Abstract
This study involved the development of an ultra-high-performance liquid chromatography-fluorescence method for rapid detection of piperazine residues in eggs. Accelerated solvent extraction, a rapid and efficient sample extraction technique, was used to extract piperazine from egg samples (whole egg, albumin and yolk). Ground eggs were subjected to accelerated solvent extraction using n-hexane in the first step and acetonitrile-formic acid (98:2, v/v) in the second step and were then purified by a solid phase extraction cartridge using polymeric strong cation sorbents. The collected eluate was placed on a nitrogen blower and dried. After reconstitution with acetonitrile, piperazine was derivatized with dansyl chloride and catalytic triethylamine. A portion of the precipitate was removed using a 0.22 μm needle filter (organic-phase) and injected into the separation system for analysis. The analyte was separated using an Acquity HSS T3 [100 × internal diameter (i.d.) 2.1 mm, 1.8 µm] ultra high performance liquid chromatography column that was coupled with a fluorescence detector. Under the optimum conditions, the linear ranges were 3.5–400.0 μg/kg for the whole egg and yolk and 4.2–400.0 μg/kg for the albumin, the extraction recoveries were ≥ 77.07%, and the precision values were ≤ 6.63%. The limit of detection and limit of quantification values were 1.05–1.32 and 3.50–4.20 μg/kg, respectively. The method was successfully applied for the analysis of piperazine in egg (whole egg, albumin and yolk) samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of antibiotics reduces mortality, improves the quality of poultry and eggs, increases production, and promotes the development of poultry farming (Ashraf et al. 2018). Piperazine is a heterocyclic antiparasitic drug with a good deworming effect on many types of nematodes that occur in pig, cattle, sheep and poultry (Adams 2001). Due to its low toxicity, low price and good insect-repelling effect, piperazine has gradually replaced the strongly toxic phenothiazine. Piperazine has a narrow antibacterial spectrum and is mainly used to treat certain nematodes such as ascarids. The hydrogen atoms on the two nitrogen atoms in the piperazine molecule are relatively active and easily substituted by a nucleophilic reagent, making piperazine an ideal pharmaceutical intermediate (Glamkowski et al. 1974; Solomon et al. 2010). Therefore, it is often provided as salts and derivatives, such as piperazine citrate, piperazine chloride, piperazine dihydrochloride, piperazine hydrochloride, 1-benzhydrylpiperazine derivatives and 4-[(1-phenyl-1H-pyrazol-4-yl) methyl] 1-piperazine (Onuaguluchi and Ghasi 2006; Kumar et al. 2009; Silva et al. 2015; Nikalje et al. 2017). These salt compounds have good elimination effects on nematodes, hookworms and aphids, and the derivatives can effectively treat some diseases (Onuaguluchi and Ghasi 2006; Kumar et al. 2009; Silva et al. 2015). However, long-term use of piperazine may also be associated with several problems, such as a shortened service life, the effects attenuated by long-term use, and piperazine residues in animal-derived foods. Furthermore, piperazine causes methemoglobinemia and affects blood oxygen carrying capacity thereby endangering human health (Staack and Maurer 2003). Thus, China, Japan, America, and the European Union (EU) have established a maximum residue limit (MRL) value for piperazine of 2 mg/kg in eggs to protect human health (Ministry of Agriculture of the People’s Republic of China 2002; The European Medicines Agency 2010; US Food and Drug Administration 2014; The Japan Food Chemical Research Foundation 2015). Animal-derived foods may contain veterinary drug residues, so efficient and simple methods are needed to detect these drugs to ensure that these foods are safe. Chromatographic methods such as high performance liquid chromatography (HPLC) (Gadzała-Kopciuch 2005; Lin et al. 2010; Navaneeswari and Reddy 2012; Montesano et al. 2013; Dong et al. 2016; Park et al. 2016; Xie et al. 2017; Liu et al. 2019; Bu et al. 2020) and gas chromatography (GC) (Skarping et al. 1986; Peters et al. 2003; Wang et al. 2017) have generally been employed to analyse piperazine or piperazine salt residues in beef, milk, eggs, chicken, pork, and human plasma. Due to the complexity of animal-derived foods, sample preparation is required prior to liquid chromatography (LC) and GC instrument analysis. Liquid–liquid extraction (LLE) is a traditional sample preparation method with simple operation but shortcomings such as requiring a long time, high reagent consumption and propensity for manual error (Sarafraz-Yazdi and Amiri 2010).
Solid phase extraction (SPE) is also complex and time-consuming and requires expensive solid-phase extraction cartridges (Płotka-Wasylka et al. 2015). Accelerated solvent extraction (ASE) is an automated and environmentally friendly extraction technology that consumes fewer reagents, requires less time and allows the processing of batch samples (Liu et al. 2019; Wang et al. 2019; Bu et al. 2020). Xie et al. (2017) used an LLE-SPE method to extract piperazine from chicken muscle and ultra-high-performance liquid chromatography (UHPLC) for analysis. Park et al. (2016) established an SPE method using a PCX cartridge combined with liquid chromatography–fluorescence detection (LC–FLD) technology to detect residues of piperazine in animal foods. Wang et al. (2017) established a method combining ASE with SPE (using a Strata-X-C cartridge) to extract piperazine residues from chicken and pig tissues and analysed them by the GC method. Bu et al. (2020) and Liu et al. (2019) developed an ASE–SPE technique coupled with LC–FLD method for the determination of piperazine in eggs, chicken tissues and pork, respectively, with SPE involving the use of a Strata-X-C cartridge to purify these samples. Since piperazine is a weak basic compound, extraction and purification under acidic conditions generally requires an SPE cartridge with polymeric strong cation sorbents. Therefore, in the present study a PCX or Strata-X-C SPE cartridge with polymeric strong cation sorbents was used. Compared with GC and LC–FLD methods, the ASE–SPE–UHPLC–FLD method has higher recovery, precision and sensitivity, and the entire experimental process is much shorter.
These chromatographic methods are generally not capable of directly detecting piperazine residues in animal foods, and the piperazine must usually be derivatized with dansyl chloride (DNS-Cl).
Piperazine is an organic heterocyclic compound with two nitrogen heteroatoms in position 1,4, classified as biologically active compound. Piperazine is used as an antiparasitic drug as an additive in feed or as a therapeutic agent. Therefore, the residue may undoubtedly be found in egg yolk. The aim of this research was to introduce a rapid and efficient sample preparation method based on ASE and SPE that uses formic acid–acetonitrile (2:98, v/v) as the extractant to extract piperazine from egg (whole egg, albumin and yolk) samples prior to analysis with UHPLC–FLD. Relatively few methods are available for the extraction and detection of piperazine residues in animal foods. Based on previous studies (Wang et al. 2017; Xie et al. 2017; Liu et al. 2019; Bu et al. 2020), this study used an ASE–SPE method and an optimized UHPLC–FLD method to detect piperazine residues in eggs. Unlike the previously reported methods, this study used an isocratic elution procedure with a short detection time (5 min). Compared with previously reported methods, this developed method greatly shortens the extraction and detection time, facilitating rapid and efficient extraction and detection of piperazine in egg samples.
2 Materials and methods
2.1 Chemicals and reagents
Piperazine (99.0% purity) and DNS-Cl (98.0% purity) were supplied by Sigma-Aldrich (St. Louis, Missouri, USA) and Yuan-Ye Biological Technology Co., Ltd. (Shanghai, China), respectively. Acetonitrile and triethylamine (99.0% purity) of HPLC grade were supplied by Thermo Fisher Scientific (Waltham, MA, USA). Analytical grade formic acid (98.0% purity), n-hexane (97.0% purity) and ammonium hydroxide were supplied by the Sinopharm Chemical Reagent Co. (Shanghai, China). The experimental water was ultrapure water.
A standard stock solution of piperazine was prepared by dissolving 10 mg of piperazine in 10 mL of acetonitrile (1.0 mg/mL) and was stored in the dark at − 70 °C, which provided stable storage for 4 months. Standard working solutions at different concentrations were prepared for calibration curves and recoveries by diluting the stock solutions of piperazine with acetonitrile. The working solutions were prepared daily and were stored at 4 °C. DNS-Cl solution was prepared daily by dissolving 10 mg of DNS-Cl standard in 10 mL of acetonitrile (1.0 mg/mL) and was stored in the dark at 4 °C.
2.2 Samples
Fifty eggs were obtained from a local supermarket in Yangzhou (Jiangsu Province, China). Other egg samples were supplied from a farm that did not use antibiotics and were used as a blank antibiotic-free samples in the optimization step of the proposed method. The eggs were separated into albumin and yolk, homogenized through an electric eggbeater and stored at − 35 °C. Fifty egg samples were kept at 4 °C after the homogenization of each egg.
2.3 Apparatus
The egg samples were analysed with a UHPLC–FLD system (Waters Corp., Milford, Massachusetts, USA) consisting of an Acquity UHPLC™ system with an online degasser, an autosampler and a fluorescence detector (excitation and emission wavelengths, 330 nm and 531 nm, respectively). Chromatographic separation of the target compound in the egg samples was performed using an isocratic elution (acetonitrile: water = 85:15, v/v) procedure using an Acquity HSS T3 UHPLC column (100 × i.d. 2.1 mm, 1.8 µm) at 25 °C. The experiment tested the separation effects of an Acquity BEH Amide UHPLC column (100 × i.d. 2.1 mm, 1.7 µm), an Acquity BEH C18 UHPLC column (100 × i.d. 2.1 mm, 1.7 µm), and an Acquity HSS T3 UHPLC column (100 × i.d. 2.1 mm, 1.8 µm) on the target compound. The flow rate was 0.2 mL/min. The injection volume was 10.0 µL. The experiment compared the effect of three different ratios of a water-acetonitrile mobile phase system (10:90, 15:85 and 20:80, v/v) on the separation of the target compound.
2.4 Sample preparation
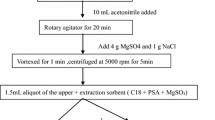
For preparing blank samples of whole egg, albumin and yolk, a blank sample (2.0 g) and diatomaceous earth (4.0 g) were placed in a mortar, and the mixture was thoroughly ground and placed in an extraction cell. The temperature, pressure, extraction cycles and extraction time were set on an ASE350 instrument (Thermo Fisher Scientific Co. Ltd.) to 80 °C, 1500 psi, 3 times, and 5 min, respectively. The first extraction was performed with n-hexane to remove the fat, and the next two extractions were performed with acetonitrile-formic acid (98:2, v/v) to extract the target compound and then collect the sample extract. The sample purification procedure uses a solid-phase extraction method with a Strata-X-C cartridge (3 mL/100 mg, Phenomenex Corp., Torrance, CA, USA) that was activated and equilibrated by the addition of 6 mL of methanol and then 6 mL of formic acid and water (2:98, v/v). After 20 mL of the extracts had been added to the Strata-X-C cartridge at a constant rate and the extract was completely drained from the cartridge, 2 mL of 0.1 mol/L hydrochloric acid solution was added immediately to the cartridge, and then 2 mL of methanol was added to wash the target compound. Finally, the target compound was eluted by adding 12 mL of 10% ammonium hydroxide in methanol to the cartridge. After the sample was purified, the eluate was collected in a 15 mL centrifuge tube and placed in a nitrogen blower to dry.
2.5 Derivatization reaction
After the extracts were dried with nitrogen, 1.0 mL acetonitrile was added to a 15 mL centrifuge tube, followed by ultrasonication for 10 min. Then, 100 μL of 0.12% triethylamine, 600 μL of 1.0 mg/mL DNS-Cl solution and 300 μL of acetonitrile were added to a 15 mL centrifuge tube and protected from light for 20 min at 50 °C. After the derivatization reaction was completed, the sample substrate fluid was vortexed for 1 min, a 0.22-μm needle filter (organic-phase) was used to remove some of the precipitated material, and the sample substrate fluid was subjected to UHPLC–FLD.
2.6 Quality parameters
The appropriate amounts of blank sample extracts were separately used to dilute the standard working solutions of piperazine to a series of concentrations (3.5/4.2, 10, 50, 100, 150, 200, and 400 μg/kg), and the derivatization reaction was then carried out.
A linear regression equation was established by measuring seven concentrations of piperazine in the blank sample, and the peak area corresponding to the piperazine concentration was determined to form a regression line equation. The four experimentally determined concentrations [limit of quantification (LOQ), 0.5 MRL, 1 MRL and 2 MRL] specified by the methodology (US Department of Health and Human Services 2001; European Commission 2002) were used to evaluate the recovery and precision, and six spiked samples were measured at the same concentration. The intra-day precision was evaluated by measuring these four concentrations again after 6 h on the same day, and the inter-day precision was evaluated by measuring these four concentrations on three days. When the signal-to-noise ratio (S/N) was ≥ 3, the corresponding piperazine concentration was the limit of detection (LOD); when the S/N was ≥ 10, the corresponding piperazine concentration was the LOQ, and the concentration met the accuracy and precision requirements [recovery ≥ 70%, relative standard deviation (RSD) ≤ 20%].
3 Results and discussion
3.1 Selection of the extraction solvent
As shown in Table 1, this study used 2% formic acid–acetonitrile to extract piperazine residues in eggs (whole egg, albumin and yolk) and achieved a good recovery rate (84.43–87.90%). Park et al. (2016) extracted piperazine from egg samples using formic acid–acetonitrile (2:98, v/v), and the results showed a recovery of piperazine of 80.9–81.4% when the concentration was 20 ng/mL and 40 ng/mL. Xie et al. (2017) reported the extraction of piperazine from chicken meat with a mixture of 5% trichloroacetic acid and acetonitrile. When the concentrations were 50 μg/kg, 100 μg/kg and 200 μg/kg, the recoveries of piperazine were between 102.93% and 111.46%. Wang et al. (2017) used acetonitrile to extract piperazine residues from chicken and pig tissues and achieved recoveries ranging from 77.46 to 96.26%. Strong acids or acidic solutions with concentrations > 5% easily corrode system piping. The present study tested methanol as an extractant; the egg extract by ASE is more turbid, which is not conducive to the purification of SPE. In addition, aqueous solutions of trichloroacetic acid and perchloric acid are not as effective as acetonitrile solutions for deproteinization. Based on our previous research (Liu et al. 2019; Bu et al. 2020), the present study compared the effects of different extraction reagents on the recovery of piperazine (Table 1), and 2% formic acid–acetonitrile was finally selected as the extractant. The obtained recovery rate was good, the extract was clear, excessive impurities were not observed, and few by-products were generated. In addition, the extraction recovery rate met the EU method validation requirements (European Commission 2002).
3.2 Selection of extraction method
This study compared the extraction recovery of piperazine in whole egg, albumin and yolk using LLE–SPE and ASE–SPE methods and revealed that the ASE–SPE methods are associated with a higher recovery and precision than the LLE–SPE methods (Table 1). Compared to the LLE–SPE method, the ASE–SPE method has the advantage of consuming smaller volumes of reagents, requiring less time and being well suited to automation (Wang et al. 2017; Liu et al. 2019; Bu et al. 2020).
3.3 Optimization of UHPLC–FLD
Piperazines are a class of relatively polar basic compounds. This study compared the separation effects of an Acquity BEH Amide UHPLC column (100 × i.d. 2.1 mm, 1.7 µm), an Acquity BEH C18 UHPLC column (100 × i.d. 2.1 mm, 1.7 µm), and an Acquity HSS T3 UHPLC column (100 × i.d. 2.1 mm, 1.8 µm). The test found that the derivative product has a reasonable retention time on the BEH C18 and BEH Amide columns, but the chromatographic peak has severe tailing, which may be caused by the sample containing impurities or column overload. The derivative product is better retained by the HSS T3 column and has no impurity peak and interference peak around the analyte peak, and no leading or trailing peaks appear. The HSS T3 column was filled with high-strength silica gel particles, which not only better retain polar substances but also do not excessively retain non-polar substances and maintain a good peak shape. The method reported by Xie et al. (2017) also uses an Acquity HSS T3 UHPLC column as a liquid chromatography (LC) column. Therefore, an Acquity HSS T3 UHPLC column was selected as the separation column for the piperazine derivatives. Furthermore, the separation effects of three different ratios of a water-acetonitrile mobile phase system (10:90, 15:85 and 20:80, v/v) were investigated. When the mobile phase ratio was 10:90, the retention time of the derivative product was short, which is not conducive to the separation of impurities; when the mobile phase ratio was 20:80, the shape of the chromatographic peak was too wide, and the longer time is not suitable for detection. At a ratio of 15:85, the retention time of the derivative product was reasonable, and there was no interference from other chromatographic peaks. Thus, a ratio of 15:85 was selected as the mobile phase for isocratic elution. Compared to Park et al. (2016) who used a gradient elution procedure (20 min), the isocratic elution procedure is simpler, and the detection time per sample is shorter (5 min).
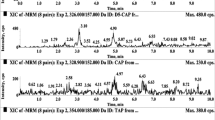
The experiment was performed with an excitation wavelength of 330 nm and an emission wavelength of 531 nm. As shown in the Figs. 1, 2, 3 and 4, under the optimized chromatographic conditions, the piperazine standard-DNS-Cl derivative product (1- DNS piperazine) was separated from the interfering impurities by UHPLC, with symmetric peak shapes and a retention time of 3.207 min. Piperazine was added to the blank whole egg at 50 μg/kg, and the retention time of the derivative products was 3.190 min.
3.4 Method validation
Blank egg samples (whole egg, albumin and yolk) were spiked with piperazine at concentrations from the LOQ to 400 μg/kg, and samples at each concentration were analysed with the optimized UHPLC–FLD method. The peak area (y) of the piperazine derivative product is linearly related to the concentration (x) of the piperazine standard working solution in different blank matrices, and the linear relationship is good. The linearity values from the analysis of piperazine in egg samples are listed in Table 2.
In addition to the samples at the LOQ level, the samples at the three concentrations of 0.5 MRL, 1 MRL and 2 MRL should be diluted 10 times with whole egg, albumin and yolk blank matrix solution before derivatization to ensure that the sample concentration is within the linear range of the standard curve. The UHPLC–FLD test was performed, and the test results were used with the standard curve to obtain concentrations. The actual measured concentration is multiplied by 10 except for the LOQ, and the ratio of these concentrations to the actual concentration is used to evaluate the recovery and precision of piperazine in whole egg, albumin and yolk samples. As shown in Table 3, the recoveries of piperazine in the blank samples were 77.07–89.90%, the RSDs were 1.73–4.90%, the intra-day RSDs were 2.44–5.36%, and the inter-day RSDs were 3.10–6.63%. The LODs for piperazine in the whole egg, albumin and yolk were 1.25, 1.32, and 1.05 μg/kg, respectively, and the LOQs were 3.50, 4.20, and 3.50 μg/kg, respectively. The study verified the reproducibility of the method by measuring six batches of samples via UHPLC–FLD. The RSD of the piperazine derivative product retention time was 0.10%, and the RSD of the peak area was 0.18%.
Matrix effects are unavoidable and can be divided into matrix enhancement and inhibition effects, mainly due to differences between the matrix components. This study investigated the matrix effects at the three concentrations of 0.5 MRL, 1.0 MRL, and 2.0 MRL in whole egg, albumin and yolk (Table 4). The matrix effect in each sample ranged from 83.83 to 92.05%, and the matrix inhibition effect in egg samples was more pronounced, probably due to the high levels of protein, fat and vitamin impurities in the eggs. In actual sample analysis, there are several ways to reduce matrix effects in a sample, including optimizing pre-treatment methods, optimizing chromatographic conditions, reducing injection volume, adding internal standards, and using matrix-matching calibration methods. The optimized pre-treatment method and matrix-matching calibration method are the simplest and most fundamental methods to reduce the matrix effect of the sample. This study optimized the ASE pre-treatment method and used the blank matrix addition method to reduce the influence of the matrix on the accuracy of the experimental results.
3.5 Method comparison
Various analytical methods, including LLE-HPLC-diode array detection (DAD) (Gadzała-Kopciuch 2005), SPE–HPLC–FLD (Park et al. 2016), LLE-HPLC-tandem mass spectrometry (MS/MS) (Lin et al. 2010), LLE–SPE–UHPLC–MS/MS (Xie et al. 2017), ASE–SPE–GC–MS/MS (Wang et al. 2017), and ASE–SPE–HPLC–FLD (Liu et al. 2019; Bu et al. 2020) have been used to determine piperazine residues in chicken, fish, eggs, pig tissues and human plasma. This work compared existing methods with the EU methodological validation requirements shown in Table 5. The proposed method has a much shorter sample preparation time and detection time (5 min) than the previously reported methods, which greatly improves the work efficiency.
The LOD and LOQ obtained by the current method are better than those of the previously reported methods, except for that by MS, which is more sensitive than DAD or FLD.
The repeatability of the proposed method is satisfactory, and the %-RSDs and extraction recovery are better than that of the SPE–HPLC–FLD, ASE–SPE–GC–MS/MS and ASE–SPE–HPLC–FLD methods. Park et al. (2016) reported an SPE–HPLC–FLD method for the qualitative analysis of piperazine residues in eggs. The linear range of quantifying the piperazine in the eggs was 20–120 μg/kg, the recoveries of piperazine were 80.9–81.4%, and the LOD and the LOQ were 6 μg/kg and 20 μg/kg, respectively. Bu et al. (2020) reported an ASE–SPE–HPLC–FLD method for the determination of piperazine residues in eggs. The recoveries of piperazine in the eggs were greater than 72.9%, and the LOD and the LOQ were 2.1 μg/kg and 6.8 μg/kg, respectively. Compared with these two methods, the ASE–SPE–UHPLC–FLD method has a lower LOD and LOQ and higher recovery and sensitivity, and the entire experimental process is much shorter.
Thus, this study optimized the sample preparation method and UHPLC–FLD method and developed an advanced method to extract and detect piperazine residues in eggs.
3.6 Actual sample analysis
To assess the feasibility of the method, this research analysed 50 egg samples from a local supermarket. Through ASE extraction, SPE purification, UHPLC–FLD detection and analysis, five egg samples containing piperazine were identified (19.45, 37.61, 43.48, 52.73 and 59.36 μg/kg; < MRL). Thus, the novel UHPLC–FLD method is feasible according to the actual sample analysis.
4 Conclusions
This research developed a method combining ASE extraction, SPE purification, and UHPLC–FLD for piperazine residue detection in eggs. The pre-treatment method of this study is fast, efficient and less time-consuming. It has advantages over the previously reported methods, as it consumes less organic reagents, has a shorter detection time, and has a higher recovery, sensitivity and precision. The proposed method was used to analyse the egg samples of local supermarkets, and the repeatability and stability of the method were verified, allowing it to be successfully applied to the analysis of piperazine residues in eggs.
References
Adams HR (2001) Veterinary pharmacology and therapeutics. Iowa State University Press, Ames
Ashraf A, Abd Rahman F, Abdullah N (2018) Poultry feed in Malaysia: an insight into the Halalan Toyyiban issues. In: Hashim NM, Shariff NNM, Mahamood SF, Harun HMF, Shahruddin MS, Bhari A (eds) Proceedings of the 3rd international halal conference (INHAC 2016). Springer, Singapore, pp 511–531
Bu X, Pang M, Wang B et al (2020) Determination of piperazine in eggs using accelerated solvent extraction (ASE) and solid phase extraction (SPE) with high-performance liquid chromatography–fluorescence detection (HPLC–FLD) and pre-column derivatization with dansyl chloride. Anal Lett 53:53–71. https://doi.org/10.1080/00032719.2019.1636386
Dong S, Yan Z, Yang H (2016) A sensitive precolumn derivatization method for determination of piperazine in vortioxetine hydrobromide using a C8 column and high-performance liquid chromatography–mass spectrometry. Anal Sci 32:1333–1338. https://doi.org/10.2116/analsci.32.1333
European Commission (2002) Commission decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, 2002/657/EC. Off J Eur Union L221:8–36
Gadzała-Kopciuch R (2005) Accurate HPLC determination of piperazine residues in the presence of other secondary and primary amines. J Liq Chromatogr Relat Technol 28:2211–2223. https://doi.org/10.1081/JLC-200064156
Glamkowski EJ, Strupczewski J, Wolf E, Woodward DL (1974) Antihypertensive activity of 1-dimethylphosphinylmethyl-4-arylpiperazines. J Med Chem 17:1008–1009. https://doi.org/10.1021/jm00255a021
Kumar CSA, Prasad SBB, Vinaya K, Chandrappa S, Thimmegowda NR, Kumar YCS, Swarup S, Rangappa KS (2009) Synthesis and in vitro antiproliferative activity of novel 1-benzhydrylpiperazine derivatives against human cancer cell lines. Eur J Med Chem 44:1223–1229. https://doi.org/10.1016/j.ejmech.2008.09.025
Lin H, Tian Y, Zhang Z, Wu L, Chen Y (2010) Quantification of piperazine phosphate in human plasma by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry employing precolumn derivatization with dansyl chloride. Anal Chim Acta 664:40–48. https://doi.org/10.1016/j.aca.2010.02.003
Liu C, Xie X, Wang B et al (2019) Optimization of ASE and SPE conditions for the HPLC–FLD detection of piperazine in chicken tissues and pork. Chirality 31:845–854. https://doi.org/10.1002/chir.23117
Ministry of Agriculture of the People ‘s Republic of China (2002) Maxium residue level of veterinary drugs in food of animal origin. Notice no. 235 (appendix 4). Ministry of Agriculture, Beijing
Montesano C, Sergi M, Moro M, Napoletano S, Romolo FS, del Carlo M, Compagnone D, Curini R (2013) Screening of methylenedioxyamphetamine- and piperazine-derived designer drugs in urine by LC–MS/MS using neutral loss and precursor ion scan. J Mass Spectrom 48:49–59. https://doi.org/10.1002/jms.3115
Navaneeswari R, Reddy PR (2012) Analytical method for piperazine in an active pharmaceutical ingredient using chemical derivatization and HPLC-UV. J Chem Pharm Res 4:2854–2859
Nikalje APG, Tiwari SV, Tupe JG, Vyas VK, Qureshi G (2017) Ultrasound assisted-synthesis and biological evaluation of piperazinylprop-1-en-2-yloxy-2H-chromen-2-ones as cytotoxic agents. Lett Drug Des Discov 14:1195–1205. https://doi.org/10.2174/1570180814666170322154750
Onuaguluchi G, Ghasi S (2006) Electrocardiographic profile of oral piperazine citrate in healthy volunteers. Am J Ther 13:43–47. https://doi.org/10.1097/01.mjt.0000151860.52074.5f
Park JA, Zhang D, Kim DS, Kim SK, Cho SH, Jeong D, Kim JS, Shim JH, El-Aty AMA, Shin HC (2016) Development of a high-performance liquid chromatography with fluorescence detection method for quantification of piperazine in animal products by using precolumn derivatization. Food Chem 196:1331–1337. https://doi.org/10.1016/j.foodchem.2015.10.081
Peters FT, Schaefer S, Staack RF, Kraemer T, Maurer HH (2003) Screening for and validated quantification of amphetamines and of amphetamine- and piperazine-derived designer drugs in human blood plasma by gas chromatography/mass spectrometry. J Mass Spectrom 38:659–676. https://doi.org/10.1002/jms.483
Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J (2015) Miniaturized solid-phase extraction techniques. TrAC Trends Anal Chem 73:19–38. https://doi.org/10.1016/j.trac.2015.04.026
Sarafraz-Yazdi A, Amiri A (2010) Liquid-phase microextraction. TrAC Trends Anal Chem 29:1–14. https://doi.org/10.1016/j.trac.2009.10.003
Silva DP, Florentino IF, Oliveira LP, Lino RC, Galdino PM, Menegatti R, Costa EA (2015) Anti-nociceptive and anti-inflammatory activities of 4-[(1-phenyl-1H-pyrazol-4-yl) methyl] 1-piperazine carboxylic acid ethyl ester: a new piperazine derivative. Pharmacol Biochem Behav 137:86–92. https://doi.org/10.1016/j.pbb.2015.08.008
Skarping G, Bellander T, Mathiasson L (1986) Determination of piperazine in working atmosphere and in human urine using derivatization and capillary gas chromatography with nitrogen- and mass-selective detection. J Chromatogr 370:245–258. https://doi.org/10.1016/S0021-9673(00)94696-X
Solomon VR, Hu C, Lee H (2010) Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approach. Bioorg Med Chem 18:1563–1572. https://doi.org/10.1016/j.bmc.2010.01.001
Staack RF, Maurer HH (2003) Toxicological detection of the new designer drug 1-(4-methoxyphenyl)piperazine and its metabolites in urine and differentiation from an intake of structurally related medicaments using gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 798:333–342. https://doi.org/10.1016/j.jchromb.2003.10.004
The European Medicines Agency (2010) Commission regulation (EU) no. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. The European Medicines Agency, Europe
The Japan Food Chemical Research Foundation (2015) Maximum residue limits (MRLs) list of agricultural chemicals in foods. The Japan Food Chemical Research Foundation, Japan
US Department of Health and Human Services (2001) Guidance for industry: bioanalytical method validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Veterinary Medicine, Washington, DC
US Food and Drug Administration (2014) CFR-code of federal regulations title 21 part 556 tolerances for residue of new animal drugs in food. US Food and Drug Administration, Rockville
Wang B, Pang M, Xie X et al (2017) Quantification of piperazine in chicken and pig tissues by gas chromatography–electron ionization tandem mass spectrometry employing pre-column derivatization with acetic anhydride. J Chromatogr A 1519:9–18. https://doi.org/10.1016/j.chroma.2017.08.079
Wang B, Zhao X, Xie X, Xie K, Zhang G, Zhang T, Liu X (2019) Development of an accelerated solvent extraction approach for quantitative analysis of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry eggs. Food Anal Methods 12:1705–1714. https://doi.org/10.1007/s12161-019-01517-4
Xie K, Liu Y, Sun L, Pang M, Xie X, Gao Q, Wang B, Zhang Y, Wang R, Zhang G, Dai G, Wang J (2017) Quantification of piperazine in chicken muscle by ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Food Anal Methods 10:1736–1744. https://doi.org/10.1007/s12161-016-0717-x
Funding
This work was financially supported by the China Agriculture Research System (CARS-41-G23), the Priority Academic Programme Development of Jiangsu Higher Education Institutions (PAPD), the Yangzhou University High-end Talent Support Programme, and the Yangzhou University International Academic Exchange Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not present any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Zhang, Y., Xie, K. et al. Automated accelerated solvent extraction coupled with ultra-high-performance liquid chromatography for the analysis of piperazine in egg samples. J Consum Prot Food Saf 15, 363–371 (2020). https://doi.org/10.1007/s00003-020-01291-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-020-01291-0