Abstract
Purpose
Androgen receptor (AR) is playing an important role in the progression of a subset of TNBC. We evaluated the impact of ERβ expression along with anti-AR drugs in AR-positive TNBC.
Methods
ERβ expression was examined in AR-positive TNBC cell line using MTT assay, scratch and Annexin V-FITC assay in the presence or absence of anti-androgens. Protein levels of involved molecules were assessed using Western blot. Receptors’ localization was detected by immunofluorescence and their physical association was examined using proximity ligation assay (PLA), which enables the visualization of interacting proteins in fixed cells and tissues.
Results
Transient transfection of ERβ in MDA-MB 453 AR-positive TNBC cell line significantly inhibited cell proliferation, metastatic potential and induced apoptosis. ERβ expression reversed the aggravating role of AR in both indirect and direct ways. Indirectly, ERβ decreased AR activation through the inhibition of PI3K/AKT signaling pathway. Directly, ERβ formed heterodimers with AR in MDA-MB 453 cells and in human tissue samples impeding AR from forming homodimers. Enzalutamide is a more potent anti-androgen in AR + TNBC compared to bicalutamide. ERβ expression increased the sensitivity of MDA-MB 453 cells to anti-androgens and especially to enzalutamide. The administration of enzalutamide enhanced AR:ERβ heterodimers formation increasing the anti-tumor capacity of ERβ.
Conclusions
Collectively, our results provide evidence for a novel mechanism by which ERβ exerts oncosuppressive effect in AR-positive TBNC through direct and indirect interactions with AR. Moreover, ERβ expression may identify a new subset of TNBC that would respond more favorable to anti-androgens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is a subset of breast cancer, which comprises approximately 15% of all breast cancers and is defined by lack of estrogen receptor-α (ER-α), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) amplification. TNBC is associated with aggressive pathology, poor clinical prognosis, and diminished overall survival (Lebert et al. 2018). Due to the lack of ER and PR receptors, patients with TNBC do not benefit from well-tolerated targeted therapies and cytotoxic chemotherapy remains the main active systemic therapy, with disappointing outcomes; TN patients are likely to develop recurrent disease within 5 years of diagnosis following chemotherapy regimens (Anestis et al. 2015; Li et al. 2018).
Recent research on gene expression has revealed a subset of TNBC where the expression of androgen receptor (AR) is increased and was proposed as luminal androgen receptor (LAR) subtype (Doane et al. 2006; Farmer et al. 2005). In TNBC, AR is expressed significantly lower compared to other breast cancer subtypes reaching approximately 10–35% (Safarpour et al. 2014). Numerous research studies have investigated the prognostic value of AR in TNBC with controversial results; some studies report improved survival, whereas others worse survival (Rahim and O’Regan 2017). The proliferative activity of androgens in preclinical models led to the studying of the therapeutic potential of anti-androgens (Cochrane et al. 2014), bicalutamide and enzalutamide, for AR-positive advanced or metastatic TNBC (Gucalp et al. 2013; Traina et al. 2018). Bicalutamide is a non-steroidal selective androgen antagonist which inhibits AR and has been approved for the treatment of advanced prostate cancer (Akaza et al. 2009). Enzalutamide is a newer generation anti-androgen with higher affinity to AR than bicalutamide approved for the treatment of metastatic castration-resistant prostate cancer (Tran et al. 2009). Phase II clinical trials for both anti-androgens in metastatic TNBC have revealed promising results and demonstrated enzalutamide’s higher clinical benefit rate (Gucalp et al. 2013; Traina et al. 2018). Based on these encouraging results, enzalutamide is currently being assessed in phase II clinical trial in early-stage AR-positive TNBC (NCT02750358).
AR promotes tumor progression in concert with a network of oncogenic signaling pathways (PI3K/AKT/mTOR, cell cycle and MAPK pathways) and key proteins. The PI3K/AKT/mTOR signaling pathway is known to be implicated in breast cancer development (Costa et al. 2018). In TNBC, PIK3CA mutations in AR-positive tumors occurred more frequently than in AR-negative TNBC (40% versus 4%, respectively) (Lehmann et al. 2011). Moreover, research data show interplay between AR and mTOR/p-AKT, both molecules of PI3K signaling pathway (Aleskandarany et al. 2011).
Although ERα is not expressed in TNBC, the second form of estrogen receptors, ERβ, has been detected in approximately 30% of TNBC patients (Wang et al. 2015). A recent meta-analysis in ERα-negative breast cancer patients correlated ERβ expression with increased disease-free survival (DFS) and overall survival (OS) (Joseph et al. 2013; Karamouzis et al. 2016; Kim et al. 2017; Lazennec 2006). In vitro, ERβ has been shown to inhibit human breast cancer cell proliferation by repressing the transcription of cell cycle genes (Reese et al. 2017). The fact that ERβ increases the expression of phosphatase and tensin homologue deleted on chromosome 10 (PTEN), the natural inhibitor of Akt, indicates a potential implication of ERβ in AR signaling (Lindberg et al. 2011; Karamouzis et al. 2016). Here, we have demonstrated that Erβ exerts oncosuppressive potential in AR + TNBC and we provide, for first time, mechanistic insights about its predictive role about the efficacy of anti-androgens in this TNBC subset.
Materials and methods
Antibodies and reagents
The following antibodies were used for Western Blot (WB), immunohistochemistry (IHC), immunofluorescence (IF), proximity ligation assay (PLA, Duolink). For WB: androgen receptor antibody (#3202), phospho-androgen receptor antibody(Ser 650) (#PA5-37479), ERβ antibody (H-150) (sc-8974), phospho-Akt (Ser 473) antibody (#9271), Akt antibody (#9272), phospho-mTOR (Ser 2448) antibody (#2971), mTOR antibody (#2972), PTEN antibody (#9552), anti-actin antibody, clone C4 MAB1501 (Millipore, MA), Histone deacetylase 1 (HDAC1) antibody (# 2062), α-tubulin antibody (#2144).For IHC and PLA: ERβ antibody (H-150) (sc-8974), anti-androgen receptor antibody (AR441) (ab9474). For IF: ERβ antibody (H-150) (sc-8974), androgen receptor antibody (#3202). The secondary antibodies were employed: WB: goat anti-mouse IgG, HRP-conjugate (12–349, Millipore), goat anti-rabbit IgG, HRP-conjugate (12–348, Millipore).For IHC: IHC: Dako Real Envision Detection System, peroxidase/DAB1, rabbit/mouse (Dako). For IF: CF 543 Donkey Anti-Rabbit IgG (H + L), Highly Cross-Adsorbed (#20308, Biotinum). The following reagents were used in this study: bicalutamide (Sigma) dissolved in DMSO at 0.1 to 10 µM concentration, enzalutamide (MDV3100 CAS 915087-33-1) dissolved in DMSO at 0.1–10 µΜ concentration, dihydrotestosterone (DHT) (Sigma) dissolved in DMSO at 0.1–100 nM concentration and 17-β-estradiol (Sigma) dissolved in absolute ethanol at 0.1–10µΜ. The DUOLINK In Situ Ligation Kit was purchased from Olink (DUO92002-Duolink In Situ PLA Probe Anti-Rabbit PLUS, DUO92004—Duolink In Situ PLA Probe Anti-Mouse MINUS, DUO92006—Duolink In Situ PLA Probe Anti-Goat MINUS, DUO92007—Duolink In Situ Detection Reagents Orange) (Olink Bioscience, Uppsala, Sweden). Nuclei were stained with DAPI (Invitrogen).
Cell culture and transfection
MDA-MB 453 cell line is used extensively in TNBC research. It has been classified as a luminal androgen receptor (LAR) subtype of TNBC cell line with high levels of AR mRNA and protein and undetectable levels of Erβ expression and thus provides a valuable in vitro model for this study (Lehmann et al. 2011; Vladusic et al. 2000). MDA-MB 453 cells were cultured in RPMI 1640 medium GlutaMAX (Gibco, Life Technologies) supplemented with 10% FBS (Gibco, Life Technologies) and 1% penicillin streptomycin. Cell cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2–95% air. MDA-MB 453 cells were transiently transfected with pEGFP-C1-ER beta plasmid (#plasmid 28237, Addgene). Transient transfection was performed using Lipofectamin 2000 (Invitrogen, CA) according to the manufacturer’s instructions. ERβ was activated when cells were exposed to 17β-estradiol 10 nM for 12 h. Then media was removed and cells were treated and harvested accordingly.
Tissue samples
The study included 55 archival TNBC tissue sample provided by the First Department of Pathology, “Laiko” Hospital, University of Athens Medical School, the Second Oncology Clinic, Saint Savvas Anti-Cancer Hospital, Athens, Greece and the Hematology Oncology Unit, Fourth Department of Internal Medicine, Attikon University Hospital. Pathologic staging and the histological grade designation of the tumors followed the principles laid down in the World Health Organization Classification. Follow-up was available for 40 patients. Paraffin-embedded TNBC tissues from those patients were selected and were further analyzed with immunohistochemistry to detect their positivity for AR and/or ERβ. All the patients have signed Informed Consent Form for the use of their biological material for research purposes.
Western blot analysis
Protein extraction was performed using ice-cold RIPA buffer (Thermo Scientific). Proteins were resolved by electrophoresis in SDS–polyacrylamide gels with several densities (8%, 10% and 12%) depending on the molecular weight of each protein. Then, they were transferred to a nitrocellulose membrane (Macherey–Nagel, Germany). The membrane was blocked for 2 h at room temperature in PBST with 5% nonfat milk and then incubated with special primary antibodies overnight at 4 °C (dilutions were 1:300 for antibody against ERβ, 1:1000 for antibodies against AR, p-AKT, AKT, p-mTOR, mTOR, PTEN and 1:2000 for antibodies against actin, a-tubulin and HDAC in PBST containing 1% nonfat milk). The detection of the immunoreactive bands was performed with the LumiSensor Chemiluminescent HRP Substrate kit (GenScript). Relative protein amounts were evaluated by a densitometry analysis using Image-J software and normalized to the corresponding actin or a-tubulin levels.
Cell proliferation assay
The cell proliferation of MDA-MB 453 rate was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Cells were starved in phenol red-free RPMI supplemented with 5% charcoal-stripped serum (CSS) for 24 h prior the treatments. Control cells were treated with the same amount of vehicle alone DMSO and did not exceed the concentration of 0.01% v/v. 24, 48 and 72 h of incubation after treatment the medium was replaced with MTT diluted in serum-free, phenol red (PR)-free medium at a final concentration of 1 mg/ml. Cells were incubated for about 3 h at 37 °C in a 5% CO2 atmosphere and the MTT-formazan product was solubilized in isopropanol. The absorbance was measured at 570 nm with a background wavelength of 690 nm. Each condition was conducted in triplicate into 96-well plate.
Apoptosis assay
To assess the cell apoptosis an annexin V-florescein isothiocyanate (FITC) apoptosis detection kit (Trevigen, Gaithersburg) was used according to the manufacturer’s instructions. Cells were seeded in six-well plate (105 cells/well) and were starved in phenol red-free RPMI supplemented with 5% charcoal-stripped serum (CSS) for 24 h prior the treatments. 72 h post-treatment cells were trypsinized, washed once with PBS, and collected by centrifugation. For FACS analysis, a total of 10,000 events were analyzed per sample in a FACSCalibur flow cytometer and analyzed with CellQuest software (Becton–Dickinson). A total of 10,000 events were measured per sample. Events in the Annexin V-positive/propidium iodide (PI)-negative quadrant were representative of apoptotic cells. Each sample was tested in duplicate.
Migration assay
MDA-MB 453 cells were seeded in 12-well plate (10 × 105 cells/well). 48 h after, the cell monolayer was scratched with a sterile 200-µl tip head marking point of zero migration. The debris was removed by washing with PBS and cells were starved for 24 h. Cells were then treated with anti-androgens and/or DHT for 0, 24, 48, and 72 h. After the incubation, the migration distance for the same scratch area was visualized and photographed at 34 and 320 magnifications using computer-assisted microscopy. The pictures were analyzed by the TScratch software (Wimasis image analysis platform). Results were expressed as percent of cell-covered areas.
Immunohistochemistry
Immunohistochemistry was carried on FFPE sections from archival TNBC sections cut at 4-µm thickness lm using the two-step peroxidase-conjugated polymer technique (Dako Envision). Paraffin-embedded sections from prostate cancer and endocervical epithelium were used as positive control for AR and ERβ, respectively. Tissues were dried at 65 °C for 20 min, deparaffinized in xylene and then rehydrate in an ethanol series. After washing with distilled H2O (dH2O), tissue sections were treated in the microwave with sodium citrate pH 6.0 retrieval solutions for 25 min and then, with 3% H2O2 (v/v) in dH2O for 30 min at room temperature to block endogenous peroxidase activity. Sections were incubated with the primary antibodies overnight at 4 °C (dilutions were 1: 100 for the antibody against AR and 1:50 for the antibody against the ERβ). Slides were incubated for 30 min with the Dako Real Envision Detection System, Peroxidase/DAB+, Rabbit/Mouse (Dako, Glostruo, Denmark), and immunolabeling was visualized with diaminobenzidine for 3 min. Hematoxylin solution was used for counterstaining (Sigma-Aldrich). Sections were dehydrated in ethanol buffers of 70, 80, 96, and 100% concentration and were mounted onto glass coverslips. PBS was used as the negative control instead of the primary antibody. Immunostaining was evaluated by the pathologist ST.
Proximity ligation assay (PLA) and immunofluorescence (IF)
PLA is used for visualization of endogenous protein–protein physical interaction by retaining the dependency of proximal binding of antibodies and providing a mean of signal amplification (Söderberg et al. 2006). MDA-MB 453 cells were cultured on glass coverslips and were transfected with pEGFP-C1-ERβ as described above. After reaching ~ 70% confluency, cells were starved for 24 h and treated accordingly for 48 h. After the fixation of cells with 4% PFA in PBS and permeabilization for 10 min at room temperature, cells were blocked with 3% BSA in TBST for 1 h in room temperature. For IF analysis, cells were incubated overnight at 4 °C with AR antibody diluted 1:600 in 1% BSA in PBST. For PLA analysis, as described in the Duolink II protocol, different set of primary antibodies was used (ERβ antibody (H-150) (sc-8974) and anti-androgen receptor antibody (AR441) (ab9474) diluted 1:50). Cells were then incubated with the PLA probes diluted 1:5 in antibody diluent (Sigma-Aldrich) in a humidified chamber for 1 h at 37 °C. Subsequent hybridization, ligation, amplification, and detection were performed as per manufacturer’s instructions (Sigma-Aldrich). Cells were then incubated for 10 min with DAPI (2:1000 in PBS) for nuclei staining and glass coverslips were mounted on the slides. Fluorescence images were acquired using a Zeiss Axiovert microscope (Carl Zeiss Microscopy, Thornwood, NY USA). At least ten random fields of view were selected and images were taken. Data analysis was performed using Duolink Image Tool Software, developed for quantification of PLA signals (Sigma-Aldrich) (Smal et al. 2010). Representative images for each condition are shown in the figures.
Proximity ligation assay in human tissue samples
Patients’ samples that were immunohistochemically positive for both AR and ERβ were selected for PLA assay. Sections were deparaffinized following a standard protocol of xylene and ethanol series, antigen retrieval (sodium citrate pH 6.0), wash with ddH20 and PBS and all other reactions (blocking, primary antibodies, PLA probes, ligation, amplification and cover slips mounting) according to manufacturer’s instructions. Fluorescence images were acquired using a Zeiss Axiovert microscope. At least ten random fields of view were selected and images were taken. Data analysis was performed using Duolink Image Tool (Olink Bioscience) (Smal et al. 2010). Representative images for each condition are shown in the figures.
Statistical analysis
All experiments were performed at least two or three times and representative results of one experiment are shown. The data are presented as mean ± SE for the number of experiments indicated and analyzed by Student’s t test. All statistical tests were two-sided. p values less than 0.05 were considered statistically significant.
Results
Overexpression of ERβ negatively relates to AR expression in AR + TNBC cells through the regulation of PI3K/Akt/mTOR pathway
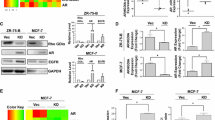
We first investigated the impact of ERβ overexpression on the AR protein expression in MDA-MB 453 TNBC cell line. Initially, we transiently transfected ERβ-bearing plasmid construct into the cells. The efficacy of transfection with the green fluorescent protein (GFP)-tagged ERβ plasmid construct was confirmed through fluorescent microscopy (Fig. 1a). As shown in Fig. 1b the expression of ERβ was significantly higher compared to that in the control (pEGFP-C1 empty vector) (p < 0.01) as quantified by immunoblotting. ERβ overexpression, resulted in significant AR downregulation compared to control (p < 0.05) (Fig. 1e). We investigated the implication of mTOR pathway in ERβ-mediated AR downregulation. Western blot analysis (Fig. 1c, d) showed that ERβ overexpression, following E2 treatment, significantly increased PTEN (p < 0.05) (Fig. 1f), the natural inhibitor of mTOR pathway resulting in a significant downregulation of phosho-AKT (Fig. 1g) compared to control (cells transfected with pEGFP-C1 vector treated with DMSO). No significant change in the expression of phosho-mTOR was observed (Fig. 1h).
Effect of ERβ along with anti-androgens bicalutamide and enzalutamide in AR signaling and PI3K signaling pathway in MDA-MB 453 cells. a Detection of ERβ by fluorescent microscopy and protein level b after the transient transfection of pEGFP-C1-ERβ plasmid vector into MDA-MB 453 cells. 24 h post-transfection, cells were treated with 17β-estradiol 10 nM for 12 h to activate ERβ and then media was removed. Immunoblots showing efficient ERβ transfection compared to mock cells transfected with empty vector (pEGFP-C1). MDA-MB 453 cells were cultured in castrate conditions (DCC) with DMSO, 10µΜ bicalutamide (Bica), 10 µΜ enzalutamide (Enza) in the presence or absence of 10 nM DHT for 48 h. c Cell lysates were analyzed by western blot for AR, PTEN, p-Akt, Akt, p-mTOR and mTOR. Actin was used as a protein loading control. The experiments were repeated two times and representative blots are presented. d The same western blot as c was carried out 48 h post transfection of MDA-MB 453 cells with pEGFP-C1-ERβ vector. The histograms represent the mean ± SD of two separate experiments in which western blot band intensities of c and d were evaluated in terms of optical density showing the fluctuation of relative protein levels of AR e, PTEN f, phopho-Akt g and phospho-mTOR h after normalized by actin levels. Data are represented as mean ± SD and were analyzed by Student’s t test. All statistical tests were two-sided. *p < 0.05
To compare the action of anti-androgens in AR + TNBC, MDA-MB 453 cells were starved and then treated with bicalutamide or enzalutamide. AR expression was evaluated with immunoblotting (Fig. 1c). Comparing to the control, AR expression was significantly suppressed (p < 0.05) when cells were treated with enzalutamide (p < 0.05) than with bicalutamide (Fig. 1e). Moreover, enzalutamide showed more suppressive effect than bicalutamide. The addition of androgens, 5 alpha dihydrotestosterone (DHT) dramatically increased AR expression (p < 0.01) but this ligand-mediated effect was significantly reversed by bicalutamide (p < 0.05) and enzalutamide (p < 0.05). The same AR expression pattern was followed with ERβ overexpression as observed with immunoblotting analysis (Fig. 1d, e). DHT does not seem to affect the PI3K pathway and, rationally, anti-androgens bicalutamide and enzalutamide did not exhibit any significant change in the expression of the mTOR pathway modulators PTEN, AKT and mTOR (Fig. 1f–h). These data suggest that ERβ dοwnregulates the expression of AR through the mTOR pathway and this effect tends to be enhanced by enzalutamide but this trend should be further investigated.
Enzalutamide inhibits cell viability and induces apoptosis in AR + TNBC. Enzalutamide’s anti-proliferative and anti-metastatic effect is enhanced with Erβ
MTT assay was performed to assess the effect of anti-androgens bicalutamide and enzalutamide on the viability of AR + TNBC cells. In the absence of DHT, enzalutamide significantly inhibited (p < 0.05) cell proliferation on 72 h post-treatment in MDA-MB 453 cells compared to the control group. DHT alone dramatically increased cell proliferation (p < 0.01). Enzalutamide retained its anti-androgenic properties after the addition of DHT (p < 0.05). In contrast, bicalutamide did not affect cell proliferation at 72 h per se. However, bicalutamide did inhibit DHT-mediated cell growth (p < 0.05) (Fig. 2a left panel). These data are in accordance with the annexin V/PI apoptosis assay results. Enzalutamide significantly induced (p < 0.05) late apoptosis in MDA-MB 453 cells with or without the addition of DHT compared to DMSO-treated control cells 72 h post-treatment. In contrast to MTT, bicalutamide significantly induces (p < 0.05) late apoptosis, also in the absence of DHT, reaching almost the same efficacy as of enzalutamide (Fig. 2b upper panel and c left panel).
Antitumor effect of ERβ along with anti-androgens bicalutamide and enzalutamide in MDA-MB 453 cells. a MTT assay in MDA-MB 453 cells transfected with pEGFP-C1-ERβ (right panel) or with empty vector (left panel). 24 h post-transfection, cells were treated with 17β-estradiol 10 nM for 12 h to activate ERβ, then media was renewed and cells were treated as indicated. Results are expressed in the histogram as the optical density (O.D. value) and represent mean ± SD of two different experiments each performed in triplicate. b Flow cytometry analysis of annexin V and propidium iodite (PI) staining of apoptotic cells after 72 h of treatment. MDA-MB 453 cells were transfected with pEGFP-C1-ERβ (lower panel) or with empty vector (upper panel) and treated as indicated. c Summary of apoptosis assay showing per cent population in different cell death modes: apoptosis, necrosis in the absence (left panel) or presence (right panel) of ERβ. Histogram σ represents the mean ± SD of three independent experiments and was analyzed by Student’s t test. All statistical tests were two-sided. *p < 0.05, *p < 0.01. d MDA-MB 453 cells were subjected to a scratch assay and then treated as indicated in the absence (left panel) or absence of ERβ (right panel). The histogram shows percent area covered at 48 h compared to the original gap at 0 h. Data represent mean ± SD of two separate experiments and were analyzed by Student’s t test. All statistical tests were two-sided. *p < 0.05, *p < 0.01. #p < 0.05 compared to DHT treated cells for each panel
Then, the effect of ERβ on the viability of AR-positive TNBC cells was evaluated under the same conditions. MDA-MB 453 transfected with pEGFP-C1-ERβ plasmid exhibited significantly lower cell viability than the control (p < 0.05) in all treatment groups following E2 treatment. In 72 h, enzalutamide significantly inhibited cell proliferation independently of DHT, whereas bicalutamide did not (Fig. 1a right panel). ERβ overexpression induced high rates of cell necrosis after the addition of enzalutamide or bicalutamide. However, only enzalutamide exhibited significantly late apoptosis rates (p < 0.05) (Fig. 1b lower panel and c right panel).
We evaluated the effect of anti-androgens and ERβ overexpression on the migration potential of AR-positive TNBC cells by wound-healing assay. Enzalutamide efficiently inhibit cell migration 48 h post-treatment with or without DHT (p < 0.05). Cell migration rate was slightly but insignificantly reduced with bicalutamide in the presence of androgens. ERβ overexpression significantly inhibited the migration capacity of cells compared to untransfected ones (p < 0.05). Notably, a dramatic inhibition of migration rate was observed when cells overexpressing ERβ were treated with enzalutamide for 48 h with or without DHT (p < 0.01) (Fig. 1d). The above findings suggest that enzalutamide impairs AR better than bicalutamide leading to lower cell viability, increased late apoptosis and lower migration rate in AR + TNBC cells. ERβ expression enhanced the in vitro anti-proliferative and anti-metastatic effect of enzalutamide.
Androgens induce the nuclear translocation of AR, which modulates the transcription of specific genes regulating the cancer progression. We compared the capacity of anti-androgens to prevent this translocation. Using confocal microscopy, immunofluorescence staining of AR in MDA-MB 543 cells showed that the endogenous localization of AR is in both nucleus and cytoplasm (control group) (Fig. 3a). An increase in AR nuclear translocation was revealed when cells were treated with DHT (Fig. 3a). This DHT-driven result was blocked after the addition of anti-androgens (Fig. 3a). Enzalutamide is a more potent AR-translocation inhibitor than bicalutamide, confirming the results of the Fig. 2. The above results were being confirmed with immunoblotting; cytoplasmic and nuclear proteins were isolated and the activated form of AR phosho-AR was probed with an AR antibody and quantified (Fig. 3b). However, when ERβ is overexpressed, a different pattern of AR nuclear translocations was observed (Fig. 3c). In the control group, AR is mainly located in the cytoplasm, implying the interaction between AR and ERβ. DHT, as expected, increased the nuclear localization of AR (Fig. 3c). However, an unexpected effect of anti-androgens was observed when ERβ is overexpressed. The androgen-specific effect was not notably reversed after the treatment with bacalutamide and enzalutamide. Moreover, the fluorescent signal of AR in the nucleus was increased with enzalutamide compared to bicalutamide (Fig. 3c), generating the hypothesis that the addition of anti-androgens, especially enzalutamide, affect not only the AR but also the crosstalk between AR and ΕRβ. The above results were being confirmed with immunoblotting; cytoplasmic and nuclear proteins were isolated and the activated form of AR phosho-AR was probed with an AR antibody (Fig. 3d). Enzalutamide further reduced the phosphorylation of AR compared to bicalutamide and, consequently, inhibited more effectively the nuclear translocation of the receptor, before and after the stimulation with DHT (Fig. 3b). Oddly, when ERβ overexpressed, the expression of nuclear AR was changed only after DHT treatment, whereas not any dramatic fluctuation in AR translocation was blotted after the addition of anti-androgens (Fig. 3d).
Effect of ERβ along with anti-androgens in nuclear translocation of AR in MDA-MB 453 cells. a MDA-MB 453 cells were cultured in castrate conditions (DCC) with DMSO, 10µΜ bicalutamide (Bica), 10 µΜ enzalutamide (Enza) in the presence or absence of 10 nM DHT for 6 h. The subcellular localization of endogenous AR, cell nucleus (DAPI) and merged images of AR and DAPI was assessed by confocal microscopy. b The inhibition of AR translocation was also confirmed by western blot analysis after cell fractionation. HDAC was used as protein loading control for nuclear extract and a-tubulin as a loading control for cytoplasmic extract. c, d Numbers indicate the blot ration between the experimental samples and the control (DMSO). The same immunofluorescence assay as a and western blot analysis as b was carried out 48 h post transfection of MDA-MB 453 cells with pEGFP-C1-ERβ vector
Physical association between AR and ERβ through the formation of heterodimers
We investigated the formation of AR and ERβ dimers to verify that the physical crosstalk occurs between the two receptors using Proximity Ligation Assay (PLA). In MDA-MB 453 transfected with Erβ cells (PLA signal scale 0–40), there is an incidence of AR:ERβ heterodimers formation in the control group (Fig. 4a, b). As expected, DHT led to AR:ERβ heterodimers decrease compared to the control (4.3 vs. 12.1) (Fig. 4a, b). DHT promotes the activation, homodimerization and nuclear translocation of AR (37). This is in accordance to our results where the addition of DHT doubled the formation of AR:AR homodimers compared to control (20.8 vs. 10) (Fig. 4d, e). Notably, compared to the control group, the addition of enzalutamide soared the formation of AR:ERβ heterodimers and no discernible difference was observed after the addition of DHT (12.1 vs. 37.2 vs. 36.6). Accordingly, bicalutamide alone increased AR/ERβ heterodimers (28.87 vs. 12.1) but this ability was weakened with DHT (12.1 vs. 28.87 vs. 19.5) (Fig. 4a, b). Moreover, after enzalutamide, 58% of the heterodimers were observed in the nucleus compared to 73% after bicalutamide and the same trend was detected after DHT (54 vs. 62.7) (Fig. 4c). PLA confirmed that enzalutamide is a more potent anti-androgen than bicalutamide. Enzalutamide decreased by half the amount of AR:AR homodimers, whereas a subtle decrease was detected with bicalutamide compared to control group (6.6 vs. 9.2 vs. 10) (Fig. 4d, e). These findings show that ERβ affects the activity and behavior of AR also directly through the heterodimers formation, thus generating new data regarding the therapeutic way we may target them.
Dimer formation pattern in MDA-MB 453 cells. MDA-MB 453 cells were transfected with pEGFP-C1-ERβ plasmid vector and treated as indicated for 48 h. Representative image of each condition is shown using confocal microscopy. Red dots represent the positive signal of AR:ERβ heterodimers (a) or AR/AR homodimers (d) using DUOLINK in situ proximity ligation assay (PLA) (scale bar 20 µΜ). b, e Histograms represent the total number of dots per cell as analyzed by Duolink Image Tool Software. The resulting “PLA signals per cell” in all groups were normalized to the mean value of the control group. c Histogram represents the distribution of the signal in the nucleus and cytoplasm
AR:ERβ heterodimer formation in TNBC patients
In human paraffin-embedded TNBC tissues, we evaluated AR and ERβ expression in a cohort of TNBC patients (No 55). AR immunostaining was observed in 32 patients. AR immunoreactivity was both nuclear and cytoplasmic. Accordingly, ERβ was detected in 22 and was mainly detected in the cytoplasm, in which result agrees with the previous reports (Söderberg 2006). Staining intensities were classified into four scores: 0 (no staining), 1 (pale yellow), 2 (deep yellow), and 3 (brown). The two receptors were co-expressed in five patient cases. To expand our data in the in vivo setting, we performed in situ PLA assay to confirm the dimerization of AR and ERβ in those samples (5/5, 100%). Representative images with double-positive TNBC are shown at Fig. 5a. Also, the number of AR:ERβ heterodimers is proportional to the staining intensity (0 vs. 2,8 vs. 11,3 vs. 15,5 heterodimers) Fig. 5b.
AR:Erβ heterodimer formation pattern in human TNBC tissues. a Immunohistochemical expression patterns of AR and ERβ in TNBC patients. b–e Representative fluorescent images of AR:ERβ heterodimer formation in TNBC patients. White arrowheads point to positive signal (red dots) of heterodimer formation using the Duolink in situ proximity ligation assay (PLA) (scale bar, 20 µm). The number of AR:ERβ heterodimers is proportional to the staining intensity. (IHC score 0 = 0 IHC score 1 = 2,8 IHC score 2 = 11,3 IHC score 3 = 15,7 heterodimers)
Discussion
ERβ has been recently detected in several tumors, such as breast cancer, prostate cancer, lung and colorectal cancer and has been associated with antitumor effect (Lazennec 2006). Although evidence points have emerged ERβ as a promising novel predictive and prognostic biomarker, at this time, it is not routinely considered in patients’ management in the clinic. New reports demonstrate that ERβ inhibits proliferation and induces apoptosis in ERα—low or negative breast cancer cell line (Murphy and Leygue 2012). In this study, ERβ transfection in MDA-MB 543 cells resulted in reduced cell proliferation after the activation of ERβ with estrogens. This finding is an extension of the previous studies demonstrating the anti-proliferative effect of ERβ in several cellular backgrounds (Reese et al. 2017; Thomas et al. 2012). This can be explained by the fact that ERβ expression causes G2 cell cycle arrest in different types of cancer cells including TNBC (Paruthiyil et al. 2004). Moreover, the decreased rates of proliferation due to the presence of ERβ contributed to lower metastatic activity in MDA-MB 453. Interestingly, programmed cell death was not changed in the presence of ERβ since the number of cells undergoing apoptosis was not significantly increased compared to when ERβ is not expressed. Previous studies have associated this finding with the lower rates of cell growth (Reese et al. 2017; Hodges-Gallagher et al. 2008). The role of ERβ in opposing proliferation and migration suggests that ERβ agonists may be used to prevent the progression of TNBC.
Although in ER-positive breast cancer, AR has been established as a favorable prognostic marker (Hu et al. 2011), in TNBC, AR clinical value remains an active area of research giving debatable results. A recent NHS study with a large cohort of breast cancer patients has demonstrated an 83% increase in overall mortality for AR-positive TNBC patients compared to AR-negative TNBC (Hu et al. 2011). Moreover, studies reported lower rate of pathological complete response (pCR) in AR-positive TNBC (12.85%, n = 358) compared to AR-negative (25.4%, n = 315) (Loibl et al. 2011).
Also, in preclinical reports, activation of AR with the AR agonist, DHT (a non-aromatizable form of testosterone), induced proliferation in MCF7 and BCK4 cell lines and epithelial-to-mesenchymal transition (EMT) in TNBC cell line (Smal et al. 2010; Söderberg 2006; Tan et al. 2016). In our study, we show that the addition of DHT increased the nuclear import of AR and led to increased proliferation, decreased apoptosis and increased metastatic potential. Interestingly, the tumorigenic effect of androgens was reversed upon the activation of ERβ with estradiol.
Given the tumor-suppressive effect of ERβ in AR-positive TNBC cells, we wondered whether ERβ acts as an anti-tumor effector also by affecting AR expression. It has been shown that ERβ mediates the expression of cyclin D1 and p21 genes in which both are important in AR signaling (Reese et al. 2017). However, in this study we investigated whether ERβ interacts with AR by non-genomic inputs, including extracellular signaling events. The association between PI3K pathway and AR activation has been established. More specifically, AR can be activated by non-genomic signaling pathway PI3K/Akt/mTOR, an intracellular signaling pathway mediating the regulation of cell cycle. PTEN exhibits its main tumor-repressive role through dephosphorylation of PIP3. PTEN is an important natural negative regulator of AKT signaling, which in turn activates AR (Costa et al. 2018; Aleskandarany et al. 2011). Moreover, the combined deletion of PTEN and p53 in mammary epithelium has been associated with the formation of TNBC (Liu et al. 2014). PI3K pathway inhibition has already been proposed as a therapeutic approach for TNBC patients, as an early-phase clinical trial evaluating the efficacy of Ipatasertib (an AKT inhibitor) combined with paclitaxel (NCT03337724) has given promising primary outcomes (Kim et al. 2017). Thus, we found it important to clarify the effect of ERβ on PTEN in AR-positive TNBC cells. Our data showed that ERβ overexpression upregulated PTEN and subsequently decreased the phosphorylation of AKT in MDA-MB 453. Consequently, the expression of AR was downregulated. This is in accordance with the previous in vivo studies where PTEN was downregulated in the ventral prostate of ERβ knockout mice. The same study revealed that the administration of ERβ agonists in wild type mice led to increased PTEN and decreased AR (Wu et al. 2017). However, the precise mechanism by which ERβ affects PTEN expression needs to be studied further. These findings illustrate the non-genomic negative interplay between ERβ and AR in TNBC through PI3K signaling pathway, suggesting that combined targeting of both receptors may be a novel therapeutic strategy for AR+/ERβ + TNBC. These data bring us closer to the hypothesis that the role of AR on TNBC might be affected by the presence or absence on ERβ.
It has been reported that ERβ expression is reduced in the nuclei of TNBC and increased in the extranuclear sites (Hamilton et al. 2015). An interesting observation of our study was that when ERβ was expressed in MDA-MB 453, AR localization was observed mainly in cytoplasm instead of being equally distributed in the subcellular compartments. Taken together, we wondered whether any physical interaction, beyond the functional one, occurs among the two receptors. Previous studies in breast cancer cell lines have reported that AR can regulate the genomic signaling of ER in MCF-7 cells and has been found to be bound in the same EREs sequences with ERα (Majumder et al. 2017; D’Amato et al. 2016). Other studies demonstrated that the amino-terminal domain of AR interacts with ligand-binding domain of the ERα (Panet-Raymond et al. 2000). However, none of these studies investigated whether AR and ER can form a heterodimer complex. This manuscript provides the initial evidence that ERβ also physically and functionally interact with AR in MDA-MB 453 cells and also this interaction occurs also in the absence of estradiol. However, we should not exclude the possibility that circulating testosterone was converted to estradiol by CYP19 and thus, activating ERβ (Gao et al. 2005). Moreover in this study, for first time, we show that the formation of AR:ERβ heterodimers is confirmed in human samples, strengthening the reliability of our findings. All these data suggest that ERβ exerts its non-genomic anti-proliferative effect on AR-positive TNBC through the direct and indirect interactions with AR suggesting ERβ as a novel predictor for better clinical outcomes in this subset of TNBC.
Since androgens are considered to promote cell growth in AR-positive TNBC, many preclinical and clinical studies show that the administration of anti-androgens would provide benefit to AR-positive TNBC. Patients, whose tumors presented ≥ 10% nuclear AR expression were treated with bicalutamide daily (150 mg) and showed clinical benefit rate (CBR) of 19% with a median progression-free survival (PFS) of 12 weeks (NCT00468715/TBCRC011) (Gucalp et al. 2013). Accordingly, enzalutamide (160 mg daily) in AR-positive metastatic TNBC has shown 33% CBR at 4 months in and 28% at 6 months with PFS of 3.3 months (Traina et al. 2018).
Mechanistically, preclinical studies have shown that androgens promote proliferation via several gene targets and impairing AR signaling with bicalutamide and enzalutamide did reverse this effect in both MCF-7 and MDA-MB 453 xenografts (Cochrane et al. 2014). In our study, although both anti-androgens impaired AR signaling, enzalutamide exhibited higher anti-androgenic effect leading to reduced cell proliferation and metastasis and increased apoptosis compared to bicalutamide. It is known that enzalutamide inhibits the nuclear translocation of AR and impairs the binding to androgen-responsive elements (ARE) with no known agonistic properties (Tran et al. 2009). The apoptotic effect of enzalutamide has been also reported in SUM159PT xenografts where enzalutamide caused 90% of necrotic tumor (Zhu et al. 2016). The most interesting finding of this study is that the expression of ERβ in MDA-MB 453 cells increased the sensitivity of the cells to anti-androgens and especially to enzalutamide. A more marked effect of enzalutamide against proliferation and migration was noticed when ERβ was expressed. Moreover, in this study we observed that the administration of enzalutamide enhances the formation of AR:ERβ heterodimers leading to more favorable outcomes. This may be explained by recent studies where enzalutamide mediates the induction of ER-regulated genes such as the chemokine SDF-1 which can activate ER via phosphorylation (Hall and Korach 2003). These findings raise concerns regarding the ongoing clinical trials studying bicalutamide (TBCR C011) and enzalutamide (MDV30100-11) suggesting that ERβ holds a predictive role for endocrine response to anti-androgens in AR-positive TNBC cells. However, a recently identified mutation in AR which provides enzalutamide needs to be considered in TNBC patients (Joseph et al. 2013). Previous studies in prostate cells have shown that enzalutamide impairs AR nuclear translocation and androgen-mediated stabilization greater than bicalutamide (Tran et al. 2009). Our study is in accordance with the previous reports as enzalutamide significantly inhibits nuclear translocation of AR whether androgen-dependent or independent compared to bicalutamide. Notably, the fact that enzalutamide which impairs nuclear translocation of AR, gives a different result when ERβ is expressed may shed a light into the role of ERβ in AR-positive TNBC. More specifically, when bound to enzalutamide, AR still translocates in the nucleus but cells are more sensitive to endocrine therapy with enzalutamide than when ERβ is absent. This may be explained by the fact that AR enters the nucleus as a heterodimer with ΕRβ and does not bind to AREs sequences to promote cell growth. This finding suggests the dominant influence of ERβ on AR enhancing the argument that ERβ may be a potential predictive biomarker for AR-positive TNBC.
Conclusions
In conclusion, ERβ seems to play a pivotal role in the progress of TNBC. The obvious anti-tumor effect of ERβ in TNBC cells along with the physical association between ERβ and AR raise hope for new therapeutic strategies for AR-positive TNBC patients. In addition of being an independent predictor of better clinical outcomes, ERβ increased the sensitivity of these cells to anti-androgens, especially to enzalutamide and thus, providing mechanistic insights regarding its predictive role for the efficacy of anti-AR agents in this TNBC group. Our preclinical data support the administration of enzalutamide for AR-positive and ERβ-positive TNBC tumors as a more potent anti-androgen against this subset of patients than bicalutamide. However, several caveats still remain and thus, further preclinical investigations are still needed.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akaza H, Hinotsu S, Usami M et al (2009) Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. https://doi.org/10.1002/cncr.24395
Aleskandarany MA, Rakha EA, Ahmed MA et al (2011) Clinicopathologic and molecular significance of phospho-Akt expression in early invasive breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-010-1012-y
Anestis A, Karamouzis MV, Dalagiorgou G, Papavassiliou AG (2015) Is androgen receptor targeting an emerging treatment strategy for triple negative breast cancer? Cancer Treat Rev 41:547
Cochrane DR, Bernales S, Jacobsen BM et al (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. https://doi.org/10.1186/bcr3599
Costa RLB, Han HS, Gradishar WJ (2018) Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-018-4697-y
D’Amato NC, Gordon MA, Babbs B et al (2016) Cooperative dynamics of AR and ER activity in breast cancer. Mol Cancer Res. https://doi.org/10.1158/1541-7786.MCR-16-0167
Doane AS, Danso M, Lal P et al (2006) An estrogen receptor-negative breast cancer subset characterized by hormonically regulated transcriptional program and response to androgens. Oncogene. https://doi.org/10.1038/sj.onc.1209415
Farmer P, Bonnefoi H, Becette V et al (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. https://doi.org/10.1038/sj.onc.1208561
Gao W, Bohl CE, Dalton JT (2005) Chemistry and structural biology of androgen receptor. Chem Rev 105:3352
Gucalp A, Tolaney S, Isakoff SJ et al (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-12-3327
Hall JM, Korach KS (2003) Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. https://doi.org/10.1210/me.2002-0438
Hamilton N, Márquez-Garbán D, Mah V et al (2015) Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer. Biomed Res Int. https://doi.org/10.1155/2015/925703
Hodges-Gallagher L, Valentine CD, Bader S El, Kushner PJ (2008) Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-007-9640-6
Hu R, Dawood S, Holmes MD et al (2011) Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-10-2021
Joseph JD, Lu N, Qian J et al (2013) A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-13-0226
Karamouzis MV, Papavassiliou KA, Adamopoulos C, Papavassiliou AG (2016) Targeting androgen/estrogen receptors crosstalk in cancer. Trends Cancer 2:35
Kim SB, Dent R, Im SA et al (2017) Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(17)30450-3
Lazennec G (2006) Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett 231:151
Lebert JM, Lester R, Powell E et al (2018) Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. https://doi.org/10.3747/co.25.3954
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig. https://doi.org/10.1172/jci45014
Li Y, Liang Y, Sang Y et al (2018) MIR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1 article. Cell Death Dis. https://doi.org/10.1038/s41419-017-0030-7
Lindberg K, Helguero LA, Omoto Y et al (2011) Estrogen receptor β represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN: implications for tamoxifen sensitivity. Breast Cancer Res. https://doi.org/10.1186/bcr2865
Liu JC, Voisin V, Wang S et al (2014) Combined deletion of Pten and p53 in mammary epithelium accelerates triple-negative breast cancer with dependency on eEF2K. EMBO Mol Med. https://doi.org/10.15252/emmm.201404402
Loibl S, Muller BM, von Minckwitz G et al (2011) Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-011-1715-8
Majumder A, Singh M, Tyagi SC (2017) Post-menopausal breast cancer: from estrogen to androgen receptor. Oncotarget. https://doi.org/10.18632/oncotarget.22156
Murphy LC, Leygue E (2012) The role of estrogen receptor-β in breast cancer. Semin Reprod Med. https://doi.org/10.1055/s-0031-1299592
Panet-Raymond V, Gottlieb B, Beitel LK et al (2000) Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. https://doi.org/10.1016/S0303-7207(00)00279-3
Paruthiyil S, Parmar H, Kerekatte V et al (2004) Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-03-2446
Rahim B, O’Regan R (2017) AR signaling in breast cancer. Cancers (Basel) 9:E21
Reese JM, Bruinsma ES, Monroe DG et al (2017) ERβ inhibits cyclin dependent kinases 1 and 7 in triple negative breast cancer. Oncotarget. https://doi.org/10.18632/oncotarget.21787
Safarpour D, Pakneshan S, Tavassoli FA (2014) Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am J Cancer Res 4:353
Smal I, Loog M, Niessen W, Meijering E (2010) Quantitative comparison of spot detection methods in fluorescence microscopy. IEEE Trans Med Imaging. https://doi.org/10.1109/TMI.2009.2025127
Söderberg O, Gullberg M, Jarvius M et al (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. https://doi.org/10.1038/nmeth947
Tan W, Li Q, Chen K et al (2016) Estrogen receptor beta as a prognostic factor in breast cancer patients: a systematic review and meta-analysis. Oncotarget. https://doi.org/10.18632/oncotarget.7219
Thomas C, Rajapaksa G, Nikolos F et al (2012) ERbeta1 represses basal-like breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. https://doi.org/10.1186/bcr3358
Traina TA, Miller K, Yardley DA et al (2018) Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2016.71.3495
Tran C, Ouk S, Clegg NJ et al (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. https://doi.org/10.1126/science.1168175
Vladusic E, Guerra-Vladusic HaE FK, et al (2000) Expression and regulation of estrogen receptor beta in human breast tumors and cell lines. Oncol Rep 7:157
Wang J, Zhang C, Chen K et al (2015) ERβ1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-015-3467-3
Wu W, Maneix L, Insunza J et al (2017) Estrogen receptor β, a regulator of androgen receptor signaling in the mouse ventral prostate. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1702211114
Zhu A, Li Y, Song W et al (2016) Antiproliferative effect of androgen receptor inhibition in mesenchymal stem-like triple-negative breast cancer. Cell Physiol Biochem. https://doi.org/10.1159/000443052
Funding
Sponsored by ASTELLAS PHARMA EUROPE LTD ISR GR-72-RG-35.
Author information
Authors and Affiliations
Contributions
AA, PS, ST, IZ, DT, AK, AK, DT, EX, and MK made substantial contributions to acquisition, analysis, and interpretation of data. AA, AGP, and MVK made substantial contributions in the conception, design, and interpretation of the data. AA, AGP, and MVK made substantial contributions in drafting the manuscript and revising it critically for important intellectual content.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The protocol of this study was approved by the National and Kapodistrian University Ethics Committee following the principles of the Declaration of Helsinki.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anestis, A., Sarantis, P., Theocharis, S. et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol 145, 1221–1233 (2019). https://doi.org/10.1007/s00432-019-02872-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02872-9