Abstract
Objective and design
The present work investigates the modulation of experimental autoimmune encephalomyelitis (EAE) using genistein before the EAE induction.
Material
Female C57BL/6 mice (n = 96 mice/experiment), 4–6 weeks old, were used to induce the EAE. The mice were divided into three experimental groups: non-immunized group, immunized group (EAE), and immunized and treated with genistein group (Genistein).
Treatment
Genistein was used at a dose of 200 mg/kg s.c. and were initiated 2 days before the immunization and continued daily until day 6 postimmunization.
Methods
Animals were monitored daily for clinical signs of EAE up to day 21. Inflammatory infiltration, demyelination, Toll-like receptor (TLR) expression, cytokines and transcription factors were analyzed in spinal cords.
Results
The present study demonstrates, for the first time, the genistein ability to modulate the factors involved in the innate immune response in the early stages of EAE. The genistein therapy delayed the onset of the disease, with reduced inflammatory infiltration and demyelination. In addition, the expression of TLR3, TLR9 and IFN-β were increased in genistein group, with reduction in the factors of TH1 and Th17 cells.
Conclusion
These findings shed light on the potential of genistein as a prophylactic strategy for multiple sclerosis (MS) prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS), a debilitating neurological autoimmune disease, is characterized by clinical exacerbation, where the damage to the myelin is due to a chronic progressive inflammation of the central nervous system (CNS) [1, 2]. Experimental autoimmune encephalomyelitis (EAE) is the main animal model to study MS and is able to reproduce the inflammatory demyelinating disease of the CNS [3].
The development and progression of EAE are affected by Toll-like receptor (TLR) expression and the autoimmune responses are limited by TLR pathways regulation [4, 5]. In this way, the TLR3 and TLR9 reduction in the spinal cord cells of EAE mice are related to cytokine enhancement and disease progression [6]. Moreover, up-regulation of TLR3 is associated with EAE improvement and increased release of IFN-β [4].
IFN-β plays an important role in MS treatment by reducing the frequency of relapses as well as new lesions appearance [2, 7, 8]. However, studies of new substances for treating or suppressing the MS development are required because of the failure of many patients to respond to existing treatments.
Evidence from basic and clinical studies supports the therapeutic potential of estrogens in MS [9]. Estrogens or selective estrogen receptors (ER) agonists showed potent neuroprotective or anti-inflammatory effects in EAE, but are associated with a number of adverse effects [10,11,12].
Phytoestrogens, including genistein, are good estrogen alternatives that keep the beneficial effects of estrogens but lack potential hazardous effects. Our previous studies show that mice treated daily with genistein after the onset of clinical signs improved clinical symptoms of the disease through down-regulation of IFN-γ, IL-6, IL-12, TNF-α, and Th17 cells, and up-regulation of IL-10 and Foxp3 + CD4+ expression, with reduced rolling of leukocytes [13, 14]. On the other hand, oral genistein treatment alleviated the clinical signs by reducing the production of IFN-γ and enhanced IL-10 secretion in splenocyte and brain if administrated at the onset of disease, but not after EAE establishment [15].
Genistein can also suppress the immune system through different molecular pathways, such as inactivation of NF-κB [16,17,18]. Moreover, genistein regulates immune response by suppressing dendritic cell activation and modulating TLRs expression in microglia, reducing TLR4 and increasing TLR2 [18,19,20,21].
The present study investigated the modulation of the immune response in the EAE using genistein before the EAE induction.
Materials and methods
Mice
Female C57BL/6 mice (n = 96 mice/experiment), 4–6 weeks old, were obtained from the Animal Care Facilities of the Federal University of Juiz de Fora (UFJF), and maintained in microisolator cages in the animal facility in the Laboratory of Immunology. All procedures were in accordance with the principles of the Brazilian Code for the Use of Laboratory Animals. The Committee on the Use of Laboratory Animals from UFJF (Protocol 037/2012) approved all protocols involving mice handling.
Eae induction
The EAE group was subcutaneously (s.c.) immunized at the tail base with 100 µg of myelin oligodendrocyte glycoprotein peptide (MOG35–55), synthesized in the Laboratory of Biophysics, Federal University of São Paulo. MOG35–55 was emulsified v/v in complete Freund’s adjuvant (CFA; Sigma Chemical Co., Saint Louis, USA) supplemented with 400 µg of attenuated M. tuberculosis H37RA (Difco, Detroit, USA). Pertussis toxin (Sigma) was injected intraperitoneally (i.p.) at 300 ng/animal on the day of immunization and 48 h later. A non-immunized control group was included, to which none of the immunization components was administered. Animals were monitored daily, and their neurological impairment was quantified.
Genistein prophylactic strategy
The mice were divided into three experimental groups: non-immunized group, immunized group (EAE), and immunized and treated with genistein group (Genistein). Genistein was used at a dose of 200 mg/kg s.c., because of its proven effects on EAE [13]. The non-immunized and the immunized groups received, s.c., only the diluent DMSO 0.1% (Sigma). Treatments with genistein were initiated 2 days before the immunization and continued daily until day 6 postimmunization. Animals were monitored daily for clinical signs of EAE up to 21 days postimmunization.
Clinical assessment
The clinical status was assessed, scoring certain parts of the mouse body individually according to a previous study [13]. The final clinical score was obtained adding all individual scores assessed.
Histological analysis
To evaluate spinal cord tissue histology, mice (n = 5/group/day analyzed) were euthanized under anesthesia, and perfused by intracardiac puncture with 5 ml 4% buffered formalin on days 7, 14, and 21 postimmunization. The thoracic/lumbar region of each spinal cord was fixed in 4% paraformaldehyde and embedded in paraffin. 5 µm thick sections were stained with hematoxylin and eosin (H&E) to assess tissue damage and inflammation. Semi-quantitative histological evaluation for inflammation was scored in a blinded fashion as 0, no inflammation; 1, immune cellular infiltration only in the perivascular area and meninges; 2, mild cellular infiltration in parenchyma; 3, moderate cellular infiltration in parenchyma; 4, severe cellular infiltration in parenchyma [22]. Also, to assess the demyelination, 10 µm thick sections of the spinal cord at day 21 postimmunization were stained with Luxol fast blue (LFB) cresyl violet. The demyelination was assessed using a scoring system described by [23]: +/- traces of perivascular or subpial demyelination (score 1); + marked perivascular or subpial demyelination (score 2); ++ confluent perivascular or subpial demyelination (score 4); +++ massive confluent demyelination (e.g., half-spinal cord completely demyelinated) (score 6); ++++ extensive demyelination (complete demyelination) (score 8).
Isolation of spinal cord cells
On day 7 after immunization, mice (n = 8/group) were euthanized under anesthesia (i.p.) and perfused through the left ventricle with phosphate buffered saline (PBS). Spinal cords were dissected, macerated, and filtered using a 70 µm cell strainer (BD Biosciences, Bedford, USA) in RPMI medium 1640 (Gibco, Grand Island, USA) with 10% fetal bovine serum (FBS) (Gibco). Dispersed spinal cord cells were incubated in RPMI containing 2 mg/mL of collagenase D (Roche, Mannheim, Germany) at 37 °C for 45 min. Spinal cord cells were collected after Percoll density gradient centrifugation [24] and washed with staining buffer (PBS, 1% FBS, 0.09% sodium azide). The cell pellet was suspended in ACK solution, centrifuged at 350×g for 5 min, counted with Trypan, and suspended in staining buffer for flow cytometry analysis.
Flow cytometry analysis
Spinal cord cells were incubated with anti-mouse F4/80-PerCP (BD Biosciences Pharmingen, San Diego, USA), anti-mouse TLR4-PE or anti-mouse TLR2-PE (eBioscience, San Diego, USA), and anti-mouse CD11c-FITC (eBioscience) for TLR4 or TLR2 expression. After 30 min at 4 °C, the cells were washed in staining buffer and kept in the dark at 4 °C until acquisition. For TLR3 or TLR9 expression, spinal cord cells were incubated with anti-mouse F4/80-PerCP and anti-mouse CD11c-FITC. After 30 min at 4 °C, the cells were washed in staining buffer and fixed/permeabilized with BD-Pharmingen Fix/Perm solution, washed in BD Pharmingen Perm/Wash Buffer, and stained with anti-mouse TLR3-PE or anti-mouse TLR9-PE (IMGENEX, San Diego, USA). After 30 min at 4 °C, the cells were washed in staining buffer and kept in the dark at 4 °C until acquisition. For IL-10 and Foxp3 expression, spinal cord mononuclear cells were incubated with anti-mouse CD4-PerCP. After 30 min at 4 °C, cells were washed in staining buffer. The cells were then permeabilized with BD Pharmingen Fix/Perm solution, washed in BD Pharmingen Perm/Wash Buffer, and stained with anti-mouse IL-10-PE and anti-mouse Foxp3-FITC (BD Biosciences). After 30 min at 4 °C, cells were washed in staining buffer and kept in the dark at 4 °C until acquisition. The cells were acquired and analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, San Diego, USA).
Cytokine assays
Spinal cord tissue samples were obtained from each group on day 7 postimmunization and weighed (n = 5 mice/group). One hundred milligrams of each tissue was homogenized in 1 ml 0.4 M NaCl containing 0.05% Tween 20 (Merck &Co., Inc., Whitehouse Station, USA), 0.5% bovine serum albumin (Sigma), 0.1 M phenylmethylsulfonyl fluoride (Sigma), 0.1 M benzethonium chloride (Sigma), 10 mM ethylenediaminetetraacetic acid (Sigma), and 20 KIU/ml aprotinin (Sigma). The homogenate was centrifuged at 2000×g for 15 min at 4 °C and supernatants were collected. The concentrations of IFN-γ, IL-6, IL-12p40, IL-17 and TGF-β were determined by ELISA using commercially available antibodies (BD Biosciences), in accordance to the procedures supplied by the manufacturer.
Quantitative real-time PCR
The spinal cords (n = 4 mice/group) were collected individually on day 7 postimmunization and stored in RNA-later (Ambion, Austin, TX) at − 80 °C. Total RNA was extracted from the spinal cords according to the manufacturer’s instructions (RNeasy kit, Qiagen, Valencia, CA). After treatment with RNase-Free DNase (Qiagen), first-strand cDNA was synthesized using Super Script III First Strand, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). For each gene, an optimal amplification condition (cDNA and primer concentration) was selected. Relative quantification was performed in duplicate using real-time PCR (Prism 7300 Sequence detection Systems; Applied Biosystems; Foster City, CA, USA). Amplification conditions consisted of 10 min at 95 °C, followed by 40 cycles (95 °C for 15 s, TA 30 s, and 60 °C for 30 s). After the run, melting curve analysis was performed for each sample to confirm that a single specific product was generated. The primer sets for each gene were as follows:
IFN-β, 5′–GGAGATGACGGAGAAGATGC–3′ (forward) and 5′–CCCAGTGCTGGAGAAATTGT–3′ (reverse); RORγt, 5′–CGCGGAGCAGACACACTTA–3′ (forward) and 5′–CCCTGGACCTCTGTTTTGGC–3′ (reverse); T-bet, 5′–GATCGTCCTGCAGTCTCTCC–3′ (forward) and 5′–AACTGTGTTCCCGAGGTGTC–3′ (reverse); β-actin, 5′–GCTGTCCCTGTATGCCTCT–3′ (forward) and 5′–GTCACGCACGATTTCCCTC–3′ (reverse). RNA was normalized to expression of β-actin and relative expression was calculated with the 2− ΔΔCt method.
Statistics
The present results are representative of three independent experiments expressed as mean ± standard deviation (SD) of n observations where n ≥ 4. The significance of the differences between groups was analyzed using a Student’s t test, Mann–Whitney test, or two-way ANOVA where appropriate using GraphPad Prism 5.00 (GraphPad Prism Software Inc., San Diego, CA USA). Differences were considered significant at p < 0.05.
Results
Genistein therapy delays the onset and suppresses the severity of EAE symptoms
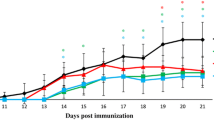
The clinical course of EAE was investigated in mice for 21 days. The EAE group showed 100% disease incidence with very typical signs characterized by an elevated clinical score and accentuated body weight loss (Fig. 1a, b). On the other hand, the group treated with genistein showed a delay of 5 days in the onset of clinical signs of disease, and much less severe clinical signs. The EAE group had a mean maximum score of 5.4 and the mean score of the genistein group was 1.4 (Fig. 1a). Maximum clinical score determination confirmed that genistein is able to reduce disease severity. Parallel to the worsening of clinical signs, severe loss of body mass was observed in animals of the EAE group, but was not observed in the group treated with genistein, which was not statistical different of the non-immunized group (Fig. 1b).
Genistein attenuates the progression of EAE disease. Clinical score (a) and weight (b) of C57BL/6 mice that were non-immunized, immunized (EAE) with 100 µg MOG35–55, or immunized and treated with genistein (Genistein) were recorded from day 0 until day 21 postimmunization. Genistein (200 mg/kg) was administered s.c. daily from day − 2 until day 6 postimmunization. Each point represents the mean ± SD. *p < 0.001 versus other groups. Results were representative of three independent experiments
Genistein therapy reduces inflammatory infiltration and demyelination during the development of EAE
The presence of inflammatory infiltrates in the spinal cord on the 7th, 14th, and 21st days postimmunization was assessed by histological analysis using H&E stain. The EAE group of mice showed cell infiltrated at all days evaluated, with the peak infiltration at day 14 postimmunization (Fig. 2a–c). The mice treated with genistein showed no inflammatory infiltration at days 7 and 14 (Fig. 2d, e). In the 21st day, this group had mild cellular infiltration in the perivascular area and meninges (Fig. 2f). The histological sections of the spinal cord of the non-immunized group were considered normal (Fig. 2g). As expected, the genistein group showed a delayed in the inflammation score compared to the EAE group (Fig. 2h).
Genistein reduces spinal cord inflammation in EAE. The inflammatory infiltrate in the spinal cord of C57BL/6 mice immunized (EAE) with 100 µg MOG35 − 55 (a–c), or immunized and treated with genistein (Genistein) (d–f), or non-immunized (g) (n = 5/group/day analyzed) was evaluated on days 7, 14, and 21 postimmunization. Micrographs show histological analysis of sections from the experimental groups stained with H&E and captured at a magnification of ×40, scale bar = 20 µm. Arrow = inflammatory infiltrate. h The inflammation score was evaluated on days 7, 14, and 21 postimmunization. Each bar represents the mean ± SD. *p < 0.001 when compared genistein and EAE groups. Results were representative of three independent experiments, each with five subjects/group/day
Demyelination in the spinal cords was observed at day 21 postimmunization by histological analysis using LFB stain. Concurrent with the delayed of inflammation score, the genistein group showed lower demyelination score (Fig. 3a). The histological sections of the spinal cord of the non-immunized group were considered normal (Fig. 3b). The LFB staining of the spinal cord was remarkably reduced in the genistein group compared to the EAE group (Fig. 3c, d).
Genistein reduces spinal cord myelin damage in EAE. The demyelination score (a) and demyelination images were evaluated in the spinal cord of C57BL/6 mice that were non-immunized (b), immunized (EAE) with 100 µg MOG35–55 (c), or immunized and treated with genistein (Genistein) (d) (n = 5/group) at day 21 postimmunization. Images show histological analysis of sections stained with Luxol Fast Blue and captured at a magnification of ×40, scale bar = 50 µm. a Each bar represents the mean ± SD. *p < 0.001 when compared genistein and EAE groups. Results were representative of three independent experiments, each with five subjects/group
Genistein therapy increased TLR3 and TLR9 expression in dendritic cells and macrophages in the spinal cord
The median fluorescence intensity (MFI) of TLR2, TLR3, TLR4, and TLR9 in dendritic cells (CD11c + F4/80−) and macrophages (CD11c + F4/80+) in the spinal cord was assessed by flow cytometry at day 7 postimmunization. The results showed an increase in TLR3 and TLR9 expression, in both cells, in the genistein group (Fig. 4b, d, f, h). The MFI of TLR2 was reduced in the DCs of the genistein group (Fig. 4a) and alterations in the MFI of TLR4 were not observed (Fig. 4c, g).
Genistein increases expression of TLR3 and TLR9. The median fluorescence intensity (MFI) of TLR2 (a, e), TLR3 (b, f), TLR4 (c, g), and TLR9 (d, h) (n = 8 mice/group) in dendritic cells (CD11c + F4/80–), or macrophages (CD11c + F4/80+) in the spinal cord of C57BL/6 mice that were non-immunized, immunized (EAE) with 100 µg MOG35–55, or immunized and treated with genistein (Genistein) at day 7 postimmunization. Bars represent mean ± SD. *p < 0.05. Representative histograms was shown: non-immunized (red); EAE (blue); Genistein (black) and isotype (gray). MHC-II-APC; CD11c-FITC; F4/80-PerCP. Representative gate strategy was shown. Results were representative of 3 independent experiments, each with eight subjects/group. (Color figure online)
Genistein increases endogenous IFN-β production in the spinal cord
Relative IFN-β mRNA expression was analyzed in supernatants of macerated spinal cords on day 7 after immunization. Interestingly, genistein was able to increase relative IFN-β mRNA expression in the spinal cord (Fig. 5). We hypothesized that the genistein could have a protective effect on EAE by enhanced levels of endogenous IFN-β in CNS.
Genistein increases the relative expression of IFN-β in EAE. The relative expression of IFN-β in the spinal cord of C57BL/6 mice that were non-immunized, immunized (EAE) with 100 µg MOG35–55, or immunized and treated with genistein (Genistein) on day 7 postimmunization (n = 4 mice/group). Bars represent mean ± SD. *p < 0.05. Results were representative of three independent experiments, each with four subjects/group. Dashed line = relative expression of the non-immunized group
Genistein therapy decreased transcription factors for Th1 and Th17 cells and up-regulated Treg cells in the spinal cord
To examine whether genistein regulated the production of cytokines and the expression of T-bet and RORγt during EAE development, the levels of cytokines IL-12p40, IFN-γ, IL-6, TGF-β, and IL-17, and the relative mRNA expression of transcription factors T-bet and RORγt were analyzed in spinal cords on day 7 after immunization. In addition, the MFI of CD4 + Foxp3+ and CD4 + IL-10+ cells were analyzed in spinal cords on day 7 after immunization.
Genistein treated mice showed lower expression of T-bet (Fig. 6c) and RORγt (Fig. 7c), in accordance with the reduced production of IL-12p40 (Fig. 6a), IFN-γ (Fig. 6b), and IL-6 (Fig. 7a) when compared to the EAE group. On the other hand, the MFI of CD4 + Foxp3+ (Fig. 8a) and CD4 + IL-10+ (Fig. 8b) cells, and the production of TGF-β (Fig. 8h) were up-regulated in genistein treated mice when compared to the EAE group.
Genistein promotes Th1 suppression. Production (n = 5 mice/group) of IL-12p40 (a) and IFN-γ (b), and relative expression (n = 4 mice/group) of T-bet (c) in the spinal cord of C57BL/6 mice that were non-immunized, immunized (EAE) with 100 µg MOG35–55, or immunized and treated with genistein (Genistein) on day 7 postimmunization. Bars represent mean ± SD. *p < 0.05. Results were representative of three independent experiments. The dashed line represents relative expression of the non-immunized group
Genistein promotes Th17 suppression. Release (n = 5 mice/group) of IL-6 (a), and IL-17A (b), and relative expression (n = 4 mice/group) of RORγt (c) in the spinal cord of C57BL/6 mice that were non-immunized, immunized (EAE) with 100 µg MOG35–55, or immunized and treated with genistein (Genistein) on day 7 postimmunization. Bars represent mean ± SD. *p < 0.05. Results were representative of three independent experiments. The dashed line represents relative expression of the non-immunized group
Genistein promotes a regulatory environment in EAE. Median fluorescence intensity (MFI) of Foxp3 and IL-10 in CD4+ cells (n = 8/group) and release of TGF-β (n = 5/group) in the spinal cord of C57BL/6 mice that were non-immunized, immunized (EAE) with 100 µg MOG35–55, or immunized and treated with genistein (Genistein) on day 7 postimmunization. a, c CD4 + Foxp3+, b, d CD4 + IL-10+, and h TGF-β. e–g Gating strategy for CD4 + cells selection. h TGF-β production. Bars represent mean ± SD. *p < 0.05. Representative histograms were shown: non-immunized (red); EAE (blue); Genistein (black) and isotype (gray). CD4-PerCP; IL-10-PE; Foxp3-FITC. Results were representative of three independent experiments. (Color figure online)
Discussion
Phytoestrogens, including genistein, act at the estradiol receptors (ER) with therapeutic activity similar to estrogen [11, 25]. In spite of the effect of genistein and estrogen in ER, their mechanisms of action are not fully understood, being able to produce estrogenic or anti-estrogenic effects [26, 27]. Besides acting in the ER, the genistein can activate different intracellular pathways, such as ERK, MAPK, NF-κB, microRNA [17, 18, 28]. In EAE model genistein modulates the immune response through reduction in the Th1 and Th17 responses, associated with an increase in Treg cells in the EAE model, as shown by our group [13, 14]. Oral treatment with genistein can only reduce the EAE severity if initiated in the early stages of the disease [15]. Due to this, the genistein prophylactic strategy was initiated before the EAE induction in the present study, with reduced EAE score result and decreases in T-bet and RORγt expression, associated with increased expression of IFN-β, TLR3, TLR9, and TGF-β. These evaluations were done in day 7 due to represent the beginning of the disease development. Previous work from our group shows that although at day 7 postimmunization the animals with EAE shows no clinical signs of disease, it is already possible to observe cellular infiltration and increased pro-inflammatory cytokines in the CNS, which are pathological characteristics of the disease course [29].
The adaptive immunity function in EAE and MS is already known; however, the role of innate immunity in disease progression is still unclear. The Toll-like receptors are pattern recognition receptors expressed in several cell types that play critical roles in initiating inflammatory responses and linking the innate to the adaptive immune response. In EAE, interactions of TLR with PRPs contribute to the activation of T cells against myelin antigen. By this way, the modulation of different TLRs modulates the established response in EAE [30,31,32].
Signaling via TLR2 and TLR4 induces IL-1, IL-6, and IL-12 production that enhance T naïve cells differentiation into Th1 and Th17 cells [30]. In addition, development of EAE is characterized by reduced TLR3 and TLR9 expression [6]. On the other hand, TLR3 and TLR9 signaling can lead to IFN-β production, which activates suppressor T cells and inhibits IL-17 and IL-23 production [33]. In the present study, genistein was able to increase the TLR3 and TLR9 MFI in the spinal cord. Previous studies report that TLR3 stimulation induces interferon regulatory factor (IRF)-3 activation, which increases the production of IFN-β and improves EAE [34].
Interferon-β is well established as a disease-modifying treatment for patients with multiple sclerosis, and is effective against EAE. Treatment efficacy has been shown by decreases in annual relapse rate, disability progression and inflammatory brain lesions [35]. In EAE, IFN-β reduces the Th17 cell response by up regulating IL-10 and IL-27 expression, and by reducing VCAM-1 and ICAM-1 expression that diminishes monocyte migration to the CNS [35,36,37]. Interestingly, genistein was able to increase the relative expression of IFN-β in the spinal cord. Together, our data showed that the suppressive effect of genistein was probably mediated by the increase in the level of endogenous IFN-β.
During EAE, the inflammatory process triggers demyelination of the neuron [38]. The results of histological analyses of spinal cord showed delayed in the inflammatory infiltration in the CNS together with lower demyelination. These data could suggest that genistein inhibits migration of activated inflammatory cells from the periphery to the CNS of C57BL/6 mice with EAE.
IL-12 and T-box transcription factor (T-bet) are involved in the differentiation of Th1 cells that were originally proposed to be the pathogenic cell population in MS and EAE [39,40,41]. Moreover, different studies show that inhibition of T-bet correlates with reduced susceptibility to EAE [42, 43]. In the present study, genistein was able to reduce the production of IL-12, IFN-γ, and the relative T-bet mRNA expression in the spinal cord, suggesting that genistein prophylactic strategy promoted reduction in the differentiation of Th0 into Th1 cells.
IL-17-producing T helper cells are considered the main population of pathogenic T cells responsible for MS development [44,45,46]. Therefore, transgenic mice that over-expressed RORγt presents an anti-MOG Th17 response more robust and develop a more severe form of EAE compared to wild type mice [47]. In the present study, the genistein prophylactic strategy reduced the relative expression of RORγt and the release of IL-6, suggesting reduced differentiation to Th17 cells profile.
In contrast, TGF-β alone drives Foxp3 expression and induces the differentiation of naïve T cells into regulatory T cells (Treg) that are central element for the maintenance of peripheral tolerance [48,49,50]. The genistein was able to reduce the production of IL-6, concomitant with the increase in TGF-β production and the MFI of CD4 + Foxp3 + and CD4 + IL-10 + cells, suggesting a favorable microenvironment for differentiation of Tregs in the CNS.
The role of genistein in the present study enhances the knowledge on the acting pathways of this compound, suggesting a possible use in a prophylactic strategy. Despite the beneficial effects of genistein, suggested in different works including the present results, is important to note that genistein may have adverse effects, probably due its different intracellular mechanisms of action and the intake of high dosages, deserving further toxicological investigations [51,52,53].
In conclusion, genistein prophylactic strategy improved the clinical signs of EAE by immunomodulatory effects on some parameters of the innate immune response that are crucial for the development of MS. Moreover, the results suggested that the increase in endogenous IFN-β could be the mechanism that allows genistein to decrease the EAE severity. This can suggest possible benefits of dietary supplementation with genistein, or the use of genistein-enriched diets, in preventing the onset of MS, or inhibiting disease relapses.
References
Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–99.
O’Gorman C, Lin R, Stankovich J, Broadley SA. Modelling genetic susceptibility to multiple sclerosis with family data. Neuroepidemiology. 2013;40:1–12.
Rao P, Segal BM. Experimental autoimmune encephalomyelitis. Methods Mol Biol. 2012;900:363–80.
Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol. 2006;11:7505–9.
Gooshe M, Abdolghaffari AH, Gambuzza ME, Rezaei N. The role of Toll-like receptors in multiple sclerosis and possible targeting for therapeutic purposes. Rev Neurosci. 2014;25:713–39.
Evangelista MG, Castro SBR, Alves CC, Dias AT, Souza VW, Reis LB, Silva LC, Castañon MC, Farias RE, Juliano MA, Ferreira AP. Early IFN-γ production together with decreased expression of TLR3 and TLR9 characterizes EAE development conditional on the presence of myelin. Autoimmunity. 2016;49:258–67.
Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004;63:15–8.
Burks J. Interferon-beta1β for multiple sclerosis. Expert Ver Neurother. 2005;5:153–64.
Goverman J. Autoimmune T cell response in the central nervous system. Nat Rev Immunol. 2009;9:393–407.
Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, de Nicola AF. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation. 2008;15:76–83.
Lélu K, Laffont S, Delpy L, Paulet PE, Périnat T, Tschanz SA, Pelletier L, Engelhardt B, Guéry JC. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386–93.
Spanier JA, Nashold FE, Mayne CG, Nelson CD, Hayes CE. Vitamin D and estrogen synergy in Vdr-expressing CD4(+) T cells is essential to induce Helios(+)FoxP3(+) T cells and prevent autoimmune demyelinating disease. J Neuroimmunol. 2015;286:48–58.
De Paula ML, Rodrigues DH, Teixeira HC, Barsante MM, Souza MA, Ferreira AP. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2008;8:1291–7.
Castro SBR, Junior COR, Alves CCS, Dias AT, Alves LL, Mazzoccoli L, Mesquita FP, Figueiredo NS, Juliano MA, Castañon MC, Gameiro J, Almeida MV, Teixeira HC, Ferreira AP. Immunomodulatory effects and improved prognosis of experimental autoimmune encephalomyelitis after O-tetradecanoyl-genistein treatment. Int Immunopharmacol. 2012;12:465–70.
Jahromi SR, Arrefhosseini SR, Ghaemi A, Alizadeh A, Sabetghadam F, Togha M. Effect of oral genistein administration in early and late phases of allergic encephalomyelitis. Iran J Basic Med Sci. 2014;17:509–15.
Dijsselbloem N, Goriely S, Albarani V, Gerlo S, Francoz S, Marine JC, Goldman M, Haegeman G, Vanden Berghe W. A critical role for p53 in the control of NF-kappa B-dependent gene expression in TLR-4-stimulated dendritic cells exposed to genistein. J Immunol. 2007;178:5048–57.
Byun EB, Sung NY, Yang MS, Lee BS, Song DS, Park JN, Kim JH, Jang BS, Choi DS, Park SH, Yu YB, Byun EH. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-κB and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food Chem Toxicol. 2014;74:255–64.
Kim DH, Jung WS, Kim ME, Lee HW, Youn HY, Seon JK, Lee HN, Lee JS. Genistein inhibits pro-inflammatory cytokines in human mast cell activation through the inhibition of the ERK pathway. Int J Mol Med. 2014;34:1669–74.
Buathong N, Poonyachoti S, Deachapunya C. Isoflavone genistein modulates the protein expression of toll-like receptors in cancerous human endometrial cells. J Med Assoc Thai. 2015;98:S31–8.
Jeong JW, Lee HH, Han MH, Kim GY, Kim WJ, Choi YH. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem Biol Interact. 2014;212:30–9.
Zhou X, Yuan L, Zhao X, Hou C, Ma W, Yu H, Xiao R. Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-Κb. Nutrition. 2014;30:90–5.
Xiao J, Liu W, Chen Y, Deng W. Recombinant human PDCD5 (rhPDCD5) protein is protective in a mouse model of multiple sclerosis. J Neuroinflammation. 2015;12:117.
Abdul-Majid KB, Wefer J, Stadelmann C, Stefferl A, Lassmann H, Olsson T, Harris RA. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4-/- and CD8-/- DBA/1 mice defines qualitative roles of different T cell subsets. J Neuroimmunol. 2003;141:10–9.
Blanco YC, Farias AS, Goelnitz U, Lopes SC, Arrais-silva WW, Carvalho BO, Amino R, Wunderlich G, Santos LM, Giorgio S, Costa FT. Hyperbaric oxygen prevents early death caused by experimental cerebral malaria. Plos One. 2008;3:e3126.
Chrzan BG, Bradford PG. Phytoestrogens activate estrogen receptor beta1 and estrogenic responses in human breast and bone cancer cell lines. Mol Nutr Food Res. 2007;51:171–7.
Moran J, Garrido P, Alonso A, Cabello E, Gonzalez C. 17beta- Estradiol and genistein acute treatments improve some cerebral cortex homeostasis aspects deteriorated by aging in female rats. Exp Gerontol. 2013;48:414–21.
Rietjens IMCM., Louisse J, Beekmann K. The potential health effects of dietary Phytoestrogens. Br J Pharmacol. 2016;174:1263–80.
de la Parra C, Castillo-Pichardo L, Cruz-Collazo A, Cubano L, Redis R, Calin GA, Dharmawardhane S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr Cancer. 2016;68:154–64.
Dias AT, Castro SBR, Alves CS, Mesquita FP, Figueiredo NS, Evangelista MG, Castanon MCMN., Juliano MA, Ferreira AP. Different MOG35-55 concentrations induce distinguishable inflammation through early regulatory response by IL-10 and TGF-β in mice CNS despite unchanged clinical course. Cell Immunol. 2015;293:87–94.
Marta M, Andersson A, Isaksson M, Kämpe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–75.
Zhang ZY, Zhang Z, Schluesener HJ. Toll-like receptor-2, CD14 and heat-shock protein 70 in inflammatory lesions of rat experimental autoimmune neuritis. Neuroscience. 2009;159:136–42.
Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109:13064–9.
Miranda-Hernandez S, Baxter AG. Role of toll-like receptors in multiple sclerosis. Am J Clin Exp Immunol. 2013;2:75–93.
Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–30.
Hegen H, Auer M, Deisenhammer F. Pharmacokinetic consideration in the treatment of multiple sclerosis with interferon-β. Expert Opin Drug Metab Toxicol. 2015;11:1803–19.
Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–90.
Khorooshi R, Mørch MT, Holm TH, Berg CT, Dieu RT, Dræby D, Issazadeh-Navikas S, Weiss S, Lienenklaus Owens T. Induction of endogenous Type I interferon within the central nervous system plays a protective role in experimental autoimmune encephalomyelitis. Acta Neuropathol. 2015;130:107–18.
Rossi B, Angiari S, Zenaro E, Budui SL, Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J Leukoc Biol. 2011;89:539–56.
Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, Lord GM, Jenner RG. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268.
Schulz EG, Mariani L, Radbruch A, Höfer A. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–83.
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69.
Bettelli B, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87.
Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immun. 2004;21:719–31.
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73.
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5.
Yang C, He D, Yin C, Tan J. Inhibition of interferon regulatory factor 4 suppresses Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Scand J Immunol. 2015;82:345–51.
Martinez NE, Sato F, Omura S, Kawai E, Takahashi S, Yoh K, Tsunoda I. RORγt, but not T-bet, overexpression exacerbates an autoimmune model for multiple sclerosis. J Neuroimmunol. 2014;276:142–9.
Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–8.
Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4 + T cells and the control of autoimmune disease. Curr Opin Immunol. 2004;16:695–701.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87.
Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Crit Rev Toxicol. 2011;41:463–506.
Chatterjee G, Roy D, Khemka VK, Chattopadhyay M, Chakrabarti S. Genistein, the isoflavone in soybean, causes amyloid beta peptide accumulation in human neuroblastoma cell line: implications in Alzheimer’s disease. Aging Dis. 2015;6:456–65.
Singh P, Sharma S, Rath SK. Genistein induces deleterious effects during its acute exposure in Swiss mice. Biomed Res Int. 2014;2014:619617.
Acknowledgements
This work was supported in part by Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico [Grant Numbers 481459/2009-0, 303369/2009-4, 306575/2012-4, 470768/2013-4, and 306768/2015-1]; Fundação de Amparo à Pesquisa do Estado de Minas Gerais [grant numbers 02236/10, PPM 0216/10 and PPM 00269-14]; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Grant Number PNPD-2882/2011].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Dias, A.T., de Castro, S.B.R., de Souza Alves, C.C. et al. Genistein modulates the expression of Toll-like receptors in experimental autoimmune encephalomyelitis. Inflamm. Res. 67, 597–608 (2018). https://doi.org/10.1007/s00011-018-1146-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1146-7