Abstract
United States regulatory and research agencies may rely upon skin sensitization test data to assess the sensitization hazards associated with dermal exposure to chemicals and products. These data are evaluated to ensure that such substances will not cause unreasonable adverse effects to human health when used appropriately. The US Consumer Product Safety Commission, the US Environmental Protection Agency, the US Food and Drug Administration, the Occupational Safety and Health Administration, the National Institute for Occupational Safety and Health, and the US Department of Defense are member agencies of the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM seeks to identify opportunities for the use of non-animal replacements to satisfy these testing needs and requirements. This review identifies the standards, test guidelines, or guidance documents that are applicable to satisfy each of these agency’s needs; the current use of animal testing and flexibility for using alternative methodologies; information needed from alternative tests to fulfill the needs for skin sensitization data; and whether data from non-animal alternative approaches are accepted by these US federal agencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin sensitizers are substances that elicit an allergic response, such as allergic contact dermatitis, following contact with the skin. Allergic contact dermatitis causes itching, swelling, and redness of the skin in susceptible individuals (Murphy et al. 2012). Skin sensitization testing identifies the potential for a substance to cause allergic contact dermatitis. US regulatory authorities require or recommend that manufacturers of chemicals and products conduct tests or review available test data to assess skin sensitization hazards. Skin sensitization test data enable appropriate classification and labeling to alert handlers and consumers to potential hazards. These data may also be used to determine levels of sensitizers that will not produce allergic sensitization.
Skin sensitization testing is traditionally performed using either human tests such as the human repeat insult patch test and the human maximization test or animal tests such as the murine local lymph node assay (LLNA), the guinea pig maximization test, and the Buehler test (OECD 1992, 2010a). However, interest in alternative non-animal approaches is increasing due to ethical concerns about animal testing, legal restrictions on the use of animal data, and scientific concerns about the relevance of animal test results to human outcomes. This interest has resulted in a number of focused activities to support the development and acceptance of alternative approaches to reduce or replace animal use in skin sensitization testing. Because the biological steps leading to skin sensitization initiated by covalent binding of sensitizing chemicals to proteins have been well characterized by international research efforts (OECD 2012a, b) and several alternative non-animal test methods have been developed (OECD 2015, 2017a, 2018c), the outlook for implementing non-animal test methods for skin sensitization testing seems promising.
The Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) is an interagency committee of the US government with representatives from 16 federal agencies that use or generate toxicological and safety testing information (42 U.S.C. § 285l–3). ICCVAM promotes the scientific validation and regulatory acceptance of testing methods that more accurately assess the safety and hazards of chemical products and that replace, reduce, or refine animal use. The National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) supports ICCVAM activities; NICEATM and ICCVAM work collaboratively to develop and evaluate new and improved testing approaches applicable to the needs of US federal agencies.
ICCVAM’s efforts to establish alternatives to skin sensitization testing are led by the ICCVAM Skin Sensitization Workgroup, which is comprised of experts from multiple member agencies and is supported by NICEATM. The group’s main activity is to evaluate and promote the use of alternative non-animal test methods for regulatory use in skin sensitization hazard assessments (Casey 2016).
As part of an effort to increase confidence in alternative methods and improve the relevance of test outcomes to human health, ICCVAM has developed a strategic roadmap for establishing new approaches for evaluating the safety of chemicals and medical products (NIEHS 2018). The roadmap implementation plan addresses the development and evaluation of alternative approaches for acute systemic toxicity testing, skin and eye irritation testing, and skin sensitization testing. A key element in development of the strategic roadmap and subsequent progress towards incorporation of replacements to animal tests is an understanding of the data needed and how they are used by government regulatory and research agencies. Six ICCVAM member agencies use skin sensitization data to satisfy the research and regulatory functions designated to them under federal laws: the US Consumer Product Safety Commission (CPSC), the US Environmental Protection Agency (EPA), the US Food and Drug Administration (FDA), the Occupational Safety and Health Administration (OSHA), the National Institute for Occupational Safety and Health (NIOSH) and the US Department of Defense (DoD). This review summarizes (and where appropriate, contrasts) the current regulatory and non-regulatory needs of these agencies for skin sensitization testing to:
-
Provide test method developers with clear targets for alternative method development by clarifying the regulatory needs for skin sensitization testing for these US agencies
-
Describe how the different agencies are satisfying their needs while reducing or eliminating animal use
-
Inform agencies and relevant stakeholders about the status of existing alternative methods as a starting point for future method development and validation efforts
Overview of US regulatory testing requirements for skin sensitization
Table 1 lists statutory requirements, regulations, and regulated products relevant to four ICCVAM member agencies that use skin sensitization data for regulatory purposes. Some regulations do not require the use or consideration of skin sensitization data specifically, but indicate that toxicity or safety assessments must be performed.
Two additional agencies, DoD and NIOSH, use skin sensitization data but are not regulatory agencies and have no statutory requirements for skin sensitization assessments. DoD generates and uses skin sensitization data to protect DoD personnel, including Warfighters, who may be exposed to chemicals during their work activities. NIOSH performs skin sensitization assessments to make recommendations for the prevention of work-related injury and illness.
In the following sections, we review the needs for, and uses of, skin sensitization data by these six federal agencies. Specifically, we address the following questions:
-
1.
What standards, test guidelines, or guidance documents apply to skin sensitization testing performed to satisfy the agency’s needs?
-
2.
Is there a specific requirement for data from animal studies, or are alternative approaches accepted?
-
3.
What information from a non-animal approach will satisfy the skin sensitization data needs? Should the alternative approach predict animal responses (which must then be extrapolated to a human response), or instead predict human responses?
-
4.
What is the path to regulatory acceptance of non-animal approaches for skin sensitization testing and assessment?
ICCVAM agency use of skin sensitization data
US Consumer Product Safety Commission
CPSC uses skin sensitization data in administering two statutes, the Federal Hazardous Substances Act (FHSA) (15 U.S.C. § 1261–1278 [1960]) and the Labeling of Hazardous Art Materials Act (LHAMA) (15 U.S.C. § 1277 [1988]).
FHSA applies to hazardous substances that are intended or packaged for use in the household. The statute excludes heating, cooking, and refrigeration fuels stored in containers; substances covered by the FDA’s Federal Food, Drug, and Cosmetic Act; and pesticides subject to the EPA’s Federal Insecticide, Fungicide, and Rodenticide Act.
According to regulations issued under the FHSA, a sensitizer is defined as “a substance that is capable of inducing a state of immunologically mediated hypersensitivity (including allergic photosensitivity) following a variable period of exposure to that substance. Hypersensitivity to a substance will become evident by an allergic reaction elicited upon re-exposure to the same substance” (CPSC 2017c). FHSA requires appropriate hazard labeling of strong sensitizers, which have significant potential to cause hypersensitivity based on the frequency of occurrence and the severity of the reaction (CPSC 2017d). CPSC specifically identifies several substances covered under the FHSA as strong sensitizers. These include (1) p-phenylenediamine and products containing it; (2) powdered orris root and products containing it; (3) epoxy resin systems containing, in any concentration, ethylenediamine, diethylenetriamine, and diglycidyl ethers of molecular weight less than 200; (4) formaldehyde and products containing at least 1% formaldehyde; and (5) oil of bergamot and products containing at least 2% oil of bergamot (CPSC 2017d).
Skin sensitization data are also used in administering LHAMA, which requires that all art materials sold in the United States be properly labeled to identify the potential to produce adverse health effects. Substances subject to the Federal Food, Drug, and Cosmetic Act are excluded from LHAMA, as are substances covered by the Federal Insecticide, Fungicide, and Rodenticide Act. Proper labeling of skin sensitizing ingredients in substances covered by LHAMA is described in the “Art Materials” regulation (CPSC 2017a). The regulation requires the manufacturer to provide a product’s formulation to a toxicologist for review and recommendation of appropriate precautionary labeling. To determine labeling, the toxicologist must consider the composition of the substance, its toxic potential, and any reasonably foreseeable uses or misuses of the product that may result in chronic adverse health effects, including skin sensitization.
The CPSC does not require the submission of skin sensitization data; rather, the agency requires that a product be labeled appropriately to reflect the associated hazards.
Standards, guidelines, and guidance for skin sensitization testing
Guidance on the methods to be used for skin sensitization evaluations for FHSA and LHAMA is provided in the relevant regulations and on the CPSC website (CPSC 2012, 2013). To determine whether a substance is a strong sensitizer, CPSC uses existing information in a weight-of-evidence approach (CPSC 2017b). The following information, in descending order of importance, is considered: well-conducted clinical and diagnostic studies; epidemiological studies, with a preference for general population studies over occupational studies; well-conducted animal studies; well-conducted in vitro test studies; cross-reactivity data; and case histories. Any skin sensitization studies should be carried out in accordance with national or international test guidelines and in compliance with good laboratory practice (CPSC 2017b).
The specific skin sensitization test guidelines that are currently accepted by CPSC include: the LLNA and subsequent LLNA method updates, including the reduced LLNA (OECD 2010a); and two non-radiolabeled versions of the LLNA, the LLNA: BrdU-ELISA and the LLNA: DA (CPSC 2012; OECD 2010b, c). CPSC also accepts the guinea pig maximization and Buehler tests (OECD 1992).
Current requirements for animal testing and flexibility for the use of alternatives
Animal testing is not mandatory under either FHSA or LHAMA. CPSC’s statement on its animal testing policy and alternatives to animal testing for substances regulated under FHSA (CPSC 2017f) encourages the use of existing non-animal alternatives whenever possible. Acceptable non-animal alternatives include prior human experience, CPSC-approved in vitro or in silico methods [which can be found on the CPSC website (CPSC 2012)], literature, and expert opinion. CPSC recommends that animal testing should only be pursued after exhausting all other information sources. If deemed necessary, CPSC recommends that animal tests use the most humane procedures and the smallest number of animals necessary to provide reliable results.
Information needed from alternative tests to fulfill the needs for skin sensitization data
CPSC requires potency information to determine whether a substance is a strong sensitizer under FHSA (15 U.S.C. § 1261(k)) (CPSC 2017b). To comply with LHAMA, a risk assessment should be performed for art materials containing known sensitizers to determine if the art material will cause skin sensitization under the conditions of expected use or foreseeable misuse. CPSC prefers reliable human data over animal data and therefore prefers that alternative methods predict human rather than animal responses.
Acceptance of alternative methods and approaches to determine skin sensitization
No non-animal methods are currently accepted by CPSC as complete replacements for in vivo skin sensitization test methods. However, data generated using such methods may be accepted to fulfill FHSA or LHAMA requirements on a case-by-case basis. CPSC may consider quantitative structure–activity relationships, in silico data, human threshold values, or other potency and sensitizer bioavailability information derived from validated methods (CPSC 2017e). CPSC may also consider data generated using in vitro tests described in test guidelines adopted by the Organisation for Economic Co-operation and Development (OECD). The OECD Mutual Acceptance of Data agreement requires test data generated in any OECD member country to be accepted for review in other member countries (CPSC 2012). However, CPSC may refuse to accept data from any test that it has not previously approved as adequate for a specific classification or labeling requirement. CPSC may also require additional data to satisfy the requirement.
US Environmental Protection Agency
Skin sensitization data are used by EPA to administer two statutes: the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) (7 U.S.C. § 136 et seq. [1996]) and the Toxic Substances Control Act (TSCA) (15 U.S.C. §§ 2601–2692 [1976]), recently amended via the Frank R. Lautenberg Chemical Safety for the 21st Century Act (Public Law 114–182 130 Stat. 448 [2016]).
The EPA Office of Pesticide Programs (OPP) regulates the supply and use of pesticides in the United States under FIFRA. Any distributor wishing to sell pesticides in the United States must register their product with OPP. Registration requires the applicant to demonstrate that the product does not cause unreasonable adverse effects to human health or the environment when used appropriately (EPA 2017). Under FIFRA, skin sensitization data are required for pesticide active ingredients and final products.
The EPA Office of Pollution Prevention and Toxics (OPPT) administers the amended TSCA, which regulates new and existing chemical substances that are imported into the United States but are not covered by other statutes such as FIFRA or the FDA’s Federal Food, Drug, and Cosmetic Act. TSCA requires a company to submit a pre-manufacture notice to OPPT before manufacturing or importing a new chemical substance or before initiating a new use of an existing chemical substance. Although TSCA does not require generation of data for skin sensitization or any other specific toxicity endpoint, any existing toxicity data in the possession or control of the submitter must be submitted to EPA with pre-manufacture notices. OPPT must evaluate the new chemical substance to make one of the following determinations:
-
1.
The new chemical substance presents an unreasonable risk of injury to human health or the environment (TSCA, as amended in § 5(a)(3)(A)) (Public Law 114–182 130 Stat. 448 [2016])
-
2.
The information on the new chemical substance is insufficient to make a reasoned evaluation of the health and environmental effects (TSCA, as amended in § 5(a)(3)(B)(i)) (Public Law 114–182 130 Stat. 448 [2016])
-
3.
The new chemical substance may present an unreasonable risk of injury to human health or the environment (TSCA, as amended in § 5(a)(B)(ii)(I)) (Public Law 114–182 130 Stat. 448 [2016])
-
4.
The new chemical substance is or will be produced in substantial quantities, and the substance either enters or may reasonably be anticipated to enter the environment in substantial quantities, or there is or may be significant or substantial human exposure to the substance (TSCA, as amended in § 5(a)(B)(ii)(II)) (Public Law 114–182 130 Stat. 448 [2016])
-
5.
The new chemical substance is not likely to present an unreasonable risk of injury to human health or the environment (TSCA, as amended in § 5(a)(3)(C)) (Public Law 114–182 130 Stat. 448 [2016])
In 2016, the Frank R. Lautenberg Chemical Safety for the 21st Century Act amended TSCA to give OPPT authority to require data for existing chemicals that are poorly characterized for toxicity. Furthermore, the new act required that OPPT construct a plan to promote the development and implementation of alternative test methods for new and existing chemical substances that reduce, refine, or replace animal testing, such as high-throughput screening methods, computational approaches, and in vitro studies (Public Law 114–182 130 Stat. 448 [2016]).
Standards, guidelines, and guidance for skin sensitization testing
To standardize test procedures and minimize variance in test data, EPA published a series of test guidelines describing methods to generate data required under FIFRA and TSCA. The skin sensitization test guideline, OPPTS 870.2600, includes three in vivo test methods: the LLNA, the guinea pig maximization test, and the Buehler test (EPA 2003). OPP also accepts data generated using the reduced LLNA (EPA 2011). Under Mutual Acceptance of Data, EPA accepts, for review, data generated using OECD test guidelines. These describe traditional and non-radiolabeled LLNA methods (OECD 2010a, b, c) and six non-animal test methods—direct peptide reactivity assay (DPRA), KeratinoSens™, LuSens, human cell line activation test (h-CLAT), IL8-Luc assay and U-SENS assay™ (OECD 2015, 2017a, 2018c). Although OPP accepts such data for review, OPP may require additional information to fulfill US pesticide data submission requirements.
Although skin sensitization testing is not a requirement under TSCA, existing data generated according to EPA and OECD test guidelines and non-guideline tests are accepted. Even though OPPT accepts such data for review, OPPT may require additional information to address this toxicity endpoint.
Current requirements for animal testing and flexibility for the use of alternatives
Although EPA’s current test guidelines include only in vivo test methods, EPA is flexible about the use of non-animal alternatives for skin sensitization assessments. OPP has a strategic vision for implementing non-animal tests to support FIFRA data requirements for the “six-pack” of acute toxicity (i.e., tests for skin sensitization, acute systemic toxicity by oral, dermal, and inhalation routes, eye irritation, and skin irritation) (EPA 2018c). In evaluating an alternative method, OPP will consider (1) the availability of an OECD test guideline describing the method or the degree of international validation of the method; (2) whether the method is suitable for individual chemicals, mixtures, or both; (3) the types of chemicals for which the method is applicable; (4) whether the method alone can properly replace an in vivo study or if it should be used as part of an integrated testing strategy; (5) additional strengths or uncertainties associated with the method (e.g., feasibility, cost, accuracy, etc.); and (6) whether the data derived from the method are sufficient for labeling and regulatory decisions (EPA 2016). When OPP finds that an alternative method is suitable for FIFRA regulatory purposes, it will publish a draft waiver guidance or draft alternative testing guideline document for public review and comment. Any feedback received will be used to address issues and revise the draft policy as needed. OPP will then issue a final policy, at which time data generated from the alternative method may be submitted to fulfill testing requirements.
Skin sensitization testing required under FIFRA can be avoided under certain conditions. Guidance for Waiving or Bridging of Mammalian Acute Toxicity Tests for Pesticides and Pesticide Products (EPA 2012) provides guidance and criteria for submitting waiver requests and bridging toxicity data. OPP may grant a waiver for skin sensitization testing if: (1) the test material is corrosive to skin, or has a pH less than 2 or greater than 11.5; (2) the product is corrosive to skin, or has a pH less than 2 or greater than 11.5 at the most dilute use concentration recommended on the label; (3) the product conditions of use do not result in repeated dermal exposure; (4) the test material is a pesticidal paint that contains strong dyes or pigments, making dermal evaluation impossible; (5) the product design prevents dermal exposure; or (6) the technical active ingredient is a known dermal sensitizer. “Bridging” refers to assessing the hazard of a chemical for which there is little or no existing data using a read-across method to evaluate data for a structurally similar chemical, thus avoiding the need to generate new test data. OPP may accept bridging of data in situations including, but not necessarily limited to, the following: (1) the toxicity profile of a proposed product matches that of the product for which the cited data were submitted and demonstrates a reduced hazard potential; or (2) a new product is essentially a water dilution of a registered product. Waiving and bridging are beneficial in reducing time and resource investments and animal testing (EPA 2012). Pesticide formulations must be tested in animals if waiving and bridging do not apply.
Animal testing is not required by OPPT for pre-manufacture notices under TSCA. If no chemical-specific data are available, OPPT uses a read-across method, when feasible, to assess toxicity of the submitted new chemical substance. If available data are inadequate for read-across, new toxicity data may be requested for new chemical substances under Sect. 5 of TSCA or for existing chemical substances under Sect. 4 of TSCA. The amended TSCA mandates a tiered approach to producing new information; all of the available and relevant data must be reviewed before animal testing is considered (Public Law 114–182 130 Stat. 448 [2016]).
Information needed from alternative tests to fulfill the needs for skin sensitization data
OPP requires that products regulated under FIFRA undergo a hazard assessment for classification as sensitizers or non-sensitizers to provide appropriate hazard information for product labels. OPPT requires a risk assessment based on hazard and exposure for chemical substances covered by TSCA. When the information provided for the risk assessment indicates that a chemical substance may or will present an unreasonable risk to human health or the environment, OPPT may recommend changes to the safety data sheet for the substance, including requirements for personal protective equipment. In addition, specific engineering or administrative controls may be required, as appropriate, to adequately protect worker health and the environment. When information provided for the risk assessment is insufficient to make a risk determination, additional testing may be required. OPP may consider models using alternative methods that predict animal skin sensitization responses; however, both OPP and OPPT envision a future where models predict human responses to skin sensitizers.
Acceptance of alternative methods and approaches to determine skin sensitization
In accordance with OPP’s strategic vision for implementing non-animal tests (EPA 2018c), OPP, in coordination with the EPA Office of Chemical Safety and Pollution Prevention and OPPT, has recently published a draft interim science policy on the acceptance of alternative approaches for skin sensitization assessments (EPA 2018a). Under this policy, OPP is now accepting submissions of data from specific defined approaches for skin sensitization hazard assessments for pesticide active ingredients or pesticide inert ingredients. A defined approach consists of a fixed data interpretation procedure (e.g., mathematical models or decision trees) applied to data (e.g., in silico predictions, in chemico, in vitro data) generated with a defined set of information sources to derive a prediction (OECD 2016a). The defined approaches accepted include the “2 out of 3” approach and the 3/1 Sequential Testing Strategy (EPA 2018a). Both defined approaches require testing in one or more in vitro or in chemico skin sensitization tests described in OECD test guidelines. OPPT currently accepts and uses non-animal approaches for skin sensitization hazard assessments, but may require additional information to address this endpoint for quantitative risk assessments.
Under Sect. 4(h) of the amended TSCA, OPPT has published a strategic plan to promote the development and implementation of alternative test methods and strategies to reduce, refine, or replace the use of vertebrate animals in toxicity testing, including skin sensitization (EPA 2018b). In accordance with this requirement, OPPT is also accepting submissions of the specific defined approaches mentioned in the EPA interim policy on the acceptance of alternative approaches for skin sensitization hazard (EPA 2018a).
US Food and Drug Administration
The Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq. [1938]) and its amendments give FDA authority to oversee the safety of several types of products, including but not limited to new drugs, drugs intended for a new use or delivery route, vaccines, blood and blood products, allergenics, cellular and tissue products, gene therapies, medical devices, food and food additives, dietary supplements, color additives, cosmetics, and animal drugs.
The FDA Center for Drug Evaluation and Research (CDER) regulates over-the-counter and prescription drugs, including biological therapeutics and generic drugs (FDA 2018b). The FDA Center for Biologics Evaluation and Research (CBER) regulates biological products for human use that are not regulated by CDER, such as vaccines, blood and blood products, allergenics, cellular and tissue products, and gene therapies and devices used to test the safety of blood and cellular products (FDA 2018d). To permit marketing in the United States, CDER and CBER generally request data, including skin sensitization data in humans for topically applied products, to demonstrate that these products are reasonably safe for human use.
The FDA Center for Devices and Radiological Health (CDRH) regulates medical devices and radiation-emitting products (FDA 2018a). CDRH recommends that tissue-contacting medical devices, including all surface, external communicating, and implanted devices, be evaluated for sensitization potential (FDA 2016). Skin sensitization testing is one mechanism some manufacturers have historically used to address this endpoint. Chemical characterization and risk assessment tools can be used to determine whether known sensitizers are likely to elute from medical devices during clinical use.
In addition to food products, food additives, and substances expected to come into contact with food, the FDA Center for Food Safety and Applied Nutrition (CFSAN) is responsible for the safety of cosmetic ingredients (including color additives) and cosmetic products (FDA 2018e). CFSAN requires cosmetics to be safe for humans when used according to the intended conditions of use or as directed on the label. However, CFSAN does not have authority to approve cosmetic products or cosmetic ingredients (other than color additives) prior to marketing and does not require skin sensitization data for cosmetic products. Manufacturers are responsible for conducting any necessary testing to ensure the safety of the cosmetics they market (FDA 2018f).
The FDA Center for Veterinary Medicine (CVM) regulates over-the-counter and prescription animal drugs, including therapeutics, production drugs (which enhance the production of edible or non-edible products, or increase the efficiency of a particular phase of life, such as reproduction), and generic animal drugs (FDA 2018c). To obtain approval to manufacture and market animal drugs in the United States, CVM generally recommends the submission of safety data for human users. CVM requires the animal drugs to be safe for animals and for humans handling the drugs when used per the intended conditions of use or as directed on the label.
Standards, guidelines, and guidance for skin sensitization testing
FDA has not issued regulations or guidelines for skin sensitization testing. Instead, FDA has offered guidance to sponsors for identifying potentially hazardous drug and biological products. For topically applied products, Guidance for Industry: Immunotoxicology Evaluation of Investigational New Drugs (FDA 2002) and Guidance for Industry: Skin Irritation and Sensitization Testing of Generic Transdermal Drug Products (FDA 1999a) are now outdated and not used by FDA. The former guidance is currently being revised. Because pivotal studies for CDER and CBER are conducted in humans, any scientifically valid screening assay/approach can be used.
With regard to the assessment of medical devices, Guidance for Industry and FDA Staff on Use of International Standard ISO 10993-1, Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process recommends a sensitization assessment for all surface, external communicating, and implanted medical devices, regardless of the duration of contact (FDA 2016). Guidance for Industry and FDA Reviewers/Staff: Premarket Notification [510(k)] Submission for Testing for Skin Sensitization to Chemicals in Natural Rubber Products (FDA 1999b) provides guidance for the preparation and evaluation of claims regarding reduced potential for inducing sensitization or reduced potential for causing sensitization reactions in sensitized individuals after exposure to medical devices made of natural rubber. The guidance also provides recommendations on testing, including human trials, to support such claims.
As part of the labeling information to address the safety of human users of animal drugs, CVM accepts skin sensitization data generated from OECD test guidelines for the LLNA, guinea pig maximization test, and Buehler test (OECD 1992, 2010a).
Current requirements for animal testing and flexibility for the use of alternatives
In data submissions for new human products for topical use, provided the products are not irritating to the skin, CDER and CBER generally request skin sensitization data from pivotal studies that test clinical formulations of these products on humans to make regulatory decisions, and prefer that data from screening tests also be included in submissions. Any scientifically valid approach may be used for screening tests, including a battery of non-animal methods (e.g., in silico, in chemico and in vitro) that assess different endpoints. Current guidance from CDER and CBER recommends using a guinea pig test for identifying potential skin sensitizing clinical formulations. Although the LLNA is not recommended for this purpose, LLNA data will be reviewed and may be accepted on a case-by-case basis. Most screening information on local toxicity can be obtained from repeat-dose toxicity studies in animals. CDER and CBER also request information regarding dermal pharmacokinetics associated with all in vivo doses.
If it is determined that testing is needed for medical devices, the guinea pig maximization test is commonly used to assess sensitization hazards. However, for certain applications, CDRH will evaluate data generated using traditional and non-radiolabeled LLNA methods (OECD 2010a, b, c). Such applications might include testing device materials in aqueous solutions or for testing metal compounds (except nickel), unless the properties of the materials used would prevent the LLNA from detecting sensitization, as might be the case for some nanomaterials, for example. For radiolabeled LLNA tests of medical devices, CDRH recommends that the ASTM F2148 standard (ASTM 2013) be followed. CDRH evaluates the use of LLNA tests for medical devices on a case-by-case basis for medical device extracts that are composed of chemical mixtures. Per ISO 10993-10, Biological evaluation of medical devices—tests for irritation and skin sensitization, topical medical devices are the only products for which the Buehler test is appropriate (ISO 2010). Clinical studies are recommended for some medical devices, particularly for those with labels claiming reduced potential for sensitizing users to rubber chemical additives or reduced potential for causing a reaction in users sensitized to rubber chemical additives (FDA 2008). Currently, CDRH is not aware of any non-animal alternative skin sensitization method that has been validated for mixtures of potentially weak skin sensitizers that may be found in medical devices; therefore, non-animal alternatives for skin sensitization tests are not generally submitted and evaluated in CDRH regulatory submissions.
Although CFSAN does not request or require skin sensitization testing for cosmetic products, CFSAN requires cosmetics to be safe for human use. Animal testing for this purpose can be minimized using existing data on individual ingredients and product formulations for a cosmetic product that is similar to the cosmetic product in question. If existing data for individual ingredients or existing formulations are insufficient to assess a cosmetic product’s safety, testing could be performed on the finished product as appropriate (FDA 1975).
To assist in assessing the safety of animal drugs to human users, CVM currently accepts animal skin sensitization tests when performed according to OECD test guidelines for the LLNA, guinea pig maximization test, and Buehler test (OECD 1992, 2010a). CVM has the flexibility to consider data from non-animal skin sensitization tests on a case-by-case basis.
Information needed from alternative tests to fulfill the needs for skin sensitization data
For drugs and biologics, CDER and CBER desire characterization of potency and the proportion of subjects sensitized. For medical devices, CDRH requests information on sensitization hazard sufficient to support appropriate labeling of any new or modified medical device that involves contact with patients. CVM uses skin sensitization data for animal drugs to assess the hazard and risk to human users. CDER, CBER, CDRH, and CVM would prefer that alternative methods for skin sensitization predict human rather than animal responses. However, from the perspective of comparative dose analysis of the complex mixtures of chemicals extracted from medical devices, some comparison to animal as well as human data may be helpful to support submissions to CDRH.
Acceptance of alternative methods and approaches to determine skin sensitization
CDER and CBER will accept a non-animal test battery that includes multiple endpoints as a screen prior to testing in humans. CDRH has not yet accepted non-animal alternatives for skin sensitization because the available methods have not been validated for mixtures of potentially weak sensitizers that may be found in medical devices. CVM will consider data from non-animal skin sensitization test methods on a case-by-case basis. Sponsors who wish to discuss their proposal for using alternative methods for skin sensitization assessments are encouraged to contact CDER, CBER, CDRH, or CVM as appropriate.
Occupational Safety and Health Administration
OSHA oversees enforcement of the Occupational Safety and Health Act (29 U.S.C. § 651 et seq. [1970]), which assures safety in the workplace, including the safe use of chemicals. Under OSHA’s Hazard Communication Standard (OSHA 2017), chemical manufacturers and importers must evaluate the hazards of the chemicals they produce or import. They must prepare labels and safety data sheets, and along with distributors, provide the safety data sheets for hazardous chemicals to downstream users to communicate information on these hazards. OSHA’s Hazard Communication Standard also requires that employers with hazardous chemicals in their workplaces make labels and safety data sheets available to their exposed workers and train workers to handle the chemicals appropriately. OSHA revised its Hazard Communication Standard in 2012 to align it with the third revision of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (UN 2009).
OSHA does not require that skin sensitization testing be conducted; it does, however, require that the available skin sensitization test data be used to fulfill its requirement for chemical hazard evaluation and communication. Chemical manufacturers and importers, not OSHA, are responsible for classifying toxicity hazards. Chemicals that meet the skin sensitizer criteria in Table 2 are required to be labeled with the same pictogram label (exclamation point), signal word (Warning), and precautionary statement regardless of whether they are classified as Category 1, 1A, or 1B (OSHA 2016).
Standards, guidelines, and guidance for skin sensitization testing
The classification criteria for skin sensitizers are described in Appendix A, A.4.2.2 of OSHA’s Hazard Communication Standard (OSHA 2017). OSHA requires that available test data from human and animal exposures be evaluated for skin sensitization classification. Substances may be allocated to sub-category 1A or 1B using a weight-of-evidence approach in accordance with the criteria in Table 2 and on the basis of reliable and high-quality evidence. Positive responses from the human repeat insult patch test or the human maximization test are considered sufficient evidence for classification in sub-categories 1A or 1B depending on the frequency and potency of the response. Positive results from the three specific animal test methods discussed below can also be used to classify skin sensitizers in sub-categories 1A and 1B. Additionally, classification can be based on epidemiological evidence.
OSHA has also published Hazard Classification Guidance for Manufacturers, Importers, and Employers (OSHA 2016), which assists manufacturers and importers of chemicals in identifying, classifying, and communicating chemical hazards. The procedure for skin sensitizer classification can be found in Chapter VII.4 of OSHA (2016).
Current requirements for animal testing and flexibility for the use of alternatives
Three animal test methods conducted according to OECD test guidelines, the LLNA, the guinea pig maximization test, and the Buehler test (OECD 1992, 2010a), can be used to classify skin sensitizers. Depending on the induction dose, test substances may meet the criteria for skin sensitization sub-categories 1A or 1B on the basis of the following test results (OSHA 2016):
-
A stimulation index of three or more in the LLNA.
-
Positive patch test response in at least 30% of animals in the guinea pig maximization test.
-
A positive patch test response in at least 15% of the animals in the Buehler test.
Other test methods may also be used to classify skin sensitizers provided they are scientifically validated. A method is considered “scientifically validated” if its reliability and relevance are established for a specific purpose (OSHA 2017). Any test that determines hazardous properties and is conducted according to recognized scientific principles can be used for a determination of health hazards. Therefore, reliance on animal testing is not mandatory, and the use of non-standard methods and data for close structural analogs can be considered as part of a weight-of-evidence approach for classification of skin sensitization on a case-by-case basis.
Information needed from alternative tests to fulfill the needs for skin sensitization data
OSHA requires chemical manufacturers, importers, and employers to use all available information, consistent with principles specified in Appendix A of the Hazard Communication Standard, to classify chemicals into the OSHA skin sensitization hazard categories. These categories are then used to determine the contents of product labels and safety data sheets (OSHA 2017).
OSHA indicates that toxic effects consistent with individual test criteria for hazard classification, whether seen in humans or animals, usually justify classification in a weight-of-evidence approach (OSHA 2017). The quality and reliability of the evidence from both sources are evaluated to resolve the question of classification. Therefore, positive results from animal studies are not necessarily negated by negative human data. Non-animal skin sensitization test methods that reliably predict human toxicity are generally more informative for OSHA’s purposes than methods that predict animal toxicity.
Acceptance of alternative methods and approaches to determine skin sensitization
Skin sensitizers are classified using a weight-of-evidence approach that either meets specific animal test method criteria (as noted above under “Current requirements for animal testing and flexibility for the use of alternatives”) or uses expert judgement to combine information from multiple sources of data. This means that chemical manufacturers, importers, and employers should consider all available information bearing on the classification for skin sensitization. This could include the results of valid in vitro tests, relevant animal data, and human experience such as epidemiological and clinical studies and well-documented case reports and observations. OSHA does not classify hazards itself and does not require or endorse particular alternative or standard approaches. Chemical manufacturers, importers, distributors, and employers are responsible for performing skin sensitization hazard assessments, classifying chemicals, and applying the appropriate labels and providing safety data sheets.
National Institute for Occupational Safety and Health
NIOSH was established as a research agency focused on worker safety and health under the Occupational Safety and Health Act of 1970 (29 U.S.C. § 671 [1970]), with the mandate to assure safe and healthful working conditions. NIOSH conducts research in occupational safety and health and recommends practices that help prevent work-related injury and illnesses. NIOSH is charged with recommending occupational safety and health standards and describing exposure levels that are safe for various periods of employment. These include but are not limited to setting limits for chemical exposures at which no employee will suffer diminished health, functional capacity, or life expectancy because of his or her work experience.
NIOSH provides information about skin sensitization potential of workplace chemicals in its authoritative recommendation documents such as Criteria Documents and Current Intelligence Bulletins. NIOSH also develops skin notations for workplace chemicals, which are hazard warnings to alert workers and employers to the health risks of skin exposure to chemicals in the workplace. The process for assigning skin notations is described in NIOSH Current Intelligence Bulletin 61: A Strategy for Assigning New NIOSH Skin Notations (NIOSH 2009). Skin notation assignments address multiple effects, including (1) systemic toxicity following dermal contact, (2) direct effects such as irritation and corrosion, and (3) immune-mediated effects such as allergic contact dermatitis. Substances identified as causing or contributing to allergic contact dermatitis or other immune-mediated responses, such as airway hyperreactivity (asthma) or systemic allergic reactions are identified with the notation “SEN”. Chemicals that have the potential to produce skin sensitization are assigned the skin designation “SK: SEN” in NIOSH skin notation profile documents (NIOSH 2009).
NIOSH also uses skin sensitization information in its evaluation of evidence during occupational risk assessment. NIOSH occupational risk assessments support NIOSH’s authoritative recommendations, including those it issues on exposure limits, controlling workplace exposures using engineering controls, and personal protective equipment.
Standards, guidelines, and guidance for skin sensitization testing
Assignment of hazard-specific skin notations and the development of occupational risk assessments are based on a weight-of-evidence approach and a critical review of available data, including (1) human health effects and exposure data, (2) in vivo toxicity study data, (3) in vitro toxicity study data, and (4) computational techniques, such as structure–activity relationships. Although epidemiologic studies, observational case reports, and clinical studies that include clinical investigations may be used as the basis of a sensitization skin notation, these data are often lacking or insufficient. For this reason, NIOSH generally relies on in vivo data that indicate the potential for allergic contact dermatitis or other immune-mediated responses associated with skin exposure. This includes the LLNA, guinea pig maximization test, the Buehler test, and the mouse ear swelling test (EPA 2003; OECD 1992). Tests that provide quantitative data for assessing the dose–response relationship are preferred.
Current requirements for animal testing and flexibility for the use of alternatives
NIOSH does not require specific tests for the development of skin notations or occupational risk assessments. NIOSH uses all available data to support its analyses supporting authoritative recommendations. Skin sensitization testing in animals may not be necessary for an assessment if data generated with scientifically validated non-animal test methods are available for both hazard identification and potency assessment. NIOSH considers a method “scientifically validated” if its accuracy, reliability, and relevance are established using recognized scientific principles. For evaluations supporting skin notations and occupational risk assessment, the use of non-standard methods and data for close structural analogs can be considered as part of a weight-of-evidence approach on a case-by-case basis.
NIOSH generally uses data from both animal and human exposures, when available, to support skin notations and occupational risk assessments. When data are not available NIOSH may generate the required data using in vivo or in vitro methods. When conflicting evidence is obtained between animal and human sources, the quality and reliability of each dataset are assessed on a case-by-case basis to determine classification. The impact of the vehicle used in each study is also considered.
Information needed from alternative tests to fulfill the needs for skin sensitization data
NIOSH has provided its criteria for information supporting a skin notation for sensitizers in its publication, “NIOSH Current Intelligence Bulletin 61: A Strategy for Assigning New NIOSH Skin Notations” (NIOSH 2009). NIOSH occupational risk assessments, on the other hand, evaluate all available data to develop conclusions about the hazards of chemicals, including sensitization potential. Information obtained from any test must be sufficient to support a finding of hazard. Alternative methods that predict human responses rather than animal responses are ranked more highly in the hierarchy of preferred data for skin sensitization assessments.
Acceptance of alternative methods and approaches to determine skin sensitization
The hierarchy of evaluated scientific data for assigning skin notations is: (1) human health effects and exposure data, (2) in vivo toxicity study data, (3) in vitro toxicity study data, and (4) computational techniques. When there are no empirical data of acceptable quality to determine sensitization potential, information from structure–activity relationships and other computational techniques for identifying hazards are considered. Since the performance and reliability of computational techniques currently remains unclear, the predictions from these techniques are not used as the primary basis for assignment of skin notations at this time.
NIOSH uses all available data in its occupational risk assessments, including valid in vivo and in vitro information. Occupational risk assessment relies on the weight of the evidence using expert judgment to determine specific hazard findings.
US Department of Defense
DoD is not a regulatory agency and has no statutory requirements mandating the collection and use of skin sensitization data. However, DoD generates and uses skin sensitization data with the goal of protecting DoD active duty and civilian personnel who may be exposed to chemicals. New substances entering the supply chain and new and hypothetical substances under research, development, testing, and evaluation must be evaluated for toxicity.
Standards, guidelines, and guidance for skin sensitization testing
DoD relies on data generated using EPA and OECD test guidelines for skin sensitization testing (EPA 2003; OECD 1992, 2010a, b, c, 2015, 2017a, 2018c). Historically, the Buehler test was preferred by the Army; however, the LLNA is currently the preferred animal test method for the Army and the Navy. To determine if an animal test is necessary, the Air Force evaluates the existing animal and class data for a chemical or the individual components of a chemical mixture. Non-animal alternative test results will be considered during evaluation. If a determination cannot be made or data are equivocal, a non-animal alternative test will be considered. If an animal test is required, the Air Force currently prefers a modified Buehler test.
Current requirements for animal testing and flexibility for the use of alternatives
DoD uses a phased approach for toxicity assessments and ultimately for Toxicity Clearances, which are required by the Army prior to the acquisition and use of new substances. Early in the development of a new substance for DoD use, in silico data or data from in vitro tests are used for skin sensitization hazard assessments. Testing is performed only when data needed to develop hazard assessments are unavailable or incomplete. Animal tests are used when potency information is needed or when exposure limits must be developed.
Information needed from alternative tests to fulfill the needs for skin sensitization data
For the early evaluation of new substances for skin sensitization potential, alternatives to animal tests for skin sensitization should provide information sufficient to develop relevant hazard assessments. Information on skin sensitization potency may also be needed for toxicity assessments of new and theoretical substances early in the research, development, testing and evaluation phase. Substance-specific exposure limits may be developed when none exist. DoD would prefer that in vitro alternative methods for skin sensitization testing predict human responses than animal responses when results are shown to be robust.
Acceptance of alternative methods and approaches to determine skin sensitization
Alternative methods are currently being used for skin sensitization hazard assessments early in the research, development, testing, and evaluation process of new substances. Toxicity assessments for early evaluations are performed by integrating data from in silico, read-across, and in vitro methods. Animal data are currently used for determination of potency and for Toxicity Clearances within the Army. Other services address toxicity and use consistent with the health hazard assessment paradigm.
Summary of agency information needs
Information from skin sensitization testing is used to manage exposure to skin allergens to minimize the occurrence of allergic contact dermatitis. Skin sensitization information is necessary across multiple agencies and regulatory statutes for hazard classification, warning label information, potency categorization, and risk assessment. Table 3 summarizes how the ICCVAM agencies contributing to this review currently use skin sensitization data for hazard classification, potency, or risk assessment.
Some US authorities prefer or suggest animal data for skin sensitization assessments (Table 3). Animal test data are preferred by EPA OPP for formulations, if waivers and bridging cannot be applied, and by FDA CDRH for medical devices (or device extracts), if qualified non-animal methods are unavailable. However, CPSC prefers that existing human data be used, when possible, over animal data. Although FDA CFSAN does not require the submission or use of skin sensitization test data for cosmetic products under its regulatory authority, manufacturers or distributors are responsible for ensuring that marketed products are safe. Manufacturers or distributors may accomplish this using either animal or non-animal tests. EPA OPPT does not require the submission of skin sensitization data; however, EPA OPPT may require the generation of this type of information on a case-by-case basis. In cases where animal data are available, OPPT may use these data to determine whether substances might produce an unreasonable health risk under expected conditions of use. OSHA does not require submission of skin sensitization test data, but it does require the chemical’s manufacturer or importer to evaluate the available skin sensitization data and classify the hazard according to specific criteria as part of its Hazard Communication Standard (Table 2). DoD uses non-animal data early in the evaluation of new substances for hazard determination and then uses animal data in cases where evaluations of potency and risk are needed.
Although most of these US authorities prefer animal data for some applications, many are flexible in the consideration and acceptance of alternative methods. CPSC considers non-animal methods on a case-by-case basis. EPA OPP accepts two non-animal defined approaches (for single chemical substances only) as well as requests for animal test waivers. EPA OPPT currently uses non-animal methods in its hazard assessments, most recently including the defined approaches accepted by OPP; however, OPPT may require in vivo data to determine whether substances might produce an unreasonable health risk. FDA CDER and CBER currently consider data from non-animal test batteries that cover multiple endpoints to screen topical products. If a method is qualified for use with medical devices, FDA CDRH is amenable to using a non-animal test battery to assess sensitization potential. OSHA allows the use of scientifically validated non-animal skin sensitization testing methods provided the relevance and reliability are established using recognized scientific principles and approaches. DoD uses non-animal methods early in the toxicity evaluation of new substances.
Obstacles and opportunities to replacing animal use
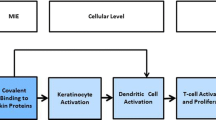
One obstacle in replacing animal tests for skin sensitization potential with non-animal tests is the complexity of the skin sensitization endpoint. This complexity is mitigated by an adverse outcome pathway (AOP) for skin sensitization, which provides a framework, and opportunity, for the targeted development of non-animal methods that measure specific activities related to the skin sensitization potential of tested substances (OECD 2012a, b). The AOP summarizes the sequence of biological events that occur from the time a chemical structure interacts with an organism through the in vivo outcome of interest. The AOP for skin sensitization initiated by covalent binding to proteins (Fig. 1) includes four key events with well-accepted biological significance: (1) binding of haptens to endogenous proteins in the skin, (2) keratinocyte activation, (3) dendritic cell activation, and (4) proliferation of antigen-specific T cells. Six non-animal methods incorporated into internationally recognized test guidelines adopted by OECD member countries assess the ability of chemicals to activate the first three key events of the skin sensitization AOP (Fig. 1).
The adverse outcome pathway for skin sensitization initiated by covalent binding to proteins, with notations indicating accepted OECD test guidelines (TG) for non-animal methods that measure key events in the AOP. Figure reprinted from Strickland et al. (2016)
-
Key Event 1, formation of a hapten–protein complex by the test substance, is measured by the in chemico DPRA (OECD 2015).
-
Key Event 2, activation of keratinocytes, is measured by the KeratinoSens and LuSens methods (OECD 2018c).
-
Key Event 3, activation of dendritic cells, is measured by the h-CLAT, the IL8-Luc assay, and the U-SENS assay (OECD 2017a).
There are currently no non-animal methods that assess the ability of substances to activate Key Event 4, the proliferation of activated T cells.
Because of the multiple key events that must occur to produce skin sensitization, it is generally believed that no single non-animal test that measures one key event can fully replace animal models for hazard identification (Rovida et al. 2015). Thus, the methods described in the non-animal OECD test guidelines are not currently accepted as stand-alone replacement methods (OECD 2015, 2017a, 2018c). Instead, they are recommended to be used as part of a weight-of-evidence approach to skin sensitization hazard assessment, although some regulatory authorities may accept positive results without additional supporting information.
Despite the availability and international acceptance of these methods, a number of obstacles still exist to the full replacement of animal use with non-animal methods for skin sensitization assessment.
Regulatory acceptance of integrated strategies
Because no specific integrated strategies, or defined approaches, have been prescribed by the OECD test guidelines, there is a degree of uncertainty about how to use and apply data obtained from these non-animal methods. The development and validation of integrated strategies would promote use of these methods by chemical sponsors and acceptance of data derived from these methods by US regulatory agencies. A number of integrated approaches have been submitted to OECD and documented in a structured format as examples to facilitate regulatory review in Annex I of OECD Guidance Document 256 (OECD 2016b). These approaches have not been validated for regulatory use, but progress is being made toward that end. A recent collaboration between NICEATM and Cosmetics Europe showed that a number of these non-animal testing strategies were comparable or superior to the LLNA when evaluated against human outcomes for over 120 chemicals (Kleinstreuer et al. 2018). The European Union Reference Laboratory for Alternatives to Animal Testing recently recommended that the published defined approaches for skin sensitization should be used where applicable instead of, or in conjunction with, the LLNA for hazard classification (JRC 2017). This recommendation is supported by OECD, as indicated by the recent approval of a project to develop a new performance-based test guideline for defined approaches and test methods for skin sensitization (OECD 2017b).
Lack of information
Another potential obstacle to the use of non-animal methods for skin sensitization assessment may be a lack of information about their availability. However, a number of government and non-government organizations distribute such information via their websites; a few are mentioned here as examples. The ICCVAM website lists a number of approaches that can reduce, replace, or refine animal use for skin sensitization testing (NTP 2018). The European Union Joint Research Centre’s DataBase service on Alternative Methods (DB-ALM) (JRC 2018) is another valuable source of information about available non-animal alternative methods. Perhaps the most well-known source internationally is the OECD. The OECD distributes alternative toxicity test guidelines that have been adopted by 36 member countries (OECD 2018a). The OECD has skin sensitization test guidelines for three animal reduction and refinement methods, the LLNA and its modifications, that still use animals (OECD 2010a, b, c) and three test guidelines for non-animal methods (OECD 2015, 2017a, 2018c).
Lack of validated non-animal alternatives for mixtures
Another obstacle to the use of non-animal methods for skin sensitization assessments is the lack of validated alternatives for the assessment of mixtures. Many substances regulated by agencies contributing to this review are mixtures rather than single substances. The non-animal test methods adopted by OECD are technically suitable for testing mixtures that are soluble or form stable dispersions (OECD 2015, 2017a, 2018c), but not for testing products with the consistency of gels, ointments, or creams. Even testing mixtures in solution is complicated for the DPRA, KeratinoSens, and LuSens because the test guidelines specify that chemicals are to be applied in molar concentrations. In limited evaluations, modifications to the DPRA for testing mixtures have met with little success (de Ávila et al. 2017), but modifications to KeratinoSens seem promising (Settivari et al. 2015). Recent efforts show that the performance of the h-CLAT for testing mixtures is also promising (Varsho et al. 2017).
Lack of validated non-animal alternatives for potency assessment
The lack of validated non-animal alternatives for the assessment of skin sensitization potency is also an obstacle to the use of such methods for skin sensitization assessments. The OECD non-animal test guidelines propose the methods for hazard assessment when used with other relevant complementary information (OECD 2015, 2017a, 2018c). The guidelines typically indicate that the methods may potentially contribute to potency assessments when integrated with other information, however, additional work should be performed to determine exactly how these methods could inform potency assessments. The test guideline for the activation of keratinocytes is more confident about the use of KeratinoSens for assessing potency in combination with additional information because a number of strategies have been evaluated in the scientific literature (OECD 2018c).
Lack of clear information about the acceptability of methods for specific regulatory purposes
A frequently overlooked consideration in the implementation of new methods is the legal framework in which data from these methods must be considered. Regulatory agencies need to clearly communicate their information needs as defined by the statues under which they operate. Though the regulatory authorities often communicate that non-animal methods may be appropriate in certain situations, guidance on the subject is generally limited to encouraging sponsors designing their testing strategies to consult directly with the agency that is responsible for regulating the test product. Specific information, such as lists of accepted non-animal methods and the purposes for which they may be used, has not been provided. Progress in this area could be achieved through improved communication between industry and government regulatory agencies to ensure clarity when defining expectations from both sides. One recent example of clear communication about acceptable non-animal methods or strategies and their application is EPA’s interim policy whereby OPP and OPPT will accept skin sensitization data submissions for two specific defined approaches for testing single chemical entities (EPA 2018a).
Communication and training
Communication within regulatory agencies is also essential to implementing alternative methods. Active training programs within federal regulatory agencies can provide information about the most recent advances in non-animal approaches and facilitate more consistent and timely decisions regarding data submission acceptance. Some regulatory agencies already have these types of programs in place. For example, EPA OPP and OPPT hold in-house training on the use of in vitro and in silico methods for toxicity assessments. ICCVAM is working with stakeholders to promote existing alternative methods to US regulatory agencies and to facilitate understanding of how they may be used. NICEATM assists in developing training materials and provides technical support to assist agencies in issuing guidance for the use of non-animal methods (NIEHS 2018).
Global harmonization
Global harmonization also influences the level of alternative method use, particularly among international companies. It is more economically practical for product sponsors to conduct a single test, usually an animal test that is internationally accepted, than to undertake duplicate testing to meet conflicting regulatory requirements of multiple agencies. This is a very real challenge now for skin sensitization assessments. For example, non-animal tests for the evaluation of cosmetics are accepted in Europe, Japan, and South Korea, but China requires animal tests, and not only for cosmetics, but for all chemical sectors (Daniel et al. 2018). The approach recently taken by the European Chemicals Agency to fulfill chemical registration requirements of the Registration, Evaluation, Authorization, and Restriction of Chemicals is directly opposite that of China. The European Chemicals Agency now requires that, in the absence of existing data, skin sensitization assessments should start with in vitro testing rather than in vivo testing (ECHA 2017).
OECD has an important role in global harmonization of test methods used for skin sensitization assessments and thereby in promoting the acceptance of non-animal methods. The OECD test guideline program uses input from member country national coordinators and experts to produce internationally agreed upon standards for regulatory toxicity testing (OECD 2018b). Per the Mutual Acceptance of Data agreement among OECD member countries, US agencies and all member countries must accept data generated using OECD test guidelines for review. However, individual agencies may require additional data to fulfill their regulatory requirements. As indicated previously, OECD member countries have recently approved a project to develop a new performance-based test guideline for defined approaches and test methods for skin sensitization (OECD 2017b). The adoption of such an OECD test guideline will encourage implementation of defined approaches and raise awareness about these approaches in chemical regulatory agencies all over the world.
Another collaborative effort aimed at facilitating worldwide acceptance of alternative methods is the International Cooperation on Alternative Test Methods (ICATM), which includes ICCVAM and governmental organizations from six other countries and regional authorities: the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM), the Japanese Centre for the Validation of Alternative Methods (JaCVAM), the Korean Center for the Validation of Alternative Methods (KoCVAM), Health Canada (the Canadian Centre for Alternatives to Animal Methods [CCAAM] and its subsidiary, the Canadian Centre for the Validation of Alternative Methods [CaCVAM], participate as partners with Health Canada in ICATM activities), the Brazilian Center for the Validation of Alternative Methods (BraCVAM) and China. ICCVAM partners with the other ICATM participants to promote enhanced international cooperation and coordination on the scientific development, validation, and regulatory use of alternative approaches. ICATM works to ensure that the alternative methods adopted internationally for regulatory use will provide equivalent or improved protection for humans, animals, and the environment while reducing, refining, or replacing animal use when possible. ICATM partners work together by sharing expertise on test method validation management teams and test method peer reviews.
A specific ICATM activity that continues to have international influence is the workshop International Regulatory Applicability and Acceptance of Alternative Non-animal Approaches to Skin Sensitization Assessment of Chemicals, which was hosted by EURL ECVAM and held in October 2016. The workshop is an example of how progress can be made internationally regarding the acceptance of alternative methods for skin sensitization. It was attended by international regulatory authorities from 14 countries, test method validation authorities, and supporting organizations, such as the OECD and the Scientific Committee on Consumer Safety, which is an independent committee that provides advice to the European Commission. Workshop participants reviewed the performance of multiple non-animal integrated strategies for skin sensitization hazard assessment from the NICEATM-Cosmetics Europe collaboration reported by Kleinstreuer et al. (2018), discussed regulatory requirements for skin sensitization testing among various global regions by chemical sector, discussed obstacles to implementing non-animal approaches, and planned the path forward for evaluating and accepting integrated approaches in lieu of animals for skin sensitization testing (Casati et al. 2018). ICATM partners followed the workshop by publishing a position paper on the standardization and use of defined approaches for regulatory purposes (Casati et al. 2018) and a paper on the skin sensitization information needs of regulatory authorities for multiple chemical sectors in the countries and regions covered by ICATM partners (Daniel et al. 2018). As an additional follow-up activity, ICATM partners ICCVAM, EURL ECVAM, and Health Canada sponsored the OECD proposal to develop the performance-based test guideline for defined approaches and test methods for skin sensitization.
Conclusion
The future of non-animal methods for skin sensitization testing seems promising; however, the next steps toward implementation and acceptance by research and regulatory authorities will require communication and interaction to establish scientific confidence in their ability to protect human health. ICCVAM is leading this effort in the United States by coordinating a strategic roadmap for establishing new approaches to evaluate the safety of chemicals and medical products; the implementation plan for the roadmap specifically addresses the development, evaluation, and acceptance of alternative approaches for skin sensitization testing (NIEHS 2018). As alternative methods are progressively considered and accepted at the national level, global harmonization will be necessary to achieve global implementation. Partnerships with international regulatory authorities and supporting organizations are critical to the widespread evaluation and implementation of new approaches.
References
ASTM (2013) F 2148-13, standard practice for evaluation of delayed contact hypersensitivity using the murine local lymph node assay (LLNA). American Society for Testing and Materials, West Conshohocken
Casati S, Aschberger K, Barroso J, Casey W, Delgado I, Kim TS, Kleinstreuer N, Kojima H, Lee JK, Lowit A, Park HK, Regimbald-Krnel MJ, Strickland J, Whelan M, Yang Y, Zuang V (2018) Standardisation of defined approaches for skin sensitisation testing to support regulatory use and international adoption: position of the international cooperation on alternative test methods. Arch Toxicol 92(2):611–617
Casey W (2016) Advances in the development and validation of test methods in the United States. Toxicol Res 32(1):9–14
CPSC (2012) Recommended procedures regarding the CPSC’s policy on animal testing. https://www.cpsc.gov/business--manufacturing/testing-certification/recommended-procedures-regarding-the-cpscs-policy-on-animal-testing Accessed 10 July 2018
CPSC (2013) CPSC staff’s strong sensitizer guidance document. https://www.cpsc.gov/s3fs-public/pdfs/blk_pdf_strongsensitizerguidance.pdf. Accessed 10 July 2018
CPSC (2017a) Art materials. Labeling of hazardous art materials act. 16 CFR 1500.14(b)(8)
CPSC (2017b) Certain statutory definitions interpreted, supplemented, or provided with alternatives. Federal Hazardous Substances Act. 16 CFR 1500.3(c)(5)
CPSC (2017c) Definition of sensitizer. Federal Hazardous Substances Act. 16 CFR 1500.3(c)(5)(i)
CPSC (2017d) Listing of “strong sensitizer” substances. Federal Hazardous Substances Act. 16 CFR 1500.13
CPSC (2017e) Significant potential for causing hypersensitivity. Federal Hazardous Substances Act. 16 CFR 1500.3(c)(5)(ii)
CPSC (2017f) Statement on animal testing policy. Federal Hazardous Substances Act. 16 CFR 1500.232
Daniel AB, Strickland J, Allen D, Casati S, Zuang V, Barroso J, Whelan M, Regimbald-Krnel MJ, Kojima H, Nishikawa A, Park HK, Lee JK, Kim TS, Delgado I, Rios L, Yang Y, Wang G, Kleinstreuer N (2018) International regulatory requirements for skin sensitization testing. Regul Toxicol Pharmacol 95:52–65
de Ávila R, Teixeira G, Veloso D, Moreira L, Lima E, Valadares M (2017) In vitro assessment of skin sensitization, photosensitization and phototoxicity potential of commercial glyphosate-containing formulations. Toxicol In Vitro 45(Pt 3):385–392
ECHA (2017) Guidance on information requirements and chemical safety assessment. Chapter R.7a: endpoint specific guidance (version 6.0). European Chemicals Agency (ECHA), Helsinki
EPA (2003) Health effects test guidelines: OPPTS 870.2600—skin sensitization. US Environmental Protection Agency, Washington, DC
EPA (2011) Expansion of the traditional local lymph node assay for the assessment of dermal sensitization potential of end use pesticide products; and adoption of a “reduced” protocol for the traditional LLNA (limit dose). Office of Pesticide Programs, Washington, DC
EPA (2012) Guidance for waiving or bridging of mammalian acute toxicity tests for pesticides and pesticide products (acute oral, acute dermal, acute inhalation, primary eye, primary dermal, and dermal sensitization) (March 1, 2012). Office of Pesticide Programs, Washington, DC
EPA (2016) Process for evaluating and implementing alternative approaches to traditional in vivo acute toxicity studies for FIFRA regulatory use. Office of Pesticide Programs, Washington, DC
EPA (2017) Summary of the Federal Insecticide, Fungicide, and Rodenticide Act. https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act. Accessed 10 July 2018
EPA (2018a) Interim science policy: use of alternative approaches for skin sensitization as a replacement for laboratory animal testing. Office of Chemical Safety and Pollution Prevention, Office of Pesticide Programs, Office of Pollution Prevention and Toxics, Washington, DC
EPA (2018b) Strategic plan to promote the development and implementation of alternative methods within the TSCA program. Office of Chemical Safety and Pollution Prevention, Washington, DC
EPA (2018c) Strategic vision for adopting 21st century science methodologies. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/strategic-vision-adopting-21st-century-science Accessed 10 July 2018
FDA (1975) Cosmetic products: warning statements/package labels. Fed Reg 40(42):8912–8916
FDA (1999a) Guidance for industry—skin irritation and sensitization testing of generic transdermal drug products (draft guidance). http://www.pharmanet.com.br/pdf/2887dft.pdf. Accessed 31 July 2018
FDA (1999b) Guidance for industry and FDA reviewers/staff: premarket notification [501(k)] submissions for testing for skin sensitization to chemicals in natural rubber products. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm073793.pdf. Accessed 31 July 2018
FDA (2002) Guidance for industry: immunotoxicology evaluation of investigational new drugs. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM079239.pdf. Accessed 31 July 2018
FDA (2008) Guidance for industry and FDA staff: medical glove guidance manual. https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm428191.pdf. Accessed 31 July 2018
FDA (2016) Guidance for Industry and Food and Drug Administration staff: use of international standard ISO 10993-1, “Biological evaluation of medical devices—part 1: evaluation and testing within a risk management process. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm348890.pdf. Accessed 31 July 2018
FDA (2018a) About the Center for Devices and Radiologic Health. https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cdrh/. Accessed 10 July 2018
FDA (2018b) About the Center for Drug Evaluation and Research. In. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/default.htm. Accessed 10 July 2018
FDA (2018c) About the Center for Veterinary Medicine (CVM). In. https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CVM/default.htm. Accessed 26 July 2018
FDA (2018d) CBER vision & mission. In. https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm122878.htm. Accessed 26 July 2018
FDA (2018e) CFSAN—what we do. https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/WhatWeDo/default.htm. Accessed 10 July 2018
FDA (2018f) Product testing. https://www.fda.gov/Cosmetics/ScienceResearch/ProductTesting. Accessed 10 July 2018
ISO (2010) ISO 10993-10: Biological evaluation of medical devices—part 10: tests for irritation and skin sensitization. International Organization for Standardization, Geneva
JRC (2017) EURL ECVAM recommendation on the use of non-animal approaches for skin sensitisation testing. Publications Office of the European Union, Luxembourg
JRC (2018) Database on alternative methods (DB-ALM). https://eurl-ecvam.jrc.ec.europa.eu/databases/database-on-alternative-methods-db-alm. Accessed 26 July 2018
Kleinstreuer N, Hoffmann S, Alepee N et al (2018) Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit Rev Toxicol 48(5):359–374
Murphy K, Travers P, Walport M, Janeway C (2012) Janeway’s immunobiology, 8th edn. Garland Science, New York
NIEHS (2018) A strategic roadmap for establishing new approaches to evaluate the safety of chemicals and medical products in the United States. https://www.ntp.niehs.nih.gov/go/natl-strategy. Accessed 10 July 2018
NIOSH (2009) A strategy for assigning new NIOSH skin notations. Current intelligence bulletin 61. DHHS (NIOSH) Publication No. 2009-147
NTP (2018) Alternative methods accepted by US agencies. https://ntp.niehs.nih.gov/pubhealth/evalatm/accept-methods/index.html. Accessed 10 July 2018
OECD (1992) Test no. 406. Skin sensitisation. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2010a) Test no. 429. Skin sensitisation: local lymph node assay. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2010b) Test no. 442A. Skin sensitization: local lymph node assay: DA. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2010c) Test no. 442B. Skin sensitization: local lymph node assay: BrdU-ELISA. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2012a) OECD series on testing and assessment no. 168. The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. Part 2: use of the AOP to develop chemical categories and integrated assessment and testing approaches. OECD Publishing, Paris
OECD (2012b) OECD series on testing and assessment no. 168. The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. Part 1: scientific assessment. OECD Publishing, Paris
OECD (2015) Test no. 442C. In chemico skin sensitization: direct peptide reactivity assay (DPRA). OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2016a) OECD series on testing and assessment no. 256. Guidance document on the reporting of defined approaches and individual information sources to be used within integrated approaches to testing and assessment (IATA) for skin sensitisation. OECD Publishing, Paris
OECD (2016b) OECD series on testing and assessment no. 256. Guidance document on the reporting of defined approaches and individual information sources to be used within integrated approaches to testing and assessment (IATA) for skin sensitisation. Annex 1. OECD Publishing, Paris
OECD (2017a) Test no. 442E. In vitro skin sensitisation. In vitro skin sensitisation assays addressing the key event on activation of dendritic cells on the adverse outcome pathway for skin sensitisation. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2017b) Work plan for the test guidelines program (TGP) as of August 2017. http://www.oecd.org/chemicalsafety/testing/TGP%20work%20plan_August%202017.pdf. Accessed 31 July 2018
OECD (2018a) OECD guidelines for the testing of chemicals, section 4, health effects. https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788. Accessed 26 July 2018
OECD (2018b) OECD test guidelines programme. http://www.oecd.org/chemicalsafety/testing/oecd-guidelines-testing-chemicals-related-documents.htm. Accessed 26 July 2018
OECD (2018c) Test no. 442D. In vitro skin sensitisation: ARE-Nrf2 luciferase test method. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OSHA (2016) Hazard communication: hazard classification guidance for manufacturers, importers, and employees. Occupational Safety and Health Administration, US Department of Labor Publication: OSHA 3844-02. https://www.osha.gov/Publications/OSHA3844.pdf. Accessed 10 July 2018
OSHA (2017) Hazard communication. Occupational Safety and Health Act. 29 CFR 1910.1200
Rovida C, Alepee N, Api AM et al (2015) Integrated testing strategies (ITS) for safety assessment. ALTEX 32(1):25–40
Settivari R, Gehen S, Amado R, Visconti N, Boverhof D, Carney E (2015) Application of the KeratinoSens™ assay for assessing the skin sensitization potential of agrochemical active ingredients and formulations. Regul Toxicol Pharmacol 72(2):350–360
Strickland J, Zang Q, Kleinstreuer N, Paris M, Lehmann DM, Choksi N, Matheson J, Jacobs A, Lowit A, Allen D, Casey W (2016) Integrated decision strategies for skin sensitization hazard. J Appl Toxicol 36(9):1150–1162
UN (2009) Globally harmonized system of classification and labelling of chemicals, vol ST/SG/AC.10/30/Rev.3, 3rd rev. edn. United Nations, New York
Varsho B, Carathers M, Vij P, DeGeorge G (2017) The human cell line activation test (h-CLAT) for assessment of dermal sensitization potency of commercially available mixtures and the OECD proficiency chemicals. ALTEX Proc 5(1):40
Acknowledgements
The authors thank Drs. B. Alex Merrick and Kristen Ryan for their thoughtful critical review of this manuscript, and Ms. Catherine Sprankle for editorial review. This project was funded in part with federal funds from the National Institute of Environmental Health Sciences, National Institutes of Health under Contract No. HHSN273201500010C to ILS in support of the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods.
Disclaimer
This article may be the work product of an employee or group of employees of the US CPSC, DoD, EPA, FDA, NIEHS, NIH, NIOSH, OSHA, or other organizations. However, the statements, opinions, or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of these organizations or the United States government. ILS staff provides technical support for NICEATM, but do not represent NIEHS, NTP, or the official positions of any federal agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Strickland, J., Daniel, A.B., Allen, D. et al. Skin sensitization testing needs and data uses by US regulatory and research agencies. Arch Toxicol 93, 273–291 (2019). https://doi.org/10.1007/s00204-018-2341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2341-6