Abstract
The number of studies which evaluated interferon-gamma release assays (IGRAs) results after anti-tuberculosis (TB) treatment has been rapidly increasing. The aim of this study was to investigate the potential use of IGRAs (QFT-GIT, T-SPOT.TB, QFT-Plus) in assessing the response to anti-TB treatment. We searched all studies in English language published from 1 October 2011 to 18 November 2018 in PubMed, Web of Science, and Scopus. Our search included the term “tuberculosis treatment AND interferon-γ release assay”. We included studies evaluating the performance of commercial IGRAs (including QFT-GIT, T-SPOT.TB and QFT-Plus) before and after the anti-TB treatment. We performed subgroup analysis based on the age (children, adults), type of TB (active, latent, active and latent, and contacts exposed to MDR defined as MDR LTBI), type of IGRAs (QFT-GIT and T-SPOT.TB), and follow-up interval (2, 3, 4, 6, 9 months). Of the 18 included studies, 12 used QFT-GIT for assessment of IGRA performance after therapy, 1 used T-SPOT.TB, and 3 used both QFT-GIT and T-SPOT.TB. Since then, only two studies have assessed the QFT-Plus performance during therapy. According to the results of the meta-analysis, the pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) following anti-TB therapy was estimated at 76% [95% CI 70–81%] and no difference was found compared to the pooled positive rate of IGRAs before initiation of therapy which was 76% [95% CI 60–89%]. The subgroup analysis showed that the pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) after anti-TB therapy was significantly higher in monitoring active TB subjects [80% (95% CI 74–88%)] than LTBI [71% (95% CI 70–81%)]. Available data are now sufficient to suggest that monitoring changes in the IGRAs (QFT-GIT and T-SPOT.TB) response during anti-TB treatment may have limited use in evaluating the effectiveness of treatment, while the monitoring changes in QFT-Plus during anti-tubercular treatment are recommended to determine treatment efficacy or for treatment monitoring. Further research is needed to establish the efficacy of this new assay as marker on a larger scale for treatment monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the latest World Health Organization (WHO) global tuberculosis report, tuberculosis (TB) is the ninth prime cause of death worldwide and has been responsible for 1.7 million deaths in 2017 (Organization 2016). On the other hand, latent TB infection (LTBI) affects one-quarter of the world’s population and can develop into active TB in almost 15% of the cases (Getahun et al. 2015; Sester et al. 2015); therefore, the accurate diagnosis and treatment of LTBI can control and eliminate a TB outbreak (Lönnroth et al. 2015). Patient-centered care is one of the main components of WHO’s End TB Strategy. According to the latest reports, it has been estimated that 54 million lives were saved through TB diagnosis and treatment between 2000 and 2017 (World Health Organization 2016).
Currently, a new biomarker to monitor treatment efficacy would be a major advance for the management of TB globally (Goletti et al. 2018). Specifically, in the case of extrapulmonary TB, it is hard to correlate the sputum result with the clinical outcome when Mycobacterium. tuberculosis (Mtb) cannot be detected in sputum. It is even more difficult in LTBI cases, where Mtb cannot be isolated despite its presence (Goletti et al. 2016). In addition to monitoring the treatment efficacy in patients, a new biomarker may allow the assessment of shorter course regimens for both LTBI and active TB treatment. A new biomarker capable of confirming adequate TB treatment would also reduce transmission from reactivation due to treatment failure.
The number of studies which evaluated interferon-gamma release assays (IGRAs) results after anti-tuberculosis (TB) treatment has been rapidly increasing. Currently, there are three commercial IGRAs available: the QuantiFERON-TB Gold In-Tube assay (QFT-GIT) (Cellestis, Valencia, CA, USA), the T-SPOT.TB assay (Oxford Immunotec, Inc, Oxfordshire, UK) and an updated version of the QFT-GIT assay termed QuantiFERON TB Plus (QFT-Plus). QFT-GIT assay is an enzyme-linked immunosorbent assay-based, whole-blood test that uses peptides from ESAT-6 and CFP-10 as well as peptides from TB7.7 [Rv2654c]. T-SPOT.TB is an enzyme-linked immunospot assay using ESAT-6 and CFP-10 peptides. QFT-Plus is the next-generation IGRA launched by Qiagen in 2015. It includes an additional antigen tube (TB2) [QIAGEN (2014). www.quantiferon.com/irm/content/package-inserts.aspx Date last accessed: March 13], which contains peptides stimulating Mtb-specific CD8+ T cells, in addition to the CD4+ T-cell response detected with QFT-GIT (Petruccioli et al. 2016).
In this systematic review and meta-analysis, we investigated the potential use of IGRAs (QFT-GIT, T-SPOT.TB, QFT-Plus) in assessing the response to anti-TB treatment.
Materials and Methods
Search Strategy
This study was performed in accordance with the PRISMA protocol (Preferred Reporting Items for Systematic reviews and Meta-analysis) (Moher et al. 2009). We searched all studies in English language published from 1 October 2011 to 18 November 2018 in PubMed, Web of Science, and Scopus. Our search included the term “tuberculosis treatment AND interferon-γ release assay”. Additional references were screened through references cited by the identified studies.
Study Selection and Data Extraction
We included studies aimed at evaluating the performance of commercial IGRAs (including QFT-GIT, T-SPOT.TB and QFT-Plus) before and after the anti-TB treatment.
The results of QFT-GIT were assessed according to the manufacturer’s criteria (Keshavarz Valian et al. 2019). The result was considered positive if the IFN-γ value in TB antigen tube was ≥ 0.35 IU/ml after correction for negative control. The result was considered negative if the response to the specific antigens was < 0.35 IU/mL and if the IFN-γ of the positive control (mitogen well) was ≥ 0.5 IU/mL.
The results of T-SPOT.TB were assessed according to the manufacturer’s criteria (Wang et al. 2012). A test was scored as positive if the ESAT-6- or CFP-10-stimulated well had at least 6 spots more than the negative control well, which had ≤ 5 spots. Also, if the negative control well had six to ten spots, the result was considered as positive when either the ESAT-6- or CFP-10-stimulated well contained at least twice as many spots.
In QFT-Plus assay, the qualitative cutoff values, and result interpretation, is identical to the QFT-GIT assay, with the key exception that if either one or both of the QFT-Plus TB antigen tubes (TB1 or TB2) are equal to or greater than 0.35 IU/mL and are at least 25% of the nil tube value, the patient is considered positive (Qiagen, Hilden, Germany). The QFT-Plus results can be stratified according to the ability of subjects to respond to both TB1 and TB2 peptides (“TB1 and TB2”), only to TB1 (“only TB1”) or only to TB2 (“only TB2”).
Patients were considered to have active TB if they had laboratory-confirmed active TB and culture isolation of Mtb and/or distinctive clinical presentation and/or symptoms as well as available radiographs and computed tomography images.
The inclusion criteria for patients with LTBI were: asymptomatic patients with both risk factors for TB infection (travel to or origin from a TB endemic country, known TB exposure, etc.), and a positive tuberculin skin test (TST) (induration ≥ 10 mm) and/or IGRAs result.
Multidrug-resistant (MDR) TB was defined as Mtb that is resistant at least to isoniazid and rifampicin (Prasad et al. 2018).
Studies were excluded when there was a lack of the number of positive or negative IGRA results before and after therapy, or included participants who did not receive treatment, or if the results did not differentiate between treated and untreated subjects. Moreover, we excluded review articles and conference abstract.
Two reviewers evaluated all titles and abstracts independently to identify potentially relevant studies. Next, one author reviewed the full text of all selected studies to determine the eligibility for inclusion and a second author repeated this evaluation separately. There was a complete agreement regarding the excluded studies. Two authors extracted data from all the included articles independently. Disagreements were resolved by consensus.
Quality Assessment
Two authors independently assessed each study based on Joanna Briggs Institute (JBI) checklist for cohort studies (Institute 2014). Any discrepancies were resolved by discussion or, if an agreement could not be reached, a third author was consulted.
Data Analysis and Synthesis
We calculated pooled estimates using random effects modeling, which provides more conservative estimates than fixed effects modeling when heterogeneity is present. Outcomes were the total rate of positive IGRAs tests following anti-TB treatment. We visually assessed for heterogeneity using forest plots, which represents the estimated prevalence and their relevant 95% confidence interval (CI). The Cochran’s heterogeneity statistic (Q test) and I2 statistic were used to examine the heterogeneity of studies. The I2 values of 25%, 50%, and 75% were considered as low, medium, and high heterogeneity, respectively.
For each individual study, we assessed all outcomes for which data were available. We generated forest plots to display the individual study estimates and their 95% confidence intervals.
We performed subgroup analysis based on the age (children, adults), type of TB (active, latent, active and latent, and contacts exposed to MDR defined as MDR LTBI), type of IGRAs (QFT-GIT and T-SPOT.TB), and follow-up interval (2, 3, 4, 6, 9 months). The Q and I2 statistics values were calculated for each subgroup to determine the effective factors on the positivity of IGRA and heterogeneity of the studies. Publication bias was evaluated by Egger’s regression test (Begg and Mazumdar 1994).
We performed all analyses using STATA software (Release 12. statistical software. College Station, Texas: STATA Corp LP).
Results
Included Studies
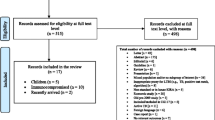
A total of 1432 unique references were identified by the initial search. After review of title and abstracts, 114 full-text articles were assessed for eligibility. Of these, 96 articles were excluded after the review of the complete texts, and 18 studies met our inclusion criteria. Figure 1 shows the study selection process, reported according to PRISMA guidelines.
Study Description
Of the 18 included studies, 12 used QFT-GIT (Bartalesi et al. 2013; Bastos et al. 2013; Bedini et al. 2016; Denkinger et al. 2013; Johnson et al. 2014; Kim et al. 2014; Le Hang et al. 2014; Lee et al. 2012; Matsushita et al. 2015; Sali et al. 2018; Sauzullo et al. 2014; Wang et al. 2013) for assessment of IGRA performance after therapy, 1 used T-SPOT.TB (Wang et al. 2012), and 3 used both QFT-GIT and T-SPOT.TB (Chiappini et al. 2012; O'Shea et al. 2014; Park and Shim 2017). Since, only two studies assessed the QFT-Plus performance during therapy (Kamada and Amishima 2017; Petruccioli et al. 2018), they were not considered in quantitative synthesis (meta-analysis). All the studies had quality score ≥ 5 (high quality).
Seven studies were conducted in Europe (Bartalesi et al. 2013; Bedini et al. 2016; Chiappini et al. 2012; O'Shea et al. 2014; Petruccioli et al. 2018; Sali et al. 2018; Sauzullo et al. 2014), one in the USA (Johnson et al. 2014), one in Canada (Denkinger et al. 2013), one in Brazil (Bastos et al. 2013) and eight in Asia (Kamada and Amishima 2017; Kim et al. 2014; Le Hang et al. 2014; Lee et al. 2012; Matsushita et al. 2015; Park and Shim 2017; Wang et al. 2012, 2013).
The mean age of the subjects was 36.45 ± 17.3 years. The number of participants after treatment follow-up in each study varied considerably (from 12 to 391); the largest studies were by Hang et al. (with 391 participants) (Le Hang et al. 2014) and Matsushita et al. (with 351 participants) (Matsushita et al. 2015). There was one study including people living with human immunodeficiency virus (Sauzullo et al. 2014); however, the number of HIV-positive patients in this work was very low (n = 13).
Classical anti-TB therapy (rifampicin, isoniazid, pyrazinamide, and ethambutol) was used in seven studies (Chiappini et al. 2012; Kamada and Amishima 2017; Kim et al. 2014; Le Hang et al. 2014; Matsushita et al. 2015; Park and Shim 2017; Sauzullo et al. 2014). Rifampicin and isoniazid were used in four studies (Bartalesi et al. 2013; Bastos et al. 2013; Lee et al. 2012; Petruccioli et al. 2018), isoniazid in three studies (Johnson et al. 2014; Sali et al. 2018; Wang et al. 2012), and rifampin in one study (Wang et al. 2013). Pyrazinamide plus levofloxacin were used as treatment choice for MDR LTBI in one study (Bedini et al. 2016).
According to the results of the meta-analysis, the pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) following anti-TB therapy was estimated at 76% [95% CI 70–81%] and no difference was found compared to the pooled positive rate of IGRAs before initiation of therapy which was 76%7 [95% CI 60–89%] (Fig. 2).
Forest plot shows percentage of patients with positive IGRA test using QFT-GIT and T-SPOT.TB. Squares and horizontal lines correspond to the recorded percentage of patients with positive results of IGRA at baseline (a) and after treatment follow-up in patients with positive IGRAs results at baseline (b). Squares reflect the weight each study contributed to the analysis. The diamond represents the pooled value, and corresponding 95% CI
Results revealed a strong heterogeneity among the selected studies (I2 = 89.1%; P < 0.001) (Fig. 2b).
The subgroup analysis was performed according to age (children, adults), type of TB (active, latent, active and latent, and MDR LTBI), type of IGRAs (QFT-GIT and T-SPOT.TB), TB burden, and follow-up interval (2, 3, 4, 6, 9 months). The results are shown in Table 2.
Studies in Children and Adults
We identified only one study which used the T-SPOT.TB assay for monitoring therapy in children (26). Instead, the QFT-GIT assay was used in two pediatric studies (21, 26) in which a total of 108 children had a longitudinal follow-up treatment. The remaining studies included only adults (Bartalesi et al. 2013; Bedini et al. 2016; Johnson et al. 2014; Kim et al. 2014; Le Hang et al. 2014; Lee et al. 2012; Matsushita et al. 2015; Park and Shim 2017; Sauzullo et al. 2014; Wang et al. 2012, 2013).
Based on the results of Egger’s regression test, the publication bias among included studies could not be ignored (P < 0.001; Fig. 3).
This subgroup analysis illustrated that the pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) following anti-TB treatment was similar among the two age groups of participants [children 77% (95% CI 44–98%); adults 76% (95% CI 69–82%)] (Table 1).
Studies in Different Types of TB
Seven studies investigated active TB patients (Denkinger et al. 2013; Kim et al. 2014; Le Hang et al. 2014; Matsushita et al. 2015; Park and Shim 2017; Sauzullo et al. 2014; Wang et al. 2013), six investigated LTBI patients (Bartalesi et al. 2013; Bastos et al. 2013; Johnson et al. 2014; Lee et al. 2012; O'Shea et al. 2014; Wang et al. 2012), two investigated both latent and active TB (Chiappini et al. 2012; Sali et al. 2018), and one investigated MDR LTBI patients (Bedini et al. 2016).
The subgroup analysis showed that the pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) after anti-TB therapy was significantly higher in monitoring of active TB subjects [80% (95% CI 74–88%)] than LTBI [71% (95% CI 70–81%)]. The pooled rate of positive IGRAs for two studies, evaluating both active and latent TB infection, was estimated at 77% [95% CI 44–98%]. Only one study assessed the performance of IGRA in MDR LTBI subjects, who displayed a positive QFT-GIT result after completion of treatment (100%) (14).
TB Burden
The pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) after anti-TB therapy was higher in high burden TB countries [80% (95% CI 75–85%)] than low/intermediate TB burden countries [73% (95% CI 62–82%)] (Table 2).
Treatment Follow-Up
The pooled rate of positive IGRAs (QFT-GIT and T-SPOT.TB) following anti-TB therapy was significantly different based on follow-up intervals (64–88%) (Table 2).
The largest and most statistically significant decreases of positive IGRAs results were observed during the first 3 months of specific treatment [64% (95% CI 55–72%)].
Studies Using QFT-GIT and T-SPOT.TB
Among the studies evaluating the performance of IGRA for monitoring therapy, there was no significant difference in the pooled rate of positive QFT-GIT (77%) and T-SPOT.TB (70%) following TB treatment (P = 0.36).
Overall, a large proportion of subjects remained IGRAs positive even after completion of specific treatment. The IFN-γ level responses generally fall after anti-TB treatment (Bartalesi et al. 2013; Bedini et al. 2016; Denkinger et al. 2013; Johnson et al. 2014; Le Hang et al. 2014; Lee et al. 2012; O'Shea et al. 2014), while two studies showed no significant change in IGRA results (Bartalesi et al. 2013; Sauzullo et al. 2014).
Studies Using QFT-Plus
We identified only two studies evaluating the effect of treatment on QFT-Plus response (Kamada and Amishima 2017; Petruccioli et al. 2018). Notably, these studies were carried out in low-burden settings. In the study of Petruccioli et al,. a cohort of subjects with LTBI (n = 46) and active TB (n = 28) was evaluated and 59% of them were BCG vaccinated (Petruccioli et al. 2018). The median age of the cases was 38 (24–50) years. The authors showed that anti-TB therapy significantly decreased IFN-γ values and number of responders to TB1 and TB2 peptides stimulation in both latent and active TB patients (Table 3). Stratifying LTBI subjects according to the type of preventive TB therapy, INH treatment, but not INH and RIF, significantly decreased IFN-γ production. In fact, among LTBI subjects treated with INH, 97% responded to both “TB1 and TB2” at baseline and 75% at treatment completion (P = 0.02). Among the “TB1” responders, 97% responded at baseline and 78% at therapy completion (P = 0.03). Among the “TB2” responders, 100% responded at baseline and 81% at therapy completion (P = 0.03).
In clinical TB patients, the positive IFN-γ response at therapy completion declined more than in microbiologically confirmed TB patients (44% vs 79%) (Petruccioli et al. 2018) (Table 4).
In the study of Kamada et al., 38 patients [mean ± SD age of 66 ± 19.3 years (29–94 years)] with confirmed TB were enrolled from October 2013 to January 2015. In this study, 36 patients (95% of the cohort) displayed a positive QFT-Plus result before therapy. Statistically significant decreases of IFN-γ responses of TB2 were observed during initiation of treatment to 3 months, but not between 3 and 6 months (Kamada and Amishima 2017) (Table 3).
Changes from a positive to negative result during treatment occurred in 26% of the cases (9 out of 35) for TB1, 14% (5 out of 36) for TB2, and 14% (5 out of 36) for TB1 or TB2.
Discussion
In recent years, numerous studies have been conducted to investigate the impact of anti-TB treatment on specific IFN-γ response, as measured by IGRAs, and controversial results in the case of both latent and active TB treatments have been shown.
It has been suggested that the progressive decline of IFN-γ response measured by IGRAs reflects the reduction of mycobacterial burden following a successful anti-TB treatment, but the clinical significance of IGRA conversions and reversions is still to be established.
Our analysis indicates that the IGRA, in particular the QFT-GIT and T-SPOT.TB, should not be used as a reliable marker for monitoring the efficacy of therapy in clinical practice. Before and after specific TB treatment, we found an equal pooled positive performance of IGRA. In addition, no significant differences between QFT-GIT and T-SPOT.TB following therapy was observed.
Conversely, the new generation of IGRA, QFT-Plus which contains novel antigens aimed at eliciting a response from CD8+ T cells and CD4+ T cells, seems to be useful as a surrogate marker for effective anti-TB monitoring (Kamada and Amishima 2017; Petruccioli et al. 2018). Further researches on a larger scale are needed to establish its clinical value as a supplemental tool for the monitoring of the efficacy of pharmacologic intervention for TB.
A review of included studies shows a general decline in quantitative IFN-γ response following anti-TB treatment, even if the majority of patients showed still a positive result at the end of therapy using especially QFT-GIT and T-SPOT.TB.
Persistent positive IGRA results were seen particularly in subjects with active TB disease, in which the pooled rate of positive IGRAs after TB therapy was significantly higher than that observed in subjects with LTBI infection, during which the bacterial burden is limited (Nikolova et al. 2013).
According to previous reports, the Mtb-specific CD8+ T-cell response, but not the CD4+ response, was different after TB treatment compared to baseline (Day et al. 2011; Nyendak et al. 2013). Interesting, Nyendak et al. showed that after 24 weeks of anti-TB therapy, the CD8+ T-cell response decreased by 58%, with an early difference observed at 8 weeks of therapy. Conversely, no significant difference in CD4+ T-cell response was observed during the treatment (Nyendak et al. 2013), suggesting that CD8+ T-cell response may be more accurate for monitoring TB treatment.
With the use of new QFT-Plus, it has been shown that IFN-γ response following TB2 stimulation decreased during TB therapy in subjects with active TB disease in parallel with decrease of Mtb load (Kamada and Amishima 2017; Petruccioli et al. 2016, 2017, 2018). It is in line with flow cytometry studies showing CD8+ T-cell response declines with anti-TB treatment (Cho et al. 2000; Day et al. 2011; Kamada and Amishima 2017; Nyendak et al. 2013).
In particular, the recent study of Petruccioli et al. (2018), no “only TB2” response was observed in active TB patients at the end of TB therapy, which suggests a loss of the CD8+ T-cell response in parallel with the decrease of mycobacterial load; therefore, investigation of the TB2 response could be served as a new tool to monitor TB therapy efficacy.
Although our meta-analysis revealed that both QFT-GIT and T-SPOT.TB assay cannot be a useful tools for monitoring therapeutic efficacy, it has been reported that decreases in the Mtb-specific antigens/phytohaemagglutinin ratios could be tested for monitoring of anti-TB treatment efficacy (Wang et al. 2018). However, future studies are needed to better characterize this approach as a potential marker for monitoring therapy.
Although IGRA performance may vary largely between adults and children (Silveira et al. 2018), our results show no differences between the pooled positive rate of IGRAs (QFT-GIT and T-SPOT.TB) following anti-TB drugs in both groups. The significant decreases of positive IGRAs during the first 3 months of treatment might be due to the greater bacterial clearance during the first 3 months (Kamada and Amishima 2017).
Several studies have evaluated the IGRAs performance (QFT-GIT and T-SPOT.TB) following anti-TB treatment in both low and high TB burden countries. Only two studies have evaluated anti-TB treatment using QFT-Plus, and both were conducted in low TB burden countries. Further studies on evaluation of QFT-Plus performance in high TB burden countries are highly recommended. It is not known if QFT-Plus can also apply to patients with HIV infection, immunosuppression, or affected by other abnormalities of the ratio CD4+ /CD8+. There are no reports on efficacy of QFT-plus in MDR-TB cases. Since observing the changes in QFT-Plus during anti-tubercular treatment might be a reliable tool for monitoring the treatment of MDR-TB, further studies on the evaluation of QFT-Plus performance following treatment of MDR-TB, especially in developing countries, are highly recommended.
Biomarker responses to TB antigens may be useful for the detection of TB infection (Mamishi et al. 2014, 2015, 2016). Changes in cytokine responses induced by TB antigens over the course of anti-tuberculous therapy can reflect immunological changes in the host due to the reduction in antigenic burden (Clifford et al. 2015); therefore, the use of cytokine responses as a biomarker of TB infection in patients treated with anti-TB warrants further investigation.
Conclusions
Available data are now sufficient to suggest that monitoring changes in the IGRAs (QFT-GIT and T-SPOT.TB) response during anti-TB treatment may have limited use in evaluating the effectiveness of treatment, while monitoring changes in QFT-Plus during anti-tubercular treatment is recommended to determine the treatment efficacy or for treatment monitoring. However, further research is needed to establish the efficacy of this new assay as a marker on a larger scale for treatment monitoring.
References
Bartalesi F, Goletti D, Spinicci M, Cavallo A, Attala L, Mencarini J, Fiori G, Gobbi FL, Mantella A, Benucci M (2013) Serial QuantiFERON TB-gold in-tube testing during LTBI therapy in candidates for TNFi treatment. J Infect 66(4):346–356
Bastos M, Menzies D, Belo M, Teixeira E, De Abreu S, Antas P, Trajman A (2013) Changes in QuantiFERON®-TB Gold In-Tube results during treatment for tuberculous infection. Int J Tuberc Lung Dis 17(7):909–916
Bedini A, Garlassi E, Stentarelli C, Petrella S, Meacci M, Meccugni B, Meschiari M, Franceschini E, Cerri S, Brasacchio A, Rumpianesi F, Richeldi L, Mussini C (2016) Multidrug-resistant tuberculosis outbreak in an Italian prison: tolerance of pyrazinamide plus levofloxacin prophylaxis and serial interferon gamma release assays. New Microbes New Infect 12:45–51
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Chiappini E, Bonsignori F, Mangone G, Galli L, Mazzantini R, Sollai S, Azzari C, de Martino M (2012) Serial T-SPOT. TB and quantiFERON-TB-Gold In-Tube assays to monitor response to antitubercular treatment in Italian children with active or latent tuberculosis infection. Pediatric Infect Dis J 31(9):974–977
Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro RJ, Flynn JL, Barnes PF, Southwood S, Celis E, Bloom BR, Modlin RL, Sette A (2000) Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci USA 97(22):12210–12215
Clifford V, Zufferey C, Germano S, Ryan N, Leslie D, Street A, Denholm J, Tebruegge M, Curtis N (2015) The impact of anti-tuberculous antibiotics and corticosteroids on cytokine production in QuantiFERON-TB Gold In Tube assays. Tuberculosis (Edinb) 95(3):343–349
Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA (2011) Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187(5):2222–2232
Denkinger CM, Pai M, Patel M, Menzies D (2013) Gamma interferon release assay for monitoring of treatment response for active tuberculosis: an explosion in the spaghetti factory. J Clin Microbiol 51(2):607–610
Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, Den Boon S, Gutierrez SMB, Bruchfeld J, Burhan EJERJ (2015) Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 46(6):1563–1576
Goletti D, Petruccioli E, Joosten SA, Ottenhoff THJIDR (2016) Tuberculosis biomarkers: from diagnosis to protection. Infect Dis Rep 8(2):6568
Goletti D, Lee MR, Wang JY, Walter N, Ottenhoff THJR (2018) Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology 23(5):455–466
Institute JBIJATJB (2014) Joanna Briggs Institute reviewers’ manual: 2014 edition
Johnson JL, Geldenhuys H, Thiel BA, Toefy A, Suliman S, Pienaar B, Chheng P, Scriba T, Boom WH, Hanekom W (2014) Effect of isoniazid therapy for latent TB infection on QuantiFERON-TB Gold In-Tube responses in adults with positive tuberculin skin test results in a high TB incidence area. Chest 145(3):612–617
Kamada A, Amishima M (2017) QuantiFERON-TB® Gold Plus as a potential tuberculosis treatment monitoring tool. Eur Respir J. https://doi.org/10.1183/13993003.01976-2016
Keshavarz Valian S, Mahmoudi S, Pourakbari B, Abdolsalehi MR, Eshaghi H, Mamishi S (2019) Screening of healthcare workers for latent tuberculosis infection in the low tuberculosis burden country: QuantiFERON-TB gold in tube test or tuberculin skin test? Arch Environ Occup Health 74(3):109–114
Kim CH, Choi KJ, Yoo SS, Lee SY, Won DI, Lim JO, Cha SI, Park JY, Lee J (2014) Comparative analysis of whole-blood interferon-γ and flow cytometry assays for detecting post-treatment Immune responses in patients with active tuberculosis. Cytom Part B Clin Cytom 86(4):236–243
Le Hang NT, Matsushita I, Shimbo T, Lien LT, Thuong PH, Cuong VC, Hijikata M, Kobayashi N, Sakurada S, Higuchi K (2014) Association between tuberculosis recurrence and interferon-γ response during treatment. J Infect 69(6):616–626
Lee S, Lee S, Yim J-J (2012) Serial interferon-gamma release assays after chemoprophylaxis in a tuberculosis outbreak cohort. Infection 40(4):431–435
Lönnroth K, Migliori GB, Abubakar I, D'Ambrosio L, De Vries G, Diel R, Douglas P, Falzon D, Gaudreau M-A, Goletti DJERJ (2015) Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 45(4):928–952
Mamishi S, Pourakbari B, Teymuri M, Rubbo PA, Tuaillon E, Keshtkar AA, Mahmoudi S (2014) Diagnostic accuracy of IL-2 for the diagnosis of latent tuberculosis: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 33(12):2111–2119
Mamishi S, Pourakbari B, Marjani M, Bahador A, Mahmoudi S (2015) Discriminating between latent and active tuberculosis: the role of interleukin-2 as biomarker. J Infect 70(4):429–431
Mamishi S, Pourakbari B, Shams H, Marjani M, Mahmoudi S (2016) Improving T-cell assays for diagnosis of latent TB infection: confirmation of the potential role of testing Interleukin-2 release in Iranian patients. Allergol Immunopathol (Madr) 44(4):314–321
Matsushita I, Le Hang NT, Lien LT, Thuong PH, Cuong VC, Hijikata M, Kobayashi N, Sakurada S, Higuchi K, Harada N (2015) Dynamics of immune parameters during the treatment of active tuberculosis showing negative interferon gamma response at the time of diagnosis. Int J Infect Dis 40:39–44
Moher D, Liberati A, Tetzlaff J, Altman DGJAOIM (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, Taskov H, Saltini C, Amicosante M (2013) Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 75(3):277–281
Nyendak MR, Park B, Null MD, Baseke J, Swarbrick G, Mayanja-Kizza H, Nsereko M, Johnson DF, Gitta P, Okwera A, Goldberg S, Bozeman L, Johnson JL, Boom WH, Lewinsohn DA, Lewinsohn DM (2013) Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment. PLoS ONE 8(12):e81564
O'Shea MK, Fletcher TE, Beeching NJ, Dedicoat M, Spence D, McShane H, Cunningham AF, Wilson D (2014) Tuberculin skin testing and treatment modulates interferon-gamma release assay results for latent tuberculosis in migrants. PLoS ONE 9(5):e97366
Park I-N, Shim TS (2017) Qualitative and quantitative results of interferon-γ release assays for monitoring the response to anti-tuberculosis treatment. Korean J Intern Med 32(2):302
Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D (2016) First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73(6):588–597
Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, Ippolito G, Goletti D (2017) Analytical evaluation of QuantiFERON-Plus and QuantiFERON-Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 106:38–43
Petruccioli E, Chiacchio T, Vanini V, Cuzzi G, Codecasa LR, Ferrarese M, Schinina V, Palmieri F, Ippolito G, Goletti D (2018) Effect of therapy on Quantiferon-Plus response in patients with active and latent tuberculosis infection. Sci Rep 8(1):15626
Prasad R, Gupta N, Banka A (2018) Multidrug-resistant tuberculosis/rifampicin-resistant tuberculosis: principles of management. Lung India 35(1):78–81
QIAGEN (2014) QuantiFERON-TB Gold Plus ELISA Package Insert. www.quantiferon.com/irm/content/package-inserts.aspx. Accessed 13 Mar
Sali M, Buonsenso D, D'Alfonso P, De Maio F, Ceccarelli M, Battah B, Palucci I, Chiacchio T, Goletti D, Sanguinetti M (2018) Combined use of quantiferon and HBHA-based IGRA supports tuberculosis diagnosis and therapy management in children. J Infect 77(6):526–533
Sauzullo I, Mengoni F, Ermocida A, Massetti AP, D'Agostino C, Russo G, Salotti A, Falciano M, Vullo V, Mastroianni CM (2014) Interferon-γ release assay in HIV-infected patients with active tuberculosis: impact of antituberculous drugs on host immune response. New Microbiol 37(2):153–161
Sester M, van Crevel R, van Leth F, Lange CJERJ (2015) Numbers needed to treat to prevent tuberculosis. Eur Respir J 46(6):1836–1838
Silveira MBV, Ferrarini MAG, Viana PO, Succi RC, Terreri MT, Costa-Carvalho B, Carlesse F, de Moraes-Pinto MI (2018) Contribution of the interferon-gamma release assay to tuberculosis diagnosis in children and adolescents. Int J Tuberc Lung Dis 22(10):1172–1178
Wang J-Y, Shu C-C, Lee C-H, Yu C-J, Lee L-N, Yang P-C (2012) Interferon-gamma release assay and rifampicin therapy for household contacts of tuberculosis. J Infect 64(3):291–298
Wang H, Li Z, Zhang Q, Lu G, Wang Q, Zhang L, Zhang C (2013) Clinical value of a whole blood interferon γ release assay for the diagnosis of Mycobacterium tuberculosis infection during antitubercular treatment. Exp Ther Med 6(2):455–458
Wang F, Yu J, Zhou Y, Luo Y, Wu S, Huang M, Yin B, Huang J, Mao L, Sun Z (2018) The use of TB-specific antigen/phytohemagglutinin ratio for diagnosis and treatment monitoring of extrapulmonary tuberculosis. Front Immunol 9:1047
World Health Organization (2016) Global tuberculosis report 2016
Acknowledgements
This study was supported by a grant (grant number: 97-03-88-40795) from Tehran University of Medical Sciences to Dr. Shima Mahmoudi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Pourakbari, B., Mamishi, S., Benvari, S. et al. Can Interferon-γ Release Assays Be Useful for Monitoring the Response to Anti-tuberculosis Treatment?: A Systematic Review and Meta-analysis. Arch. Immunol. Ther. Exp. 68, 4 (2020). https://doi.org/10.1007/s00005-020-00568-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00005-020-00568-4