Abstract
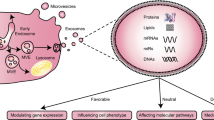
Characterized by coronary artery obstruction or stenosis, ischemic cardiovascular diseases as advanced stages of coronary heart diseases commonly lead to left ventricular aneurysm, ventricular septal defect, and mitral insufficiency. Extracellular vesicles (EVs) secreted by diverse cells in the body exert roles in cell–cell interactions and intrinsic cellular regulations. With a lipid double-layer membrane and biological components such as DNA, protein, mRNA, microRNAs (miRNA), and siRNA inside, the EVs function as paracrine signaling for the pathophysiology of ischemic cardiovascular diseases and maintenance of the cardiac homeostasis. Unlike stem cell transplantation with the potential tumorigenicity and immunogenicity, the EV-based therapeutic strategy is proposed to satisfy the demand for cardiac repair and regeneration while the circulating EVs detected by a noninvasive approach can act as precious biomarkers. In this chapter, we extensively summarize the cardioprotective functions of native EVs and bioengineered EVs released from stem cells, cardiomyocytes, cardiac progenitor cells (CPCs), endothelial cells, fibroblast, smooth muscle cells, and immune cells. In addition, the potential of EVs as robust molecule biomarkers is discussed for clinical diagnosis of ischemic cardiovascular disease, attributed to the same pathology of EVs as that of their origin. Finally, we highlight EV-based therapy as a biocompatible alternative to direct cell-based therapy for ischemic cardiovascular diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ischemic cardiovascular diseases
- Native extracellular vesicles
- Bioengineered extracellular vesicles
- Biomarkers
1 Background
Ischemic cardiovascular diseases including myocardial infarction (MI) and heart failure are the leading causes of morbidity and mortality worldwide [1, 2]. The cardiovascular system supplies blood to all tissues in the body during which oxygen and nutrients are simultaneously carried to cells. Decrease or block of local blood perfusion leads to ischemia which also deprives cells of oxygen and nutrients such as glucose and growth factors [3]. Once myocardial ischemia occurs, cardiomyocytes would undergo cell death (apoptosis, pyroptosis, and necrosis) within 20 min, followed by complete necrosis at 2–4 h after persistent coronary arterial occlusion [4, 5]. Clinical therapy of ischemic cardiovascular diseases is timely re-establishing blood flow in the ischemic cardiac tissue. However, reperfusion increases oxidative stress and causes paradoxical cardiomyocyte impairment, which is named ischemia–reperfusion (I/R) and determines the final infarct size after successful revascularization following MI [6, 7].
Without sufficient blood supply to the heart, myocardial ischemia could hinder the supply of glucose and oxygen, as well as delay the clearance of metabolic by-products such as lactic acid and CO2 [8]. Although hypoxia is a key determinant to induce cellular damage in ischemic conditions, other abnormal events involving acidosis, increases in oxidative stress, disturbed calcium homeostasis, decreased levels of adenosine triphosphate (ATP), and mitochondrial and DNA damage exert significant effects on inflammation, neovascularization, and collateral formation [9]. Anaerobic glycolysis consuming glycogen storage allows short periods of ischemia. Glucose transporters (Glut1 and Glut4) are carried to the muscle fiber membrane to facilitate additional glucose uptake [10]. Ischemia could lead to acidosis and decrease the cardiac pH from approximately 7.2 to 6.5, which is necessary to alter the activities of various enzymes including phosphofructokinase and phospholipid burning enzymes. Besides, acidosis can be further enhanced by inhibition of mitochondrial NADH oxidation and vacuolar proton ATPase, increased CO2 levels in tissues, excessive glycogen conversion, anaerobic glycolysis, and ATP hydrolysis [11,12,13,14]. As a result, intracellular acidosis triggers apoptosis of cardiomyocytes via cavitation proton ATPase [15].
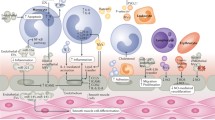
Cell-to-cell communication is essential for maintaining tissue homeostasis and disease progression. Two classic pathways are direct cell-to-cell contact with short-range cell crosstalk and long-distance communication of cytokines or hormones [16]. Another intercellular communication mechanism emerges as the intercellular transfer of extracellular vesicles (EVs), which could deliver various biological signaling to receptor cells at an exceeded level than that of soluble factor signaling, attributed to a large number of bioactive molecules, surface receptors, and genetic information in EVs [17]. EVs are extracellular structures surrounded by lipid bilayers and secreted by almost all known cell types. According to their sizes, EVs have been classified into three categories involving exosomes (sizes of 30–150 nm), apoptotic bodies (sizes of 50 nm–10 μm), and microparticles or microvesicles (sizes of 100–1000 nm) [18, 19]. Exosomes are intracavinal vesicles that are formed through the membrane invagination of multivesicular endosomes and released into the extracellular space followed by the fusion of multivesicular endosomes with cell membranes [20]. Microvesicles (50–1000 nm) are heterogeneous EVs characterized by their origin and secretion to the exobud through the plasma membrane. Apoptotic bodies are released by dying cells after apoptosis. Responsible for intercellular communication, EVs carry molecules such as DNA, proteins, lipids, RNA, and/or microRNAs (miRNAs) [21]. These functional components vary from cell origin and specific pathophysiological conditions at the time of EV packaging and secretion. Extensive evidence suggests that EVs get involved in diverse cardiovascular physiological and pathological processes including regulation of angiogenesis and blood pressure, cardiomyocyte hypertrophy, apoptosis/survival, and cardiac fibrosis. Also, due to their wide distribution in human plasma, bronchoalveolar fluid, serum, saliva, urine, semen, bile, cerebrospinal fluid, amniotic fluid, tumor effusion, ascites, and milk, EVs have been employed as potential biomarkers for cardiovascular disease [22,23,24,25,26,27,28]. Cell-to-cell communication transferred by EVs between cardiomyocytes and vessel cells has an impact on cardiovascular pathology, diagnosis, and therapy [29,30,31,32].
In this chapter, we would summarize a variety of natural/engineered EVs derived from different types of cells, with an emphasis on their biogenesis and cargo formation, and performances as biomarkers of ischemic cardiovascular disease for cardiac repair and regeneration. Finally, we would discuss the potential and challenges of EVs in clinical therapy of ischemic cardiovascular disease.
2 Different Sources of Extracellular Vesicles for Therapy of Ischemic Cardiovascular Diseases
Current treatments for ischemic cardiovascular diseases mainly focus on slowing the progression of diseases, rather than repairing and regenerating damaged heart muscle [33]. Although cell transplantation is considered one of the most promising ways to promote the proliferation of cardiomyocytes, it suffers from immunogenicity, risk of post-transplant arrhythmia and tumorigenesis, and uncertain differentiation and retention rate of cells [34]. A series of cytokines and growth factors, such as VEGF, HGF, Ang-1, SDF-1A, IGF-1, SFRP-2, TGF-β, and eNOS/iNOS, are involved in the paracrine effect of cell-based therapy [35]. These factors are beneficial to protect cardiomyocytes from apoptosis and necrosis, promote angiogenesis in infarcted myocardium, delay interstitial remodeling, and increase the recruitment of circulating progenitor cells [36]. With the same bioactive factors as their source cells, EVs can function as an alternative to cell transplantation. EVs are isolated from all major cell types found in the heart ranging from primary adult cardiomyocytes, primary cardiac endothelial cells, primary cardiac fibroblasts, and vascular smooth muscle cells to cardiac progenitor cells (CPCs) [37]. The majority of EVs in healthy people’s plasma is derived from platelets and red blood cells, but plasma EVs are also released from white blood cells, endothelial cells, monocytes, neutrophils, and lymphocytes [38].
-
1.
Cardiomyocyte-derived EVs: Cardiomyocytes may be an important type of parent cells to secrete EVs, especially under stress conditions such as myocardial ischemia and failure. Cardiomyocytes can release EVs containing heat shock proteins HSP70 and HSP90 and HSP60 in response to hypoxia and reoxygenation injury in vitro [39]. EVs containing tumor necrosis factor (TNF)-α can also be released by cardiomyocytes for inflammatory response. Glucose deprivation induces the loading of functional glucose transporters and glycolytic enzymes into EVs that are derived from neonatal rat cardiomyocytes [40]. Thus, cardiomyocytes can specifically regulate the function of neighboring cells by releasing specific EVs to respond to environmental stress.
-
2.
Cardiac progenitor cell-derived EVs: a kind of cells with the ability to proliferate and differentiate into cardiomyocytes are called CPCs [41]. There were 857 unique gene products and 150 miRNAs in CPC-derived EVs, compared to CPCs. The miR-22 in CPC-derived EVs could inhibit methylCpG binding protein 2 and reduce apoptosis of the ischemic cardiomyocytes [42]. Hypoxia stimulates CPCs to release EVs which upregulate the expression of proangiogenic genes, anti-fibrosis genes, and a cluster of miRs (miR-210, miR-132, and miR-146a-3p), as well as increase their capacity to improve cardiac function after I/R injury in rats. After being inoculated into the ischemic/reperfusion heart, the CPC-derived EVs could elevate ATP and NADH levels in vivo [43]. With matrix metalloproteinases (MMPs) and extracellular matrix metalloproteinases (ECMPs) inside, EVs derived from CPCs could mediate proangiogenic efficiency. Therefore, CPC-derived EVs may play key roles in the EV-based therapy of ischemic cardiovascular disease [44].
-
3.
Endothelial cell-derived EVs: The endothelial cells can release EVs containing miR-146a to stimulate angiogenesis [45]. Hypoxia changes the composition of mRNA and protein in EVs released from cultured endothelial cells in vitro. Exosomal intercellular adhesion protein expression was increased after TNF-α treatment of endothelial cells. These findings exemplify the protective function of endothelium-derived EVs against ischemic cardiovascular disease, which may also make them biomarkers for cardiac stress and diseases [46].
-
4.
Fibroblast and smooth muscle cell-derived EVs: Cardiac fibroblasts secrete miRNA-27a*-enriched EVs into the extracellular space in response to stimulation of Angiotensin II, which inhibits PDLIM5 translation, thereby leading to the expression of hypertrophic gene in cardiomyocytes [47]. EVs released by cardiac fibroblasts contain high levels of miR-21-3p/miR-21 which can induce cardiomyocyte hypertrophy [48]. EVs released by smooth muscle cells are associated with vascular calcification and atherosclerosis. Different conditions such as ischemia, stress, and volume overload are able to induce fibroblasts, cardiomyocytes, endothelial cells, and inflammatory cells to regulate mast cell responses through EVs-mediated intercellular communication.
-
5.
Mesenchymal stem cell-derived EVs: Mesenchymal stem cells (MSCs) are present in almost all tissues and play a major role in tissue repair and regeneration. Many signaling molecules from mesenchymal stem cells are associated with self-renewal and differentiation, which also have been found in the EVs derived from MSCs. Thus, these MSC-derived EVs could influence cell cycle, proliferation, cell adhesion, cell migration, and cell morphogenesis. Similarly, miRNAs shuttling through MSC-derived EVs mainly in their precursor form drive downstream signaling pathways. In addition, MSC-derived EVs carry anti-inflammatory cytokines such as interleukin-10 and tumor growth factor (TGF)-β to affect the lymphocyte proliferation [49].
-
6.
Immune cell-derived EV: B cells and dendritic cells in immune cells mediate secretion of EVs with major histocompatibility complex (MHC)-dependent immune responses. These EVs express specific adhesion molecules to target specific receptor cells. NK cells-derived EVs surrounding perforin and granulase B could mediate antitumor activity in vitro and in vivo. Taking into consideration that macrophages can release IL-1β during inflammasome activation, the EVs secreted by macrophages exert roles in pro-inflammatory activity and initiating immune response [50].
-
7.
Platelet-derived EVs: Studies have shown that increased cell adhesion factors, thrombopoietic factors, and inflammatory factors in EVs that are released by platelet in vascular plaques, thrombosis, and atherosclerosis can promote the delivery of platelet EVs to endothelial cells and macrophages in the vascular lesion sites. Platelet-derived EVs also stimulate angiogenesis, and intramuscular injection may improve vascular remodeling after ischemia [51].
3 Native Extracellular Vesicles for Ischemic Cardiovascular Therapeutics
The first study on EVs as a potential therapeutic intervention for cardiovascular disease was published in 2010. Since then, different types of cell transplantation were found to repair the infarcted heart. Besides, exploration of the underlying mechanism revealed that the protective effect of cell transplantation is mainly through the paracrine mechanism, especially EVs secreted by surviving cells, instead of directly mediation by cells [52]. EVs are naturally suitable for the transport of proteins and nucleic acids as well as cell-to-cell crosstalk, which makes them particularly attractive as drug delivery agents. In addition, due to their biophysical properties, EVs are easy to isolate while their contents such as RNA and protein can be easily manipulated [53]. Although EVs must contend with low cardiac osmotic and endocytosis rates, they can overcome poor transplantation by being directly internalized by recipient cells when compared to cells [54]. The limitations of EVs in cardiovascular therapy are the lack of effective target for the damaged myocardium. Optimizing the storage, isolation, and purification procedures for EVs is challenging to move EV-based therapy from the laboratory bench to the clinic [55].
The therapeutic effect of EVs in recipient cells is mainly attributed to the delivery of proteins and/or non-coding RNAs, especially miRNAs. For example, miRNA-19, miRNA-21, miRNA-24, and miRNA-210 have been reported to get involved in cardiovascular repair, while several novel miRNAs (miRNA-22, miRNA-29a, miRNA-143, miRNA-146, miRNA-181b, miRNA-222, miRNA-294-3p, and miR-126) favor cardiovascular protective effects of exosomes [56]. Another important component of EVs, which is also related to their bioactivity, is proteins such as platelet-derived growth factor D and pregnancy-associated plasma protein A. Highly expressed in exosomes, pregnancy-associated plasma protein A has been shown to mediate cardiac protection and angiogenesis [57]. EVs could regulate autophagy, activate pro-survival signaling pathways, and reduce oxidative stress, thereby improving the survival rate of cardiomyocytes and endothelial cells. In addition, it can regulate the inflammatory response and cytokine secretion, as well as increase the activation of CD4 positive T cells by affecting the polarization of immune cells [58].
It has been confirmed that EVs can be secreted by cultured heart and vascular cells, stem cells. EVs have been shown to mediate communication between endothelial cells and smooth muscle cells, endothelial cells (ECs) and pericytes, cardiomyocytes and ECs, and fibroblasts and cardiomyocytes [59]. Smooth muscle cells play important roles in the formation of atherosclerotic plaque which can lead to MI. Studies have indicated that ECs release microvesicles rich in miR-143/145, which are absorbed by smooth muscle cells and regulate gene expression in receptor cells. Injection of EVs containing miR-143/145 into a mouse model of atherosclerosis reduced the formation of atherosclerotic lesions [60]. Cardiac fibroblasts have been demonstrated to secrete miR-21-rich EVs as key paracrine signaling mediators for cardiac hypertrophy. MiR-21 is shuttled to cardiomyocytes and affects the expression of its miR-21 target genes, thereby leading to cell hypertrophy [61]. EVs in the rat heart after ischemic preconditioning were responsible for the transmission of remote conditioning signals to protect the heart. The proangiogenic activity of pericytes is partially dependent on the released miR-132, especially in response to hypoxia. Pericyte-derived miR-132 was absorbed by ECs, thereby resulting in a higher proangiogenic capacity [62]. A recent report suggested that both ischemic and healthy human and mouse cardiomyocytes might release exosomal-like vesicles in vivo [63]. In mice with acute MI, circulating miRNA-1 was released into the bloodstream via EVs to inhibit the expression of the SDF-1 receptor CXCR-4 in bone marrow mononuclear cells [64]. These studies reveal that EV-mediated communication mechanisms can effectively favor cardiac repair and regeneration.
4 Bioengineered Extracellular Vesicles for Ischemic Cardiovascular Therapeutics
Although native EVs function as a delivery modality with their unique characteristics, they have inherent limitations of unclear heterogeneity and lack of targeting. The bioengineering operation can endow the EVs with improved target as therapeutic tools for the treatment of cardiovascular disease [65]. In detail, engineered EVs overcome their limitations by addressing the bioactivity, stability, internalization, and targeting of EVs. Exosome modifications are classified from a technical point of view depending on whether they are performed before EVs are secreted by donor cells or after the purification of EVs from culture medium or liquid. From a biological point of view, these modifications occur on the membrane or in the cavity of EVs [66]. Regulation of EVs-secreting cells has two different procedures: culture under stress conditions (hunger, hypoxia, inflammation) and transfection of exogenous compounds such as miRNAs, plasmid DNA, and small molecules to enhance their bioactivity. Some cell platforms have been customized to enrich EVs with specific proteins and RNAs [67]. Additionally, few studies have used cellular mechanisms to design EVs with specific epitopes to the heart. Although the biophysical properties of EVs can be kept relatively intact, overexpression may produce unforeseen biological consequences that ultimately interfere with the biogenesis of EVs [68]. Tracking EVs and their biological distribution in vivo are important for the full evaluation of their therapeutic potential for cardiovascular disease. Most EVs have been isolated and labeled with fluoro groups, luminescent reporters, or radioactive tracers. In a few cases, reporter genes are expressed by transgenic treatment of EVs-secreting cells. EVs can be monitored in vitro or in vivo by luminescence or fluorescence [69]. To enhance the targeting of EVs, EVs have been modified with exogenous peptides (such as integrin αVβ3 high-affinity cyclic RGD peptides, ischemic targeting peptides, and cardiomyocyte-specific peptides), proteins (such as streptavidin), or lipids [70]. To enhance the internalization and endosomal escape of EVs, vesicles were modified with cationic lipids, pH-sensitive peptides, and cell-penetrating peptides. Taken together, these studies demonstrate the possibility of improving the bioactivity, tracer, targeting, and internalization of engineered EVs, compared with naked EVs [71].
5 EVs as Potential Biomarkers of Ischemic Cardiovascular Diseases
EVs originate from different subcellular compartments and are released in the extracellular space. By transferring their cargos to targeted cells and tissues, they act as new regulators of cell-to-cell communication between adjacent and distal cells. Since their vesicle composition, biological content, and protein markers are the individual characteristics of cell activation and damage, most EVs detected in serum and saliva can be concentrated as diagnostic and prognostic biomarkers which could suggest the occurrence of ischemic cardiovascular diseases [72,73,74]. Their isolation within the membrane also protects proteins, RNA, and DNA from degradation [75]. The features of circulating EVs or non-vesicular binding nucleic acids are valuable tools for the diagnosis and monitoring of cardiovascular disease, recently referred to as liquid biopsy. In epidemiological investigations, circulating EVs provide a noninvasive and nearly continuous flow of information about disease status [76,77,78]. Finally, the cell-specific application of genetic engineering and EVs may provide a new therapeutic approach for the treatment of ischemic cardiovascular diseases, offering hope for the application of EVs in ischemic cardiovascular disease [79].
With a variety of proteins, lipids, mRNAs, non-transcriptional RNAs, miRNAs, and small RNAs that represent their cellular origin and reflect the pathology of their source cells, EVs have potential as biomarkers for clinical diagnosis of ischemic cardiovascular disease [80]. The possibility of isolating and characterizing EVs from body fluids makes them very attractive as diagnostic markers [81]. CPC-derived EVs possess several cardioprotective and angiogenic microRNAs, such as miR-132, miR-210, and miR-146. Compared with CPC itself, CPC-derived EVs contained a portion of specific miRNAs that were specifically enriched in miR-146a, suggesting that miRNA enrichment into EVs may have occurred through a specific mechanism rather than random selection [82]. Recently, the elevated serum miR-192 levels (specifically EVs) were significantly associated with patients who developed heart failure within 1 year after MI, in comparison with a matched control group that did not develop a cardiovascular event after discharge [83]. In addition, miR-133 and miR-328 in plasma were elevated in patients with MI, both of which are considered novel biomarkers for acute MI [84]. A study of EVs in patients with acute coronary syndrome showed that miRNA-208a expression was significantly upregulated in serum EVs from patients with acute coronary syndrome [85]. In addition, survival was reduced in patients with high miRNA-208A expression, suggesting that exosomal miRNA-208A can be employed for early diagnosis and prognosis of acute coronary syndromes. Another study revealed an increase in the number of EVs binding cardiac miRNAs after coronary artery bypass surgery [86]. In the future, diverse EVs can function as new biomarkers of persistent myocardial ischemia, vascular injury without cell death, non-infarct or asymptomatic myocardial ischemia, and different types of angina and microvascular angina. Besides, a biomarker of myocardial ischemia with low persistence and no cell death would help identify the disease at an early stage [87]. Similarly, the diagnosis of acute coronary syndromes needs to be improved, especially at the early time point after MI, microvascular angina, and non-ST-segment elevation of acute coronary syndrome (ACS) [88]. Given the wide range of cardiac cell types that are able to secrete EVs, circulating EVs which originate from coronary and peripheral arteries could provide a potentially significant identifying biomarker to support diagnosis and reflect the formation of coronary thrombotic occlusion in patients of MI [89]. However, the practical application of exosomal-derived proteins or miRNAs as biomarkers has not been implemented in clinical practice despite the presence of a large number of exosomes in biological fluids, mainly due to the lack of a rapid and effective method to process large numbers of biological samples. Several commercial companies have begun to develop EV-based cancer diagnostics, while EVs as biomarkers of cardiovascular diseases are still an unexplored world that we are committed to pioneering [90].
6 Conclusion and Future Perspectives
Circulating EVs can be detected in the plasma of patients with cardiovascular diseases, thus EVs expression patterns can be used as diagnostic and prognostic biomarkers for a variety of cardiovascular diseases. The multiple functional efficiencies of EVs on the progression of ischemic cardiovascular disease vary from the origin of cells, the functional status of source cells, and the transport capacity of functional bioactive molecules in the vesicle [91].
Since EVs get involved in the physiological and pathological processes of ischemic cardiovascular disease, research about their biogenesis, contents, and functional effects on target cells can provide new diagnostic and prognostic information for patients with cardiovascular diseases [92, 93]. Firstly, EVs are known to exert therapeutic effects on ischemic cardiovascular disease in preclinical MI models, which lights the future of EV-based therapy for cardiovascular diseases. Secondly, with advantages of modifiability, high viability, and inherency, EVs has similar therapeutic effect when compared to stem cell/progenitor cells. Thirdly, the therapeutic effect of EVs can be enhanced by increasing the stability and targeting of EVs, enriching their therapeutic content, improving their internalization and intracellular transport, and controlling their spatial and temporal release in biomaterials [94]. The implementation of standard separation and characterization of EVs are urgently required to explore EVs as a potential approach for ischemic cardiovascular disease [95,96,97]. Autologous EVs have the advantage of immune compatibility, but they cannot be collected on demand, and are difficult to be standardized as clinical products based on individual factors such as the donor’s disease and age. EVs provided by exogenous sources have the advantage of being easier to standardize and bulk storage [98, 99]. In addition, loading exogenous molecules into EVs and controlling their delivery in vivo provides many opportunities to enhance the bioactivity of EVs [100]. Therefore, targeted technologies that increase the accumulation of EVs in the cardiovascular system to reduce the necessary injected dose, as well as strategies that enrich specific biomolecules in EVs, may be the key approaches to unlocking its clinical application. In the future, EVs can become clinical biomarkers for ischemic cardiovascular disease due to the specificity of their inclusivity. Native and engineered EVs represent a promising cell-free, safe, and customizable therapeutic approach to improving the therapeutic efficiency of cardiovascular diseases.
References
Adamiak M, Sahoo S (2018) Exosomes in myocardial repair: advances and challenges in the development of next-generation therapeutics. Mol Ther 26(7):1635–1643
Aikawa E, Blaser MC (2021) 2020 Jeffrey M. Hoeg award lecture: calcifying extracellular vesicles as building blocks of microcalcifications in cardiovascular disorders. Arterioscler Thromb Vasc Biol 41(1):117–127
Albino D, Falcione M, Uboldi V, Temilola DO, Sandrini G, Merulla J, Civenni G, Kokanovic A, Sturchler A, Shinde D, Garofalo M, Mestre RP, Constancio V, Wium M, Burrello J, Baranzini N, Grimaldi A, Theurillat JP, Bossi D, Barile L, Henrique RM, Jeronimo C, Zerbini LF, Catapano CV, Carbone GM (2021) Circulating extracellular vesicles release oncogenic miR-424 in experimental models and patients with aggressive prostate cancer. Commun Biol 4(1):119
Almeida Paiva R, Martins-Marques T, Jesus K, Ribeiro-Rodrigues T, Zuzarte M, Silva A, Reis L, da Silva M, Pereira P, Vader P, Petrus Gerardus Sluijter J, Goncalves L, Cruz MT, Girao H (2019) Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J Cell Mol Med 23(2):1137–1151
Balbi C, Lodder K, Costa A, Moimas S, Moccia F, van Herwaarden T, Rosti V, Campagnoli F, Palmeri A, De Biasio P, Santini F, Giacca M, Goumans MJ, Barile L, Smits AM, Bollini S (2019) Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting using the human amniotic fluid stem cell secretome. Int J Cardiol 287:87–95
Cesselli D, Parisse P, Aleksova A, Veneziano C, Cervellin C, Zanello A, Beltrami AP (2018) Extracellular vesicles: how drug and pathology interfere with their biogenesis and function. Front Physiol 9:1394
Chen CW, Wang LL, Zaman S, Gordon J, Arisi MF, Venkataraman CM, Chung JJ, Hung G, Gaffey AC, Spruce LA, Fazelinia H, Gorman RC, Seeholzer SH, Burdick JA, Atluri P (2018) Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res 114(7):1029–1040
Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, Bolis S, Altomare C, Matteucci M, Di Silvestre D, Brambilla F, Fertig TE, Torre T, Demertzis S, Mauri P, Moccetti T, Vassalli G (2018) Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-a. Cardiovasc Res 114(7):992–1005
Balbi C, Vassalli G (2020) Exosomes: beyond stem cells for cardiac protection and repair. Stem Cells 38(11):1387–1399
Barile L, Milano G, Vassalli G (2017) Beneficial effects of exosomes secreted by cardiac-derived progenitor cells and other cell types in myocardial ischemia. Stem Cell Investig 4:93
Batista-Almeida D, Martins-Marques T, Ribeiro-Rodrigues T, Girao H (2020) The role of proteostasis in the regulation of cardiac intercellular communication. Adv Exp Med Biol 1233:279–302
Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J (2017) Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol 112(4):38
Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J (2019) Correction to: exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol 114(6):44
Belostotskaya G, Hendrikx M, Galagudza M, Suchkov S (2020) How to stimulate myocardial regeneration in adult mammalian heart: existing views and new approaches. Biomed Res Int 2020:7874109
Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith RR, Marban L, Marban E (2017) Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 9(3):337–352
Chen G, Xu C, Gillette TG, Huang T, Huang P, Li Q, Li X, Li Q, Ning Y, Tang R, Huang C, Xiong Y, Tian X, Xu J, Xu J, Chang L, Wei C, Jin C, Hill JA, Yang Y (2020) Cardiomyocyte-derived small extracellular vesicles can signal eNOS activation in cardiac microvascular endothelial cells to protect against ischemia/reperfusion injury. Theranostics 10(25):11754–11774
Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, Chen M (2021) Targeted delivery of extracellular vesicles in heart injury. Theranostics 11(5):2263–2277
Chen Q, Wang ZY, Chen LY, Hu HY (2017) Roles of high mobility group box 1 in cardiovascular calcification. Cell Physiol Biochem 42(2):427–440
de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L (2020) Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol 17(11):685–697
Chung JJ, Han J, Wang LL, Arisi MF, Zaman S, Gordon J, Li E, Kim ST, Tran Z, Chen CW, Gaffey AC, Burdick JA, Atluri P (2020) Delayed delivery of endothelial progenitor cell-derived extracellular vesicles via shear thinning gel improves postinfarct hemodynamics. J Thorac Cardiovasc Surg 159(5):1825–1835.e1822
de Boer C, Calder B, Blackhurst D, Marais D, Blackburn J, Steinmaurer M, Woudberg NJ, Lecour S, Lovett J, Myburgh K, Bezuidenhout D, Human P, Davies NH (2021) Analysis of the regenerative capacity of human serum exosomes after a simple multistep separation from lipoproteins. J Tissue Eng Regen Med 15(1):63–77
de Couto G, Jaghatspanyan E, DeBerge M, Liu W, Luther K, Wang Y, Tang J, Thorp EB, Marban E (2019) Mechanism of enhanced MerTK-dependent macrophage efferocytosis by extracellular vesicles. Arterioscler Thromb Vasc Biol 39(10):2082–2096
De Vita A, Liverani C, Molinaro R, Martinez JO, Hartman KA, Spadazzi C, Miserocchi G, Taraballi F, Evangelopoulos M, Pieri F, Bongiovanni A, Mercatali L, Tasciotti E, Ibrahim T (2021) Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer. Sci Rep 11(1):5107
Deddens JC, Vrijsen KR, Colijn JM, Oerlemans MI, Metz CH, van der Vlist EJ, Nolte-’t Hoen EN, den Ouden K, Jansen Of Lorkeers SJ, van der Spoel TI, Koudstaal S, Arkesteijn GJ, Wauben MH, van Laake LW, Doevendans PA, Chamuleau SA, Sluijter JP (2016) Circulating extracellular vesicles contain miRNAs and are released as early biomarkers for cardiac injury. J Cardiovasc Transl Res 9(4):291–301
Dekker M, Waissi F, van Bennekom J, Silvis MJM, Timmerman N, Bank IEM, Walter JE, Mueller C, Schoneveld AH, Schiffelers RM, Pasterkamp G, Grobbee DE, de Winter RJ, Mosterd A, de Kleijn DPV, Timmers L (2020) Plasma extracellular vesicle proteins are associated with stress-induced myocardial ischemia in women presenting with chest pain. Sci Rep 10(1):12257
Dusing P, Zietzer A, Goody PR, Hosen MR, Kurts C, Nickenig G, Jansen F (2021) Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. J Mol Med (Berl) 99(3):335–348
Eguchi S, Takefuji M, Sakaguchi T, Ishihama S, Mori Y, Tsuda T, Takikawa T, Yoshida T, Ohashi K, Shimizu Y, Hayashida R, Kondo K, Bando YK, Ouchi N, Murohara T (2019) Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J Biol Chem 294(31):11665–11674
Emanueli C, Shearn AI, Angelini GD, Sahoo S (2015) Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol 71:24–30
Escate R, Padro T, Suades R, Camino S, Muniz O, Diaz-Diaz JL, Sionis A, Mata P, Badimon L (2021) High miR-133a levels in the circulation anticipates presentation of clinical events in familial hypercholesterolaemia patients. Cardiovasc Res 117(1):109–122
Femmino S, Penna C, Margarita S, Comita S, Brizzi MF, Pagliaro P (2020) Extracellular vesicles and cardiovascular system: biomarkers and cardioprotective effectors. Vascul Pharmacol 135:106790
Firoozi S, Pahlavan S, Ghanian MH, Rabbani S, Barekat M, Nazari A, Pakzad M, Shekari F, Hassani SN, Moslem F, Lahrood FN, Soleimani M, Baharvand H (2020) Mesenchymal stem cell-derived extracellular vesicles alone or in conjunction with a SDKP-conjugated self-assembling peptide improve a rat model of myocardial infarction. Biochem Biophys Res Commun 524(4):903–909
Fu Y, Zhang Y, Khoo BL (2021) Liquid biopsy technologies for hematological diseases. Med Res Rev 41(1):246–274
Fujimoto S, Fujita Y, Kadota T, Araya J, Kuwano K (2020) Intercellular communication by vascular endothelial cell-derived extracellular vesicles and their MicroRNAs in respiratory diseases. Front Mol Biosci 7:619697
Greco S, Gaetano C, Martelli F (2014) HypoxamiR regulation and function in ischemic cardiovascular diseases. Antioxid Redox Signal 21(8):1202–1219
Huang G, Garikipati VNS, Zhou Y, Benedict C, Houser SR, Koch WJ, Kishore R (2020) Identification and comparison of hyperglycemia-induced extracellular vesicle transcriptome in different mouse stem cells. Cell 9(9):2098
Huang P, Tian X, Li Q, Yang Y (2016) New strategies for improving stem cell therapy in ischemic heart disease. Heart Fail Rev 21(6):737–752
Grover SP, Mackman N (2020) Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis 307:80–86
Herrera-Zelada N, Zuniga-Cuevas U, Ramirez-Reyes A, Lavandero S, Riquelme JA (2021) Targeting the endothelium to achieve cardioprotection. Front Pharmacol 12:636134
James-Allan LB, Devaskar SU (2021) Extracellular vesicles and their role in gestational diabetes mellitus. Placenta 113:15
Jansen F, Nickenig G, Werner N (2017) Extracellular vesicles in cardiovascular disease: potential applications in diagnosis, prognosis, and epidemiology. Circ Res 120(10):1649–1657
Jimenez-Avalos JA, Fernandez-Macias JC, Gonzalez-Palomo AK (2021) Circulating exosomal MicroRNAs: new non-invasive biomarkers of non-communicable disease. Mol Biol Rep 48(1):961–967
Kang S, Yang JW, Jeong JY, Park J, An HJ, Koh HM, Jang SM, Lee YJ, Song DH (2019) Size distribution of serum extracellular vesicles in mice with atherosclerosis. Pathol Res Pract 215(10):152574
Kato M, Nakamoto R, Ishizuka M, Watanabe N (2021) Facile and simple purification method for small extracellular vesicles obtained from a culture medium through cationic particle capture. Anal Bioanal Chem 413:2523
Kervadec A, Bellamy V, El Harane N, Arakelian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Perier MC, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagege A, Ruel M, Boulanger CM, Silvestre JS, Menasche P, Renault NK (2016) Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 35(6):795–807
Kollmann D, Linares-Cervantes I, Ganesh S, Rosales R, Hamar M, Goto T, Urbanellis P, Tessandier N, Boilard E, Bruguera C, Wiebe A, Bartczak A, Yip P, Adeyi O, Selzner M, Selzner N (2020) Normothermic ex vivo liver perfusion prevents intrahepatic platelet sequestration after liver transplantation. Transplantation 104(6):1177–1186
Kraus L, Mohsin S (2020) Role of stem cell-derived microvesicles in cardiovascular disease. J Cardiovasc Pharmacol 76(6):650–657
Laksono S, Setianto B, Prawara AS, Dwiputra B (2021) Highlighting exosomes’ function in cardiovascular diseases. Curr Cardiol Rev 18:e241121191159
Lebedeva AM, Shpektor AV, Vasilieva EY, Margolis LB (2018) Cytomegalovirus infection in cardiovascular diseases. Biochemistry (Mosc) 83(12):1437–1447
Li B, Huang Q, Lin C, Lu R, Wang T, Chen X, Liu Z, Liu Y, Wu J, Wu Y, Liao S, Ding X (2021a) Increased circulating CD31+/CD42b-EMPs in Perthes disease and inhibit HUVECs angiogenesis via endothelial dysfunction. Life Sci 265:118749
Li Q, Song Y, Wang Q, Chen J, Gao J, Tan H, Li S, Wu Y, Yang H, Huang H, Yu Y, Li Y, Zhang N, Huang Z, Pang Z, Qian J, Ge J (2021b) Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics 11(8):3916–3931
Lindoso RS, Sandim V, Collino F, Carvalho AB, Dias J, da Costa MR, Zingali RB, Vieyra A (2016) Proteomics of cell-cell interactions in health and disease. Proteomics 16(2):328–344
Liu Q, Piao H, Wang Y, Zheng D, Wang W (2021) Circulating exosomes in cardiovascular disease: novel carriers of biological information. Biomed Pharmacother 135:111148
Logue SE, Gustafsson AB, Samali A, Gottlieb RA (2005) Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol 38(1):21–33
Madonna R, Pieragostino D, Rossi C, Guarnieri S, Nagy CT, Giricz Z, Ferdinandy P, Del Boccio P, Mariggio MA, Geng YJ, De Caterina R (2020) Transplantation of telomerase/myocardin-co-expressing mesenchymal cells in the mouse promotes myocardial revascularization and tissue repair. Vascul Pharmacol 135:106807
Maiullari F, Chirivi M, Costantini M, Ferretti AM, Recchia S, Maiullari S, Milan M, Presutti D, Pace V, Raspa M, Scavizzi F, Massetti M, Petrella L, Fanelli M, Rizzi M, Fortunato O, Moretti F, Caradonna E, Bearzi C, Rizzi R (2021) In vivoorganized neovascularization induced by 3D bioprinted endothelial-derived extracellular vesicles. Biofabrication 13:035014
Maring JA, Lodder K, Mol E, Verhage V, Wiesmeijer KC, Dingenouts CKE, Moerkamp AT, Deddens JC, Vader P, Smits AM, Sluijter JPG, Goumans MJ (2019) Cardiac progenitor cell-derived extracellular vesicles reduce infarct size and associate with increased cardiovascular cell proliferation. J Cardiovasc Transl Res 12(1):5–17
Maron BA, Wang RS, Shevtsov S, Drakos SG, Arons E, Wever-Pinzon O, Huggins GS, Samokhin AO, Oldham WM, Aguib Y, Yacoub MH, Rowin EJ, Maron BJ, Maron MS, Loscalzo J (2021) Individualized interactomes for network-based precision medicine in hypertrophic cardiomyopathy with implications for other clinical pathophenotypes. Nat Commun 12(1):873
Martins-Marques T, Ribeiro-Rodrigues T, de Jager SC, Zuzarte M, Ferreira C, Cruz P, Reis L, Baptista R, Goncalves L, Sluijter JP, Girao H (2020) Myocardial infarction affects Cx43 content of extracellular vesicles secreted by cardiomyocytes. Life Sci Alliance 3(12):e202000821
Minghua W, Zhijian G, Chahua H, Qiang L, Minxuan X, Luqiao W, Weifang Z, Peng L, Biming Z, Lingling Y, Zhenzhen W, Jianqing X, Huihui B, Xiaozhong W, Xiaoshu C (2018) Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death Dis 9(3):320
Penna C, Femmino S, Alloatti G, Brizzi MF, Angelone T, Pagliaro P (2021) Extracellular vesicles in comorbidities associated with ischaemic heart disease: focus on sex, an overlooked factor. J Clin Med 10(2):327
Pezzana C, Agnely F, Bochot A, Siepmann J, Menasche P (2021) Extracellular vesicles and biomaterial design: new therapies for cardiac repair. Trends Mol Med 27(3):231–247
Pironti G, Andersson DC, Lund LH (2021) Mechanistic and therapeutic implications of extracellular vesicles as a potential link between Covid-19 and cardiovascular disease manifestations. Front Cell Dev Biol 9:640723
Potz BA, Scrimgeour LA, Pavlov VI, Sodha NR, Abid MR, Sellke FW (2018) Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. J Am Heart Assoc 7(12):e008344
Prattichizzo F, Matacchione G, Giuliani A, Sabbatinelli J, Olivieri F, de Candia P, De Nigris V, Ceriello A (2021) Extracellular vesicle-shuttled miRNAs: a critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics 11(3):1031–1045
Reddy LVK, Murugan D, Mullick M, Begum Moghal ET, Sen D (2020) Recent approaches for angiogenesis in search of successful tissue engineering and regeneration. Curr Stem Cell Res Ther 15(2):111–134
Rosand O, Hoydal MA (2021) Cardiac exosomes in ischemic heart disease- a narrative review. Diagnostics (Basel) 11(2):269
Saheera S, Jani VP, Witwer KW, Kutty S (2021) Extracellular vesicle interplay in cardiovascular pathophysiology. Am J Physiol Heart Circ Physiol 320:H1749
Schaub T, Janke D, Zickler D, Lange C, Girndt M, Schindler R, Dragun D, Hegner B (2021) High cut-off dialysis mitigates pro-calcific effects of plasma on vascular progenitor cells. Sci Rep 11(1):1144
Schurgers LJ, Akbulut AC, Kaczor DM, Halder M, Koenen RR, Kramann R (2018) Initiation and propagation of vascular calcification is regulated by a concert of platelet- and smooth muscle cell-derived extracellular vesicles. Front Cardiovasc Med 5:36
Scrimgeour LA, Potz BA, Aboul Gheit A, Liu Y, Shi G, Pfeiffer M, Colantuono BJ, Sodha NR, Abid MR, Sellke FW (2020) Intravenous injection of extracellular vesicles to treat chronic myocardial ischemia. PloS One 15(9):e0238879
Scrimgeour LA, Potz BA, Aboul Gheit A, Shi G, Stanley M, Zhang Z, Sodha NR, Ahsan N, Abid MR, Sellke FW (2019) Extracellular vesicles promote arteriogenesis in chronically ischemic myocardium in the setting of metabolic syndrome. J Am Heart Assoc 8(15):e012617
Semenza GL (2011) Oxygen sensing, homeostasis, and disease. N Engl J Med 365(6):537–547
Silvestre JS (2012) Pro-angiogenic cell-based therapy for the treatment of ischemic cardiovascular diseases. Thromb Res 130(Suppl 1):S90–S94
Sluijter JPG, Davidson SM, Boulanger CM, Buzas EI, de Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K, Ferdinandy P (2018) Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the working group on cellular biology of the heart of the European Society of Cardiology. Cardiovasc Res 114(1):19–34
Song Y, Zhang C, Zhang J, Jiao Z, Dong N, Wang G, Wang Z, Wang L (2019) Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 9(8):2346–2360
Terenzi DC, Trac JZ, Teoh H, Gerstein HC, Bhatt DL, Al-Omran M, Verma S, Hess DA (2019) Vascular regenerative cell exhaustion in diabetes: translational opportunities to mitigate cardiometabolic risk. Trends Mol Med 25(7):640–655
Uil M, Hau CM, Ahdi M, Mills JD, Kers J, Saleem MA, Florquin S, Gerdes VEA, Nieuwland R, Roelofs J (2021) Cellular origin and microRNA profiles of circulating extracellular vesicles in different stages of diabetic nephropathy. Clin Kidney J 14(1):358–365
Vacchi E, Burrello J, Burrello A, Bolis S, Monticone S, Barile L, Kaelin-Lang A, Melli G (2021) Profiling inflammatory extracellular vesicles in plasma and cerebrospinal fluid: an optimized diagnostic model for Parkinson’s disease. Biomedicine 9(3):230
Vagida MS, Arakelyan A, Lebedeva AM, Grivel JC, Shpektor AV, Vasilieva EY, Margolis LB (2016) Analysis of extracellular vesicles using magnetic nanoparticles in blood of patients with acute coronary syndrome. Biochemistry (Mosc) 81(4):382–391
van Kralingen JC, McFall A, Ord ENJ, Coyle TF, Bissett M, McClure JD, McCabe C, Macrae IM, Dawson J, Work LM (2019) Altered extracellular vesicle MicroRNA expression in ischemic stroke and small vessel disease. Transl Stroke Res 10(5):495–508
Vanhaverbeke M, Gal D, Holvoet P (2017) Functional role of cardiovascular exosomes in myocardial injury and atherosclerosis. Adv Exp Med Biol 998:45–58
Vilades D, Martinez-Camblor P, Ferrero-Gregori A, Bar C, Lu D, Xiao K, Vea A, Nasarre L, Sanchez Vega J, Leta R, Carreras F, Thum T, Llorente-Cortes V, de Gonzalo-Calvo D (2020) Plasma circular RNA hsa_circ_0001445 and coronary artery disease: performance as a biomarker. FASEB J 34(3):4403–4414
Villa Del Campo C, Liaw NY, Gunadasa-Rohling M, Matthaei M, Braga L, Kennedy T, Salinas G, Voigt N, Giacca M, Zimmermann WH, Riley PR (2021) Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer. Cardiovasc Res 118:597
Villanueva M, Michie C, Parent S, Kanaan GN, Rafatian G, Kanda P, Ye B, Liang W, Harper ME, Davis DR (2019) Glyoxalase 1 prevents chronic hyperglycemia induced heart-explant derived cell dysfunction. Theranostics 9(19):5720–5730
Wang B, Zhang M, Urabe G, Shirasu T, Guo LW, Kent KC (2021) PERK inhibition promotes post-angioplasty re-endothelialization via modulating SMC phenotype changes. J Surg Res 257:294–305
Wang L, Wei J, Da Fonseca FA, Wang H, Zhang L, Zhang Q, Bellio MA, Chu XM, Khan A, Jayaweera D, Hare JM, Dong C (2020) Rejuvenation of senescent endothelial progenitor cells by extracellular vesicles derived from mesenchymal stromal cells. JACC Basic Transl Sci 5(11):1127–1141
Ward MR, Abadeh A, Connelly KA (2018) Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Transl Med 7(7):543–550
Wider J, Undyala VVR, Whittaker P, Woods J, Chen X, Przyklenk K (2018) Remote ischemic preconditioning fails to reduce infarct size in the Zucker fatty rat model of type-2 diabetes: role of defective humoral communication. Basic Res Cardiol 113(3):16
Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, Yu X, Tan J, Liu S, Zhang P, Tiang Z, Chen Z, Foo RS, Yang HT (2020) Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis 11(5):354
Xiong YY, Gong ZT, Tang RJ, Yang YJ (2021) The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics 11(3):1046–1058
Yadid M, Lind JU, Ardona HAM, Sheehy SP, Dickinson LE, Eweje F, Bastings MMC, Pope B, O'Connor BB, Straubhaar JR, Budnik B, Kleber AG, Parker KK (2020) Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci Transl Med 12(565):eaax8005
Yuan C, Ni L, Zhang C, Hu X, Wu X (2021) Vascular calcification: new insights into endothelial cells. Microvasc Res 134:104105
Zhang L, Graf I, Kuang Y, Zheng X, Haupt M, Majid A, Kilic E, Hermann DM, Psychogios MN, Weber MS, Ochs J, Bahr M, Doeppner TR (2021a) Neural progenitor cell-derived extracellular vesicles enhance blood-brain barrier integrity by NF-kappaB (nuclear factor-kappaB)-dependent regulation of ABCB1 (ATP-binding cassette transporter B1) in stroke mice. Arterioscler Thromb Vasc Biol 41(3):1127–1145
Zhang YX, Tang RN, Wang LT, Liu BC (2021b) Role of crosstalk between endothelial cells and smooth muscle cells in vascular calcification in chronic kidney disease. Cell Prolif 54(3):e12980
Zhao X, Jia Y, Chen H, Yao H, Guo W (2019) Plasma-derived exosomal miR-183 associates with protein kinase activity and may serve as a novel predictive biomarker of myocardial ischemic injury. Exp Ther Med 18(1):179–187
Zhu Z, Shen Y, Chen Y, Shi H, Shi Y (2021) The exosome of platelet endothelial cell adhesion molecule-1 (PECAM1) protein: a potential risking star in high blood pressure patients (HBPP). Medicine (Baltimore) 100(4):e21370
Alfaidi M, Wilson H, Daigneault M, Burnett A, Ridger V, Chamberlain J, Francis S (2015) Neutrophil elastase promotes interleukin-1beta secretion from human coronary endothelium. J Biol Chem 290(40):24067–24078
Beltrami C, Angelini TG, Emanueli C (2015) Noncoding RNAs in diabetes vascular complications. J Mol Cell Cardiol 89(Pt A):42–50
Das S, Halushka MK (2015) Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 24(4):199–206
Gill R, Kuriakose R, Gertz ZM, Salloum FN, Xi L, Kukreja RC (2015) Remote ischemic preconditioning for myocardial protection: update on mechanisms and clinical relevance. Mol Cell Biochem 402(1–2):41–49
Acknowledgment
This work was supported by the National Natural Science Foundation of China (22003038 to X.C.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhu, Y., Wang, S., Chen, X. (2023). Extracellular Vesicles and Ischemic Cardiovascular Diseases. In: Xiao, J. (eds) Extracellular Vesicles in Cardiovascular and Metabolic Diseases. Advances in Experimental Medicine and Biology, vol 1418. Springer, Singapore. https://doi.org/10.1007/978-981-99-1443-2_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-1443-2_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1442-5
Online ISBN: 978-981-99-1443-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)