Abstract
Amphiphilic polymeric block copolymers find frequent applications due to their ability to deliver hydrophobic moieties to the physiological system. Their ability to solubilize water-insoluble drugs in the core has improved the pharmacokinetics and therapeutic potential of such drugs. The hydrophobic core of the polymeric micelles provides protection to degradation-prone drugs. The thermodynamically stable polymeric micelles can be engineered to liberate the loaded cargo in various fashions. Drug release patterns such as biphasic, stimuli-responsive, and physiological/pathological environment-responsive have been reported to improve the therapeutic potential in multiple diseases such as neurological disorders and cancer. The advantages of polymeric micelles over conventional drug delivery systems have been explained. The concepts behind engineering polymeric micelles with a particular drug-releasing phenomenon will be deliberated throughout the chapter. Additionally, the need for functionalization and the development of functionalized micelles in various diseases have been discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
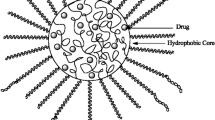
Polymeric micelles are colloidal drug delivery systems consisting of supra molecular nano-constructs of size ~10 to 100 nm. Self-assembly of amphiphilic molecules, mainly di- or tri-block copolymers, forms micelles (Trivedi and Uday 2012). These amphiphilic molecules exist as separate entities at lower concentrations. Whereas at higher concentrations, these can self-assemble and form micelles. The concentration where amphiphilic molecules start forming micelles is called critical micellar concentration (CMC). At CMC, the interface and bulk phase are saturated with monomers; hence, further increment in their concentration causes aggregation of monomers resulting in micelle formation, which in turn is evidenced by liberating the system’s free energy (Patist et al. 2001). The formed micellar structures comprise two parts: the core (the inner structure of micelles) and the shell (the outer surface of micelles). In most cases, the hydrophilic portion of block copolymers forms the shell/outer surface of micelles, and the hydrophobic portion includes micelles’ core/inner structure. In contrast, the converse is the case with reverse micelles. The most commonly employed hydrophilic polymers for micelle formulation are: poly(ethylene oxide)/poly(ethylene glycol), poly(vinyl pyrrolidone), and poly(N-isopropyl acryl amide). The polymers that form hydrophobic core structure include poly(l-lactide), poly(lactide-co-glycolic acid), poly(ε-caprolactone), poly(propylene oxide), poly(l-aspartic acid), poly(l-histidine) (Aliabadi and Lavasanifar 2006). The critical parameters that affect micelle formation are (i) the composition of the amphiphilic structure, (ii) the molecular weight of hydrophobic and/or hydrophilic moieties, (iii) the extent of hydrophilicity/phobicity, (iv) wetting property, (v) hydrophilic–hydrophobic balance of the copolymer, (vi) temperature, (vii) solvent system employed, (viii) concentration of amphiphilic molecules, (ix) aggregation number of amphiphiles, (x) process of micellization, (xi) solvation ability of amphiphilic moieties, etc. (Trivedi and Uday 2012; Patist et al. 2001; Aliabadi and Lavasanifar 2006; Förster and Plantenberg 2002; Hwang et al. 2020; Lombardo et al. 2015). These parameters, in turn, govern the size, shape, drug entrapment efficiency, and drug release kinetics of polymeric micelles (Aliabadi and Lavasanifar 2006; Förster and Plantenberg 2002; Zhang et al. 2017).
Polymeric micelles have evolved as potential carriers owing to their small size, ease of preparation, ability to enhance the solubility of hydrophobic drugs, and facilitate the transport of drugs across cellular barriers. The hydrophobic nature of the micellar core can facilitate the solubilization of hydrophobic drug moieties by interacting via weak Van der walls and ionic or hydrophobic interactions. As a result, micelles enhance the oral bioavailability of hydrophobic drugs (Xu et al. 2013). Further, the hydrophilic shell of micelles also plays a vital role in preventing opsonization to reduce immune clearance (Jhaveri and Torchilin 2014). Due to their small size and optimal surface characteristics, nanomicelles can endocytose easily and get transported across cellular barriers, potentially improving therapeutic agents’ bioavailability in various tissues (Gaurav et al. 2013).
Further, composition, the pattern of self-assembly of block copolymers, and their physicochemical properties govern the release kinetics (i.e., immediate release/sustained release/controlled or stimuli-responsive release) (Ghezzi et al. 2021). In conventional micelle-based formulations, drug release takes place via diffusion. Such diffusion-based drug release may occur from intact micelles or after disassociation/disassembly/distortion of micelle structure. Literature reveals that the drug release kinetics from polymeric micelles can be varied by altering the interaction between therapeutic agents and block copolymers used to fabricate micelles. Since premature drug release before reaching the intended site in the body leads to compromised efficiency of micelle-based delivery systems, appropriate strategies have been adopted to achieve drug release, preferably in the area of interest. Such strategies include cross-linking polymeric micelles and using stimuli-responsive polymers (Ghezzi et al. 2021; Zhou et al. 2018a; Kim et al. 2021; Barve et al. 2020; Shi et al. 2017).
5.2 Drug Release from Micelles
Drug release profile plays a vital role in governing the pharmacokinetics and pharmacodynamics of administered therapeutic agents. Therefore, appropriate formulation parameters must be adopted during the development of micelle-based formulations to achieve the desired release rate. Since the drug release is majorly directed by the composition of the micelles and the pattern of arrangement of amphiphilic block copolymers, the polymeric materials employed for the fabrication of micelles play a pivotal role in governing drug release from micelles. Further, drug entrapment and release kinetics can also be governed by the type of micelles (normal/reverse) employed for drug delivery applications. In general, conventional or normal micelles are composed of hydrophilic shell and hydrophobic core and hence, widely employed to entrap and deliver hydrophobic drugs.
Conversely, reverse micelles are composed of a hydrophobic shell and hydrophilic core and are employed to deliver hydrophilic drugs. Reverse micelles showed promising results in protecting sensitive hydrophilic moieties such as proteins, enzymes, antibodies, and nucleic acids (Qiu et al. 2007). Since the biological environment contains aqueous fluids, normal micelles can show predictable drug release profiles compared to reverse micelles (Vrignaud et al. 2011). The structure of normal and reverse micelles have been depicted in the Fig. 5.1.
The major drawback associated with polymeric micelles is premature drug release before they reach the site of interest. To overcome this challenge, scientists explored the stabilization process, i.e., the stabilization of polymeric core by forming reversible or weak bonding between the therapeutic agent and micellar structure (Xu et al. 2013; Talelli et al. 2015; Lu et al. 2018). Although this approach offered satisfactory results, the emergence of advanced strategies such as stimuli-responsive drug delivery gained more attention. Stimuli-responsive micelles can help to achieve spatio-temporal controlled drug release at the tissue of interest. The stimuli-responsive drug delivery strategies explored thus far include enzyme-responsive micelles, light-responsive micelles, magnetic field-responsive micelles, pH-responsive micelles, redox-responsive micelles, thermoresponsive micelles, ultrasound responsive micelles, or multi responsive micelles (Ghezzi et al. 2021; Zhou et al. 2018a; Kim et al. 2021; Barve et al. 2020). Various studies demonstrated improved efficacy via stimuli-responsive micellar systems for treating various diseases. In a study, Barve et al. demonstrated enhanced efficacy of enzyme-responsive polymeric micelles for tumor management. In this study, authors formulated matrix metalloproteinase-2 (MMP-2) enzyme-responsive polymeric micelles for targeted delivery of cabazitaxel to prostate cancer cells. The micelles were fabricated using two amphiphilic block copolymers (PEG, an enzyme-responsive peptide, cholesterol; and PEG, cholesterol, a targeting ligand). Since MMP-2 expression is higher in prostate cancer cells, the micelles released a higher amount of drug in the tumor microenvironment and showed improved therapeutic efficacy in the prostate cancer xenograft mice model compared to free cabazitaxel and unmodified micelles (Barve et al. 2020).
Further, light-responsive polymeric micelles showed promising results in cancer therapy by controlling drug release at the specific site, as demonstrated by Kim et al., where light-responsive micelles conjugated with diazonaphthoquinone (DNQ) were fabricated that could change their polarity, i.e., hydrophobic micelles to hydrophilic micelles under UV light. Such light responsiveness resulted in enhanced release of encapsulated therapeutic agent, docetaxel, and as a result, improved cytotoxicity toward breast cancer cells (Kim et al. 2021). Another stimuli-responsive system, pH-responsive polymeric micelles were developed by Zhou et al. for delivery of doxorubicin to the tumor. Lipid modified polymer, namely 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] conjugated poly(β-amino esters) was employed for pH sensitive release and demonstrated enhanced cytotoxicity of micelles toward tumor cells such as B16F10, HepG2, and HeLa cell lines (Zhou et al. 2018a). Taken together, stimuli-responsive micelle-based delivery systems show promise for spatio-temporal controlled release of entrapped therapeutic agents. The drug loading and drug release from micelle based- delivery systems have been depicted in the Fig. 5.2.
The micelles are stable above CMC and the structure disassembles when the concentration of the amphiphilic molecules is diluted below the CMC (Owen et al. 2012). Along with CMC, the kinetic stability index of micelles also plays an important role in the disassembly of micelles postdilution which is described as the tendency of micelles to disassemble over time when the concentration of block copolymers is reduced below CMC (Owen et al. 2012). Polymeric micelles demonstrate the advantage of low CMC and higher kinetic stability, enabling them to form stable structures with the ability to deliver drugs to the desired target organ (Wakaskar 2017). In comparison low molecular weight surfactants lose their structures in the range of microseconds, disabling the successful delivery of the drug. Drug release from polymeric micelles follows two mechanisms, either diffusion of the drug from the core of micelles or drug release due to the disassembly of the micellar structure (Fig. 5.3) (Ghezzi et al. 2021). To achieve site-specific drug release various stimuli-responsive micelles have been developed. Such stimuli-responsive micelles may aid in developing tolerability to toxic and potent drugs such as chemotherapeutics (Zhou et al. 2018b). The tumor microenvironment demonstrates various atypical conditions such as high levels of glutathione, acidic pH, and hypoxia (Whiteside 2008). Recent literature demonstrate that redox-, pH-, and hypoxia-sensitive micelles have demonstrated better anticancer activity and lower side effects (Sikder et al. 2022; Son et al. 2021; Feng et al. 2020). Stimuli-responsive micelles are disassembled in the presence of specific stimuli which leads to the release of loaded drugs.
5.3 Role of Micelles in Protecting Drugs from the Biological Environment
The biological system consists of complex cellular and extracellular tissue compartments, aqueous fluids with varying ionic concentration and/or pH, and other miscellaneous units, each performing a particular physiological function. Such a complex physiological environment may adversely affect the solubility, stability, pharmacokinetics, and pharmacodynamics of administered therapeutic agents, ultimately leading to compromised therapeutic efficacy. These drawbacks can be minimized by employing a suitable drug delivery system, wherein drugs are either entrapped, encapsulated, conjugated, or adsorbed on a suitable material (Allen and Cullis 2004; Vargason et al. 2021). Micelles can protect entrapped therapeutic agents from the harsh biological environment (such as acid and/or alkali-mediated degradation or denaturation, enzyme-mediated degradation, etc.) and offer site-specific drug delivery (Aliabadi and Lavasanifar 2006). Since micelles are composed of amphipathic/amphiphilic moieties (that contain hydrophilic and hydrophobic domains), they tend to aggregate by entropy effect, which results in the incorporation of drugs into their structure. The resultant hydrophilic exterior surface stabilizes the structure of the micelle by forming weak interactions (such as hydrogen bonds) with the surrounding microenvironment/aqueous fluids.
Further, such arrangement of micelles helps to (i) protect entrapped therapeutic agents from harsh microenvironments such as gastric pH, (ii) reduce the extent of metabolism of rapidly metabolizing drugs, and (iii) minimize immune clearance of entrapped drugs (Hwang et al. 2020). Therefore, micelles can be explored as drug delivery vehicles for delivering sensitive therapeutic agents across harsh biological microenvironments. In a study, Almeida et al. demonstrated the potential of amphiphilic chitosan micelle-based drug delivery system in preventing camptothecin degradation. Literature reveals that camptothecin exists in two forms based on surrounding pH conditions: the active lactone form (at acidic pH) and the inactive carboxylated form (at alkaline pH). Since alkaline pH solutions/fluids convert camptothecin to inactive carboxylated form, it is desirable to maintain the stability of camptothecin in vivo at alkaline pH conditions using suitable drug delivery strategies. In an attempt to resolve this issue, a novel copolymer consisting of O-methyl-O′-succinylpolyethylene glycol 5000 (mPEG)-chitosan-oleic acid and the resultant copolymer conjugate (i.e., chitosan conjugated with hydrophobic and hydrophilic moieties) was used for fabrication of camptothecin loaded micelles. The stability of free camptothecin and micelle-entrapped camptothecin was studied in simulated gastric fluid (SGF, pH 1.2) and simulated intestinal fluid (SIF, pH 6.8). Since camptothecin is unstable in SIF (alkaline pH cause degradation/carboxylation of camptothecin), authors compared the stability of free camptothecin and micelle-entrapped camptothecin in SIF. It was observed that free camptothecin was degraded in SIF, whereas micelle-entrapped camptothecin was stable. Therefore, authors inferred that the fabricated chitosan micelles protected the structure of camptothecin (active lactone form) by preventing its hydrolysis to inactive carboxylated form when incubated in simulated intestinal fluid (Almeida et al. 2020).
Furthermore, micelles can be employed not only for improving the stability and targetability of chemical moieties but also for protein or oligonucleotide-based therapeutic moieties (such as plasmid DNA, siRNA, and mRNA) as well. Since many nucleic acid-based entities require supraphysiological ionic strengths to maintain their integrity and are susceptible to degradation by nucleases, it is desirable to formulate them in an appropriate delivery system, such as micelles, for efficient in vivo delivery. In a study, Agarwal et al. employed cationic poly(ethylene glycol)-polylysine block copolymer as a coating material for different DNA origami structures. The authors coated DNA origami structures via electrostatic interactions and named them DNA origami polyplex micelles (DOPMs). The stability of resultant DOPM and intact DNA origami structures was studied by incubating them with a buffer containing DNase I or nucleases in fetal bovine serum-supplemented RPMI media. The agarose gel electrophoresis study revealed that the polyplex structures were stable and protected against DNAse I, whereas control DNA origami structures were completely degraded. This study demonstrated that the stability of nucleic acid-based structures was improved while using micelles as a delivery system (Agarwal et al. 2017).
In addition to their protective role, micelle-based delivery systems can minimize the metabolism of drugs in the physiological environment. Cheng et al. improved the circulatory half-life of a rapidly metabolizing drug, docetaxel, by employing a micelle-based drug delivery system. In this study, authors prepared docetaxel nanocrystals by high-pressure homogenization. Subsequently, the resultant nanocrystals were employed to fabricate nanocrystal-loaded micelles using methoxy polyethylene glycol-poly(d,l-lactide) block copolymer [mPEG-PLA]. When administered to rats, the resulting amphiphilic copolymer-based docetaxel-loaded micelles prolonged the retention time of docetaxel in the blood (as evidenced by enhanced mean residence time and half-life) when compared to free docetaxel and docetaxel nanocrystals. These results inferred that micelle-based drug delivery systems could be employed for reducing the rate and extent of metabolism of rapidly metabolizing drugs. As a consequence, micelles can potentially improve the circulation time and bioavailability of drugs (Cheng et al. 2021).
Further, numerous studies demonstrated that micelles, due to the presence of a hydrophilic shell, can escape immune recognition by the reticuloendothelial system (RES), as a result, prolong in vivo circulation time of entrapped therapeutic agents. In a study, Shen et al. demonstrated the ability of cholesterol-conjugated polyoxyethylene sorbitol oleate (CPSO) micelles to evade phagocytosis by escaping from macrophages. In this study, rhodamine B (RhB) and paclitaxel (PTX) loaded CPSO micelles were fabricated using a dialysis-ultrasonic method, and the resultant optimized formulation was evaluated for intracellular uptake in human alveolar basal epithelial cells (A549 cells) and phagocytosis escape in alveolar macrophages (NR8383 cells). The confocal laser scanning microscopy study revealed that RhB-loaded CPSO micelles get endocytosed by A549 cells (as evidenced by enhanced fluorescence intensity) and escape phagocytosis by NR8383 cells (as evidenced by lower fluorescence intensity). This study revealed micelles could be designed to escape phagocytosis by macrophages (Shen et al. 2020).
5.4 Factors Affecting the Solubilization of Drugs in Micelles
Since micelles are the structures of copolymers or surfactant, they helps in the solubilization of hydrophobic drugs in the aqueous environment (Kulthe et al. 2012). According to the literature, authors came to know that nowadays, whatever drug substances were invented or synthesized, most of them are hydrophobic. Therefore, micelles play an essential role in achieving higher bioavailability of hydrophobic drugs (Xu et al. 2013). Some dominant factors which affect the solubilization of hydrophobic drug substances by the micellar structures are as follows: surfactant concentration above the CMC, the addition of salt, temperature, pH, nature of solubilizate, the polarity of the solubilizate, molecular structure of solubilizate, use of buffers in the formulation of micelles, use of surfactant mixture instead of single surfactant (Tehrani-Bagha and Holmberg 2013).
5.4.1 Use of Concentration of Surfactant above the CMC Level
Solubilization of hydrophobic drugs by the micelles is dependent on surfactant concentration. The concentration of surface-active agents below the CMC level is unable to solubilize the hydrophobic drug because below CMC level. Beneath the CMC level, surfactants were present in the molecular form as a single moiety. These individual structures of the surface-active agents are not capable of solubilizing the hydrophobic drugs. Therefore, for the solubilization of water-hating drug substances, the concentration of the surfactant should be above the CMC level; at/above the CMC level, surfactant significantly affects the solubility of the hydrophobic drug substances. As the concentration of the surfactants increases above the CMC level, solubilization of the hydrophobic drug substance increases. An increase in solubility with an increase in surfactant concentration above the CMC level was observed up to some extent. After a specific concentration, the viscosity of the solution starts to elevate, which is considered an indication of rod shape micelles formation. So, beyond this point, the linear relation between the increased surfactant concentration and hydrophobic drug solubilization does not exist. So, we can say that up to some extent increase in the concentration of surface-active agents will help in the improvement of drug solubilization (Tehrani-Bagha et al. 2007). Anura S. indulkar et al. observed the impact of nature and surfactant concentration on the amorphous and crystalline solubility of atazanavir supersaturated solution. Findings from the study suggested that the crystalline solubility of the atazanavir increased in sodium dodecyl sulfate (SDS) compared to the absence of SDS in the supersaturated solution of the atazanavir. From observed results, researchers concluded that the concentration of surfactant, i.e., SDS, and the addition of other additives would positively affect the solubility of the drug substance (Indulkar et al. 2017).
5.4.2 Addition of Salt or Electrolyte
Innumerable studies confirm that adding salts or electrolytes positively affects the micellar solubilization of hydrophobic drugs. Micelles possess positively charged head groups on the surface and negatively charged tails in the inner core. Due to the presence of charged groups on the micelles, there is a possibility of ionic interactions between electrolytes and the charged moieties (that were present on the micellar structure).
In the case of micelles prepared from ionic surfactant, the addition of electrolyte causes ionic reactions between the charged groups present on the surface of the micelles. The reaction between the surface charge of micelles and electrolytes results in the neutralization of the micellar surface charge. This shielding or neutralization of surface charge leads to a more hydrophobic surfactant. Hydrophobic surfactants can form micelles at the lowest concentration compared to the other surfactant, which will help manufacture more stable micellar formation. Generally, low CMC value surfactants will create a more stable micellar formulation.
After a conclusive literature search, authors observed that the addition of NaCl salt in the polymeric solution of dodecyl trimethylammonium chloride (DTAC) led to an increase in the solubility of Sudan IV (hydrophobic dye), which is two times higher than the polymeric solution in the absence of NaCl (Ikeda et al. 1980).
Adding salt to the surfactant solution increases the solubilization power and aggregation number of the surfactant. In contrast, it decreases the CMC concentration, which fulfills the requirement of a standard surfactant for stable micelle formation. An increase in the concentration of NaCl in the surfactant solution increases the solubilization power up to a specific concentration. Once the concentration of NaCl crosses 1.5 M, then the shape of micelles transfers from spherical to rod or worm shape, which may cause an increase in the viscosity of the solution. Beyond this limit, adding more salt did not work, and the solubilization of the hydrophobic drug did not increase. Therefore an appropriate amount of electrolytes or salts must be added (Ozeki and Ikeda 1980).
5.4.3 Temperature
In typical solutions, an increment in temperature positively increases the solubility of the drug substance. Similarly, in the case of micelles also rise in temperature leads to improved micellar solubilization of hydrophobic drug substances. Different surfactants like non-ionic surfactants (Penta(ethylene glycol) monoundecyl ether), anionic surfactants (SDS), and cationic surfactants (dodecyl trimethylammonium bromide (DTAB)) are the temperature sensitive surfactants which are mainly used for the micelles formulation to solubilize the hydrophobic drugs. Generally, non-ionic surfactants prominently act as thermoresponsive surfactants in micellar formulations. Researchers conducted an experiment on the solubilization of hydrophobic dye to identify thermoresponsive properties of different polymers like anionic, cationic, and non-ionic polymers. After completing the study, researchers found that non-ionic surfactant shows prominent temperature-dependent solubility of the hydrophobic dye compared to cationic and anionic types of surfactants (Datyner 1978).
Due to an increase in temperature of the non-ionic surfactant solution, the polyoxyethylene chain of the surfactant lost the hydration water, which led to a rise in critical packing parameters of the surfactant, which is considered the leading cause for the translation of spherical-shaped micelles into elongated micelles (Gharanjig et al. 2011). Increment in the micellar solubilization of hydrophobic drugs is mainly due to the elongated micelles.
5.4.4 Effect of pH
The relationship between pH and micellar solubilization has not been explored much. It is believed that there is no relation between the micellar solubilization of the drug and the pH of the medium, but this is not true most of the time as we increase the pH of the polymeric solution of DTAB, SDS, and penta(ethylene glycol) monoundecyl ether above 8, an increase in solubilization of hydrophobic dyes, i.e., Sudan I was observed. It only happens with cationic surfactants, whereas in the case of non-ionic and anionic surfactants, no significant difference was observed in the micellar solubilization of surfactant. An increase in solubilization of this dye is observed due to the deprotonation of the phenolic hydroxyl group. Deprotonation causes the ionization of functional groups in the dye, which further makes ionic bonding with cations on the cationic surfactant. Due to electrostatic forces, bond formation between dye and surfactant will happen, which is the predominant region for the increment of solubilization of hydrophobic dye in micellar solution with an increase in pH of the solution.
In some cases, this high pH of the micellar solution causes the ionization of dye which behaves as an anionic surfactant. So due to the formation of an anionic surfactant formation of mixed micelles takes place, which is responsible for the reduction of the CMC value and the enhancement in the solubilization power of the surfactant. This deprotonation will not be possible in the case of non-ionic and anionic surfactants. Hence the increase in pH value did not positively affect the solubilization power of the surfactant (Tehrani-Bagha et al. 2013).
5.4.5 Position of Solubilizate in Micellar Structure
In micelles, we can see the existence of the polarity gradient, i.e., the outer portion of the micellar structure is polar. In contrast, the inner part consists of lipophilic tails of the surfactant. According to the like-dissolve-like phenomenon, polar substances retain on the outer portion of the micelles, i.e., near the head portion of the surfactant or in the palisade region of the micelles. Similarly, non-polar or lipophilic drugs reside in the inner core of the micelles. It is not similar in all cases; sometimes, the drugs having functional groups like OH or NH2 may interact with the head portion of the surfactant. Due to these interactions, the drug substance resides in the palisade region, i.e., just beneath the outer part of the micelles (Gharanjig et al. 2011).
5.4.6 Use of Mixed Micelles
The surfactant having a low CMC level is always preferred. It will help in the formulation of more stable micelles as compared to the other surfactants. In the formation of mixed micelles, more than one surfactant is used, which will give the synergetic effect or improve the performance of the surfactant, which will usually be more than the summation of the individual surfactant performance in the micellar formulation. A combination of more than one surfactant will help reduce the CMC level of the surfactant and increase the solubilization power of the surfactant. The mixed micelles concept helps to reduce the concentration of cationic surfactants, most of which are toxic in higher amounts. In some cases, a mixture of ionic surfactants will negatively affect the solubilization power of micelles due to changes in the environment of the micelles (Muto et al. 1988).
5.4.7 Structure of Surfactant
Solubilization of any hydrophobic or hydrophilic drugs prominently depends on the solubilization power of the surface-active agents used in the formulation of the micelles. Solubilization of any drug or substance in the micellar formulation mutually depends on the structural properties of surface-active agents used in the formulation and the drug substance that has to be solubilized. The functional groups present on the surface-active agents, as well as functional groups present on the drug substance, decide the orientation of the drug substance in the micellar structure. Molecular interaction between functional groups of drugs and surfactants determines the solubilization power of the surfactant for the specific drug substance.
According to some case studies, it was observed that the solubilization power of the cationic surfactant is directly proportional to the number of carbon atoms present in the alkyl chain. But in some instances, it was observed that the replacement of the CH3 group by CF3 from the DTAB surfactant led to a decline in the solubility of the hydrophobic dye in an aqueous solution of DTAB. Fluorination of the DTAB leads to the formation of the unfavorable inner core of the micellar structure and decreases the aggregation number, which hampers the solubilization power of the surfactant (Tehrani-Bagha et al. 2013).
5.5 Advantages over Conventional Drug Delivery
Conventional drug delivery strategies majorly aim to deliver therapeutic agents into the body using drug solutions, suspensions, or emulsion-based delivery systems (that have particle/globule dimensions in a few micrometers range). The major drawback associated with such drug delivery systems is (i) compromised stability of the drug/dosage form resulting in less shelf life; (ii) suitability for systemic administrations (suspensions and emulsions are not suitable for systemic administration); (iii) susceptibility of drugs to the harsh physiological environment; (iv) low bioavailability; (v) non-specific drug distribution in body tissues; and (vi) (Allen and Cullis 2004; Vargason et al. 2021; Wen et al. 2015) no targeting ability. Such disadvantages of conventional drug delivery formulations can be addressed using micelles. Nanomicelles, owing to their small size and high surface area to volume ratio, can potentially offer advantages such as (i) high penetrability across cellular barriers; (ii) providing high stability of entrapped therapeutic agents by preventing their degradation in harsh biological environments; (iii) enhance the solubility of lipophilic drugs; (iv) extend the circulatory half-life of entrapped therapeutic agents by minimizing their recognition by the immune system; (v) enable targeted drug delivery to a specific tissue site; (vi) amenable for conjugation of targeting moieties; and (vii) offer controlled or sustained drug delivery, stimuli (pH, temperature, ultrasound, light, magnetism, redox potential) responsive delivery, etc. (Aliabadi and Lavasanifar 2006; Hwang et al. 2020; Srinivasarao et al. 2019). Nanomicelles are popularly known for enhancing the solubility of lipophilic drugs such as paclitaxel. In a study, Zhang et al. illustrated enhancement in paclitaxel solubility using micelles fabricated using Solutol HS15 and tocopherol polyethylene glycol succinate (TPGS). The resultant micelles not only improved the solubility of paclitaxel but also enhanced cell uptake efficiency and cytotoxicity in drug-resistant MCF-7/Adr cells compared to taxol-based formulations.
Further, these micelles enhanced the bioavailability of paclitaxel after oral administration and inhibited tumor growth in a rodent model (Zhang et al. 2017). In addition, micelles can also improve the retention of drugs in body tissues such as the eye, resulting in enhanced bioavailability of administered drugs (Ghezzi et al. 2022). Ghezzi et al. demonstrated that cyclosporin-loaded non-ionic amphiphilic micelles composed of tocopherol polyethylene glycol 1000 succinate (TPGS) and Solutol® HS15 offered enhanced permeation across superficial ocular layers and improved micelle retention in cornea and scleral tissues. Such enhanced permeation and improved retention of cyclosporin micelles in ocular tissues showed a reservoir effect (for cyclosporin) and sustained presentation of the drug in other ocular tissues. Therefore, micelle-based delivery systems can help to improve patient compliance by offering sustained drug delivery (Ghezzi et al. 2022).
Further, micelle-based drug delivery carriers have shown promising applications in targeted drug delivery, as illustrated by Zhang et al. by fabricating docetaxel and anti-Nucleostemin siRNA entrapped polyethylene oxide (PEO)-polycaprolactone (PCL) micelles decorated with DCL {N-[N-[(S)-1,3-dicarboxypropyl] carbamoyl]-(S)-lysine} and TAT ligands. DCL ligand can target (active targeting) the micelles to prostate cancer (PCa) cells, whereas TAT ligand helps enhance micelles’ penetration across cell membranes of PCa cells. The resultant micelles were evaluated for their in vivo efficacy in the castration-resistant prostate cancer (CRPC) mice tumor model. The study results revealed that the surface-modified micelle-based dual drug delivery system suppressed CRPC tumor proliferation by inhibiting the G1/S and G2/M mitotic cycle via synergistic interaction. Consequently, micelle-based drug delivery systems effectively stopped tumor growth (Zhang et al. 2021). Together, micelles showed enhanced efficacy in improving the pharmacokinetics and pharmacodynamics of entrapped therapeutic agents for better alleviating pathological conditions.
5.6 Role of Functionalized Micelles in Various Diseases
Micelles are one of the prominent delivery systems because of their ability to encapsulate and improve the pharmacokinetic profiles of hydrophobic drugs and their stability in circulation (Xu et al. 2013). Despite several advantages offered by polymeric micelles, they face issues like toxicity and immunogenicity, poor selectivity, less efficient drug delivery, and low membrane disrupting capabilities, which can be addressed by functionalization with appropriate ligands to improve the targeting capabilities (Figueiras et al. 2022). Targeted therapy is needed to improve patient outcomes by enhancing therapeutic effectiveness and reducing side effects, and functionalization is key to targeted therapy. Functionalization of micelles is a widely explored domain in various diseases wherein various options are available for functionalization but are not limited to peptides, antibodies, polymers, glycoproteins, folate, transferrin, and aptamers (Jhaveri and Torchilin 2014; Sutton et al. 2007).
5.6.1 Cancer
Cancer is a disease characterized by the uncontrolled proliferation of cells. Despite numerous studies and research, none of the available therapies can eliminate cancer or prevent its relapse. Functionalizing micelles with ligands that bind to overexpressed receptors on cancer cells is a popular approach. Several receptors, like folate receptors, are overexpressed on various cancer cells, including ovarian, breast, brain, and lung (Fernández et al. 2018). It is well established that exploiting targeting ligands endow micelles with superior capabilities in terms of improved cellular uptake, reduced off-target effects, and prolonged circulation time (Jhaveri and Torchilin 2014). Zhang et al. utilized folate modification on the surface of micelles containing superparamagnetic iron oxide nanoparticles (SPIONS) and sorafenib to target hepatocellular carcinoma, where these micelles specifically reached cancer cells overexpressing folate receptors. Folate-functionalized micelles displayed greater cellular internalization by HepG2 cells compared to naked micelles. Moreover, functionalized micelles had a higher apoptosis rate of about 17.01%, whereas naked micelles had only an 11.04% apoptotic rate. Moreover, micelles could be visualized by MRI because of the presence of SPIONS (contrast agent) and could assist in image-guided chemotherapy (Zhang et al. 2013).
Metastasis is a major reason that contributes to treatment complications and a major contributor to mortality (Guan 2015). Developing micelles that deliver the drugs at the metastatic sites could improve cancer management. A more significant fibronectin expression characterizes metastatic sites compared to primary tumors (Wang and Hielscher 2017). Given the above strategy, Gong et al. developed PEGylated polymeric micelles encapsulating doxorubicin and vinorelbine, functionalized micelles with cysteine-arginine-glutamic acid-lysine-alanine (CREKA) to target fibronectin-expressing metastatic tumors. These engineered micelles had superior targeting capabilities and efficiently inhibited fibronectin expression compared to non-functionalized micelles. Modified micelles prolonged the circulatory half-life of doxorubicin and vinorelbine, where 51.48% of doxorubicin and 58.69% of vinorelbine were detected in the blood. Still, only 18.36% of doxorubicin and 45.30% of vinorelbine were detected after 2 hours of administration of non-functionalized micelles. CREKA-modified micelles efficiency suppressed metastatic tumors compared to non-modified micelles (Gong et al. 2020). In addition to functionalization, this combination of drugs effectively suppressed the metastasis.
Multi-drug resistant cancer is another setback limiting the treatment of cancers. Several siRNA has been shown to minimize or overcome tumor resistance by downregulating P-gp (Navarro et al. 2012; Liu et al. 2019). Combining siRNA with chemotherapy displays synergistic effects in addition to reducing drug resistance (Sun et al. 2018). Yalamarty et al. developed 2C5 antibody functionalized dendrimer-based mixed micelles (MDM) encapsulating small interfering RNA (siRNA) and doxorubicin. Western blot analysis confirmed the downregulation of P-gp by siRNA where 2C5-functionalized MDM compared to non-functionalized MDM owing to targeted delivery. Additionally, greater cellular uptake was observed in MDA-MB-231 and SKOV-3TR cells compared to non-functionalized micelles (Yalamarty et al. 2022).
5.6.2 Neurological Disorders
Polymeric micelles have widely been explored for brain diseases due to their ability to traverse across BBB by enhancing membrane fluidity and steric stabilization due to PEGylation (Waris et al. 2022). The ease of functionalization makes them an attractive carrier for drugs in various CNS-related diseases, including epilepsy, psychosis, Alzheimer’s disease, Parkinson’s disease, glioblastoma, and cerebral ischemic injury (Kaur et al. 2022). Lactoferrin receptors are overexpressed on the surface of the brain endothelial cells in Alzheimer’s and Parkinson’s disease and could be effectively utilized for brain targeting (Le Gao et al. 2010). Agwa and co-workers used lactoferrin functionalized conjugated linoleic acid (CLA) micelles to deliver CLA in Alzheimer’s. In vivo biodistribution studies revealed a more significant accumulation of functionalized micelles in brain tissue than in other organs owing to the presence of Lactoferrin receptors on the surface of brain endothelial cells. Furthermore, it reduced brain oxidative stress, inflammation, apoptosis and acetylcholine esterase activity, and improved cognitive capabilities (Agwa et al. 2020). CLA-based micelles could be a potential carrier system to deliver drugs across BBB to treat neurodegenerative disorders like Alzheimer’s. A major obstacle to treating brain disorders is the passage of drugs across the BBB. Strategies such as nose-to-brain delivery have been shown to circumvent BBB (Giunchedi et al. 2020). Interestingly, functionalization with TAT peptide enabled nose-to-brain targeting of mPEG-PDLLA micelles (Ahmad et al. 2020).
5.6.3 Infectious Diseases
Combating intracranial infections is challenging due to the complexity and selective permeability of the blood–brain barrier, which poses a hurdle for brain targeting (Ziai and Lewin 2006). Management of CNS infections is more challenging than peripheral infections due to the limited stability of drugs in blood circulation and poor permeability across BBB (Kasinathan et al. 2015). Glucose transporter-1 (GLUT1) is abundantly expressed on the surface of BBB, transporting D-glucose and other hexoses across BBB (Koepsell 2020). Shao and co-workers developed a micellar delivery system for improving the permeability of Itraconazole (first line antifungal drug) across the BBB. Micelles comprised of PEG-b-poly(l-lysine)-b-poly(l-phenylalanine) (PEG-pLys-pPhe). Additionally, a disulfide linkage was introduced at the pLys end to impart stimuli responsiveness (Shao et al. 2015). Then endowing it with brain targeting capabilities by functionalization with dehydroascorbic acid (DHA), having a high affinity toward GLUT1 on BBB. The functionalized micelles remarkably prolonged the circulatory half-life in blood and improved the delivery of Itraconazole in the brain, effectively managing intracranial infection (Shao et al. 2015).
Amphotericin B is another important agent for managing systemic fungal infections; however, it exerts infusion-related and chronic toxicities (Cavassin et al. 2021). Wang et al. encapsulated Amphotericin B in PEG-PC micelles functionalized with urea (PEG-PUC) and PEG-PC micelles functionalized with phenylboronic acid (PEG-PBC) for reducing toxicity. To test the formulation’s effectiveness, it was compared with the marketed product Fungizone®. PEG-PBC micelles showed sustained release, whereas PEG-PUC micelles displayed burst release. Both micelles showed comparable antifungal activity to free Amphotericin B or Fungizone®. PEG-PBC micelles caused very little or no hemolysis, whereas PEG-PBC micelles caused slightly lower hemolysis compared to free Amphotericin B and Fungizone®. Interestingly, when PEG-PUC and PEG-PBC were mixed in equimolar ratios to form micelles, these micelles had similar antifungal activity to free Amphotericin B and Fungizone® and remarkably decreased the nephrotoxicity (Wang et al. 2016).
5.6.4 Pulmonary Diseases
Tuberculosis is one of the deadly diseases whose treatment is complicated, and the emergence of resistance to therapy further limits the treatment options. Rifampicin, one of the most effective drugs for treating tuberculosis, possesses high hydrophobicity, a barrier to drug delivery (Mazlan et al. 2021). Tripodo et al. developed inulin-based micelles functionalized with vitamin E (INVITE) to deliver Rifampicin in treating mycobacterium tuberculosis infection. Compared to Rifampicin, INVITE prolonged the drug release for up to seven days. Furthermore, INVITE displayed antimicrobial activity comparable to free Rifampicin (Tripodo et al. 2019). INVITE could be a potential platform for delivering hydrophobic drugs against Mycobacterium.
5.6.5 Imaging and Diagnosis
Various imaging techniques like magnetic resonance imaging (MRI), nuclear imaging, and computed tomography scan (CT) play a crucial role in diagnosing and evaluating the response to the therapies, where the contrast agent is administered that highlights the area of interest (Oerlemans et al. 2010). However, imaging agents lack specificity, which limits their distribution at desired sites. To address the above issue, Yoo et al. fabricated peptide amphiphile micelles using DSPE-PEG2000-DTPA(Gd), functionalized with peptide CREKA to target fibrin; hence imaging fibrin-containing atherosclerotic plaques.. Clot-binding assays revealed that CREKA-based micelles targeted clots eight times higher than non-functionalized micelles. Additionally, in vivo MRI and optical imaging studies of the aortas and hearts confirmed fibrin targeting conferred by the CREKA peptide (Yoo et al. 2016).
Inflammation is an innate host defense mechanism that protects against harmful stimuli or foreign substances (Chen et al. 2018). Various cell adhesion molecules (VCAM-1, ICAM-1, E, and P selectin) mediate this complex process (Panés et al. 1999). Pagoto and co-workers, to improve the visualization of inflammation, developed DSPE-PEG2000 micelles loaded with MRI contrast agent Gd-DOTAMA(C18)2; the micelles were functionalized with a cyclic peptide specific to VCAM-1 receptor (a biomarker of endothelium activated by inflammation) (Pagoto et al. 2016). This delivery system improved the MRI visualization of inflammation. Various studies that employed functionalized micelles for targeted therapeutic applications have been presented in the Table 5.1.
5.7 Summary
Micelles have successfully enabled the solubilization and disposition of hydrophobic drugs. The loading of drugs in the core of micelles protects the degradation-prone drugs from various degrading stimuli. Polymeric micelles demonstrate advantages such as low CMC and high kinetic stability enabling efficient drug delivery to various physiological compartment. Low CMC value of the amphiphilic block copolymers is important for stability of micellar structure post-dilution in the physiological fluids. The main mechanisms behind drug release from micelles are diffusion and disassembly of micelles in the physiological system. Micelles can be efficiently functionalized with polymers and ligands to engineer the dispositioning of micelles in the physiological system. The development of stimuli-responsive micelles has demonstrated excellent results in the neoplastic and inflammatory diseases due to the site-specific fashion of drug release.
References
Trivedi R, Uday K (2012) Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond) 100:130–134
Patist SG, Oh R, Leung DO (2001) Shah, kinetics of micellization: its significance to technological processes. Colloids Surfaces A Physicochem Eng Asp 176:3–16. https://doi.org/10.1016/S0927-7757(00)00610-5
Aliabadi HM, Lavasanifar A (2006) Polymeric micelles for drug delivery. Expert Opin Drug Deliv 3:139–162. https://doi.org/10.1517/17425247.3.1.139
Förster S, Plantenberg T (2002) From self-organizing polymers to nanohybrid and biomaterials. Angew Chemie Int Ed 41:688–714. https://doi.org/10.1002/1521-3773(20020301)41:5<688::aid-anie688>3.0.co;2-3
Hwang D, Ramsey JD, Kabanov AV (2020) Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev 156:80–118. https://doi.org/10.1016/j.addr.2020.09.009
Lombardo D, Kiselev MA, Magazù S, Calandra P (2015) Amphiphiles self-assembly: basic concepts and future perspectives of supramolecular approaches. Adv Condens Matter Phys 2015:1–22. https://doi.org/10.1155/2015/151683
Zhang T, Luo J, Fu Y, Li H, Ding R, Gong T, Zhang Z (2017) Novel oral administrated paclitaxel micelles with enhanced bioavailability and antitumor efficacy for resistant breast cancer. Colloids Surfaces B Biointerfaces 150:89–97. https://doi.org/10.1016/j.colsurfb.2016.11.024
Xu W, Ling P, Zhang T (2013) Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv 2013:1–15. https://doi.org/10.1155/2013/340315
Jhaveri AM, Torchilin VP (2014) Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol 5:77. https://doi.org/10.3389/fphar.2014.00077
Gaurav S, Elena VB, Alexander VK (2013) Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjug Chem 19:51–57
Ghezzi M, Pescina S, Padula C, Santi P, Del Favero E, Cantù L, Nicoli S (2021) Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release 332:312–336. https://doi.org/10.1016/j.jconrel.2021.02.031
Zhou XX, Jin L, Qi RQ, Ma T (2018a) PH-responsive polymeric micelles self-assembled from amphiphilic copolymer modified with lipid used as doxorubicin delivery carriers. R Soc Open Sci 5:171654. https://doi.org/10.1098/rsos.171654
Kim KN, Oh KS, Shim J, Schlaepfer IR, Karam SD, Lee JJ (2021) Light-responsive polymeric micellar nanoparticles with enhanced formulation stability. Polymers (Basel) 13:1–11. https://doi.org/10.3390/polym13030377
Barve A, Jain H, Liu Z, Zhao K (2020) Cheng, enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater 113:501–511. https://doi.org/10.1016/j.actbio.2020.06.019
Shi Y, Lammers T, Storm G, Hennink WE (2017) Physico-chemical strategies to enhance stability and drug retention of polymeric micelles for tumor-targeted drug delivery. Macromol Biosci 17. https://doi.org/10.1002/mabi.201600160
Qiu L, Zhang J, Yan M, Jin Y, Zhu K (2007) Reverse self-assemblies based on amphiphilic polyphosphazenes for encapsulation of water-soluble molecules. Nanotechnology 18:475602. https://doi.org/10.1088/0957-4484/18/47/475602
Vrignaud S, Anton N, Gayet P, Benoit JP, Saulnier P (2011) Reverse micelle-loaded lipid nanocarriers: a novel drug delivery system for the sustained release of doxorubicin hydrochloride. Eur J Pharm Biopharm 79:197–204. https://doi.org/10.1016/j.ejpb.2011.02.015
Talelli M, Barz M, Rijcken CJF, Kiessling F, Hennink WE, Lammers T (2015) Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today 10:93–117. https://doi.org/10.1016/j.nantod.2015.01.005
Lu Y, Zhang E, Yang J, Cao Z (2018) Strategies to improve micelle stability for drug delivery. Nano Res 11:4985–4998. https://doi.org/10.1007/s12274-018-2152-3
Owen SC, Chan DPY, Shoichet MS (2012) Polymeric micelle stability. Nano Today 7:53–65. https://doi.org/10.1016/J.NANTOD.2012.01.002
Wakaskar RR (2017) Polymeric micelles and their properties. J Nanomed Nanotechnol 8:433. https://doi.org/10.4172/2157-7439.1000433
Zhou Q, Zhang L, Yang TH, Wu H (2018b) Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int J Nanomedicine 13:2921. https://doi.org/10.2147/IJN.S158696
Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904. https://doi.org/10.1038/ONC.2008.271
Sikder G, Vambhurkar E, Amulya D, Bagasariya P, Famta S, Shah DK, Khatri SB, Singh VR, Sinha S (2022) Srivastava, advancements in redox-sensitive micelles as nanotheranostics: a new horizon in cancer management. J Control Release 349:1009–1030. https://doi.org/10.1016/J.JCONREL.2022.08.008
Son Y, Lee J, Baek M, Park D, Han SK, Min D, Lee BS (2021) Kim, PH-responsive amphiphilic polyether micelles with superior stability for smart drug delivery. Biomacromolecules 22:2043–2056. https://doi.org/10.1021/ACS.BIOMAC.1C00163/SUPPL_FILE/BM1C00163_SI_001.PDF
Feng H, Chu D, Yang F, Li Z, Fan B, Jin L, Li J (2020) Hypoxia-responsive polymeric micelles for enhancing cancer treatment. Front Chem 8:742. https://doi.org/10.3389/FCHEM.2020.00742/BIBTEX
Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303:1818–1822. http://science.sciencemag.org/content/sci/303/5665/1818.full.pdf
Vargason AM, Anselmo AC, Mitragotri S (2021) The evolution of commercial drug delivery technologies. Nat Biomed Eng 5:951–967. https://doi.org/10.1038/s41551-021-00698-w
Almeida A, Araújo M, Novoa-Carballal R, Andrade F, Gonçalves H, Reis RL, Lúcio M, Schwartz S, Sarmento B (2020) Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater Sci Eng C 112:110920. https://doi.org/10.1016/j.msec.2020.110920
Agarwal NP, Matthies M, Gür FN, Osada K, Schmidt TL (2017) Block copolymer micellization as a protection strategy for DNA origami. Angew Chemie 129:5552–5556. https://doi.org/10.1002/ange.201608873
Cheng M, Liu Q, Gan T, Fang Y, Yue P, Sun Y, Jin Y, Feng J, Tu L (2021) Nanocrystal-loaded micelles for the enhanced in vivo circulation of docetaxel. Molecules 26:4481. https://doi.org/10.3390/molecules26154481
Shen D, Shen Y, Chen Q, Huang B, Mi Y, Shan Y, Yu G, Webster TJ (2020) Macrophage escape by cholesterol-polyoxyethylene sorbitol oleate micelles for pulmonary delivery. Nanomedicine 15:489–509. https://doi.org/10.2217/nnm-2019-0376
Kulthe SS, Choudhari YM, Inamdar NN, Mourya V (2012) Polymeric micelles: authoritative aspects for drug delivery. Des Monomers Polym 15:465–521. https://doi.org/10.1080/1385772X.2012.688328
Tehrani-Bagha AR, Holmberg K (2013) Solubilization of hydrophobic dyes in surfactant solutions. Materials (Basel). 6:580–608. https://doi.org/10.3390/ma6020580
Tehrani-Bagha AR, Bahrami H, Movassagh B, Arami M, Amirshahi SH, Menger FM (2007) Dynamic adsorption of gemini and conventional cationic surfactants onto polyacrylonitrile. Colloids Surfaces A Physicochem Eng Asp 307:121–127. https://doi.org/10.1016/j.colsurfa.2007.05.011
Indulkar AS, Mo H, Gao Y, Raina SA, Zhang GGZ, Taylor LS (2017) Impact of micellar surfactant on supersaturation and insight into solubilization mechanisms in supersaturated solutions of atazanavir. Pharm Res 34:1276–1295. https://doi.org/10.1007/s11095-017-2144-0
Ikeda S, Ozeki S, Tsunoda MA (1980) Micelle molecular weight of dodecyldimethylammonium chloride in aqueous solutions, and the transition of micelle shape in concentrated NaCl solutions. J Colloid Interface Sci 73:27–37. https://doi.org/10.1016/0021-9797(80)90117-4
Ozeki S, Ikeda S (1980) The viscosity behavior of aqueous NaCl solutions of dodecyldimethylammonium chloride and the flexibility of its rod-like micelle. J Colloid Interface Sci 77:219–231. https://doi.org/10.1016/0021-9797(80)90434-8
Datyner (1978) The solubilization of disperse dyes by dispersing agents at 127 °C. J Soc Dye Colour 94:256–260. https://doi.org/10.1111/j.1478-4408.1978.tb03417.x
Gharanjig K, Sadeghi-Kiakhani M, Tehrani-Bagha AR, Khosravi A, Menger FM (2011) Solubility of two disperse dyes derived from n-alkyl and n-carboxylic acid naphthalimides in the presence of gemini cationic surfactants. J Surfactant Deterg 14:381–389. https://doi.org/10.1007/s11743-011-1253-8
Tehrani-Bagha AR, Singh RG, Holmberg K (2013) Solubilization of two organic dyes by anionic, cationic and nonionic surfactants. Colloids Surfaces A Physicochem Eng Asp 417:133–139. https://doi.org/10.1016/j.colsurfa.2012.10.006
Muto Y, Asada M, Takasawa A, Esumi K, Meguro K (1988) The efficiency of solubilization in mixed micelles of nonionic and anionic surfactants. J Colloid Interface Sci 124:632–638. https://doi.org/10.1016/0021-9797(88)90200-7
Wen H, Jung H, Li X (2015) Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J 17:1327–1340. https://doi.org/10.1208/s12248-015-9814-9
Srinivasarao DA, Lohiya G, Katti DS (2019) Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 11:e1548. https://doi.org/10.1002/wnan.1548
Ghezzi M, Ferraboschi I, Delledonne A, Pescina S, Padula C, Santi P, Sissa C, Terenziani F, Nicoli S (2022) Cyclosporine-loaded micelles for ocular delivery: investigating the penetration mechanisms. J Control Release 349:744–755. https://doi.org/10.1016/j.jconrel.2022.07.019
Zhang Y, Wang Y, Meng L, Huang Q, Zhu Y, Cui W, Cheng Y, Liu R (2021) Targeted micelles with chemotherapeutics and gene drugs to inhibit the G1/S and G2/M mitotic cycle of prostate cancer. J Nanobiotechnol 19:17. https://doi.org/10.1186/s12951-020-00756-6
Figueiras A, Domingues C, Jarak I, Santos AI, Parra A, Pais A, Alvarez-Lorenzo C, Concheiro A, Kabanov A, Cabral H, Veiga F (2022) New advances in biomedical application of polymeric micelles. Pharmaceutics 14:1700. https://doi.org/10.3390/pharmaceutics14081700
Sutton D, Nasongkla N, Blanco E, Gao J (2007) Functionalized micellar Systems for Cancer Targeted Drug Delivery. Pharm Res 24:1029–1046. https://doi.org/10.1007/s11095-006-9223-y
Fernández M, Javaid F, Chudasama V (2018) Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci 9:790–810. https://doi.org/10.1039/C7SC04004K
Zhang L, Gong F, Zhang F, Ma J, Zhang P, Shen J (2013) Targeted therapy for human hepatic carcinoma cells using folate-functionalized polymeric micelles loaded with superparamagnetic iron oxide and sorafenib in vitro. Int J Nanomedicine 8:1517–1524. https://doi.org/10.2147/IJN.S43263
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5:402–418. https://doi.org/10.1016/j.apsb.2015.07.005
Wang JP, Hielscher A (2017) Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer 8:674–682. https://doi.org/10.7150/jca.16901
Gong Z, Chen M, Ren Q, Yue X, Dai Z (2020) Fibronectin-targeted dual-acting micelles for combination therapy of metastatic breast cancer. Signal Transduct Target Ther 5:12. https://doi.org/10.1038/s41392-019-0104-3
Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP (2012) P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine 7:65–78. https://doi.org/10.2217/nnm.11.93
Liu W, Lo Y-L, Hsu C, Wu Y-T, Liao Z-X, Wu W-J, Chen Y-J, Kao C, Chiu C-C, Wang L-F (2019) CS-PEI/Beclin-siRNA downregulate multidrug resistance proteins and increase paclitaxel therapeutic efficacy against NSCLC. Mol Ther Nucleic Acids 17:477–490. https://doi.org/10.1016/j.omtn.2019.06.017
Sun W, Chen X, Xie C, Wang Y, Lin L, Zhu K, Shuai X (2018) Co-delivery of doxorubicin and anti-BCL-2 siRNA by pH-responsive polymeric vector to overcome drug resistance in in vitro and in vivo HepG2 hepatoma model. Biomacromolecules 19:2248–2256. https://doi.org/10.1021/ACS.BIOMAC.8B00272/SUPPL_FILE/BM8B00272_SI_001.PDF
Yalamarty SSK, Filipczak N, Li X, Pathrikar TV, Cotter C, Torchilin VP (2022) Co-delivery of siRNA and chemotherapeutic drug using 2C5 antibody-targeted dendrimer-based mixed micelles for multidrug resistant cancers. Pharmaceutics 14:1470. https://doi.org/10.3390/pharmaceutics14071470
Waris A, Ali AU, Khan M, Asim D, Zamel K, Fatima A, Raziq MA, Khan N, Akbar A, Baset MAS (2022) Abourehab, applications of various types of nanomaterials for the treatment of neurological disorders. Nanomater 12:2140. https://doi.org/10.3390/NANO12132140
Kaur J, Gulati M, Kapoor B, Jha NK, Gupta PK, Gupta G, Chellappan DK, Devkota HP, Prasher P, Ansari MS, Aba Alkhayl FF, Arshad MF, Morris A, Choonara YE, Adams J, Dua K, Singh SK (2022) Advances in designing of polymeric micelles for biomedical application in brain related diseases. Chem Biol Interact 361:109960. https://doi.org/10.1016/J.CBI.2022.109960
Le Gao H, Pang ZQ, Fan L, Hu KL, Wu BX, Jiang XG (2010) Effect of lactoferrin- and transferrin-conjugated polymersomes in brain targeting: in vitro and in vivo evaluations. Acta Pharmacol Sin 31:237. https://doi.org/10.1038/APS.2009.199
Agwa MM, Abdelmonsif DA, Khattab SN, Sabra S (2020) Self- assembled lactoferrin-conjugated linoleic acid micelles as an orally active targeted nanoplatform for Alzheimer’s disease. Int J Biol Macromol 162:246–261. https://doi.org/10.1016/j.ijbiomac.2020.06.058
Giunchedi P, Gavini E, Bonferoni MC (2020) Nose-to-brain delivery. Pharmaceutics 12:138. https://doi.org/10.3390/pharmaceutics12020138
Ahmad E, Lv Y, Zhu Q, Qi J, Dong X, Zhao W, Chen Z, Wu W, Lu Y (2020) TAT modification facilitates nose-to-brain transport of intact mPEG-PDLLA micelles: evidence from aggregation-caused quenching probes. Appl Mater Today 19:100556. https://doi.org/10.1016/j.apmt.2020.100556
Ziai WC, Lewin JJ (2006) Advances in the Management of Central Nervous System Infections in the ICU. Crit Care Clin 22:661–694. https://doi.org/10.1016/j.ccc.2006.11.009
Kasinathan N, Jagani HV, Alex AT, Volety SM, Rao JV (2015) Drug delivery strategies for drug delivery to the central nervous system by systemic route strategies for drug delivery to the central nervous system by systemic route. Drug Deliv 22:243–257. https://doi.org/10.3109/10717544.2013.878858
Koepsell H (2020) Glucose transporters in brain in health and disease. Pflugers Arch 472:1299–1343. https://doi.org/10.1007/s00424-020-02441-x
Shao K, Zhang Y, Ding N, Huang S, Wu J, Li J, Yang C, Leng Q, Ye L, Lou J, Zhu L, Jiang C (2015) Functionalized nanoscale micelles with brain targeting ability and intercellular microenvironment biosensitivity for anti-intracranial infection applications. Adv Healthc Mater 4:291–300. https://doi.org/10.1002/adhm.201400214
Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F (2021) Sixty years of amphotericin B: An overview of the Main antifungal agent used to treat invasive fungal infections. Infect Dis Ther 10:115–147. https://doi.org/10.1007/s40121-020-00382-7
Wang Y, Ke X, Voo ZX, Yap SSL, Yang C, Gao S, Liu S, Venkataraman S, Obuobi SAO, Khara JS, Yang YY, Ee PLR (2016) Biodegradable functional polycarbonate micelles for controlled release of amphotericin B. Acta Biomater 46:211–220. https://doi.org/10.1016/j.actbio.2016.09.036
Mazlan MKN, Tazizi MHDM, Ahmad R, Noh MAA, Bakhtiar A, Wahab HA, Gazzali AM (2021) Antituberculosis targeted drug delivery as a potential future treatment approach. Antibiotics 10:1–19. https://doi.org/10.3390/antibiotics10080908
Tripodo G, Perteghella S, Grisoli P, Trapani A, Torre ML, Mandracchia D (2019) Drug delivery of rifampicin by natural micelles based on inulin: physicochemical properties, antibacterial activity and human macrophages uptake. Eur J Pharm Biopharm 136:250–258. https://doi.org/10.1016/j.ejpb.2019.01.022
Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE (2010) Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res 27:2569–2589. https://doi.org/10.1007/s11095-010-0233-4
Yoo SP, Pineda F, Barrett JC, Poon C, Tirrell M, Chung EJ (2016) Gadolinium-functionalized peptide amphiphile micelles for multimodal imaging of atherosclerotic lesions. ACS Omega 1:996–1003. https://doi.org/10.1021/acsomega.6b00210
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204. https://doi.org/10.18632/ONCOTARGET.23208
Panés J, Perry M, Granger DN (1999) Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br J Pharmacol 126:537. https://doi.org/10.1038/SJ.BJP.0702328
Pagoto R, Stefania F, Garello F, Arena G, Digilio S, Aime E (2016) Terreno, paramagnetic phospholipid-based micelles targeting VCAM-1 receptors for MRI visualization of inflammation. Bioconjug Chem 27:1921–1930. https://doi.org/10.1021/acs.bioconjchem.6b00308
Qiu X, Qu Y, Guo B, Zheng H, Meng F, Zhong Z (2022) Micellar paclitaxel boosts ICD and chemo-immunotherapy of metastatic triple negative breast cancer. J Control Release 341:498–510. https://doi.org/10.1016/j.jconrel.2021.12.002
Pawar A, Singh S, Rajalakshmi S, Shaikh K, Bothiraja C (2018) Development of fisetin-loaded folate functionalized pluronic micelles for breast cancer targeting. Artif Cells Nanomed Biotechnol 46:347–361. https://doi.org/10.1080/21691401.2018.1423991
Situ J-Q, Wang X-J, Zhu X-L, Xu X-L, Kang X-Q, Hu J-B, Lu C-Y, Ying X-Y, Yu R-S, You J, Du Y-Z (2016) Multifunctional SPIO/DOX-loaded A54 homing peptide functionalized dextran-g-PLGA micelles for tumor therapy and MR imaging. Sci Rep 6:35910. https://doi.org/10.1038/srep35910
Bi Y, Liu L, Lu Y, Sun T, Shen C, Chen X, Chen Q, An S, He X, Ruan C, Wu Y, Zhang Y, Guo Q, Zheng Z, Liu Y, Lou M, Zhao S, Jiang C (2016) T7 peptide-functionalized PEG-PLGA micelles loaded with Carmustine for targeting therapy of glioma. ACS Appl Mater Interfaces 8:27465–27473. https://doi.org/10.1021/acsami.6b05572
Wu B, Sai S, Li K, Sun X, Han J, Tian B (2022) Maleimide-functionalized phospholipid/Pluronic F127 mixed micelles for efficient ophthalmic delivery of voriconazole against Candida albicans. Colloids Surfaces B Biointerfaces. 209:112180. https://doi.org/10.1016/J.COLSURFB.2021.112180
Ahn YS, Baik HJ, Lee BR, Lee ES, Oh KT, Lee DH, Youn YS (2010) Preparation of multifunctional polymeric micelles for antiviral treatment. Macromol Res 18:747–752. https://doi.org/10.1007/s13233-010-0802-8
Hisey B, Ragogna PJ, Gillies ER (2017) Phosphonium-functionalized polymer micelles with intrinsic antibacterial activity. Biomacromolecules 18:914–923. https://doi.org/10.1021/acs.biomac.6b01785
Acknowledgments
The authors would like to acknowledge the research funding support by Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India to National Institute of Pharmaceutical Education and Research (NIPER) Hyderabad, INDIA.
Declaration of Interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vambhurkar, G., Jain, N., Srinivasarao, D.A., Famta, P., Singh, S.B., Srivastava, S. (2023). Drug Solubilization and Drug Release from Polymeric Micelles. In: Singh, S.K., Gulati, M., Mutalik, S., Dhanasekaran, M., Dua, K. (eds) Polymeric Micelles: Principles, Perspectives and Practices. Springer, Singapore. https://doi.org/10.1007/978-981-99-0361-0_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-0361-0_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0360-3
Online ISBN: 978-981-99-0361-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)