Abstract
This manuscript describes a simple, reliable, and sensitive approach that utilizes high-performance liquid chromatography-electrospray ionization tandem triple quadrupole mass spectrometry (HPLC-ESI/MS/MS) for the selective determination of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry eggs (hen eggs, duck eggs, and goose eggs). Samples were extracted with methanol-ammonium hydroxide-ultrapure water (97:2:1, v/v) via accelerated solvent extraction (ASE) at 80 °C and 1500 psi and defatted with hexane saturated with acetonitrile. The experimental method uses ESI (+) and ESI (−) combination mode, full-scan qualitative analysis, and multiple reaction monitoring (MRM) combined with an internal standard for quantitative analysis. This optimized method was validated according to the requirements defined by the European Union and the Food and Drug Administration. The poultry eggs were spiked with the target compounds at four levels, and the average recoveries were all higher than 88.3%, and the relative standard deviations did not exceed 3.9%. The limits of detection and limits of quantification were 0.04–0.5 μg/kg and 0.1–1.5 μg/kg, respectively. The decision limits were 0.37–102 μg/kg, and the detection capabilities were 0.44–103 μg/kg. Finally, the new approach was successfully applied to the quantitative determination of these analytes in 120 commercial poultry eggs from local supermarkets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chloramphenicol (CAP), thiamphenicol (TAP), and florfenicol (FF) belong to the same class of chloramphenicol drugs (CAPs), which have similar antibacterial mechanisms and have effects on both Gram-positive and Gram-negative bacteria. Their effects on Gram-negative bacteria are stronger than those on Gram-positive bacteria, and these drugs are widely used in livestock production (Holt et al. 1998; Festing et al. 2001; Du et al. 2004; Graziani et al. 2008). However, chloramphenicol can cause aplastic anemia in humans and animals; thiamphenicol is less toxic to the blood system but inhibits the production of red blood cells, white blood cells and platelets; florfenicol exhibits embryo toxicity, and its main metabolite in animals is florfenicol amine, which is a residual marker for florfenicol (Hanekamp and Bast 2015). Thus, considering the dangers of these drugs and the health and safety of consumers, China, the USA, and the European Union have list CAP as a banned drug and set a minimum required performance limit (MRPL) for CAP of 0.3 μg/kg and have set maximum residue limits (MRL values) for TAP of 50 μg/kg and for FF of 100–3000 μg/kg in animal-derived foods (Ministry of Agriculture of the People’s Republic of China 2002; The European Medicines Agency 2010; US Food and Drug Administration 2014). These drugs remain in animal-derived foods, and long-term consumption of foods with these drugs that exceed the maximum residue limit can cause the above conditions in consumers. The improper use of TAP and FF is often reported in animal-derived foods. Therefore, it is necessary for us to test and analyze animal-derived foods on the market to ensure the health and safety of consumers. In addition, governmental agencies should increase efforts to stop illegal business practices to prevent excessive use of CAP antibiotics in animal-derived foods.
To date, many methods, including liquid chromatography tandem triple quadrupole mass spectrometry (LC-MS/MS) (Zhang et al. 2008; Luo et al. 2010; Sichilongo et al. 2014; Tao et al. 2014; Fedeniuk et al. 2015; Barreto et al. 2016; Liu et al. 2016; Xie et al. 2018), ultra-performance liquid chromatography tandem triple quadrupole mass spectrometry (UPLC-MS/MS) (Alechaga et al. 2012; Pan et al. 2015; Xiao et al. 2015; Faulkner et al. 2016; Orlando et al. 2016), high-performance liquid chromatography with fluorescence detection (HPLC-FLD) (Xie et al. 2011), gas chromatography-negative chemical ionization mass spectrometry (GC-NCI/MS) (Shen et al. 2009) and indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) (An et al. 2016), for detecting chloramphenicol residues in animal-derived foods have been reported. Barreto et al. (2016) established a liquid-liquid extraction (LLE) approach for extracting CAP, TAP, FF, and FFA from poultry, swine, bovine, and fish tissues using LC-MS/MS. The validated LC-MS/MS method was successfully applied for the determination of CAP, TAP, FF, and FFA in animal-derived foods. Xiao et al. (2015) reported a subcritical water extraction method for trace analysis of CAP, TAP, FF, and FFA in poultry tissues using UPLC-MS/MS, and the method was fast, sensitive, and water-based and thus more environmentally acceptable, making it a suitable replacement for conventional organic solvent extractions of veterinary drug residue analysis. Xie et al. (2011) developed an HPLC-FLD method for detecting TAP, FF, and FFA in eggs; however, this analytical method can only analyze drugs with fluorophores. Thus, CAP cannot be detected by HPLC-FLD because it does not contain a fluorophore. Compared to FLD detection, MS/MS can effectively eliminate matrix interference, reduce the detection limit, simplify the required sample purification steps and improve the sample recovery. In addition, MS/MS methods are a good solution to this problem and are well suited to confirming the presence of drugs.
In two previous studies, we developed LC-MS/MS (Xie et al. 2018) and HPLC-FLD (Xie et al. 2011) methods for the selective determination of TAP, FF, and FFA and CAPs and FFA in eggs. The sample preparation techniques adopted in these two methods involved liquid-liquid extraction with ethyl acetate-acetonitrile-ammonium hydroxide (49:49:2, v/v) as the extractant, as this solvent could effectively extract the target compounds in eggs. Using liquid-liquid extraction, we improved the pretreatment method used for these samples. In this study, we used accelerated solvent extraction (ASE) to pretreat the poultry egg samples, and methanol:ammonium hydroxide:water (97:2:1, v/v) was used to extract the chloramphenicol residues from the poultry eggs, and the fat was removed with n-hexane. The ASE method can be successfully applied to the extraction of multiple chloramphenicol residues in poultry eggs. The developed ASE-HPLC-MS/MS method was validated by measuring a series of quality parameters according to the guidelines established by the European Union (EU) (The European Communities 2002) and the Food and Drug Administration (FDA) (US Department of Health and Human Services et al. 2001).
Materials and Methods
Chemicals and Reagents
CAP (99.3% standard), TAP (99.0% standard), FF (99.0% standard), and deuterated chloramphenicol-d5 (d5-CAP, 99.0% standard, 100 μg/ml; used as an internal standard) were purchased from Labor Dr. Ehrenstorfer Gmbh (Augsburg, Germany). FFA (99.3% standard) was obtained from WITEGA Laboratorien Berlin-Adlershof Gmbh (Augsburg, Germany). Acetonitrile and methanol (HPLC grade) were purchased from Tedia Company Inc. (Fairfield, OH, USA). Analytical-grade ammonium acetate, ammonium hydroxide, hexane, and ethyl acetate were acquired from Sinopharm Chemical Reagent Co. (Shanghai, China). Water was obtained from a PURELAB Option-Q synthesis system (ELGA Lab Waters, High Wycombe, Bucks, UK).
Preparation of the Standard Stock and Working Solutions
Standard stock solutions of CAP, TAP, and FF at 200 μg/ml were prepared in pure acetonitrile. A standard stock solution of FFA at 200 μg/ml was prepared in 1 ml of ultrapure water and 9 ml of pure acetonitrile. A 250-ng/ml d5-CAP internal standard solution was prepared by dissolving the contents of the ampoule, which was at a concentration of 100 μg/ml, in acetonitrile. Standard working solutions at different concentrations were prepared by diluting the stock solutions of CAP, TAP, FF, and FFA with acetonitrile-water (40:60, v/v) solution and then used to determine the calibration curves, recoveries, and precisions. The standard stock solutions were stable for 5 months at − 70 °C, and the standard working solutions were stable for 1 month at − 34 °C. A 250-ng/ml d5-CAP internal standard solution was prepared from the 100-μg/ml standard stock solution before use.
HPLC-MS/MS Analysis
The liquid chromatographic separation and mass spectrometric detection were performed on a Waters Alliance e2695 separation module (Waters Corp., Milford, MA, USA) coupled to a triple quadrupole mass spectrometer (AB SCIEX Triple Quad™ 5500, AB SCIEX Corp., Framingham, MA, USA), and Analyst version 1.6.1 software (AB SCIEX Pte. Ltd., Concord, Ontario, Canada) was used for the analysis. The chromatographic separation was performed at 25 °C on a Waters XBridge™ C18 column (250 mm × 4.6 mm; i.d. 5 μm). The mobile phase used in the gradient elution was composed of water (A) and acetonitrile (B) both containing 2 mM ammonium acetate. The flow rate was 0.8 ml/min, and the injection volume was 10 μl. The gradient started with 40% of B, which was increased to 90% in 3 min, kept for 5 min and decreased to 40% in 2 min, and then the HPLC system was re-equilibrated.
The tandem mass spectrometry system was equipped with an electrospray ionization (ESI) source and used in multiple reaction monitoring (MRM) modes. CAP, TAP, FF, and d5-CAP were analyzed in negative ionization mode (ESI−), whereas FFA was analyzed in positive ionization mode (ESI+). The typical mass spectrometry parameters are as follows: electrospray ionization voltage, 5500 V (ESI+) and 4500 V (ESI−); ion source temperature, 500 °C; the pressures of the spray gas, auxiliary gas (nitrogen), curtain gas, and collision gas were 45, 50, 15, and 8 psi (ESI+) and 50, 35, 20, and 8 psi (ESI−), respectively. The data were acquired in MRM mode, and the corresponding cone voltage and collision energy are presented in Table 1.
Preparation of the Samples
Homogenized poultry eggs (5.0 g; hen eggs, duck eggs, and goose eggs) were precisely weighed and placed in a mortar. Then, 4.0 g of diatomaceous earth was added to the mortar. To achieve the best extraction efficiency, the samples were ground into the smallest particles possible and transferred to a 22-ml stainless steel extraction cell.
The stainless steel extraction cell was placed in an ASE350 instrument (Thermo Fisher Scientific Co. Ltd., MA, USA) at a set temperature of 80 °C and a pressure of 1500 psi, and the static extraction process was conducted for 5 min. Methanol:ammonium hydroxide:ultrapure water (97:2:1, v/v) was used as the extractant, and the extraction solvent and sample account for 40% of the extraction cell volume. Each sample was automatically rinsed once, then purged with nitrogen for 60 s followed by a static extraction. The extract was collected in a 60-ml collection bottle, transferred to a 50-ml centrifuge tube, and concentrated to 1–2 ml in a 50 °C centrifugal concentrator (2000×g) (Scan Speed Vac40, Labogene Corp., Denmark).
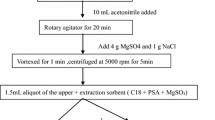
After evaporation and concentration, the residual solution was reconstituted by adding 1 ml of pure acetonitrile and then vortexed for 1 min. Then, 10 ml of hexane saturated with pure acetonitrile was added to the centrifuge tube, and the mixture was vortexed for 1 min. After standing for 5 min, the n-hexane layer was removed, and the fat-removal process was repeated. The extract was concentrated to dryness under a nitrogen stream at 50 °C and was maintained at − 4 °C until analysis. Before analysis, the residue was reconstituted in 5 ml of acetonitrile-water (40:60, v/v), centrifuged at 12100×g for 15 min and passed through a 0.22-μm organic phase needle filter into an HPLC-MS/MS system.
Quality Parameters
The linearity of this method was evaluated by establishing calibration curves, which were constructed using the analyte/internal standard peak area ratio versus the analyte concentration. Matrix-matched calibration curves were prepared at eight spiking levels over the ranges of 0.1–25 μg/kg for CAP, 1.0–150 μg/kg for TAP, 0.3–250 μg/kg for FF and 1.5–250 μg/kg for FFA. The correlation coefficients (R2 values) were determined, and these values should all be ≥ 0.999 2.
The developed method was validated by measuring the linearity, recovery, precision, limit of detection (LOD), limit of quantitation (LOQ), decision limit (CCα), and detection capability (CCβ) values as established by the EU (The European Communities 2002) and the FDA (US Department of Health and Human Services et al. 2001).
Results and Discussion
Sample Clean-up
Several methods, such as liquid-liquid extraction (LLE) (Luo et al. 2010; Xie et al. 2011; Sichilongo et al. 2014; Barreto et al. 2016; Xie et al. 2018), LLE-solid-phase extraction (SPE) (Zhang et al. 2008; Shen et al. 2009; Fedeniuk et al. 2015; Xiao et al. 2015; Orlando et al. 2016), matrix solid-phase dispersion extraction (MSPD) (Tao et al. 2014; Pan et al. 2015), and QuEChERS (quick, easy, cheap, effective, rugged, and safe) extraction (Liu et al. 2016), have been developed for the extraction of CAP, TAP, FF, and FFA from animal-derived foods. An ASE method for extracting CAP, TAP, FF, and FFA from poultry eggs (hen eggs, duck eggs, and goose eggs) has not been reported. In this study, an ASE method was optimized to further reduce the matrix effects and enhance extraction recovery for the quantification of amphenicol antibiotics (CAP, TAP, FF, and FFA) in poultry eggs (hen eggs, duck eggs, and goose eggs).
In this study, we compared the effects of different extractants on the recovery of the target compounds from poultry eggs. FFA is a weakly basic drug that is extracted under alkaline conditions and typically has a high recovery rate (Barreto et al. 2016; Xie et al. 2018). Thus, ethyl acetate-acetonitrile-ammonium hydroxide (49:49:2, v/v), ethyl acetate-ammonium hydroxide (98:2, v/v), and ethyl acetate-ammonium hydroxide (97:3, v/v) were used to optimize the extraction efficiency at 40–120 °C and 1500 psi. The test results showed that the recovery rate in the ASE method using alkaline ethyl acetate is generally low, and it does not meet the requirements for recovery verification. Based on previous studies, the recoveries of the target compounds from poultry eggs at 40–120 °C at 1500 psi using alkaline methanol solutions were tested. The alkaline methanol solutions included methanol:ammonium hydroxide:ultrapure water (97:2:1, v/v), methanol:ammonium hydroxide (97:3, v/v), methanol:ammonium hydroxide (98:2, v/v) and methanol:ammonium hydroxide:ultrapure water (97:1:2, v/v), because the composition of poultry eggs, especially the yolk, is more complex than the compositions of other matrices. Meanwhile, Xie et al. (2018) reported that the LLE method was used to extract CAP, TAP, FF, and FFA from eggs, and the delipidation used two times of hexane saturated with pure acetonitrile (10 mL each time), which can remove fat very well. Based on this research, this experiment used two manual degreasing cycles. The test first selects an extractant, extracts once or twice at each temperature (40 °C, 60 °C, 80 °C, 100 °C, and 120 °C) and 1500 psi, static extraction for 5 min at a time, and manually degreases twice, and then combine different extraction steps and number them. After extraction, the hen egg samples from different extraction steps were separately analyzed by HPLC-MS/MS. The other two extractants repeat the above steps. After optimizing the temperature (40 °C, 60 °C, 80 °C, 100 °C, and 120 °C), and the number of extractions, the recoveries of target compounds from hen eggs obtained using different proportions of alkaline methanol solutions were compared. The test showed that at 80 °C, 1500 psi, methanol:ammonium hydroxide:ultrapure water (97:2:1, v/v) as the extractant, static extraction for 5 min, one static cycle, two manual degreasing cycles the greatest extraction efficiency was achieved.
Optimization of HPLC-MS/MS Analysis
The chromatographic conditions were primarily optimized in terms of different mobile phase compositions, gradient elution procedures, and column.
The reported studies on CAP, TAP, FF, and FFA determination mostly use C18 columns and involve animal-derived foods (Zhang et al. 2008; Barreto et al. 2016; Xie et al. 2018). Therefore, this experiment used a Waters XBridgeTM C18 column (250 mm × 4.6 mm; i.d. 5 μm) to determine CAP, TAP, FF, and FFA in poultry eggs. However, when acetonitrile-water was used as the mobile phase, an FFA peak was not observed. To solve this problem, we added 2 mM ammonium acetate to the mobile phase to improve the elution of FFA without affecting the responses of CAP, TAP, and FF. The gradient elution procedure was optimized as follows: the gradient started with 40% B, increased to 90% in 3 min, kept for 5 min, and decreased to 40% in 2 min, and then the HPLC system was re-equilibrated. The equilibration time between each run was proven to be critical: 5 min of equilibrium using the initial conditions of gradient was necessary to obtain reproducible retention times.
Full scans of 50 ng/ml standard solutions were conducted in ESI (−) mode and ESI (+) mode, the precursor ions for CAP, TAP, FF, and d5-CAP were the deprotonated molecular ions [M − H] − in ESI (−) mode, and the precursor ion for FFA was the protonated molecular ion [M + H] + in ESI (+) mode. These modes were used because ESI (−) mode has high sensitivity for CAP, TAP, FF, and d5-CAP, and ESI (+) mode has high sensitivity for FFA (Zhang et al. 2008; Barreto et al. 2016; Xie et al. 2018). In the MS/MS mode, two product ions were chosen for the confirmation of each analyte, and the most abundant of these was used for quantitative purposes in compliance with the 2002/657/EC Decision (The European Communities 2002). The chromatograms from total ion chromatography (TIC) and extracted ion chromatography (XIC) of CAP, TAP, FF, FFA, and d5-CAP are shown in Fig. 1 for blank hen egg sample. The TIC and XIC chromatograms of the quantitative ions of blank hen egg spiked with 0.3 μg/kg for CAP, 5.0 μg/kg for TAP, 10.0 μg/kg for FF and FFA, and 5.0 μg/kg for d5-CAP are shown in Fig. 2. Compared with the mass spectrum of the blank hen egg sample, the peak shape of each target compound in the spiked sample is a sharp and symmetric with no tailing. In addition, the blank samples did not contain any of the target compounds. The cone voltage and collision energy were optimized based on the monitored ion pairs of CAP, TAP, FF, FFA, and d5-CAP, and the MRM method was used to determine the target compounds.
Bioanalytical Method Validation
The method was validated by determining its linearity, recovery, precision (intraday precision and interday precision), LOD, LOQ, CCα, and CCβ according to European Union Regulation 2002/657/EC (The European Communities 2002) as well as some important parameters from the FDA guidelines (US Department of Health and Human Services et al. 2001), and the specificity of the method was tested by analyzing blank poultry egg samples.
The blank hen egg, duck egg, and goose egg samples were extracted and purified by the above sample preparation method to obtain a blank matrix extract. The standard working solution was serially diluted to a range of concentrations using the appropriate amounts of different blank matrix extracts. The linearity of the method was determined by analyzing the standard plots associated with an 8-point standard calibration curve. The linear ranges of CAP, TAP, FF, and FFA are 0.1–25 μg/kg, 1.0–150 μg/kg, 0.3–250 μg/kg, and 1.5–250 μg/kg, respectively, and the internal standard (d5-CAP) concentration was 5.0 μg/kg. The concentration curve of each analyte in the blank matrix sample is plotted on the abscissa (X), and the ratio of the peak area of the quantified ion of each analyte to the quantitative ion of the isotopically labeled internal standard is plotted on the ordinate (Y). The calibration curves were fitted to equation Y = aX + b by least-squares linear regression, and the resulting correlation coefficients (R2 values) were all greater than 0.999 2, as listed in Table 2. According to the EU guidelines (The European Communities 2002), the recovery and precision of this method were verified at four different concentrations (LOQ, 0.5, 1.0, and 2 MRL/MRPL) for each drug in the poultry egg samples. As shown in Tables 3, 4, and 5, the recoveries of CAP, TAP, FF, and FFA in the blank hen egg, duck egg, and goose egg samples were 88.3–107%, the relative standard deviations (RSDs) were 1.5–3.9%, the intraday RSDs were 1.6–4.7%, and the interday RSDs were 2.1–5.9%.
The LOQ is defined as the lowest concentration at which the method yields recovery and precision values that fall within the ranges recommended by the EU (The European Communities 2002). The LOQ was estimated from the peak height-to-averaged background noise ratio, which was based on the peak-to-peak baseline near the analyte peaks identified in the MRM chromatograms. The LOQ was then calculated based on a signal-to-noise (S/N) ratio of 10 according to the FDA guidelines (US Department of Health and Human Services et al. 2001). The LOD values are defined as the lowest concentrations of CAP, TAP, FF, and FFA at which it is possible to detect the presence of the analytes with reasonable statistical certainty using a detector with a signal-to-noise (S/N) ratio of at least 3. The LOQ values of CAP, TAP, FF and FFA in poultry eggs are 0.1, 1.0, 0.3, and 1.5 μg/kg, respectively, and the LOD values of CAP, TAP, FF, and FFA are 0.04, 0.4, 0.1, and 0.5 μg/kg, respectively. A total of 60 blank hen eggs, duck eggs, and goose eggs (20 of each type) were determined, and CAP, TAP, FF, and FFA were added to the samples at 1 MRPL/MRL, and the samples were re-evaluated. The results of CCα and CCβ are shown in Table 2. The CCα and CCβ values were close to 1 MRPL or MRL, which meet the requirements of the EU, the FDA and the Ministry of Agriculture of the People’s Republic of China and fully met the requirements for veterinary drug residue analysis.
Comparison of Different Detection Methods
Various analytical methods, including HPLC-FLD (Xie et al. 2011), LC-MS/MS (Tao et al. 2014; Barreto et al. 2016; Liu et al. 2016; Xie et al. 2018), and UPLC-MS/MS (Pan et al. 2015; Xiao et al. 2015), have been used to measure CAP, TAP, FF, and FFA in shrimp, fish, milk, honey, poultry tissues and eggs. In previous studies, Xie et al. (2011) developed an HPLC-FLD method for the simultaneous determination of TAP, FF, and FFA in eggs. LC-MS/MS allowed the simultaneous confirmation and quantification of CAP, TAP, FF, and FFA in eggs (Xie et al. 2018). Compared with HPLC-FLD methods, the LC-MS/MS method has higher selectivity and sensitivity, and it can efficiently separate target compounds.
Barreto et al. (2016) established an LC-MS/MS method that showed better recovery and precision in the detection of CAP, TAP, FF, and FFA in poultry, swine, and bovine and fish muscle. Xie et al. (2018) reported a widely applicable method for the simultaneous determination of CAP, TAP, FF, and FFA in eggs. The method uses LLE to extract the target compounds, and ethyl acetate-acetonitrile-ammonium hydroxide (49:49:2, v/v) is used as the extractant, and acetonitrile-saturated n-hexane is used to remove the fat. The LODs and LOQs of this method were 0.04–0.5 μg/kg and 0.1–1.5 μg/kg in eggs, respectively. The average recoveries of CAP, TAP, FF, and FFA from egg samples were 90.8–108%, and RSDs were less than 9.6%. The corresponding intraday and interday RSD values were found to be less than 8.1% and 11%, respectively. Xiao et al. (2015) established a UPLC-MS/MS method to detect CAP, TAP, FF, and FFA in poultry tissues, and the results were compared with those obtained with conventional shaking extraction, ultrasonic extraction, and pressurized liquid extraction. Decreasing the polarity of the water by successively increasing the extraction temperature from 50 to 200 °C at moderate pressure enabled selective, highly effective extractions. The average recoveries of the four analytes from fortified samples ranged between 86.8 and 102% and showed RSDs lower than 7.7%. The LODs and LOQs for the target compounds were 0.03–0.5 μg/kg and 0.1–2.0 μg/kg, respectively. We compared the sensitivity and recovery of target compounds obtained using different detection and extraction methods. As shown in Table 6, because it uses mass spectrometry, the LLE method is simpler, more efficient and has higher recovery than the LLE-SPE, MSPD, and QuEChERS methods.
In previous studies, we used the LLE method to pretreat the samples and detected the target compound in egg by HPLC-FLD and LC-MS/MS. Based on previous research, we optimized the gradient elution procedure and sample extraction method. In this study, an ASE method was used to extract poultry egg samples, methanol-ammonium hydroxide-ultrapure water (97:2:1, v/v) as the extractant. Compared with the LLE method, the ASE method has the advantages of high extraction efficiency, reduced consumption of reagents and time, and reduced error associated with manual extraction, making it suitable for batch processing of samples. Thus, this study established an ASE-HPLC-MS/MS method to detect CAP, TAP, FF, and FFA in poultry eggs and provided new techniques and scientific basis for the detection of chloramphenicol residues in poultry eggs.
Real Sample Analysis
After validating the method in fortified samples, we then applied the method to real samples. For the analysis of real sample, we purchased 40 hen eggs, 40 duck eggs, and 40 goose eggs from a local supermarket. Each poultry egg was subjected to extraction and purification by the above sample preparation method and then analyzed by HPLC-MS/MS. The experiment showed that the target compounds could not be detected in duck eggs and goose eggs, and 30–55 μg/kg FF and 53–70 μg/kg FFA were detected in three hen eggs; however, none of the samples exceeded the MRL of 100 μg/kg (FDA standard). Therefore, the optimized ASE-HPLC-MS/MS method can be applied to quantify CAP, TAP, FF, and FFA in poultry egg samples.
Conclusions
The experimental results in this work represent one of the very few case studies of ASE extraction of CAP, TAP, FF, and FFA residues from poultry eggs. The ASE method is suitable for the batch processing of samples, saving time, and reducing reagent consumption, thereby avoiding lengthy and labor intensive sample preparation steps prior to HPLC-MS/MS analysis. As compared with the conventional extraction methods, the proposed ASE presented here is quick (rapid extraction and shorter sample preparation) and sensitive (lower LODs and LOQs), higher recovery (exceed 88.3%) and precision (RSD ≤ 3.9%). In addition, the optimized ASE-HPLC-MS/MS method validation parameters met the requirements of the EU, the FDA and the Ministry of Agriculture of the People’s Republic of China, and this novel method was successfully used to analyze 120 poultry egg samples, demonstrating the applicability and reliability of this method.
References
Alechaga E, Moyano E, Galceran MT (2012) Ultra-high performance liquid chromatography-tandem mass spectrometry for the analysis of phenicol drugs and florfenicol-amine in foods. Analyst 137:2486–2494. https://doi.org/10.1039/c2an16052h
An L, Wang Y, Pan Y, Tao Y, Chen D, Liu Z, Yang W, Peng D, Yuan Z (2016) Development and validation of a sensitive indirect competitive enzyme-linked immunosorbent assay for the screening of florfenicol and thiamphenicol in edible animal tissue and feed. Food Anal Methods 9:1–10. https://doi.org/10.1007/s12161-016-0436-3
Barreto F, Ribeiro C, Hoff RB, Costa TD (2016) Determination of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in poultry, swine, bovine and fish by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1449:48–53. https://doi.org/10.1016/j.chroma.2016.04.024
Du X, Xia C, Shen J, Wu B, Shen Z (2004) Characterization of florfenicol resistance among calf pathogenic Escherichia coli. FEMS Microbiol Lett 236:183–189. https://doi.org/10.1016/j.femsle.2004.05.013
Faulkner D, Cantley M, Walker M, Crooks S, Kennedy D, Elliott C (2016) Evidence of non-extractable florfenicol residues: development and validation of a confirmatory method for total florfenicol content in kidney by UPLC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33:983–994. https://doi.org/10.1080/19440049.2016.1175187
Fedeniuk RW, Mizuno M, Neiser C, O’Byrne C (2015) Development of LC-MS/MS methodology for the detection/determination and confirmation of chloramphenicol, chloramphenicol 3-O-beta-d-glucuronide, florfenicol, florfenicol amine and thiamphenicol residues in bovine, equine and porcine liver. J Chromatogr B 991:68–78. https://doi.org/10.1016/j.jchromb.2015.04.009
Festing MF, Diamanti P, Turton JA (2001) Strain differences in haematological response to chloramphenicol succinate in mice: implications for toxicological research. Food Chem Toxicol 39:375–383. https://doi.org/10.1016/S0278-6915(00)00149-6
Graziani C, Busani L, Dionisi AM, Lucarelli C, Owczarek S, Ricci A, Mancin M, Caprioli A, Luzzi I (2008) Antimicrobial resistance in Salmonella enterica serovar Typhimurium from human and animal sources in Italy. Vet Microbiol 128:414–418. https://doi.org/10.1016/j.vetmic.2007.10.017
Hanekamp JC, Bast A (2015) Antibiotics exposure and health risks: chloramphenicol. Environ Toxicol Pharmacol 39:213–220. https://doi.org/10.1016/j.etap.2014.11.016
Holt DE, Andrews CM, Payne JP, Williams TC, Turton JA (1998) The myelotoxicity of chloramphenicol: in vitro and in vivo studies: II: in vivo myelotoxicity in the B6C3F1 mouse. Hum Exp Toxicol 17:8–17. https://doi.org/10.1177/096032719801700102
Liu HY, Lin SL, Fuh MR (2016) Determination of chloramphenicol, thiamphenicol and florfenicol in milk and honey using modified QuEChERS extraction coupled with polymeric monolith-based capillary liquid chromatography tandem mass spectrometry. Talanta 150:233–239. https://doi.org/10.1016/j.talanta.2015.12.045
Luo P, Chen X, Liang C, Kuang H, Lu L, Jiang Z, Wang Z, Li C, Zhang S, Shen J (2010) Simultaneous determination of thiamphenicol, florfenicol and florfenicol amine in swine muscle by liquid chromatography-tandem mass spectrometry with immunoaffinity chromatography clean-up. J Chromatogr B 878:207–212. https://doi.org/10.1016/j.jchromb.2009.10.002
Ministry of Agriculture of the People’s Republic of China (2002) Maxium residue level of veterinary drugs in food of animal origin. Notice no. 235 (appendix 4). Ministry of Agriculture, Beijing
Orlando EA, Roque AGC, Losekann ME, Simionato AVC (2016) UPLC-MS/MS determination of florfenicol and florfenicol amine antimicrobial residues in tilapia muscle. J Chromatogr B 1035:8–15. https://doi.org/10.1016/j.jchromb.2016.09.013
Pan X-D, Wu P-G, Jiang W, Ma B-J (2015) Determination of chloramphenicol, thiamphenicol, and florfenicol in fish muscle by matrix solid-phase dispersion extraction (MSPD) and ultra-high pressure liquid chromatography tandem mass spectrometry. Food Control 52:34–38. https://doi.org/10.1016/j.foodcont.2014.12.019
Shen J, Xia X, Jiang H, Li C, Li J, Li X, Ding S (2009) Determination of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry and porcine muscle and liver by gas chromatography-negative chemical ionization mass spectrometry. J Chromatogr B 877:1523–1529. https://doi.org/10.1016/j.jchromb.2009.03.040
Sichilongo KF, Kolanyane P, Masesane IB (2014) A sensitive LC-MS/MS method employing a THF–water solvent system for the determination of chloramphenicol, thiamphenicol and florfenicol in bovine muscle. Anal Methods 6:7015–7021. https://doi.org/10.1039/C4AY01135J
Tao Y, Zhu F, Chen D, Wei H, Pan Y, Wang X, Liu Z, Huang L, Wang Y, Yuan Z (2014) Evaluation of matrix solid-phase dispersion (MSPD) extraction for multi-fenicols determination in shrimp and fish by liquid chromatography-electrospray ionisation tandem mass spectrometry. Food Chem 150:500–506. https://doi.org/10.1016/j.foodchem.2013.11.013
The European Communities (2002) Commission decision 2002/657/EC of 12 august 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun 221:8–36
The European Medicines Agency (2010) Commission regulation (EU) No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. The European Medicines Agency, Canary Wharf
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine (2001) Guidance for industry: bioanalytical method validation. US Department of Health and Human Services, Washington, DC
US Food and Drug Administration (2014) CFR-code of federal regulations title 21 part 556 tolerances for residues of new animal drugs in food. US Food and Drug Administration. Silver Spring, Maryland
Xiao Z, Song R, Rao Z, Wei S, Jia Z, Suo D, Fan X (2015) Development of a subcritical water extraction approach for trace analysis of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry tissues. J Chromatogr A 1418:29–35. https://doi.org/10.1016/j.chroma.2015.09.047
Xie K, Jia L, Yao Y, Xu D, Chen S, Xie X, Pei Y, Bao W, Dai G, Wang J, Liu Z (2011) Simultaneous determination of thiamphenicol, florfenicol and florfenicol amine in eggs by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B 879:2351–2354. https://doi.org/10.1016/j.jchromb.2011.06.027
Xie X, Wang B, Pang M, Zhao X, Xie K, Zhang Y, Wang Y, Guo Y, Liu C, Bu X, Wang R, Shi H, Zhang G et al (2018) Quantitative analysis of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in eggs via liquid chromatography-electrospray ionization tandem mass spectrometry. Food Chem 269:542–548. https://doi.org/10.1016/j.foodchem.2018.07.045
Zhang S, Liu Z, Guo X, Cheng L, Wang Z, Shen J (2008) Simultaneous determination and confirmation of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in chicken muscle by liquid chromatography-tandem mass spectrometry. J Chromatogr B 875:399–404. https://doi.org/10.1016/j.jchromb.2008.09.035
Funding
This research was financially supported by the China Agriculture Research System (CARS-41-G23), the Priority Academic Programme Development of Jiangsu Higher Education Institutions (PAPD), the Project of Drafting and Revision of the National Agriculture Industry Standard for Quality and Safety of Agricultural Products (181721301092371104), the Project of Jiangsu Agricultural Science and Technology Independent Innovation Fund (CX (18) 3022), the Yangzhou University High-end Talent Support Programme and the Yangzhou University International Academic Exchange Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bo Wang declares that he has no conflict of interest. Xia Zhao declares that he has no conflict of interest. Xing Xie declares that he has no conflict of interest. Kaizhou Xie declares that he has no conflict of interest. Genxi Zhang declares that he has no conflict of interest. Tao Zhang declares that he has no conflict of interest. Xuezhong Liu declares that he has no conflict of interest.
Ethical Approval
This article does not present any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Zhao, X., Xie, X. et al. Development of an Accelerated Solvent Extraction Approach for Quantitative Analysis of Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol Amine in Poultry Eggs. Food Anal. Methods 12, 1705–1714 (2019). https://doi.org/10.1007/s12161-019-01517-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01517-4