Abstract
The identification of retinal lesions in fundus pictures is necessary for an automatic telemedicine system for computer-assisted screening and grading of diabetic retinopathy. This study defines and validates an entirely new method for automatically identifying each microaneurysm and haemorrhage in colour fundus images. The most significant contribution has come from a new set of form options known as “dynamic form options,” which do not require precise segmentation of the regions to be classified. These options allow you to differentiate between lesions and vessel segments while also displaying how the morphology changed during image flooding. Six databases are used in the technique, four of which are open to the public. It is valid for each lesion on each image. It shows strength in image quality, resolution, and acquisition system variability. The method comes in fourth place with a FROC score of 0.420, according to the Retinopathy Online Challenge data. The planned method successfully achieves a section of 0.899 for detecting photos with diabetic retinopathy. Messidor data show that traditional methods underperform human consultants’ scores and love the score of traditional methods.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In Diabetes retinopathy (DR) is a diabetes complication that can cause vision impairment and even blindness. It is the leading cause of blindness in people of working age. One in every three diabetics has signs of DR, and one in every ten has its most severe and vision-threatening form [1,2,3]. DR can be managed with existing treatments, which are effective if detected early. Because DR is asymptomatic until late in its progression, regular eye fundus examinations are required to monitor any changes in the retina [4,5,6].
By 2025, there are projected to be 333 million diabetic individuals globally who need annual retinal exams due to the increased prevalence of diabetes and an ageing population. Automation of screening is urgently needed to cover the enormous diabetes population and lessen the clinical stress on retina specialists due to the shortage of ophthalmologists. Automating the detection of DR cases as well as the grading of these cases is achievable on two different levels [7, 8]. In fact, DR grading allows for more accurate and consistent referral to treatment facilities by determining the severity level.
2 Literature Review
The authors in [1] proposed a method based on two approaches. The first strategy entails creating a new pixel-based red lesion candidate detection system. This approach allows for the separation of vascularity and red lesions from the background of the image. The remaining items are thought to be potential red lesions after the linked vasculature is eliminated. Spencer-characteristics Frames are supplemented by a plethora of new ones in the Second Approach. The discovered candidate objects are categorised using a k-nearest neighbour classifier. Some of these candidate objects are not picked up by the mathematical morphology-based candidate extraction processes or the pixel categorization.
The Researchers in [2] proposed a method for an automatic method to detect microaneurysms in retinal photographs. The most typical and frequently the initial lesions that appear as a result of diabetic retinopathy are microaneurysms. They must therefore be found in order to perform both pathology screening and follow-up (progression measurement). We suggest finding them by regionally pairing a lesion template in wavelet-transformed image sub-bands. To enhance method performance, we looked for the wavelet that best suited the lifting scheme structure. Not all issues are fully resolved by the procedure. Since they are grouped together or too close to huge vessels, some MAs are missed.
The authors of [3] proposed a method for automatically spotting microaneurysms (MAs) in coloured retinal pictures is suggested in this research. By analysing directional cross-section profiles centred on the local maximum pixels of the pre-processed image, this technique finds MA. Each profile is subjected to peak detection, and as a result, a number of parameters, including maximum size, height, and form, are computed. This strategy was put to the test in the Retinopathy Online Challenge, where it was discovered to be on par with modern techniques. This approach did not include any further vessel or optical disc detecting steps.
In [4], the authors proposed a method on automatic detection of microaneurysms and haemorrhages in fundus pictures. It begins with a pre-processing phase that involves denoising, contrast enhancement, and shade correction. Second, all candidate regional minima are those that have been extracted and have sufficient contrast. Third, as a function of intensity, an image flooding approach computes a fresh collection of dynamic form features. The candidates are then categorised as having lesions or not by a random forest. Only photos with resolutions comparable to the achieved score of 0.233, which is 40% less than our project technique, were used to validate this method.
The researchers in [5] proposed a method to recognise the optic nerve in photographs of the ocular fundus. We employ a unique approach termed fuzzy convergence to identify the source of the blood vascular network. With symptoms ranging from twisted arteries to choroidal neovascularization and haemorrhages that fully cover the real nerve, we evaluated our approach on 50 sick retinas and 31 healthy retinas. Other researchers examining comparable techniques are welcome to utilise any of our photographs or data without restriction. However, edge detection methods are not always precise.
Existing Methodology
An infinite active contour model that utilises the hybrid region information of the image is employed to address this problem. More particularly, the L2 Lebesgue measure of border neighbourhood's infinite perimeter regularisation makes it possible to detect minor oscillatory (branching) structures more effectively than traditional models based on the length of a feature's boundaries (i.e., the H1 Hausdorff measure) [9]. Diabetes-related retinopathy (DR) is a condition brought on by blood vessel damage in the light-sensitive tissue at the back of the eye. When there is too much sugar in the blood, the tiny blood vessels become blocked, cutting off the blood supply to the eye, which then tries to develop new blood vessels [10].
Non-proliferative diabetic retinopathy is the name for early DR, in which no new blood vessels are developing but the blood vessel walls begin to weaken, causing minute bulges to protrude from the vessel walls and occasionally exudates from blood and fluid leaks into the retina. The diameter of larger vessels changes as a result of dilation. Blood vessels become blocked as the illness worsens, and the retina swells. As a result, the retina begins to develop abnormally new blood vessels. These new blood vessels may cause leakage and the development of scar tissue in the eye, which may ultimately result in retinal detachment and glaucoma. a picture showing different diabetic retinopathy situations [11, 12].
Thus, a crucial diagnostic challenge in DR is the detection of such exudates, scars, and aberrant blood vessels. Normal manual diagnosis requires specialised expert input and takes 7–14 days.
By using severity scores based on high-resolution retinal scans, this study aims to predict DR and perform automated analysis. In addition to speeding up manual diagnosis, this will offer assistance in cases where professionals are not present [13].
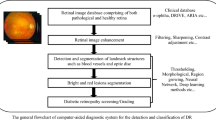
3 Proposed Methodology
3.1 Spatial Calibration
We employ a spatial calibration technique to adjust to various image resolutions. There is no image resizing. Instead, the diameter of the ROI is used as a size invariant (after the black backdrop is removed). This theory is plausible given that the majority of the images used for DR screening are obtained with a 45-degree field of view (FOV) (Fig. 1).
3.2 Image Preprocessing
Local variations in luminance and contrast result from the retina's frequently uneven light. In areas with low brightness and/or poor contrast, lesions may not be at all evident. Also, the colour and quality of photographs vary in a telemedicine setting. Pre-processing procedures are therefore necessary to address these problems [14].
3.3 Disc Removal
We initially determine where the OD's centre is by using an entropy-based method to start with the pre-processed image. In essence, the OD is situated in an area of high intensity where the vessels have the highest directional entropy. The next round of optimization then calculates the OD's radius and fine-tunes its location. This involves applying a multi-scale ring-shaped matching filter with a radius equal to one-third of the ROI's radius to the picture in a sub-ROI that is centred on the initial estimation of the OD's centre [15].
3.4 Candidate Extraction
MAs and HEs can be seen as structures with local minimal intensities in the green channel. Extraction of all the regional minima would be a brute-force method. A set of connected pixels with a constant intensity is referred to as a “regional minimum” when all of the adjacent pixels have strictly higher values. Sadly, this approach is rather noise-sensitive. The number of regional minima can therefore be very significant, depending on how smooth the image [16].
3.5 Classification
We employ a support vector machine (SVM) classifier to differentiate between lesions and non-lesions. Because of its many benefits, this potent method has been extensively applied in computer vision during the past few years. For non-linear classification of noisy, high-dimensional data, it is practical. It is resistant to over fitting and outliers [17]. Additionally, it includes a stage for implicitly selecting features.
4 Simulation Results
The simulation results of the suggested work are shown in this section. In this work, various retinal photographs are gathered from databases that are accessible to the general public and used as input to identify retinal lesions in abnormal images. For both normal and pathological retinal pictures, this job is done.
4.1 Normal Images
4.2 Abnormal Images
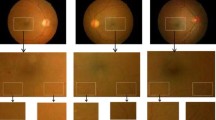
Figure 2 displays a typical retinal image that has been pre-processed to make it amenable for further processing using techniques like lighting, denoising, equalisation, and colour normalization. Any classification strategy must start with feature extraction. Feature extraction determines whether or not a pixel belongs to an actual blood artery by using a feature vector, which is a pixel representation in terms of some quantifiable measurements that can be conveniently employed in the classification stage. Figures 3 and 4 displays the optic disc removal image and candidate Extracted pictures respectively. In order to classify the input image and identify the lesions, an SVM classifier is employed in the last step.
Figure 5 shows a conversation box that says “Normal” if the image is normal; otherwise, Figs. 5, 6, 7, 8 and 9 shows a dialogue box that says “Abnormal” if the image is abnormal.
5 Conclusion
A brand-new technique for detecting red lesions was presented and tested on six different databases using a brand-new collection of shape features called the DSFs. The results show that the suggested method performs well in detecting both MAs and HEs in fundus images of various resolutions, qualities, and acquisition technologies. The technique performs better than several cutting-edge techniques, both for each lesion and for each individual image. DSFs have proven to have reliable characteristics with a high degree of lesion and vascular segment discrimination. DSFs could be used in different applications, especially when the items that need to be recognised lack distinct borders and are challenging to segment precisely.
References
Adali T, Anderson M, Fu G (2014) Diversity in independent component and vector analyses: identifiability, algorithms, and applications in medical imaging. IEEE Signal Process Mag 31(3):18–33
Akram M, Khalid S, Khan S (2013) Identification and classification of microaneurysms for early detection of diabetic retinopathy. Pattern Recogn 46(1):107–116
Gaddam DKR, Ansari MD, Vuppala S, Gunjan VK, Sati MM (2022) A performance comparison of optimization algorithms on a generated dataset. In: ICDSMLA 2020: Proceedings of the 2nd international conference on data science, machine learning and applications. Springer Singapore, pp 1407–1415
Khandelwal K, Chahar B, Chahar PS (2022) Problems and challenges of social entrepreneurship from an entrepreneur’s perception. In: Choudhury A, Singh TP, Biswas A, Anand M (eds) Evolution of digitized societies through advanced technologies. Advanced technologies and societal change. Springer, Singapore. https://doi.org/10.1007/978-981-19-2984-7_6
Ahmed M, Ansari MD, Singh N, Gunjan VK., Santhosh Krishna BV, Khan M (2022) Rating-based recommender system based on textual reviews using IOT smart devices. Mob Inf Syst
Chavali A, Biradar V (2022) Digitalization of education—challenges and measures for promotion. In: Choudhury A, Singh TP, Biswas A, Anand M (eds) Evolution of digitized societies through advanced technologies. Advanced technologies and societal change. Springer, Singapore. https://doi.org/10.1007/978-981-19-2984-7_4
Shaik F, Sharma AK, Ahmed SM (2016) Detection and analysis of diabetic myonecrosis using an improved hybrid image processing model. In: 2016 2nd International conference on advances in electrical, electronics, information, communication and bio-informatics (AEEICB), Chennai, India, 2016, pp 314–317. https://doi.org/10.1109/AEEICB.2016.7538298
Gaddam DKR, Ansari MD, Vuppala S, Gunjan VK, Sati MM (2022). Human facial emotion detection using deep learning. In: ICDSMLA 2020: proceedings of the 2nd international conference on data science, machine learning and applications. Springer Singapore, pp 1417–1427
Shaik F, Sharma AK, Ahmed SM (2016) Hybrid model for analysis of abnormalities in diabetic cardiomyopathy and diabetic retinopathy related images. Springerplus 23(5):507. https://doi.org/10.1186/s40064-016-2152-2.PMID:27186471;PMCID:PMC4842195
Sivayamini L, Venkatesh C, Fahimuddin S, Thanusha N, Shaheer S, Sree PS (2017) A novel optimization for detection of foot ulcers on infrared images. In: 2017 International conference on recent trends in electrical, electronics and computing technologies (ICRTEECT), Warangal, India, 2017, pp 41–43. https://doi.org/10.1109/ICRTEECT.2017.36
Gunjan VK, Shaik F, Kashyap A, Kumar A (2017) An interactive computer aided system for detection and analysis of pulmonary TB. Helix J 7(5):2129–2132. ISSN 2319–5592
Gunjan VK, Singh N, Shaik F, Roy S (2022) Detection of lung cancer in CT scans using grey wolf optimization algorithm and recurrent neural network. Heal Technol 12(6):1197–1210
Galimyanov AF, Garifyanov NF, Minnegalieva CB (2020) Testing of streaming data clustering algorithm effectiveness. Helix—The Sci Explorer | Peer Rev Bimonthly Int J 10(05):124–128
Tikhomirov DA, Rastimeshin SA, Trunov SS, Ukhanova VY, Kuzmichev AV (2020) Mathematical modelling and energy accounting of heaters for growing stock. Helix—The Sci Explorer | Peer Rev Bimonthly Int J 10(04):13–20
Merugu S, Kumar A, Ghinea G (2023) Hardware, component, description. In: Track and trace management system for dementia and intellectual disabilities. Advanced technologies and societal change. Springer, Singapore. https://doi.org/10.1007/978-981-19-1264-1_5
Gunjan VK, Vijayalata Y, Valli S, Kumar S, Mohamed MO, Saravanan V (2022) Machine learning and cloud-based knowledge graphs to recognize suicidal mental tendencies. Comput Intell Neurosci
Merugu S, Kumar A, Ghinea G (2023) A review of some assistive tools and their limitations. In: Track and trace management system for Dementia and intellectual disabilities. Advanced technologies and societal change. Springer, Singapore. https://doi.org/10.1007/978-981-19-1264-1_3
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Venkata Dasu, M., Chandrika, C., Vasanth Kumar, K.M., Sreelatha, D., Akhileswar Reddy, K. (2024). An Automatic Detection of Retinal Lesions for Screening of Diabetic Retinopathy. In: Kumar, A., Mozar, S. (eds) Proceedings of the 6th International Conference on Communications and Cyber Physical Engineering . ICCCE 2024. Lecture Notes in Electrical Engineering, vol 1096. Springer, Singapore. https://doi.org/10.1007/978-981-99-7137-4_68
Download citation
DOI: https://doi.org/10.1007/978-981-99-7137-4_68
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-7136-7
Online ISBN: 978-981-99-7137-4
eBook Packages: EngineeringEngineering (R0)