_0000732 could significantly inhibit the cell migration and invasion accelerated by overexpression of ERβ2 (P < 0.001, Fig. 3A, B). At the same time, we detected the expression levels of EMT-related factors in both BT-549 and SUM-159 cells. Compared with overexpression of ERβ2, overexpression of ERβ2 superposition knockdown hsa_circ_0000732 can significantly reduce the mRNA and protein expression levels of MMP-2 and MMP-9, and significantly increase the mRNA and protein expression levels of TIMP-1 and TIMP-2 (P < 0.001, Fig. 3C, D). These results suggest that ERβ2 promotes TNBC cell migration and invasion by regulating hsa_circ_0000732.
ERβ2 promoted TNBC cell migration and invasion by upregulation of hsa_circ_0000732. A ERβ2 promoted TNBC cell migration by up-regulating hsa_circ_0000732; B ERβ2 promoted TNBC cell invasion by up-regulating hsa_circ_0000732; C ERβ2 promoted the mRNA expression of migration and invasion related factors by up-regulating hsa_circ_0000732; D ERβ2 promoted the protein expression of migration and invasion related factors by up-regulating hsa_circ_0000732; ***P < 0.001 compared with Ctrl, ###P < 0.001 compared with ERβ2 + si-NC
ERβ2 regulated hsa_circ_0000732 expression by binding to SCARF1 promoter
To further verify the mechanism that ERβ2 regulates hsa_circ_0000732 and participates in the malignant progression of TNBC cells, we used JASPAR to predict the binding sites of ERβ2 and hsa_circ_0000732. As shown in Fig. 4A, there are two binding sites (− 659 to − 645 and − 436 to − 422) between ERβ2 and the promoter of gene symbol SCARF1 of hsa_circ_0000732. Then, we used dual-luciferase gene reporter assay to verify these two binding sites in BT-549 and MDA-MB-231 cells, respectively. Results as shown in Fig. 4B–E, ERβ2 binds to hsa_circ_0000732 at these two sites in both BT-549 and MDA-MB-231 cells (P < 0.001). Thus, we confirm that ERβ2 promotes the malignant proliferation and metastasis of TNBC by regulating hsa_circ_0000732.
ERβ2 regulated hsa_circ_0000732 expression by binding to SCARF1 promoter. A JASPAR predicted that there were two binding sites between ERβ2 and the promoter of gene symbol SCARF1 of hsa_circ_0000732; B the overexpression of ERβ2 in BT549 cells confirmed the binding of ERβ2 to hsa_circ_0000732 at site 1; C the overexpression of ERβ2 in MDA-MB-231 cells confirmed the binding of ERβ2 to hsa_circ_0000732 at site 1; D the overexpression of ERβ2 in BT549 cells confirmed the binding of ERβ2 to hsa_circ_0000732 at site 2; E the overexpression of ERβ2 in MDA-MB-231 cells confirmed the binding of ERβ2 to hsa_circ_0000732 at site 2. ***P < 0.001 compared with pcDNA3.1 in B and D, compared with si-NC in C and E
Hsa_circ_0000732 targeted regulation of miR-1184 in TNBC cells
CircRNA usually plays a role by sponging with miRNA. We further explored the downstream regulatory factors of hsa_circ_0000732. The miRNAs targeted by hsa_circ_0000732 were predicted by the Circular RNA Interactome and Circbank database, and there were five overlapped miRNAs: miR-1184, miR-361-3p, miR-663b, miR-885-3p, and miR-885-5p (Fig. 5A). When we’re knocked down of hsa_circ_0000732 in the MDA-MB-231 cells, the levels of three miRNA, miR-1184, miR-361-3p, and miR-663b, were significantly increased, among which miR-1184 was the most elevated (P < 0.001, Fig. 5B). In TNBC tissues, the expression level of miR-1184 was significantly decreased (P < 0.001, Fig. 5C), and was significantly correlated with the level of hsa_circ_0000732 (P < 0.01, Fig. 5D). In MDA-MB-231 and MDA-MB-468 cells, the expression level of miR-1184 was significantly lower than that in MCF-10A (P < 0.001, Fig. 5E). Subsequently, we verified the binding sites of miR-1184 and hsa_circ_0000732 in MDA-MB-231 and MDA-MB-468 cells using dual luciferase and RIP experiments, respectively. The results showed that there was a binding relationship between miR-1184 and hsa_circ_0000732 and that miR-1184 and hsa_circ_0000732 were enriched in compounds deposited with anti-AGO2 antibodies (P < 0.001, Fig. 5F–H). In addition, the expression level of hsa_circ_0000732 was significantly increased and the expression level of miR-1184 was significantly decreased after transfection with a miR-1184 inhibitor in both MDA-MB-231 and MDA-MB-468 cells (P < 0.001, Fig. 5I).
Hsa_circ_0000732 targeted regulation of miR-1184 in TNBC cells. A The miRNAs targeted by hsa_cira_0000732 were predicted by Circular RNA Interactome and Circbank database, and there were five overlapped miRNAs; B the expression levels of miR-1184 and miR-663b were significantly increased in MDA-MB-231 cells with hsa_circ_0000732 knockdown, *P < 0.05 and ***P < 0.001 compared with si-NC; C the expression of miR-1184 was significantly decreased in TNBC tissues, ***P < 0.001 compared with non-tumor; D the expression of miR-1184 was negatively correlated with hsa_circ_0000732 in TNBC tissues, P < 0.01; E MiR-1184 was low expressed in TNBC cells MDA-MB-231 and MDA-MB-468, ***P < 0.001 compared with MCF-10A; F schematic diagram of binding sites of hsa_circ_0000732 and miR-1184; G the results of dual luciferase assay showed that there was a binding relationship between hsa_circ_0000732 and miR-1184, ***P < 0.001 compared with mimic NC; H RIP results showed that hsa_circ_0000732 and miR-1184 were enriched in compounds precipitated by anti-AGO2 antibodies, ***P < 0.001 compared with IgG; I the expression level of hsa_circ_0000732 was significantly increased in TNBC cells with miR-1184 knockdown, ***P < 0.001 compared with miR-NC inhibitor
Knockdown of hsa_circ_0000732 inhibited TNBC cell proliferation, migration, and invasion by up-regulating miR-1184
Hsa_circ_0000732 was knocked down by transfection with si-circ in MDA-MB-231 and MDA-MB-468 cells significantly increased the expression level of miR-1184 (P < 0.001, Fig. 6A). In MDA-MB-231 and MDA-MB-468 cells co-transfected with si-circ and miR-1184 inhibitor, inhibition of miR-1184 significantly improved cell viability, proliferation, CyclinD1, and PCNA level induced by knockdown of hsa_circ_0000732 (P < 0.05 or P < 0.001, Fig. 6B–D). The results suggested that hsa_circ_0000732 promoted the viability and proliferation of TNBC cells by inhibiting miR-1184.
Knockdown of hsa_circ_0000732 inhibited TNBC cell viability and proliferation by up-regulating miR-1184. A Knockdown of hsa_circ_0000732 by si-circ significantly increased the level of miR-1184 in TNBC cells, and miR-1184 inhibitor transfection effectively reduced the level of miR-1184; B knockdown of hsa_circ_0000732 inhibited TNBC cell activity by upregulating miR-1184; C knockdown of hsa_circ_0000732 inhibited TNBC cell proliferation by upregulating miR-1184; D knockdown of hsa_circ_0000732 inhibited the expression of proliferation-related factors, CyclinD1 and PCNA, by upregulating miR-1184, ***P < 0.001 compared with si-NC, #P < 0.05 and ###P < 0.001 compared with si-circ + miR-NC inhibitor
Inhibition of miR-1184 significantly increased the reduction of cell migration and invasion induced by knockdown of hsa_circ_0000732 in both MDA-MB-231 and MDA-MB-468 cells (P < 0.05 or P < 0.01 or P < 0.001, Fig. 7A, B). Moreover, inhibition of miR-1184 could counteract the changes in mRNA and protein expression levels of EMT-related genes by knockdown of hsa_circ_0000732 (P < 0.01 or P < 0.001, Fig. 7C, D). The results suggested that hsa_circ_0000732 promoted the migration and invasion of TNBC cells by inhibiting miR-1184.
Knockdown of hsa_circ_0000732 inhibited TNBC cell migration and invasion by up-regulating miR-1184. A Knockdown of hsa_circ_0000732 inhibited TNBC cell migration by up-regulating miR-1184; B knockdown of hsa_circ_0000732 inhibited TNBC cell invasion by up-regulating miR-1184; C knockdown of hsa_circ_0000732 inhibited the mRNA expression of migration and invasion related factors by up-regulating miR-1184; D knockdown of hsa_circ_0000732 inhibited the protein expression of migration and invasion-related factors by up-regulating miR-1184; ***P < 0.001 compared with si-NC, #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with si-circ + miR-NC inhibitor
Discussion
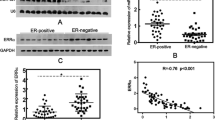
TNBC has more aggressive behavior and worse disease-specific outcomes than ERα-positive BC. Although TNBC accounts for only about 15% of all BC cases, it accounts for more than 50% of mortality [2, 3]. Because TNBC is more aggressive, early and accurate diagnosis can choose a more appropriate to determine the prognosis and treatment methods for more time and opportunity Because of the lack of target receptors, patients with TNBC does not apply to hormone or by bead sheet resistance to treatment, based on the current main use of treatment is used alone or a combination of surgery and cytotoxic chemotherapy [17, 18]. Various reports have shown that ERβ is expressed in TNBC, accounting for 15–30% of tissue samples [19,20,21]. Few studies have described the nature and role of ERβ in TNBC, but available data suggest that its role may be different from that observed in the presence of ERα [22]. Direct expression of multiple ERβ subtypes at both RNA and protein levels has been reported in BC [23].
Following the discovery of different subtypes of ERβ, the initial studies focused on differences in ERβ2 expression levels in different types of BC. Park et al. believed that the reduction of ERβ2 expression was a key event in the development of breast cancer [24]. ERβ2 expression levels were significantly reduced in tumors, especially in tumors with ERα expression, compared with corresponding normal tissues. Zhao et al. found that ERβ2 negatively regulates ERα-mediated effects related to breast cancer development, including hormonal responses [25], which may be one of the reasons for its influence on breast cancer development. The relationship between ERβ2 expression level and prognosis of BC has also been reported by more scholars. In patients with late-onset breast cancer, ERβ2 expression levels are specifically associated with tumor size, and higher ERβ2 levels are associated with a better prognosis than ERβ1 subtypes [26]. Nuclear ERβ2 is significantly correlated with disease-free survival (DFS) and overall survival (OS) and can predict the response to endocrine therapy [27]. Clinical studies suggested that ERβ2 might be an independent prognostic marker and therapeutic target for BC [28]. The study evaluated ERβ1 and ERβ2 expression in 101 BC patients and found that ERβ2 expression rate was 50.5% (51/101) in primary breast cancer tissues. DFS and OS were significantly worse in patients whose tumors expressed ERβ2, and ERβ2 expression was significantly associated with shorter DFS in patients treated with tamoxifen [28]. There are also some reports on the role of ERβ2 in TNBC, which ERα does not express. ERβ2 and ERβ5 increase aggressiveness of TNBC by regulation of hypoxic signaling [29]. ERβ2 is the main endogenous form of TNBC tumors and cell lines, and a high ERβ2 level predicts poor clinical prognosis. Endogenous ERβ2 can promote cell proliferation, migration and invasion, and expression of surviving [30]. Bialesova et al. also found that estrogen receptor β2 induced TNBC cell proliferation and invasion is related to the regulation of PHD3 and HIF-1α [31]. Our study found that ERβ2 was up-regulated in TNBC and promoted the growth, migration, and invasion of TNBC cells, which was consistent with previous studies.
In a study of the circRNA profile of breast cancer brain metastasis, hsa_circ_0000732 was identified to increase brain metastatic breast cancer cell line 231-BR by RNA-seq compared with its parental involvement nonspecific metastatic cell line MDA-MB-231 [32]. Our study found that hsa_circ_0000732 was up-regulated in TNBC tissues and positively correlated with ERβ2 level. Using RNA interference and dual-luciferase gene reports, we identified the targeting relationship between ERβ2 and hsa_circ_0000732 and confirmed that ERβ2 positively regulates hsa_circ_0000732 to promote TNBC cell growth, migration, and invasion. There are few reports about hsa_circ_0000732, and this study is the first to find the mechanism of its involvement in BC development.
CircRNAs usually play a regulatory role through sponge miRNAs, and we predicted and verified that hsa_circ_0000732 promotes cell growth and metastasis through sponge miR-1184 in TNBC cells. MiR-1184 has been reported as a tumor suppressor in a variety of cancers, such as miR-1184 regulates the proliferation and apoptosis of colon cancer cells by targeting CSNK2A1 [33]. Multiple reports have confirmed that multiple circRNAs are involved in tumor development through sponge miR-1184 in bladder cancer: circBC048201 inhibits the proliferation, migration, and invasion of bladder cancer cells by miR-1184/ITGA3 axis [34]; silencing circRNA VANGL1 inhibits bladder cancer progression by regulating the miR-1184/IGFBP2 axis [35]; circCA12 promotes malignant processes in bladder cancer through sponge miR-1184 and upregulated RAS family [36]. Besides, hsa_circ_0128846 promotes tumorigenesis of colorectal cancer by sponging miR-1184 and releasing AJUBA, and inactivating Hippo/YAP signaling [37]. These studies have demonstrated that miR-1184 can inhibit cancer development and metastasis. In BC cells, hsa_circ_0001598 promotes programmed death ligand-1 (PD-L1)-mediated immune escape and trastuzumab resistance by sponge phagocytosis of miR-1184 [38]. The circ_0001598/miR-1184/PD-L1 signaling pathway plays a key role in the progression of BC and the regulation of trastuzumab resistance phenotype [38]. Our results suggest that miR-1184 has inhibitory effects on cell growth, migration, and invasion in TNBC cells, which are offset by the effects of the hsa_circ_0000732 sponge. This is similar to previous studies, but we confirmed for the first time the antitumor effect of miR-1184 in TNBC and the mechanism of miR-1184 in hsa_circ_0000732 sponge.
Subsequent studies can continue to explore the downstream target genes of miR-1184, or turn to explore the role and potential mechanism of other subtypes of ERβ in TNBC.
Conclusion
Our present study found that ERβ2 is upregulated in some TNBC cell lines and promotes TNBC cell growth, migration, and invasion by regulating hsa_circ_0000732 targeting miR-1184. ERβ2 regulates hsa_circ_0000732 expression by binding to SCARF1 promoter, and hsa_circ_0000732 promotes TNBC cell proliferation, migration and invasion through sponge miR-1184. The special role of ERβ2 in TNBC may be the breakthrough of a targeted treatment strategy for TNBC.
References
Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Morris GJ, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results Database. Cancer. 2007;110(4):876–84.
Foulkes WD, Smith IE, Reis JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48.
Dent R, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–34.
Fox EM, Davis RJ, Shupnik MA. ER beta in breast cancer - onlooker, passive player, or active protector? Steroids. 2008;73(11):1039–51.
Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neopl. 2000;5(3):271–81.
Speirs V, et al. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol. 2002;55(5):371–4.
Alexandrova E et al (2020) Small non-coding RNA profiling identifies miR-181a-5p as a mediator of estrogen receptor beta-induced inhibition of cholesterol biosynthesis in Triple-Negative Breast Cancer. Cells 9(4)
Anestis A, et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145(5):1221–33.
Austin D, et al. Estrogen receptor-beta is a potential target for triple negative breast cancer treatment. Oncotarget. 2018;9(74):33912–30.
Sellitto A et al (2020) Insights into the Role of Estrogen Receptor beta in Triple-Negative Breast Cancer. Cancers 12(6)
Zhao C, et al. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67(8):3955–62.
Bozkurt KK, Kapucuoglu N. Investigation of immunohistochemical ERalpha, ERbeta and ERbetacx expressions in normal and neoplastic breast tissues. Pathol Res Pract. 2012;208(3):133–9.
Chantzi MI, et al. Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha-negative breast carcinoma. J Cancer Res Clin Oncol. 2013;139(9):1489–98.
Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–14.
Han Z, et al. ERβ-mediated alteration of circATP2B1 and miR-204-3p signaling promotes invasion of clear cell renal cell carcinoma. Cancer Res. 2018;78(10):2550–63.
Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19(5):312–21.
Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12(2):106–16.
Alexandrova E, et al. Interaction proteomics identifies ERbeta association with chromatin repressive complexes to inhibit cholesterol biosynthesis and exert an oncosuppressive role in triple-negative breast cancer. Mol Cell Proteom. 2020;19(2):245–60.
Novelli F et al (2008) A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res 10(5).
Skliris GP, et al. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95(5):616–26.
Skliris GP, et al. Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target. J Steroid Biochem Mol Biol. 2008;109(1–2):1–10.
Leung YK, et al. Estrogen receptor-beta and breast cancer: Translating biology into clinical practice. Steroids. 2012;77(7):727–37.
Park BW, et al. The changes of estrogen receptor-beta variants expression in breast carcinogenesis: Decrease of estrogen receptor-beta 2 expression is the key event in breast cancer development. J Surg Oncol. 2006;93(6):504–10.
Zhao CY, et al. Estrogen receptor beta 2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67(8):3955–62.
Mandusic V, et al. Different associations of estrogen receptor beta isoforms, ER beta 1 and ER beta 2, expression levels with tumor size and survival in early- and late-onset breast cancer. Cancer Lett. 2012;321(1):73–9.
Shaaban AM, et al. Nuclear and cytoplasmic expression of ER beta 1, ER beta 2, and ER beta 5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14(16):5228–35.
Baek JM, et al. The potential role of estrogen receptor β2 in breast cancer. Int J Surg. 2015;14:17–22.
Faria M, et al. The ERbeta4 variant induces transformation of the normal breast mammary epithelial cell line MCF-10A; the ERbeta variants ERbeta2 and ERbeta5 increase aggressiveness of TNBC by regulation of hypoxic signaling. Oncotarget. 2018;9(15):12201–11.
Yan S, et al. Contrasting activities of estrogen receptor beta isoforms in triple negative breast cancer. Breast Cancer Res Treat. 2021;185(2):281–92.
Bialesova L, et al. Estrogen receptor beta2 induces proliferation and invasiveness of triple negative breast cancer cells: association with regulation of PHD3 and HIF-1alpha. Oncotarget. 2017;8(44):76622–33.
Fu B, et al. Circular RNA profile of breast cancer brain metastasis: identification of potential biomarkers and therapeutic targets. Epigenomics. 2018;10(12):1619–30.
Chen S et al (2020) miR-1184 regulates the proliferation and apoptosis of colon cancer cells via targeting CSNK2A1. Mol Cell Probes 53:101625
Jiang P, et al. Interference with circBC048201 inhibits the proliferation, migration, and invasion of bladder cancer cells through the miR-1184/ITGA3 axis. Mol Cell Biochem. 2020;474(1–2):83–94.
Yang D, et al. Silencing circular RNA VANGL1 inhibits progression of bladder cancer by regulating miR-1184/IGFBP2 axis. Cancer Med. 2020;9(2):700–10.
Jiang L et al (2021) CircCA12 promotes malignant process via sponging miR-1184 and upregulating RAS family in bladder cancer. Front Genet 12:663982
Wang X, et al. Hsa_circ_0128846 promotes tumorigenesis of colorectal cancer by sponging hsa-miR-1184 and releasing AJUBA and inactivating Hippo/YAP signalling. J Cell Mol Med. 2020;24(17):9908–24.
Huang L, Ma J, Cui M (2021) Circular RNA hsa_circ_0001598 promotes programmed death-ligand-1-mediated immune escape and trastuzumab resistance via sponging miR-1184 in breast cancer cells. Immunol Res
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, D., Wang, M., Zhang, H. et al. Estrogen receptor β2 (ERβ2)-mediated upregulation of hsa_circ_0000732 promotes tumor progression via sponging microRNA-1184 in triple-negative breast cancer (TNBC). Inflamm. Res. 71, 255–266 (2022). https://doi.org/10.1007/s00011-021-01536-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01536-0