Abstract
Cosmetics and personal care products induce skin sensitization, and allergies are very common skin-related issues in people worldwide. Therefore, dermal safety assessment is a mandatory requirement before marketing these products. After the ban on animal testing for cosmetics, the safety assessment of these products is now very challenging. Integrated approaches to testing and assessment are the best option and can be used for the prediction of the toxicity or safety of chemicals based on the integration of multiple pieces of information generated via non-testing and testing methods for regulatory purposes. Moreover, along with traditional tools (in vivo and in vitro), IATA is widely including high-throughput screening, and computational approaches as new approach methods (NAM) for data generation, interpretation, and integration. Identifying the AOP for skin allergy also aids in the development of IATA for regulatory decision-making. This chapter describes the role of IATA in the safety and toxicity prediction of cosmetics and personal care products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
10.1.1 Cosmetics and Personal Care Products
Personal care products and cosmetics use are very common everywhere in the globe. It has a long list of chemicals such as soaps, hair dyes, emulsifiers, fragrances, ultraviolet absorbers, and preservatives (Hamilton and de Gannes 2011). Cosmetics and personal care products contain various ingredients such as colorants, fragrances, and preservatives to make them attractive and safe for consumers. However, these ingredients are a major cause of skin sensitization and skin allergy (Tan et al. 2014). Generally, dermal and ocular safety testing of cosmetics and personal care products is sufficient for commercial use. Therefore, before getting regulatory approval skin irritation, corrosion, phototoxicity, skin sensitization, eye irritation, corrosion, and skin absorption testing are compulsory (Fig. 10.1). If any chemical has deep penetration and is reaching systemic circulation, then systemic toxicity evaluation is important to avoid any organ-specific toxicity.
Carcinogenicity, genotoxicity, developmental toxicity, reproductive toxicity, and endocrine disruption testing are necessary to prevent systemic toxicity of cosmetics and personal care products and their constituents (Fig. 10.2).
The animal-based study was the gold standard for skin sensitization assessment of cosmetics and personal care products. However, after the ban on animal use for cosmetics and personal care product safety in 2013 by European Union and in 2014 by the Indian government, it was very difficult to assess the safety of these products. To overcome this, different alternative models were developed and approved by OECD to assess the skin sensitization/skin allergy of these products. The individual method was not sufficient to draw any regulatory decision. Therefore, integrated approaches are most appropriate in testing and safety assessment of cosmetics and personal care products.
10.1.1.1 IATA
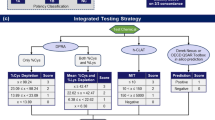
IATA is used to combine various existing statistics and properties including physical and chemical properties, non-testing (QSAR), and testing methods (in vivo and in vitro and in chemico) based on information for regulatory decision (Browne et al. 2017, 2020; Sakuratani et al. 2018). Chemical regulation authorities are confronted due to intensive testing approaches including expensive, utilization of a large number of chemicals, time-consuming, and use of live animals to evaluate all chemicals in the development of personal healthcare products (Tollefsen et al. 2014). The need for robust and effective strategies for the evaluation of threats was imposed by the chemicals in humans via different routes including dermal, inhalation, and systemic exposure (Abd et al. 2016; Wills et al. 2016; Shah et al. 2018; Conway et al. 2020). Though the number of methods are existing for safety assessment, the single method for the prediction of toxicity/safety of chemical is not sufficient for regulatory decision (Pfuhler et al. 2020, 2021; Tollefsen et al. 2014). Therefore, considering the need for a combined approach via the use of IATA is more reliable for the prediction of toxicity of any chemical. Additionally, to evaluate proper risk assessment, there are progressive approaches to substituting methods for animal use in toxicology and refining to incorporate new approach method (NAM) (Brannen et al. 2016; OECD 2016a, b, 2017). Applying NAM as a solution to toxicological endpoints is included in IATA, e.g., defined approaches for testing and assessment and integrated testing strategies (Casati 2018; Eskes 2019). With acceleration, the artificial intelligence is imparting to incorporate and mix various streams (Fig. 10.3).
10.1.1.2 Non-Testing Methods
In silico approaches (QSAR, read across) are used to assess the safety/toxicity of cosmetics and personal care products.
10.1.1.3 Testing Methods
There are a variety of testing methods that can serve as important components of an IATA, including in vivo, in vitro, and in chemo experiments. IATA needs to move away from relying on in vivo/animal-based data and test components such as toxicogenomic and high-content/high-throughput screening (HC/HT) to address one or more adverse outcomes.
10.2 New Approach Methods
Various alternative methods such as omics technology (genomics, proteomics, and metabolomics), in silico models, and other advanced biotechnological and computational models can be considered as new approach methods. These new approach methods support the IATA in the exact prediction of the toxicity of chemicals.
10.2.1 IATA in Skin Sensitization
Skin sensitization has been a regulatory endpoint, needed in various chemical sectors like industrial chemicals, cosmetics, and pesticides, being the center of concerted efforts for replacing animal testing over the years (Henning et al. 2009; Casati 2018 ; de Ávila et al. 2019; Kandarova and Hayden 2021). Various guinea–pig assay was migrated to reduced and distinguished LLNA, to demonstrate the dominance of in vitro and in silico methods (Basketter 2016). EURL organization for alternatives to animal models for testing has implemented strategies for skin sensitization. It has been of key importance in the assurance of the translation of the customary non-animal methods for skin sensitization into the internationally approved test guidelines (European Parliament and the Council of the European Union 2009; Rauscher et al. 2012; Chapman 2015). Consequently, from 2015 to 2017, in vitro and in chemico models and mechanisms are approved by the OECD under the first three crucial events of adverse outcome pathway for skin sensitization (Kandárová et al. 2006; Hecker et al. 2011; OECD 2022a, b). The current test methods cannot fulfill all regulatory requirements regarding skin sensitization potential and chemical potency in comparison with those that are provided by regulatory animal tests. LLNA (OECD TG 429) (OECD 2010a, b) or non-radioactive variants, like LLNA: BrdU-ELISA (OECD TG 442B) (OECD 2018), and LLNA: DA (OECD TG 442A). Considering this reason, data from the direct peptide reactivity assay, the ARE-Nrf2 luciferase assay, and the three protocols of dendritic cell activation (h-CLAT, interleukin-8, and Luc assay) must be considered for IATA. Moreover, combining other relevant information like physical–chemical properties, facts for other important measures of skin sensitization adverse outcome pathways along with non-testing methods, which includes the read across from chemical analogs (OECD 2022a, b).
In recent years, several defined approaches integrating information and facts from many non-animal methods and other information are developed for skin sensitization hazard assessment, or/and potency categorization. EURL ECVAM gave a project proposal to OECD to develop guidance for harmonized reporting of the defined approaches facilitating their application and assessment in IATA for regulatory purposes, on behalf of the European Commission (Hartung et al. 2004, 2013; Kinsner-Ovaskainen et al. 2009). OECD task force on hazard assessment reported in OECD guidance documents (GD-255 and GD 256, OECD 2016a, b, c) describes defined approaches. This provides a consistent format for describing a defined approach for regulatory purpose (OECD 1997, 2015, 2016a, b).
10.2.2 IATA Methodologies
The discrepancy between IATA and the defined approach is a key concept from the OECD. IATA is known as approaches, which are used in the prediction of risk or chemical hazard assessment by integrating the existing information and new information generated by the testing strategies. IATA is an iterative approach for answering a question in a defined regulatory context. The total evaluation process within the IATA mainly depends on weight of evidence, essentially implying an expert judgment for evaluating different pieces of information (OECD 2019, 2020, 2021a, b).
The intended design of IATA renders adaptability for particular regional requirements or regulatory statutes. The defined approaches can be considered a comparable alternative for the in vivo data if a similar kind of information is considered within the decision context of the IATA. As per the EURL ECVAM workshop, collaborated with ICATM, it was aimed to enrich the regulatory considered and adopted individual test methods, with the acceptance of defined approaches (although recognized for requirement differences in several sectors and jurisdictions) as an alternative for non-animal methodologies to skin sensitization evaluation by the chemicals used in several areas (Blaauboer et al. 1999; Clothier et al. 1999; Spielmann et al. 2006; Daniel et al. 2018; Strickland et al. 2019). This followed a consensus to maximize regulatory acceptance of data in defined approaches, for which international harmonization and standardization were necessary. This could be accomplished by developing an evaluation outline that allows an independent assessment of the defined approaches, wherein the reproducibility, relevance/predictive capacity, providing sufficient and equivalent information, mechanistically and biologically relevant, transparently described comparable to the reference animal test. Moreover, independent assessment by third parties and conflicting results and uncertainty in vivo data and defined approaches should also be considered, with predictions in the context of IATA (OECD 2016a, b).
10.3 International Cooperation in the Development of Alternative Test Methods
ICATM was promoting the use of DAs in the field of skin sensitization, and a framework was (Hoffmann 2015; Dumont et al. 2016; Roberts et al. 2016) defined based on the aforementioned criteria. Recent publications that evaluated the variability in the recorded animal datasets could be added to a meta-analysis (Kleinstreuer et al. 2018). Furthermore, the human data will be assembled and LLNA enactment against human data will be matched based on chemical data and variables (Basketter et al. 2014; Bell et al. 2017). The decision of definitions and a clear understanding of defined approaches and IATA were made to create information that brought all who were involved in the related area to a similar understanding. Many stakeholders were identified as important for progress in the field of DAs for skin sensitization assessment. Several scientists from research institutions, industry, and NGOs experienced with in vitro and in silico methods were also noted to form the building blocks of DAs. The US EPA, and other partners, has initiated many data-sharing pilots for encouraging industry stakeholders and other testing laboratories for easy access to non-animal methods and in vitro methods, respectively, for the areas related to toxicology and the broader scientific community, by the availability of such internal resources (https://www.aahp-abhp.org/node/1224). CAAT commissioned report suggests utilizing the existing data and monitoring future testing in toxicology by integrated testing strategies (Jaworska and Hoffmann 2010). OECD workshop and previous work (Jaworska et al. 2010) outlined the conceptual requirements as transparent, consistent, and hypothesis-driven (Jaworska et al. 2010).
10.4 Conclusion
IATA has the ability to reduce the usage of animal testing for safety studies of cosmetics and personal care products by utilizing testing and non-testing methods with integration with new approach methods.
References
Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S et al (2016) Skin models for the testing of transdermal drugs. Clin Pharm 8:163
Basketter D (2016) Skin sensitisation, adverse outcome pathways and alternatives. Altern Lab Anim 44(5):431–436
Basketter DA, Alépée N, Ashikaga T, Barroso J, Gilmour N, Goebel C, Hibatallah J, Hoffmann S, Kern P, Martinozzi-Teissier S, Maxwell G, Reisinger K, Sakaguchi H, Schepky A, Tailhardat M, Templier M (2014) Categorization of chemicals according to their relative human skin sensitizing potency. Dermatitis 25(1):11–21
Bell SM, Phillips J, Sedykh A, Tandon A, Sprankle C, Morefield SQ, Shapiro A, Allen D, Shah R, Maull EA, Casey WM, Kleinstreuer NC (2017) An integrated chemical environment to support 21st-century toxicology. Environ Health Perspect 125(5):054501
Blaauboer BJ, Barratt MD, Houston JB (1999) The integrated use of alternative methods in toxicological risk evaluation: ECVAM integrated testing strategies task force report 1. Altern Lab Anim 27(2):229–237
Brannen KC, Chapin RE, Jacobs AC, Green ML (2016) Alternative models of developmental and reproductive toxicity in pharmaceutical risk assessment and the 3Rs. ILAR J 57(2):144–156
Browne P, Noyes PD, Casey WM, Dix DJ (2017) Application of adverse outcome pathways to US EPA’s endocrine disruptor screening program. Environ Health Perspect 125(9):096001
Browne P, Van Der Wal L, Gourmelon A (2020) OECD approaches and considerations for regulatory evaluation of endocrine disruptors. Mol Cell Endocrinol 504:110675
Casati S (2018) Integrated approaches to testing and assessment. Basic Clin Pharmacol Toxicol 123(S5):51–55
Chapman PM (2015) Including or excluding toxicity test data for development of a geometric mean. Environ Toxicol Chem 34(8):1691–1692
Clothier R, Willshaw A, Cox H, Garle M, Bowler H, Combes R (1999) The use of human keratinocytes in the EU/COLIPA international in vitro Phototoxicity test validation study and the ECVAM/COLIPA study on UV filter chemicals. Altern Lab Anim 27(2):247–259
Conway GE, Shah UK, Llewellyn S, Cervena T, Evans SJ, Al Ali AS, Jenkins GJ, Clift MJD, Doak SH (2020) Adaptation of the in vitro micronucleus assay for genotoxicity testing using 3D liver models supporting longer-term exposure durations. Mutagenesis 35(4):319–330
Daniel AB, Strickland J, Allen D, Casati S, Zuang V, Barroso J, Whelan M, Régimbald-Krnel MJ, Kojima H, Nishikawa A, Park H-K, Lee JK, Kim TS, Delgado I, Rios L, Yang Y, Wang G, Kleinstreuer N (2018) International regulatory requirements for skin sensitization testing. Regul Toxicol Pharmacol 95:52–65
de Ávila RI, Lindstedt M, Valadares MC (2019) The 21st century movement within the area of skin sensitization assessment: from the animal context towards current human-relevant in vitro solutions. Regul Toxicol Pharmacol 108:104445
Dumont C, Barroso J, Matys I, Worth A, Casati S (2016) Analysis of the local lymph node assay (LLNA) variability for assessing the prediction of skin sensitisation potential and potency of chemicals with non-animal approaches. Toxicol in Vitro 34:220–228
Eskes C (2019) The usefulness of integrated strategy approaches in replacing animal experimentation. Ann Ist Super Sanita 55(4):400–404
European Parliament and the council of the European Union (2009) Regulation (EC) No 1223/2009 of the European parliament and of the council. 342: 59
Hamilton T, de Gannes GC (2011) Allergic contact dermatitis to preservatives and fragrances in cosmetics. Dermatitis 14:16
Hartung T, Bremer S, Casati S, Coecke S, Corvi R, Fortaner S et al (2004) A modular approach to the ECVAM principles on test validity. Altern Lab Anim 32(5):467–472
Hartung T, Luechtefeld T, Maertens A, Kleensang A (2013) Integrated testing strategies for safety assessments. ALTEX 30(1):3–18
Hecker M, Hollert H, Cooper R, Vinggaard AM, Akahori Y, Murphy M, Nellemann C, Higley E, Newsted J, Laskey J, Buckalew A, Grund S, Maletz S, Giesy J, Timm G (2011) The OECD validation program of the H295R steroidogenesis assay: phase 3. Final inter-laboratory validation study. Environ Sci Pollut Res Int 18(3):503–515
Henning A, Schaefer UF, Neumann D (2009) Potential pitfalls in skin permeation experiments: influence of experimental factors and subsequent data evaluation. Eur J Pharm Biopharm 72(2):324–331
Hoffmann S (2015) LLNA variability: an essential ingredient for a comprehensive assessment of non-animal skin sensitization test methods and strategies. ALTEX 32(4):379–383
Jaworska J, Hoffmann S (2010) Integrated testing strategy (ITS)–opportunities to better use existing data and guide future testing in toxicology. ALTEX 27(4):231–242
Jaworska J, Gabbert S, Aldenberg T (2010) Towards optimization of chemical testing under REACH: a Bayesian network approach to integrated testing strategies. Regul Toxicol Pharmacol 57(2):157–167
Kandarova H, Hayden PJ (2021) Standardised reconstructed skin models in toxicology and pharmacology: state of the art and future development. In: Schäfer-Korting M, Maria-Engler SS, Landsiedel R (eds) Organotypic models in drug development. Springer, Cham, pp 57–71
Kandárová H, Liebsch M, Spielmann H, Genschow E, Schmidt E, Traue D, Guest R, Whittingham A, Warren N, Gamer AO, Remmele M, Kaufmann T, Wittmer E, De Wever B, Rosdy M (2006) Assessment of the human epidermis model SkinEthic RHE for in vitro skin corrosion testing of chemicals according to new OECD TG 431. Toxicol in Vitro 20(5):547–559
Kinsner-Ovaskainen A, Akkan Z, Casati S, Coecke S, Corvi R, Negro GD, De Bruijn J, De Silva O, Gribaldo L, Griesinger C, Jaworska J, Kreysa J, Maxwell G, McNamee P, Price A, Prieto P, Schubert R, Tosti L, Worth A, Zuang V (2009) Overcoming barriers to validation of non-animal partial replacement methods/integrated testing strategies: the report of an EPAA–ECVAM workshop. Altern Lab Anim 37(4):437–444
Kleinstreuer NC, Hoffmann S, Alépée N, Allen D, Ashikaga T, Casey W et al (2018) Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit Rev Toxicol 48(5):359–374
OECD (1997). Test No 473: in vitro mammalian chromosome aberration test
OECD (2010a) Test no. 429: skin sensitisation
OECD (2010b) Test no. 442A: skin sensitization
OECD (2015) Test no. 404: acute dermal irritation/corrosion
OECD (2016a) Guidance document on the reporting of defined approaches to be used within integrated approaches to testing and assessment, organization for economic co-operation and development, Paris
OECD (2016b) Test no. 476: in vitro mammalian cell gene mutation tests using the Hprt and xprt genes
OECD (2017) Guidance document for the use of adverse outcome pathways in developing integrated approaches to testing and assessment (IATA). OECD Publishing
OECD (2018) Test no. 442B: skin sensitization
OECD (2019) Second edition–guidance document on integrated approaches to testing and assessment (IATA) for serious eye damage and eye irritation
OECD (2020) Test no. 437: bovine corneal opacity and permeability test method for identifying i) chemicals inducing serious eye damage and ii) chemicals not requiring classification for eye irritation or serious eye damage
OECD (2021a) Test no. 405: acute eye irritation/corrosion
OECD (2021b) Test no. 439: in vitro skin irritation: reconstructed human epidermis test method
OECD (2022a) Test no. 442E: in vitro skin sensitisation
OECD (2022b) Test no. 456: H295R steroidogenesis assay
Pfuhler S, van Benthem J, Curren R, Doak SH, Dusinska M, Hayashi M, Heflich RH, Kidd D, Kirkland D, Luan Y, Ouedraogo G, Reisinger K, Sofuni T, van Acker F, Yang Y, Corvi R (2020) Use of in vitro 3D tissue models in genotoxicity testing: strategic fit, validation status and way forward. Report of the working group from the 7th international workshop on genotoxicity testing (IWGT). Mutat Res Genet Toxicol Environ Mutagen 850–851:503135
Pfuhler S, Downs TR, Hewitt NJ, Hoffmann S, Mun GC, Ouedraogo G, Roy S, Curren RD, Aardema MJ (2021) Validation of the 3D reconstructed human skin micronucleus (RSMN) assay: an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis 36(1):1–17
Rauscher H, Sokull-Klüttgen B, Stamm HJN (2012) The European commission’s recommendation on the definition of nanomaterial makes an impact. Nanotoxicology 7(7):1195–1197
Roberts DW, Api AM, Aptula AO (2016) Chemical applicability domain of the local lymph node assay (LLNA) for skin sensitisation potency. Part 2. The biological variability of the murine local lymph node assay (LLNA) for skin sensitisation. Regul Toxicol Pharmacol 80:255–259
Sakuratani Y, Horie M, Leinala E (2018) Integrated approaches to testing and assessment: OECD activities on the development and use of adverse outcome pathways and case studies. Basic Clin Pharmacol Toxicol 123(S5):20–28
Shah UK, de Oliveira Mallia J, Singh N, Chapman KE, Doak SH, Jenkins GJS (2018) A three-dimensional in vitro HepG2 cells liver spheroid model for genotoxicity studies. Mutat Res Genet Toxicol Environ Mutagen 825:51–58
Spielmann H, Seiler A, Bremer S, Hareng L, Hartung T, Ahr H et al (2006) The practical application of three validated in vitro embryotoxicity tests: the report and recommendations of an ECVAM/ZEBET workshop (ECVAM workshop 57). Altern Lab Anim 34(5):527–538
Strickland J, Daniel AB, Allen D, Aguila C, Ahir S, Bancos S, Craig E, Germolec D, Ghosh C, Hudson NL, Jacobs A, Lehmann DM, Matheson J, Reinke EN, Sadrieh N, Vukmanovic S, Kleinstreuer N (2019) Skin sensitization testing needs and data uses by US regulatory and research agencies. Arch Toxicol 93(2):273–291
Tan CH, Rasool S, Johnston GA (2014) Contact dermatitis: allergic and irritant. Clin Dermatol 32(1):116–124
Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, Garcia-Reyero N, Hartung T, Worth A, Patlewicz G (2014) Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA). Regul Toxicol Pharmacol 70(3):629–640
Wills JW, Hondow N, Thomas AD, Chapman KE, Fish D, Maffeis TG, Penny MW, Brown RA, Jenkins GJS, Brown AP, White PA, Doak SH (2016) Genetic toxicity assessment of engineered nanoparticles using a 3D in vitro skin model (EpiDerm™). Part Fibre Toxicol 13(1):50
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Patel, S.K., Gaur, P., Pandey, A., Yadav, A.K., Sahu, R.S., Mishra, B.N. (2023). Integrated Approaches to Testing and Assessment (IATA) for Safety of Cosmetics and Personal Care Products. In: Pant, A.B., Dwivedi, A., Ray, R.S., Tripathi, A., Upadhyay, A.K., Poojan, S. (eds) Skin 3-D Models and Cosmetics Toxicity. Springer, Singapore. https://doi.org/10.1007/978-981-99-2804-0_10
Download citation
DOI: https://doi.org/10.1007/978-981-99-2804-0_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2803-3
Online ISBN: 978-981-99-2804-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)