Abstract
Background
The major mechanism of sepsis is immunosuppression caused by host response dysfunction. It has been found that α-Mangostin (α-M) is a potential candidate as a treatment for multiple inflammatory and immune disorders. To date, the role of α-M in host response during sepsis remains unexplored.
Methods and results
Herein, we examined the effect of α-M on macrophages-mediated host response in the presence of lipopolysaccharide (LPS), and the vital organ function, inflammatory response, and survival rate in septic mice. In murine peritoneal macrophages, α-M induced autophagy and then inhibited LPS-stimulated NLRP3 inflammasome activation, as well as interleukin-1β (IL-1β) production. Moreover, α-M improved phagocytosis and killing of macrophages, and increased M2 macrophages numbers after LPS stimulation. Furthermore, in vivo experiment suggested that α-M reduced serum levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-1β, alanine transaminase (ALT), aspartate transaminase (AST), and creatinine (Cr), whilst increased that of interleukin-10 (IL-10) in caecal ligation and puncture (CLP) mice.
Conclusion
Taken together, these findings showed that α-M-mediated macrophages autophagy contributed to NLRP3 inflammasome inactivation and α-M exerted organ protection in septic mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is redefined to the life-threatening organ dysfunction caused by a dysregulated host response to infection [1, 2]. Despite decades of scientific advances, the prognosis of severe sepsis and septic shock remain poor [3, 4]. Sepsis leads to the imbalance of pro-inflammatory and anti-inflammatory responses and cell-mediated immunosupression plays a central role in this process [5,6,7,8,9]. Importantly, macrophages can recognize and remove the invading pathogens at the early stage of sepsis. However, impaired macrophages can lead to dysfunction of innate immunity and then aggravate sepsis.
With the improved understanding of the mechanisms underlying this immune dysfunction, researchers suggested that the regulation of immune status might be useful in patients with sepsis [10,11,12,13,14,15,16]. α-Mangostin (α-M), a polyphenolic xanthone extracted from the pericarp and bark, has extensive biological activities and pharmacological properties, including anti-inflammation, antibacterial, anti-malarial, anti-tumor and anti-oxidation [17,18,19,20,21,22,23,24]. α-M can protect and improve the survival of cerebral cortical neurons and inhibit the migration and invasion of cancer cells without obvious cytotoxic effects [25, 26]. α-M was usually used as a common medicine against dysentery, suppurative wound infection and ulcer in several tropical countries [17]. Furthermore, α-M could attenuate dextran sulfate sodium (DSS)-induced colitis by inhibiting NF-κB and MAPK pathways [22]. Recently, You et al. reported that α-M exerted anti-biofilm effects on multidrug-resistance staphylococci [24]. However, the effects of α-M on macrophages in sepsis were still unclear. In the current study, we explored the effects of α-M on LPS-treated macrophages and the vital organ function in septic mice with underlying mechanism.
Materials and methods
Animals and reagents
BALB/c mice (male, 6–8 weeks old, 20–25 g) were obtained from Experimental Animal Center, Zhejiang University, Hangzhou, China (Assurance Number: SCXK2014-0004). All animals were bred and housed in separate cages under pathogen-free conditions, as well as appropriate temperature and a 12-h light/12-h dark cycle. All of the experimental procedures were performed in accordance with the Zhejiang University Guidelines for the Welfare of Experimental Animals Hangzhou, Zhejiang province China.
α-Mangostin (α-M) was obtained from Hangzhou Bomai MediTech Co., Ltd., Hangzhou, China. LPS and Bafilomycin A1 were purchased from Sigma-Aldrich, St. Louis, MO. Rabbit polyclonal antibodies against LC3II, Beclin1, P62, NLPR3, ASC, Caspase 1, β-actin, and IL-1β were obtained from Cell Signaling Technology Inc., Beverly, MA, USA. Anti-mouse F4/80 PE-Cyanine 7, anti-mouse CD11c APC, anti-mouse CD206 PE were purchased from eBioscience, San Diego, CA. ELISA kits for mouse IL-1β, IFN-γ, TNF-α, IL-10, TGF-β1, ALT, AST, and Creatinine were obtained from ExCell Biology Inc., Shanghai, China. Beclin1 siRNA and control siRNA were purchased from Shanghai Genechem Co., Ltd., Shanghai, China. The L1210 cell line was purchased from the Cell Resource Center of Shanghai Institutes of Biological Sciences, Shanghai, China.
Cell isolation, cell culture and treatment
Briefly, mice were injected intraperitoneally of 5 ml cold PBS. After shaking, macrophages were harvested from peritoneal lavage by plastic adherence. All cell counts were performed on a hemocytometer using trypan blue exclusion assay. Macrophages were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. After 2-h incubation, floating cells were removed and adherent macrophages were used for experiments. For in vitro treatment, macrophages were pretreated with or without α-M (40 μmol/L) for 2 h, and treated with LPS (1 μg/mL).
Lentiviral infection
According to the manufacturer’s instructions, macrophages were transfected with 100 nM of either Beclin1 RNA interference silence (siRNA) or control siRNA. Then, the transfected cells were incubated for 12 h in medium. Next, the cells were washed and used for experiments.
Flow cytometric analysis
Macrophages in different groups were stained by anti-mouse F4/80 PE-Cyanine 7, anti-mouse CD11c APC, or anti-mouse CD206 PE. Then, flow cytometry analysis was determined by a FACScan flow cytometer. Data were analyzed by FlowJo software (Tree Star, Ashland, OR, USA). Each sample was at least 10,000 events.
Laser scanning confocal microscopy (LSCM)
Macrophages in different groups were incubated with polyclonal rabbit anti-LC3II antibody. The secondary antibodies, FITC-conjugated affinipure goat anti-rabbit IgG (1:100), were used and then applied at 37 °C for 1 h followed by staining with the nuclei with 4, 6-diamidino-2-phenylindole (DAPI). Macrophages were photographed using a laser scanning confocal microscopy.
Cell viability assay
Cell Counting Kit-8 (CCK8) was applied to assess the number of viable cells. The peritoneal macrophages were plated in 96-well plates at a density of 2 × 105 cells/well and then cultured in complete medium in the presence or absence of LPS and α-M (10, 20, 40, 80 μmol/L). The OD450 was tested at 24 h using a microtiter plate reader (Molecular Devices, Menlo Park, CA).
Phagocytosis assay
2 × 105 peritoneal macrophages were incubated with neutral red dye for 30 min at 37 °C. Cells were then washed by PBS buffer, followed by visualization using microscopy and the OD540 test.
Bacterial killing assays analysis
For the determination of bacterial killing by macrophages, peritoneal macrophages (1 × 105 cells) were intubated with L1210 cell (macrophages:L1210 cell = 5:1) at 37 °C for 24 h, and the OD450 was then analyzed by CCK8 method.
Quantitative reverse transcription PCR (q-PCR) analysis
Macrophages mRNA was isolated by RNeasy Mini Kit (Qiagen, Valencia, CA) and then reverse transcribed by a iScript™ kit (BioRad, Hercules, CA) according to the manufacturer’s instructions. The Concentration of mRNA was determined using absorbance at 260 and 280 nm. cDNAs was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies, USA). q-PCR was performed using a CFX96TM Real-Time PCR Detection System (BioRad, Hercules, CA). SYBR Green Master Mix (Bio-Rad) was used for all q-PCR reactions. β-actin was used as an endogenous control. The amplification efficiency was 0.90–0.99.
Western blot analysis
Cells were lysed with lysis buffer on ice and then centrifuged at 13,200 rpm for 20 min at 4 °C. The protein concentration was determined by the bicinchoninic acid (BCA) method. Next, proteins were separated by sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked and subsequently incubated with polyclonal rabbit anti-LC3II (1:1000), anti-Beclin1 (1:1000), anti-P62 (1:1000), anti-NLPR3 (1:500), anti-ASC (1:1000), anti-IL-1β (1:500) or anti-β-actin (1:1000) overnight. Next, PVDF members were incubated with horseradish peroxidase-conjugated polyclonal goat anti-rabbit (1:5000) or monoclonal rabbit anti-mouse secondary antibodies (1:5000) at room temperature for 1 h. The western blot bands were visualized by ECLPlus (Amersham Biosciences, Piscataway, NJ) and analyzed by the ImageQuantTM LAS 4000 biomolecular imager (GE Healthcare Life Sciences, Waukesha, WI), as well as ImageJ software (Image Processing and Analysis in Java software, National Institutes of Health, MD).
Transmission electron microscopy (TEM)
After being collected, macrophages were fixed with 2.5% glutaraldehyde and stored at 4 °C. According to the manufacturer’s instructions, each specimen was performed by a series of manipulation, including fixation, dehydration, embedding, curing, biopsy, and dyeing. The autophagic vacuoles were observed by TEM (JEOL, Tokyo).
Animal sepsis model and treatment
BALB/c mice were subjected to Cecal ligation and puncture (CLP) to establish a model of polymicrobial sepsis. In brief, mice were anesthetized intraperitoneally with 0.3% pentobarbital sodium and the cecum was exposed, ligatured at its external third, and punctured through with a 22-gauge needle. Luminal content was pressed out via puncture sites to ensure patency. Next, the cecum was returned and incisions were closed. Mice in sham group underwent the same procedure without ligation and puncture of the cecum. Postoperatively, mice were injected subcutaneously with 1 mL normal saline (NS). For record of survival rate, mice were observed twice daily for 7 days. In the first 6 h, mice presented with piloerection, lethargy and diarrhea, suggesting the successful establishment of sepsis model.
Each mouse was injected intravenously with 3 mg/kg of α-M 2 h before CLP, immediately, or 2 h after CLP. NS was administrated in a similar fashion as a control. At the end of the survival experiment, all live mice were euthanized by 0.3% pentobarbital sodium followed by cervical dislocation.
Serum biochemistry serum and inflammatory markers assay
Analysis of inflammatory cytokines including IL-1β, IFN-γ, TNF-α, IL-10, TGF-β1 ALT, AST, and Creatinine was performed using ELISA kits according to the manufacturers’ instructions.
Tissue histology
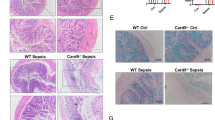
Lungs, livers, and kidneys were harvested and fixed in 4% formalin, stained with hematoxylin–eosin (H&E), and tested by light microscopy. Pathology scores were analyzed by a pathologist blinded for groups.
Statistical analysis
All data are presented as mean ± standard deviation at least three experiments. Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL). The differences between groups were analyzed by the Brown-Forsythe test and, when appropriate, by one-way ANOVA followed by Dunnett’s test. Survival was analyzed with Kaplan–Meier survival curves and compared with the log-rank test. A p value of less than 0.05 was considered significant.
Results
α-Mangostin attenuated LPS-stimulated inflammasome activation in murine peritoneal macrophages
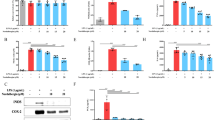
α-Mangostin was extracted from fruit Gracinia Manostatna (Fig. 1a). The structure formula of or Mangostin was shown in Fig. 1b. To identify the effects of α-M on LPS-stimulated inflammasome activation in murine peritoneal macrophages, we first determined NLRP3 inflammasome activation in the absence or presence of LPS. As shown in Fig. 1, LPS (1 μg/ml) priming greatly improved mRNA and protein expressions of NLRP3, ASC, Caspase1, IL-1β in macrophages at 24 h, compared with the PBS control group. Next, to evaluate the inhibitory effect of α-M on NLRP3 activation in the presence or absence of LPS were treated α-M (10, 20, 40, 80 μmol/L) for 24 h. α-M inhibited increased mRNA levels of NLRP3, ASC, Caspase1, and IL-1β induced by LPS (Fig. 2a). Moreover, the increased protein levels of these genes were also reversed by α-M treatment(Fig. 2b, c).
α-Mangostin led to autophagy induction in murine peritoneal macrophages
To illuminate the effects of α-M (10, 20, 40, 80 μmol/L) on autophagy activity, we treated the murine peritoneal macrophages with α-M from 0 to 24 h and determined the levels of LC3-II/LC3-II, the autophagic flux, the subcellular localization of LC3-II and formation of autophagosomes. On the basal autophagy, the ratio LC3-II/LC3-I, Beclin1, and P62 levels were low in macrophages (Fig. 3a). Macrophages treated with α-M represented elevated levels of the ratio LC3-II/LC3-I, Beclin1, and P62, especially 40 mM at 24 h. Besides that, we determined LC3 punctuation (Fig. 3c) and autophagic vacuoles (Fig. 3d) to monitor autophagosome formation, which showed an increase after α-M treatment for 24 h.
To further investigate a potential role for α-M-induced autophagy, we examined the autophagic flux. Generally, bafilomycin A1 (Baf A1) was used to block the autophagosome degradation for measurement of autophagic flux. As expected, the expression of LC3-II/LC3-I, Beclin1, P62 and the number of LC3II-positive cells were further enhanced by α-M treatment combined with Baf A1 (Fig. 3b, c).
Macrophage autophagy deficiency abrogated the effect of α-Mangostin in LPS-stimulated inflammasome inactivation
As described above, α-M reversed LPS-induced increased expression of NLRP3, ASC, Caspase1, and IL-1β in murine peritoneal macrophages. Next, we further explored the role of autophagy in LPS-stimulated NLRP3 inflammasome inactivation in the presence or absence of Beclin1 RNA interference silence (siRNA) (Fig. 4a). Autophagy inhibition by Beclin1 siRNA significantly enhanced protein expression of NLRP3, ASC, Caspase 1, and IL-1β, compared with LPS + α-M group (Fig. 4b). As expected, the above result suggested that autophagy significantly abolished the effect of α-M in the LPS-induced inactivation.
α-Mangostin enhanced phagocytosis and regulated M2 macrophages polarization after LPS stimulation
Here, we examined whether α-M could regulate the intrinsic antibacterial functions and viability of murine peritoneal macrophages after LPS stimulation. α-M had no effect on survival and function of normal macrophages. After LPS stimulation, the viability and immune functions of macrophages were decreased. In vitro, pretreatment with α-M significantly enhanced phagocytosis and killing of peritoneal macrophages (Fig. 5a). However, α-M had little effect on the viability of macrophages (Fig. 5a). Moreover, we isolated murine peritoneal macrophages, sorted them into M1 (F4/80+ CD11c+) into M2 (F4/80+ CD206+) populations. After LPS stimulation, M1 macrophages numbers obviously increased and the number of M2 populations were low. In contrast, the numbers of M2 populations were much higher that of M1 macrophages in LPS + α-M group, compared with LPS + PBS group (Fig. 5b, c).
α-Mangostin inhibited inflammation and protects organ function in sepsis
In vitro, we further evaluated whether α-M exerted a protective effect on septic mice. At 24 h after CLP, pre-treatment (2 h before surgery) with α-M reduced circulating levels of TNF-α, IFN-γ, and IL-1β, whilst elevated levels of IL-10, which was also reflected by decreased pathology scores compared with PBS-treated mice (Fig. 6a). In addition, α-M-treated mice revealed markedly decreased serum levels of creatinine, a marker for renal dysfunction (Fig. 6a). The markers for hepatocellular injury, serum levels of ALT and AST were also reduced after α-M pretreatment in mice (Fig. 6b, c).
The effect of α-Mangostin on septic animal survival
Furthermore, we also studied the survival rate of septic mice after α-M treatment. The mortality rate was 50–70% in the CLP group. In vivo, pre-treatment (2 h prior to CLP or immediately) with α-M significantly improved survival rate (Fig. 7), compared with the normal saline (NS) control group. However, treatment (2 h post CLP) had little effect on the survival rate of CLP. Therefore, these results indicated that early treatment of α-M could promote the prognosis of septic mice.
Discussion
Immunosuppression refers to dysregulated host response to infection in sepsis, resulting in multiple organ dysfunction and high mortality in these patients. Thus, immunosuppression is a promising therapeutic target in the management of septic patients [27,28,29,30]. Impaired function of macrophages was associated with increased severity and mortality in sepsis [31,32,33,34]. Linch et al. reported that the protection effects of IL-5 against polymicrobial sepsis were mediated by macrophages [35]. In addition, macrophages were required for the protective effect of leukocyte cell-derived chemotaxin 2 (LECT2) in sepsis [36]. Conversely, inhibition of peritoneal macrophage phagocytosis would aggravate sepsis. In the current study, we identified the α-M could induce autophagy and then inhibit NLRP3 inflammasome signaling. Moreover, pretreatment of α-M protected vital organ function and inhibited inflammatory cytokines.
α-M, a traditional Chinese medicine, was the major constituent of the fruit hull of the Garcinia Mangostana [18, 19]. More importantly, α-M was well known for a wide range of biological activities and pharmacological effects, such as anti-inflammation, anti-oxidation effects, antitumor effects and COX-2 inhibition [20,21,22,23,24]. It was reported that α-M inhibited brain inflammation and improved the survival of cerebral cortical neurons against Aβ oligomers-induced toxicity in rats [37]. Recently, it was reported that α-M protects against LPS induced cytotoxicity through the regulation of SIRT-1/NF-κB Signaling [23]. Although α-M had been used commonly as a curative agent in diverse disorders, the mechanism of its effects was still unclear. In particular, the effects of α-Mangostin on macrophages in sepsis had not yet been reported.
In this study, we examined the effects of α-M on the expression of the components of NLRP3 inflammasome in LPS-stimulated macrophages. We evaluated the cytotoxic effect of α-M on primary murine macrophages, implying that α-M (concentration from 10 to 80 μmol/L) did not significantly influence cell survival. Previous studies had shown that LPS strongly activated NLRP3 inflammation signaling and then promoted pro-inflammatory cytokines release, leading to the innate immune dysfunction. Moreover, NLRP3 inflammasome activation was strongly associated with sepsis-induced multiple organ injuries and immune dysfunction [38,39,40]. Therefore, NLRP3 inflammasome was a key player in innate immunity of sepsis. Consistent to the previous studies, stimulation of LPS (1 μg/mL) obviously enhanced the expressions of NLRP3, ASC, Caspase 1 and IL-1β in primary murine macrophages at 24 h. Meanwhile, we also found that phagocytosis and killing of macrophages decreased, implying partly inhibition of innate immune functions. Our results further indicated that α-M pretreatment significantly reduced LPS-induced expression of NLRP3, ASC, Caspase 1 and IL-1β, as well as increased phagocytosis and killing of macrophages.
Autophagy was homoeostasis process through invading microorganism as well as degradation of dysfunctional or unnecessary cellular components [13, 14]. Among various essential regulators of autophagy, Beclin1 was required for the initiation of autophagy. LC3II was often used to demonstrate active autophagy. P62 as a LC3-binding protein and modulated the formation of the protein aggregates [16, 41, 42]. To date, it had been shown that NLRP3 inflammasome activation was associated with the regulation of autophagy. Our findings showed that genetic deletion of Beclin1 could abolish NLRP3 inflammasome inactivation caused by α-M pretreatment in LPS-stimulated macrophages. These results indicated that inhibition of NLRP3 inflammasome by α-M was mediated by autophagy induction in macrophages, which offered a new target for inflammatory and immune homoeostasis in sepsis.
The current study also identified α-M as a negative regulator of CLP-induced inflammation and vital organ injuries. Pretreatment of α-M blunted circulatory TNF-α, IFN-γ, and IL-1β, whilst enhanced systemic IL-10 and TGF-β1 at 24 h after CLP. Moreover, α-M downregulated the systemic levels of ALT, AST, and Cr at 24 h in CLP mice, suggesting a protective effect on sepsis. However, the detailed regulatory mechanism of α-M on the expression of cytokines and organ protection during sepsis required further studies.
Taken together, α-M could improve organ injury and prevented from sepsis-induced lethality. The study provided novel insights into the α-M mediated protective effect via autophagy induction and NLPR3 inactivation in macrophages.
References
Shankar-Hari M, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis ans septic shock (sepsis-3). JAMA. 2016;315:775–87.
Sartelli M, et al. Raising concerns about the sepsis-3 definitions. World J Emerg Surg. 2018;13:6.
Hotchkiss RS, et al. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045.
Angus DC, Van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74.
Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126:23–31.
Cohen J, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614.
Jensen IJ, Sjaastad FV, Griffith TS, Badovinac VP. Sepsis-induced T cell immunoparalysis: the ins and outs of impaired T cell immunity. J Immunol. 2018;200:1543–53.
Savelkoel J, Claushuis TAM, vanEngelen TSR, Scheres LJJ, Wiersinga WJ. Global impact of World Sepsis Day on digital awareness of sepsis: an evaluation using Google Trends. Crit Care. 2018;22:61.
Anthony JL, Timothy RB, Matthew RR. Biology and metabolism of sepsis: innate immunity, bioenergetics, and autophagy. Surg Infect. 2016;17:286–93.
Zhang L, Ai YH, Tsung A. Clinical application: restoration of immune homeostasis by autophagy as a potential therapeutic target in sepsis (review). Exp Ther Med. 2016;12:1159–67.
Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: always two sides to a problem. J pathol. 2012;226:255–73.
Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–62.
Chen YQ, Klionsky DJ. The regulation of autophagy–unanswered questions. J Cell Sci. 2011;124:161–70.
Watanabe E, et al. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab Invest. 2009;9:549–61.
Ho J, et al. Autophagy in sepsis: degradation into exhaustion? Autophagy. 2016;12:1073–82.
Chen G, Li Y, Wang W, Deng L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: a review. Expert Opin Ther Pat. 2018;3:1–13.
Scolamiero G, Pazzini C, Bonafè F, Guarnieri C, Muscari C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int J Med Sci. 2018;15:23–30.
Liu T, et al. Alpha-mangostin attenuates diabetic nephropathy in association with suppression of acid sphingomyelianse and endoplasmic reticulum stress. Biochem Biophys Res Commun. 2018;496:394–400.
Pan T, et al. Alpha-Mangostin suppresses interleukin-1β-induced apoptosis in rat chondrocytes by inhibiting the NF-κB signaling pathway and delays the progression of osteoarthritis in a rat model. Int Immunopharmacol. 2017;52:156–62.
Pimchan T, Maensiri D, Eumkeb G. Synergy and mechanism of action of α-mangostin and ceftazidime against ceftazidime-resistant Acinetobacter baumannii. Lett Appl Microbiol. 2017;65:285–91.
You BH, et al. α-Mangostin ameliorates dextran sulfate sodium-induced colitis through inhibition of NF-κB and MAPK pathways. Int Immunopharmacol. 2017;49:212–21.
Franceschelli S, et al. A novel biological role of α-mangostin in modulating inflammatory response through the activation of SIRT-1 signaling pathway. J Cell Physiol. 2016;231:2439–51.
Sivaranjani M, et al. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl Microbiol Biotechnol. 2017;101:3349–59.
Chen ZL, et al. Transferrin-modified liposome promotes α-mangostin to penetrate the blood-brain barrier. Nanomedicine. 2016;12:421–30.
Catorce MN, et al. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J Neuroimmunol. 2016;297:20–7.
Patil NK, Bohannon JK, Sherwood ER. Immunotherapy: a promising approach to reverse sepsis-induced immunosuppression. Pharmacol Res. 2016;111:688–702.
Yadav H, Cartin-Ceba R. Balance between hyperinflammation and immunosuppression in sepsis. Semin Respir Crit Care Med. 2016;37:42.
Fattahi F, Ward PA. Understanding immunosuppression after sepsis. Immunity. 2017;47:3.
Venet F, Rimmelé T, Monneret G. Management of sepsis-induced immunosuppression. Crit Care Clin. 2018;34:97.
Cavaillon JM, Adib-Conquy M. Monocytes/macrophages and sepsis. Crit Care Med. 2005;33(Suppl):S506–9.
Rabani R, et al. Mesenchymal stem cells enhance NOX2 dependent ROS production and bacterial killing in macrophages during sepsis. Eur Respir J. 2018;8:1702021.
Liu Y, et al. Scutellarin Suppresses NLRP3 inflammasome activation in macrophages and protects mice against bacterial sepsis. Front Pharmacol. 2018;8:975.
Xing L, et al. Role of M2 Macrophages in Sepsis-Induced Acute Kidney Injury. Shock. 2017;1:233–9.
Linch SN, Danielson ET, Kelly AM, Lee JJ, Gold JA. The effect of IL-5 on macrophages and PMNs in sepsis. Am J Resp Crit Care. 2009;179:A1024.
Lu XJ, et al. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med. 2013;210:5–13.
Wang Y, et al. Alpha- mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates beta-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology. 2012;62:871–81.
Jin L, Batra S, Jeyaseelan S. Deletion of Nlrp3 augments survival during polymicrobial sepsis by decreasing autophagy and enhancing phagocytosis. J Immunol. 2017;198:1253–62.
Long H, Xu B, Luo Y, Luo K. Artemisinin protects mice against burn sepsis through inhibiting NLRP3 inflammasome activation. Am J Emerg Med. 2016;34:772–7.
Wu D, et al. Intermedin1-53 protects cardiac fibroblasts by inhibiting NLRP3 inflammasome activation during sepsis. Inflammation. 2018;41:505–14.
Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23.
Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–70.
Author information
Authors and Affiliations
Contributions
YG, MH guaranteed integrity of the entire study. YG designed the research, wrote the paper, analyzed the data. XX, QL,YX conducted experiments. MH contributed to revise the paper. All authors critically reviewed the manuscript for important intellectual content and approved the final version.
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, Y., Xu, X., Liang, Q. et al. α-Mangostin suppresses NLRP3 inflammasome activation via promoting autophagy in LPS-stimulated murine macrophages and protects against CLP-induced sepsis in mice. Inflamm. Res. 68, 471–479 (2019). https://doi.org/10.1007/s00011-019-01232-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01232-0