Abstract
Brain tumour is a serious issue caused in the brain, where a group of unwanted tissues grows in unrestrained manner. The image-processing field has gained outstanding growth in the region of medical applications with the discovery of various approaches in baseline methods. Convolutional Neural Networks (CNN) is applied in the process of brain tumour identification and categorization due to their significant performance in image processing. The main limitation of existing approaches is that it has a large count of constraints and produced elevated implementation period and requires high specification for system implementation. Hence, this paper aims to design the efficient brain tumour classification with the help of various technologies. At first, the data is collected from online resources. Next, it is pre-processed by the ‘median filtering and the Contrast Limited Adaptive Histogram Enhancement (CLAHE) techniques’. Then, the pre-processed images are inputted to the final classification phase, where the final output is classified by the parameter optimized CNN by Dingo Optimizer (DOX). The results on the dataset have revealed the best performance in comparison with several traditional methods.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Brain Tumour Classification
- Convolutional Neural Network
- Contrast Limited Adaptive Histogram Enhancement
- Dingo Optimizer
- Parameter optimized CNN
1 Introduction
The brain is a vital organ in human body with compound structures [1]. The brain is surrounded with the skull region that makes it difficult to detect activities of brain. Abnormality of brain would lead in affecting structure of brain and cause brain cancer. The report of World Health Organization (WHO) has specified that 9.6 million peoples overall died of cancer in 2018 [2]. For analysing the brain tumour, ‘Magnetic resonance imaging (MRI)’ is usually used to spot the brain tumours with high-quality images. The MRI provides the best visualization of both contrasts and spatial resolution [3].
Brain is a complicated organ, which play an essential part in our daily behaviours and the uncontrollable, unequal tissue growth in the brain is named as brain tumour that is a dreadful disease [4]. The most widely detected tumour types are Pituitary, Meningioma, and Glioma [5]. The Glioma tumours are high-grade tumours usually present in cerebral hemisphere named as attocycles, meningioma is low-grade tumours in skull, Tumours in pituitary gland are called pituitary tumour [6].
The recent researches were undergone to implement machine learning to detect the Region of Interest (ROI) in data in an efficient manner than other traditional methods [7]. Baseline algorithms have the capability to identify different ranges of data depiction for the detection and prediction task. CNN is a type of deep neural networks that is utilized effectively on image-processing technique that involves object detection, segmentation, and classification of image [8]. The CNN is concentrated mostly in object detection and classification. Numerous researches have executed in the region of brain tumour identification, segmentation, categorization and various positive results have attained based on this techniques. The other techniques like ‘Statistical Region Merging, K-means clustering and Fuzzy Clustering’ are new approaches in predictive analysis for the identification and categorization of brain tumour, and these approaches are performed better than other conventional approaches [9]. The adoption of deep learning methods is often allocated with high computational cost and reduction in accuracy [10]. Moreover, it has less prediction certainty that keeps the life of human in loop, which is dangerous for medical field. The main contribution of brain tumour classification method is explained below. To design an efficient brain tumour classification by inputting MRI brain images with CNN for classification and optimization-based algorithm for parameter tuning. Categorize the brain images with CNN and optimization of hidden neurons and count of epochs using DOX algorithm and by increasing the accuracy and precision of brain tumour classification. Demonstrate the performance of the introduced method over various existing methods by analysing numerous paradigm measures.
2 Literature Survey
2.1 Related Works
In 2020, Ge et al. [11] have investigated the issues in the classification of brain tumour with MRI from various scanning procedures like ‘T2 weighted, T1 weighted’, FLAIR and contrast improved with T1-weighted images. The currently available glioma dataset was different in dimension and mostly associated with unfinished MRIs in dissimilar modalities. To overcome the issues related to insufficient dataset and unfinished modality of brain images, a new Generative Adversarial Network (GAN) method was proposed to expand the brain tumour dataset. The GAN was capable to produce artificial MRIs in different procedures. The post-processing method was introduced to detect the glucose level of patients. The attributes of glioma were extracted with two phase GAN-improved MRIs. The effectiveness of the suggested scheme was estimated and the experimental were performed on brain tumour dataset for glioma classification. The simulation results have achieved good performance than other existing approaches.
In 2021, Habib et al. [12] have planned for the segmentation of brain tumour on MRI images with threshold segmentation and then brain tumour images were classified, and then the features were extracted with various classifiers. The suggested methodology has involved four phases like ‘image acquisition, image pre-processing, image segmentation, and feature extraction’. The brain tumours datasets were classified accurately with various classifiers. The conclusions have specified that the recommended framework has attained high accuracy in brain tumour detection. In 2021, Khairandish et al. [13] have categorized the MRI brain images using CNN in standard dataset to categorize Malignant and Benign tumours. The deep learning models have achieved high performance in image classification in recent years. The CNN approach was implemented to extract the features from images and has attained high accuracy regarding classification and for the threshold-based segmentation regarding classification.
In 2021, Kesav and Jibukumar [14] have suggested a novel framework for brain tumour object recognition and categorization via RNN approach, which was analysed on two standard datasets from Kaggle and figshare. The proposed work has planned to reduce the training period of RNN with less complex scheme and implemented a novel framework for brain detection. The CNN was adopted to categorize the healthy and unhealthy tumours in MRI images. Then, the attributes were extracted with RCNN to identify the brain tumour regions with MRI images and further, the region of tumour was enclosed with bouncing boxes. Therefore, this approach has expanded to notice various types of tumours. Hence, the introduced model has attained low training time than other existing techniques. In 2020, Afshar et al. [15] have suggested ‘Bayesian CapsNet’ scheme that was utilized to predict the brain tumour and also measure the improbability in prediction. The obtained results have shown that the removing the improbability in forecast has enhanced the precision of the recommended method. The removal of undefined prediction was a suitable approach for enhancing the network interpretability.
2.2 Problem Statement
The CNNs, which were the most image-based applications, are susceptible to lose essential spatial details among image occurrences. Because of the lethal nature of this malignancy and the repercussions of tumour misclassification, brain tumour classification is of fundamental relevance in the field of medical imaging difficulties. GAN [11] is robust and efficient and the performance of classification is better. But multiple datasets are not applied. Hybrid algorithm [12] utilizes the datasets in an appropriate manner and the accuracy is very high. Still, the exact location and size of the tumour is not considered. CNN-SVM [13] offers much efficient and enhancement approaches for the classification and high classification output rate is attained. Yet, various optimization algorithms are not considered. RCNN [14] supports in the real time processing and can be incorporated in handheld tools having lesser computational facilities. But it has the drawback of object detection. BayesCap [15] increases the trust into the decisions and the accuracy is enhanced. Still, the aleatoric uncertainty is not included. Hence, it is needed to include new deep structured learning approaches for efficient brain tumour classification.
2.3 Proposed Model- Brain Tumour Classification Enabled by Proposed Deep Learning
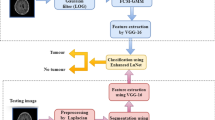
Brain is the majority compound organ with billions of neuron cells. The brain tumours are formed when the uncontrolled cells divide into abnormal cell groups. The brain tumours are categorized into high grade and low grade tumours. The MRI method is usually used to detect the tumour cells in brain and it has a great capability to analyse the brain structures. However, the high grade cancers are difficult to detect because it is formed under the skull regions. Various learning methods were adopted to recognize the image accurately. CNN is used for detecting and classifying the brain tumours automatically. Therefore, optimized CNN is adopted in this proposed method for accurate classification of brain tumours. The structural representation of brain tumour classification is represented in Fig. 1.
The proposed method covers three stages like, ‘(a) data collection, (b) pre-processing, and (c) classification’. The dataset is selected from model dataset. At first the input images are subjected to pre-processing phase, which is employed with median filtering and CLAHE. Later, the pre-processed image is given to classification task using CNN. In the classification phase, the parameters such as hidden neuron and epoch count are optimized using DOX with the function of increasing the precision and accuracy of the brain tumour classification model.
2.4 Brain Tumour Classification Dataset
The dataset is selected from ‘https://www.kaggle.com/mateuszbuda/lgg-mri-segmentation’. The brain MRI segmentation dataset compiles of MRI brain images with FLAIR and segmentation masks. The input images are gathered from ‘The Cancer Imaging Archive (TCIA)’, which is related to 110 adults with lower and higher grade glioma. The composed data are referred as Bit, where t = 1,2,3,…,T and the overall number of collected data is referred as T. The sample image of brain tumour is illustrated in Fig. 2.
3 Pre-processing and Classification of Brain Tumour Using Parameter Optimized CNN
3.1 MRI Image Pre-processing
The pre-processing is a crucial step in any processing of image models to enhance the contrast of the images and for the illumination changes. It is essential to progress the quality of image and amplify the noises present in the image. The pre-processing of MRI images is done with median filtering and CLAHE.
Median filtering: It is applied to the images to neglect the noise and to restrain the huge or small frequencies to further enhancement or to detect the edges of the images clearly. A non-linear filter is used in this filtering process. The main purpose of the median filter is to eliminate the noisy pixels by the median values of the surrounding pixels and removes unwanted things present in the in the segmented image. Some isolated pixels if image contains very low and high intensity values, this impulse noise is removed by median filtering. The median filtered images are referred as Bimedian.
CLAHE: The median filtered image Bimedian is inputted to CLAHE. The contrast enhancement is the effective technique utilized to amplify the noises troubles and to boost the contrast in the image. CLAHE is widely used in contrast enhancement and it is more effective in medical image processing. This technique works well than other regular histogram equalization, the value of the filter which is given on the neighboring pixel is computed and it does not count the global filter value. The final preprocessed images are derived as Bipre.
3.2 CNN-Based Classification
The brain tumour images are classified using CNN. It is a deep learning architecture used to recognize the special designs in 2D images. It has many layers like “convolutional layer, pooling layer, sample layer, input layer and output layer”. CNN model consists of pooling region, fully connected region and convolutional region. The convolution region is dispersed with pooling region to decrease the consumption period and to construct enhanced spatial and configuration variance. Convolutional region has a rectangular rid with multiple neurons. The convolutional layer uses different segmented images as input. There is a pooling layer after convolutional layer, which is illustrated from the prior convolutional layer.
Convolutional layer: The convolutional layers evaluate the input images and act as a feature extractor. The neurons in convolutional layer are determined as feature maps. Both weights and inputs are combined together to initialize the features map and passed through the activation functions. The feature maps presented in the same region has distinct weights, thus from each regions, various features are extracted.
Pooling layer: It is also named as down sampling region. The input distortions and spatial invariance are obtained in extraction process. All input average values presented in a small image are moved to the next layer.
Fully connected region: It prefers the n dimensional input data, the term n establish the count of categories gathered for the program. The class possibility is represented with n dimensional matrix in all ranges. The precise probability from different classes is attained by fully connected region.
3.3 DOX-CNN-Based Classification
The proposed DOX-CNN-based classification is performed to categorize the MRI images with CNN and to optimize the parameters using DOX. The suggested method utilizes the DOX owing to its ability to solve all types of optimization issues and it selects best options for real life issues. The effectiveness of procedures is enhanced and has high convergence speed. DOX is inspired by the dingoes hunting activity. Usually, the dingoes hunt in cluster grouping. The hunting method is classified into three stages like ‘approaching, chasing, encircling and attacking’. The major phases of DOX are exploitation and exploration phase. The optimal solutions are selected using the exploitation stage.
The location of the preys is easily detected by dingo. The alpha encircles the prey after finding its site. The prey is targeted by the preeminent agent and the surrounding activity of dingoes is derived here.
Here, the variable \(\vec{k}_r (u + {1})\) represents the novel location of the search agent, n denotes the random integer, the sub set of candidate solution is indicated a φo(u), the population generated is taken as U, the current candidate solution is termed as \(\vec{k}_r (u)\), the best candidate solution from the prior iteration is denoted as \(\vec{k}_* (u)\), the uniformly produced random number is represented as β1 at the time interval − 2 to 2.
The dingo usually chases the smaller victim and it follows the victim until it is trapped alone. The following activity of the dingo is expressed in Eq. (2).
The variables \(\vec{k}_r (u + {1})\) denotes the dingoes movement, \(\vec{k}_r (u)\) explains the present iteration, the best candidate solution from the prior iteration is denoted as \(\vec{k}_* (u)\), the arbitrary number β1 lies in the gap − 1 to 1 and the chosen candidate solution is referred as \(\vec{k}_e (u)\).
The activity of scavenger is the tradition of dingo eating the victim, the forager activity of dingo is expressed in Eq. (3).
The dingo’s survival range is referred in Eq. (4).
The variables ftmax and ftmin are indicated as the worst and optimal fitness values in the present iteration. The variable ft(r) indicates the present robustness value of rth search agent. The standardized fitness value is available in the survival matrix at bounding limit [0, 1]. Mention about Eq. 5.
The survival range of the candidate solution is represented as \(\vec{k}_r (u)\), the terms e2 and e1 is the generated random numbers, respectively, \(\vec{k}*(u)\) is indicated as the best candidate solution from the former iteration and the term σ is the binary number. The pseudo code for DOX is shown in Algorithm 1.
Algorithm 1: Introduced DOX |
|---|
Population the initialization |
Create the optimal location based on fitness solution |
Estimate the fitness for all resolution |
Conclude the constraints |
Upgrade the latest candidate solution by Eq. (1) |
Upgrade the chasing activity with Eq. (2) |
Upgrade the activity of scavenger with Eq. (3) |
Update the survival range with Eq. (4) |
Find the preeminent solution |
end |
The pre-processed MRI images are classified with CNN and the parameters like hidden neuron number and number of epochs is tuned with DOX algorithm with the principle of enhancing the precision and accuracy of the categorization. The main objective of the suggested method is derived in Eq. (6).
In above formula, the term Hn is denoted as hidden neuron count, the count of epochs is termed as Ne. The hidden neuron is taken among [5–255] and the epochs count ranges from [0.01–0.99]. “Accuracy referred as to how closely the measured value of a quantity corresponds to its true value”.
The variables, ab, an, ac, ad are referred as “true positive, true negative, false positive and false negative”. “Precision is the positive predictive value or the fraction of the positive predictions that are actually positive”.
Thus, the proposed DOX-CNN-based classification phase increases the accuracy and precision of the suggested method.
4 Results and Discussions
4.1 Experimental Analysis
The introduced brain tumour categorization was executed in Python and the analyses of the experiments were established. The performance of the projected method was evaluated with various traditional method regarding various metrics such as “accuracy, sensitivity, specificity, precision, Net Present Value (NPV), F1 Score, False-positive rate (FPR), false-negative rate (FNR), and False Discovery Rate (FDR)”. The suggested approach is evaluated with other algorithms like PSO [16], DHOA [17], SSA [18], EHO [19] and further, evaluated with LSTM [20], SVM [21], DNN [5], CNN [22].
4.2 Image Results
The image results for the introduced brain tumour classification is given in Fig. 3.
4.3 Performance Measures
The performance measures of the introduced brain tumour classification are taken from “https://en.wikipedia.org/wiki/Sensitivity_and_specificity: Access Date: 2022-03-21”.
4.4 Analysis of Performance on Algorithms
The analysis of modified brain tumour classification is assessed with algorithms by differing the learning rate is shown in Fig. 4. The accuracy of the modified DOX-CNN is 16% higher than PSO-CNN, 2% higher than DHOA-CNN, 3% higher than SSA-CNN, 4% higher than EHO-CNN at learning percentage 40%. While comparing the evaluated results, the precision of implemented DOX-CNN is 3% improved than PSO-CNN, 5% improved than DHOA-CNN, 4% improved than SSA-CNN, 6% improved than EHO-CNN at learning rate 50%. Thus, the proposed DOX-CNN is attaining best results than other traditional approaches.
4.5 Performance on Traditional Learning Models
The analysis measures of introduced brain tumour classification is executed with prior existing learning methods by differentiating learning rate as shown in Fig. 5. The accuracy of the implemented DOX-CNN is 44% improved then LSTM, 29% improved than SVM, 19% improved than DNN, 6% improved than CNN at the given learning rate 30%. Moreover, at learning rate 40%, the specificity of the suggested DOX-CNN is 48% higher than LSTM, 31% higher than SVM, 15% higher than CNN and 12% higher than DNN. Thus, the suggested method has gained high classification accuracy.
4.6 Performance on Algorithms
The analysis of introduced brain tumour classification is assessed with different algorithms is given in Table 1. The accuracy of the introduced DOX-CNN is 15% progressed than PSO-CNN, 12% progressed than DHOA-CNN, 13% progressed than SSA-CNN and 14% progressed than EHO-CNN. The precision of implemented DOX-CNN is 13% progressed than PSO-CNN, 15% progressed than DHOA-CNN, 14% progressed than SSA-CNN and 16% progressed than EHO-CNN. Thus, the proposed DOX-CNN has gained best results than other algorithms.
4.7 Performances on Traditional Learning Models
The analysis metrics of introduced brain tumour classification is evaluated with other existing learning methods by differentiating learning rate as shown in Table 2. The accuracy of the suggested DOX-CNN is 4% higher then LSTM, 9% higher than SVM, 6% higher than DNN, 8% higher than CNN. The specificity of the suggested DOX-CNN is 8% enhanced than LSTM, 3% enhanced than SVM, 11% enhanced than DNN and 15% enhanced than CNN. Thus, the suggested method has gained high classification accuracy.
5 Conclusion
The proposed brain tumour classification was implemented with a CNN enabled with optimization algorithm. The collected data was pre-processed with median filtering and CLAHE. Then, the pre-processed images were classified with CNN and the parameters like hidden neuron count and epochs count were tuned using DOX and the main aim of the introduced method was to increase the accuracy and precision of the categorization phase. The accuracy of the proposed DOX-CNN was 15% enhanced than PSO-CNN, 12% enhanced than DHOA-CNN, 13% enhanced than SSA-CNN and 14% enhanced than EHO-CNN. Hence, the suggested brain tumour classification has attained high accuracy rate.
References
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. Amer J Neuroradiol 27(3):475–487
Zulpe N, Pawar V (2012) GLCM textural features for brain tumor classification. Int J Comput Sci Issues 9:354–359
El-Dahshan EA et al (2014) Computer-aided diagnosis of human brain tumor through MRI: a survey and a new algorithm. Expert Syst Appl 41(11):5526–5545
Liu M et al (2018) Anatomical landmark based deep feature representation for MR images in brain disease diagnosis. IEEE J Biomed Health Inf 22(5):1476–1485
Mohsen H, El-Dahshan ESA, El-Horbaty ESM (2018) Classification using deep learning neural networks for brain tumors. Future Comput Inform J 3(1):68–71
Cheng J et al (2015) Enhanced performance of brain tumor classification via tumor region augmentation and partition. PloS One 10(12)
Abbadi NKE, Kadhim NE (2017) Brain cancer classification based on features and artificial neural network. Int J Adv Res Comput Commun Eng 8(1):123–134
Ali S, Ismael A, Mohammed A, Hefny H (2020) Artificial intelligence in medicine an enhanced deep learning approach for brain cancer MRI images classification using residual networks. Artificial Intell Med 102
Anaraki AK, Ayati M, Kazemi F (2018) Magnetic resonance imaging-based brain tumor grades classification and grading via convolutional neural networks and genetic algorithms. Integrative Med Res 39(1):63–74
Çinar A, Yildirim M (2020) Detection of tumors on brain MRI images using the hybrid convolutional neural network architecture. Med Hypotheses 139
Ge C, Gu IYH, Jakola AS, Yang J (2020) Enlarged training dataset by pairwise GANs for molecular-based brain tumor classification. IEEE Access 8:22560–22570
Habib H, Amin R, Ahmed B, Hannan A (2021) Hybrid algorithms for brain tumor segmentation, classification and feature extraction. J Ambient Intell Human Comput
Khairandish MO, Sharma M, Jain V, Chatterjee JM, Jhanjhi NZ (2021) A hybrid CNN-SVM threshold segmentation approach for tumor detection and classification of MRI brain images. IRBM. Available online, June 2021
Kesav N, Jibukumar MG (2021) Efficient and low complex architecture for detection and classification of brain tumor using RCNN with two channel CNN. Available online, May 2021
Afshar P, Mohammadi A, Plataniotis KN (2020) BayesCap: a Bayesian approach to brain tumor classification using capsule networks. IEEE Signal Process Lett 27:2024–2028
Pedersen MEH, Chipperfield AJ (2010) Simplifying particle swarm optimization. Appl Soft Comput 10(2):618–628
Brammya G, Praveena S, Ninu Preetha NS, Ramya R, Rajakumar BR, Binu D (2019) Deer hunting optimization algorithm: a new nature-inspired meta- heuristic paradigm. 24 May 2019
Jain M, Singh V, Rani A (2019) A novel nature-inspired algorithm for optimization: squirrel search algorithm. Swarm Evol Comput 44:148–175
Yilmaz S, Sen S (2020) Electric fish optimization: a new heuristic algorithm inspired by electrolocation. Neural Computing and Applications 15
Amin J, Sharif M, Raza M, Saba T (2020) Brain tumor detection: a long short-term memory (LSTM)-based learning model. Neural Comput Appl 32:15965–15973
Rao CS, Karunakara K (2022) Efficient detection and classification of brain tumor using kernel-based SVM for MRI. Multimedia Tools and Applications 81:7393–7417
Devi S (2020) Performance prediction using deep learning technique in education sector. The International Journal of Analytical and Experimental Modal Analysis
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Aishwarya, R., Sumathi, G., RathisBabu, T.K.S. (2023). Efficient Brain Tumour Classification Using Parameter Optimized CNN with Dingo Optimizer Concept. In: Kumar, A., Ghinea, G., Merugu, S. (eds) Proceedings of the 2nd International Conference on Cognitive and Intelligent Computing. ICCIC 2022. Cognitive Science and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-2742-5_54
Download citation
DOI: https://doi.org/10.1007/978-981-99-2742-5_54
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2741-8
Online ISBN: 978-981-99-2742-5
eBook Packages: Computer ScienceComputer Science (R0)