Abstract
The B cell lymphoma-2 (BCL-2) family of proteins plays a critical role in the intrinsic pathway of apoptosis. It is therefore not surprising that this pathway is frequently dysregulated in numerous malignancies, including acute myeloid leukemia (AML), in order to evade apoptosis. In the last 25 years, research into the pathobiology of AML has focused intensely on the antiapoptotic proteins, BCL-2, and myeloid cell leukemia-1 (MCL-1), whose overexpressions are associated with enhanced survival and chemoresistance of leukemic cells. In light of this, BCL-2 and MCL-1 have been attractive targets in the development of novel agents to treat AML. Many BCL-2 and MCL-1 inhibitors have yielded promising results in preclinical trials and are currently undergoing evaluation in clinical trials. Recently, venetoclax, a first-in-class selective oral BCL-2 inhibitor, was approved for upfront treatment of AML in the unfit or elderly population and had revolutionized the therapeutic landscape of AML. In this chapter, we will review the role of BCL-2 and MCL-1 in AML as well as the preclinical and clinical data supporting the use of BCL-2 and MCL-1 inhibitors in AML treatment. Furthermore, we will discuss the mechanisms of resistance to BCL-2 inhibitors and highlight ongoing clinical trials of combination therapies aimed at overcoming such resistance pathways.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Role of the BCL-2 Family of Proteins in Apoptosis
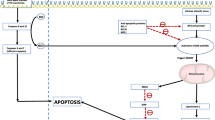
Programmed cell death, or apoptosis, is a tightly regulated process critical to the maintenance of cellular homeostasis through carefully orchestrated elimination of senescent and genetically aberrant cells [1]. It is governed by two interconnected pathways, the extrinsic pathway, which is activated by external signalling proteins such as TNF-α and FAS-L, and the intrinsic pathway, which is strictly regulated by the BCL-2 family of proteins [1, 2]. In the resting state, suppressor proteins (BCL-2, MCL-1, BCL-xL, and BCL-w) bind to effector (BAX and BAK) and activator proteins (tBID and BIM) and inhibit their activity, thereby preventing downstream apoptotic signalling. Conversely, in response to cellular stress signals, such as DNA damage from cytotoxic agents, the intrinsic apoptotic pathway is activated, leading to production of sensitizers and activators. Sensitizer proteins (e.g., PUMA, NOXA, and BAD) antagonize the actions of antiapoptotic BCL-2 proteins through interactions with their BCL-2 homology 3 (BH3) domains [3]. Thus, increased expression of sensitizers liberates activators and effectors from the inhibitory effects of antiapoptotic BCL-2 proteins. Consequently, activators bind to effectors and induce a conformational change, resulting in the creation of pores in the outer mitochondrial membrane. This causes mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome C from the intermembrane space into the cytoplasm. Cytochrome C complexes with Apaf-1 to form an apoptosome, which recruits and activates downstream caspases to initiate apoptosis (Fig. 10.1) [1, 3].
Role of BCL-2 in apoptosis. In the resting state, suppressor proteins (BCL-2, BCL-xL, BCL-W, and MCL-1) bind to and inhibit effectors and activators, thus preventing apoptosis. Cellular stressors activate the intrinsic pathway and induce the production of sensitizers (PUMA, NOXA, BAD) and activators (BIM, BID). Sensitizers inhibit suppressor proteins, thus freeing activators from their inhibitory effects. This allows activators to bind to and activate effectors (BAX, BAK), resulting in mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome C. This binds to Apaf-1 to form an heptameric complex that recruits caspase 9 to become an apoptosome. This, in turn, activates effector caspases 3 and 7 to induce apoptosis. Key BCL-2 family inhibitors (within purple boxes) and their respective targets are highlighted by the red brackets
10.2 Role of BCL-2 in AML
BCL-2 was first discovered as a partner of the immunoglobulin heavy chain in the translocation of chromosome 14 and 18, which is the oncogenic hallmark of follicular lymphoma [4]. However, its role in AML was not established until the 1990s, when a number of key studies confirmed that BCL-2 overexpression promotes leukemogenesis [5, 6], therapeutic resistance and poor responses to chemotherapy in AML [7], and leukemic stem cells (LSC) [8]. These findings led to the development of an array of BCL-2 inhibitors. The following section will expand on their development, efficacy, and usage in AML, with a focus on those in clinical use or undergoing clinical trial.
10.2.1 Oblimersen
Oblimersen was the first anti-BCL-2 agent explored in AML. It is an antisense oligonucleotide that targets human BCL2 mRNA and leads to decreased BCL-2 protein expression. In preclinical models, this was shown to increase apoptosis of leukemic cells [9, 10]. This finding led to a Phase 1 trial of oblimersen in combination with fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG) salvage chemotherapy in patients with relapsed and refractory AML or acute lymphoblastic leukemia (ALL) [11]. Overall, 20 patients were recruited, of which 17 had AML. Complete responses (CR) were observed in 5 of 17 (29%) AML patients, with two additional patients (12%) achieving CR with incomplete hematological responses (CRi). Another Phase 1 trial performed by the same group combined oblimersen with chemotherapy in previously untreated older patients with AML [12]. Fourteen of 29 patients (48%) achieved a CR, with a decrease in BCL2 mRNA copies observed in responders. The drug was considered tolerable compared to standard induction therapy.
Following on from these Phase 1 trials, the Cancer and Leukemia Group B (CALGB) performed a Phase 3 randomized controlled trial in treatment-naïve patients with AML who were older than 60 years of age [13]. Patients were treated with standard “3 + 7” (daunorubicin + infusional cytarabine) induction chemotherapy followed by high-dose cytarabine (HiDAC) consolidation with or without oblimersen. Unfortunately, there was no difference in CR rate (48% vs. 52%; p = 0.75) or overall survival (OS). Thus, no further trials of this drug were carried out in AML.
10.2.2 Obatoclax
Obatoclax was the first BH3-mimetic to enter clinical trials for AML. Preclinical models confirmed its ability to inhibit BCL-2 and related family members including BCL-xL, MCL-1, BCL-w, A1, and BCL-B [14]. In AML cell lines and primary AML samples, obatoclax was shown to induce apoptosis and impair leukemic proliferation [15]. In a Phase 1 trial of 44 patients with advanced hematological malignancies, including patients with refractory AML (57%), myelodysplastic syndrome (MDS) (32%), chronic lymphocytic leukaemia (CLL) (9%), and ALL (2%), obatoclax monotherapy was well tolerated but responses were modest given only one case of refractory AML achieved CR [16]. A follow-up Phase 1/2 study using obatoclax in older patients with untreated AML was performed based on the safety profile in the previous trial [17]. Nineteen patients were recruited with a median age of 81 years. None of the patients achieved a CR, and only four patients showed stable disease. Based on these disappointing responses, the drug was not further developed in AML.
10.2.3 ABT-737/ABT-263 (Navitoclax)
ABT-737 is another BH3-mimetic that inhibits BCL-2, BCL-xL, and BCL-w with high potency. Preclinical studies demonstrated that ABT-737 was effective in inducing apoptosis of AML cell lines and LSCs, inhibiting the growth of AML progenitor cells, and reducing leukemia burden in murine xenograft models of AML [18]. High MCL-1 expression was shown to confer resistance to ABT-737, with restoration of drug activity upon MCL-1 knockdown [18, 19]. Interestingly, this study also demonstrated that AML cells that were exquisitely sensitive to the pharmacological blockade of BCL-2 were those already “primed” for apoptosis. That is, effector proteins such as BAX were already assembled on the outer mitochondrial membrane, but were kept in check by inhibitory BCL-2 proteins. Thus, once the inhibitory effects of these proteins were neutralized by ABT-737, these cells were rendered exquisitely susceptible to apoptosis. BH3 profiling demonstrated widespread dependence on BCL-2 in the mitochondria of blasts from AML patient samples. Importantly, normal hematopoietic stem and progenitor cells were found to be less reliant on BCL-2, suggesting an exploitable therapeutic index for the inhibition of BCL-2 in AML. Despite these promising data, its lack of oral bioavailability and water insolubility hampered the translation of this drug into clinical practice. These limitations led to the development of ABT-263 (navitoclax). Navitoclax is an orally bioavailable BH3 mimetic with a similar spectrum of inhibitory activity as ABT-737 and induces apoptosis through disruption of interactions between BCL-2/BCL-xL and proapoptotic proteins [20]. As with ABT-737, navitoclax demonstrated preclinical efficacy in AML models [21,22,23,24]. However, no clinical trials with navitoclax have been undertaken in AML, in part, because it can potentially worsen thrombocytopenia through its ‘on-target’ inhibitory effects on BCL-xL which is required for platelet survival.
10.2.4 Venetoclax
Venetoclax is a BH3-mimetic that was engineered based on the structure of navitoclax. Critical modifications were made to enhance selectivity for BCL-2 while decreasing its affinity for BCL-xL and BCL-w, thus sparing platelets while maintaining antileukemic activity [25]. The efficacy of venetoclax was demonstrated in AML cell lines and in primary AML samples treated ex vivo and in murine xenograft models [26, 27]. Based on these preclinical findings and early safety data in CLL patients [28], venetoclax was swiftly transitioned to the clinical arena for treatment of AML.
10.2.4.1 Venetoclax Monotherapy in Relapsed/Refractory Patients
The first trial of venetoclax in AML was conducted in 32 patients with R/R AML or treatment-naive patients unfit for intensive chemotherapy [29]. In those with R/R AML, venetoclax monotherapy demonstrated only modest activity with a CR/CRi rate of 19% (6% CR and 13% CRi). However, this response was achieved rapidly, within 4 weeks in all but one patient, with a median duration of CR of 48 days. Moreover, those with IDH1 or IDH2 mutations achieved a higher CR/CRi rate of 33%. This is consistent with preclinical studies showing that IDH1/2 mutations, via the oncometabolite (R)-2-hydroxyglutarate, inhibit the activity of cytochrome C oxidase in the mitochondrial electron transport chain, which in turn lowers the mitochondrial threshold to trigger apoptosis upon BCL-2 inhibition [30]. Importantly, venetoclax was well tolerated, with the most common Grade 3/4 adverse events (AEs) being febrile neutropenia, hypokalemia, pneumonia, hypotension, and urinary tract infections, all of which would be expected in this cohort of patients [31, 32]. There were no reported episodes of tumor lysis syndrome.
10.2.4.2 Venetoclax + Hypomethylating Agents (HMA) in Treatment-Naïve Patients
Venetoclax was subsequently studied in combination with HMAs, either azacitidine or decitabine, in treatment-naïve elderly patients or those unfit for intensive chemotherapy. This was driven by preclinical studies demonstrating a synergistic effect between BH3-mimetics and HMAs in AML cell lines and AML patient samples [33]. Furthermore, azacitidine has been shown to reduce MCL-1 levels, an antiapoptotic protein not targeted by venetoclax, and thus, potential source of drug resistance. A phase Ib/II escalation and expansion study was conducted in 145 patients with untreated AML over the age of 65 years (median age 74 years). Venetoclax (400 mg once daily) in combination with either azacitidine or decitabine demonstrated remarkable CR/CRi rates of 71% and 74%, median duration of response of 21.2 and 15.0 months, and median OS of 16.9 and 16.2 months, for azacitidine and decitabine, respectively [34]. Efficacy was observed among all AML subgroups, including patients with secondary AML, those with adverse-risk cytogenetics, and across the genomic landscape of the disease [35].
These findings were confirmed by results of the Phase III VIALE-A trial published in 2020. In this trial, 433 patients (median age 76 years) underwent a 2:1 randomization to either azacitidine and venetoclax (400 mg once daily) (AZA + VEN) or azacitidine and placebo. Median OS was 14.7 months in the AZA + VEN group, compared with 9.6 months in the control group (p < 0.001). CR and CR/CRi rates were superior in the treatment arm at 36.7% versus 17.9% (p < 0.001) and 66.4% versus 28.3% (p < 0.001), respectively. Responses were both rapid and durable. Median time to first response was 1.0 month in the AZA + VEN group compared with 2.6 months in the control arm. Similarly, median duration of response was superior with AZA + VEN (17.5 vs. 13.4 months). Notably, the CR/CRi was improved across all AML genomic risk groups, including patients with adverse cytogenetic risk, secondary AML, and high-risk molecular mutations. These improvements in responses also translated into an increased OS in many of the evaluated subgroups, most notably among patients with either de novo or secondary AML, intermediate cytogenetic risk, and IDH1 or IDH2 mutations [36].
10.2.4.3 Venetoclax + Low Dose Cytarabine in Treatment-Naïve Patients
Venetoclax was also shown to be safe and effective in combination with low-dose cytarabine (LDAC) in upfront treatment of AML [37]. The rationale for this combination emerged from preclinical studies demonstrating venetoclax/LDAC synergy and reduced MCL-1 protein levels with combination therapy compared to venetoclax monotherapy [38, 39]. A Phase III, randomized, double-blinded trial (VIALE-C) examined this combination in 211 patients (median age 76 years) with treatment-naïve AML ineligible for intensive chemotherapy or ≥75 years of age, or both [40]. Subjects were randomized in a 2:1 fashion to receive either venetoclax 600 mg once daily or placebo plus LDAC (20 mg/m2 subcutaneously daily on days 1–10). Prior HMA exposure was permitted unlike the VIALE-A trial. In total, 38% had secondary AML, 20% had prior HMA treatment, and 32% had poor-risk cytogenetic features. Patients who received venetoclax and LDAC showed an improved composite CR rate of 48% compared with 13% in those who received LDAC alone. This translated to an improved median OS of 8.4 versus 4.1 months and median event-free survival (EFS) of 4.7 versus 2.0 months in the venetoclax + LDAC and control arms, respectively. Similar to the VIALE-A trial, responses were also achieved more rapidly with the addition of venetoclax, with CR/CRi before initiation of cycle 2 observed in 34% of patients in the venetoclax arm, compared with only 3% of patients in the control arm. Venetoclax + LDAC was also associated with a higher rate of red cell and platelet transfusion independence (37% vs. 16%), as well as superior patient-reported outcomes, especially regarding fatigue and quality of life. Again, subgroup analyses showed superior rates of composite CR in those treated with venetoclax + LDAC compared with those who received LDAC alone. In addition, survival outcome was particularly promising for subgroups with NPM1c (median OS not reached) and IDH1/2 mutations (median OS 19.4 months). Toxicities were primarily hematological, as expected, with febrile neutropenia, neutropenia, and thrombocytopenia representing the most common Grade ≥ 3 AEs. Although these were numerically higher in the venetoclax group, the rates of AEs leading to discontinuation (24% vs. 25%) and the rates of serious AEs such as pneumonia (13% vs. 10%) or sepsis (6% each arm) were nearly identical between the venetoclax and control arms, respectively.
In summary, these promising results have led to the FDA approval of venetoclax in combination with HMAs or LDAC as therapeutic options for treatment-naïve AML in elderly patients or those unfit for intensive chemotherapy.
10.2.4.4 Venetoclax + HMA/LDAC in Relapsed/Refractory Patients
Although it has not been directly tested in a clinical trial, there are a number of retrospective studies examining the role of venetoclax + HMAs or LDAC in R/R AML. In a series of 33 patients who received prior HMAs (61%) or allogeneic stem cell transplants (39%), the combination of venetoclax and either azacitidine or decitabine produced a CR/CRi rate of 33% [41]. In another series of 24 patients treated with the combination of venetoclax + HMA (n = 8) or venetoclax + LDAC (n = 16), the composite CR rate was 24% [42]. In yet another series of 43 patients with R/R myeloid neoplasms of which 91% had AML, the CR/CRi rate was a dismal 12%, with a median OS of only 3 months [43]. This is comparable to the 19% CR/CRi observed with single-agent venetoclax [29]. Hence, with the available data, the value of venetoclax as a salvage therapy, either alone or in combination with HMAs/LDAC, appears comparable with standard salvage regimens albeit with likely reduced toxicities [44].
10.3 Current Clinical Trials of Venetoclax in AML
10.3.1 Venetoclax + Intensive Chemotherapy
Trials are currently underway combining venetoclax with intensive chemotherapeutic regimens, including FLAG-IDA (fludarabine, cytarabine, filgrastim, idarubicin) (NCT03214562), “3 + 7” (NCT03709758), and CPX-351 (NCT03629171) (Table 10.1). Only preliminary data are available for the FLAG-IDA + venetoclax trial (FLAG-V-I) [45], which is recruiting fit patients with R/R AML over the age of 18. In an interim analysis of 11 patients, 8 patients (73%) achieved a CR/CRi. The safety profile was acceptable with no early mortality or severe AEs expected for such an intensive regimen. The median time to neutrophil recovery was 28 days, which is comparable to the recovery time for FLAG-IDA alone.
The efficacy of combining venetoclax with intensive chemotherapy in the elderly AML population has also been studied. In a phase Ib trial (CAVEAT) by Wei and colleagues [46], patients were treated with venetoclax and a modified cytarabine and idarubicin induction and consolidation regimen. Patients received 14 days of venetoclax with each cycle of chemotherapy, followed by 7 cycles of venetoclax monotherapy as maintenance. The overall CR/CRi rate was 71%, with an impressive 95% rate observed in de novo AML cases. The best responses were observed in patients with NPM1 (100%), RUNX1 (90%), IDH1/2 (89%), and RAS (90%) mutations, while those with TP53 (33%) mutations fared the worst. Remarkably, NPM1 MRD negativity was demonstrated in 83% of patients with NPM1 mutations. However, the question that remains is whether such high-intensity treatment is required, given the high responses observed by combining venetoclax with lower-intensity therapies, such as HMAs and LDAC.
10.3.2 Venetoclax + FLT3 Inhibitors
Venetoclax is also being explored in combination with FLT3 inhibitors. The rationale for this combination arises from preclinical models in which a synergistic effect was seen between BCL-2 inhibitor, ABT-737, and the FLT3 inhibitors, sunitinib and SU5614, in AML cell lines and primary AML blasts [47]. Ongoing trials include a Phase 1 study combining venetoclax with gilteritinib in R/R FLT3-mutated AML patients (NCT03625505) and a Phase 1/2 trial combining venetoclax with quizartinib in a similar cohort (NCT03735875).
In addition, a recent paper highlighted the synergistic effect of venetoclax combined with midostaurin or gilteritinib in vivo using a murine FLT3-ITD AML cell line-derived xenograft model [48]. Midostaurin and gilteritinib were shown to downregulate MCL-1 expression, which may, in part, explain the synergistic cytotoxicity observed. The combination of quizartinib and venetoclax has also been explored, with increased survival observed in a murine FLT3-ITD AML model [49]. The authors demonstrated reduced expression of MCL-1 and BCL-xL, but not BCL-2, in FLT3-ITD cell lines following treatment with quizartinib. In summary, the combination of venetoclax and FLT3 inhibitors is in early development, with preliminary safety data being awaited. Given that FLT3 mutations have been associated with an inferior response to HMA/LDAC + venetoclax combinations, it will be interesting to see if exchanging an HMA/LDAC for a FLT3 inhibitor will result in improved responses.
10.3.3 Venetoclax + IDH1/2 Inhibitors
IDH1- and IDH2-mutant primary AML cells are more sensitive to venetoclax inhibition compared with wild-type IDH1/2 cells due to the accumulation of 2-hydroxyglutarate [30], with durable responses and superior OS seen in IDH-mutated patients treated with venetoclax-based regimens [29, 36]. Preclinical studies using patient-derived xenograft AML models have demonstrated that concurrent therapy with enasidenib and venetoclax is superior to monotherapy in IDH2-mutated AML [50], with efficacy achieved through enasidenib-induced differentiation and venetoclax-mediated reduction in BCL-2. Building on these promising results, a phase Ib/II study of venetoclax in combination with enasidenib in IDH2-mutated AML (ENAVEN-AML; NCT04092179) is currently ongoing. Similarly, there is a separate phase Ib/II study investigating the combination of venetoclax and ivosidenib (with or without the incorporation of azacitidine) for patients with IDH1-mutated MDS and AML in both the R/R and treatment-naïve setting [51]. To date, 19 patients have been enrolled and interim results show an impressive composite CR (CR/CRi/CRh) of 78%, of which 50% achieved MRD negativity by flow cytometry.
10.3.4 Venetoclax + JAK Inhibitors (Ruxolitinib)
A preclinical study by Karjalainen et al. analyzed the ex vivo responses of primary AML blasts to various agents, including venetoclax and ruxolitinib, incubated in either bone marrow stroma-derived or standard culture conditions [52]. The authors demonstrated that bone marrow stroma-derived conditions protected AML blasts from the effects of BCL-2 inhibition, while this cytoprotection was reversed in the presence of ruxolitinib. Mechanistically, the bone marrow stroma appears to confer venetoclax resistance by reducing the BCL-2 dependency of primary AML cells through downregulation of BCL-2 expression, while increasing the expression of other antiapoptotic proteins such as BCL-xL and BCL-xS. The upstream effectors of this appear to be G-CSF and GM-CSF secreted from the stromal cells, which leads to increased phosphorylation of STAT5, and consequently, activation of JAKs. JAK inhibition with ruxolitinib, therefore, maintains BCL-2 dependency in AML blasts through suppression of the JAK-STAT pathway. Based on this preclinical work, a Phase I trial is currently exploring the effectiveness of this combination in R/R AML (NCT03874052).
10.3.5 Venetoclax + MCL-1 Inhibitors
Venetoclax is being trialled in combination with novel direct MCL-1 inhibitors, S64315 (NCT03672695) and AMG 176 (NCT03797261), in R/R AML patients. It is also being investigated in combination with indirect MCL-1 inhibitors, including the MEK inhibitor, cobimetinib (NCT02670044), and the cyclin-dependent kinase (CDK) inhibitors, dinaciclib (NCT03484520) and alvocidib (NCT03441555). Given its selectivity for BCL-2, an intrinsic mechanism of venetoclax resistance is due to increased AML blast dependency on other antiapoptotic proteins, such as MCL-1 and BCL-xL. In a Phase II trial of venetoclax monotherapy in R/R AML, increased BCL-xL and MCL-1 expression levels negatively correlated with response to venetoclax [29]. A number of novel therapies tested in preclinical models in combination with venetoclax have demonstrated synergistic effects by downregulating MCL-1 expression [21, 53,54,55]. Indeed, azacitidine has also been shown to reduce MCL-1 expression [56]. Hence, direct targeting of MCL-1 makes logical sense in combination with venetoclax in AML. As proof of concept, a recent study investigated the role of BCL-2 and MCL-1 in AML survival by combining inducible lentiviral vectors expressing BH3-only proteins [38]. Targeting BCL-2 and MCL-1 improved survival in a mouse xenograft model, whereas other combinations including BCL-2/BCL-xL/BCL-w or MCL-1 alone did not. Hence, combining venetoclax with an MCL-1 inhibitor is an exciting prospect for the treatment of patients with AML. Preliminary results from the Phase Ib trial combining cobimetinib and venetoclax showed overall responses of 18% in a heavily pretreated population, with gastrointestinal toxicity being the major adverse toxicity [57]. There are no preliminary results from the combination of the direct MCL-1 inhibitors, S64315 and AMG 176, and venetoclax, to date.
10.3.6 Venetoclax + MDM2 Inhibitors
Idasanutlin is a novel MDM2 inhibitor that is being tested in combination with venetoclax (NCT02670044). MDM2 is a negative regulator of wild-type p53 (WT-p53). In AML, TP53 mutations occur in only 7–8% of de novo cases, whereas inactivation of WT-p53 occurs in almost all subsets, making disruption of the MDM2 and WT-p53 interaction a promising target [58]. Preclinical models have shown that approximately two thirds of AML cell lines and primary AML blasts respond to MDM2 inhibition, with resistance observed in the TP53 mutant samples, as expected [59, 60]. The combination of venetoclax and MDM2 inhibitors has exhibited synergistic responses in vitro and in vivo models of AML [55, 61]. Preliminary results from the Phase Ib trial, which combines venetoclax and Idasanutlin, demonstrated a 38% overall response in the higher dose cohort, while no responses were seen in patients with theTP53 mutation [57].
10.4 Role of MCL-1 in AML
MCL-1 is an antiapoptotic protein that binds to the proapoptotic effectors, BAK and BAX, to prevent cell death. In AML cell lines, MCL-1 has been shown to play an important role in cell survival [62,63,64]. In the clinical setting, overexpression of MCL-1 in human leukemia cells has been demonstrated in nearly all bone marrow samples from patients with newly diagnosed AML [65]. This has been implicated in resistance to chemotherapy [66] and BH3-mimetics targeting BCL-2/BCL-xL [67, 68], as well as in the setting of relapsed AML [64]. Hence, targeting MCL-1 represents a promising, novel approach in the treatment of AML.
10.4.1 MCL-Inhibitors
Several Phase I clinical trials of MCL-1 inhibitors in AML are ongoing: AZD5991 [NCT03218683], S64315 [NCT02979366, NCT03672695], AMG 176 [NCT03797261, NCT02675452], and AMG 397 [NCT03465540]. Despite evidence of their efficacy in preclinical studies, the search for a safe, effective, and selective MCL-1 inhibitor has proven formidable for two reasons: (1) MCL-1 plays an important physiologic role in normal cells, including cardiac and hepatic tissues [69, 70], pluripotent stem cells [71], and brain cells [72]. Thus, it has been challenging to create an inhibitor with a sufficiently wide therapeutic index that does not cause unacceptable side effects; (2) The key binding site on MCL-1 is shallow and relatively inflexible compared with the binding sites on BCL-2 and BCL-xL. Thus, early MCL-1 inhibitors lacked specificity and were ineffective. Nonetheless, a number of selective MCL-1 inhibitors have been developed and are currently in various stages of clinical development [62, 63, 73, 74].
AMG 176 is a potent and selective MCL-1 inhibitor that has been shown to induce rapid and robust apoptosis in tumor xenografts after a single dose [75]. Similarly, it resulted in a dose-dependent reduction in tumor burden in an orthotopic model of AML in mice [73]. These data led to the initiation of two Phase I trials. The first trial examined the safety and tolerability of AMG 176 monotherapy in R/R multiple myeloma and AML (NCT02675452), while the second trial studied AMG 176 in combination with venetoclax in patients with R/R AML, non-Hodgkin lymphoma (NHL), or diffuse large B cell lymphoma [NCT03797261].
Similar to AMG 176, AMG 397 is an oral small-molecule inhibitor of MCL-1. It is the only oral MCL-1 inhibitor to reach the clinic thus far [76]. Preclinical data in the literature are sparse; however, clinical evaluation is underway. Unfortunately, the phase I dose-finding clinical studies involving AMG 176 (NCT02675452) and AMG 397 (NCT03465540) in patients with multiple myeloma, NHL, or AML are currently on hold for investigation of AEs related to cardiac toxicity [77]. The dose-finding combination trial of AMG 176 and venetoclax (NCT03797261) is also currently suspended based on this safety signal [78].
Another MCL-1 inhibitor, S64315, is also under clinical evaluation in a Phase I trial in patients with AML or MDS (NCT02979366). This non-randomized, non-comparative study aims to investigate the safety, tolerability, and incidence of dose-limiting toxicities of the drug. The study started in March 2017 and is estimated to finish in October 2020. Another study is also planned to assess S64315 in combination with venetoclax in patients with AML (NCT03672695) [79].
In addition to compounds that cause apoptosis through direct MCL-1 inhibition, there is an array of compounds that cause apoptosis, in part, through a reduction in MCL-1 cellular levels by reducing expression of MCL1 or by increasing protein degradation. Therefore, in addition to direct MCL-1 inhibition, disruption of key proteins involved in MCL-1 regulation may offer potential therapeutic targets for cancer treatment. Among these indirect MCL-1 inhibitors, CDK9 inhibitors have most recently entered the clinic. CDK9 is an enzyme critical for transcriptional activation of MCL-1. Dinaciclib, a new generation CDK9 inhibitor, has demonstrated efficacy in hematological malignancies [80,81,82] and is currently being studied in combination with venetoclax in R/R AML in a Phase I trial (NCT03484520).
In summary, therapies targeting MCL-1 could offer a novel treatment approach for patients with disease resistant to other therapies. MCL-1 inhibitors could potentially synergize with other targeted agents or conventional chemotherapeutic agents to enhance their antileukemic efficacy. However, it remains to be seen if this can be achieved without causing unacceptable levels of toxicities to normal tissues.
10.5 Resistance Mechanisms to BCL-2 Inhibitors
Although venetoclax-based regimens have become a powerful addition to the AML treatment armamentarium, drug resistance remains a veritable barrier to maintaining durable responses. Therefore, understanding the mechanisms that lead to BCL-2 resistance is crucial to the development of strategies in overcoming this problem. The following section will highlight the salient mechanisms underpinning resistance to BCL-2 inhibition.
10.5.1 Increased Expression of MCL-1
The best described mechanism of venetoclax resistance is through increased expression of antiapoptotic proteins other than BCL-2, most notably, MCL-1. This has given rise to numerous clinical trials investigating the effect of direct and indirect MCL-1 inhibition on overcoming resistance to BCL-2 inhibition. As direct MCL-1 inhibitors have been discussed previously, this section will elaborate on the role of indirect MCL-1 inhibitors.
Indirect MCL-1 inhibitors comprise a large group of agents with a diverse range of mechanisms. The vast majority of these are still being evaluated in preclinical studies. These include: MEK1/2 inhibitors, which subvert the MAPK pathway that stabilizes MCL-1. These have been shown to synergistically enhance the proapoptotic effects of venetoclax in AML cell lines and reduce leukemia burden in AML xenograft models through increased levels of BIM [83]. Similarly, bromodomain extra-terminal protein inhibitors (BETi) reduce MCL-1 and BCL-xL levels while increasing BIM levels. They also synergize with venetoclax to induce apoptosis in AML cell lines, reduce leukemia burden, and improve survival in AML-engrafted mice [84]. Other indirect MCL-1 inhibitors include: CDK9 inhibitors, which impair the transcription of MCL-1 [85]; FLT3 inhibitors, which downregulate MCL-1 to increase venetoclax activity [48]; CUDC-907, a dual PI3K and histone deacetylase inhibitor that downregulates MCL-1 while upregulating BIM to cause apoptosis [86]; MDM2 inhibitors, which restore TP53 activation and downregulation of MCL-1 through inhibition of the MAPK pathway [87]; PI3K inhibitors, which induce BAX-dependent mitochondrial apoptosis in AML cells when coadministered with venetoclax [88]; selinexor, an XPO1-selective inhibitor [89]; inhibitors of the Nedd8-activating enzyme and 3-hydroxy-3-methylglutaryl coenzyme A reductase, which lead to upregulation of NOXA and PUMA, respectively, resulting in neutralization of MCL-1 and increased activity of venetoclax [21, 90]; ibrutinib, a Bruton tyrosine kinase inhibitor, and ArQule 531, a multi-kinase inhibitor of SRC family kinases, have also been shown to synergize with venetoclax and overcome BCL-2 inhibition through MCL-1 inhibition [91, 92].
10.5.2 Dysregulation of Mitochondrial Energy Metabolism
One mechanism by which venetoclax kills AML cells is through inhibition of mitochondrial respiration. Thus, disruption of mitochondrial energy metabolism is implicated in the resistance of AML to venetoclax. Using a genome-wide CRISPR knockout screen, Sharon et al. [93] found that inactivation of genes involved in mitochondrial protein synthesis restored sensitivity of resistant AML cells to venetoclax. Pharmacologic inhibition of mitochondrial protein synthesis with antibiotics that target the ribosome, including tedizolid and doxycycline, can enhance the anti-AML effect of venetoclax and azacitidine in vivo and in vitro, thus potently reversing venetoclax resistance [93, 94]. In leukemic stem cells, mutated TP53 disrupted mitochondrial homeostasis by dysregulating activation of transcription factor, DP-1, and translocation of PMAIP1 into the mitochondria, hence impairing the effector function of BAX and BAK [95]. Moreover, TP53 mutation also impedes BCL-2 expression, thus directly decreasing the target of venetoclax and leading to drug resistance [95].
10.5.3 Disruption of Mitochondrial Architecture
The mitochondrial architecture plays an important role in apoptosis. Its organization and function are maintained by various proteins, including the mitochondrial chaperone, CLPB, whose function is to maintain mitochondrial cristae structure through interaction with the cristae-shaping protein, OPA1 [96]. When this interaction is disrupted, the structural integrity of the mitochondria is damaged, leading to stress responses and induction of apoptosis. Preclinical studies by Chen et al. demonstrated that CLPB is upregulated in human AML cells and its expression is induced upon acquisition of venetoclax resistance. Using a genome-wide CRISPR screen, they found that inactivation of this gene sensitized AML cells to venetoclax, and thus to apoptosis [96]. Thus, targeting the mitochondrial structure represents another novel approach of disarming venetoclax resistance.
10.6 Conclusion
Upregulation of antiapoptotic proteins in the BCL-2 family as a means of evading apoptosis is a key mechanism of treatment resistance and disease relapse in AML. Therefore, targeted inhibition of these proteins, especially BCL-2 and MCL-1, represents a compelling therapeutic approach in the management of AML. While this strategy has shown promising antileukemic activity in preclinical studies, only venetoclax has demonstrated efficacy in the clinical setting and is approved for use in AML. As confirmed by two recent Phase III trials, venetoclax in combination with a HMA or LDAC improves OS in treatment-naïve elderly patients, or patients unfit for intensive chemotherapy. Unfortunately, MCL-1 inhibitors have not yet generated the same success in clinical trials, in part due to their on-target but off-tissue toxicities.
While BCL-2 inhibition with venetoclax has revolutionized the therapeutic landscape in a cohort of AML patients who would otherwise have limited treatment options, there are a number of obstacles that remain. Overcoming resistance to BCL-2 inhibitors will be crucial in prolonging responses, and therefore, long-term survival. Although the mechanisms of resistance are being characterized in preclinical studies, the primary mechanisms of resistance in vivo remain unclear. Current research is focusing on combination strategies and appear promising. However, determining which drug combinations will provide optimal clinical efficacy and in which clinical setting will be an important goal moving forward.
The role of venetoclax and other BH3 mimetics in standard induction and consolidation therapy in younger patients or the fit elderly is also of great interest. The use of venetoclax combination therapies could replace “3 + 7” as the new standard induction regimen for all AML patients. Furthermore, there are ongoing clinical trials looking at the use of venetoclax and HMAs as maintenance therapy after consolidation chemotherapy or allogeneic stem cell transplant (Table 10.1). Determining the subgroups of patients who are most likely to benefit from BH3 mimetics is also of vital pertinence and requires further investigation.
In summary, it is currently an exciting time to be treating AML, especially with the approval of venetoclax, which represents a promising and significant advance in targeted treatment approaches in AML. Targeting other antiapoptotic proteins, such as MCL-1 and BCL-xL, in combination with venetoclax, is also an exciting prospect, and if successful, will be key in overcoming resistance to BCL-2 inhibition.
References
Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63.
Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811.
Kollek M, Müller A, Egle A, Erlacher M. Bcl-2 proteins in development, health, and disease of the hematopoietic system. FEBS J. 2016;283(15):2779–810.
Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440–2.
Russell NH, Hunter AE, Bradbury D, Zhu YM, Keith F. Biological features of leukaemic cells associated with autonomous growth and reduced survival in acute myeloblastic leukaemia. Leuk Lymphoma. 1995;16(3–4):223–9.
Delia D, Aiello A, Soligo D, Fontanella E, Melani C, Pezzella F, et al. Bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992;79(5):1291–8.
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091–6.
Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–41.
Campos L, Sabido O, Rouault J, Guyotat D. Effects of BCL-2 antisense oligodeoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood. 1994;84(2):595–600.
Cotter FE, Johnson P, Hall P, Pocock C, Al Mahdi N, Cowell JK, et al. Antisense oligonucleotides suppress B-cell lymphoma growth in a SCID-hu mouse model. Oncogene. 1994;9(10):3049–55.
Marcucci G, Byrd JC, Dai G, Klisovic MI, Kourlas PJ, Young DC, et al. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 2003;101(2):425–32.
Marcucci G, Stock W, Dai G, Klisovic RB, Liu S, Klisovic MI, et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol. 2005;23(15):3404–11.
Marcucci G, Moser B, Blum W, Stock W, Wetzler M, Kolitz JE, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. J Clin Oncol. 2007;25(18_Suppl):7012.
Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci. 2007;104(49):19512–7.
Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology Domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 2008;68(9):3413–20.
Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(24):8295–301.
Schimmer AD, Raza A, Carter TH, Claxton D, Erba H, DeAngelo DJ, et al. A multicenter Phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS One. 2014;9(10):e108694.
Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88.
van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–99.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–8.
Knorr KLB, Schneider PA, Meng XW, Dai H, Smith BD, Hess AD, et al. MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ. 2015;22(12):2133–42.
Airiau K, Prouzet-Mauléon V, Rousseau B, Pigneux A, Jeanneteau M, Giraudon M, et al. Synergistic cooperation between ABT-263 and MEK1/2 inhibitor: effect on apoptosis and proliferation of acute myeloid leukemia cells. Oncotarget. 2015;7(1):845–59.
Kontro M, Kumar A, Majumder MM, Eldfors S, Parsons A, Pemovska T, et al. HOX gene expression predicts response to BCL-2 inhibition in acute myeloid leukemia. Leukemia. 2017;31(2):301–9.
Kivioja JL, Thanasopoulou A, Kumar A, Kontro M, Yadav B, Majumder MM, et al. Dasatinib and navitoclax act synergistically to target NUP98-NSD1+/FLT3-ITD+ acute myeloid leukemia. Leukemia. 2019;33(6):1360–72.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8.
Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–75.
Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279):279ra40.
Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–96.
Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–17.
Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong W-J, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21(2):178–84.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5.
Pinto A, Zagonel V, Ferrara F. Acute myeloid leukemia in the elderly: biology and therapeutic strategies. Crit Rev Oncol Hematol. 2001;39(3):275–87.
Bogenberger JM, Kornblau SM, Pierceall WE, Lena R, Chow D, Shi CX, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28(8):1657–65.
Pollyea DA, Pratz KW, Jonas BA, Letai A, Pullarkat VA, Wei A, et al. Venetoclax in combination with hypomethylating agents induces rapid, deep, and durable responses in patients with AML ineligible for intensive therapy. Blood. 2018;132(Suppl 1):285.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–29.
Wei AH, Strickland SA Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–84.
Teh TC, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32(2):303–12.
Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of released Bim to mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res. 2016;22(17):4440–51.
Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–45.
Ibrahim A, Dongyun Y, Ahmed A, Haris A, Karamjeet S, Monzr MAM, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404–e7.
Goldberg AD, Horvat TZ, Hsu M, Devlin SM, Cuello BM, Daley RJ, et al. Venetoclax combined with either a hypomethylating agent or low-dose cytarabine shows activity in relapsed and refractory myeloid malignancies. Blood. 2017;130(Suppl 1):1353.
DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401–7.
Kantarjian HM, DiNardo CD, Nogueras-Gonzalez GM, Kadia TM, Jabbour E, Bueso-Ramos CE, et al. Results of second salvage therapy in 673 adults with acute myelogenous leukemia treated at a single institution since 2000. Cancer. 2018;124(12):2534–40.
DiNardo CD, Albitar M, Kadia TM, Naqvi K, Vaughan K, Cavazos A, et al. Venetoclax in combination with FLAG-IDA chemotherapy (FLAG-V-I) for fit, relapsed/refractory AML patients: interim results of a phase 1b/2 dose escalation and expansion study. Blood. 2018;132(Suppl 1):4048.
Wei AH, Chua CC, Tiong IS, Fong CY, Ting SB, Macraild S, et al. Molecular patterns of response and outcome in the chemotherapy and venetoclax in elderly AML trial (CAVEAT study). Blood. 2018;132(Suppl 1):333.
Kohl TM, Hellinger C, Ahmed F, Buske C, Hiddemann W, Bohlander SK, et al. BH3 mimetic ABT-737 neutralizes resistance to FLT3 inhibitor treatment mediated by FLT3-independent expression of BCL2 in primary AML blasts. Leukemia. 2007;21(8):1763–72.
Ma J, Zhao S, Qiao X, Knight T, Edwards H, Polin L, et al. Inhibition of Bcl-2 synergistically enhances the antileukemic activity of midostaurin and gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin Cancer Res. 2019;25(22):6815–26.
Mali R, Lasater EA, Doyle K, Malla R, Boghaert E, Souers A, et al. Abstract B052: FLT3-ITD activation mediates resistance to the BCL-2 selective antagonist, venetoclax, in FLT3-ITD mutant AML models. Mol Cancer Therap. 2018;17(1 Suppl):B052.
Cathelin S, Sharon D, Subedi A, Cojocari D, Phillips DC, Leverson JD, et al. Combination of enasidenib and venetoclax shows superior anti-leukemic activity against IDH2 mutated AML in patient-derived xenograft models. Blood. 2018;132(Suppl 1):562.
Lachowiez CA, Borthakur G, Loghavi S, Zeng Z, Kadia TM, Masarova L, et al. Phase Ib/II study of the IDH1-mutant inhibitor ivosidenib with the BCL2 inhibitor venetoclax +/− azacitidine in IDH1-mutated hematologic malignancies. J Clin Oncol. 2020;38(15_Suppl):7500.
Karjalainen R, Pemovska T, Popa M, Liu M, Javarappa KK, Majumder MM, et al. JAK1/2 and BCL2 inhibitors synergize to counteract bone marrow stromal cell–induced protection of AML. Blood. 2017;130(6):789–802.
Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26(4):778–87.
Mohamed R, Mandy Mayo A, Elisa H, Rebecca EP, Masey R, Maciej K, et al. Co-administration of the mTORC1/TORC2 inhibitor INK128 and the Bcl-2/Bcl-xL antagonist ABT-737 kills human myeloid leukemia cells through mcl-1 down-regulation and AKT inactivation. Haematologica. 2015;100(12):1553–63.
Lehmann C, Friess T, Birzele F, Kiialainen A, Dangl M. Superior anti-tumor activity of the MDM2 antagonist idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type acute myeloid leukemia models. J Hematol Oncol. 2016;9(1):50.
Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91(12):1861–70.
Daver N, Pollyea DA, Yee KWL, Fenaux P, Brandwein JM, Vey N, et al. Preliminary results from a phase Ib study evaluating BCL-2 inhibitor venetoclax in combination with MEK inhibitor cobimetinib or MDM2 inhibitor idasanutlin in patients with relapsed or refractory (R/R) AML. Blood. 2017;130(Suppl 1):813.
Marcucci G, Haferlach T, Döhner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29(5):475–86.
Long J, Parkin B, Ouillette P, Bixby D, Shedden K, Erba H, et al. Multiple distinct molecular mechanisms influence sensitivity and resistance to MDM2 inhibitors in adult acute myelogenous leukemia. Blood. 2010;116(1):71–80.
Weisberg E, Halilovic E, Cooke VG, Nonami A, Ren T, Sanda T, et al. Inhibition of wild-type p53-expressing AML by the novel small molecule HDM2 inhibitor CGM097. Mol Cancer Ther. 2015;14(10):2249–59.
Saiki AY, Caenepeel S, Yu D, Lofgren JA, Osgood T, Robertson R, et al. MDM2 antagonists synergize broadly and robustly with compounds targeting fundamental oncogenic signaling pathways. Oncotarget. 2014;5(8):2030–43.
Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, et al. Discovery of mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9(1):1–14.
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–82.
Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26(2):120–5.
Zhang Z, Liu Y, Song T, Xue Z, Shen X, Liang F, et al. An antiapoptotic Bcl-2 family protein index predicts the response of leukaemic cells to the pan-Bcl-2 inhibitor S1. Br J Cancer. 2013;108(9):1870–8.
Michels J, Obrist F, Vitale I, Lissa D, Garcia P, Behnam-Motlagh P, et al. MCL-1 dependency of cisplatin-resistant cancer cells. Biochem Pharmacol. 2014;92(1):55–61.
Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471(7336):110–4.
Williams MM, Lee L, Hicks DJ, Joly MM, Elion D, Rahman B, et al. Key survival factor, mcl-1, correlates with sensitivity to combined Bcl-2/Bcl-xL blockade. Mol Cancer Res. 2017;15(3):259–68.
Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013;27(12):1365–77.
Hikita H, Takehara T, Shimizu S, Kodama T, Li W, Miyagi T, et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50(4):1217–26.
Rasmussen ML, Kline LA, Park KP, Ortolano NA, Romero-Morales AI, Anthony CC, et al. A non-apoptotic function of MCL-1 in promoting Pluripotency and modulating mitochondrial dynamics in stem cells. Stem Cell Rep. 2018;10(3):684–92.
Hasan SMM, Sheen AD, Power AM, Langevin LM, Xiong J, Furlong M, et al. Mcl1 regulates the terminal mitosis of neural precursor cells in the mammalian brain through p27Kip1. Development. 2013;140(15):3118–27.
Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui D, et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 2018;8(12):1582–97.
Hird AW, Secrist JP, Adam A, Belmonte MA, Gangl E, Gibbons F, et al. Abstract DDT01–02: AZD5991: a potent and selective macrocyclic inhibitor of mcl-1 for treatment of hematologic cancers. Washington, DC: AACR; 2017.
Caenepeel SR, Belmontes B, Sun J, Coxon A, Moody G, Hughes PE. Preclinical evaluation of AMG 176, a novel, potent and selective mcl-1 inhibitor with robust anti-tumor activity in mcl-1 dependent cancer models. Washington, DC: AACR; 2017.
Hird AW, Tron AE. Recent advances in the development of mcl-1 inhibitors for cancer therapy. Pharmacol Ther. 2019;198:59–67.
Amgen. Amgen Highlights New Data from Kyprolis (carfilzomib) and Oncology Pipeline at IMW 2019. 2019. https://www.amgen.com/media/news-releases/2019/09/amgen-highlights-new-data-from-kyprolis-carfilzomib-and-oncology-pipeline-at-imw-2019/.
A study of venetoclax and AMG 176 in patients with relapsed/refractory hematologic malignancies. https://clinicaltrials.gov/ct2/show/NCT03797261.
Phase I Study of S64315 administered intravenously in patients with acute myeloid leukaemia or myelodysplastic syndrome. https://clinicaltrials.gov/ct2/show/NCT02979366.
Flynn J, Jones J, Johnson AJ, Andritsos L, Maddocks K, Jaglowski S, et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2015;29(7):1524–9.
Gojo I, Sadowska M, Walker A, Feldman EJ, Iyer SP, Baer MR, et al. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol. 2013;72(4):897–908.
Kumar SK, LaPlant B, Chng WJ, Zonder J, Callander N, Fonseca R, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015;125(3):443–8.
Han L, Zhang Q, Dail M, Shi C, Cavazos A, Ruvolo VR, et al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica. 2020;105(3):697–707.
Fiskus W, Cai T, DiNardo CD, Kornblau SM, Borthakur G, Kadia TM, et al. Superior efficacy of cotreatment with BET protein inhibitor and BCL2 or MCL1 inhibitor against AML blast progenitor cells. Blood Cancer J. 2019;9(2):4.
Cidado J, Boiko S, Proia T, Ferguson D, Criscione SW, San Martin M, et al. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin Cancer Res. 2020;26(4):922–34.
Xinyu L, Yongwei S, Katie H, Gerard M, Holly E, Tristan K, et al. The HDAC and PI3K dual inhibitor CUDC-907 synergistically enhances the antileukemic activity of venetoclax in preclinical models of acute myeloid leukemia. Haematologica. 2021;106(5):1262–77.
Pan R, Ruvolo V, Mu H, Leverson JD, Nichols G, Reed JC, et al. Synthetic lethality of combined Bcl-2 inhibition and p53 activation in AML: mechanisms and superior antileukemic efficacy. Cancer Cell. 2017;32(6):748–60.e6.
Rahmani M, Nkwocha J, Hawkins E, Pei X, Parker RE, Kmieciak M, et al. Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML cells. Cancer Res. 2018;78(11):3075–86.
Luedtke DA, Su Y, Liu S, Edwards H, Wang Y, Lin H, et al. Inhibition of XPO1 enhances cell death induced by ABT-199 in acute myeloid leukaemia via mcl-1. J Cell Mol Med. 2018;22(12):6099–111.
Lee JS, Roberts A, Juarez D, Vo TT, Bhatt S, Herzog LO, et al. Statins enhance efficacy of venetoclax in blood cancers. Sci Transl Med. 2018;10(445):eaaq1240.
Eide CA, Kurtz SE, Kaempf A, Long N, Agarwal A, Tognon CE, et al. Simultaneous kinase inhibition with ibrutinib and BCL2 inhibition with venetoclax offers a therapeutic strategy for acute myeloid leukemia. Leukemia. 2020;34(9):2342–53.
Elgamal OA, Mehmood A, Jeon JY, Carmichael B, Lehman A, Orwick SJ, et al. Preclinical efficacy for a novel tyrosine kinase inhibitor, ArQule 531 against acute myeloid leukemia. J Hematol Oncol. 2020;13(1):8.
Sharon D, Cathelin S, Mirali S, Di Trani JM, Yanofsky DJ, Keon KA, et al. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci Transl Med. 2019;11(516):eaax2863.
Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859–66.
Nechiporuk T, Kurtz SE, Nikolova O, Liu T, Jones CL, D’Alessandro A, et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 2019;9(7):910–25.
Chen X, Glytsou C, Zhou H, Narang S, Reyna DE, Lopez A, et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 2019;9(7):890–909.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tang, K., Chan, S.M. (2023). The Role of BCL-2/MCL-1 Targeting in Acute Myeloid Leukemia. In: Gill, H., Kwong, YL. (eds) Pathogenesis and Treatment of Leukemia. Springer, Singapore. https://doi.org/10.1007/978-981-99-3810-0_10
Download citation
DOI: https://doi.org/10.1007/978-981-99-3810-0_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3809-4
Online ISBN: 978-981-99-3810-0
eBook Packages: MedicineMedicine (R0)