Abstract
The development of pathogens and parasites resistant to synthetic drugs has created the need for developing alternative approaches to fight vector-borne diseases. In this research, we fabricated green-synthesized silver nanoparticles (AgNP) using Musa paradisiaca stem extract as a reducing and stabilizing agent. AgNP showed plasmon resonance reduction under UV–Vis spectrophotometry, SEM and XRD highlighted that they were crystalline in nature with face centered cubic geometry. The FTIR spectrum of AgNP exhibited main peaks at 464.74, 675.61, 797.07, 1059.42, 1402.58, 1639.69, 2115.61 and 3445.75 cm−1. AgNP showed growth inhibition activity against bacteria and fungi of public health relevance. AgNP were a valuable candidate for treatment of diabetes in STZ-treated rat by normalizing glucose, galactose and insulin. AgNP were toxic against larvae and pupae of the malaria vector Anopheles stephensi, with LC50 of 3.642 (I), 5.497 (II), 8.561 (III), 13.477 (IV), and 17.898 ppm (pupae), respectively. Furthermore, the antiplasmodial activity of nanoparticles was evaluated against CQ-resistant (CQ-r) and CQ-sensitive (CQ-s) strains of Plasmodium falciparum, IC50 were 84.22 μg/ml (CQ-s) and 89.24 μg/ml (CQ-r), while chloroquine IC50 were 86 μg/ml (CQ-s) and 91 μg/ml (CQ-r). Overall, we add knowledge on the multipurpose effectiveness of green-fabricated nanoparticles in medicine and parasitology, which can be potentially helpful to develop newer and safer antiplasmodial agents and vector control tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquitoes (Diptera: Culicidae) pose a major threat to millions of people worldwide, as they vector important parasites and pathogens, including malaria, dengue, filariasis and Zika virus [1]. Malaria mortality rates have fallen by 47 % globally since 2000 and by 54 % in the African region, but it is still a major problem. Most deaths occur among children living in Africa, where a child dies every minute from malaria [2, 3]. Mosquito eggs, larvae, and pupae are usually targeted using organophosphates, insect growth regulators, and microbial control agents [4]. However, these chemicals have negative effects on human health and the environment, and induce resistance in a number of mosquito species [5]. In recent years, a large number of botanical products, including plant extracts, essential oils and pure metabolites have been proposed for eco-friendly control mosquito vectors and other blood-sucking arthropods [6, 7].
Musa paradisiaca L., commonly known as banana or plantain in English and Kela in Hindi languages, belongs to the family Musaceae. It is a perennial tree-like herb indigenously growing in the tropics and subtropics and cultivated for its fruits. Traditionally, the leaves, fruits and stem of M. paradisiaca are used for dressing of wound sand ulcers, as well as to treat eye diseases, anemia, cachexia, hemorrhages, dysmenorrheal, menorrhagia, inflammation and diabetes [8], diarrhea and dysentery, intestinal colitis and antilithic [9], inflammation, pain and snakebite and protein metabolic disorders [10], they also showed antimicrobial [11], antiulcerogenic, anthelmintic [12] hypoglycemic [13] and antioxidant properties [14].
Diabetes is a metabolic disease characterized by hyperglycemia and disturbances in fat and protein metabolism that results from defects in insulin secretion and/or insulin action [15]. Earlier report on the role of green plantain products in the control of hyperglycaemia has been discussed [16]. In particular, new therapeutic approaches are needed to simplify the joint treatment of diabetes and malaria.
Nanoparticles may cover a vast application in pharmaceutical, industrial and biotechnological fields [17]. In recent years, nanoparticle composites have become important owing to their small size and large surface area and because they exhibit unique properties not seen in bulk materials with useful applications in photovoltaic cells, optical and biological sensors, conductive materials, and coating formulations [18]. In recent years, there is lot of interest shown in the environmentally benign synthesis of nanoparticles that do not use any toxic chemicals or extreme conditions in the synthesis process [19]. Silver nanoparticles (AgNPs) are emerging as one of the fastest growing materials due to their unique physical, chemical and biological properties [20–22]. On this basis, here the AgNP were fabricated using the stem extract of M. paradisiaca and characterized by UV–Vis spectrophotometry, FTIR, SEM, TEM and EDX. Then, the multipurpose biological effectiveness of AgNP was evaluated, including: (a) the antimicrobial potential against different pathogenic bacteria and fungi; (b) the antidiabetic potential, with AgNP administered to STZ-treated diabetic rat; (c) the larvicidal and pupicidal potential of AgNP, against the malaria vector Anopheles stephensi; (d) the growth inhibition potential on chloroquine-sensitive (CQ-s) and chloroquine-resistant (CQ-r) strains of Plasmodium falciparum parasites.
Materials and Methods
Preparation of Musa paradisiaca Stem Extract
The stem of M. paradisiaca was collected at the Bharathiar University campus garden and was authenticated at the Department of Botany, Bharathiar University, Coimbatore, India. The green color stem was peeled off and its white inner portion was cut into small pieces. The pieces were mechanically crushed and 5.0 L of juice were extracted and considered as stock standard solution.
Green Synthesis of Silver Nanoparticles
The M. paradisiaca stem juice extract was prepared adding 10 g M. paradisiaca stem in a 300-mL Erlenmeyer flask filled with 100 mL of sterilized double distilled water and then boiling the mixture for 5 min, before finally decanting it. The extract was filtered using Whatman filter paper n. 1, stored at −4 °C and tested within 5 days. The filtrate was treated with aqueous 1 mM AgNO3 (Sigma Aldrich, Mumbai) solution in an Erlenmeyer flask and incubated at room temperature. A brown-yellow solution indicated the formation of AgNP.
Characterization of Green-Synthesized Silver Nanoparticles
Synthesis of AgNP was confirmed by sampling the reaction mixture at regular intervals and the absorption maxima was scanned by UV–Vis spectra, at the wavelength of 200–800 nm in UV-3600 Shimadzu spectrophotometer at 1 nm resolution. Furthermore, the reaction mixture was subjected to centrifugation at 15,000 rpm for 20 min, resulting pellet was dissolved in deionized water and filtered through Millipore filter (0.45 µm).
The surface groups of the AgNP were qualitatively confirmed by FTIR spectroscopy [23], with spectra recorded by a Perkin-Elmer Spectrum 2000 FTIR spectrophotometer; in addition, EDX assays confirmed the presence of metals in analyzed samples. The structure and composition of freeze-dried purified AgNP was analyzed by using a 10 kV ultra high-resolution scanning electron microscope with 25 µl of sample was sputter coated on copper stub and the images of nanoparticles were studied using a FEI QUANTA-200 SEM. TEM was performed using a JEOL model 1200 EX instrument operating at an accelerating voltage of 120 kV. Samples were prepared by placing drops of AgNP suspension on carbon-coated TEM grids. The film on TEM grid was allowed to dry for 5 min in laboratory condition. XRD analysis of drop-coated films on glass substrates from the AOT-capped AgNP was carried out on a Phillips PW1830 instrument operating at 40 kV and current of 30 mA with Cu Kα radiation.
Anopheles stephensi Rearing
Eggs of A. stephensi were collected from water reservoirs in Coimbatore, Tamil Nadu, India using an “O” type brush. Batches of 100–110 eggs were transferred to 18 cm × 13 cm × 4 cm enamel trays containing 500 ml of water, where eggs were allowed to hatch in laboratory conditions (27 ± 2 °C and 75–85 % R. H.; 14:10 (L:D) photoperiod. A. stephensi larvae were fed daily with 5 g of ground dog biscuits (Pedigree, USA) and hydrolyzed yeast (Sigma-Aldrich, USA) in a 3:1 ratio. Newly emerged larvae and pupae were collected and used in the experiments [24].
Larvicidal and Pupicidal Potential
Twenty-five A. stephensi larvae (I, II, III and IV instar) or pupae were placed for 24 h in a glass beaker filled with 250 ml of dechlorinated water in a 500 mL glass beaker, and 1 mL of the desired concentration of AgNP was added and replicated for five times against all instars. Larval food (0.5 mg) was provided for each tested concentration [25]. Control mosquitoes were exposed for 24 h to the corresponding concentration of the solvent. Percentage mortality was calculated as follows:
In Vitro Cultivation of Plasmodium falciparum
CQ-sensitive strain 3D7 and CQ-resistant strain INDO of P. falciparum were used in vitro blood stage culture to test the anti-malarial efficacy of AgNP. The culture was maintained at G. Kuppuswamy Naidu Memorial Hospital (Coimbatore, India). P. falciparum culture was maintained according to the method described by Trager and Jensen [26], with minor modifications. P. falciparum (3D7) cultures were maintained in fresh O+ve human erythrocytes suspended at 4 % hematocrit in RPMI 1640 (Sigma Aldrich, India) containing 0.2 % sodium bicarbonate, 0.5 % albumax, 45 μg/l hypoxanthine and 50 μg/l gentamycin and incubated at 37 °C under a gas mixture of 5 % O2, 5 % CO2 and 90 % N2. Every day, infected erythrocytes were transferred into a fresh complete medium to propagate the culture. For P. falciparum (INDO strain) in culture medium, albumax was replaced by 10 % pooled human serum.
Antiplasmodial Potential
Control stock solutions of CQ were prepared in water (milli-Q grade); the tested extracts were prepared in dimethyl sulfoxide (DMSO). All stocks were diluted with culture medium to achieve the required concentrations (in all cases except CQ, the final solution contained 0.4 % DMSO (which was found to be non-toxic to the parasite). Then, AgNP treatments were placed in 96-well flat-bottom tissue culture-grade plates.
AgNP were evaluated for anti-malarial activity against P. falciparum strains 3D7 and INDO. For drug screening, SYBR green I-based fluorescence assay was used following the method by Smilkstein et al. [27]. Sorbitol-synchronized parasites were incubated under normal culture conditions at 2 % hematocrit and 1 % parasitemia in the absence or presence of increasing concentrations of AgNP where CQ was used as positive control. After 48 h of incubation, 100 μl of SYBR Green I solution {0.2 μl of 10,000 X SYBR Green I (Invitrogen)/ml} in lysis buffer [Tris (20 mM; pH 7.5), EDTA (5 mM), saponin (0.008 %; w/v) and Triton X-100 (0.08 %; v/v)] was added to each well and mixed gently twice with a multi-channel pipette and incubated in the dark at 37 °C for 1 h. Fluorescence was measured with a Victor fluorescence multi-well plate reader (Perkin Elmer) with excitation and emission wavelength bands centered at 485 and 530 nm, respectively. The fluorescence counts were plotted against the drug concentration and the 50 % inhibitory concentration (IC50) was determined by an analysis of dose–response curves. Results were validated microscopically by the examination of Giemsa-stained smears of extract-treated parasite cultures [28].
Anti-Microbial Potential
The bacteria, Bacillus subtilis, Bacillus thuringiensis, Escherichia coli, and fungal species Candida albicans, Fusarium solani and Aspergillus sp. used in this study were purchased by Microbial Type Culture Collection and Gene Bank Institute of Microbial Technology Sector 39-A, Chandigarh-160036 (India). Disc diffusion method: Antimicrobial activity of AgNP was tested against the selected Gram-positive and Gram-negative bacteria and fungal strains using disc diffusion method [29]. The species were incubated in the nutrient broth and incubated at 28 ± 2 °C for 24 h. These bacteria (on nutrient agar) and fungi (on Potato dextrose agar) were grown on their respective media. 20 ml of medium was poured into the plates to obtain uniform depth and allowed to solidify. The standard inoculum suspension (106 CFU/ml) was streaked over the surface of the media using sterile cotton swab to ensure confluent growth of the organisms. 6 mm diameter discs were prepared with Whatman n. 1 paper and used for the study. 10 μl of AgNP was diluted with two volumes of 5 % dimethyl sulfoxide (DMSO) and impregnated on the filter paper discs, placed on the surface of the plates with sterile forceps and gently pressed to ensure contact with the inoculated agar surface. The Petri plates were kept for incubation at room temperature (27 °C ± 2) for 24 h. After incubation, plates were observed for zones of inhibition (millimeters) were measured using a photomicroscope (Leica ES2, Germany) and compared with the standards tetracycline (bacteria) and fluconazole (fungi).
Antidiabetic Potential
Male albino rats of Sprague–Dawley strain (8–10 weeks of age, body weight 120 ± 20 g) was procured from the animal colony of Central Drug Research Institute, Lucknow, India. Animals were acclimatized under standard laboratory conditions at 25◦ ± 2 °C and normal photoperiod (12 h light: dark cycle). The animals were fed with standard rat chow and water ad libitum. The food was withdrawn 18–24 h before the experiment. Research on animals was conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) formed by the Government of India. The CPCSEA with the registration number 34/99/CPCSEA approved on 11th March 1999 and renewed up to 2014. After 1 week of acclimatization period, the animals were divided into four groups with six animals in each.
-
Group I: Control rats fed with standard pellet diet and water.
-
Group II: Rats treated with nicotinamide (110 mg/kg body weight) followed by streptozotocin
-
(60 mg/kg body weight), intraperitoneally
-
Group III: Diabetic rats treated with AgNP orally (50 µg/kg body weight for 8 weeks)
-
Group IV: Rats treated with standard drug glibenclamide orally (600 µg/kg body weight for 8 weeks)
After the experimental regimen, the animals were sacrificed by cervical dislocation under mild chloroform anesthesia. Blood was collected by an incision made in the jugular veins and the serum was separated by centrifugation at 2000 rpm for 20 min. The liver was excised immediately and thoroughly washed in ice-cold physiological saline. A 10 % homogenate of the washed tissue was prepared in 0.1 M TrisHCl buffer (pH 7.4) in a potter homogenizer filled with a Teflon plunger at 600 rpm for 3 min. Blood glucose was estimated by the method of Beach and Turner [30], serum insulin by the method of Anderson [31], hemoglobin by the method of Drabkin and Austin [32], glycosylated hemoglobin was estimated following the method by Sudhakar and Pattabiraman [33], liver glycogen was estimated by the method of Morales et al. [34].
Data Analysis
SPSS software package 16.0 version was used for all analyses. Data from larvicidal and pupicidal experiments were analyzed by probit analysis, calculating LC50 and LC90 [35]. Antiplasmodial assays, all values were expressed as percentage growth inhibition. The concentrations causing 50 % inhibition of parasite growth (IC50) were calculated from the drug concentration response curves. In anti-diabetic trials, the values were analyzed by one-way ANOVA followed by Tukey’s HSD test. All the results were expressed as mean ± SD for six replicates in each group, P < 0.05 were considered as significant.
Results and Discussion
Synthesis and Characterization of Silver Nanoparticles
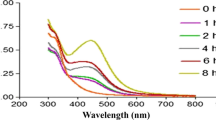
In order to confirm the formation of AgNP, the M. paradisiaca stem extract treated with 1 mM AgNO3 solution was monitored for 120 min by UV–Vis absorption spectrum in the range of 400– 600 nm, then the obtained samples were subjected to FTIR, SEM, TEM and EDX analyses. UV–visible spectroscopy is an important technique to determine the formation and stability of AgNP in aqueous suspension. UV–Vis absorption spectrum (Fig. 1) of the AgNP showed a peak at 410 nm which is probably linked with the surface plasmon resonance of the nanoparticles in the suspension. The reaction mixture showed color changes by adding various concentrations of metal ions and AgNP formation led to a plasmon vibrations peak at around 410 nm. These color changes may be due to the excitation of surface plasmon vibrations in AgNP [22, 36]. The findings were in agreement with Dinesh et al. [24], which fabricated AgNP using Aloe vera extracts. FTIR spectroscopy was used to shed light on the different functional groups from plant-borne molecules (e.g. flavonoids, triterpenoids and polyphenols) that may act as reducing and capping agents of the bio-fabricated AgNP [37].
The FTIR spectrum of the synthesized AgNP is shown in Fig. 2, and reveals various stretching peaks at 464.74, 675.61, 797.07, 1059.42, 1402.58, 1639.69, 2115.61 and 3445.75 cm−1. The peak located at 1639.69 cm−1 may be attributed to carbonyl (C=O) stretching frequency and the peak at 1402.58 cm−1 might be due to the N–H stretching vibrations due to the presence of amide groups. A broad intense band at 3445.75 cm−1 in the spectrum could be assigned to the N–H stretching frequency. The FTIR spectrum of M. paradisiaca-synthesized AgNP revealed the possible biomolecules present in the aqueous medium, which is accountable for the reduction of silver ions. The carbonyl (C=O) stretching frequency was also detected in M. paradisiaca stem extract. The N–H stretching vibrations due to the presence of amide group and the broad intense spectrum can be assigned to the N–H stretching frequency arising from peptide linkages present in the proteins of the banana extract [38]. By these stretching frequencies, it was confirmed that the M. paradisiaca mediated the reduction and capping of AgNP.
SEM and TEM micrographs (Figs. 3, 4, respectively) of the green-synthesized AgNP showed spherical shapes with an average size of 30–60 nm. The shape of nanoparticles was mostly spherical which yielded polydisperse particles both with spherical and flat plate-like morphology, 5–35 nm in size, in accordance with [24] and Shankar et al. [39]. Figure 5 shows a standard energy-dispersive X-ray (EDX) spectrum recorded on the examined SEM samples. In the middle part of the spectrum, two peaks were located between 2.8 and 4 kV, where silver is present. Both were related to the silver characteristic lines K and L. Quantitative analysis showed high oxygen content (63.34 %) in the examined samples; Ag content was about 20.85 %.
Larvicidal and Pupicidal Potential
In laboratory assays, the stem extract of M. paradisiaca and green-synthesized AgNP were assessed for mosquitocidal activity against A. stephensi. The stem extract was toxic against larval instars (I–IV) and pupae of A. stephensi, with LC50 values of 117.254 (I instar), 137.058 (II instar), 162.989 (III instar), 190.296 (IV instar), and 239.595 ppm (pupae) (Table 1). Higher toxicity was reported for AgNP, LC50 were 3.642 (I instar), 5.497 (II instar), 8.561 (III instar), 13.477 (IV instar), and 17.898 ppm (pupae) (Table 2). The toxicity of M. paradisiaca-synthesized AgNP against A. stephensi young instars may be due to the small size of nanoparticles, which penetrate into the cells where they interfere with molting and other physiological processes. A dose-dependent effect was found, as previously described for other plant-borne compounds [1, 3, 7]. Govindarajan et al., [40] postulated that the silver nanocrystals synthesized using Malva sylvestris were effective against A. stephensi larvae. Similarly, poly-dispersed silver nanocrystals fabricated using Carissa spinarum was toxic against larvae of A. stephensi [41]. The present study showed that M. paradisiaca-synthesized AgNP can be considered further as potential mosquito control tools, over current pesticides, reducing damages to the environment [4].
Antiplasmodial Potential
In antiplasmodial assays, AgNP showed higher activity against P. falciparum over chloroquine (Fig. 6). AgNPIC50 were 84.22 μg/ml (CQ-s) and 89.24 μg/ml (CQ-r), while chloroquine IC50 were 86 μg/ml (CQ-s) and 91 μg/ml (CQ-r). Moreover, in antiplasmodial assays, M. paradisiaca-synthesized AgNP showed higher activity against P. falciparum over chloroquine. In agreement with our results, Rajakumar et al. [42] also showed the antiplasmodial activity of palladium nanoparticles synthesized using the leaf aqueous extract of E. prostrata against a NK65 strain of Plasmodium berghei.
In Vivo Anti-Diabetic Potential
Streptozotocin-induced hyperglycemia in rodents is considered a good model for the preliminary screening of agents active against diabetes mellitus [43]. Table 3 reports the levels of blood glucose, serum insulin and liver glycogen of control and experimental animals. A significant increase in glucose level and decrease in insulin and glycogen was observed in the diabetic group II when compared to the control. The treatment with AgNP decreased the levels of elevated blood glucose and simultaneous increased insulin and glycogen levels (group III). The tested parameters were found to be normal as like that of control with the standard drug treatment in group IV. Streptozotocin induction causes destruction of the pancreatic cells, which tends to increase the glucose levels in diabetic group animals at the same time it increases glycogenesis, inhibiting gluconeogenesis in the liver or inhibiting the absorption of glucose from the intestine in order to lower the blood glucose levels. The mode of action of the active compound(s) of the plant material is probably mediated through enhanced secretion of insulin from the β-cells of Langerhans or through extra pancreatic mechanism [44]. Previous data shows that ferulic acid, a phenolic compound, and increases insulin release in clonal β-cells RIN-5F [45]. M. paradisiaca-synthesized AgNP treatment normalized the condition that might have the efficacy in activating the glucose uptake by the cells and might induced insulin hormones. Hence, by lowering the levels of blood glucose levels it was showed that the AgNP are a suitable candidate for the treatment of diabetic mellitus, in accordance with [46].
In diabetes, the glycation and subsequent browning (glycoxidation) reactions are enhanced by elevated glucose levels and there is some evidence that glycation itself may induce the formation of oxygen-derived free radicals [47]. Studies have shown that HbA1C comprises 3.4–5.8 % of total hemoglobin in normal red cells, but it is elevated in patients with diabetes mellitus [48]. HbA1C levels are monitored as a reliable index of glycemic control in diabetes. In our findings the levels of hemoglobin and glycosylated hemoglobin was assed and indexed in Table 4. Form the results, it has been confirmed that induction of streptozotocin altered the levels of hemoglobin and glycosylated hemoglobin respectively in group II and the condition was normalized in group III treated with M. paradisiaca-synthesized AgNP. Inadequate secretion of insulin hormones was the reason behind the depletion and enhancement of hemoglobin levels. Total hemoglobin decreased in the diabetic group, possibly due to the increased formation of HbA1C. This result was well correlated with an earlier report of decreased hemoglobin levels in experimentally diabetic rats. The increase in hemoglobin levels in animals receiving M. paradisiaca-synthesized AgNP may have been due to the decreased blood glucose levels. In this context, several medicinal plants have also been reported to have the ability to reduce HbA1C levels in diabetic rats [49].
Antimicrobial Potential
AgNP antimicrobial activity was tested against different Gram-positive and Gram-negative bacterial (B. subtilis, B. thuringiensis, and E. coli) and fungal species C. albicans, F. solani, and Aspergillus sp. In a dose-dependent manner, the maximum inhibitory zone (mm) was obtained testing 150 mg/ml of AgNP on B. subtilis (90.25 mm) followed by Escherichia coli and Bacillus thuringiensis (Table 5). As regards to fungi, the maximum inhibitory zone was obtained testing 150 mg/ml of AgNP on Candida albicans, (70.00 mm) followed by F. solani and Aspergillus sp. (Table 5), in comparison with positive control fluconazole (1 mg/ml). However, the exact mechanism of the inhibition is still unknown. It has been formulated that the inhibition is due to ionic binding of the AgNP on the surface of the bacteria, which creates a great intensity of the proton motive force. In addition, the AgNP could invade bacterial cells and bind to the vital enzymes containing thiol groups [21].
Conclusions
Overall, this study highlights the multipurpose effectiveness M. paradisiaca-synthesized AgNP. M. paradisiaca-synthesized AgNP are hydrophilic in nature, able to disperse uniformly in water, stable over time, and highly effective as toxic against the tested vectors, parasites and pathogens. M. paradisiaca-synthesized AgNP employed at low dosages, strongly reduce the populations of malarial vector An. stephensi and pathogenic microbes. M. paradisiaca-synthesized AgNP were also a potent drug against STZ-induced diabetes mellitus in in vivo rat model at 50 µg/kg of body weight. Therefore, we believe that M. paradisiaca-synthesized AgNP are worthy of further research attention in programs aimed at mosquito and Plasmodium control as well as for their pharmacological potential as antibiotic and antidiabetic drugs.
References
G. Benelli (2016). Parasitol Res. 115, 23–34.
WHO (2014), Lymphatic filariasis, Fact sheet No 102.
G. Benelli and H. Mehlhorn (2016). Parasitol Res. 115, 1747–1754.
G. Benelli, K. Murugan, C. Panneerselvam, P. Madhiyazhagan, B. Conti, and M. Nicoletti (2015). Parasitol Res. 114, 391–397.
G. Benelli (2015). Parasitol Res. 114, 2801–2805.
S. Azizi, M. B. Ahmad, F. Namvar, and R. Mohamad (2014). Mater Lett. 116, 275–277.
K. Murugan, G. Benelli, S. Ayyappan, D. Dinesh, C. Panneerselvam, M. Nicoletti, J. S. Hwang, P. Mahesh Kumar, J. Subramaniam, and U. Suresh (2015a), Parasitol Res. 114, 2243–2253.
A. K. Nadkarni The Indian materia medica (Popular Prakashan, Bombay, 2002), pp. 822–827.
K. V. Prasad, K. Bharathi, and K. K. Srinivasan (1993). Indian J Physiol Pharmacol. 37, 337–341.
P. K. Agarwal, A. Singh, K. Gaurav, G. Shalini, H. D. Khanna, and R. K. Goel (2009). Indian J Exp Biol. 47, 32-40.
A. Hussain, M. N. Khan, Z. Iqbal, M. S. Sajiid, and M. K. Khan (2011). Vet Parasitol. 179, 92–99.
A. V. Karne (2011). J Curr Sci. 16, 165–175.
P. K. Rai, D. Jaiswal, N. K. Rai, S. Pandhija, A. K. Rai, and G. Watal (2009). Lasers Med Sci. 24, (5), 761–768.
N. Loganayaki, D. Rajendrakumaran, and S. Manian (2010), Food Sci Biotechnol. 19, 1251–1258.
C. C. Teixeira, C. A. Rava, P. M. Da-Silva, R. Melchior, R. Argenta, F. Anselmi, C. R. Almeida, and F. D. Fuchs (2000), J Ethnopharmacol. 71, 343–347.
H. A. Oboh and V. G. Erema (2010). Afr J Food Sci. 4, 514–521.
T. Y. Suman, S. R. Rajasree, A. Kanchana, and S. S. BeenaElizabeth (2013). Colloids Surf B Biointerf. 106, 74–78.
A. C. Templeton, W. P. Wuelfing, and R. W. Murray (2000). AccChem Res. 33, 27–36.
J. Jain, S. Arora, J. M. Rajwade, P. Omray, S. Khandelwal, and K. M. Paknikar (2011). Mol Pharm. 6, 1388–1400.
A. Panacek, L. Kvitek, R. Prucek, M. Kolar, and R. Vecerova (2006). J Phys Chem B. 110, 16248–16253.
M. Govindarajan, S. L. Hoti, G. Benelli. (2016a), Enzyme Microb Technol. doi: 10.1016/j.enzmictec.2016.05.005.
M. Govindarajan, G. Benelli. (2016), J Asia-Pacif Entomol. 19, 377–385.
B. H. Stuart Polymer analysis (John Wiley and Sons, Kent, 2002).
K. Murugan, P. Madhiyazhagan, C. Panneerselvam, M. Nicoletti, W. Jiang, G. Benelli, B. Chandramohan, and U. Suresh (2015). Parasitol Res. 114, 1519–1529.
K. Kovendan, K. Murugan, S. Vincent, and D. R. Barnard (2012). Parasitol Res. 110, 195–203.
W. Trager and J. B. Jensen (1976). Science 193, 673–675.
M. Smilkstein, N. Sriwilaijaroen, J. X. Kelly, P. Wilairat, and M. Riscoe (2004). Antimicrob Agents Chemother. 48, 1803–1806.
A. Bagavan, A. A. Rahuman, N. K. Kaushik, and D. Sahal (2011). Parasitol Res. 108, 15–22.
A. W. Bauer, W. M. Kirby, J. C. Sherris, and M. Turek (1996). Am J Clin Pathol. 44, 493–496.
E. F. Beach and J. J. Turner (1958). Clin Chem. 4, 462–468.
L. B. Anderson, P. N. Dinesen, F. P. Jorgesen, and M. F. Roder (1993). Clin Chim Acta 38, 578.
D. L. Drabkin and J. M. Austin (1932). J Biol Chem. 98, 719–733.
N. S. Sudhakar and T. N. Pattabiraman (1981). Clin Chim Acta. 109, 267–274.
M. A. Morales, A. J. Jabbagy, and H. F. Terenzi (1973). Neurospora News Lett. 20, 24–25.
D. J. Finney Probit analysis (Cambridge University Press, Cambridge, 1971). 333.
N. Thakkar, S. Snehitmhatre, and Y. Rasesh Parikh (2010), Biology and Medicine 2, 257–262.
N. Asmathunisha and K. Kathiresan (2013). Int J Pharma Bio Sci 4, (1), 334–344.
P. Mukherjee, M. Roy, B. P. Mandal, G. K. Dey, P. K. Mukherjee, J. Ghatak, A. K. Tyagi, and S. P. Kale (2008). Nanotechnology 19, 75–103.
S. Shankar, A. Rai, A. Ahmad, and M. Sastry (2004). J Colloid Inter Sci. 275, 496–502.
M. Govindarajan, S. L. Hoti, M. Rajeswary, G. Benelli. (2016), Parasitol Res. doi: 10.1007/s00436-016-5038-x.
M. Govindarajan, M. Nicoletti, G. Benelli. (2016), J. Clust Sci. 27, 745–761.
G. Rajakumar, A. A. Rahuman, I. M. Chung, A. Vishnu Kirthi, S. Marimuthu, and K. Anbarasan (2015), Parasitol Res. 114, 1397–1406.
M. D. Ivorra, M. Paya, and A. Villar (1989). J Ethnopharmacol 27, 243–275.
M. A. Akhtar, M. Rashid, M. I. I. Wahed, M. R. Islam, S. H. Shaheen, A. Islam, M. S. Amran, and M. Ahmed (2007). Res J Med Medical Sci. 2, 29–34.
E. Nomura, A. Kashiwada, A. Hosoda, K. Nakamura, H. Morishita, T. Tsuno, and H. Taniguchi (2003). Bioorg Med Chem. 11, 3807–3813.
P. Bhuvaneswari and S. Krishnakumari (2002). Int J Pharm Pharmaceut Sci. 4, 527–531.
L. A. Trivelli, H. M. Ranney, and I. I. T. Lai (1971). New England J Med. 284, 353–357.
S. Venkateswaran and L. Pari (2002). Pharmaceutical Biol. 40, 165–170.
A. Sereemaspun, P. Hongpiticharoen, R. Rojanathanes, R. Maneewattanapinyo, S. Ekgasit, and W. Warisnoicharoen (2008). Int J Pharmacol. 4, (6), 492–495.
Acknowledgments
Prof. C. M. Lukehart and the anonymous reviewers improved an earlier version of our manuscript. The Authors are grateful to the Department of Science and Technology (New Delhi, India) for providing financial support (Project No. DST/SB/EMEQ-335/2013). Dr. A. Jaganathan is grateful to the University Grant Commission(New Delhi, India), Project No. PDFSS-2014-15-SC-TAM-10125.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anbazhagan, P., Murugan, K., Jaganathan, A. et al. Mosquitocidal, Antimalarial and Antidiabetic Potential of Musa paradisiaca-Synthesized Silver Nanoparticles: In Vivo and In Vitro Approaches. J Clust Sci 28, 91–107 (2017). https://doi.org/10.1007/s10876-016-1047-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1047-2