Abstract
More contemporary synthetic approaches have been superseded by affordable and ecologically friendly nanoparticle (NP) production technologies. Green chemistry is environmentally safe, nontoxic, biocompatible, and financially productive. In the past, numerous research teams have produced silver and silver oxide nanoparticles from diverse plant extracts, and their findings have been widely reported. Green synthesis methods utilize microorganisms, plant extracts, or proteins as bio-capping and bio-reducing agents. They function as bio-nanofactories for material synthesis at the nanoscale size to create biogenic nanomaterials. Here, we have outlined the creation process, characterization, and composition of these plant-mediated silver oxide and silver nanoparticles made by plant-mediated synthesis. Green synthesis of silver and silver oxide nanoparticles depends on variety of parameters, such as extract concentration, exposure time, temperature, and pH. UV–visible spectroscopy, DLS, TEM, SEM, XRD, EDX, FTIR, etc. can successfully characterize plant-mediated silver and silver oxide nanoparticles. Future studies must concentrate on understanding the intricate mechanism of metal and metal oxide nanoparticle synthesis and designing nanoparticles that are less hazardous, healthier, and have precisely controlled dimensions and form. Additionally, silver release is more likely to occur in small-sized spherical silver nanoparticles. This review concludes that further research into the synthesis and characterization of metallic silver and silver oxide nanoparticles made via green synthesis is necessary to bring green synthesized products to the market.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanoscience is one of the encouraging research fields in novel science with marvelous applications in biology, physics, chemistry, and material science. Nanoscience and nanotechnology is a branch of science and engineering defined as the design, synthesis, characterization, and application of materials whose shape and size have been manipulated at the nanoscale. Particles smaller than 100 nm in any one dimension are usually referred to as “nanoparticles” (Husen & Iqbal, 2019; Kouvaris et al., 2012). High surface area-to-volume ratio, variable pore size, and high reactivity are some of the nanostructured materials' improved or pronounced characteristics that change particles' chemical, physical, thermal, mechanical, catalytic, and electromagnetic characteristics (Bachheti et al., 2019; Iravani et al., 2014; Painuli et al., 2020; Pal et al., 2011). Metallic nanoparticles (MNPs) play a crucial role in reforming human life and its environment. This draws immense interest from researchers owing to its large variety of potential applications in optical, biomedical, water treatment, electronics, cosmetics, agriculture fields, food technology, mechanical engineering, energy, polymer science, agriculture and environmental and health science (Bachheti et al., 2020, 2020a, 2020b, 2021, 2022; Chawla et al., 2023; Fu & Fu, 2015; Husen et al. 2019; Husen & Jawaid, 2020; Husen, 2022a, 2023a, 2023b, 2023c; Husen 2023a, 2023b; Husen & Siddiqi, 2023; Mandal et al., 2023; Taghiyari et al., 2023). Today, a significant portion of the nanomaterials employed in many industries utilize chemical and physical processes. Existing synthesis techniques are known to be costly and to involve chemicals that are harmful to the environment and human health, which include solvents made from organic materials, reduction agents, and stabilizers, which limits the practical application of these nanoparticles in therapeutic and scientific sectors (Shume et al., 2020). In order to reduce or altogether avoid the use of dangerous chemicals and prevent the development of superfluous and toxic byproducts, several research organizations are now engaged in finding affordable and environmentally safe nanoparticle synthesis procedures. The green synthesis technique offers the most advantageous replacement for the current chemical and physical ways of synthesis since it is less expensive, safer to use, and environmentally benign (Abate et al., 2023; Godeto et al., 2023; Gonfa et al., 2023; Zebeaman et al., 2023). Because botanical extracts contain biomolecules including protein, carbohydrates, and coenzyme precursors that can reduce metal ions, using them as reducing substances has also started to look interesting. Additionally, the developed nanoparticles are stabilized and capped by these biomolecules. In comparison to microbe-mediated production, plant-mediated synthesis is more scalable and quicker. It has also been shown to be effective, affordable, and easily accessible. Because of the aforementioned factors, manufacturing nanoparticles from plant sources has emerged as the most desirable and popular technique (Danish et al., 2021; Elemike et al., 2017).
Metallic NPs (silver, gold, zinc oxide, silver oxide, copper oxide, and titanium oxide) have been synthesized, studied, and utilized by researchers in numerous/frequent fields (Joshi et al., 2019; Leyu et al., 2023). Silver and silver oxide nanoparticles are an excellent choicer in nanotechnology in the field of biological systems for medical imaging and for treating diseases among different metallic nanoparticles (Gonfa et al., 2022; Jeevanandam et al., 2018). Silver oxide and silver NP have been extensively used for cellular delivery because of their versatile characteristics/properties like wide availability, deep functionality, good compatibility, potential for targeted drug delivery, and moderate release of drugs. Besides medical and pharmaceutical applications, metallic NPs widely used in everyday applications (Christian et al., 2008).
Recently, scientists' attention has switched to the use of plant extracts in the production of nanoparticles. This approach is attracting more interest than other biological systems because it offers a clear, practical, economical, secure, environmentally friendly, practical, and advantageous path to synthesizing MNPs of desired shape and size. The fact that plant extract-mediated synthesis of NPs is easier to maintain, more readily available, and safe to handle than microbial synthesis of NPs is a significant advantage. Different nanoparticles (Ag, Au, silver oxide, and TiO2) of various shapes and sizes have been produced using various plant components, including leaves, peels, roots, stems, seeds, fruits, and flowers (Jana et al., 2000). The main benefit of plant-mediated MNP synthesis over chemical synthesis is that no additional protective agent is required in the former (Shekhawat et al., 2014). Moreover, it is easy to get plant extracts to develop a successful and environmentally friendly method for manufacturing industrial quantities of uniform metallic nanoparticles. Thus, the aim of this chapter is to provide in-depth information about the plant-mediated synthesis and characterization of ZnO nanoparticles.
2 Classification of Metallic Nanoparticles
Nanoparticles can be widely classified into two categories, namely, organic nanoparticles, which comprise carbon NPs (fullerenes), and inorganic NPs, comprising magnetics NPs of noble metals (like gold, silver, platinum) and semi-conductor NPs (like titanium oxide and zinc oxide, etc.). At present, interest in inorganic metallic nanoparticles (silver, gold, platinum, titanium oxide, silver oxide, zinc oxide) is growing intensively as they provide better material properties with functional adaptability and have been tested as potential tools for medical imaging for dealing with diseases because of their size features and superiority over chemical images (Khan et al., 2019).
Metallic NPs (silver, gold, zinc oxide, silver oxide silver oxide, copper oxide, titanium oxide) have been prepared, studied, and utilized by researchers, scholars, and scientists in numerous fields. Ag and silver oxide-based nanoparticles are of excellent choice and have excellent scope in nanotechnology and biological systems for medical imaging and treating diseases among the different metallic nanoparticles (Jeevanandam et al., 2018). Silver oxide and silver NP have been extensively utilized for cellular delivery because of their adaptable characteristics/properties like widespread accessibility, deep functionality, good compatibility, the potential for targeted drug administration, and moderate absorption of drugs. Besides medical and pharmaceutical applications, metallic NPs widely used in everyday applications (Christian et al., 2008).
The number of dimensions of materials that are outside the nanoscale (100 nm) range allows for the classification of nanoparticles into three categories, as follows (Tiwari et al., 2012).
-
(I)
Zero-dimensional nanostructured materials (O D)
All three dimensions are measured within the nanoscale in zero-dimensional (0 D) nanostructured materials, i.e., all dimensions are smaller than 100 nm. Zero-dimensional NSMs can be either amorphous or crystalline. It can exhibit various shapes and forms. For example, nanoparticles, quantum dots, nanolenses, hollow spheres, and uniform particle arrays.
-
(II)
One-dimensional nanostructured materials (1 D)
Only one dimension is outside the nanoscale in one-dimensional nanostructured materials, which may result in needle-like nanomaterials. They substantially impact nanoelectronics, alternative energy sources, and nanocomposite materials. For example, nanotubes, nanobelts, nanoribbons, nanowires, and nanorods.
-
(III)
Two-dimensional nanostructured materials (2 D)
In this class, two dimensions are outside the nanoscale. They can exhibit plate-like shapes. Examples are nanofilms, nanowalls, nanoplates, nanodisks, nanoprisms, nanolayers, nanocoatings, and graphene. They are especially interesting not only for understanding the mechanism of nanoparticle growth but also for their investigation and applications in sensors, nanoreactors, photocatalysts, nanocontainers, and templates for two-dimensional structures for other materials.
3 General Methods of Metallic and Metal Oxide Nanoparticle Synthesis
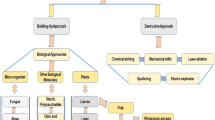
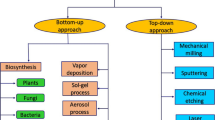
Currently, various methods, including physical, chemical, and biological, are used to synthesize metallic nanoparticles (Mittal et al., 2013). From the structural point of view, there are two basic approaches for nanoparticles synthesis which involve “top-down” and “bottom-up” (Fig. 1). “Top-down” technique is usually achieved by physical methods, in which the size of starting bulk material is reduced by removing material until the desired nanoparticles are obtained. However, the serious drawback of this approach is the imperfection of obtained structure. The other method is “bottom-up” (atom by atom or molecule by molecule), usually achieved by chemical and biological methods, in which the atoms and molecules randomly assemble themselves into the desired nanoparticles (Arole & Munde, 2014). The main advantage of the bottom-up method is that the synthesized nanoparticles are free of structural defects. Scheme 1 shows several methods and strategies used to synthesize metallic NPs (Singh et al., 2018).

Adopted from Alsaiari et al. (2023)
Top-down and bottom-up approach for nanoparticles synthesis.
The physical methods used to synthesize MNPs include evaporation–condensation, spray pyrolysis, laser ablation, pulse wire discharge, sputtering, electron beam lithography, plasma, gamma radiation, and microwave irradiation (Tran & Le, 2018). The chemical methods such as chemical reduction of metallic salt, template method, reverse micelles method, polyol process, sonochemical method, electrochemical method, hydrolysis, wet chemical method, and tollens methods are generally used to synthesize metallic NPs. A stabilizing agent must be added in chemical approaches to stabilize dispersive nanoparticles. The use of toxic chemicals and high radiation levels harmful to the environment is a severe drawback of the chemical and physical approaches (Khandel & Shahi, 2018). Therefore, researchers need to develop green routes for the synthesis of MNPs.
Green chemistry, also called sustainable chemistry, is the scheme, development, and execution of chemical reactions and products that minimizes or eradicates the hazardous and toxic substances harmful to social life and the environment. It also focuses on energy-efficient processes. In recent years, the researcher has shifted their focus toward the biosynthesis of nanoparticles as it is green, cost-effective, feasible, rapid, sustainable, and environmentally benign. Biosynthesis of metallic NPs (bottom-up approach) is a bio-reduction method that uses naturally occurring eco-friendly substances such as plant extracts, vitamins, proteins, polysaccharides, biodegradable polymers, amino acids, microalgae, and microorganisms, including fungi and bacteria, as reducing, capping, and stabilizing agents. Bio-inspired methods are increasing steadily as they do not require hazardous toxic chemicals, high pressure, temperature, and energy (Heera & Shanmugam, 2015). The bio-enthused approach is very much close to the principles of nature, and it allows nature to synthesize stable MNPs.
4 Plant-Mediated Synthesis of Silver and Silver Oxide Nanoparticles
Recently, researchers have shifted their focus toward the biosynthesis of MNPs using plant extracts. This method is gaining more attention than other biological systems because it provides a clean, cost-effective, safe, eco-friendly, convenient, and beneficial route to synthesizing MNPs of desired shape and size. The significant advantages of plant extract-mediated synthesis of NPs over the microbial synthesis of NPs are that these are easy to maintain, readily available, and safe to handle. Different parts of plants, such as leaves, peel, roots, stem, seeds, fruits, and flowers, have been used to produce different nanoparticles (Ag, Au, silver oxide, TiO2) of different shape and size (Jana et al., 2000). The major advantage of plant-mediated synthesis of MNPs over the chemical method is that in the former, there is no need for an additional protective agent (Shekhawat et al., 2014).
Several FTIR spectroscopic studies of plant-mediated NPs indicate that phytochemicals like flavonoids, alkaloids, terpenoids, polyphenols, polysaccharides, protein, and chlorophyll present in plant extracts act as reducing, capping, and stabilizing agents, and thus were believed to be responsible for the synthesis of Ag and silver oxide nanoparticles (Liu et al., 2017). The synthesis of bio-inspired nanoparticles results from the interaction of metallic salt with the plant extracts, which only takes place at optimum temperature, time, and pH (Kredy, 2018).
The most effective green synthesis technique for Ag and Ag2O–NPs is using plant sources. The production of Ag and Ag2O–NPs using plant-mediated synthesis takes less time and results in more stable nanoparticles than Ag and Ag2O–NPs produced through microbe-mediated generation. The fact that botanical sources may be used easily, cheaply, and safely is crucial. They also include flavonoids that can stabilize the resultant nanoparticles (Husen et al. 2023d; Abbasi et al. 2020). The use of plant-derived materials has advanced to this point as more and more research teams have investigated the potential application of various plants for the manufacture of nanoparticles through the use of extracts of both aerial and beneath-the-ground parts, such as the roots, leaves, fruits, vegetables, and others (Ghotekar et al., 2020).
Silver nanoparticles were synthesized by using common and standard plant-mediated methods. At room temperature, aqueous plant extract and AgNO3 solution were mixed in a fixed ratio in a 2 L Erlenmeyer conical flask. Then, the mixed solution was kept in a dark place for 72 h so that the synthesis of silver nanoparticles could take place. The solution turned a dark crimson-red color after 72 h, indicating the creation of Ag nanoparticles. To eliminate unreacted or uncoordinated components, the resulting solution was centrifuged for 20 min at 7500 rpm, rinsed with deionized distilled water, and then washed with ethanol. After being mashed in a mortar and pestle to create fine and homogeneous powdered graying black nanoparticles, the material was finally dried in an oven at 50 ℃. Phytochemicals in plants are considered to mediate the synthesis of silver oxide nanoparticles. AgOH is produced initially as a result of the reaction among the hydroxyl groups (–OH) of the phytochemicals and the Ag+ from AgNO3 before being changed into Ag2O (Ravichandran et al., 2016; Sharma & Srivastava, 2020).
The roots and leaves of the plant contained various phytochemicals which plays an important role in reducing, capping, and stability of silver and silver oxide nanoparticles. For instance, Ricinus communis roots and leaves contained a variety of phenolic and flavonoid chemicals, including gallic acid, kaempferol-3-o-b-d-xylopyranoside, diethyl phthalate, kaempferol-3-o-b-d-xylopyranoside, 2-methyl, l-valine, triethyl citrate, quercetin-3-o-p-d-glucopyranoside, octadecanoic acid, 1-hexadecanol, and n-hexadecenoic acid; however, indole-3-acetic acid is the main component in root extract. Although due to the complicated chemical makeup of plants (extract), the precise method of metal nanoparticles by employing plant extract is uncertain. However, based on the above finding, a generic strategy for the creation of silver nanoparticles using three phytochemicals is given (see Fig. 2). With the release of hydrogen (reactive), keto form of the indole-3-actetic acid (present in the root extract) transforms into the enol form; the enol form, however, was unstable since it had two hydroxyl groups on the same carbon, and it changed back into keto form. Thus, silver nanoparticles are created when Ag+ and Ag0 combine as a result of the reactive hydrogen that has been released. Similar to this, triethyl citrate and flavonoid (quercetin-3-o-p-d-glucopyranoside) were regarded as reducing agents in the case of leaf extract. The phytochemicals (1-valine amino acid) and phenolic compounds play a significant role in the stability of metal nanoparticles, thus stabilized the silver nanoparticles (Huang et al., 2010; Rakhi & Gopal, 2012).

Adopted from Gul et al. (2021)
Probable mechanism for plant-mediated synthesis of silver nanoparticles.
5 Characterization of Metallic Nanoparticles
Several techniques, such as UV–visible spectroscopy, SEM, XRD, DLS, EDX, TEM, FTIR, and TGA, can characterize metallic nanoparticles (Moond et al., 2023; Shaik et al., 2018).
In the preliminary stage, the formation of metallic nanoparticles is characterized by UV–visible spectroscopy. Metallic nanoparticles show the bright color and characteristic absorption band in the UV–visible region of the electromagnetic spectrum owing to a process known as surface plasmon resonance (SPR). Green-synthesized Ag and silver oxide nanoparticles show characteristic color and absorption UV–visible band, which is mainly dependent on their shape and size. Different surface plasmon resonance absorption bands in the range 300–700 nm are generally used to characterize different Ag (410–480 nm) and silver oxide (310–390 nm) nanoparticles of size less than 100 nm. A study revealed that the UV–visible spectrum of plant-mediated silver nanoparticles using Allium cepa extract has a sharp peak at around 413 nm. In another study, SPR peaks at around 422 and 447 nm were reported for the plant-mediated silver oxide NPs using Vitex negundo extract (Zargar et al., 2011).
X-ray diffraction (XRD) is a widely used technique for characterizing and identifying metallic nanoparticles' phase, crystalline nature, and crystalline structure). In X-ray diffraction, X-rays enter the powdered sample, resulting in a diffraction pattern according to Bragg's equation n \(\lambda =2dSin\theta\). This diffraction pattern is compared with the standard data to obtain the crystal structures. In XRD pattern consists of diffraction peaks as a function of the diffraction angle 2 \(\theta\). The size of metallic nanoparticles is determined by the Debye–Scherrer equation:
where D is the average crystallite size, λ is the X-ray wavelength, β is the full width at half maxima (FWHM), K is Scherrer's constant, and θ is Bragg's diffraction angle (Fig. 3).
XRD spectra of silver NP using Origanum vulgare leaf extract. Adopted from Shaik et al. (2018)
Scanning electron microscopy (SEM) is a type of electron microscope that is used to produce the image of a sample and thus gives the morphology of metallic nanoparticles (Arjunan et al., 2012). In SEM, electron beams are generated from a filament (tungsten) and then accelerated toward a condenser lens by anode, where these are converged and then projected onto the specimen by the objective lens. These beams scan the surface sample in a raster pattern. The electron beam interacts with the atom in the sample, producing various signals and giving information about the surface morphology and composition of samples (Pradeep, 2007).
Energy-dispersive X-ray (EDX) analysis is a chemical microanalysis technique used to characterize the elemental composition of nanoparticles. SEM coupled with energy-dispersive X-ray spectroscopy (SEM–EDX) is widely used for surface analysis and elemental composition. EDX spectra measure the relative abundance of emitted X-rays from samples against their energy (Fig. 4). EDX spectrum shows the peaks at around 0.5 keV for oxygen and 3 keV for the elemental silver (Alaraidh et al., 2014).
a SEM image of Ag nanoparticles b EDX image of Ag nanoparticles using Trigonella foenum-graceum leaf extract. Adopted from Moond et al. (2023)
Transmission electron microscopy (TEM) is a valuable and prominent microscopy approach that involves passing an electron beam through a specimen to create an image. Transmission electron microscopes can image at a substantially higher resolution than other microscopes due to the smaller de Broglie wavelength of electrons (Fig. 5). TEM can reveal the finest details of internal structure. High-resolution TEM can be used to analyze the structure and constitutional analysis of Ag and silver oxide nanoparticles (Ankamwar et al., 2005).
TEM images of Ag nanoparticles using Trigonella foenum-graceum leaf extract. Adopted from Moond et al. (2023)
Fourier-transform infrared (FTIR) spectroscopy is used to identify the phytochemicals responsible for the capping, reducing, and effective stabilization of metallic nanoparticles. It is a technique that produces an IR spectrum of absorption or emission of a solid, gas, and liquid over a wide spectral range of 4000–400 cm−1 (Fig. 6).
FTIR spectrum of Ag nanoparticles using Trigonella foenum-graceum leaf extract. Adopted from Moond et al. (2023)
For instance, silver nanoparticles were synthesized from the leaf extract of Moringa oleifera, and their antimicrobial and catalytic properties were evaluated (Sathyavathi et al., 2011). The absorption peak of silver nanoparticles at \(\lambda\) max = 460 nm was recorded by UV–visible spectrophotometer, XRD and FRSEM results confirmed the average size of NPs was less than 40 nm. FTIR study showed several stretching peaks at 3308, 1629, 1069, and 1029 cm−1 indicating the presence of several functional groups acting as capping and stabilizing agents. In another study, Roy et al. (2017) reported the synthesis of silver NPs from the leaf extract of Azadirachta indica and the screening of these NPs for antioxidant and antibacterial activities. SPR peaks of these nanoparticles at \(\lambda\) max = 430 nm were recorded by a spectrophotometer, particle size analyzer (PSA) showed the average size range of nanoparticles was 21.07 nm, and FTIR study confirmed the presence of phenolic compounds. In another report, spherical silver NPs were synthesized from Abutilon indicum leaf extract, and their antioxidant, antibacterial, and anticancer activity was investigated (Mata et al., 2015). TEM imaging showed the formation of spherical NPs with an average size of 70–75 nm, and XRD results revealed wurtzite hexagonal silver oxide NPs. FTIR spectrum showed a peak at 3291 cm−1 due to the presence of hydroxyl functional groups, while other peaks at 2930, 1663, 1530, 1239, and 459 cm−1 confirmed the presence of C-H, amide I and II groups, C-O bond, and metal–oxygen groups.
In order to create Ag2O–NPs, Rashmi et al. (2020) combined separately milled plant powders of Centella Asiatica and Tridax procumbens using AgNO3. Gotu kola, or Centella Asiatica, is part of the Apiaceae family. It is important in medicine and cosmetics because of the pentacyclic triterpenoids, which are asiaticoside, brahmoside, Asiatic acid, and Brahmic acid that it is said to possess. These substances can stabilize the resultant nanoparticles as well as decrease AgNO3 to Ag2O. The synthesized Ag2O nanoparticles appear to have outstanding electrochemical capabilities, good photocatalytic activity for AO-8 under UV light, and antimicrobial properties. The generated nanoparticles, with a diameter of particles of 11–12 nm, are suggested by the results to have these qualities.
Using the hydroethanolic leaf extracts of the Ranunculaceae family's medicinal plant, Helleborus odors, Sutan et al. (2021) created Ag2O–NPs in a bottom-up manner. Using a 1:1 mixture of crude hydroethanolic leaf extracts and AgNO3, the Ag2O–NPs were created. The reaction was allowed to run its course for 24 h in the dark at room temperature.
The gathered nanoparticles were identified as Cytogen toxic leaf extracts of papaya (Carica papaya) and fenugreek (Trigonella foenum-graecum). Separate mixtures of fenugreek leaf and papaya leaf extracts were combined with AgNO3, which was then agitated at room temperature for 8 h to produce Ag2O–NPs with a particle size range of 19.4–30.4 nm. These Ag2O-NPs were then tested for hemolytic activity.
Shah et al. (2019) used Paeonia emodi leaf extracts combined with AgNO3 while constantly stirring at 55 ℃ for 1 h 30 min. The resultant nanomaterials were investigated for their potential as photocatalytic and antibacterial agents. According to Suresh et al. (2018), the plant Pavetta indica Linn was employed to create Ag2O–NPs. In order to create distorted cubic-shaped nanomaterials with a size less than 50.5 nm, leaf extracts were combined with an AgNO3 solution and agitated continuously for 24 h at 100–120 ℃. These particles were then examined for their ability to reduce inflammation and for their phytochemical content.
As a reaction mediator and capping agent, Muthukumar et al. (2022) used Amaranthus sp. leaf extract to create Ag2O–NPs in the shape of husks. Aqueous leaf extract of Amaranthus sp. was gradually added to AgNO3 solution with constant magnetic stirring at 35 °C for 15–20 min to produce Ag2O–NPs. According to Muthukumar et al. (2022), Amaranthus sp. leaf extract was utilized as a capping agent and reaction mediator in forming Ag2O nanoparticles in the shape of husks. Other reports of green synthesis of Ag and silver oxide NPs from the various parts of plant extract are illustrated in Table 1.
6 Challenges and Future Perspectives
The most frequently studied and utilized nanomaterials are undoubtedly silver nanoparticles. Green synthesis approaches have garnered a lot of interest recently among the various ways to produce silver nanoparticles since they are easy, effective, economical, and ecologically safe. In order to effectively use the biosynthesized silver nanoparticles for potential applications, it is necessary to obtain insight into the various inevitable challenges that must be addressed in the future. Metal and metal oxide nanoparticles have various properties that can be greatly influenced by their physical and chemical properties, especially their size, shape, and composition. This, in turn, can have a positive impact on their health benefits. Additionally, the metallic nanoparticle’s typical size has a direct impact on both their catalytic and antibacterial properties (Chi et al., 2019; Ferdous & Nemmar, 2020; Hong et al., 2016; Yin et al., 2020). Although plant-mediated synthesis has received greater attention recently because it is a green strategy, it is still a novel concept that is in the early stages of research. In order to effectively use the plant-mediated silver and silver oxide nanoparticles for potential applications, it is necessary to obtain insight into the following inescapable issues that must be addressed in the future.
The shape and size of the silver nanoparticles produced by plants have varied greatly. To effectively manage the shape, size, and monodispersity of nanoparticles, it is necessary to experiment with and validate a variety of parameters, such as extract concentration, exposure time, temperature, and pH. Additionally, silver release is more likely to occur in small-sized spherical silver nanoparticles. Silver nanoparticles less than 10 nm are easily able to infiltrate bacterial cells, alter cell permeability, and cause cell damage. A significant issue in addition to this is the toxicity of metal nanoparticles. According to reports, the use of metal NPs in various industries has had extremely damaging impacts on both human health and the environment. For instance, prolonged exposure to silver can cause eye and skin discoloration and bluish-gray skin. Additionally, recent studies appear to support the idea that some bacteria are less susceptible to silver nanoparticles or are resistant to them. The mechanism behind the mechanism of silver nanoparticles is still poorly understood and now in a dormant state. In the future, attention should be paid to thorough investigations that identify the biochemistry of the bioactive molecules involved in capping and reducing during the creation of nanoparticles. In plant-mediated biosynthesis, different outcomes from the extract of the same plant species gathered from different parts of the world are expected due to differences in their makeup. This problem can be solved by correctly identifying the variety of phytochemicals present in plant extract and then researching how they affect capping and decreasing. Studies on the production of silver nanoparticles to date have solely used laboratory-scale experiments. For mass or industrial production to be possible, more optimization effort is required. The potential of biosynthesized silver nanoparticles for various applications has been shown in several research. Future research is necessary to assess the toxicity of biosynthesized silver nanoparticles, their biocompatibility (with cells and tissues), their safety for the environment (soil, water, and air), their accumulation in plants and animals, and their long-term effects on human and agricultural crop health. In comparison to conventional techniques, biogenic production of nanoparticles is more affordable. However, there is still room to cut costs, which are mostly caused by metal salts, microbial media, and downstream processing in biosynthesis. This can be resolved in the future by taking waste recycling into account as a different cost-saving measure.
7 Conclusion
Silver and silver oxide nanoparticles are the most desirable nanomaterials for various applications, including electrical, catalytic, antibacterial, and other biological activities. The literature for understanding the production and characterization of silver and silver oxide nanoparticles utilizing plant extracts is summarized in the current review. The use of plant extract in the synthesis of silver and silver oxide nanoparticles is advantageous due to its lower environmental impact and ability to create vast numbers of nanoparticles. Plant extracts may function in creating silver and silver oxide nanoparticles as both reducing and stabilizing agents. The use of plant extract in synthesizing silver oxide nanoparticles has advantages over other physical and chemical approaches since it is secure, environmentally benign, and easy to implement. UV–visible spectroscopy, DLS, TEM, SEM, XRD, EDX, and FTIR can successfully characterize these nanoparticles. Plants have a huge potential for producing silver and silver oxide nanoparticles with the desired shape and size that have a vast potential for use. In a nutshell, future studies must concentrate on understanding the intricate mechanism of metal and metal oxide nanoparticle synthesis and designing nanoparticles that are less hazardous, healthier, and have precisely controlled dimensions and form. Additionally, silver release is more likely to occur in small-sized spherical silver nanoparticles. Furthermore, the necessity to use plant-based metallic NPs more frequently in related disciplines must also be taken into consideration.
References
Abate, L., Megra, M. B., Nigussie, Y., Zebeaman, M., Tadesse, M. G., Bachheti, A., Bachheti, R. K., & Husen, A. (2023). Medicinally important seed extract and seed oil-mediated nanoparticles synthesis and their role in drug delivery and other applications. In R. K. Bachheti & A. Bachheti (Eds.), Secondary metabolites from medicinal plants (pp. 199–212). CRC Press.
Abbasi, B. A., Iqbal, J., Nasir, J. A., Zahra, S. A., Shahbaz, A., Uddin, S., Hameed, S., Gul, F., Kanwal, S., & Mahmood, T. (2020). Environmentally friendly green approach for the fabrication of silver oxide nanoparticles: Characterization and diverse biomedical applications. Microscopy Research and Technique, 83(11), 1308–1320.
Ahmed, S., Saifullah, A. M., Swami, B. L., & Ikram, S. (2016). Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. Journal of Radiation Research and Applied Sciences, 9(1), 1–7.
Ahsan, A., Farooq, M. A., Ahsan Bajwa, A., & Parveen, A. (2020). Green synthesis of silver nanoparticles using Parthenium hysterophorus: Optimization, characterization and in vitro therapeutic evaluation. Molecules, 25(15), 3324.
Alaraidh, I. A., Ibrahim, M. M., & El-Gaaly, G. A. (2014). Evaluation of green synthesis of Ag nanoparticles using Eruca sativa and Spinacia oleracea leaf extracts and their antimicrobial activity. Iranian Journal of Biotechnology, 12(1), 50–55.
Alsaiari, N. S., Alzahrani, F. M., Amari, A., Osman, H., Harharah, H. N., Elboughdiri, N., & Tahoon, M. A. (2023). Plant and microbial approaches as green methods for the synthesis of nanomaterials: Synthesis, applications, and future perspectives. Molecules, 28(1), 1–38.
Anbazhagan, P., Murugan, K., Jaganathan, A., Sujitha, V., Samidoss, C. M., Jayashanthani, S., Amuthavalli, P., Higuchi, A., Kumar, S., Wei, H., Nicolett, M., Canale, A., & Benelli, G. (2017). Mosquitocidal, antimalarial and antidiabetic potential of Musa paradisiaca-synthesized silver nanoparticles: In vivo and in vitro approaches. Journal of Cluster Science, 28(1), 91–107.
Anjum, S., Jacob, G., & Gupta, B. (2019). Investigation of the herbal synthesis of silver nanoparticles using Cinnamon zeylanicum extract. Emergent Materials, 2(1), 113–122.
Ankamwar, B., Damle, C., Ahmad, A., & Sastry, M. (2005). Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer, and transmetallation in an organic solution. Journal of Nanoscience and Nanotechnology, 5(10), 1665–1671.
Arjunan, N. K., Murugan, K., Rejeeth, C., Madhiyazhagan, P., & Barnard, D. R. (2012). Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector-Borne and Zoonotic Diseases, 12(3), 262–268.
Arole, V. M., & Munde, S. V. (2014). Fabrication of nanomaterials by top-down and bottom-up approaches-an overview. Journal of Materials Science, 1, 89–93.
Ashokraja, C., Sakar, M., & Balakumar, S. (2017). A perspective on the hemolytic activity of chemical and green-synthesized silver and silver oxide nanoparticles. Materials Research Express, 4(10), 105406.
Bachheti, A., Bachheti, R. K., Abate, L., & Husen, A. (2022). Current status of Aloe-based nanoparticle fabrication, characterization and their application in some cutting-edge areas. South African Journal of Botany, 147, 1058–1069.
Bachheti, R. K., Fikadu, A., Bachheti, A., & Husen, A. (2020). Biogenic fabrication of nanomaterials from flower-based chemical compounds, characterization and their various applications: A review. Saudi Journal of Biological Sciences, 27(10), 2551–2562.
Bachheti, R. K., Sharma, A., Bachheti, A., Husen, A., Shanka, G. M., & Pandey, D. P. (2020b). Nanomaterials from various forest tree species and their biomedical applications. In A. Husen, M. Jawaid (Eds.), Nanomaterials for agriculture and forestry applications (pp. 81–106). Elsevier. https://doi.org/10.1016/B978-0-12-817852-2.00004-4
Bachheti, R. K., Abate, L., Bachheti, A., Madhusudhan, A., & Husen, A. (2021). Algae-, fungi-, and yeast-mediated biological synthesis of nanoparticles and their various biomedical applications. In Handbook of greener synthesis of nanomaterials and compounds (pp. 701–734). Elsevier. https://doi.org/10.1016/B978-0-12-821938-6.00022-0
Bachheti, R. K., Konwarh, R., Gupta, V., Husen, A., & Joshi, A. (2019). Green synthesis of iron oxide nanoparticles: Cutting edge technology and multifaceted applications. In Nanomaterials and plant potential (pp. 239–259), Springer. https://doi.org/10.1007/978-3-030-05569-1_9
Bachheti, R. K., Godebo, Y., Bachheti, A., Yassin, M. O., & Husen, A. (2020a). Root-based fabrication of metal/metal-oxide nanomaterials and their various applications. In A. Husen, M. Jawaid (Eds.), Nanomaterials for agriculture and forestry applications (pp.135–166). Elsevier. https://doi.org/10.1016/B978-0-12-817852-2.00006-8
Balashanmugam, P., Santhosh, S., Mukesh Kumar, D. J., Anbazhakan, S., & Kalaichelvan, P. T. (2014). Synthesis of plant-mediated silver nanoparticles using Aerva lanata leaf aqueous extract and evaluation of its antibacterial activities. Indo-American Journal of Pharmaceutical Research, 4, 475–480.
Balavijayalakshmi, J., & Ramalakshmi, V. (2017). Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. Journal of Applied Research and Technology, 15(5), 413–422.
Behravan, M., Panahi, A. H., Naghizadeh, A., Ziaee, M., Mahdavi, R., & Mirzapour, A. (2019). Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. International Journal of Biological Macromolecules, 124, 148–154.
Bharathi, D., Josebin, M. D., Vasantharaj, S., & Bhuvaneshwari, V. (2018). Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. Journal of Nanostructure in Chemistry, 8(1), 83–92.
Bose, D., & Chatterjee, S. (2015). Antibacterial activity of green synthesized silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract. Indian Journal of Microbiology, 55(2), 163–167.
Bouqellah, N. A., Mohamed, M. M., & Ibrahim, Y. (2019). Synthesis of eco-friendly silver nanoparticles using Allium sp. and their antimicrobial potential on selected vaginal bacteria. Saudi Journal of Biological Sciences, 26(7), 1789–1794.
Chawla, S., Singh, S., & Husen, A. (2023). Smart nanomaterials targeting pathological hypoxia. Springer Nature Singapore Pte Ltd. ISBN: 978–981–99–1717–4. https://doi.org/10.1007/978-981-99-1718-1
Chi, M., Qi, M., Wang, P., Weir, M. D., Melo, M. A., Sun, X., Dong, B., Li, C., Wu, J., Wang, L., & Xu, H. H. (2019). Novel bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. International Journal of Molecular Sciences, 20(2), 1–29.
Christian, P., Von der Kammer, F., Baalousha, M., & Hofmann, T. (2008). Nanoparticles: Structure, properties, preparation, and behavior in environmental media. Ecotoxicology, 17(5), 326–343.
Chunfa, D., Fei, C., Xianglin, Z., Xiangjie, W., Xiuzhi, Y., & Bin, Y. (2018). Rapid and green synthesis of monodisperse silver nanoparticles using mulberry leaf extract. Rare Metal Materials and Engineering, 47, 1089–1095.
Danish, M. S. S., Estrella, L. L., Alemaida, I. M. A., Lisin, A., Moiseev, N., Ahmadi, M., Nazari, M., Wali, M., Zaheb, H., & Senjyu, T. (2021). Photocatalytic applications of metal oxides for sustainable environmental remediation. Metals, 11(1), 1–25.
El-Ghmari, B., Farah, H., & Ech-Chahad, A. (2021). A new approach for the green biosynthesis of Silver Oxide nanoparticles Ag2O, characterization and catalytic application. Bulletin of Chemical Reaction Engineering and Catalysis, 16(3), 651–660.
El-Refai, A. A., Ghoniem, G. A., El-Khateeb, A. Y., & Hassaan, M. M. (2018). Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. Journal of Nanostructure in Chemistry, 8(1), 71–81.
Elemike, E. E., Onwudiwe, D. C., Ekennia, A. C., Sonde, C. U., & Ehiri, R. C. (2017). Green synthesis of Ag/Ag2O nanoparticles using aqueous leaf extract of Eupatorium odoratum and its antimicrobial and mosquito larvicidal activities. Molecules, 22(5), 1–15.
Fatimah, I., & Mutiara, N. A. L. (2016). Biosynthesis of silver nanoparticles using Putri Malu (Mimosa pudica) leaves extract and microwave irradiation method. Molekul, 11(2), 288–298.
Fayyadh, A. A., & Jaduaa Alzubaidy, M. H. (2021). Green synthesis of Ag2O nanoparticles for antimicrobial assays. Journal of the Mechanical Behavior of Materials, 30(1), 228–236.
Ferdous, Z., & Nemmar, A. (2020). Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. International Journal of Molecular Sciences, 21(7), 1–31.
Fu, L., & Fu, Z. (2015). Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceramics International, 41(2), 2492–2496.
Ghotekar, S., Dabhane, H., Pansambal, S., Oza, R., Tambade, P., & Medhane, V. (2020). A review of biomimetic synthesis of Ag2O nanoparticles using plant extract, characterization, and its recent applications. Advanced Journal of Chemistry-Section B, 2(3), 102–111.
Godeto, Y. G., Ayele, A., Ahmed, I. N., Husen, A., & Bachheti, R. K. (2023). Medicinal plant-based metabolites in nanoparticles synthesis and their cutting-edge applications: An overview. In: R. K. Bachheti, A. Bachheti (Eds.), Secondary metabolites from medicinal plants (pp. 1–34). CRC Press.
Gomathi, M., Rajkumar, P. V., Prakasam, A., & Ravichandran, K. (2017). Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resource-Efficient Technologies, 3(3), 280–284.
Gonfa, Y. H., Tessema, F. B., Tadesse, M. G., Bachheti, A., & Bachheti, R. K. (2023). Medicinally important plant roots and their role in nanoparticles synthesis and applications. In R. K. Bachheti & A. Bachheti (Eds.), Secondary metabolites from medicinal plants (pp. 227–242). CRC Press.
Gonfa, Y. H., Tessema, F. B., Bachheti, A., Tadesse, M. G., Eid, E. M., Abou Fayssal, S., Adelodun, B., Choi, K. S., Širić, I., Kumar, P., & Bachheti, R. K. (2022). Essential oil composition of aerial part of Pluchea ovalis (Pers.) DC., Silver nanoparticles synthesis, and larvicidal activities against Fall Armyworm. Sustainability, 14(23),15785.
Gul, A., Fozia, Shaheen, A., Ahmad, I., Khattak, B., Ahmad, M., Ullah, R., Bari, A., Ali, S.S., Alobaid, A., Asmari, M.M. & Mahmood, H. M. (2021). Green synthesis, characterization, enzyme inhibition, antimicrobial potential, and cytotoxic activity of plant mediated silver nanoparticle using Ricinus communis leaf and root extracts. Biomolecules, 11(2), 1–15.
Haq, S., Yasin, K. A., Rehman, W., Waseem, M., Ahmed, M. N., Shahzad, M. I., Shahzad, N., Shah, A., Rehman, M. U., & Khan, B. (2021). Green synthesis of silver oxide nanostructures and investigation of their synergistic effect with moxifloxacin against selected microorganisms. Journal of Inorganic and Organometallic Polymers and Materials, 31, 1134–1142.
Heera, P., & Shanmugam, S. (2015). Nanoparticle characterization and application: An overview. International Journal of Current Microbiology & Applied Science, 4(8), 379–386.
Hong, X., Wen, J., Xiong, X., & Hu, Y. (2016). Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environmental Science and Pollution Research, 23, 4489–4497.
Huang, X., Wu, H., Liao, X., & Shi, B. (2010). One-step, size-controlled synthesis of gold nanoparticles at room temperature using plant tannin. Green Chemistry, 12(3), 395–399.
Husen, A. (2022). Engineered nanomaterials for sustainable agricultural production, soil improvement and stress management. Elsevier Inc https://doi.org/10.1016/C2021-0-00054-7
Husen, A. (2023a). Secondary metabolites based green synthesis of nanomaterials and their applications. Springer Nature Singapore Pte Ltd. https://doi.org/10.1007/978-981-99-0927-8
Husen, A. (2023b). Nanomaterials from agricultural and horticultural products. Springer Nature. https://springerlink.bibliotecabuap.elogim.com/book/9789819934348
Husen, A. (2023c). Nanomaterials and nanocomposites exposures to plants (Response, Interaction, Phytotoxicity and Defense Mechanisms). Springer Nature. https://doi.org/10.1007/978-981-99-2419-6
Husen, A., Bachheti, R. K., & Bachheti, A. (2023d). Current trends in green nano-emulsions (Food, Agriculture and Biomedical Sectors). Springer Nature Singapore Pte Ltd.
Husen, A., & Iqbal, M. (2019). Nanomaterials and plant potential. Springer International Publishing AG. https://doi.org/10.1007/978-3-030-05569-1
Husen, A., & Jawaid, M. (2020). Nanomaterials for agriculture and forestry applications. Elsevier Inc. https://doi.org/10.1016/C2018-0-02349-X
Husen, A., & Siddiqi, K. S. (2023). Advances in smart nanomaterials and their applications. Elsevier Inc. https://doi.org/10.1016/C2021-0-02202-1
Husen, A., Rahman, Q. I., Iqbal, M., Yassin, M. O., & Bachheti, R. K. (2019). Plant-mediated fabrication of gold nanoparticles and their applications. In Nanomaterials and plant potential (pp. 71–110). Springer. https://doi.org/10.1007/978-3-030-05569-1_3
Iravani, S., Korbekandi, H., Mirmohammadi, S. V., & Zolfaghari, B. (2014). Synthesis of silver nanoparticles: Chemical, physical and biological methods. Research in Pharmaceutical Sciences, 9(6), 385–406.
Jana, N. R., Wang, Z. L., Sau, T. K., & Pal, T. (2000). Seed-mediated growth method to prepare cubic copper nanoparticles. Current Science-Bangalore, 79(9), 1367–1369.
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., & Danquah, M. K. (2018). Review on nanoparticles and nanostructured materials: History, sources, toxicity, and regulations. Beilstein Journal of Nanotechnology, 9(1), 1050–1074.
Joshi, A., Sharma, A., Bachheti, R. K., Husen, A., & Mishra, V. K. (2019). Plant-mediated synthesis of copper oxide nanoparticles and their biological applications. In Nanomaterials and plant potential (pp. 221–237). Springer. https://doi.org/10.1007/978-3-030-05569-1_8
Khan, I., Saeed, K., & Khan, I. (2019). Nanoparticles: Properties, applications, and toxicities. Arabian Journal of Chemistry, 12(7), 908–931.
Khandel, P., & Shahi, S. K. (2018). Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. Journal of Nanostructure in Chemistry, 8(4), 369–391.
Kokila, N. R., Mahesh, B., Roopa, K. P., Prasad, B. D., Raj, K., Manjula, S. N., Mruthunjaya, K., & Ramu, R. (2022). Thunbergia mysorensis mediated nano silver oxide for enhanced antibacterial, antioxidant, anticancer potential and in vitro hemolysis evaluation. Journal of Molecular Structure, 1255, 132455.
Kouvaris, P., Delimitis, A., Zaspalis, V., Papadopoulos, D., Tsipas, S. A., & Michailidis, N. (2012). Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Materials Letters, 76, 18–20.
Kredy, H. M. (2018). The effect of pH, Temperature on the green synthesis and biochemical activities of silver nanoparticles from Lawsonia inermis extract. Journal of Pharmaceutical Sciences and Research, 10(8), 2022–2026.
Krishnaraj, C., Jagan, E. G., Rajasekar, S., Selvakumar, P., Kalaichelvan, P. T., & Mohan, N. J. C. S. B. B. (2010). Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water-borne pathogens. Colloids and Surfaces b: Biointerfaces, 76(1), 50–56.
Kumar, B., Smita, K., Cumbal, L., & Debut, A. (2016b). Ficus carica (Fig) fruit mediated green synthesis of silver nanoparticles and its antioxidant activity: a comparison of thermal and ultrasonication approach. BioNanoScience, 6(1), 15–21.
Kumar, B., Smita, K., Debut, A., & Cumbal, L. (2016a). Extracellular green synthesis of silver nanoparticles using Amazonian fruit Araza (Eugenia stipitata McVaugh). Transactions of Nonferrous Metals Society of China, 26(9), 2363–2371.
Leyu, A. M., Debebe, S. E., Bachheti, A., Rawat, Y. S., & Bachheti, R. K. (2023). Green synthesis of gold and silver nanoparticles using invasive alien plant Parthenium hysterophorus and their antimicrobial and antioxidant activities. Sustainability, 15(12), 9456.
Liu, H., Zhang, H., Wang, J., & Wei, J. (2017). Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arabian Journal of Chemistry, 13, 1011–1019.
Maheshwaran, G., Bharathi, A. N., Selvi, M. M., Kumar, M. K., Kumar, R. M., & Sudhahar, S. (2020). Green synthesis of Silver oxide nanoparticles using Zephyranthes Rosea flower extract and evaluation of biological activities. Journal of Environmental Chemical Engineering, 8(5), 104137.45.
Mandal, A. K., Ghorai, S., & Husen, A. (2023). Functionalized smart nanomaterials for point-of-care testing. Springer Nature Singapore Pte Ltd.
Mani, M., Harikrishnan, R., Purushothaman, P., Pavithra, S., Rajkumar, P., Kumaresan, S., Farraj, D. A. A., Elshikh, M. S., Balasubramanian, B., & Kaviyarasu, K. (2021). Systematic green synthesis of silver oxide nanoparticles for antimicrobial activity. Environmental Research, 202, 111627.
Mata, R., Nakkala, J. R., & Sadras, S. R. (2015). Biogenic silver nanoparticles from Abutilon indicum: Their antioxidant, antibacterial and cytotoxic effects in vitro. Colloids and Surfaces b: Biointerfaces, 128, 276–286.
Mittal, A. K., Chisti, Y., & Banerjee, U. C. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnology Advances, 31(2), 346–356.
Moldovan, B., David, L., Achim, M., Clichici, S., & Filip, G. A. (2016). A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L. fruits extract and their antioxidant activity. Journal of Molecular Liquids, 221, 271–278.
Moond, M., Singh, S., Sangwan, S., Rani, S., Beniwal, A., Rani, J., Kumari, A., Rani, I., & Devi, P. (2023). Phytofabrication of silver nanoparticles using trigonella foenum-graceum L. Leaf and evaluation of its antimicrobial and antioxidant activities. International Journal of Molecular Sciences, 24(4), 3480.
Muthukumar, H., Palanirajan, S. K., Shanmugam, M. K., Arivalagan, P., & Gummadi, S. N. (2022). Photocatalytic degradation of caffeine and E. coli inactivation using silver oxide nanoparticles obtained by a facile green co-reduction method. Clean Technologies and Environmental Policy, 24(4), 1087–1098.
Oluwaniyi, O. O., Adegoke, H. I., Adesuji, E. T., Alabi, A. B., Bodede, S. O., Labulo, A. H., & Oseghale, C. O. (2016). Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruviana Juss and its antimicrobial activities. Applied Nanoscience, 6(6), 903–912.
Painuli, S., Semwal, P., Bacheti, A., Bachheti, R. K., & Husen, A. (2020). Nanomaterials from nonwood forest products and their applications. In: A. Husen, M. Jawaid (Eds.), Nanomaterials for agriculture and forestry applications (pp. 15–40). Elsevier. https://doi.org/10.1016/B978-0-12-817852-2.00002-0
Pal, S. L., Jana, U., Manna, P. K., Mohanta, G. P., & Manavalan, R. (2011). Nanoparticle: An overview of preparation and characterization. Journal of Applied Pharmaceutical Science, 1(6), 228–234.
Patel, H., & Joshi, J. (2023). Green and chemical approach for synthesis of Ag2O nanoparticles and their antimicrobial activity. Journal of Sol-Gel Science and Technology, 105, 814–826.
Patil, A. B., & Mengane, S. K. (2016). Green synthesis of silver nanoparticles from Clerodendrum serratum and antimicrobial activity against human pathogens. Journal of Bionanoscience, 10(6), 491–494.
Phatak, R. S., & Hendre, A. S. (2015). Sunlight-induced green synthesis of silver nanoparticles using sundried leaves extract of Kalanchoe pinnata and evaluation of its photocatalytic potential. Der Pharmacia Lettre, 7(5), 313–324.
Pradeep, T. (2007). Nano: the essentials. Tata McGraw-Hill Education.
Pradheesh, G., Suresh, S., Suresh, J., & Alexramani, V. (2020). Antimicrobial and anticancer activity studies on green synthesized silver oxide nanoparticles from the medicinal plant Cyathea nilgiriensis Holttum. International Journal of Pharmaceutical Investigation, 10(2), 146–150.
Rakhi, M., & Gopal, B. B. (2012). Terminalia arjuna bark extract mediated size controlled synthesis of polyshaped gold nanoparticles and its application in catalysis. International Journal of Research in Chemistry and Environment, 2(4), 338–344.
Rashmi, B. N., Harlapur, S. F., Avinash, B., Ravikumar, C. R., Nagaswarupa, H. P., Kumar, M. A., Gurushantha, K., & Santosh, M. S. (2020). Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic, and biological studies. Inorganic Chemistry Communications, 111, 107580.
Ravichandran, S., Paluri, V., Kumar, G., Loganathan, K., & Kokati Venkata, B. R. (2016). A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. Journal of Experimental Nanoscience, 11(6), 445–458.
Roy, P., Das, B., Mohanty, A., & Mohapatra, S. (2017). Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Applied Nanoscience, 7(8), 843–850.
Sathyavathi, R., Krishna, M., & Rao, D. N. (2011). Biosynthesis of silver nanoparticles using Moringa oleifera leaf extract and its application to optical limiting. Journal of Nanoscience and Nanotechnology, 11(3), 2031–2035.
Selvam, K., Sudhakar, C., Govarthanan, M., Thiyagarajan, P., Sengottaiyan, A., Senthilkumar, B., & Selvankumar, T. (2017). Eco-friendly biosynthesis and characterization of silver nanoparticles using Tinospora cordifolia (Thunb.) Miers and evaluate its antibacterial, antioxidant potential. Journal of Radiation Research and Applied Sciences, 10(1), 6–12.
Shah, A., Haq, S., Rehman, W., Waseem, M., Shoukat, S., & Rehman, M. U. (2019). Photocatalytic and antibacterial activities of Paeonia emoji mediated silver oxide nanoparticles. Materials Research Express, 6(4), 045045.
Shaik, M. R., Khan, M., Kuniyil, M., Al-Warthan, A., Alkhathlan, H. Z., Siddiqui, M. R. H., Shaik J. P., & Adil, S. F. (2018). Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities. Sustainability, 10(4), 913.
Sharma, S. N., & Srivastava, R. (2020). Silver oxide nanoparticles synthesized by green method from Artocarpus Hetrophyllus for antibacterial and antimicrobial applications. Materials Today: Proceedings, 28, 332–336.
Shekhawat, M. S., Ravindran, C. P., & Manokari, M. (2014). Biosynthesis of zinc oxide nanoparticles from Passiflora foetida L. extracts and their characterization. International Journal of Green and Herbal Chemistry, 3, 518–523.
Shetty, P., Supraja, N., Garud, M., & Prasad, T. N. V. K. V. (2014). Synthesis, characterization, and antimicrobial activity of Alstonia scholaris bark-extract-mediated silver nanoparticles. Journal of Nanostructure in Chemistry, 4(4), 161–170.
Shume, W. M., Murthy, H. A., & Zereffa, E. A. (2020). A review on synthesis and characterization of Ag2O nanoparticles for photocatalytic applications. Journal of Chemistry, 2020, 1–15.
Singh, J., Dutta, T., Kim, K. H., Rawat, M., Samddar, P., & Kumar, P. (2018). ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. Journal of Nanobiotechnology, 16(1), 1–24.
Soman, S., & Ray, J. G. (2016). Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) Mill: Characterization and assessment of antibacterial activity. Journal of Photochemistry and Photobiology b: Biology, 163, 391–402.
Srinithya, B., Kumar, V. V., Vadivel, V., Pemaiah, B., Anthony, S. P., & Muthuraman, M. S. (2016). Synthesis of biofunctionalized AgNPs using medicinally important Sida cordifolia leaf extract for enhanced antioxidant and anticancer activities. Materials Letters, 170, 101–104.
Sujatha, V., Kaviyasri, G., Venkatesan, A., Thirunavukkarasu, C., Acharya, S., Dayel, S. B., Ghamdi, S.A., Abdelzaher, M.H., Shahid, M., & Ramesh, T. (2023). Biomimetic formation of silver oxide nanoparticles through Diospyros montana bark extract: Its application in dye degradation, antibacterial and anticancer effect in human hepatocellular carcinoma cells. Journal of King Saud University-Science, 102563.
Suresh, S., Pradheesh, G., & Ramani, V. A. (2018). Biosynthesis and characterization of CuO, MgO, and Ag2O nanoparticles, anti-inflammatory activity and phytochemical screening of the ethanolic extract of the medicinal plant Pavetta indica Linn. Journal of Pharmacognosy and Phytochemistry, 7(4), 1984–1990.
Şuţan, N. A., Fierăscu, I., Şuţan, C., Soare, L. C., Neblea, A. M., Somoghi, R., & Fierăscu, R. C. (2021). In vitro mitodepressive activity of phytofabricated silver oxide nanoparticles (Ag2O-NPs) by leaves extract of Helleborus odorus Waldst. & Kit. ex Willd. Materials Letters, 286, 129194.
Taghiyari, H. R., Morrell, J. F., Husen, A. (2023). Emerging nanomaterials (Opportunities and Challenges in Forestry Sectors) (Vol. 11, pp. 6330). Springer Nature. https://doi.org/10.1007/978-3-031-17378-3
Talabani, R. F., Hamad, S. M., Barzinjy, A. A., & Demir, U. (2021). Biosynthesis of silver nanoparticles and their applications in harvesting sunlight for solar thermal generation. Nanomaterials, 11(9), 2421.
Thirunavoukkarasu, M., Balaji, U., Behera, S., Panda, P. K., & Mishra, B. K. (2013). Biosynthesis of silver nanoparticle from leaf extract of Desmodium gangeticum (L.) DC. and its biomedical potential. Spectrochimica Acta Part a: Molecular and Biomolecular Spectroscopy, 116, 424–427.
Tiwari, J. N., Tiwari, R. N., & Kim, K. S. (2012). Zero-dimensional, one-dimensional, two-dimensional, and three-dimensional nanostructured materials for advanced electrochemical energy devices. Progress in Materials Science, 57(4), 724–803.
Tran, Q. H., & Le, A. T. (2018). Corrigendum: Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Advances in Natural Sciences: Nanoscience and Nanotechnology, 9(4), 1–20.
Velsankar, K., Parvathy, G., Sankaranarayanan, K., Mohandoss, S., & Sudhahar, S. (2022). Green synthesis of silver oxide nanoparticles using Panicum miliaceum grains extract for biological applications. Advanced Powder Technology, 33(7), 103645.
Vélez, E., Campillo, G., Morales, G., Hincapié, C., Osorio, J., & Arnache, O. (2018). Silver nanoparticles obtained by aqueous or ethanolic aloe Vera extract an assessment of the antibacterial activity and mercury removal capability. Journal of Nanomaterials, 7215210, 1–7.
Yang, N., Li, F., Jian, T., Liu, C., Sun, H., Wang, L., & Xu, H. (2017). Biogenic synthesis of silver nanoparticles using ginger (Zingiber officinale) extract and their antibacterial properties against aquatic pathogens. Acta Oceanologica Sinica, 36(12), 95–100.
Yin, I. X., Zhang, J., Zhao, I. S., Mei, M. L., Li, Q., & Chu, C. H. (2020). The antibacterial mechanism of silver nanoparticles and its application in dentistry. International Journal of Nanomedicine, 2555–2562.
Zargar, M., Hamid, A. A., Bakar, F. A., Shamsudin, M. N., Shameli, K., Jahanshiri, F., & Farahani, F. (2011). Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules, 16(8), 6667–6676.
Zebeaman, M., Bachheti, R. K., Bachheti, A., Pandey, D. P., & Husen, A (2023). Green and cost-effective nanoparticles synthesis from some frequently used medicinal plants and their various applications. In Secondary metabolites from medicinal plants (pp. 287–304). CRC Press.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kandwal, A., Parveen, S., Bachheti, R.K., Bachheti, A., Khajuria, A.K. (2024). Green Synthesis of Silver and Silver Oxide Nanoparticles From Plants and Their Characterization. In: Bachheti, R.K., Bachheti, A., Husen, A. (eds) Metal and Metal-Oxide Based Nanomaterials. Smart Nanomaterials Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-7673-7_1
Download citation
DOI: https://doi.org/10.1007/978-981-99-7673-7_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-7672-0
Online ISBN: 978-981-99-7673-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)