Abstract
Vibrio spp. are ubiquitous bacteria that are frequently discovered in aquatic environments. Globally, they are recognized as the primary cause of seafood-related illnesses. Over decades, vibrios have been a major health concern, and the number of cases is on the rise due to unhygienic eating habits and increasing demand for raw seafood. Among the 2 groups of Vibrio bacteria, the non-cholera Vibrio bacteria group mainly associate with seafood-borne illness. Though ~ 12 species have been recognized as causative agents of diseases in humans, horizontal gene transfer has attributed to an increase in emerging human pathogenic Vibrio spp. The assortment of virulence determinants contributes to the pathogenicity of vibrios. They carry specific genes to produce toxins and hemolysin, which are correlated with pathogenicity. In addition, the expanding antimicrobial use in humans and aquaculture resulted in a surge of resistant Vibrio strains found in shellfish. This has adversely affected the therapeutic results in the case of Vibrio infection. Thus, this article provides insight into the potential public health threat that may pose to seafood consumers as a consequence of the presence of virulence factors and antimicrobial resistance determinants in molluscan shellfish-borne vibrios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The aquaculture industry consists of various species, including aquatic plants and animals, with total production estimated over US$ 406 billion (FAO 2022). Over 65 different mollusk species are worth US$ 30.4 billion to the industry (FAO 2019). Mollusks represent the second largest group by weight, estimated at 17.7 million tonnes and valued at US$ 29.8 billion (FAO 2022). Oysters constitute the largest species group, accounting for 32.8% of the total mollusk, followed by clams, cockles and ark shells (FAO 2019). Mollusks are filter feeders that can filter out a great quantity of water while feeding on suspended materials in the water, due to this process they may bioaccumulate a high number of microorganisms in their tissues (Baker-Austin et al. 2017).
Vibrio spp. are naturally occurring Gram-negative bacteria characterized by their curved or comma-shaped morphology; originated from aquatic and marine ecosystems (Baker-Austin et al. 2017; Farmer et al. 2015). These bacteria can be found in warm and slightly salty water, and they actively mirror environmental temperatures. Vibrios are accountable for most human infections concerning the microbiota associated with seafood and aquatic environments (Baker-Austin et al. 2018). Ingestion of raw or undercooked contaminated seafood with Vibrio spp. may result in a variety of infections in humans. The number of Vibrio spp. infections vary with seasonal distribution, where most incidents take place during warmer months (Altekruse et al. 2000). Vibrios are widely recognized for their ability to cause a wide range of clinical symptoms and diseases, including digestive, ophthalmological, dermatological and otorhinolaryngological infections (Oliver 2005). The 2 major groups of human pathogenic vibrios are cholera and non-cholera Vibrio spp. Vibrio cholerae causes a severe diarrhoeal illness known as Cholera which is frequently caused by the intake of contaminated food or water (Howard-Jones 1984). Non-cholera vibrios like Vibrio parahaemolyticus and Vibrio vulnificus are the etiological agents of vibriosis and cause infections that can result in diverse clinical manifestations which vary depending on the specific pathogen involved, the route of infection, and the susceptibility of the host. (Baker-Austin et al. 2018). Generally, non-cholera vibrios dominate in moderate or high salinity aquatic habitats and thus can originate with seafood more frequently. V. parahaemolyticus has been widely acknowledged as the primary cause of food-borne illnesses in various Asian countries including Japan, Korea, and China (Wong et al. 2000; Lee et al. 2001; Alam et al. 2002; Liu et al. 2004). In addition, V. cholerae and V. vulnificus are also recognized as significant pathogens responsible for causing food-borne illnesses (Sawabe et al. 2007, 2013). Furthermore, V. alginolyticus and V. fluvialis have also been categorized as food-borne infectious agents as they have caused food poisoning in humans (Liang et al. 2013).
A complex group of genes is associated with the virulence of vibrios, and expression of these virulence factors is crucial for the host infection process (Lida et al. 1998; McCarthy et al. 1999). In addition, antimicrobial resistance plays a major role during infection and has become a public and animal health threat. These resistances are often carried on bacteria plasmids encoded by respective resistance genes (Balsalobre et al. 2010). The overuse or mishandling of antimicrobials can be a cause of the emergence of multidrug-resistant bacteria in different environments. Considering these factors, studies have suggested an association between resistance and virulence factors, where traits that confer a particular benefit will be chosen and become fixed with time (Beceiro et al. 2013). Therefore, the purpose of this review is to present comprehensive evidence regarding the virulence factors and antimicrobial resistance determinants found in Vibrio spp. associated with mollusks for consumer awareness and public health safety.
2 Vibriosis
Vibrio spp. are common bacteria found in different varieties of aquatic and marine habitats that cause human infections. Although over 100 Vibrio spp. have been described, ~ 12 Vibrio spp. have been identified as a source of human infections (Baker-Austin et al. 2018). Vibriosis affects a considerable number of people in the United States annually. Specifically, according to Centers for Disease Control and Prevention (CDC), roughly 80,000 individuals contract this bacterial infection, which results in the death of around 100 individuals each year (CDC 2023). A severe diarrheal disease caused by V. cholerae can be fatal if untreated, and it usually spreads rapidly through polluted water and personal contact. Vibriosis is caused by non-cholerae vibrios (for example, V. parahaemolyticus, V. alginolyticus and V. vulnificus) when exposed to seawater or eating raw or undercooked contaminated seafood (Di et al. 2017). Several symptoms of non-cholerea bacteria lead to the most common mild self-limiting gastroenteritis. Particularly, V. vulnifius infection can be a reason for fatal wound infections that may induce septicemia (Coerdt and Khachemoune 2021; Torres et al. 2002).
2.1 Vibrio parahaemolyticus
V. parahaemolyticus has a wide distribution in temperate and tropical coastal regions across the globe (Shen et al. 2009). V. parahaemolyticus infection was limited to the areas of Japan until late 1960. Afterward, it was reported in other parts of the countries, comprising the Atlantic, Pacific, Gulf states and Hawaii in the USA (Barker et al. 1974). During 2007–2012, V. parahaemolyticus, serotype O3: K6 strains turn out to be the main reason to cause bacterial infectious diarrhea in the region of southern China (Li et al. 2014). V. parahaemolyticus can cause gastroenteritis in humans eating raw or undercooked contaminated seafood, particularly bivalve shellfish, such as oysters and ready-to-eat food (Park et al. 2018; Pang et al. 2019). Other symptoms of V. parahaemolyticus comprise abdominal cramps, diarrhea, vomiting, and nausea (Daniels et al. 2000). Moreover, V. parahaemolyticus infections are accountable for a significant number of food-borne infections in the USA (Iwamoto et al. 2010). In 2018, a seafood-related outbreak of V. parahaemolyticus infections resulted in 26 cases of illness and 9 individuals requiring hospitalization (Seelman et al. 2023). In Korea, 9 outbreaks occurred in 2017 with 354 patients that were infected with V. parahaemolyticus (KCDC 2016; KMFDS 2017).
2.2 Vibrio vulnificus
This pathogen is commonly found in estuarine waters and various environmental sources, such as seawater, sediment, and seafood products (DePaola et al. 1994; Baker-Austin et al. 2010). V. Vulnificus infections are caused by two main sources; intake of contaminated seafood, specifically oysters, leading to gastroenteritis or bacterial infections; or exposure of wounds to seawater or contaminated seafood products, resulting in the development of wound infections and secondary septicemia (Jones and Oliver 2009). V. vulnificus infections are reported to have the highest fatality rate (50%) among food-borne microorganisms. Compared to V. parahaemolyticus, V. vulnificus is a lethal human pathogen evidenced to be responsible for more than 95% of seafood-related deaths in the USA (Oliver and Bockian 1995). Furthermore, about 20% of individuals with a V. vulnificus infection will not survive, and death can occur within a short period of 24–48 h after the onset of illness (CDC 2023). V. vulnificus can cause severe wound infections. The infection can progress rapidly, requiring intensive care and sometimes even limb amputations (CDC 2023). In South Korea, a total of 257 cases were reported as a result of V. vulnificus infection from 2017 to 2022 (KCDC 2022).
2.3 Vibrio alginolyticus
This bacterium can be found in seawater worldwide and is known to cause various infections, including wound and ear infections such as otitis media and otitis externa. These infections can be effectively treated using appropriate antimicrobial agents. Occasionally, these infections can be aggravated to cause septicemia and necrotizing fasciitis, especially in people with a compromised immune system (Baker-Austin et al. 2016). The incidence of these infections significantly increases during the warmer months. A French study revealed that V. alginolyticus is accountable for nearly 34% of all vibriosis infections for the last 19 years (Hoefler et al. 2022). In Spain, the occurrence of V. alginolyticus infected wounds of 2 patients exposed to contaminated seawater in 2019 (Fernández-Bravo et al. 2019). In many reports, V. alginolyticus wound infections happened in patients that had cuts and abrasions exposed to contaminated seawater (Reilly et al. 2011). In the US, 20% of all vibriosis infections were caused by V. alginolyticus, including 131 cases in Florida (Weis et al. 2011). Another study has reported that 96% of V. alginolyticus infections happen in coastal states (Slifka et al. 2017). V. alginolyticus was found in a stool sample of a patient with gastroenteritis due to the ingestion of diseased abalone (Liu et al. 2001). In Europe, several studies have reported the incidence of V. alginolyticus (Suñén et al. 1995; Barbieri et al. 1999; Hervio-Heath et al. 2002).
2.4 Other Vibrio spp.
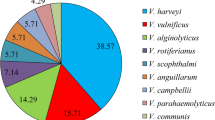
Human infections due to other Vibrio spp. including V. mimicus, V. cincinnatiensis, V. hollisae, V. furnissii, V. fluvialis and V. metschnikovii have also been documented. These infections are relatively rare, although these pathogens are important for clinical treatment decisions and to distinguish the exact causative agents. However, V. fluvialis is widely recognized as an emerging food-borne pathogen that poses a public health concern (Igbinosa et al. 2010; Ramamurthy et al. 2014). An infection with V. fluvialis was reported in a 75-year old man with diarrhea and abdominal rash after consumption of uncooked clams (Arora and McHargue 2021). Another case report dealt with a wound infection caused by V. fluvialis, acquired through an impalement injury in shallow waters of the Baltic Sea (Hecht et al. 2022). In Korea, mollusk seafood has been found to harbor V. diabolicus, V. harveyi, V. antiquarius, V. anguillarum and V. aestuarianus strains with virulent determinants and multidrug-resistant characteristics (Dahanayake et al. 2020b; De Silva et al. 2019; Hossain et al. 2020). Figure 1 summarizes the virulence factors and infections caused by the Vibrio spp.
3 Virulence factors of Vibrio spp.
3.1 TDH (thermostable direct hemolysin), TRH (TDH- related hemolysin) and toxR gene
Generally, V. parahaemolyticus strains are well-known to produce various virulence factors during pathogenesis (Letchumanan et al. 2014). Among those virulence factors, the toxins TDH (thermostable direct hemolysin) and TRH (TDH- related hemolysin) are encoded by tdh and trh genes, respectively. The tdh gene is responsible for kanagawa, with produces β hemolysis on Wagatsuma agar (Honda et al. 1988; Nishibuchi and Kaper 1995). These 2 virulence factors cause similar biological activities such as enterotoxicity, cytotoxicity and hemolytic activity (Park et al. 2004). The presence of both TDH and TRH toxins plays a major role during infections, however, irregularity among cases are reported. In most common scenarios, the tdh gene has been found in only 1–2% of environmental strains (Honda and Iida 1993). Vibrio parahaemolyticus isolated from different marine products showed the presence of tdh and trh genes (Ryu et al. 2019; Ashrafudoulla et al. 2021; Zhong et al. 2022). In Korea, a study reported 2 (2.4%) and 8 (9.5%) of 84 strains with tdh and trh genes positive samples isolated from shellfish (Oh et al. 2011). Previously, the presence of tdh and trh genes in other Vibrio spp, including V. cholerae non-O1/non-O139, V. diabolicus and V. alginolyticus had been studies by Raghunath (2015). Dahanayake et al. (2020a) found tdh in V. diabolicus isolates from cockles and V. alginolyticus isolates from abalone in Korean seafood. And V. parahaemolyticus isolated from oysters and clams harbored tdh and trh genes (Robert-Pillot et al. 2004; Vongxay et al. 2008). Tdh and trh genes in V. parahaemolyticus and V. vulnificus were isolated from retail raw oysters in Thailand (Changchai and Saunjit 2014). Ahmed et al. (2018) found tdh and trh genes in V. parahaemolyticus isolated from crustaceans in Egypt. V. parahaemolyticus strains isolated from mussels, clams and cockles expressed trh and tdh genes (Jingjit et al. 2021). According to Jang et al. (2020), the incidence of the trh gene was identified in V. parahaemolyticus strains isolated from shellfish and shrimp samples collected from the West Coast of Korea.
The toxR virulence gene is known to regulate the expression of virulence genes in Vibrio species and also produces chemotaxis proteins that aid in colonization in the intestine (Mey et al. 2015). This gene was found prevalent among V. alginolyticus isolates in mollusk seafood in Korea (Dahanayake et al. 2018, 2020b). V. parahaemolyticus isolated from clams, oysters and scallops in Poland expressed toxR genes (Lopatek et al. 2015). V. cholerae isolates from mussels were positive for toxR and hlyA genes (Ottaviani et al. 2009). Tan et al. (2020) identified the toxR gene in V. parahaemolyticus isolated from surf clams and blood clams in Malaysia. V. parahaemolyticus isolated from oysters and mussels express toxR gene (Stratev et al. 2023). Vibrio parahaemolyticus isolates from shellfish in India expressed tdh, trh and toxR genes (Narayanan et al. 2020). Vibrio species carrying the tdh and trh genes are acknowledged as pathogenic, capable of inducing acute infections in humans (Robert-Pillot et al. 2004). Table 1 summarizes the virulence factors of Vibrio spp. isolated from shellfish.
3.2 Extracellular enzymes
The extracellular enzymes of Vibrio spp. actively participate in potential virulence as secretions (Soto-Rodriguez et al. 2003; Elavarashi et al. 2017). These enzymes are health risk indicators of Vibrio spp. and present in clinical, environmental and food sources (Lafisca et al. 2008). All mussel-borne Vibrio isolates showed DNase and gelatinase activities while most of the isolates showed caseinase, phospholipase and lipase activities (Hossain et al. 2020). Furthermore, Vibrio isolates from oysters, manila clams, scallops, cockles and abalones were positive for most of the enzyme activities (Dahanayake et al. 2018, 2020a). The extracellular DNase enzymes facilitate DNA hydrolysis and reproduction of the bacterial strains (Vergis et al. 2002). V. parahaemolyticus isolated from fresh oysters were positive for lipase, phospholipase, protease, gelatinase, caseinase and Dnase activities (Costa et al. 2013). In another study, V. parahaemolyticus isolated from clams, sea mussels and oyster showed hemolytic, lipase, protease, gelatinase, and Dnase activities (Ashrafudoulla et al. 2021). Narayanan et al. (2020) and Silvester et al. (2022) studied β-hemolytic activity (KP) of V. parahaemolyticus isolated from shellfish, shrimps and crabs in India. Jingjit et al. (2021) also observed the hemolytic activity of V. parahaemolyticus strains isolated from mussels, clams and cockles. Cytotoxic, proteolytic, DNase, mucinase, lecithinase and lipase activities of V. vulnificus isolated from oyster was reported by Quiñones-Ramírez et al. (2010). Gelatinase and caseinase enzymes are related to gelatin and protein deterioration, respectively. Lipase helps to obtain nutrients by deteriorating the lipid membrane of host cells, while phospholipase involves in hemolysis and lecithinase activity (Fiore et al. 1997; Cocchiaro et al. 2008). V. parahaemolyticus, V. cholerae, V. parahaemolyticu, V. ponticus, V. litoralis, V. metschnikovii and V. crassostreae isolated from fresh and processed mollusk showed positive activities for lipase, phospholipase, gelatinase, caseinase, DNase, amylase and β-hemolysin (Silva et al. 2018). Shellfish can be vehicles of Vibrio spp. that can express extracellular enzymes associated with bacterial pathogenesis. Cooking is recommended in case of human consumption.
3.3 Other virulence factors and virulence genes
V. parahaemolyticus binds to the host cell during infection with the use of adhesion factors such as fibronectin and phosphatidic acid (Wang et al. 2015). Vongxay et al. (2008) observed the adherence activity of V. parahaemolyticus isolated from clams and mussels to Caco-2 cells. The adherence ability of V. vulnificus strains from oysters was reported by Quiñones-Ramírez et al. (2010). Vibrio spp. can form biofilm and can survive in stressful conditions resulting in increased infectivity and transmission (Song et al. 2017). Sadat et al. (2021) reported the biofilm-forming ability of V. parahaemolyticus and V. alginolyticus isolated from seafood. V. cholerae strains isolated from crustaceans also displayed biofilm formation (Ahmed et al. 2018). The biofilm-forming ability of V. parahaemolyticus isolated from mussels was shown by Ashrafudoulla et al. (2019).
V. vulnificus consists of various virulence factors that facilitate invasion and growth in the host environment. The V. vulnificus hemolysin gene (vvh) is one of the multiple virulence factors of V. vulnificus. Ingestion of contaminated seafood can also cause V. vulnificus human infection, which results in 1% of food-related deaths around the globe (Miyoshi 2006; Giltner et al. 2012). V. vulnificus strains from oysters in Thailand showed the presence of vvh gene (Changchai and Saunjit 2014). Castello et al. (2022) also studied the vvh gene in V. vulnificus strains isolated from oysters, clams and mussels.
According to the results obtained from whole-genome sequencing studies, the pathogenic isolates of V. parahaemolyticus encode 2 types of type III secretion systems (T3SS) known as T3SS1 and T3SS2 (Makino et al. 2003; Ritchie et al. 2012). The vopB, vscP, vopS, vscK, vscF, vopB2, vscC2, vscS2, vopT, and vopD genes are involved in translocating effector proteins and are essential for the performance of T3SS (Dietsche et al. 2016). V. parahaemolyticus isolated from mussels expressed biofilm genes VP950 (encoding a lipoprotein-related protein), type VI secretion T6SS (VP1409), VP952, and VP962 (encoding a hypothetical protein), Type I pilus (VP1510), pathogenicity Island-2 (VPaI-2), and VPaI-6 (VP1253) genes (Ashrafudoulla et al. 2019). Cytotoxicity and serious diseases are caused by transporting distinct effectors and toxins into the cytoplasm via T3SS1 and T3SS2 systems (Wang et al. 2015). Hcp, vasH, vgrG and PAAR genes encode proteins that are a key component of type VI secretion system (T6SS) (Church et al. 2016). Hcp, vasH, vgrG, vopB, vopD, proA, pvsA (ferric Vibrioferrin receptor), pvuA, wza (capsular polysaccharide) and lafA (lateral flagella’s flagellin) genes were reported in V. alginolyticus isolated from oysters in Mexico City (Hernández-Robles et al. 2016). V. parahaemolyticus isolated from fresh oysters, clams and shrimps expressed vscP, vopS, vscK, vscF, vopB2, vscC2, vscS2 and vopT genes (Chen et al. 2018). The pirABvp genes, which are the Photorhabdus insect-related toxin genes, are located on a plasmid in Vibrio parahaemolyticus. These genes have been recognized as virulence factors implicated in the development of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Hong et al. (2020) reported the presence of these genes in Vibrio parahaemolyticus isolated from molluscan shellfish and shrimps in Vietnam. The pirABvp genes identified in Vibrio parahaemolyticus strains that were isolated from shellfish and shrimp collected from the West Coast of Korea (Jang et al. 2020).
Álvarez-Contreras et al. (2021) reported the presence of different virulence genes. vppC (collagenase), tlh (thermolabile hemolysin), vvhA (hemolytic cytolysin) and vmh (hemolysin), pvsA, wza and lafA genes were displayed in V. parahaemolyticus, V. mimicus, V. vulnificus and V. alginolyticus isolated from oysters, mussels and clams. The pvsA and pvsD genes that play role in the siderophore formation have been reported in V. alginolyticus (Sha et al. 2013). V. cholerae pathogenicity island (VPI) is known to harbor several virulence genes at the same location, mainly in epidemic strains was prevalent in molluscan seafood in Korea (Hossain et al. 2020; Wickramanayake et al. 2020). Ahmed et al. (2018) studied ctx (cholera toxin) and hlyA (hylA-Class) genes of V. cholerae strains isolated from crustaceans. V. cholerae strains isolated from oysters, mussels and clams expressed ctx and hlyAET genes (Castello et al. 2022). Presence of several virulence genes in Vibrio spp. isolated from shellfish indicate that these strains possess potential virulence characteristics and can act as reservoirs of pathogenic Vibrio in humans.
4 Use of antimicrobials and disinfectants in the fish industry
Fish farms contribute to the pollution of the environment through the release of antimicrobials and other therapeutic agents, leading to environmental contamination. Most of the administrated antimicrobials are released into the surrounding aquatic environments as effluents such as untreated seawater, pollutants such as excessive feed and fish excretions (Jang et al. 2018). The excessive use of antimicrobials as therapeutic agents has resulted in the emergence of multidrug-resistant bacterial strains and has facilitated the transfer of antimicrobial resistance genes through horizontal gene transfer mechanisms. This has contributed to the proliferation of antimicrobial-resistant bacteria in various environments (Son et al. 1997; Levy 2001; Manjusha et al. 2005).
4.1 Occurrence of multidrug-resistant Vibrio spp. in molluscan shellfish
Among the list of extensively used antimicrobials, cefotaxime, amikacin, gentamicin, tetracycline and trimethoprim–sulfamethoxazole are being used to treat Vibrio infections (Daniels et al. 2000; Shaw et al. 2014). In a previous study, all Manila clam-borne vibrios showed resistance to at least two antimicrobials. Of the resisted antimicrobials, high resistance rates among isolates were observed particularly for ampicillin, piperacillin, rifampicin, colistin sulfate and cephalothin (Dahanayake et al. 2018; De Silva et al. 2019). V. alginolyticus, V. parahaemolyticus, V. harveyi, V. vulnificus and V. cholerae harbored from oysters, Japanese carpet shells, cockles, clams (Venus gallina/Chamelea gallina) and mussels (Mytilus galloprovinciallis and Modiolus barbatus) were resistant to tetracycline, ceftazidime, piperacillin, amoxicillin/clavulanic acid, azithromycin, cefoxitin, and streptomycin (Mancini et al. 2023).
Previous studies have reported high ampicillin resistance among Vibrio spp. isolated from coastal areas, estuaries, fish, and shellfish farms (Kim et al. 2005; Lee et al. 2009; Oh et al. 2009). Vibrio spp. isolated from clams and oysters in Nigeria showed resistance to Trimethoprim-Sulfamethoxazole (Udoekong et al. 2021). Particularly, V. parahaemolyticus isolates were found resistant to ampicillin in most of the mollusk’s Vibrio isolates (Jun et al. 2012). V. parahaemolyticus isolated from short-necked clams (Venerupis philippinarum) and oysters (Crassostrea gigas) showed resistance to ampicillin, cefotaxime, cefeprime, cephalothin, kanamycin, streptomycin and vancomycin (Jo et al. 2020). Resistance for ampicillin, cephalothin, carbenicillin, trimethoprim-sulfamethoxazole and gentamycin was reported in V. parahaemolyticus, V. mimicus, V. vulnificus and V. alginolyticus isolated from oysters, mussels and clams (Álvarez-Contreras et al. 2021). Castello et al. (2022) found ampicillin, cephalothin, ceftriaxone, ceftazidime, gentamicin, tetracycline, kanamycin, cefazolin and streptomycin-resistant V. parahaemolyticus, V. vulnificus, V. cholerae NCV and V. alginolyticus isolated from clams, mussels and oysters in Sicily. V. parahaemolyticus isolated from bivalve shellfish were found resistant to ampicillin, cefixime, streptomycin, trimethoprim and amikacin (Hu and Chen 2016; Ryu et al. 2019). In a study by Lopatek et al. (2015), V. parahaemolyticus from shellfish; which were sold in Polish markets and originated from France, Norway, Italy and Netherlands, were found resistant to ampicillin, streptomycin and gentamicin. Resistant for ampicillin, streptomycin and colistin was observed in molluscan shellfish and shrimps borne Vibrio parahaemolyticus in Vietnam (Hong et al. 2020). Mok et al. (2019a) reported ampicillin, cefazolin, aztreonam, amikacin, streptomycin, trimethoprim, kanamycin, gentamicin, aztreonam resistant strains of V. parahaemolyticus and V. cholerae isolated from oyster, ark shell and mussel from Korea. V. parahaemolyticus and V. alginolyticus strains collected from common cockle (Cerastoderma edule) and white leg shrimp in Egypt were resistant to ampicillin, trimethoprim-sulfamethoxazole, erythromycin, tetracycline, penicillin, gentamicin, ciprofloxacin and nalidixic acid (Sadat et al. 2021). Stratev et al. (2023) observed ampicillin, cefepime, and ceftazidime resistance in V. parahaemolyticus isolated from oysters and mussels. The multiple antimicrobial resistance (MAR) index > 0.2 is considered a high-risk source of contamination where antimicrobials are frequently used (Krumperman 1983). Over 50% of the Vibrio isolates from oysters, Manila clams, scallops and cockles showed > 0.2 (MAR) index (Sadat et al. 2021; Stratev et al. 2023). Table 2 summarizes the resistant antibiotic of Vibrio spp. isolated from shellfish.
The production of extended-spectrum β-lactamases (ESBL) facilitates the isolates to be resistant against β-lactam antimicrobials. Among beta-lactamase enzyme groups, ESBLs are associate with hydrolyzing penicillins, extended-spectrum cephalosporins and carbapenems. ESBLs are most frequently reported in vibrios isolated from a variety of sources such as clinical, environmental and food sources (Jun et al. 2012). A study reported 88.9% of the vibrios carrying beta-lactam resistance among marine or coastal environmental isolates (Zanetti et al. 2001). In previous studies, ESBL gene blaCTX was reported as the most prevalent among the Vibrio spp. from molluscan seafood. The blaCTX−M gene was identified in Vibrio spp. isolated from Manila clams (87%), cockles (78%), mussels (87.5%), and abalone (85%). The blaTEM gene was present in Manila clams (55%), cockles (40%), mussels (40.6%), and abalone (10%) Vibrio isolates. In addition, few molluscan Vibrio isolates carrying aphA-IAB, strA-strB (regulate the kanamycin and streptomycin resistance mechanism, respectively), blaSHV, tetA, tetB (responsible for tetracycline resistance) and Class 1 integron-related Integrase 1 (intI1) resistance genes were also detected (Dahanayake et al. 2020a; Wickramanayake et al. 2020). Silvester et al. (2019) studied the presence of blaCTX−M and blaNDM−1 genes in Vibrio spp. isolated from seafood. The intI1 gene act as an exhibitor which helps to identify different environmental pressures and is mostly engaged in the dissemination of antimicrobial resistance genes (Gillings et al. 2015). Rojas et al. (2011) reported blaTEM gene in V. parahaemolyticus isolated from oysters and mussels. V. parahaemolyticus isolated from oysters in Thailand demonstrated the presence of qnr (quinolones), strB (streptomycin), sul2 (sulfamethoxazole), tetA (tetracycline), ermb (erythromycin) and blaTEM genes (Jeamsripong et al. 2020). Nsikan et al. (2021) studied the qnrB, VIM (Vimentin) and SHV (sulfhydryl reagent variable) genes in Vibrio spp. isolated from shellfish in Nigeria. V. splendidus, V. kanaloae, V. hemicentroti, V. neocaledonicus and V. jasicida strains isolated from razor shells and clams expressed cat (C acetyltransferase genes) and tet genes (Dubert et al. 2016). Vibrio resistance to antibiotics is not only mediated by chromosomal gene transfer but also by plasmids transfer. Manjusha and Sarita (2013) reported the plasmid-mediated transfer of amoxicillin, ampicillin, amikacin, carbenicillin, cefuroxime, furazolidone, streptomycin, chloramphenicol, trimethoprim and tetracycline in Vibro spp. isolated from molluscan and shrimp. In aquaculture farming, judicious exploitation of antibiotics should be followed to prevent antibiotic resistance in pathogenic bacteria. Resistance of shellfish-associated pathogenic Vibro spp. to antibiotics and expression of antibiotic resistance genes indicate a potential risk to treat vibrios infection in humans. Consuming raw or undercooked seafood, especially shellfish, poses a significant threat to consumers.
5 Control measures to minimize Vibrio spp. in molluscan shellfish
Shellfish offer a nutritious and valuable source of high-quality proteins, beneficial polyunsaturated fatty acids, essential vitamins, and minerals contributing to a balanced and healthful diet (Prester 2011). But the prevalence of different multidrug resistance pathogens including Vibrio spp. increases the incidence of infection by seafood for consumers (Dubert et al. 2016; Mancini et al. 2023). Several measures should be taken to avoid or minimize the potential threat of multidrug resistance, pathogenic Vibrio spp. in molluscan shellfish. Implementation of good aquaculture practices including the maintenance of proper water quality, minimizing pollution or contamination and ensuring appropriate nutrition and feeding practices can help reduce the risk of Vibrio spp. contamination (Vaiyapuri et al. 2021). Application of proper harvesting and handling techniques, use of clean equipment and ensuring proper temperature control during storage and transportation can minimize contamination risks (Tan et al. 2020). High-temperature processing of molluscan shellfish can effectively reduce the level of pathogenic bacteria (CDC 2023). Raising awareness among consumers, food handlers, and producers about the risks associated with multidrug-resistant Vibrio can promote safe handling and consumption practices (Serwecińska 2020). Implementing and enforcing regulatory controls, such as regular monitoring and testing of shellfish for Vibrio contamination, can help ensure compliance with safety standards (Tan et al. 2020). Conducting regular surveillance of Vibrio strains present in shellfish can help identify potential multidrug-resistant strains. This information can guide appropriate interventions and preventive measures (Bayliss et al. 2017). Furthermore, the utilization of probiotics and prebiotics, immunostimulants, quorum quenching bacteria, phytochemicals (essential oils) and antimicrobial peptides against Vibrio spp. as viable alternatives to antibiotics have demonstrated promising potential in the field. By incorporating them into various treatment approaches, the risk of Vibrio infections and the emergence of multidrug-resistant strains can be significantly reduced (Vaiyapuri et al. 2021).
6 Conclusions
This review emphasizes the significance of molluscan shellfish as potential reservoirs of Vibrio spp., which can spread to seafood, humans, and the surrounding environment. Vibriosis, caused by various Vibrio species, is a significant public health concern associated with the consumption of contaminated seafood, particularly mollusks. V. parahaemolyticus, V. vulnificus and V. alginolyticus are among the most common Vibrio species implicated in human infections. The presence of virulence genes and antimicrobial resistance among Vibrio spp. isolates from shellfish indicate that pathogenic and resistant strains of Vibrio spp. are already distributed in seafood and are a matter of concern for public health. It is recommended that enhanced monitoring for examining the occurrence patterns of potentially pathogenic seafood production, improved sanitation practices, and the development of rapid diagnostic methods to detect virulence markers more efficiently can aid in the early detection and control of Vibrio contamination. In addition, new strategies involving effective and safer antimicrobials or alternative non-antimicrobial compounds should be developed to overcome this issue. It emphasizes the need for continued research, surveillance, and education to ensure the safety of seafood consumers and mitigate the impact of vibriosis on public health. By implementing comprehensive strategies, we can strive towards reducing the incidence of Vibrio infections and safeguarding the well-being of individuals who consume mollusks and other seafood products.
Data availability
Data sharing does not apply to this article as no new data were created or analysed in this study.
References
Ahmed HA, El Bayomi RM, Hussein MA, Khedr MH, Remela EM, El-Ashram AM (2018) Molecular characterization, antibiotic resistance pattern and biofilm formation of Vibrio parahaemolyticus and V. cholerae isolated from crustaceans and humans. Int J Food Microbiol 274:31–37. https://doi.org/10.1016/j.ijfoodmicro.2018.03.013
Alam MJ, Tomochika KI, Miyoshi SI, Shinoda S (2002) Environmental investigation of potentially pathogenic Vibrio parahaemolyticus in the Seto-Inland Sea, Japan. FEMS Microbiol Lett 208:83–87. https://doi.org/10.1111/j.1574-6968.2002.tb11064.x
Altekruse SF, Bishop RD, Baldy LM, Thompson SG, Wilson SA, Ray BJ, Griffin PM (2000) Vibrio gastroenteritis in the US Gulf of Mexico region: the role of raw oysters. Epidemiol Infect 124:489–495. https://doi.org/10.1017/S0950268899003714
Álvarez-Contreras AK, Quiñones-Ramírez EI, Vázquez-Salinas C (2021) Prevalence, detection of virulence genes and antimicrobial susceptibility of pathogen Vibrio species isolated from different types of seafood samples at “La Nueva Viga” market in Mexico City. Antonie van Leeuwenhoek. Int J Gen Mol Microbiol 114:1417–1429. https://doi.org/10.1007/s10482-021-01591-x
Arora H, McHargue C (2021) 28353 hemorrhagic cellulitis secondary to vibrio fluvialis infection. J Am Acad Dermatol 85:AB184. https://doi.org/10.1016/j.jaad.2021.06.750
Ashrafudoulla M, Mizan MF, Park H, Byun KH, Lee N, Park SH, Ha SD (2019) Genetic relationship, virulence factors, drug resistance profile and biofilm formation ability of Vibrio parahaemolyticus isolated from mussel. Front microbiol 10:513. https://doi.org/10.3389/fmicb.2019.00513
Ashrafudoulla Md, Na KW, Hossain MI, Mizan MF, Nahar S, Toushik SH, Roy PK, Park SH, Ha SD (2021) Molecular and pathogenic characterization of Vibrio parahaemolyticus isolated from seafood. Mar Pollut Bull 172:112927. https://doi.org/10.1016/j.marpolbul.2021.112927
Baker-Austin C, Stockley L, Rangdale R, Martinez‐Urtaza J (2010) Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environ Microbiol Rep 2:7–18. https://doi.org/10.1111/j.1758-2229.2009.00096.x
Baker-Austin C, Trinanes JA, Salmenlinna S, Löfdahl M, Siitonen A, Taylor NG, Martinez-Urtaza (2016) Heat wave–associated vibriosis, Sweden and Finland, 2014. Emerg Infect Dis 22:1216. https://doi.org/10.3201/eid2207.151996
Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J (2017) Non-cholera vibrios: the microbial barometer of climate change. Trends Microbiol 25:76–84. https://doi.org/10.1016/j.tim.2016.09.008
Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J (2018) Vibrio spp. infections. Nat Rev Dis Primers 4:1–9
Balsalobre LC, Dropa M, de Oliveira DE, Lincopan N, Mamizuka EM, Matté GR, Matté MH (2010) Presence of blaTEM-116 gene in environmental isolates of Aeromonas hydrophila and Aeromonas jandaei from Brazil. Braz J Microbiol 41:718–719. https://doi.org/10.1590/S1517-83822010000300023
Barbieri E, Falzano L, Fiorentini C, Pianetti A, Baffone W, Fabbri A, Matarrese P, Casiere A, Katouli M, Kühn I, Möllby R (1999) Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl Environ Microbiol 65:2748–2753. https://doi.org/10.1128/AEM.65.6.2748-2753.1999
Barker WHJ (1974) International symposium on Vibrio parahaemolyticus. Saikon Publishing Co., Ltd, Tokyo, pp 47–52
Bayliss SC, Verner-Jeffreys DW, Bartie KL, Aanensen DM, Sheppard SK, Adams A, Feil EJ (2017) The promise of whole genome pathogen sequencing for the molecular epidemiology of emerging aquaculture pathogens. Front microbiol 8:121. https://doi.org/10.3389/fmicb.2017.00121
Beceiro A, Tomás M, Bou G (2013) Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev 26:185–230. https://doi.org/10.1128/CMR.00059-12
Castello A, Alio V, Sciortino S, Oliveri G, Cardamone C, Butera G, Costa A (2022) Occurrence and molecular characterization of potentially pathogenic Vibrio spp. in seafood collected in Sicily. Microorganisms 11:53. https://doi.org/10.3390/microorganisms11010053
CDC Centers for Disease Control and Prevention (2023) Available at: https://www.cdc.gov/foodsafety/communication/oysters-and-vibriosis.html
Changchai N, Saunjit S (2014) Occurrence of Vibrio parahaemolyticus and Vibrio vulnificus in retail raw oysters from the eastern coast of Thailand. Southeast Asian J Trop Med Public Health 45:662
Chen X, Zhu Q, Yu F, Zhang W, Wang R, Ye X, Jin L, Liu Y, Li S, Chen Y (2018) Serology, virulence and molecular characteristics of Vibrio parahaemolyticus isolated from seafood in Zhejiang province. PLOS One 13:e0204892. https://doi.org/10.1371/journal.pone.0204892
Church SR, Lux T, Baker-Austin C, Buddington SP, Michell SL (2016) Vibrio vulnificus type 6 secretion system 1 contains anti-bacterial properties. PLOS One 11:e0165500. https://doi.org/10.1371/journal.pone.0165500
Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH (2008) Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci 105:9379–9384. https://doi.org/10.1073/pnas.0712241105
Coerdt KM, Khachemoune A (2021) Vibrio vulnificus: review of mild to life-threatening skin infections. Cutis 107:E12–E17
Costa RA, Amorim LM, Araújo RL, Vieira RH (2013) Múltiples perfiles enzimáticos de cepas de Vibrio parahaemolyticus aisladas de ostras. Rev Argent Microbiol 45:267–270
Dahanayake PS, De Silva BC, Hossain S, Shin GW, Heo GJ (2018) Occurrence, virulence factors, and antimicrobial susceptibility patterns of Vibrio spp. isolated from live oyster (Crassostrea gigas) in Korea. J Food Saf 38:e12490. https://doi.org/10.1111/jfs.12490
Dahanayake PS, Hossain S, Wickramanayake MV, Heo GJ (2020a) Prevalence of virulence and extended-spectrum β‐lactamase (ESBL) genes harbouring Vibrio spp. isolated from cockles (Tegillarca granosa) marketed in Korea. Lett Appl Microbiol 71:61–69. https://doi.org/10.1111/lam.13232
Dahanayake PS, Hossain S, Wickramanayake MV, Wimalasena SH, Heo GJ (2020b) Manila clam (Ruditapes philippinarum) marketed in Korea as a source of vibrios harbouring virulence and β-lactam resistance genes. Lett Appl Microbiol 71:46–53. https://doi.org/10.1111/lam.13229
Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L (2000) Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis 181:1661–1666. https://doi.org/10.1086/315459
De Silva BC, Hossain S, Dahanayake PS, Kang TM, Heo GJ (2019) Vibrio spp. from Yesso scallop (Patinopecten yessoensis) demonstrating virulence properties and antimicrobial resistance. J Food Saf 39:e12634. https://doi.org/10.1111/jfs.12634
DePaola AN, Capers GM, Alexander D (1994) Densities of Vibrio vulnificus in the intestines of fish from the US Gulf Coast. Appl Environ Microbiol 60:984–988. https://doi.org/10.1128/aem.60.3.984-988.1994
Department FAOF, Information F, Data and Statistics Unit (2019) FishStatJ, a tool for fishery statistics analysis. Release: 3.5.0, Universal Software for Fishery Statistical Time Series. Global aquaculture production: quantity 1950–2017; Value 1950–2017; global capture production. FAO, Rome, Italy, pp 1950–2017
Di DY, Lee A, Jang J, Han D, Hur HG (2017) Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Appl Environ Microbiol 83:e02680–e02616. https://doi.org/10.1128/AEM.02680-16
Dietsche T, Tesfazgi Mebrhatu M, Brunner MJ, Abrusci P, Yan J, Franz-Wachtel M, Schärfe C, Zilkenat S, Grin I, Galan JE, Kohlbacher O (2016) Structural and functional characterization of the bacterial type III secretion export apparatus. PLOS Pathog 12:e1006071. https://doi.org/10.1371/journal.ppat.1006071
Dubert J, Osorio CR, Prado S, Barja JL (2016) Persistence of antibiotic resistant Vibrio spp. in shellfish hatchery environment. Microb Ecol 72:851–860. https://doi.org/10.1007/s00248-015-0705-5
Elavarashi E, Kindo AJ, Rangarajan S (2017) Enzymatic and non-enzymatic virulence activities of dermatophytes on solid media. J Cli Diagn Res 11:DC23. https://doi.org/10.7860/JCDR/2017/23147.9410
FAO. Food and agriculture organization (2022) Retrieved from https://www.fao.org/3/cc0461en/online/sofia/2022/world-fisheries-aquaculture-production.html. Accessed on 2 February 2023
Farmer Iii JJ, Michael Janda J, Brenner FW, Cameron DN, Birkhead KM (2015) Vibrio. Bergey’s manual of systematics of archaea and bacteria. Wiley, Hoboken
Fernández-Bravo A, Ballester F, Pujol I, Gomez-Bertomeu F, Martí C, Rezusta A, Ferrer-Cerón I, Aspiroz C, Puyod MJ, Figueras MJ (2019) Vibrio alginolyticus infections: report of two cases from Spain with literature review. J Med Microb Diagn 8:2161–0703
Ferrini AM, Mannoni V, Suffredini E, Cozzi L, Croci L (2008) Evaluation of antibacterial resistance in Vibrio strains isolated from imported seafood and italian aquaculture settings. Food Anal Methods 1:164–170. https://doi.org/10.1007/s12161-007-9011-2
Fiore AE, Michalski JM, Russell RG, Sears CL, Kaper JB (1997) Cloning, characterization, and chromosomal mapping of a phospholipase (lecithinase) produced by Vibrio cholerae. Infect Immun 65:3112–3117. https://doi.org/10.1128/iai.65.8.3112-3117.1997
Gillings MR, Gaze WH, Pruden A, Smalla K, Tiedje JM, Zhu YG (2015) Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J 9:1269–1279. https://doi.org/10.1038/ismej.2014.226
Giltner CL, Nguyen Y, Burrows LL (2012) Type IV pilin proteins: versatile molecular modules. Microbiol Mol Biol R 76:740–772. https://doi.org/10.1128/MMBR.00035-12
Hecht J, Borowiak M, Fortmeier B, Dikou S, Gierer W, Klempien I, Nekat J, Schaefer S, Strauch E (2022) Case report: Vibrio fluvialis isolated from a wound infection after a piercing trauma in the Baltic Sea. Access Microbiol. https://doi.org/10.1099/acmi.0.000312
Hernández-Robles MF, Álvarez-Contreras AK, Juárez-García P, Natividad-Bonifacio I, Curiel-Quesada E, Vázquez-Salinas C, Quiñones-Ramírez EI (2016) Virulence factors and antimicrobial resistance in environmental strains of Vibrio alginolyticus. Int Microbiol 19:191–198. https://doi.org/10.2436/20.1501.01.277
Hervio-Heath D, Colwell RR, Derrien A, Robert‐Pillot A, Fournier JM, Pommepuy M (2002) Occurrence of pathogenic vibrios in coastal areas of France. J Appl Microbiol 92:1123–1135. https://doi.org/10.1046/j.1365-2672.2002.01663.x
Hoefler F, Pouget-Abadie X, Roncato-Saberan M, Lemarié R, Takoudju EM, Raffi F, Corvec S, Le Bras M, Cazanave C, Lehours P, Guimard T (2022) Clinical and Epidemiologic Characteristics and Therapeutic Management of Patients with Vibrio Infections, Bay of Biscay, France, 2001–2019. Emerg Infect Dis 28:2367–2373
Honda T, Iida T (1993) The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev Med Microbiol 4:106–113
Honda TA, Ni YX, Miwatani TO (1988) Purification and characterization of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infect Immun 56:961–965. https://doi.org/10.1128/iai.56.4.961-965.1988
Hong To TT, Yanagawa H, Khanh Thuan N, Hiep DM, Cuong DV, Khai LT, Taniguchi T, Kubo R, Hayashidani H (2020) Prevalence of Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease of shrimp in shrimp, molluscan shellfish and water samples in the Mekong Delta. Vietnam Biol 9:312. https://doi.org/10.3390/biology9100312
Hossain S, Wickramanayake MV, Dahanayake PS, Heo GJ (2020) Occurrence of virulence and extended-spectrum β-lactamase determinants in Vibrio spp. isolated from marketed hard-shelled mussel (Mytilus coruscus). Microb Drug Resist 26:391–401. https://doi.org/10.1089/mdr.2019.0131
Howard-Jones N (1984) Robert Koch and the cholera Vibrio: a centenary - Br Med J. (Clinical research ed.) 288:379. https://doi.org/10.1136/bmj.288.6414.379
Hu Q, Chen L (2016) Virulence and antibiotic and heavy metal resistance of Vibrio parahaemolyticus isolated from crustaceans and shellfish in Shanghai, China. J Food Prot 79:1371–1377. https://doi.org/10.4315/0362-028X.JFP-16-031
Igbinosa EO, Okoh AI (2010) Vibrio fluvialis: an unusual enteric pathogen of increasing public health concern. Int J Environ Res Publ Health 7:3628–3643. https://doi.org/10.3390/ijerph7103628
Iwamoto M, Ayers T, Mahon BE, Swerdlow DL (2010) Epidemiology of seafood-associated infections in the United States. Clin Microbiol Rev 23:399–411. https://doi.org/10.1128/CMR.00059-09
Jang HM, Kim YB, Choi S, Lee Y, Shin SG, Unno T, Kim YM (2018) Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea. Environ Pollut 233:1049–1057. https://doi.org/10.1016/j.envpol.2017.10.006
Jang GI, Park JI, Oh EG, Kim S (2020) The relationship between Acute Hepatopancreatic Necrosis Disease (AHPND) in shrimp Litopenaeus vannamei and Vibrio parahaemolyticus strains isolated from shellfish and shrimp of the West Coast of Korea in 2019. Korean J Fish Aquat Sci 53:752–760. https://doi.org/10.5657/KFAS.2020.0752
Jeamsripong S, Khant W, Chuanchuen R (2020) Distribution of phenotypic and genotypic antimicrobial resistance and virulence genes in Vibrio parahaemolyticus isolated from cultivated oysters and estuarine water. FEMS Microbiol Ecol 96:fiaa081. https://doi.org/10.1093/femsec/fiaa081
Jiang Y, Chu Y, Xie G, Li F, Wang L, Huang J, Zhai Y, Yao L (2019) Antimicrobial resistance, virulence and genetic relationship of Vibrio parahaemolyticus in seafood from coasts of Bohai Sea and Yellow Sea, China. Int J Food Microbiol 290:116–124. https://doi.org/10.1016/j.ijfoodmicro.2018.10.005
Jingjit N, Preeprem S, Surachat K, Mittraparp-Arthorn P (2021) Characterization and analysis of clustered regularly interspaced short palindromic repeats (CRISPRs) in pandemic and non-pandemic Vibrio parahaemolyticus isolates from seafood sources. Microorganisms 9:1220. https://doi.org/10.3390/microorganisms9061220
Jo S, Shin C, Shin Y, Kim PH, il Park J, Kim M, Park B, So JS (2020) Heavy metal and antibiotic co-resistance in Vibrio parahaemolyticus isolated from shellfish. Mar Pollut Bull 156:111246. https://doi.org/10.1016/j.marpolbul.2020.111246
Jones MK, Oliver JD (2009) Vibrio vulnificus: disease and pathogenesis. Infect immun 77:1723–1733. https://doi.org/10.1128/IAI.01046-08
Jun JW, Kim JH, Choresca CH Jr, Shin SP, Han JE, Han SY, Chai JY, Park SC (2012) Isolation, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus in korean seafood. Foodborne Pathog Dis 9:224–231. https://doi.org/10.1089/fpd.2011.1018
Kang CH, Shin Y, Jang S, Jung Y, So JS (2016) Antimicrobial susceptibility of Vibrio alginolyticus isolated from oyster in Korea. Environ Sci Pollut Res 23:21106–21112. https://doi.org/10.1007/s11356-016-7426-2
Kang CH, Shin Y, Jang S, Yu H, Kim S, An S, Park K, So JS (2017) Characterization of Vibrio parahaemolyticus isolated from oysters in Korea: resistance to various antibiotics and prevalence of virulence genes. Mar Pollut Bull 118:261–266. https://doi.org/10.1016/j.marpolbul.2017.02.070
Kang CH, Shin Y, Yu H, Kim S, So JS (2018) Antibiotic and heavy-metal resistance of Vibrio parahaemolyticus isolated from oysters in Korea. Mar Pollut Bull 135:69–74. https://doi.org/10.1016/j.marpolbul.2018.07.007
KCDC. Korea Center for Disease Control (2022) Available at: http://www.cdc.go.kr
KCDC. Korea Center for Disease Control (2016) Available at: http://www.cdc.go.kr
KIM SH, Sin YM, Lee MJ, Shin PK, Kim MC, Cho JS, Lee CH, Lee YJ, Chae KR (2005) Isolation of major foodborne pathogenic bacteria from ready-to-eat seafoods and its reduction strategy. J Life Sci 15:941–947
Krumperman PH (1983) Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol 46:165–170. https://doi.org/10.1128/aem.46.1.165-170.1983
Lafisca A, Pereira CS, Giaccone V, Rodrigues DD (2008) Enzymatic characterization of Vibrio alginolyticus strains isolated from bivalves harvested at Venice lagoon (Italy) and Guanabara Bay (Brazil). Rev Inst Med Trop Sao Paulo 50:199–202. https://doi.org/10.1590/S0036-46652008000400002
Lee WC, Lee MJ, Kim JS, Park SY (2001) Foodborne illness outbreaks in Korea and Japan studied retrospectively. J Food Prot 64:899–902
Lee HW, Lim SK, Kim MN (2009) Characteristics of ampicillin-resistant Vibrio spp. isolated from a west coastal area of Korean Peninsula. Korean J Fish Aquat Sci 42:20–25. https://doi.org/10.5657/kfas.2009.42.1.020
Letchumanan V, Chan KG, Lee LH (2014) Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol 5:705. https://doi.org/10.3389/fmicb.2014.00705
Letchumanan V, Ab Mutalib NS, Wong SH, Chan KG, Lee LH (2019) Determination of antibiotic resistance patterns of Vibrio parahaemolyticus from shrimp and shellfish in Selangor, Malaysia. Prog Microbes Mol Biol. https://doi.org/10.36877/pmmb.a0000019
Levy SB (2001) Antibiotic resistance: consequences of inaction. Clin Infect Dis 33:124–129. https://doi.org/10.1086/321837
Li Y, Xie X, Shi X, Lin Y, Mou J, Chen Q, Lu Y, Zhou L, Jiang M, Sun H, Ma H (2014) Vibrio parahaemolyticus, southern coastal region of China, 2007–2012. Emerg Infect Dis 20:685. https://doi.org/10.3201/eid2004.130744
Liang P, Cui X, Du X, Kan B, Liang W (2013) The virulence phenotypes and molecular epidemiological characteristics of Vibrio fluvialis in China. Gut Pathog 5:1–1. https://doi.org/10.1186/1757-4749-5-6
Lida T, Park KS, Suthienkul O, Kozawa J, Yamaichi Y, Yamamoto K, Honda T (1998) Close proximity of the tdh, trh and ure genes on the chromosome of Vibrio parahaemolyticus. Microbiology. https://doi.org/10.1099/00221287-144-9-2517
Liu PC, Chen YC, Lee KK (2001) Pathogenicity of Vibrio alginolyticus isolated from diseased small abalone Haliotis diversicolor supertexta. Microbios 104:71–77
Liu X, Chen Y, Wang X, Ji R (2004) Foodborne disease outbreaks in China from 1992 to 2001 national foodborne disease surveillance system. Wei sheng yan jiu 33:725–727
Lopatek M, Wieczorek K, Osek J (2015) Prevalence and antimicrobial resistance of Vibrio parahaemolyticus isolated from raw shellfish in Poland. J Food Prot 78:1029–1033. https://doi.org/10.4315/0362-028X.JFP-14-437
Lopatek M, Wieczorek K, Osek J (2018) Antimicrobial resistance, virulence factors, and genetic profiles of Vibrio parahaemolyticus from seafood. Appl Environ Microbiol 84:e00537–e00518. https://doi.org/10.1128/AEM.00537
Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y (2003) Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. The Lancet 361:743–749. https://doi.org/10.1016/S0140-6736(03)12659-1
Mancini ME, Alessiani A, Donatiello A, Didonna A, D’Attoli L, Faleo S, Occhiochiuso G, Carella F, Di Taranto P, Pace L, Rondinone V (2023) Systematic survey of Vibrio spp. and Salmonella spp. in Bivalve Shellfish in Apulia Region (Italy): prevalence and Antimicrobial resistance. Microorganisms 11:450. https://doi.org/10.3390/microorganisms11020450
Manjusha S, Sarita GB (2013) Characterization of plasmids from multiple antibiotic resistant Vibrios isolated from molluscan and crustacean of Kerala. Int Food Res J 20:77–86
Manjusha S, Sarita GB, Elyas KK, Chandrasekaran M (2005) Multiple antibiotic resistances of Vibrio isolates from coastal and brackish water areas. Am J Biochem Biotechnol 1:201–206
McCarthy SA, DePaola A, Cook DW, Kaysner CA, Hill WE (1999) Evaluation of alkaline phosphatase-and digoxigenin‐labelled probes for detection of the thermolabile hemolysin (tlh) gene of Vibrio parahaemolyticus. Lett Appl Microbiol 28:66–70. https://doi.org/10.1046/j.1365-2672.1999.00467.x
Mey AR, Butz HA, Payne SM (2015) Vibrio cholerae CsrA regulates ToxR levels in response to amino acids and is essential for virulence. MBio 6:e01064–e01015. https://doi.org/10.1128/mBio.01064-15
Miyoshi S (2006) Vibrio vulnificus infection and metalloprotease. Int J Dermatol 33:589–595. https://doi.org/10.1111/j.1346-8138.2006.00139.x
Mok JS, Ryu A, Kwon JY, Park K, Shim KB (2019a) Abundance, antimicrobial resistance, and virulence of pathogenic Vibrio strains from molluscan shellfish farms along the korean coast. Mar Pollut Bull 149:110559. https://doi.org/10.1016/j.marpolbul.2019.110559
Mok JS, Ryu A, Kwon JY, Kim B, Park K (2019b) Distribution of Vibrio species isolated from bivalves and bivalve culture environments along the Gyeongnam coast in Korea: virulence and antimicrobial resistance of Vibrio parahaemolyticus isolates. Food Control 106:106697
My Alothrubi S (2014) Antibiotic resistance of Vibrio parahaemolyticus isolated from cockles and shrimp sea food marketed in Selangor, Malaysia. Clin Microbiol 3:148. https://doi.org/10.4172/2327-5073.1000148
Narayanan SV, Joseph TC, Peeralil S, Mothadaka MP, Lalitha KV (2020) Prevalence, virulence characterization, AMR pattern and genetic relatedness of Vibrio parahaemolyticus isolates from retail seafood of Kerala, India. Front Microbiol 11:592. https://doi.org/10.3389/fmicb.2020.00592
Nishibuchi M, Kaper JB (1995) Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect Immun 63:2093–2099
Nsikan SU, Bassey EB, Anne EA, Otobong DA, Casmir ICI (2021) Multi-Drug Resistance genes associated with some GramNegative Bacterial isolated from Shellfish in Iko and Douglas River Estuaries, in Nigeria. Int J Res Stud Med Health Sci 6:11–23. https://doi.org/10.22259/ijrsmhs.0604004
Oh EG, Son KT, Ha KS, Yoo HD, Yu HS, Shin SB, Lee HJ, Kim JH (2009) Antimicrobial resistance of Vibrio strains from brackish water on the coast of Gyeongsangnamdo. Korean J Fish Aquat Sci 42:335–343. https://doi.org/10.5657/kfas.2009.42.4.335
Oh EG, Son KT, Yu H, Lee TS, Lee HJ, Shin S, Kwon JY, Park K, Kim J (2011) Antimicrobial resistance of Vibrio parahaemolyticus and Vibrio alginolyticus strains isolated from farmed fish in Korea from 2005 through 2007. J Food Prot 74:380–386. https://doi.org/10.4315/0362-028X.JFP-10-307
Oliver JD (2005) Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol Infect 133:383–391. https://doi.org/10.1017/S0950268805003894
Oliver JD, Bockian R (1995) In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol 61:2620–2623. https://doi.org/10.1128/aem.61.7.2620-2623.1995
Ottaviani D, Leoni F, Rocchegiani E, Santarelli S, Masini L, Di Trani V, Canonico C, Pianetti A, Tega L, Carraturo A (2009) Prevalence and virulence properties of non-O1 non-O139 Vibrio cholerae strains from seafood and clinical samples collected in Italy. Int J Food Microbiol 132:47–53. https://doi.org/10.1016/j.ijfoodmicro.2009.03.014
Pang R, Xie T, Wu Q, Li Y, Lei T, Zhang J, Ding Y, Wang J, Xue L, Chen M, Wei X (2019) Comparative genomic analysis reveals the potential risk of Vibrio parahaemolyticus isolated from ready-to-eat foods in China. Front Microbiol 10:186. https://doi.org/10.3389/fmicb.2019.00186
Park KS, Ono T, Rokuda M, Jang MH, Okada K, Iida T, Honda T (2004) Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun 72:6659–6665. https://doi.org/10.1128/IAI.72.11.6659-6665.2004
Park K, Mok JS, Ryu AR, Kwon JY, Ham IT, Shim KB (2018) Occurrence and virulence of Vibrio parahaemolyticus isolated from seawater and bivalve shellfish of the Gyeongnam coast, Korea, in 2004–2016. Mar Pollut Bull 137:382–387. https://doi.org/10.1016/j.marpolbul.2018.10.033
Parthasarathy S, Das SC, Kumar A, Chowdhury G, Miyoshi SI, Dutta S, Mukhopadhyay AK (2021) Molecular characterization and antibiotic resistance of Vibrio parahaemolyticus from indian oyster and their probable implication in food chain. World J Microbiol Biotechnol 37:145. https://doi.org/10.1007/s11274-021-03113-3
Prester L (2011) Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam: Part A 28:1547–1560. https://doi.org/10.1080/19440049.2011.600728
Quiñones-Ramírez EI, Bonifacio IN, Betancourt-Rule M, Ramirez-Vives F, Vázquez-Salinas C (2010) Putative virulence factors identified in Vibrio vulnificus strains isolated from oysters and seawater in Mexico. Int J Environ Health Res 20:395–405. https://doi.org/10.1080/09603123.2010.491856
Raghunath P (2015) Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front Microbiol 5:805. https://doi.org/10.3389/fmicb.2014.00805
Ramamurthy T, Chowdhury G, Pazhani GP, Shinoda S (2014) Vibrio fluvialis: an emerging human pathogen. Front Microbiol 5:91. https://doi.org/10.3389/fmicb.2014.00091
Reilly GD, Reilly CA, Smith EG, Baker-Austin C (2011) Vibrio alginolyticus-associated wound infection acquired in british waters, Guernsey, July 2011. Eurosurveillance 16:19994. https://doi.org/10.2807/ese.16.42.19994-en
Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, Davis BM, Bronson RT, Waldor MK (2012) Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLOS Pathog 8:e1002593. https://doi.org/10.1371/journal.ppat.1002593
Robert-Pillot A, Guénolé A, Lesne J, Delesmont R, Fournier JM, Quilici ML (2004) Occurrence of the tdh and trh genes in Vibrio parahaemolyticus isolates from waters and raw shellfish collected in two French coastal areas and from seafood imported into France. Int J Food Microbiol 91:319–325
Rojas MV, Matté MH, Dropa M, Da Silva ML, Matté GR (2011) Caracterização de Vibrio parahaemolyticus isolados de ostras e mexilhões em São Paulo, Brasil. Rev Inst Med Trop Sao Paulo 53:201–205. https://www.revistas.usp.br/rimtsp/article/view/31406
Ryu AR, Park K, Kim SH, Ham IT, Kwon JY, Kim JH, Yu HS, Lee HJ, Mok JS (2017) Antimicrobial resistance patterns of Escherichia coli and Vibrio parahaemolyticus isolated from shellfish from the west coast of Korea. Korean J Fish Aquat Sci 50:662–668. https://doi.org/10.5657/KFAS.2017.0662
Ryu AR, Mok JS, Lee DE, Kwon JY, Park K (2019) Occurrence, virulence, and antimicrobial resistance of Vibrio parahaemolyticus isolated from bivalve shellfish farms along the southern coast of Korea. Environ Sci Pollut Res 26:21034–21043. https://doi.org/10.1007/s11356-019-05426-1
Sadat A, El-Sherbiny H, Zakaria A, Ramadan H, Awad A (2021) Prevalence, antibiogram and virulence characterization of Vibrio isolates from fish and shellfish in Egypt: a possible zoonotic hazard to humans. J Appl Microbiol 131:485–498. https://doi.org/10.1111/jam.14929
Sawabe T, Fujimura Y, Niwa K, Aono H (2007) Vibrio comitans sp. nov., Vibrio rarus sp. nov. and Vibrio inusitatus sp. nov., from the gut of the abalones Haliotis discus discus, H. gigantea, H. madaka and H. rufescens. Int J Syst Evol Micr 57:916–922. https://doi.org/10.1099/ijs.0.64789-0
Sawabe T, Ogura Y, Matsumura Y, Feng G, Amin AR, Mino S, Nakagawa S, Sawabe T, Kumar R, Fukui Y, Satomi M (2013) Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front Microbiol 4:414. https://doi.org/10.3389/fmicb.2013.00414
Seelman SL, Whitney BM, Stokes EK, Elliot EL, Griswold T, Patel K, Bloodgood S, Jones JL, Cripe J, Cornell J, Luo Y (2023) An outbreak investigation of Vibrio parahaemolyticus Infections in the United States linked to crabmeat imported from Venezuela: 2018. Foodborne Pathog Dis 20:123–131. https://doi.org/10.1089/fpd.2022.0078
Serwecińska L (2020) Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water 12:3313. https://doi.org/10.3390/w12123313
Sha J, Rosenzweig JA, Kozlova EV, Wang S, Erova TE, Kirtley ML, van Lier CJ, Chopra AK (2013) Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis. Microbiology 159:1120–1135. https://doi.org/10.1099/mic.0.063495-0
Shaw KS, Rosenberg Goldstein RE, He X, Jacobs JM, Crump BC, Sapkota AR (2014) Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Chesapeake Bay and Maryland Coastal Bays. PlOS One 9
Shen X, Cai Y, Liu C, Liu W, Hui Y, Su YC (2009) Effect of temperature on uptake and survival of Vibrio parahaemolyticus in oysters (Crassostrea plicatula). Int J Food Microbiol 136:129–132. https://doi.org/10.1016/j.ijfoodmicro.2009.09.012
Silva IP, de Souza Carneiro C, Saraiva MA, de Oliveira TA, de Sousa OV, Evangelista-Barreto NS (2018) Antimicrobial resistance and potential virulence of Vibrio parahaemolyticus isolated from water and bivalve mollusks from Bahia, Brazil. Mar Pollut Bull 131:757–762. https://doi.org/10.1016/j.marpolbul.2018.05.007
Silvester R, Pires J, Van Boeckel TP, Madhavan A, Balakrishnan Meenakshikutti A, Hatha (2019) Occurrence of β-lactam resistance genes and plasmid-mediated resistance among Vibrios isolated from Southwest Coast of India. Microb Drug Resist 25:1306–1315. https://doi.org/10.1089/mdr.2019.0031
Silvester R, Saji A, Divakaran AR, Dilshana PM, Nair R, Hatha M, Harikrishnan M (2022) Increased incidence and antimicrobial resistance among Vibrio parahaemolyticus in shellfishes from major fish markets in Cochin, South India: Seafood risk assessment. Ann Anim Sci 22:1105–1114. https://doi.org/10.2478/aoas-2021-0077
Slifka KJ, Newton AE, Mahon BE (2017) Vibrio alginolyticus infections in the USA, 1988–2012. Epidemiol Infect 145:1491–1499
Son R, Rusul G, Sahilah AM, Zainuri A, Raha AR, Salmah I (1997) Antibiotic resistance and plasmid profile of Aeromonas hydrophila isolates from cultured fish, Telapia (Telapia mossambica). Lett Appl Microbiol 24:479–482. https://doi.org/10.1046/j.1472-765X.1997.00156.x
Song X, Ma Y, Fu J, Zhao A, Guo Z, Malakar PK, Pan Y, Zhao Y (2017) Effect of temperature on pathogenic and non-pathogenic Vibrio parahaemolyticus biofilm formation. Food Control 73:485–491. https://doi.org/10.1016/j.foodcont.2016.08.041
Soto-Rodriguez SA, Roque A, Lizarraga-Partida ML, Guerra-Flores AL, Gomez-Gil B (2003) Virulence of luminous vibrios to Artemia franciscana nauplii. Dis Aquat Organ 53:231–240. https://doi.org/10.3354/dao053231
Stratev D, Fasulkova R, Krumova-Valcheva G (2023) Incidence, virulence genes and antimicrobial resistance of Vibrio parahaemolyticus isolated from seafood. Microb Pathog 177:106050. https://doi.org/10.1016/j.micpath.2023.106050
Suñén E, Acebes M, Fernández-Astorga A (1995) Occurrence of potentially pathogenic vibrios in bivalve molluscs (mussels and clams) from retail outlets in the north of Spain. J Food Saf 15:275–281. https://doi.org/10.1111/j.1745-4565.1995.tb00139.x
Tan CW, Rukayadi Y, Hasan H, Thung TY, Lee E, Rollon WD, Hara H, Kayali AY, Nishibuchi M, Radu S (2020) Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi J Biol Sci 27:1602–1608. https://doi.org/10.1016/j.sjbs.2020.01.002
Torres L, Escobar S, López A, Marco M, Pobo V (2002) Wound infection due to Vibrio vulnificus in Spain. Eur J Clin Microbiol 21:537–538. https://doi.org/10.1007/s10096-002-0767-4
Udoekong NS, Bassey BE, Asuquo AE, Akan OD, Ifeanyi CI (2021) Multi-Drug Resistance genes associated with some Gram-Negative Bacteria isolates from Shellfish in Iko and Douglas River Estuaries, in Nigeria. Eur J Biol Biotechnol 2:19–27
Vaiyapuri M, Pailla S, Rao Badireddy M, Pillai D, Chandragiri Nagarajarao R, Prasad Mothadaka M (2021) Antimicrobial resistance in Vibrios of shrimp aquaculture: incidence, identification schemes, drivers and mitigation measures. Aquac Res 52:2923–2941. https://doi.org/10.1111/are.15142
Vergis EN, Shankar N, Chow JW, Hayden MK, Snydman DR, Zervos MJ, Linden PK, Wagener MM, Muder RR (2002) Association between the presence of enterococcal virulence factors gelatinase, hemolysin, and enterococcal surface protein and mortality among patients with bacteremia due to Enterococcus faecalis. Clin Infect Dis 35:570–575. https://doi.org/10.1086/341977
Vongxay K, Wang S, Zhang X, Wu B, Hu H, Pan Z, Chen S, Fang W (2008) Pathogenetic characterization of Vibrio parahaemolyticus isolates from clinical and seafood sources. J Food Microbiol 126:71–75. https://doi.org/10.1016/j.ijfoodmicro.2008.04.032
Wang R, Zhong Y, Gu X, Yuan J, Saeed AF, Wang S (2015) The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front Microbiol 6:144. https://doi.org/10.3389/fmicb.2015.00144
Weis KE, Hammond RM, Hutchinson R, Blackmore CG (2011) Vibrio illness in Florida, 1998–2007. Epidemiol Infect 139:591–598. https://doi.org/10.1017/S0950268810001354
Wickramanayake MV, Dahanayake PS, Hossain S, De Silva BC, Heo GJ (2020) Characterisation of pathogenic Vibrio spp. isolated from live Pacific abalone (Haliotis discus hannai Ino, 1953) marketed in South Korea. Indian J Fish 67:105–113. https://doi.org/10.21077/ijf.2019.67.1.96348-14
Wong HC, Liu SH, Ku LW, Lee IY, Wang TK, Lee YS, Lee CL, Kuo LP, Shih DY (2000) Characterization of Vibrio parahaemolyticus isolates obtained from foodborne illness outbreaks during 1992 through 1995 in Taiwan. J Food Prot 63:900–906. https://doi.org/10.4315/0362-028x-63.7.900
Yu H, Oh EG, Shin SB, Park YS, Lee HJ, Kim JH, Song KC (2014) Distribution and antimicrobial resistance of Vibrio parahaemolyticus isolated from korean shellfish. Korean J Fish Aquat Sci 47:508–515
Zanetti S, Spanu T, Deriu A, Romano L, Sechi LA, Fadda G (2001) In vitro susceptibility of Vibrio spp. isolated from the environment. Int J Antimicrob Agents 17:407–409
Zhong X, Pan Z, Mu Y, Zhu Y, Zhang Y, Ma J, Yang M, Yao H (2022) Characterization and epidemiological analysis of Vibrio parahaemolyticus isolated from different marine products in East China. Int J Food Microbiol 380:109867
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
PSD, SM and G-JH contributed to the conception and design of the study. The literature search was performed by PSD, SM, PMK, and G-JH. The first draft of the manuscript was written by PSD and SM, and all authors contributed to reviewing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sepala Dahanayake, P., Majeed, S., Kumarage, P.M. et al. Molluscan shellfish: a potential source of pathogenic and multidrug-resistant Vibrio spp.. J Consum Prot Food Saf 18, 227–242 (2023). https://doi.org/10.1007/s00003-023-01445-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-023-01445-w