Abstract
Metastasis is a leading cause of mortality in cancer patients. The journey of a detached primary tumour cell to a secondary homing site is a multi-step process with several intercellular interactions. In this process, a metastatic cell overcomes many barriers and adapts to the changing microenvironment. Our understanding of motility, invasion and epithelial to mesenchymal transition has led to the identification of targetable signalling networks and regulatory pathways. Emerging research has also identified genetic heterogeneity and variable microenvironment in the primary tumour cell as different from its metastatic progeny, leading to differential immune response. Therapeutic regimes with both standard chemotherapy and, in some cases, targeted therapy have only marginally improved the overall survival in metastatic cancer patients. It is for this reason that the current research strives to understand metastatic cancer, its origin and the role of the tumour microenvironment in genesis and persistence of metastasis. The hypoxic tumour microenvironment is known to arise in such growing primary tumours, where the vasculature is unable to keep pace with the high rate of proliferation, forming hypoxic niche within the tumours. Hypoxia, therefore, will always precede metastasis, thus implicating that the former plays a larger role in the development of the aggressive phenotype. Some of the leading research of our times is trying to understand latency of cancer cells, immune-metastatic cell interaction and epigenetic changes that promote metastasis and chemoresistance. With exciting possibilities of immunotherapeutic and other intervention strategies, a comprehensive understanding of the role of hypoxia in promoting metastasis will be useful. In this review, we dissect how hypoxia, hypoxia-inducible factors and other associated molecules dynamically modulate various stages of metastasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hypoxia
- Metastasis
- HIF
- Invasion
- Tumour-microenvironment
- EMT
- Signalling
- Immune evasion
- Epigenetics
- Anti-cancer therapy
- Chemoresistance
- CTCs
9.1 Life of a Cancer Cell in the Hypoxic Tumour Microenvironment and Beyond
Metastasizing cells appear to be a life of their own. Surviving and adapting just as we do, sometimes under adverse circumstances, often alone. While fully aware of the dreadful consequences associated with this disease, one cannot help but marvel at the skillful resilience and adaptations of this entity.
Metastasis is defined as the sequential event in which cancer cells break off from their site of origin and colonise new sites. This propensity of cells to be motile is frequently seen in normal cells too, where cells migrate in response to chemical attractants such as that observed in morphogenesis during embryo development and movement of immune cells to site of infection. A normal cell, therefore, has all the molecular components that can be triggered to initiate these events, and a cancer cell is endowed to be even more receptive to triggers from its neighbouring tumour microenvironment (TME). Over the years, several new paradigms on cancer progression and molecular underpinnings of cancer metastasis have been extensively delineated. With these studies, researchers have overcome the challenge of detection limits, where the small fraction of disseminated circulating tumour cells has been identified in the backdrop of millions of RBCs and WBCs in blood (Hong and Zu 2013; Bankó et al. 2019). The entire field of circulating tumour cells emerged with the effort to look for early dissemination of cancer cells with increasing sensitivity and also specific for certain tumour-associated mutations.
9.1.1 Manifestation of Metastasis
Metastatic disease spread is progressive and fatal. Most cancer-related deaths are said to occur due to metastasis, and in a majority of patients, the disease is detectable only in autopsy. In a significant number of patients with metastatic disease (not related to skin), a primary tumour has not been identified – tumours of unknown primary source. While it is generally reported that 90% of cancer-related deaths are due to metastasis, these numbers may vary. A study from the data of cancer registry at Norway reported 67% mortality as being caused by cancer over a period of 10 years (Dillekås et al. 2019). Chemotherapy-induced metastasis has also been reported wherein cytotoxic chemotherapy inflicts tissue damage, hypoxia and cancer cell apoptosis, enforcing the release of proinflammatory cytokines and chemokines locally as well as systemically, leading to immunosuppression and acquisition of metastatic properties (Karagiannis et al. 2019).
Cancer metastasis is considered to be a secondary event and a delayed step in tumour progression associated with tumours of diameter more than 2 cm (Koscielny et al. 1984). Initially it was assumed that removal of such tumours should dramatically improve the prognosis and survival; however this was not always observed to be true (Bedenne et al. 2007). Recurrence was a frequent event in certain breast cancers which were equal or less than 1 cm (Gonzalez-Angulo et al. 2007). Further, many of the breast cancer tumours at T1 and T2 (Zimmer and Steeg 2015) including some with clinically undetectable tumours (Mauri et al. 2005) already exhibited metastasis. This led researchers to believe that metastasis may be an early, clinically undetectable event in the development of cancer. Thereon, researchers have looked at smaller tumours and their molecular profiles carefully. Critically, investigations on the role of the tumour microenvironment around the primary tumour cell, particularly hypoxia, gained momentum.
9.1.2 Cellular Characteristics of a Metastatic Cancer Cell
Cancer cells acquire the potential to migrate, exploit the fluid nature of the circulatory and lymphatic system to move greater distance and utilise the presence of conducive external growth factors at distant sites to foster their second homes. The malignant cells of a growing tumour undergo epithelial to mesenchymal transition (EMT), a cell redevelopment program, in which the cell loses its epithelial characteristics and gains mesenchymal properties (Thiery 2002). Acquisition of these attributes enable cells to invade other tissues and colonise them. A tumour cell with its newly acquired skill-set interacts with a neighbouring vessel. Crosstalk between these EMT transitioned tumour cells and endothelial cells of the blood vessels allow the upskilled tumour cells to breach the basement membrane of these blood vessels and intravasate into them. Subsequently, this metastatic cell shall overcome challenges from immune cells and unfamiliar environments to establish itself elsewhere. Only a few cancer cells are successful in acquiring these properties, and even fewer survive the harsh vascular environment. The migrating cells that succeed reach distant tissues and become harbingers of micrometastases. Once established, micrometastases undergo proliferation leading to macrometastases, and subsequently secondary tumour colonies emerge. The series of steps that the cells undergo is known as metastatic cascade. Once the cells exit their site of origin, they may either be destroyed in blood or sustain for long enough to get lodged in capillary beds of other tissues. If sustained, the cells would then extravasate into a new niche for the formation of a potential tumour colony (Fidler and Paget 2003). However, the colonisation in this niche is dependent on the fate of the cell.
Tumour metastasis is the major cause of mortality associated with cancers, leading up to 90% of cancer patient deaths. The uncontrolled cell growth causes various microenvironment stresses such as inflammation, hypoxia and deprivation of nutrients. The hypoxic microenvironment thus developed helps in advancement of the tumour into phases of metastasis such as local invasion, EMT, intravasation, extravasation, formation of micrometastasis and ultimately colonisation via macrometastasis (Majidpoor and Mortezaee 2021). Several new paradigms have emerged on the evolution of cancers that put forth comprehensive discourse on when and how these metastatic cells arise. However, we do know that the metastatic cancer cell fate is indelibly linked to cancer evolution.
9.1.3 Fate of a Cancer Cell
There are many theories proposed for delineating cancer evolution. The understanding of cancer biology has grown by leaps and bounds from the initial reductionist view on cancer cells (Hanahan and Weinberg 2000; Hanahan and Weinberg 2011) to that of heterotypic cell biology that regard tumour as a complex tissue. A series of mutations paired with proliferation of dominant cell types results in tumour growth and development that gives rise to a favourable tumour microenvironment by virtue of different growth signals and intercellular interactions. The latter view suggests that a tumorous mass comprises proliferating cancer cells; cancer stem cells along with tumour-associated immune cells together make a tumour self-sufficient with self-renewal properties, enabling it to invade niches foreign to the cancer cells (Hanahan and Weinberg 2000; Hanahan and Weinberg 2011). The understanding of the interaction of cancer cells with the tumour microenvironment is still developing, and many interesting aspects have emerged including that of cancer stem cell niche.
Displacement and spread of cancer cells away from the primary tumour was observed and termed as metastasis in 1829 by Joseph-Claude-Anthelme Récamier, a surgical oncologist. The identification of epithelial cells in blood circulation came a few decades later in 1869 by Thomas Ashworth and in the lymphatic system and lymph nodes in 1881 by William Hampstead (Akpe et al. 2020; Galvis et al. 2021). Different theories have attempted to explain the emergence of migrating cancer that eventually finds a second home. Rudolf Virchow’s chronic irritation theory suggested that the new site of growth is due to an inflammatory stimulus (Balkwill and Mantovani 2001). Ewing’s mechanical hypothesis postulates that the pattern of circulation determines the secondary tumour location and the lungs are most prone as it is the first organ encountered in circulation. Contrary to this, Stephen Paget’s seed and soil hypothesis, deduced from autopsies of 735 breast cancer patients with high incidence of metastases in the liver, ovary and bone, suggests that a cancer is a ‘seed’ that circulates in the body and upon finding a microenvironment conducive for its development, the circulating cancer cell lodges itself in the fertile ‘soil’ (Paget and Paget 1989). A large meta-data study on 179,581 patients has shown that anatomical/mechanical constraints can contribute to site determination and the two theories are not always mutually exclusive (Riihimäki et al. 2018). These circulating tumour cells face new microenvironments with different growth signals and regulatory factors, evolving and adapting through sequential mutations and selection. However, upon encountering a hostile environment at site, the cells can undergo dormancy, or its complete eradication may occur at any point of tumour development and metastasis. Microfluidic chips are widely used to validate the metastatic potential of cells and in turn also help in identifying potential homing sites for cancers in vivo (Aleman et al. 2019). This versatile approach has been utilised to constitute an in vivo-like microenvironment, including low oxygen, to study differentiation of stem cells (Chen et al. 2013) as well as simulate the extravasation and micrometastasis of breast cancer cells to bones (Bersini et al. 2014). It has further been used to demonstrate the ability of mesenchymal stromal cells (MSCs) to undergo homing in presence of hepatoma cells, enhanced under influence of TGF-β and suppression through physical constraints of the 3D microstructures (Yang et al. 2017).
9.1.4 The Dormant Cancer Cell and Plastic Cancer Stem Cell
We now know that cancer cells can remain dormant in both the primary site and the metastatic secondary site. The dormant cells can reawaken, proliferate and cause tumour relapse. They are clinically undetectable and contribute to the therapeutic resistance. They are actively undergoing ‘editing’, accumulating genetic and epigenetic changes that could improve their adaptation in the environment. Quiescent cells have been shown to evade immunity by forming an immunosuppressive niche (Baldominos et al. 2022). In their quiescent state, the cancer cells dynamically interact with the tumour microenvironment, modulating their characteristics until they can lead to tumour outgrowth and dissemination. After treatment, some cancer cells may become quiescent or latent and form clinically undetectable minimal residual disease. Increasing evidence shows that this repertoire of cells that can switch between dormant and proliferative states, enabling greater metastatic potential, is cancer stem cells (Reya et al. 2001; Giancotti 2013). They also exhibit chemoresistance and actively engage with the microenvironment. Parallels have been drawn between the state of cancer cell dormancy and the quiescent cancer stem cell with experimental evidence of being regulated by common signalling pathways such as mTOR, Wnt and Notch (Reya et al. 2001; Schewe and Aguirre-Ghiso 2008; Oskarsson et al. 2011; O'Connell et al. 2011). It has been possible to populate cancer stem cells from dormant cancer cell populations by invoking certain signalling pathways (Ranganathan et al. 2006; Gao et al. 2012). Regardless, the two terms cannot be used interchangeably because not all dormant cancer cells are cancer stem cells and vice versa. Importantly, the role of hypoxia and tumour microenvironment in cellular adaptations undertaken by the quiescent cancer stem cells cannot be undermined.
Studies have been conducted to determine whether hypoxia emerges as an independent prognostic factor for patient outcome and disease progression. Several studies have demonstrated that patients with hypoxic tumours have an increased risk of metastasis and mortality. One such study on 247 patients of prostate cancer measured tumour hypoxia before radiotherapy and reported that the median O2 was 10mmHg (~1.3% O2) which was 10-12 times lower than muscle tissue O2 (Milosevic et al. 2012). Taking prostate-specific antigen as a marker, they determined that degree of hypoxia in the tumours before radiotherapy was a predictor of quicker biochemically measured relapse (Milosevic et al. 2012). Similarly in breast cancers, a compilation of 125 studies of pre-treatment oxygenation status of solid tumours showed that the mean partial pressure of oxygen (pO2) in breast tumours ranges from 2.5 to 28 mm of mercury (Hg), with a median value of 10 mm Hg, as compared with 65 mm Hg in normal human breast tissue (Vaupel et al. 2007). In pancreatic cancers, which are highly metastatic and usually unresectable, tracer molecules such as 18F-fluoroazomycin arabinoside (Metran-Nascente et al. 2016) as well as electrodes (Koong et al. 2000) have been used to measure hypoxia. In a pancreatic ductal adenocarcinoma mice model, the highly metastatic pancreatic ductal adenocarcinoma subpopulation is enriched for hypoxia-induced genes (Chiou et al. 2017).
9.1.5 Role of Hypoxia in Determining the Fate of a Cancer Cell
Hypoxia forms an important part of the tumour microenvironment and cancer stem cell niche. The unhindered growth of cancer cells and absence of adequate vascular support gives rise to oxygen-starved cells and tissues. The oxygen starvation is commonly known as hypoxia. While genetic defects and cellular damage by reactive oxygen species (ROS) remain overarchingly dominant, hypoxia works at the gene-environment interface. Under high oxygen tension, hypoxia-inducible factors (HIFs) are constantly inactivated and prepared for ubiquitination by prolyl hydroxylases (PHDs) and factor-inhibiting HIF (FIH). The stabilisation and activity of HIFs is an inevitable response under the prevailing reduced oxygen tension. HIFs are hetero-dimeric transcription factors, consisting of constitutively expressed β-subunit and oxygen tension-regulated α-subunit. The action of HIF transcription factors is dependent upon its binding affinity to specific sites in the promoter region of hypoxia-responsive genes. Hypoxia, through HIF-dependent signalling, affects myriads of life processes such as vascularisation, metabolism, proliferation, apoptosis, invasion, and metastasis (Semenza 2013; Schito and Semenza 2016; Kaelin and Ratcliffe 2008; Pugh and Ratcliffe 2003).
The first encounter of a cancer cell with a hypoxic microenvironment is likely to occur after the primary cell population has expanded at rates faster than the vasculature to form areas of low perfusion. A cancer cell in a proliferating population is likely to go through many rounds of ischemia (low oxygen) and reperfusion (normal tissue oxygen). A cancer cell facing transient acute hypoxia affects the metabolic processes that involve oxygen and are likely to activate autophagy. In such cells, proliferation and autophagy may co-exist. Ensuing rapid genetic, molecular and biochemical changes promote cells with increased heterogeneity and proliferative and adaptive capabilities. HIF-1α is known to be the key transcription factor that is stabilised in acute hypoxia (Hu et al. 2003). Importantly, HIF-1α null cells show markedly reduced growth and proliferation (Guimarães-Camboa et al. 2015).
A growing tumour will also have cells exposed to chronic hypoxia as a result of an imbalance of oxygen supply and demand in areas at a distance greater than 70-150um from the blood vessels (Cairns et al. 2001; Cairns and Hill 2004). The cells in this region are likely to stabilise the isoform HIF-2α best known for its role in maintaining the vasculature and vascular remodelling (Kapitsinou et al. 2016; Skuli et al. 2012). As a transcription factor, it promotes expression of many genes that help cancer cells to adapt to the changing microenvironment, different from those regulated by HIF-1α. In particular, HIF-2α has been implicated to be at an oncogenic axis, while both HIF-1α and HIF-2α participate in cancer stem cell maintenance. The quiescent cancer cells, therefore, are likely to interact with the hypoxic tumour microenvironment and invoke HIF to strategise a cellular adaptation program that will eventually lead to metastasis and chemoresistance. These cancer stem cells, under the influence of hypoxia and HIF, exhibit epithelial to mesenchymal transformation, invasion and metastasis.
An angiogenic shift marks the adaptation of cancer cells to low oxygen that results in dysregulation of genes associated with chaotic vasculature consisting mostly of truncated and stubbed vessels. The anomalous vasculature along with the secretion of abnormal signalling molecules leads to localised hypoxic niches or pockets of hypoxia further affecting angiogenesis in normal embryogenesis as well as pathophysiological context as in solid tumours. HIF-2α is highly expressed in the vascular endothelial cells and upregulates the expression of an array of target genes that can influence vascular remodelling as well as angiogenesis. The vascular endothelial growth factor, VEGF, is a major protein required in normal cells for generating new vasculatures such as that observed in foetal tissue, corpus luteum and placenta. In abnormal growth such as cancer, VEGF and other related proteins such as Angiopoietin, Ang2 are responsive to the tumour microenvironment and particularly hypoxia. It is reported that similar to cancer dormancy, quiescent cells also promote angiogenic dormancy. These cells are not proliferative and exist as a clinically undetectable population.
Every cancer type has a preferential site or destination of secondary metastasis. These have been mentioned in Table 9.1, along with the respective change in hypoxia of tumour tissue compared to normal. Interestingly, few recent reports on breast cancer have shown that hypoxia and HIFs can influence the choice in the site of metastasis. Initial studies showed that HIF-1α expression in breast cancer cells promotes lung dissemination in genetic models (Liao et al. 2007). Hypoxia-induced angiogenesis and angiogenic genetic signature were shown to promote both primary tumour growth and lung metastasis but was not essential for bone metastasis (Lu and Kang 2010). HIF-1α and TGFB together were reported to promote bone metastasis (Dunn et al. 2009). Recently published study (Todd et al. 2021) generated a spontaneous murine mammary carcinoma model where mammary-specific deletion of HIF-1α, HIF-2α or von Hippel-Lindau factor (Vhl) was done using the Cre-Lox system. Their work shows a bias in the preferred site of metastasis in HIFs and associated molecules, VHL. While HIF-1α or HIF-2α deletion in the primary tumour decreased metastatic tumour burden in the bone marrow, Vhl deletion increased it. Surprisingly, there was a marked increase of the metastatic burden in the lung with only HIF-1α and not HIF-2α or Vhl. These reports, although early, suggest the exciting possibility of redirecting the site of metastasis to a preferred destination by modulating the tumour microenvironment through specific targeting of HIF and HIF regulatory molecules.
9.2 Hypoxia Orchestrates the Metastatic Cascade
The process of metastasis initiates before cells attain the capability to migrate to a secondary site. Primary cells with metastatic potential acquire an invasive phenotype which begins with the resistance to anoikis; polarisation and elongation of migrating cell(s); the protrusion of cells in the form of lamellipodia, pseudopodia or invadopodia; and its attachment to the extracellular matrix (ECM) components. Further, adhesion and de-adhesion of the leading edge and the cell body respectively generates a contraction force that propels the entire cell forward. In this section, the contribution of hypoxia in various steps and events of metastatic disease manifestation are described.
9.2.1 The Invasive Phenotype and EMT
EMT is one of the major steps in metastasis characterised by the loss of adherent properties of epithelial cells and acquiring new mesenchymal features (Kalluri and Weinberg 2009). Activation of the EMT pathway can prime the epithelial cells to detach, gain motility as well as transform the transcriptome in such a way that it favours its invasion through the lymphatic and the circulatory system. Cell adhesions are facilitated by intracellular proteins called cadherins. Preponderance of adherent junctions declines as a result of loss or downregulation of E-cadherin and upregulation of N-cadherin. This transition of E-cadherin to N-cadherin is a molecular characteristic of EMT. On the other hand, mesenchymal proteins like actin, fibronectin and vimentin rearrange the cytoskeleton to stimulate cell motility. Several gene families play an important role in invasion – of which matrix metalloproteinases (MMPs), urokinase plasminogen activator/receptor (uPAR), integrins and cathepsins are some of the prominent ones. These proteins function to modify the surrounding microenvironment, making it more suitable for cells to move around and out of their niche. The MMPs function to alter the extracellular matrix by degrading the components of the ECM and the basement membrane (Quintero-Fabián et al. 2019). uPAR converts plasminogen to plasmin, leading to a series of proteolytic cascades that result in ECM degradation (Kwaan and Lindholm 2019). Cathepsins, a family of proteases, are often overexpressed on the cancer cell surface from where they attack and digest E-cadherin, dissolving the adherent junctions (Mijanović et al. 2019). These ECM disrupting enzymes, tightly controlled in normal development, are significantly dysregulated in cancers with poor outcome. Apart from the loss of E-cadherin, the gain of molecular markers such as N-cadherin, vimentin and fibronectin are also evident in EMT (Loh et al. 2019). The changes in gene expression while undergoing EMT is the result of signalling cascade(s) regulated by transcriptional signatures consisting of genes such as SNAIL, SLUG, ZEB and TWIST (Comijn et al. 2001; Batlle et al. 2000; Hajra et al. 2002). The EMT transcription factors act downstream of key molecular pathways such as JAK-STAT, NF-κB, Notch and TGFβ and regulate metastatic progression (Gonzalez and Medici 2014).
9.2.2 Local Invasion of Cancer Cells
In primary solid tumour microenvironment, hypoxia enhances the motility and invasiveness of cancer cells by encouraging EMT. Hypoxia via HIF-dependent mechanisms regulates the transcription of Snail, Zeb1, Twist and Tcf3 in cancer cells which further helps in reducing the expression of E-cadherin and subdues the epithelial phenotype of cancer cells (Lu and Kang 2019). HIF-1 also promotes EMT by enhanced signalling of tyrosine kinase receptors pathway (Rankin et al. 2014), TGF-β pathway (Mingyuan et al. 2018) and the Wnt/ β-catenin signalling pathway (Shi et al. 2022). In gliomas, a significantly worsened prognosis is associated with the highly malignant glioblastoma in which the primary tumour aggressively invades surrounding normal brain tissue (Lah et al. 2020). Hypoxia is implicated as a major contributor to such aggressive tumours (Monteiro et al. 2017), often leading to differential expression of signature proteins that in turn regulate critical pathways of invasion (Abou Khouzam et al. 2021; Zou et al. 2019; Yang et al. 2021a; Bhushan et al. 2021). In breast cancer cells, there is an interplay between HIF-1α, Notch and GPER which activates the HIF-1α-NOTCH-SNAIL pathway to present an enhanced EMT phenotype (De Francesco et al. 2018). hFAT1 is reported to upregulate HIFs and promote glioma invasion and stemness (Srivastava et al. 2018; Irshad et al. 2021) while being under the transcription regulation of NFkB (Srivastava et al. 2020). Several studies on different cancers with hypoxia-mediated molecules that promote EMT are known (Tam et al. 2020). As a potent ECM remodeler, hypoxia via HIF-1 and HIF-2 can induce the transcription of integrin subunits α5 and β1 (Ju et al. 2017) and integrin α6 (Brooks et al. 2016). Hypoxia can also modulate important proteins such as fibronectin receptor, syndecan-4, in a HIF-independent manner (Koike et al. 2004).
The migrating cells require collagen deposition and biogenesis. The collagen architecture is thick and linear in tumours and curly/smooth and relatively thin, in normal tissue (Fang et al. 2014). The role of collagen in different phases of metastasis is evident while exposing extracellular matrix, stimulating adhesion to the endothelium as well as mediating cancer cell aggregation in blood vessels (Spivey et al. 2012). Procollagen-proline dioxygenase (also known as Prolyl 4-hydroxylase) is an essential enzyme for collagen deposition vital in tumour fibrosis and maintenance of stiffness, and procollagen-lysine 2-oxoglutarate 5-dioxygenases (PLOD1 and PLOD2) are required for cross-linking of collagen fibres (Qi and Xu 2018; Wolf et al. 2009). Reports suggest that HIF-1 induces the expression of domain protein, prolyl 4-hydroxylase α-subunit isoform 1 and 2 (P4HA1 and P4HA2), and procollagen-lysine 2-oxoglutarate 5-dioxygenase 1 and 2 (Gilkes et al. 2013) thus playing a key role in the biogenesis of collagen. In fact, recent studies showed that HIF-mediated expression of PLOD1 contributes to a malignant form of glioblastoma (Wang et al. 2021a), while stabilisation of HIF-1 by P4H involvement in stabilisation of HIF-1α subunit induces chemoresistance in triple-negative breast cancer (TNBC) (Xiong et al. 2018).
9.2.3 Intravasation into Blood Vessels
How does it feel, how does it feel?
To be on your own, with no direction home
A complete unknown, like a rolling stone
~ Bob Dylan (June, 1965)
Nobel prize in Literature, 2016
The detached cancer cell finally crosses the endothelial layer of the lymphatic or the blood and ventures out on its own or in a group in a process termed cellular intravasation. This step, crucial for distant metastases, is affected by the tumour microenvironment in addition to the molecular signals, proteases and the tissue vasculature. Hypoxia via HIF-1-dependent mechanism is associated with an increased expression of type IV collagen-degrading enzymes – MMPs (MMP2, MMP9 and MMP14) (Gilkes et al. 2014; Turunen et al. 2017; Petrella et al. 2005). Apart from degradation of collagen by matrix metalloproteinases, HIF-1 also induces the expression of a membrane receptor, PLAUR (urokinase plasminogen activator surface receptor), which degrades the extracellular matrix components (ECM). The altered expression of PLAUR changes the interactions between integrins and ECM which further helps in tumour invasion (Shyu et al. 2007).
The cancer cells in the lumen of lymphatic or blood vessels (now called circulating tumour cells, CTCs) will move to a new location. These CTCs may travel alone or in clusters in the blood and lymphatic vessels (Aceto et al. 2014). The CTC clusters have been shown to have greater success in micrometastases compared to the individual cell, possibly because of more pronounced cancer stem cell properties, such as CD44 expression in breast cancer cell-derived CTCs in mouse models (Liu et al. 2019). Circulating tumour cells (CTCs) have been established as the early indicators of metastasis and relapse. In the absence of a clinically detectable secondary metastasis, detection of CTCs becomes of utmost importance. Present in negligible numbers, CTCs carrying the specific mutations associated with a cancer cell have driven a new field of research and biomedical engineering. From methods of isolation of CTCs (Bankó et al. 2019) to facilitating detection on sensors of new and improved nanomaterials (Sun et al. 2021; Pallares et al. 2019), the urgency to detect poor cancer progression outcome early and with ease has seen a multidisciplinary approach like never before. Some of the work from our lab has attempted to utilise the multidrug-resistant p-glycoprotein as a bait to capture the CTCs in leukaemia with fair success (Gulati et al. 2018a, b, 2019). CTCs are representative of the tumour cell heterogeneity and allow oncologists a peep into the biology of the tumour as well as prediction of the genetics of the metastatic lesion. They have been shown to reflect hypoxia-induced heterogeneity and represent hypoxia-enriched gene signatures. The study of CTCs has exposed dramatic intra-patient and inter-patient heterogeneity and their evolution over time (Micalizzi et al. 2017).
9.2.4 Moving to a New Home: Extravasation from Blood Vessel to Secondary Site
Extravasation is a remarkable stage in the journey of the migratory cancer cell. The rogue cell escapes the circulatory or lymphatic system and searches for a new address to finally establish a home and proliferate in a challenging microenvironment quite different from its site of origin. In order to escape, the ECM is remodelled which aids the cells in crossing the basement membrane barrier. HIF-1 stimulates the expression of angiopoietin-like 4 (ANGPTL4), a protein that helps in disruption of intercellular endothelial cell (EC) interaction, and L1 cell adhesion molecule (L1CAM) helps in adherence of breast cancer cells to the EC monolayers (Zhang et al. 2021a). The elevated expression of ANGPTL4 and L1CAM facilitates the hypervascular metastasis of breast cancer cells to the lungs (Zhang et al. 2012). HIF-1-mediated expression of ANGPTL4 has also been shown to promote cancer cell growth, resistance to anoikis and progression of scirrhous gastric cancer (SGC) (Baba et al. 2017). Hypoxia also promotes the EFNA3 protein accumulation by engaging the previously unknown long noncoding RNAs from EFNA3 locus. By promoting the overexpression of ENFA3, hypoxia contributes to the extravasation process and metastatic potential of breast cancer cells to surrounding tissues. Hypoxia thus promotes metastasis by helping cancer cells to extravasate to new locations and form micrometastatic lesions.
9.2.5 Pre-metastatic Niche Formation
Reversal of the EMT process, the transitioning from mesenchymal to epithelial phenotype of the recently migrated cells, precedes the formation of pre-metastatic niche at the homing site. This process is called colonisation. Establishing themselves as a secondary growth is challenging as the cells program themselves to adapt and survive in unfamiliar tissue microenvironments. Though unfamiliar, the ‘soil’ is not always unwilling to accommodate the ‘seed’; therefore despite the absence of familial growth factors as in the primary tumour site, the metastasising cells attempt to colonise. There can be many outcomes associated with this event. Without the familiar molecular and physiological support, the metastasising cells may not survive at all in the host tissue. However, when they do survive for a small period of time, they may form small clumps, known as micrometastases, and further disseminate widely throughout the body of a cancer patient, leading to poor overall and disease-free survival outcomes. Finally, these micrometastases can increase in size to form larger lesions, macrometastases.
During this stage, the bone marrow-derived cells, such as tumour-associated macrophages (TAMs) and tumour-associated neutrophils (TANs), are recruited to the pre-metastatic niches. These cells are a major source of ECM remodelling proteases at the metastasis site that leads to angiogenesis. Hypoxia via HIF-1α mediates the recruitment of neutrophils (Du et al. 2008), the major source of MMP9 inducing angiogenesis (Deryugina et al. 2014), whereas TAMs employed in hypoxic conditions provide proteins leading to upregulated synthesis and assembly of collagen (type I, IV and XIV) for deposition, cross-linking and linearisation of the collagen fibres near invasive cancerous cells (Afik et al. 2016). Furthermore, these cells also constitute collagen cross-linking enzyme, lysyl oxidase (LOX), which stimulates metastasis in breast cancer. LOX-mediated cross-linking of collagen stimulates ECM stiffness and activates the integrin-dependent invasive phenotype in breast cancer (Levental et al. 2009). Expressions of LOX and LOX-like family proteins (LOXL1 and LOXL2) are greatly influenced by HIF-1α (Erler et al. 2006).
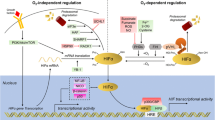
Studies have shown that hypoxic primary tumour cells release exosomes and microvesicles (MVs) which support the formation of a conducive pre-metastatic niche (King et al. 2012; Jafari et al. 2020; Peinado et al. 2011). For the recruitment of bone marrow-derived cells, these transport vesicles provide vascular permeability and fulfil the requirement of biomolecules like mRNAs, miRNAs, proteins, lipids, etc. HIF induces the expression of a small GTPase molecule RAB22A, important for the formation of budding vesicles for intracellular trafficking; thus hypoxia regulates the biogenesis of exosomes and MVs (Wang et al. 2014). Interestingly, the transcriptome and proteome analysis of exosomes isolated from glioblastoma multiforme (GBM) patients reveal the presence of HIF-regulated mRNAs and proteins (like MMPs, LOX, PDGF, IL-8, etc.) (Kucharzewska et al. 2013). Thus, hypoxia not only induces the exosomes biogenesis but also manipulates the exosome cargo loading to promote the cancer progression. Interestingly, these pre-metastatic niche promoting exosomes also determine a bias towards host sites and organotropism (Syn et al. 2016). Figure 9.1 depicts some of the key signalling networks which contribute to hypoxia-mediated promotion of metastasis.
Hypoxia-inducible factor-mediated signalling pathways of metastasis. Stabilisation of HIF proteins in the hypoxic tumour microenvironment results in transcriptional activation of genes of key signalling pathways of JAK/STAT, NFκB, Wnt/ β-catenin and Notch. These signalling pathways affect various cellular functions which are crucial for sustaining cancer progression and metastasis
9.2.6 Metabolic Reprogramming During Metastasis
A century ago, the German biochemist Otto Warburg first explained the connection between tumour progression and dysfunctional metabolism (Warburg et al. 1927). He also explained how hypoxia cells favourably convert glucose into lactose instead of its oxidation in mitochondria despite abundance of glucose. Metabolic rewiring and the metastatic cascade are highly intertwined processes, and together, they promote multiple steps of cancer metastasis (Wei et al. 2020). During metastasis, both the cancer cells of the primary tumour and the cells in the metastatic niche face multiple metabolic challenges, particularly nutrient and O2 deprivation, amid high metabolic requirements. The cells undergo metabolic reprogramming to increase cellular adaptation. Hypoxic tumour microenvironment greatly influences cellular metabolism by modulating cellular processes like glycolysis and lipid metabolism. Most of the glycolytic enzymes and glycolysis proteins are regulated by HIF-1 leading to higher uptake of glucose in tumour cells than normal cells. The following glycolytic protein isoforms are known to be transcriptionally regulated by HIF-1α and overexpressed in malignant cells compared to non-malignant cells – adenylate kinase-3; aldolase-A,C (ALDA,C); carbonic anhydrase-9; enolase-1 (ENO1); glyceraldehyde phosphate dehydrogenase (GAPDH); hexokinase 1,2 (HK1,2); phosphofructokinase L (PFKL); phosphoglycerate kinase 1 (PGK1); and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphate-3 (PFKFB3) (Semenza 2010; Ke and Costa 2006). HIF-1 directly regulates the expression of glucose transporters (GLUT1 and GLUT3) (Liu et al. 2009; Calvert et al. 2006) and upregulates the lactose secretion by promoting the expression of LDHA (Cui et al. 2017). The hallmark of hypoxia-induced metabolic reprogramming is a shift in ATP production from OXPHOS to glycolysis (Colwell et al. 2017). Tumours with isocitrate dehydrogenase (IDH1) mutations acquire gain-of-function and convert alpha-ketoglutarate to the oncometabolite 2-hydroxyglutarate (Sasaki et al. 2012). Similarly hypoxia is also known to induce the production of the L-enantiomer of 2-HG, independent of IDH1/2 mutations that performs similar functions (Intlekofer et al. 2015). Similar to 2-HG, increased L-2HG in ccRCC suppresses the methylcytosine dioxygenase (TET) and KDM6A, leading to hypermethylation of DNA and histone (Shelar et al. 2018). Therefore it appears that in hypoxia, the frequently mutated IDH1 cells undergo epigenetic reprogramming to cancer stem cell state, driven by the altered metabolic activities. Additionally, other metabolites such as succinate, fumarate and glutamine are implicated to play a role in EMT, while metabolism of fatty acids and fructose supports MET.
9.2.7 Chemoresistance and Poor Prognosis
The quest for a magic bullet against cancer has seen several hopeful candidates but none that have truly succeeded. The effort of researchers and oncologists is to move towards targeted therapy powered by personalised medicine, far different from the common cure that is envisaged with every discovery. Recently, immunotherapy that attempts to block the immune evasion tactics of the cancer cell have been awarded and found clinical success, though in limited cancers. The standard of care continues to be surgical interventions, radiotherapy and chemotherapy. Among other strategies such as surgery, immunotherapy, combination therapy, targeted therapy and others, chemotherapy is the most accepted therapy owing to the vast data and widespread use, with no clear replacements. Some targeted therapies have dramatically improved progression-free outcome such as those using trastuzumab (against HER2 in breast cancer) and imatinib or Gleevec (targeting BCR-ABL tyrosine kinase in chronic myelogenous leukaemia).
Drug resistance is the principal impediment in response to chemotherapy. Most of the known chemotherapies rely on oxygen-dependent generation of reactive oxygen species, and in the hypoxic interiors of most solid tumours, their action is limited. Due to the poor vasculature and inaccessible niches, the interiors of the solid tumours do not get sufficient drug. Sub-lethal dose of drugs, together with hypoxia-induced upregulation of channels that evict drugs, leads to chemoresistance. The problem is compounded by spiralling tumour burden, highly variable growth kinetics, genetic heterogeneity, fallible physical barriers, fallow immune system and the hypoxic microenvironment (Vasan et al. 2019). Though drug resistance is governed by a large number of factors, our focus in this section will be upon ‘chemoresistance’ as a result of the interaction between hypoxic microenvironment and metastasis.
The tumour microenvironment (TME) is of paramount importance in the initiation and progression of cancer. Consisting of many cellular and non-cellular components, it acts as a bedrock for the induction of proliferation, angiogenesis, inhibition of apoptosis, suppression of the immune system and evasion from immune surveillance (Deepak et al. 2020). Hypoxia promotes the selection of apoptosis-resistant cell clones and induction of tumour metastasis (Li et al. 2018), along with all the aforementioned processes. Further, the continuous communication, in this hypoxic TME, between cancer cells and the surrounding stromal cells also encourages the progression of cancer to metastatic stages of disease through epithelial to mesenchymal transition. The accumulation of mutation acts as a precursor for chemoresistance; though the mutations are mostly random, their subsequent snowballing gives rise to clonal expansion, genetic diversification and clonal selection. These iterative processes affect the tissue ecosystems leading to expansion of resistant variants.
Hypoxia reduces drug-induced susceptibility to apoptosis by chemotherapeutic agents, by reducing ROS formation and modulating mitochondrial function (Kim et al. 2021; Ge et al. 2018). These drug-resistant clones may adopt the properties of mesenchymal cells to circulate, invade and metastasise. As described earlier, the key molecular contributors in the signalling pathway for the regulation of metastasis are widely known. Hypoxic stress and therefore HIF signalling pathway drive the tumour aggressiveness by affecting the steps within the metastatic cascade and creating conducive pre-metastatic niche conditions. According to a study by Schietke et al., hypoxia acts upon and reduces the expression of E-cadherin and thus promotes EMT, by inducing the LOX (Lysyl oxidase) and LOXL2 (Lox-like 2); LOX and LOXL2 are implicated as direct target of HIF-1 (Schietke et al. 2010). Hypoxia, through the master-regulator HIF-1α, remodels the vessels with increased expression of matrix metalloproteinases MMP1 and MMP2 that helps intravasation. In ovarian cancer cells, hypoxia increases the exosome secretion by upregulating Rab27a and downregulating Rab7, LAMP1/2 and NEU-1 and promotes metastasis and thus chemoresistance (Dorayappan et al. 2018). As the ambit of cancer research is expanding, researchers are exploring the role of noncoding genes as a mediator of chemoresistance in cancer cells. Induction of lncrna MALAT1 is involved in the promotion of migration and invasion in A549 lung adenocarcinoma cells (Hu et al. 2018). In colorectal cancer, chemoresistance is shown to be one of the two effects meted out by hypoxia-responsive lncRNA NORAD (Zhang et al. 2018).
9.2.8 Immune Evasion by Programming
Hypoxia paves the way for tumour cells to evade immune surveillance by activating transcription of certain genes through HIFs (You et al. 2021). The infiltration of CD8+ cytotoxic T cells (adaptive) and NK cells (innate) makes up the primary antitumoural immunity. However, transcriptional activation by HIF allows tumour cells to activate genes that impair the functioning of the immune system in the tumour microenvironment and prevent flooding of immune cells (Vaupel and Multhoff 2018). Quiescent cancer cells (QCCs) are suspended in the G0 phase, but possess the ability to re-enter cell cycle and assist in tumour growth. Since QCCs have the capacity to evade the immune system and revert themselves to a mitotic phase, these play a major role in cancer recurrence and metastasis. They form an “immunosuppressive niche” (Baldominos et al. 2022). Notably, QCCs are different from dormant tumour cells, in the sense that QCCs are present in the active primary tumour, whereas dormant cells achieve their dormancy after disseminating from the tumour site of origin. The immunosuppressive niches initiated by QCC include exhausted T cells, QCC, hypoxic tumour cells and suppressive fibroblasts. Despite being in close proximity to T cells, QCCs are able to evade them by downregulating the surface antigen presentation of MHC Class I at the transcript and protein level (Agudo et al. 2018). In another possible way, tumour cells escape entrapment from NK cells by altering the expression of surface ligands for NK cell receptors or by altering expression of receptors on NK cells themselves. NK cells have certain inhibiting and activating receptors, which include NCR, NKp30, NKp44, NKp46 and NKG2D. TGFβ is known to downregulate the expression of NKp30 and NKG2D on NK cells, which suppresses their cytolytic activity, thereby indirectly contributing to evasive mechanisms (Lee et al. 2004). Apart from these direct interactions between tumour cells and immune cells, escaping the immune system of the body is also mediated by tumour-associated macrophages (TAMs), which lie outside of the tumour microenvironment. Multiple studies suggest that TAMs are major promoters of metastasis and assist cancer cells in all the stages of metastasis (Komohara et al. 2014; Ruffell and Coussens 2015). TAMs produce various proteolytic enzymes and immune checkpoint inhibitors, thereby debilitating T cells from exhibiting any immunologic properties near a TME. In hypoxia, activation of HIF-1α induces expression of PD-L1 on macrophages, which binds to its receptor PD1 on T cells, thus deactivating their immune effector properties (Henze and Mazzone 2016). Tumour cells, therefore, escape immune surveillance and undergo further proliferation and metastasis, most of which is mediated by hypoxia-inducible factors.
9.2.9 Epigenetic Changes Regulating Metastasis
Epigenetic alterations, together with genetic aberrations and biochemical changes, can drive tumour development and progression (Timp and Feinberg 2013). Interdependence between hypoxia and proteins that modulate epigenome thus promotes metastasis. Experiments suggest that epigenetic mechanisms promote HIF-α stabilisation; and hypoxia, through HIF-dependent mechanism, leads to epigenetic changes in critical signalling molecules, further fostering the metastatic potential of the tumour. Figure 9.2 shows some of the genes regulated by hypoxia-mediated epigenetic reprogramming. Jumonji C (JmjC) domain-containing oxygenases are histone demethylases that are known to introduce changes to global methylation of histones and work as a downstream effector of HIF-α (Beyer et al. 2008). Decreased JMJD1A is associated with reduced N-cadherin and Twist and increased E- cadherin, all making the non-invasive epithelial phenotype (Yamada et al. 2012; Krieg et al. 2010). Inhibition of HDAC1 by Scriptaid synergised with Cisplatin in hypoxia is known to reduce metastasis, effectively leading to cell death of hypoxic lung cancer cells (Pradhan et al. 2016). DNA hypermethylation leads to silencing of BNIP3 which is involved in hypoxia-related cell death, which indicates that the cell bypass apoptosis in hypoxia by suppressing BNIP3 (Okami et al. 2004). DNA hypomethylation is maintained in hypoxic glioma cells via increase in ten-eleven translocase (TET) expression, as well as decreased expression of DNA methyltransferases. TET1 and TET3 are known to bind to Oct4 and Nanog in their regulatory regions and induce pluripotency associated with glioma stem cells and invasion (Prasad et al. 2017). Histone deacetylase HDAC1 induces angiogenesis by suppressing the tumour suppressor p53 and VHL under the influence of low oxygen tension. HDAC1 also increases the expression of VEGF (Kim et al. 2001). The increased expression of JMJD1A is linked to increase in expression of genes linked to invasion (Krieg et al. 2010). All these reports are supportive of the fact that epigenetics in conjunction with hypoxia is playing an important role in procreating conditions suitable for metastasis to occur.
HIF signalling co-opts epigenetic mechanisms to promote metastasis. By regulating various downstream molecules, hypoxia-mediated changes in the epigenome by directly modifying epigenetic modulators such as TET demethylase, Jumonji domain-containing histone demethylases (JMJDs) and histone deacetylases (HDACs), HIF can modulate downstream genes that finally promote metastasis. HIF can also cause epigenetic changes to downregulate its own suppressor, VHL, thereby promoting other HIF-regulated events such as upregulation of CXCR4 and CYTIP through aberrant DNA methylation and histone demethylation, respectively
9.3 Case Study: Hypoxia in Breast Cancer Metastasis
A large body of work has implicated that reduced O2 availability exerts a significant effect in the progression of breast cancer. More than 50% of breast cancers foster regions of low oxygen, particularly the triple negative breast cancers (TNBC). The probability of metastasis increases especially in patients with elevated HIF expression in primary tumour biopsies. Orthotopic mouse models with null HIF-1α and HIF-2α have revealed a marked decrease in the metastasis to the lymph nodes (Schito et al. 2012). HIF-1α knockdown also resulted in decreased bone metastasis (Dunn et al. 2009) and lung metastasis (Zhao et al. 2014) in mouse models. Recent work by Godet et al. (2019) mechanistically evaluated the potential of post-hypoxic tumour cells to individually study all steps in the metastatic cascade separately (Godet et al. 2019). They demonstrated that hypoxia-exposed breast cancer cells (BCCs) have an enhanced ability to invade/intravasate and to survive in the bloodstream, but they are not more efficient at extravasating or proliferating at the metastatic site compared with their oxygenated counterparts. Their work revealed that cells exposed to hypoxia have increased metastatic potential and a ROS-resistant phenotype providing a survival advantage to the metastasising cell. Hypoxia induces the HIF-dependent expression of placental growth factor (PGF) by BCCs, which binds to VEGF receptor 1 on mesenchymal stromal cells (MSCs) and promotes their recruitment to primary breast tumours (Chaturvedi et al. 2013). They also showed that chemokine CXCL10 secreted by MSCs binds to its receptor CXCR3 on the BCCs; this triggers the release of chemokine CXCL16 by the BCCs which in turn can bind to the receptor CXCR6. PGF, CXCR3 and CXCL16 released by BCCs are known to be induced by hypoxia in a HIF-dependent manner. Increased expression of Na+-driven bicarbonate transporters (NDBTs) in response to hypoxia was observed in TNBC (McIntyre et al. 2016; Parks and Pouyssegur 2015), and recently S0859, an inhibitor targeting (NDBTs), has shown promise in curbing metastasis in vivo (Carroll et al. 2022). Epithelial cells are generally stiff and rigid; however, for motility, these cells will have to become pliable and undergo epithelial and mesenchymal transformation (EMT) (Lindsey and Langhans 2014). Hypoxia is known to activate extracellular matrix (ECM) remodelling in BCCs which results in increased expression of collagen and ECM stiffening that assists in cell motility. Hypoxia is sufficient to activate RhoA-mediated FAK signalling, which is the basis for BCC motility (Gilkes et al. 2014). Hypoxia also leads to an increase in the levels of ADAM12 in a HIF-dependent manner, leading to increased ectodomain shedding of HB-EGF, activation of EGFR signalling to focal adhesion kinase (FAK) and increased cell motility and basement membrane invasion, making ADAM12 a promising drug target (Wang et al. 2021b). Recent work on desmoglein2, an intercellular adhesion factor, showed that its downregulation in hypoxic tumours allowed single tumour cell dissemination, while DSG2-expressing tumours generated more CTC clusters (Chang et al. 2021). Multiple studies have attempted to recreate the hypoxia-induced molecular changes by verifying hypoxia gene signatures in different subtypes of breast cancers (Ye et al. 2018; Zhang et al. 2021b; Sun et al. 2020). Similar signatures have also been established for hypoxia-mediated noncoding RNAs (particularly, miRNA and lncRNA) (Kulshreshtha et al. 2007; Dhawan et al. 2018; Macharia et al. 2019). Figure 9.3 illustrates the journey of a primary breast cancer cell to the site of secondary lung metastases.
The metastatic cascade of a primary breast cancer cell to site of secondary lung metastases. A growing tumour with hypoxic interiors at the primary site acquires metastatic potential as a consequence of its adaptations. Mesenchymal stromal cells (MSCs) from the bone marrow (BM), upon entry in the tumour tissue, become cancer-associated MSCs, which induce luminal breast tumour cells to metastasise. These circulating tumour cells extravasate in a new niche and proliferate to form metastases of varying degrees (micrometastases and macrometastases). Genes upregulated and downregulated in response to hypoxia are highlighted in red and green, respectively
9.4 Advances in Hypoxia-Related Drug Development Research Against Metastatic Cancers
Technological advances in research and imaging have facilitated our understanding of cancer. Despite our current understanding, the treatment remains a challenge, and metastasis continues to be the main cause of mortality and failure of disease-free survival outcome. Most patients coming to the clinic for treatment have advanced stage cancer. Therefore, a significant body of work is required towards development of drugs that can target metastatic progression.
9.4.1 Treatment Challenges in Metastatic Cancers
Eradicating the metastatic cancer cells is challenging. Contrary to localised tumours, it is a systemic disease requiring a targeted therapeutic approach. Metastatic cancers have a low response to conventional treatment approaches and a high mortality rate. When primary tumours acquire phenotypic plasticity, they become resistant to treatment and metastasise (Bhutia et al. 2016). Hypoxia plays an important role in the cancer evolution and development of the resistant clone. Even a small population of cancer cells that resist therapy may lead to an aggressive disease. Various factors interfere with drug delivery to the targeted site such as solubility, absorption, metabolism and clearance of drugs. These factors further complicate the therapeutic approach to this disease (Ganesh and Massagué 2021).
The important implications of a successful drug treatment are slower tumour growth, survival benefit and decreased chance of relapse. Although there are certain side effects of chemotherapeutic treatment, treatment relies on trade-off between benefit and side effects. Most of the potential drugs fail in the different phases of clinical trials, leaving only a few choices ahead. It is for this reason that drug development is moving towards precision medicine and targeted therapies, hopeful of translation from bench to bedside.
Some of the challenges are (1) detecting metastatic burden in the course of the disease and treatment, (2) finding a way to predict which patients are likely to have metastasis, (3) understanding the biology of the metastatic program and (4) finding ways to target metastasis.
9.4.2 Recent Advances in Technology
Tools such as deep learning image analysis algorithms, which can determine the presence of cancer cells in tissues, and amalgamate them into clinically established pipelines are working towards improvement of sensitivity and reproducibility of pathological metastasis staging (Ehteshami Bejnordi et al. 2017; Bi 2019).
Detection of CTCs continues to be developed as a marker for early minimal residual disease markers as well as tumour biomarker profiling strategy. The low number of CTCs has hampered quick advancement in this field. In turn, ctDNA-based assays have shown more promise and have led to a plethora of sequencing-based analysis that have given insight into the disseminated tumour biology.
Continuously updated ‘omics’ databases and its attached annotations are another collection of scientific tools to bioinformatically analyse the relevant information such as drug response. Growth factors, cell surface antigens, cell death, receptors or signal transduction pathways which regulate cell cycle progression, angiogenesis and metastasis are promising targets (Lee et al. 2018). There is cogent evidence presenting AKT, a serine threonine kinase involved in key critical pathways like apoptosis and angiogenesis, as one of the novel targets for breast cancer and ovarian cancer treatment (Shariati and Meric-Bernstam 2019). The clinical management of paediatric low-grade glioma is dependent upon the mutations in several genes, such as BRAF and MAP2K1 (de Blank et al. 2020); human epidermal growth factor 2 (HER2) overexpression has been implicated in gastric cancer recently, and trastuzumab, a recombinant humanised IgG1 monoclonal antibody targeting HER2 by antibody-dependent cytotoxicity, has been tested (Patel and Cecchini 2020). Many of these molecules are known to be overexpressed in hypoxia. The novel targets identified in in vitro studies are earmarked for use in clinical settings, with stringent pre-clinical and clinical phase trials. Some direct inhibitors of the hypoxia-inducible factor have also been used in clinical trials and research (Table 9.2). Similarly ongoing research hypoxia-based gene signatures also reveal an exciting possibility of finding new drug targets.
9.4.3 Recent Advancements in Drug Development in Context of Hypoxia-Driven Metastasis
Strategies around pursuit of understanding cancer cell metastasis and hypoxia mechanisms will provide great insight for developing a new line of treatment. There are many revolutionary ideas being explored by researchers; however, in this chapter we will review some possibilities that either target hypoxia or some that have found promise in targeting hypoxia-regulated genes important for metastasis.
9.4.3.1 Nanomaterial
Nanoparticles improve the stability, solubility, half-life, and concentration of drugs. Many nanomaterials have been developed in recent times, and these have opened exciting new possibilities with biomedical applications including bioimaging, drug delivery to target tissues and therapeutic delivery (Senapati et al. 2018). A nanoparticle typically consists of core material, surface modifications, and a therapeutic payload that may be a drug, nucleic acid, protein, antibody, antibody-drug conjugate, or combination of multiple agents for cancer therapy. Nanoparticles accumulate in tissues thereby reducing the dose and toxicity of the drug being delivered which in turn lowers the chances of cancer patients developing drug resistance (Ediriwickrema and Saltzman 2015). Diminishing oxygen dependence for hypoxic tumour therapy has also been postulated as a therapeutic option in recent years, e.g., by taking advantage of penetration of therapeutic gas or generation of toxic substances in situ in hypoxic tumour (Zou et al. 2021). Oxygen nanobubbles have been used to suppress expression of hypoxia-inducible factor-1α (HIF-1α) (Song et al. 2020) albeit with some caveats (Cavalli et al. 2016). Similarly, oxygen could be generated in tumours by using catalase-loaded hydrogel, and this could help overcome the immunosuppressive tumour microenvironment (Meng et al. 2019). However, these strategies need to be further explored before they can be applied clinically (Ruan et al. 2021).
9.4.3.2 Antibodies
Monoclonal antibodies (MAb) are commonly used in antibody therapy to target cancer cells. Antibodies can target cancer cells using various mechanisms including inhibition of immune checkpoints, signalling disruption, neutralising target receptors, downregulation of receptors, antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity (Hafeez et al. 2020). MAbs that are most commonly used for targeting cancer cells include avelumab (anti-PD-L1), cemiplimab (anti-PD-1), ipilimumab (targeting CTLA-4), nivolumab (anti-PD-1), etc. (Yang et al. 2021b). Recently, two new antibodies, CA9hu-1 and CA9hu-2, have been developed against carbonic anhydrase IX (CA IX) to specifically target hypoxic cells (Zatovicova et al. 2022). Monoclonal antibodies can be conjugated with cytotoxic drugs, radioisotopes and immunotoxins. Researchers are exploring methods to increase their effectiveness.
9.4.3.3 Antibody Drug Conjugate
During the conjugation process, it is necessary to preserve the cytotoxic property of the drug and the specificity of the monoclonal antibody. An antibody-drug conjugate (ADCs) remains stable until it reaches the target site. The conjugates are biodegradable and non-immunogenic. Once the conjugate carrier and target cell interact, the drug is released. A few of the ADCs that are available include brentuximab vedotin (targets CD30), gemtuzumab ozogamicin (targets CD33), moxetumomab pasudotox (targets CD22), trastuzumab deruxtecan (targets HER2), etc. (Dahlgren and Lennernäs 2020). A study revealed that HER2 signalling results in elevated expression of HIF-1α and HIF-2α, which plays a key role in hypoxia in cancer (Laughner et al. 2001). HER2 treatment with ADCs can downregulate the HIF-1 and HIF-2 expression levels, thereby targeting cancer cells in a hypoxia-sensitive manner. The major disadvantage of this type of therapy is the amount of drug delivered to the target site. As each antibody is conjugated with a single drug molecule, its potency is reduced in most cases. Presently, several antibody-drug conjugates are available, but either the specificity of the antibody or the cytotoxicity of the drug is compromised.
9.4.3.4 Prodrugs
Hypoxic tumours have radio- and chemoresistant niche within, posing great challenges for effective drug delivery and outcome. Hypoxia-activated prodrugs, also known as HAPs or bioreductive drugs, are designed and used to target hypoxia. Some well-known HAPs and their descriptions are listed in Table 9.3. Under hypoxic conditions, HAPs can be reduced with the help of specific reductases into cytotoxic compounds that can target the hypoxic cancer cells precisely with little or no effect on surrounding normal tissues (Wilson and Hay 2011). Hetero-aromatic N-oxides, quinones, nitroaromatics and aliphatic N-oxides are currently among the classes developed (Li et al. 2021).
9.4.3.5 Drugs Targeting Hypoxia
Targeting molecular mechanisms such as HIF and unfolded protein response (UPR) aids hypoxia and tumour cell adaptation to hypoxia. HIF transcription factor activates various downstream genes that help tumour cells survive. Various drugs have been designed to specifically target HIFs to counter the hypoxia, including flavopiridol, rapamycin, KC7F2, digoxin, EZN-2968, etc. (Shirai et al. 2021). Due to severe hypoxia in TME, there is an increased concentration of unfolded proteins in the endoplasmic reticulum (ER) resulting in protein synthesis reduction due to the loss of mRNA translation. Abetment of a few members of eukaryotic initiation factors (eIFs) also contributes to the mRNA translation loss (Hammond et al. 2014b). Salicylaldimine, ONC201, bortezomib, eeyarestatin I, epigallocatechin gallate, etc. are a few drugs that target these UPRs (Ojha and Amaravadi 2017).
9.4.3.6 Biomedical Devices
With the help of biomaterial chips, implantable devices are being designed that directly deliver drugs to the targeted site. Chips impregnated with drug molecules can also be used in conjunction with chemotherapy and gene therapy. Different countries have approved implantable devices for the treatment of malignant cancer after successful clinical studies (Pial et al. 2022).
Another technique for targeted delivery of drugs uses infrared radiation. Near-infrared fluorescence (NIRF) imaging agents can be used for imaging and targeting cancer cells. NIRF conjugated with cytotoxic drugs can be successfully delivered to the target site using imaging at NIR wavelength (700–10,000 nm). These agents are specific to cancer cells, and their uptake is mediated by tumour hypoxia. Activated hypoxia-inducible factor 1α (HIF-1α) or organic anion-transporting polypeptides (OATP) signalling axis is the primary contributor. When OATP is present, NIRF dye can easily cross the BBB/BTB and enter cancer cells even in the absence of hypoxia. In addition to crossing the blood-brain barrier, these dyes target the cancer site without harming healthy cells. In mice models, the NIRF signal intensity has also been shown to quantify tumour burden. A study is currently underway to evaluate its use in male cancer patients (Wu et al. 2015).
9.4.4 Promising Strategies for Drug Development
A new study or clinical trial is conducted every day that either seeks to detect cancer earlier or to develop treatments to combat metastasis and hypoxia. The need for interdisciplinary approaches has become paramount in treating this disease. This section discusses the upcoming and promising methods, approaches and treatments for metastatic cancer and hypoxia. METPlatform is a new drug screening platform designed to identify metastatic drug vulnerabilities in vivo and ex vivo. This method involves testing candidate drugs before treatment using organotypic cultures derived from patients. This study also helps predict patient response to a specific treatment, making it one of the highly desirable methods for designing personalised medicine (Zhu et al. 2022). Bacteria are single-cell living organisms that can proliferate and translocate. It is possible to biosynthesise bacteria that can transport therapeutic substances directly to cancer cells. CAP, which coats bacteria and protects them from the human immune system, can be biosynthesised to improve therapeutic load delivery in vivo (Cress et al. 2014). In situ trafficking and high drug dosages can be used to increase therapeutic efficacy and safety. Thus far, this method has only been tested successfully on mice. Further studies are currently underway on human models (Harimoto et al. 2022). Hypoxia aids the tumour cells in evading immune cells by promoting immune escape and inhibiting the antitumour effector cells. These immune cells are affected by a mild thermal effect. Application of mild thermal therapy (hyperthermia) to the tumour microenvironment increases the vascular perfusion, thereby increasing the oxygen concentration at the tumour site. Reduction of hypoxia in TME provides an opportunity to treat the tumour with chemotherapy, radiotherapy or immunotherapy which is currently being explored in some clinical studies (Lee et al. 2010).
9.5 Concluding Remarks
We are only just beginning to understand the extent of heterogeneity exhibited by the tumour cells including the circulating tumour cells. It is apparent that the tumour microenvironment which includes hypoxic regions play a very important role in determining the outcome of a developing cancer. Hypoxia modulates the interaction of the primary tumour cells with its surroundings by mobilising immune cells. Cellular adaptation in the hypoxic niche equips the cells with metastatic and immuno-evasive capabilities that allow it to migrate and metastasise at one or more secondary sites. Modelling tumour hypoxia using in vitro, in vivo and patient-derived cells have their advantages and utility for advancing our basic knowledge. Enriching cancer stem cells in these model systems will allow us to assemble molecular pathways of reprogramming and pluripotency. Together, artificial intelligence, imaging genomics and spatial and molecular profiling are poised to give greater insights and breakthrough discoveries in the use of hypoxia as an early predictive marker of relapse and metastasis. Emerging possibilities include prediction of metastatic sites and modulation of hypoxia-regulated site specificity of metastasis. Epigenetic modulators that can reverse hypoxia-induced gene signatures and antibody/inhibitors against hypoxia-regulated molecular targets show promise in therapeutic value. Collective and multidisciplinary efforts are required to improve quality of life and survival outcome in metastatic disease burden.
References
Abi-Jaoudeh N et al (2021) Phase I trial on arterial embolization with hypoxia activated tirapazamine for unresectable hepatocellular carcinoma. J Hepatocell Carcinoma 8:421–434
Abou Khouzam R, Rao SP, Venkatesh GH et al (2021) An eight-gene hypoxia signature predicts survival in pancreatic cancer and is associated with an immunosuppressed tumor microenvironment. Front Immunol 12:680435
Aceto N, Bardia A, Miyamoto DT et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158:1110–1122
Afik R, Zigmond E, Vugman M et al (2016) Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med 213:2315–2331
Agudo J, Park ES, Rose SA et al (2018) Quiescent tissue stem cells evade immune surveillance. Immunity 48:271–285.e5
Akpe V, Shiddiky MJA, Kim TH et al (2020) Cancer biomarker profiling using nanozyme containing iron oxide loaded with gold particles. J R Soc Interface 17:20200180
Aleman J et al (2019) Deconstructed microfluidic bone marrow on-a-chip to study normal and malignant hemopoietic cell-niche interactions. Small (Weinheim an der Bergstrasse, Germany) 15:43
Baba K, Kitajima Y, Miyake S et al (2017) Hypoxia-induced ANGPTL4 sustains tumour growth and anoikis resistance through different mechanisms in scirrhous gastric cancer cell lines. Sci Rep 7:11127
Baldominos P, Barbera-Mourelle A, Barreiro O et al (2022) Quiescent cancer cells resist T cell attack by forming an immunosuppressive niche. Cell. https://doi.org/10.1016/j.cell.2022.03.033
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow. Lancet (London, England) 357:539–545
Bankó P, Lee SY, Nagygyörgy V et al (2019) Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol 12:48
Batlle E, Sancho E, Francí C et al (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
Bedenne L, Michel P, Bouché O et al (2007) Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25:1160–1168
Bersini S et al (2014) A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35:8
Beyer S, Kristensen MM, Jensen KS et al (2008) The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem 283:36542–36552
Bhushan A, Kumari R, Srivastava T (2021) Scouting for common genes in the heterogenous hypoxic tumor microenvironment and their validation in glioblastoma. 3 Biotech 11:451
Bhutia YD, Babu E, Ganapathy V (2016) Re-programming tumour cell metabolism to treat cancer: no lone target for lonidamine. Biochem J 473:1503–1506
Bi WL et al (2019) Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 69:2
de Blank P et al (2020) Molecular markers and targeted therapy in pediatric low-grade glioma. J Neuro-oncol 150:1
Brenner AJ et al (2021) Phase 2 trial of hypoxia activated evofosfamide (TH302) for treatment of recurrent bevacizumab-refractory glioblastoma. Sci Rep 11:12306
Brooks DL, Schwab LP, Krutilina R et al (2016) ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol Cancer 15:26
Cairns RA, Hill RP (2004) Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res 64:2054–2061
Cairns RA, Kalliomaki T, Hill RP (2001) Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res 61:8903–8908
Calvert JW, Cahill J, Yamaguchi-Okada M et al (2006) Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1alpha and its downstream target genes. J Appl Physiol (Bethesda, Md. : 1985) 101:853–865
Carroll CP, Bolland H, Vancauwenberghe E et al (2022) Targeting hypoxia regulated sodium driven bicarbonate transporters reduces triple negative breast cancer metastasis. Neoplasia (New York, N.Y.) 25:41–52
Cavalli R et al (2016) Nanobubbles: a promising efficient tool for therapeutic delivery. Therapeutic delivery 7:2
Chang PH, Chen MC, Tsai YP et al (2021) Interplay between desmoglein2 and hypoxia controls metastasis in breast cancer. Proc Natl Acad Sci USA 118:e2014408118
Chaturvedi P, Gilkes DM, Wong CC et al (2013) Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Investig 123:189–205
Chen J, Chen D, Xie Y et al (2013) Progress of microfluidics for biology and medicine. Nano-Micro Lett 5:66–80
Chiou SH, Risca VI, Wang GX et al (2017) BLIMP1 induces transient metastatic heterogeneity in pancreatic cancer. Cancer Discov 7:1184–1199
Chitneni SK et al (2013) 18F-EF5 PET imaging as an early response biomarker for the hypoxia-activated prodrug SN30000 combined with radiation treatment in a non-small cell lung cancer xenograft model. J Nucl Med 54:8
Colwell N, Larion M, Giles AJ et al (2017) Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro-oncology 19:887–896
Comijn J, Berx G, Vermassen P et al (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7:1267–1278
Courtney KD et al (2018) Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J Clin Oncol 36:9
Cress BF et al (2014) Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev 38:4
Cui XG, Han ZT, He SH et al (2017) HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 8:24840–24852
Dahlgren D, Lennernäs H (2020) Antibody-drug conjugates and targeted treatment strategies for hepatocellular carcinoma: a drug-delivery perspective. Molecules (Basel, Switzerland) 25(12):2861
De Francesco EM, Maggiolini M, Musti AM (2018) Crosstalk between Notch, HIF-1α and GPER in breast cancer EMT. Int J Mol Sci 19:E2011
Deepak KGK, Vempati R, Nagaraju GP et al (2020) Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res 153:104683
Deng K, Yang C, Tan Q et al (2018) Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol 150(3):460–465
Deryugina EI, Zajac E, Juncker-Jensen A et al (2014) Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia (New York, N.Y.) 16:771–788
Dhawan A, Scott JG, Harris AL et al (2018) Pan-cancer characterisation of microRNA across cancer hallmarks reveals microRNA-mediated downregulation of tumour suppressors. Nat Commun 9:5228
Dillekås H, Rogers MS, Straume O (2019) Are 90% of deaths from cancer caused by metastases. Cancer Med 8:5574–5576
Dorayappan KDP, Wanner R, Wallbillich JJ et al (2018) Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene 37:3806–3821
Du R, Lu KV, Petritsch C et al (2008) HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13:206–220
Duan J-X et al (2008) Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem 51:8
Dunn LK, Mohammad KS, Fournier PG et al (2009) Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PloS one 4:e6896
Ediriwickrema A, Saltzman WM (2015) Nanotherapy for cancer: targeting and multifunctionality in the future of cancer therapies. ACS Biomater Sci Eng 1(2):64–78
Ehteshami Bejnordi B et al (2017) Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318:22
Erler JT, Bennewith KL, Nicolau M et al (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440:1222–1226
Fang M, Yuan J, Peng C et al (2014) Collagen as a double-edged sword in tumor progression. Tumour Biol 35:2871–2882
Fidler IJ, Paget S (2003) The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature reviews. Cancer 3:453–458
Galvis MM, Romero CS, Bueno TO et al (2021) Toward a new era for the management of circulating tumor cells. Adv Exp Med Biol 1286:125–134
Ganesh K, Massagué J (2021) Targeting metastatic cancer. Nat Med 27:34–44
Gao H, Chakraborty G, Lee-Lim AP et al (2012) The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150:764–779
Ge X, Pan MH, Wang L, Li W et al (2018) Hypoxia-mediated mitochondria apoptosis inhibition induces temozolomide treatment resistance through miR-26a/Bad/Bax axis. Cell Death Dis 9:1128
Giancotti FG (2013) Mechanisms governing metastatic dormancy and reactivation. Cell 155:750–764
Gilkes DM, Bajpai S, Chaturvedi P et al (2013) Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem 288:10819–10829
Gilkes DM, Xiang L, Lee SJ et al (2014) Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci USA 111:E384-93
Godet I, Shin YJ, Ju JA et al (2019) Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun 10:4862
Gonzalez DM, Medici D (2014) Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7:re8
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN (2007) Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol 608:1–22
Guimarães-Camboa N, Stowe J, Aneas I et al (2015) HIF1α represses cell stress pathways to allow proliferation of hypoxic fetal cardiomyocytes. Dev Cell 33:507–521
Gulati P, Kaur P, Rajam MV et al (2018a) Single-wall carbon nanotube based electrochemical immunoassay for leukemia detection. Anal Biochem 557:111–119
Gulati P, Kaur, Rajam MV et al (2019) Vertically aligned multi-walled carbon nanotubes based flexible immunosensor for extreme low level detection of multidrug resistant leukemia cells. Sensors and Actuators
Gulati P, Kaur, Rajam MV et al (2018b) Leukemia biomarker detection by using photoconductive response of CNT electrode: analysis of sensing mechanism based on charge transfer induced Fermi level fluctuation. Sens Actuators B Chem 270
Hafeez U et al (2020) Antibody-drug conjugates for cancer therapy. Molecules (Basel, Switzerland) 25(20):4764
Hajra KM, Chen DY, Fearon ER (2002) The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 62:1613–1618
Hammond EM, Asselin MC, Forster D et al (2014a) The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol) 26(5):277–288. https://doi.org/10.1016/j.clon.2014.02.002. Epub 2014
Hammond EM et al (2014b) The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol (Great Britain)) 26:5
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Harimoto T, Hahn J, Chen YY et al (2022) A programmable encapsulation system improves delivery of therapeutic bacteria in mice. Nat Biotechnol
Hay M et al (2017) Discovery of the hypoxia-activated Prodrug SN30000. Comprehensive Medicinal Chemistry III. Elsevier
Hendricksen K et al (2012) Phase 2 study of adjuvant intravesical instillations of apaziquone for high risk nonmuscle invasive bladder cancer. J Urol 187:4
Henze AT, Mazzone M (2016) The impact of hypoxia on tumor-associated macrophages. J Clin Investig 126:3672–3679
Hong B, Zu Y (2013) Detecting circulating tumor cells: current challenges and new trends. Theranostics 3:377–394
Hu CJ, Wang LY, Chodosh LA et al (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23:9361–9374
Hu L, Tang J, Huang X et al (2018) Hypoxia exposure upregulates MALAT-1 and regulates the transcriptional activity of PTB-associated splicing factor in A549 lung adenocarcinoma cells. Oncol Lett 16:294–300
Hunter FW et al (2012) Homologous recombination repair-dependent cytotoxicity of the benzotriazine di-N-oxide CEN-209: comparison with other hypoxia-activated prodrugs. Biochem Pharmacol 83:5
Intlekofer AM, Dematteo RG, Venneti S et al (2015) Hypoxia induces production of L-2-hydroxyglutarate. Cell Metab 22:304–311
Irshad K, Malik N, Arora M et al (2021) The quest for ligands and binding partners of atypical cadherin FAT1. Transl Oncol 14:101097
Jafari R, Rahbarghazi R, Ahmadi M et al (2020) Hypoxic exosomes orchestrate tumorigenesis: molecular mechanisms and therapeutic implications. J Transl Med 18:474
Jonasch E et al (2021) Belzutifan for renal cell carcinoma in von hippel-lindau disease. N Engl J Med 385:22
Ju JA, Godet I, Ye IC et al (2017) Hypoxia selectively enhances integrin α5β1 receptor expression in breast cancer to promote metastasis. Mol Cancer Res 15:723–734
Kaelin WG, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119:1420–1428
Kanimozhi G, Prasad NR (2015) Chapter 73—Anticancer effect of caffeic acid on human cervical cancer cells. In: Preedy VR (ed) Coffee in Health and Disease Prevention. Academic Press
Kapitsinou PP, Rajendran G, Astleford L et al (2016) The endothelial Prolyl-4-Hydroxylase Domain 2/Hypoxia-Inducible Factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 36:1584–1594
Karagiannis GS, Condeelis JS, Oktay MH (2019) Chemotherapy-induced metastasis: molecular mechanisms, clinical manifestations, therapeutic interventions. Cancer Res 79:4567–4576
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–1480
Kim MC, Hwang SH, Yang Y et al (2021) Reduction in mitochondrial oxidative stress mediates hypoxia-induced resistance to cisplatin in human transitional cell carcinoma cells. Neoplasia (New York, N.Y.) 23:653–662
Kim MS, Kwon HJ, Lee YM et al (2001) Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 7:437–443
King HW, Michael MZ, Gleadle JM (2012) Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12:421
Koike T, Kimura N, Miyazaki K et al (2004) Hypoxia induces adhesion molecules on cancer cells: a missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci U S A 101:8132–8137
Komohara Y, Jinushi M, Takeya M (2014) Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 105:1–8
Konopleva M et al (2015) Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica 100:7
Koong AC, Mehta VK, Le QT et al (2000) Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys
Koscielny S, Tubiana M, Lê MG et al (1984) Breast cancer: relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer 49:709–715
Krieg AJ, Rankin EB, Chan D et al (2010) Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol Cell Biol 30:344–353
Kucharzewska P, Christianson HC, Welch JE et al (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA 110:7312–7317
Kulshreshtha R, Ferracin M, Wojcik SE et al (2007) A microRNA signature of hypoxia. Mol Cell Biol 27:1859–1867
Kwaan HC, Lindholm PF (2019) Fibrin and fibrinolysis in cancer. Semin Thromb Hemost 45:413–422
Laderoute K et al (1988) Molecular mechanisms for the hypoxia-dependent activation of 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233). Biochem Pharmacol 37:8
Lah TT, Novak M, Breznik B (2020) Brain malignancies: glioblastoma and brain metastases. Semin Cancer Biol 60:262–273
Laughner E et al (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21:12
Lee C-T et al (2010) Hypoxia-driven immunosuppression: a new reason to use thermal therapy in the treatment of cancer? Int J Hyperthermia 26:3
Lee JC, Lee KM, Kim DW et al (2004) Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol (Baltimore, Md. : 1950) 172:7335–7340
Lee K, Kim HM (2011) A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharmacalog Res 34:10
Lee YT et al (2018) Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol 834
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Li Y, Patel SP, Roszik J et al (2018) Hypoxia-driven immunosuppressive metabolites in the tumor microenvironment: new approaches for combinational immunotherapy. Front Immunol 9:1591
Li Y et al (2021) Targeting hypoxia: hypoxia-activated prodrugs in cancer therapy. Front Oncol 11:700407
Liao D, Corle C, Seagroves TN et al (2007) Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res 67:563–572
Lindsey S, Langhans SA (2014) Crosstalk of oncogenic signaling pathways during epithelial-mesenchymal transition. Front Oncol 4:358
Liu X, Taftaf R, Kawaguchi M et al (2019) Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov 9:96–113
Liu Y, Li YM, Tian RF et al (2009) The expression and significance of HIF-1alpha and GLUT-3 in glioma. Brain Res 1304:149–154
Loh CY, Chai JY, Tang TF et al (2019) The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells 8:E1118
Lu W, Kang Y (2019) Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell 49:361–374
Lu X, Kang Y (2010) Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16:5928–5935
Macharia LW, Wanjiru CM, Mureithi MW et al (2019) MicroRNAs, hypoxia and the stem-like state as contributors to cancer aggressiveness. Front Genet 10:125
Majidpoor J, Mortezaee K (2021) Steps in metastasis: an updated review. Med Oncol (Northwood, London, England) 38:3
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Institute 97:188–194
McIntyre A, Hulikova A, Ledaki I et al (2016) Disrupting hypoxia-induced bicarbonate transport acidifies tumor cells and suppresses tumor growth. Cancer Res 76:3744–3755
Meng Z et al (2019) Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater (Deerfield Beach, Fla.) 31:24
Metran-Nascente C, Yeung I, Vines DC et al (2016) Measurement of tumor hypoxia in patients with advanced pancreatic cancer based on 18F-Fluoroazomyin arabinoside uptake. J Nucl Med 57:361–366
Micalizzi DS, Maheswaran S, Haber DA (2017) A conduit to metastasis: circulating tumor cell biology. Genes Dev 31:1827–1840
Mijanović O, Branković A, Panin AN et al (2019) Cathepsin B: a sellsword of cancer progression. Cancer Lett 449:207–214
Milosevic M, Warde P, Ménard C et al (2012) Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res 18(7):2108–2114. https://doi.org/10.1158/1078-0432.CCR-11-2711. Epub (2012)
Mingyuan X, Qianqian P, Shengquan X et al (2018) Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget 9:3188–3197
Monteiro AR, Hill R, Pilkington GJ et al (2017) The role of hypoxia in glioblastoma invasion. Cells 6:E45
Muz B, de la Puente P, Azab F et al (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 3:83–92
O'Connell JT, Sugimoto H, Cooke VG et al (2011) VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108:16002–16007
Ojha R, Amaravadi RK (2017) Targeting the unfolded protein response in cancer. Pharmacol Res 120
Okami J, Simeone DM, Logsdon CD (2004) Silencing of the hypoxia-inducible cell death protein BNIP3 in pancreatic cancer. Cancer Res 64:5338–5346
Oskarsson T, Acharyya S, Zhang XH et al (2011) Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17:867–874
Paget S, Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101
Pallares RM, Thanh NTK, Su X (2019) Sensing of circulating cancer biomarkers with metal nanoparticles. Nanoscale 11:22152–22171
Papadopoulos KP et al (2008) A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res 14:21
Parks SK, Pouyssegur J (2015) The Na(+)/HCO3(-) Co-Transporter SLC4A4 plays a role in growth and migration of colon and breast cancer cells. J Cell Physiol 230:1954–1963
Patel TH, Cecchini M (2020) Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol 21(9):70
Patterson LH (1993) Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev 12:2
Peinado H, Lavotshkin S, Lyden D (2011) The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Sem Cancer Biol 21:139–146
Petrella BL, Lohi J, Brinckerhoff CE (2005) Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene 24:1043–1052
Pial MMH et al (2022) Implantable devices for the treatment of breast cancer. J Nanotheranostics 3:1
Pradhan S, Mahajan D, Kaur P et al (2016) Scriptaid overcomes hypoxia-induced cisplatin resistance in both wild-type and mutant p53 lung cancer cells. Oncotarget 7:71841–71855
Prasad P, Mittal SA, Chongtham J et al (2017) Hypoxia-mediated epigenetic regulation of stemness in brain tumor cells. Stem Cells (Dayton, Ohio) 35:1468–1478
Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9:677–684
Qi Y, Xu R (2018) Roles of PLODs in collagen synthesis and cancer progression. Front Cell Dev 6:66
Quintero-Fabián S, Arreola R, Becerril-Villanueva E et al (2019) Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol 9:1370
Ranganathan AC, Zhang L, Adam AP (2006) Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res 66:1702–1711
Rankin EB, Fuh KC, Castellini L et al (2014) Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci USA 111:13373–13378
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Riihimäki M, Thomsen H, Sundquist K et al (2018) Clinical landscape of cancer metastases. Cancer Med 7:5534–5542
Ruan C et al (2021) Nanomaterials for tumor hypoxia relief to improve the efficacy of ros-generated cancer therapy. Front Chem 9:649158
Ruffell B, Coussens LM (2015) Macrophages and therapeutic resistance in cancer. Cancer Cell 27:462–472
Salem A et al (2018) Targeting hypoxia to improve non-small cell lung cancer outcome. J Natl Cancer Institute 110:1
Sasaki M, Knobbe CB, Munger JC et al (2012) IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488:656–659
Sato M, Hirose K, Kashiwakura I et al (2015) LW6, a hypoxia-inducible factor 1 inhibitor, selectively induces apoptosis in hypoxic cells through depolarization of mitochondria in A549 human lung cancer cells. Mol Med Rep 12:3
Schewe DM, Aguirre-Ghiso JA (2008) ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci USA 105:10519–10524
Schietke R et al (2010) The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1. J Biol Chem 285:9
Schito L, Semenza GL (2016) Hypoxia-inducible factors: master regulators of cancer progression. Trends cancer 2:758–770
Schito L et al (2012) Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci USA 109(40):E2707-16. https://doi.org/10.1073/pnas.1214019109
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20:51–56
Semenza GL (2013) HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Investig 123:3664–3671
Senapati S et al (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3(7):16
Shariati M, Meric-Bernstam F (2019) Targeting AKT for cancer therapy. Expert Opin Investig Drugs 28:11
Shelar S, Shim EH, Brinkley GJ et al (2018) Biochemical and epigenetic insights into l-2-hydroxyglutarate, a potential therapeutic target in renal cancer. Clin Cancer Res 24:6433–6446
Shi Y, Wang S, Yang R et al (2022) ROS promote hypoxia-induced keratinocyte epithelial-mesenchymal transition by inducing SOX2 expression and subsequent activation of Wnt/β-Catenin. Oxidative Med Cell Longevity 2022:1084006
Shirai Y et al (2021) An overview of the recent development of anticancer agents targeting the HIF-1 Transcription Factor. Cancers 13:11
Shyu KG, Hsu FL, Wang MJ et al (2007) Hypoxia-inducible factor 1alpha regulates lung adenocarcinoma cell invasion. Exp Cell Res 313:1181–1191
Singleton RS et al (2009) DNA cross-links in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism, and cytotoxicity. Cancer Res 69:9
Skuli N, Majmundar AJ, Krock BL et al (2012) Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J Clin Investig 122:1427–1443
Song L et al (2020) Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomaterialia 108
Spivey KA, Chung I, Banyard J et al (2012) A role for collagen XXIII in cancer cell adhesion, anchorage-independence and metastasis. Oncogene 31:2362–2372
Srivastava C, Irshad K, Dikshit B et al (2018) FAT1 modulates EMT and stemness genes expression in hypoxic glioblastoma. Int J Cancer 142:805–812
Srivastava C, Irshad K, Gupta Y et al (2020) NFкB is a critical transcriptional regulator of atypical cadherin FAT1 in glioma. BMC Cancer 20:62
Sun J, Zhao T, Zhao D et al (2020) Development and validation of a hypoxia-related gene signature to predict overall survival in early-stage lung adenocarcinoma patients. Ther Adv Med Oncol 12:1758835920937904
Sun ZF, Chang Y, Xia N (2021) Recent development of nanomaterials-based cytosensors for the detection of circulating tumor cells. Biosensors 11:281
Syn N, Wang L, Sethi G et al (2016) Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci 37:606–617
Tam SY, Wu VWC, Law HKW (2020) Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1α and Beyond. Front Oncol 10:486
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nature reviews. Cancer 2:442–454
Timp W, Feinberg AP (2013) Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer 13:497–510
Todd VM, Vecchi LA, Clements ME et al (2021) Hypoxia inducible factor signaling in breast tumors controls spontaneous tumor dissemination in a site-specific manner. Commun Biol 4:1122
Turunen SP, Tatti-Bugaeva O, Lehti K (2017) Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim Biophys acta Mol Cell Res 1864:1974–1988
Vasan N et al (2019) A view on drug resistance in cancer. Nature 575:7782
Vaupel P, Höckel M, Mayer A (2007) Detection and characterization of tumor hypoxia using pO2 histography. Antioxidants Redox Signaling 9:1221–1235
Vaupel P, Multhoff G (2018) Hypoxia-/HIF-1α-driven factors of the tumor microenvironment impeding antitumor immune responses and promoting malignant progression. Adv Exp Med Biol 1072:171–175
Wang R, Godet I, Yang Y, Salman S, Lu H, Lyu Y, Zuo Q, Wang Y, Zhu Y, Chen C, He J, Gilkes DM, Semenza GL (2021b) Hypoxia-inducible factor-dependent ADAM12 expression mediates breast cancer invasion and metastasis. Proc Natl Acad Sci U S A 118(19):e2020490118
Wang T et al (2014) Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA 111(31):E3234-42. https://doi.org/10.1073/pnas.1410041111
Wang Z, Shi Y, Ying C et al (2021a) Hypoxia-induced PLOD1 overexpression contributes to the malignant phenotype of glioblastoma via NF-κB signaling. Oncogene 40:1458–1475
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J General Physiol 8:519–530
Wei Q, Qian Y, Yu J et al (2020) Metabolic rewiring in the promotion of cancer metastasis: mechanisms and therapeutic implications. Oncogene 39:6139–6156
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nature reviews. Cancer 11:6
Wolf K, Alexander S, Schacht V et al (2009) Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20:931–941
Wu JB et al (2015) Near-infrared fluorescence heptamethine carbocyanine dyes mediate imaging and targeted drug delivery for human brain tumor. Biomaterials 67
Xiong G, Stewart RL, Chen J et al (2018) Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat Commun 9:4456
Yamada D, Kobayashi S, Yamamoto H et al (2012) Role of the hypoxia-related gene, JMJD1A, in hepatocellular carcinoma: clinical impact on recurrence after hepatic resection. Ann Surg Oncol 19(Suppl 3):S355–S364
Yang H et al (2021b) Targeting cancer metastasis with antibody therapeutics. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol 13:4
Yang X et al (2017) 3D microstructure inhibits mesenchymal stem cells homing to the site of liver cancer cells on a microchip. Genes 8(9):218
Yang Y, Li Y, Qi R et al (2021a) Constructing a novel 5 hypoxia genes signature for cervical cancer. Cancer Cell Int 21:345
Ye IC, Fertig EJ, DiGiacomo JW et al (2018) Molecular portrait of hypoxia in breast cancer: a prognostic signature and novel HIF-regulated genes. Mol Cancer Res 16:1889–1901
You L, Wu W, Wang X et al (2021) The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev 41:1622–1643
Zatovicova M et al (2022) Novel humanized monoclonal antibodies for targeting hypoxic human tumors via two distinct extracellular domains of carbonic anhydrase IX. Cancer Metabol 10(1):3
Zhang H, Wong CC, Wei H et al (2012) HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 31:1757–1770
Zhang H, Wong CCL, Wei H et al (2021a) Correction: HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 40:1552–1553
Zhang J, Li XY, Hu P et al (2018) lncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res 26:1411–1418
Zhang Y, Zhang H, Wang M et al (2021b) Hypoxia in breast cancer-scientific translation to therapeutic and diagnostic clinical applications. Front Oncol 11:652266
Zhao T, Zhu Y, Morinibu A et al (2014) HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci Rep 4:3793
Zhu L et al (2022) A clinically compatible drug-screening platform based on organotypic cultures identifies vulnerabilities to prevent and treat brain metastasis. EMBO Mol Med 14:3
Zimmer AS, Steeg PS (2015) Meaningful prevention of breast cancer metastasis: candidate therapeutics, preclinical validation, and clinical trial concerns. J Mol Med (Berlin, Germany) 93:13–29
Zou M-Z et al (2021) Advances in nanomaterials for treatment of hypoxic tumor. Natl Sci Rev 8:2
Zou YF, Rong YM, Tan YX et al (2019) A signature of hypoxia-related factors reveals functional dysregulation and robustly predicts clinical outcomes in stage I/II colorectal cancer patients. Cancer Cell Int 19:243
Acknowledgement
Funding from University of Delhi IoE-Faculty Research Program and Science and Engineering Research Board to TS are gratefully acknowledged. TS, JC, SG, SK (1), DC, SK (2) and PG wrote and edited the manuscript. SK (1), DC, SK (2) and PG created the illustrations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gandhi, S. et al. (2023). Hypoxia and the Metastatic Cascade. In: Mukherjee, S., Kanwar, J.R. (eds) Hypoxia in Cancer: Significance and Impact on Cancer Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-99-0313-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-99-0313-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0312-2
Online ISBN: 978-981-99-0313-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)