Abstract
Many a times, a very thin line exists between physiology and pathology. Airway obstruction seen in the asthmatic subjects is exemplary of such phenomena. Physiologically, airway obstruction is needed to guard the master regulator of lung homeostasis, i.e. the alveolar epithelial cells, against the massive entry of exogenous irritants like noxious particles, allergens and microbes into the airway. Surprisingly, genetic predisposition with prolonged exposure to these exogenous irritants sets off the pathological clock causing mild-moderate- severe asthma. With time, numerous innate and adaptive immune cell types along with cytokines have been pinpointed which participates in the pathogenesis of asthma. Though Th2 cytokines form the foundation of the majority of the asthmatic situation, researchers started dividing the patients as Th2-high and Th2-low asthma. The recent concept of classifying asthma based on its endotypes is a more granular approach to avert the progression of asthma rather than the phenotypic classification. The identification of novel biomarkers based on the -omics technology will leverage the molecular data and provide a precision-based care. Here, in this chapter, we have described about the various immune cells responsible for the heterogeneity of the disease such that it can be targeted for substantial improvisation of asthma, along with current lacuna in the understanding of asthma pathogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
2.1.1 Rationale to the Study
Asthma causes a significant socioeconomic burden as well as family burden. The major symptoms are wheezing (vibrations in the small airways that are almost closed off due to obstruction), chest tightening, shortness of breath, cough with or without sputum production and night waking due to combination of the above symptoms. Asthma is an important global public health problem as 339 million people are affected worldwide as of the 2016 report from World Health Organization (WHO) [1]. It is one of the very few diseases that predominantly affects the people in developed nations. This is in accordance with the hygiene hypothesis proposed by Strachan in 1989 [2]. However, a dramatic increase in the prevalence, morbidity and mortality due to asthma has been noted in developing countries according to a recent report from the Global Initiative for Asthma [3] in spite of advanced understandings and therapeutic strategies.
2.1.2 Asthma: A Global Health Burden
Despite emergence of newer therapeutic strategies, the prevalence of asthma keeps on increasing [4]. It is the leading cause of hospitalisation among children. Asthma had secured the 16th and 28th position worldwide in causing years lived with disability and adjusted life years, respectively [2]. Its incidence varies from country to country. It has been estimated that 34% of the man days in India are lost due to airway-related disorders, of which asthma is the major cause [5]. It is also important to note that higher mortality is observed in patients having features of refractory asthma. This is due to limited therapeutic options for different pathophysiological features such as greater involvement of neutrophils and increased airway remodelling.
2.1.3 Asthma: GINA Definition
Asthma can be defined as ‘a heterogeneous disease, usually characterised by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation’.
Since Hippocrates time, the concept of asthma had changed from psychological disease to smooth muscle disease to inflammatory disease to current airway remodelling. Parallel to change in the concept, the therapeutic strategy has also changed from mind calming to bronchodilators to anti-inflammatory drugs to thermoplasty. Though all these available traditional therapy are mostly non-specific approach, the knowledge we obtained in the last two decades converts this mere non-specific approach to combined non-specific coupled with pathway-specific approach. In this chapter, we are going to witness the same.
2.2 Traditional Understanding and Parallel Non-specific Therapy
2.2.1 Major Types of Asthma
There are two major types of asthma: atopic and non-atopic. Asthma which gets triggered by allergens is called extrinsic or atopic asthma [6]. Atopic asthma is characterised by heightened expression of cytokines like interleukin-4 (IL-4), interleukin-5 (IL-5) by T helper 2 (Th2) cell, interleukin-2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in bronchoalveolar lavage (BAL) fluid [7]. These atopic asthma patients have increased levels of allergen-specific immunoglobulin E (IgE) in serum and skin prick positivity to susceptible allergens [8]. Intrinsic, non-atopic, asthma that sets off by running, exercise, etc. generally appears at a later life and is non-allergic in nature. Occupational asthma, one subtype of non-atopic asthma, is triggered by the exposure to irritants found in workplace.
2.2.2 Allergens Are Major Inducers for Atopic Asthma
The inducers for the asthma symptoms vary depending on the type of asthma. The inducers for atopic asthma are various types of allergens like different types of common pollens, cockroach allergen, house dust mite, danders of cat and dog, etc. Importantly, these allergens sensitise only allergy-prone individuals who are having genetic tendency. After sensitisation, when they are exposed to same allergens again, it leads to bronchoconstriction. In general, other environmental factors like air pollutants may not instigate asthma development in such atopic individuals, but they can aid in promoting the asthma initiation. For example, by damaging airway epithelial barrier, cigarette smoke is seen to increase the accessibility of allergens. As a result, air pollution could potentiate the allergen uptake following which they get processed by antigen-presenting cells. On the other hand, in non-atopic asthma, both indoor and outdoor pollutants could initiate the asthma development and this leads to cause occupational asthma. Thus, children residing in the urban areas have an increased tendency to develop asthma than non-urban children [9].
2.2.3 Pathophysiology
In Greek language, the word asthma means panting and being out of breath. Initially, asthma was misunderstood to have a psychosomatic history. But actually, this is not completely a misunderstanding because neurogenic inflammation does happen in asthma pathogenesis. Asthma is a complex disease commonly identified as hyperactivity in the bronchial airways. Such hyperresponsiveness in the bronchial region increases before the onset of the allergic trigger and reverts only after treatment. There is an increase in the infiltration of inflammatory cells like eosinophils. This eosinophil-dominant airway inflammation is associated with goblet cell metaplasia with overproduction of mucus in the airways and hypertrophy and hyperplasia of airway smooth muscles [10]. Airway hyperresponsiveness occurs due to the increased sensitivity of the sensory nerves to allergen. In case of serious asthmatic conditions, mucus plugs are formed which are made up of plasma proteins and mucus glycoprotein [11]. Most of these asthma features are believed to be mediated by Th2 response. Repeated airway inflammation also causes irreversible structural changes in the airways. All these features, termed ‘airway remodelling’, is characterised by airway wall thickening due to deposition of extracellular matrix proteins underneath the airway epithelial layer. Such increase in the wall tissue of the airways further causes more narrowing of the walls of the airways.
2.2.4 Symptomatic Therapy
Bronchodilators and corticosteroids are the two major types of anti-asthma drugs that are in use for a long time. Both of these drugs fall under the category of symptomatic treatment. There are other drugs like antihistamine agents and leukotriene modifiers that are considered as additional supplementary therapy. Though leukotriene modifiers showed promising results at the initial times of launching, all asthmatics did not show good improvement in lung function.
Bronchodilators are group of drugs which function by relaxing the tightened airway muscles. This facilitates intake of air and improves breathing. They symptomatically act in managing asthma [12]. Depending on their nature, bronchodilators can be subdivided into two categories. These are beta-2-agonist drug and anticholinergic drug. Based on the time of action, beta-2-agonist drugs can be further classified as long-acting beta-2-agonist (LABA) and short-acting beta-2-agonist (SABA) [13]. Ultra-LABA is also used [12]. Tiotropium and theophylline are long-acting muscarinic antagonists (LAMA). Salmeterol and formoterol are long-acting beta-2- agonists that are administered by inhalation. Theophylline, whose chemical name is dimethylxanthine, has been used for asthma therapeutics for a long time. It works by inhibiting phosphodiesterase 3 (PDE3). Activation of PDE4 and histone deacetylase-2 is also inhibited by theophylline. However, long-term theophylline use can pose some harmful effects. Low dose is recently recommended to avoid therapeutic resistance [14].
Inhaled corticosteroids (ICS) are common drugs to manage acute asthma [15]. Combination therapy of both beta-2-agonists and ICS is the frequently used strategy to control moderate to severe asthmatic conditions. LABA in combination with ICS proves to be an effective treatment for asthma [12]. Corticosteroids inhibit the inflammatory activities through transactivation and/or transrepression. The former leads to the transcription of numerous anti-inflammatory mediators, whereas the latter suppresses the transcription of pro-inflammatory mediators. However, 5–10% asthmatic patients do not respond to corticosteroids, and this condition is called steroid-insensitive condition. This has been discussed in a separate chapter in this book.
Anti-leukotriene drugs work by inhibiting the leukotriene receptors. Though lot of expectations were there on leukotriene modifiers when these were launched, the clinical trial findings were disappointing. However, leukotriene receptor antagonists have been shown to be beneficial in certain asthmatic conditions like aspirin-sensitive asthma, obese asthma, senile asthma and smoking-associated asthma [16]. The effects of these leukotriene modifiers on airway remodelling and identification of biomarkers of good responders are yet to be investigated [16].
The above-mentioned drugs are non-specific symptomatic therapies available in asthma, except leukotriene modifiers. Though this non-specific therapy can be successful in controlling the disease for a short term, it seems that this may not be sufficient in a considerable number of patients when they have to control the disease for a long time. With the emergence of multiple endotypes in asthma including neutrophilic asthma, steroid-resistant asthma, obese asthma and so on, the asthma field needs to be revisited. This would provide a better understanding of the disease pathogenesis which would in turn aid in formulating more effective therapeutic strategies. In this chapter, we have attempted to achieve the same. In this journey, one can realise how non-specific therapy can be complemented with specific, personalised strategy in asthma management.
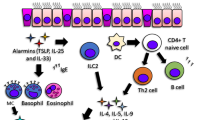
2.3 Current Understanding and Steps Towards Pathway-Specific Therapy (Fig. 2.1)
2.3.1 Cells Involved in Asthma Pathogenesis and Respective Targets
2.3.1.1 Airway Epithelium
The respiratory organ is lined by epithelial cells. Being a frontline defence barrier, the airway epithelium acts as a protective interface between the lung and environment [17]. Earlier, it was believed that airway epithelium is a mere victim of most of the recruited immune cells. However, recent reports indicate that airway epithelium can govern airway inflammation. For example, airway epithelium secretes Th2-polarising cytokines on being exposed to mild irritants like allergen but secretes interleukin-17 (IL-17) when they are exposed to severe irritants like cigarette smoke [17]. The entire epithelial layer present in the airways provides two major barriers. They act as physical barrier by forming tightly connected airway epithelial layer that does not allow foreign particles like allergens to enter. Airway epithelia also function as a chemical barrier. The epithelial layer secretes a number of innate immune cytokines that can further act as a signal to initiate the acquired immune response [17]. This will then bring the right type of immune cells to the site of insult that can lead to resolution. The protective mechanism also includes the mucus layer which has two layers: gel and sol. These layers are responsible for entrapping foreign particles. Such particles will be sent upwards in the airways by ciliary beating to be removed from the airway [17]. In addition, the airways also have other physiological mechanisms like sneezing and coughing. They are primarily responsible for elimination of foreign particles from the airway with the goal of protecting the alveoli, the functional unit of lungs. Indeed, the partial closure of the airways, bronchoconstriction, is meant for the prevention of massive entry of allergens and other toxic particles from the environment into the airways. Thus, the airway epithelial layer acts as a security force to protect the alveoli.
2.3.1.2 Dendritic Cells
Dendritic cells recognise foreign unknown agents which have a role in promoting asthma. When the airway lumen is exposed to any antigen or allergen, they are sampled by these dendritic cells. Dendritic cells insert their teeth-like projections between two subsequent bronchial epithelia. Upon identifying allergens, dendritic cell has the job to initiate allergic responses. They process the antigens and then present them to the naïve T helper (Th0) cell. They also decide whether the response would be vigorous or can be tolerated [18]. Studies have shown that dendritic cells have a part in the differentiation of the CD4+ T cells. In response to aeroallergen, dendritic cells dominate eosinophilic inflammation by regulating the activation of lung Th2 cells. They may also have a role in tuning the immune balance between T regulatory cell (Treg) and Th2 cells [19].
2.3.1.2.1 Dendritic Cell Modulator as a Target
It is known that successful migration of lymphocytes from the lymph node to the site of inflammation is crucial for the development of active immune response. This migration is facilitated by a number of events, and one such event is the gradient of sphingosine-1-phosphate (S1P) [20, 21]. In general, the levels of S1P are always high in both lymph nodes and blood compared to the tissues. Upon its receptor interaction, S1P can inhibit the exit of lymphocytes from the lymph node. Applying this strategy, fingolimod (FTY720), a synthetic sphingolipid, was developed. Upon phosphorylation, fingolimod phosphate acts as an analogue of S1P. Fingolimod prevents the movement of DCs present in the lung to mediastinal lymph nodes and can thus function as an immunosuppressant in asthma [20, 21]. However, it could cause lymphopenia that has important adverse effects. Studies have reported that inhalation of FTY720 ameliorates the asthma features without causing lymphopenia [20]. Though fingolimod seemed to be successful in murine asthma, its effects in asthmatic patients are controversial. Thus, more investigation is needed to see whether it provides beneficial effects in asthma.
2.3.1.3 Lymphocytes
Lymphocytes have a crucial role in asthma pathogenesis and both B and T cells are under its umbrella. Naïve T cells endorse T cell receptor (TCR) which, upon recognising any antigen, differentiates and proliferates into effector T cells like T helper 1 (Th1), Th2, T helper 17 (Th17) and Treg cells. Th2 lymphocytes lead to the commencement and maintenance of allergic inflammation. Such phenomena occur in asthma and it is dependent on various environmental factors like allergen exposure. CD25+ Treg, a subclass of T lymphocytes, also has a part in asthma progression. Treg cells play an integral role in managing the allergy coupled asthmatic response through immunotherapy [22]. They subdue the cytokine production and proliferating ability of CD4+ CD25- T cells. Treg cells also produce interleukin-10 (IL-10) that have a role in safeguarding atopic sensitivity in humans [22].
Current understanding and steps towards pathway- or molecule-specific therapy in asthma. Upon allergen exposure, the allergens are sampled by dendritic cells that are located in between the two subsequent airway epithelia in the mucosal layer, processed and presented to naïve T cells for the conversion of Th2 cells. These Th2 cells release various Th2 cytokines like IL-4, interleukin-13 (IL-13) and IL-5. This leads to various asthma features like IgE class switching in B lymphocytes followed by release of IgE that further binds to IgE receptors present in basophils and mast cells to release various bronchoconstrictors, goblet cell metaplasia, sub-epithelial fibrosis and recruitment of eosinophils. In contrast to traditional symptomatic therapy in asthma, current understanding in asthma pathogenesis emphasised the presence of various endotypes in asthma patients and also pave the way for pathway-oriented therapy or cytokine-oriented biologic to control asthma symptoms. However, the combination of both symptomatic therapy and cytokine-targeted therapy are complimentary. It is important to note that cytokine-targeted therapy may not be universal, but it depends on molecular involvement of individual patients. This laid a foundation of personalised approach in current therapeutic strategy. IL, interleukin; CD, cluster of differentiation; IgE, immunoglobulin E
2.3.1.4 T Helper Type 2 (Th2) Cells
They are dominantly involved in atopic asthmatic condition [23]. However, its involvement is also reported in non-atopic asthma. GATA-3, the transcription factor for Th2 cell subtype, gets overexpressed in asthma. In contrast, the transcription factor for Th1 cell is T-bet whose expression gets reduced in asthma. The cytokines released by Th2 cell like IL-13 have the capacity to induce airway hyperresponsiveness (AHR), and subsequently inflammation in asthma, independently without IgE and eosinophils. Allergic asthma which gets instigated by the Th2 cells is called eosinophilic asthma.
Th2 cytokines like IL-4, IL-5 and IL-13, which are secreted by these Th2 cells, are responsible for most of the asthma features. IL-4 is also released from mast cells upon initial allergen exposure, and this event is crucial for the conversion of naïve T cells to Th2 cells. IL-4 secreted by Th2 cells leads to a number of asthma features. Since both IL-4 and IL-13 share a common receptor, IL-13Rα1/IL-4Rα complex, most of the events, like signal transducer and activator of transcription 6 (STAT-6) phosphorylation and IgE class switching, are common in IL-4 and IL-13 signalling. In addition, IL-4 also leads to IL-13-independent effects, thanks to IL-4-specific receptor subunit that is not shared with IL-13 [24].
IL-13 is a key Th2 cytokine that regulates major airway inflammatory events in asthma. Genetic polymorphisms in IL-13, its receptor components, and its downstream molecules such as STAT-6 have been shown to be associated with asthma. Therapies have been developed which will target IL-13/IL-4/STAT-6 pathway and control this heterogeneous airway disease amount [25]. Mere administration of recombinant IL-13 seems to be sufficient to induce most of the asthma features in naïve mice even without allergen exposure. This indicates the dominant nature of IL-13 in asthma pathogenesis. In addition to its participation in allergic airway inflammation, IL-13 signalling also leads to airway remodelling changes like goblet cell metaplasia and sub-epithelial fibrosis.
2.3.1.4.1 Th2 Cytokines as Target
In order to block the interaction between IL-13 and its receptors, development of some monoclonal antibodies, that are presently having asthma based clinical trials, took place. Anrukinzumab monoclonal antibody (mAb) is of human origin that works by blocking IL-13 and inhibiting the activation of IL-13 receptor subunits IL-13Rα1 and IL-13Rα2 [26]. Lebrikizumab, which is also used in the treatment of atopic dermatitis, is a human immunoglobulin G4 (IgG4) mAb that has high affinity for IL-13, binds to IL-13 and inhibits signalling pathway that occurs through the IL-4Rα/IL-13Rα1 heterodimeric complex. Humanised IgG4 mAb, tralokinumab, that specifically targets and neutralises IL-13, is used for treating severe asthma. It affects the mRNA level of IL-13 regulated dipeptide peptidase-4 (DPP-4) in serum [27].
Inhibition of IL-4 can be done either by direct blocking of the IL-4 cytokine or by inhibiting IL-4/IL-13 receptor. One such drug is dupilumab. Being a mAb, dupilumab blocks IL-4Rα/IL-13Rα1 receptor complex and thus prevents interaction between IL-4 with IL-4R and IL-13 with IL-13Rα1. Another such IL-4Rα/IL-13Rα1 antagonist is pitrakinra [28]. AMG17 is another humanised monoclonal antibody which binds to IL-4Rα and inhibits both IL-4 and IL-13 pathways. However, it is not effective for all groups of asthmatic patients [29]. Antagonists can also be designed in the laboratory. By joining crystallisable IL-13Rα2 fragment with general antagonist mu11B11 through SCORPIO method, a research group developed dual IL-4/IL-13 antagonist that has a role in inhibiting both the cytokine and lung inflammation in OVA-challenged mice [30]. IL-13 also shares signalling pathway with thymic stromal lymphopoietin (TSLP). A research group came up with bispecific nature anti-IL-13/anti-TSLP antibodies for therapeutic benefit in Th2 asthma. These antibodies are called Zweimab and Doppelmab which are monovalent bispecific and bivalent bispecific, respectively. Both of them are very potent and target both IL-13 and TSLP [31]. It is important to note that various IL-13 and IL-4 targeting therapeutic molecules cannot be useful in all asthmatic patients. But it would be useful in a subset of patients who are having eosinophilic asthma phenotype along with increased levels of periostin [32].
2.3.1.5 B Lymphocytes and IgE
Allergen-induced T cells induce and activate B lymphocytes that further release IgE after class switching. Indeed, atopicity is determined mainly by the existence of higher IgE levels. The receptor proteins involved in such IgE signalling pathway are high-affinity Fc receptor for IgE (FcεRI) and CD23 which has low affinity. Apart from basophils and mast cells that constitutively express FcεRI protein, dendritic cells also express FcεRI. Expression of FcεRI protein is also found in the airway smooth muscle cells and airway epithelial cells of asthmatic individuals [33]. Apart from IgE, low-affinity CD23 receptor has other ligands like CD21 and integrin. CD23 causes transfer of IgE to the mucosal tissue. Concentration of IgE is maintained in tissue as masts cells express FcεRI in high amount and the rate of dissociation of IgE from FcεRI receptor protein is low [34].
Importantly, anti-IgE therapy reduces asthma features significantly. Omalizumab, a humanised monoclonal anti-IgE antibody, selectively binds to IgE and prevents the binding of IgE to its receptor [35]. This diminishes both early and late allergic responses. IgE-stimulated allergic reaction response is also inhibited. It provides a long-term effect and reduces the use of inhaled corticosteroids. Administration of omalizumab reduces the expression of FcεRI in basophils. IgE can also bind to its low-affinity receptor found on B cells. Membrane-bound IgE on the B lymphocytes, IgE+ B cell, has a critical role in controlling synthesis of IgE. Currently under clinical trial, quilizumab is an immunoglobulin G1 (IgG1) mAb that acts on the IgE+ B cells and further regulates the generation of IgE. It thus reduces the quantity of serum IgE in allergic asthma [36]. However, IgE therapy may not be effective for treating non-allergic asthma. Even in allergic asthma, it is being prescribed mostly to patients who are having severe refractory asthma as it is very expensive. More importantly, IgE mAb therapy would be useful in a subset of patients who are having very high IgE levels [32].
2.3.1.6 Mast Cells
They belong to the haematopoietic lineage and are located near the airway epithelium in the mucosal region. In airway mucosa, they are present at the junction point where the foreign antigen enters the host. When activated, degranulation of mast cells happens, following which, the progenitors of mast cells are recruited to inflammatory sites [37]. There is a significant difference in the mast cells found near airway smooth muscle between asthmatic patients and normal individuals. In asthma, airway smooth muscle cross talks with infiltrating mast cells [38]. Airway smooth muscle uses chemokine (C-X-C motif) ligand 10 (CXCL10) or chemokine (C-X-C motif) ligand 3 (CXCR3) axis for the recruitment of mast cells. Degranulation of the infiltrated mast cells is positively correlated with airway obstruction by mucus inside the airway lumen. Inhibiting the secretion by mast cells can be a therapeutic treatment for asthmatic patient [39].
Type 1 hypersensitivity-mediated mast cell degranulation in asthma is controlled by mast cell stabilising agents. Cromolyn sodium, a chemical derived from khellin, obtained from the medicinal plant Ammi visnaga, is a stabilising compound that blocks the release of inflammation-mediating agents from the mast cells [40]. Atopic asthmatic individuals who undergo pre-treatment with cromolyn sodium show decrease in asthma features [41]. Cromolyn, which is given in aerosolised form via nebuliser, acts on both early and late phases of asthma. Nedocromil sodium, an agonist of G protein-coupled receptor 35 (GPCR35), is another mast cell stabilising compound [42] that works by acting against the flux of chloride ion in the mast cells. Though it is not used commonly in asthmatic patients, it is the main therapy in case of other allergies like ocular allergy [42].
2.3.2 Th2-High Eosinophilic Asthma Versus Th2-Low Neutrophilic Asthma
Depending on the nature of infiltrated cells inside the airways, there can be two major types of asthma. One is the allergic mild to moderate eosinophilic asthma which is regulated by Th2 cells. It has an early onset and occurs in children at a young age. The other more severe type is the neutrophilic asthma which is controlled by Th1 and Th17 cells. They are mediated by two different pathways and they regulate different cytokines and co-stimulatory molecules [43]. It is also called Th2-high asthma and Th2-low asthma. The person having neutrophilic Th2-low asthma will not respond when they are given the same drug which is used for treating eosinophilic inflammation. Therefore, the approach for treating asthma is gradually changing from one size for all to personalised therapeutic. Hence, it is very important to study the pathobiology in asthma.
The most commonly defined phenotype of asthma is the IgE-regulated eosinophilic asthma.
2.3.2.1 Eosinophilic Asthma/Th2-High Asthma
Airway eosinophilia accompanied with shredding of the bronchial epithelium is the hallmark of asthma. The major basic protein (MBP) found inside the eosinophil granules is toxic to the respiratory epithelial barrier. It results in desquamation followed by destruction of the ciliated cells. Heightened MBP levels in the sputum are an important marker of asthma [44]. Eosinophilia can be identified by measuring the eosinophil count in the sputum. Reduction in eosinophilia also indicates decrease in exacerbations that happen in asthma [45]. Reduced apoptosis of eosinophils is noted in asthma. GM-CSF production is primarily the reason behind this. In vitro studies have showed that β2-agonist has a role in lengthening the lifespan of eosinophils and inhibits apoptosis [46]. Hence, it is concluded that in asthma, eosinophilia occurs both in blood and in tissue. Eosinophil maturation that happens in the bone marrow, recruitment of eosinophils by chemokine receptor 3 agonists, eosinophil transition and their survival are the steps that occur in asthma. Therapeutic strategies aimed at inhibiting eosinophil maturation and trafficking are some strategies to combat asthma [47].
Though it was believed that Th2 response is entirely due to Th2, acquired immune cells, the similar response can also be generated through type 2 innate lymphoid cell (ILC2) that secretes Th2 cytokines. However, type 2 response in asthma via ILC2 cells is carried out by interleukin-33 (IL-33) and interleukin-25 (IL-25) cytokines and TSLP. These cytokines, airway epithelial signature proteins, stimulate the ILC2 cells residing in the pulmonary region to produce more IL-5 and IL-13. IL-5 has the major role for mediating the maturation, growth, migration and viability of eosinophils. Cytokine profiles can cluster population in various subsections as they possess diverse patterns in inflammation. The subgroups can be either mixed granulocytic ‘IL-5-high and IL-17F-high’ or eosinophilic ‘IL-4- or IL-13-high’ [48].
This Th2-high asthma accounts for about 50% of asthma which can be of mild to moderate severity. They are receptive to corticosteroids. Overall, this Th2-high asthma encompasses the following features: hypersensitiveness to aeroallergen by IgE, epithelial barrier lining the airways get activated and effector cells like mast cells and basophils come into action which in turn lead to airway remodelling. The second type is Th2-low asthma that shows neutrophilic inflammation. They are non-reactive to glucocorticoids and also not receptive to Th2-high treatment [49]. Th2-low asthma is regulated by Th17 cells. This subgroup of T cell causes emission of cytokine IL-17 and interleukin-22 (IL-22) in the bronchial region. It leads to AHR and hypersecretion of mucus followed by obstruction in the airways. Degree of IL-17 is correlated with the seriousness of disease [50]. These cytokines in Th2-low asthma elevates the growth of airway smooth muscle cell and propels deposition of collagen in the airways. Interleukin-8 (IL-8) guides the recruitment of neutrophils. Transient receptor potential vanilloid 1 (TRPV1) channel expressed in the bronchial region is activated, and this incites the production of more neutrophils [51].
IL-13 signature genes like periostin, chloride channel accessory 1 and serpin β2 are considered to be biomarker in Th2-high asthma. Th2-high asthma shows a unique gene phenotype in airway epithelial cells (AECs). Th2 asthmatic patients have high level of exhaled nitric oxide fraction (FeNO). They also exhibit increased level of eosinophils in blood and sputum. Analysis has showed that there is not much difference in the IL-4, IL-5 and IL-13 levels in both Th2-high and Th2-low asthma. Mucosal C-C motif chemokine ligand 26 (CCL26) gene expression was higher in Th2 asthma. Statistical studies showed that still better biomarkers are required for easy detection at bedside level [52].
Interleukin-5, a central Th2 cytokine, helps egress of immature eosinophils from the bone marrow, activates them and recruits them into the airways, increases their survival and also causes the eosinophil degranulation upon allergen induction. IL-5 is another therapeutic target in asthma. Mepolizumab is an anti-IL-5 monoclonal antibody which is of human origin. Mepolizumab administration is safe and causes a decline in the number of circulating eosinophils in tissues and peripheral blood [53]. Mepolizumab got a FDA approval for treating eosinophilic phenotype asthma. Reslizumab is another FDA-approved IL-5 antagonist, monoclonal anti-IL-5 antibody given in severe asthma [54]. Compared to placebo, IL-5 neutralising reslizumab causes reduction in the eosinophilic counts and also improves the airway obstruction happening in eosinophilic asthma [55]. Another drug undergoing clinical trial is benralizumab. Being a humanised mAb, benralizumab acts on the α-subunit of IL-R receptor IL-5αR. Upon administration, benralizumab also causes a decrease in eosinophilic amount in sputum and peripheral blood. A decrease in asthma exacerbations is also observed [56].
2.3.2.2 Neutrophilic Asthma
In case of severe asthma, the Th1 and Th17 pathways also get activated. This then brings about neutrophilic inflammatory asthmatic response [43]. Upon facing insults from different allergens, various chemotactic factors are released. They recruit the inflammatory cells to the site of injury in the lung. The different types of structural cells in the lung like epithelial cell, endothelial cell and fibroblast also release some mediators that promote inflammation. In asthma, both branches of immune response get involved. B cell and T lymphocytes provide the adaptive immunity. Innate immunity is provided by eosinophils, neutrophils, mast cells, macrophages and dendritic cells [11]. Acute asthma often occurs by viruses. In respiratory virus-induced asthma, increased neutrophilic inflammation is observed. There are incidences of heightened neutrophilic degranulation in sputum of asthmatic patients. Increase in cell lysis was also observed [57]. Interleukin-8 (IL-8) has a role in the neutrophil infiltration and is upregulated in severe asthma. Upon IL-8 stimulation, neutrophils cause transmembrane migration of eosinophils. This in turn leads to neutrophil accumulation in the airways [58]. Detailed analysis of patient’s sputum has showed that there are some sub-phenotypes in asthma. Multivariate analysis of clusters was performed. In mild to moderate sub-phenotype of asthma, there is more eosinophil. However, in case of moderate to severe asthma, there is more predominance of neutrophils [59]. Monocyte-derived immune cells, macrophages, have an unclear role in asthma. They can secrete both pro-inflammatory and anti-inflammatory cytokines. However, it is predicted that the anti-inflammatory cytokine is reduced in asthma [11]. There is another study which showed that alveolar macrophages (AM) are the source of pro-inflammatory cytokine Th17. In OVA-induced allergic mice model, the IL-17 positive cells were primarily CD11b+F4/80+ macrophages. Neutralising IL-17 or depletion of AM diminishes the effect of OVA-induced asthma inflammation and reduces the infiltration of inflammatory cells [60]. Thus, AM has a variety of roles in the context of immune regulation in asthma. It is like a double-edged player, where, on the one hand, it can promote inflammation and on the other hand its anti-inflammatory role can maintain lung homeostasis. Further studies are required to figure out macrophage’s function in different phenotypes of asthma [61].
2.3.3 Asthma: A Complex Syndrome with Multiple Endotypes
Depending on the types of various inflammatory cells in asthmatic sputum, asthma is differentiated into different phenotypes. In addition to eosinophilic and neutrophilic asthma phenotypes, the third phenotype is mixed granulocytic asthma and the last one is steroid-insensitive paucigranulocytic asthma (PGA) [62]. But why do we need such phenotypic and endotypic classifications? In non-Th2 asthma, Th2 cell-targeted asthma therapeutics may not be beneficial [63]. Thus, phenotyping asthma helps in planning better treatment procedure. For doing it, understanding the molecular and cellular mechanism behind this disease is of utmost importance. Thus, a better understanding of these asthma phenotypes will aid in the development of specific biomarkers for each of the phenotypes [64], and phenotype-specific molecules or mechanisms can be targeted for treating asthma.
There are some comorbidities that can further heighten the asthma triggers. Additional disease pathologies found in the upper airway tract are chronic rhinosinusitis, polyposis in nasal areas, allergic rhinitis and so on. These comorbidities in the upper airway tract augment complications and take part in worsening asthma [65]. Hence, it becomes very important to deeply understand the complex pathophysiology of this heterogeneous airway inflammatory disease. Owing to various risk factors, sometimes asthma therapy becomes complicated. It becomes refractory to the standard treatments. One such refractory condition is called steroid-resistant asthma, and this is elaborately discussed in the chapter entitled ‘Targeting Cellular and Molecular Mechanisms in Steroid-Resistant Asthma’ in this book.
Among various comorbid conditions, obesity is found to be an important condition in recent times, thanks to westernisation in food and lifestyle [66]. In mice model, it is noted that the obese mice have airway hyperresponsiveness at an innate level. Such mice also show a heightened allergic response to several asthma-triggering agents [67]. There are many emerging studies which showed that there is some association between asthma and obesity. During obesity, a systemic pro-inflammatory response gets triggered in the adipose tissue. The lung volume capacity is also decreased in obese people with reduction in the peripheral airway diameter, which can further contribute to airway hyperresponsiveness. Alteration in the smooth muscle function was also observed [68]. Another study proposed that the oxidative stress that happens during obesity has a role in augmenting the airway inflammation. These also reduce the efficacy of the inhaled corticosteroids, which are commonly given drugs in asthma [69]. Asthma has also become partly a lifestyle disease.
Western lifestyle also introduced a hygiene hypothesis that has a role in an individual’s susceptibility to asthma. Evidences have shown that children who were given antibiotics at a sensitive childhood age have a genetic predisposition to develop asthma [70].
2.4 Nitro-Oxidative Stress in Asthma Pathogenesis
2.4.1 Oxidative Stress
The superoxide (O2−), hydroxyl radical and hypochlorous acid that are reactive oxygen species (ROS) are constantly formed in the lung for physiological reasons to guard against a number of environmental irritants including microbes. However, in asthmatic condition, there is a release of more ROS into the airways by various recruited inflammatory cells like Th2 cells, eosinophils, etc. The dominant role of ROS in causing various asthma features has been demonstrated in earlier literature [71, 72]. On the other hand, the lung also produces antioxidants. Once the balance between oxidants and antioxidants is disturbed, radicals can both damage the cell and initiate, amplify the inflammatory response. In the cell, these radicals damage DNA, lipids, proteins and carbohydrates. The lipid peroxidation product, 8-isoprostane, is a known bronchoconstrictor [71]. Similarly, oxidative DNA damage product, 8-hydroxydeoxyguanosine, also leads to bronchoconstriction. Excessive oxidative free radicals can also induce apoptosis of airway epithelium. This could further induce the airway remodelling by activating epithelial mesenchymal trophic unit (EMTU) to cause further airway hyperresponsiveness. In addition, oxidative free radicals combine with nitric oxide (NO) free radicals to form peroxynitrite, which is a powerful bronchoconstrictor. Overall, oxidative stress seems to play a detrimental role in asthma pathogenesis. Despite being beneficial in animal models of asthma, exogenous antioxidants have not shown any drastic improvement in asthma. Such discrepancy could either be due to species difference or it could be also due to physiological benefits of ROS. In any event, using antioxidants in lung diseases becomes controversial as it may also lead to development of lung cancer.
2.4.2 Mitochondrial Dysfunction in Asthma
On the one hand, oxidative stress has a major role in causing airway epithelial injury, and on the other hand, mitochondria are the major sources of reactive oxygen species. So theoretically, one can expect dysfunctional mitochondria in asthma pathogenesis. Our lab and others have demonstrated such mitochondrial dysfunction in bronchial epithelium of asthmatic subjects [73, 74]. Further, we have demonstrated how 15-lipoxygenase (15-LOX) leads to mitochondrial dysfunction. Though 5-LOX has been studied in detail in asthma pathogenesis, detailed studies of 15-LOX revealed more interesting observation in asthma pathogenesis [75, 76]. Further, 15-LOX inhibitors like esculetin and baicalein have attenuated asthma features [74]. Also, we have further demonstrated that mesenchymal stem cell can replace the dysfunctional mitochondria present in airway epithelia which can in turn cause attenuation of asthmatic features [77]. All these indicate that improvement of mitochondrial function in airway epithelia could be an attractive therapeutic strategy. This also emphasises the importance of airway epithelia such that epithelial protective agents could be an effective therapeutic strategy in the future. Traditionally, airway epithelium was considered as a victim of immune cells. In contrast to earlier belief, a number of recent literatures indicate that a healthy airway epithelium is crucial to have a healthy lung.
2.4.3 Nitric Oxide (NO)
Nitric oxide is produced from L-arginine, catalysed by constitutive nitric oxide synthases (cNOS), namely, endothelial NOS (eNOS) and neuronal NOS (nNOS), and their isoform, inducible nitric oxide synthase (iNOS). Under normal physiological conditions, NO produced from cNOS functions as a bronchodilator and has an anti-inflammatory action in the airways through the cyclic guanosine monophosphate (cGMP) pathway [78]. NO produced by iNOS reacts with superoxide and leads to peroxynitrite radical formation which has negative effects like inflammation and bronchoconstriction in the airways [78]. L-arginine is also utilised as a substrate by arginase, the last hepatic urea cycle enzyme, which catalyses the conversion of L-arginine to L-ornithine and urea. It also occurs in non-hepatic tissues like the airways and has two isoforms – arginase1 and arginase 2. cNOS, iNOS and arginase vie for their common substrate L-arginine. A rise in iNOS expression in asthmatic condition limits the bioavailability of L-arginine for cNOS. In asthma, there is also an upregulation of arginase by transforming growth factor β (TGFβ) and Th2 cytokines like IL-4 and IL-13, reducing the substrate availability of cNOS [79]. This competition for substrate causes depletion of L-arginine in the asthmatic airways. Previous work from our lab has shown how L-arginine supplementation at high doses can alleviate allergic airway inflammation [78]. The harmful form of NO, made from arginine utilisation by iNOS, causes L-arginine depletion and promotes peroxynitrite production. Peroxynitrite causes mitochondrial dysfunction by haem degradation in cytochrome c oxidase, a crucial mitochondrial electron transport chain enzyme. Our lab has demonstrated that L-arginine administration is able to lower peroxynitrite generation and rescue the impaired mitochondrial function in allergic airway inflammation [80].
Asymmetric dimethylarginine (ADMA), an endogenous NOS antagonist, is increased in asthmatic condition. It is a product of protein methylation formed during post-translational modification catalysed by protein methyltransferases. ADMA is a naturally occurring uncoupler for the all NOS isoforms, halting NO production by the NOS, which promotes superoxide production. Besides, an increased level of ADMA is also found to upregulate arginase activity [81]. Rise in arginase activity boosts L-ornithine production that functions as a precursor for polyamines and L-proline. Polyamines are involved in cell proliferation, whereas L-proline promotes collagen production and fibrosis [79]. Indeed, ADMA-induced arginase upregulation is found to contribute to collagen deposition and airway hyperresponsiveness in murine lungs [81].
NO, derived from iNOS activity, is highly prevalent in asthmatics. However, considerable amount of variation has been found in the FeNO in asthma patients, with some patients having FeNO levels within normal range. Nevertheless, a major population of asthmatics with high FeNO levels represent a distinct phenotype defined by their higher arginine metabolism and a more severe form of the disease. The higher arginine metabolism is attributed to the increased levels of iNOS and arginase present in their airways [82]. This highlights the clinical relevance of understanding the role of arginine biology in asthma.
2.5 Airway Remodelling in Asthma: The Knowledge that Will Shape the Future Treatment
Now, it is very clear that the airway epithelium works beyond mere mechanical barrier in the airways. It indeed maintains healthy lung homeostasis by protecting against obnoxious particles entering the airways [83]. The injury to the airway epithelium is traditionally believed as a converging event of various inflammatory pathways. In contrast to this view, recent evidences indicate that epithelial injury and its subsequent airway remodelling changes can be parallel set of events happening along with airway inflammation. This is the possible reason why anti-inflammatory agents could not reduce the initiation of irreversible airway remodelling changes. The challenges faced by the epithelium every minute are commendable, and its structural alteration leads to ‘airway remodelling’ [83].
Defining ‘airway remodelling’ is very challenging. It is a collective term to indicate the overall changes happening in the airway wall. Almost every cell type in the airways increases in number and size that further causes obstruction in the airway diameter. Airway narrowing, which occurs in early stages of asthma, seems to be physiological and temporary, with smooth muscle contraction and mucosal oedema. However, thanks to the airway remodelling changes, airway narrowing that occurs in late stages of asthma seems to be a combination of physiological and anatomical changes. As a result, even without allergen exposure, at a later stage, asthmatic patients constantly have airway narrowing. As a consequence, there occurs a continuous difficulty in breathing in later stage compared to earlier stages. Airway remodelling is thus a characteristic hallmark of asthma that has a notable functional implication in asthma pathogenesis.
Epithelial alterations in asthmatic airway remodelling are characterised by features like epithelial cell shedding, goblet cell hyperplasia and metaplasia, loss of ciliated cells [84] and compensatory hypertrophy and hyperplasia of epithelial cells. Clinically, the extent of epithelial injury is correlated to airway hyperresponsiveness [83]. Sub-epithelial fibrosis, another important feature of airway remodelling, is due to the dense deposition of a variety of extracellular matrix (ECM) proteins like proteoglycan, tenascin and collagens, predominantly in the lamina reticularis [85]. The deposition of ECM proteins in sub-epithelial regions is facilitated by the transformation of fibroblasts to myofibroblasts. Myofibroblasts are a kind of hybrid cells that have the functions of both muscle and fibroblasts. These hybrid cells are majorly responsible for the overall thickening of airway wall. The imbalance between proteases and antiproteases may favour a profibrotic balance. A number of matrix metalloproteinases (MMPs) like MMP-2, MMP-3, MMP-9 and MMP-12 are seen to have a role in asthmatic airway remodelling [84]. Hypertrophy and hyperplasia of airway smooth muscle cells (ASM) are other key features in airway remodelling. This leads to prominence of smooth muscle mass that has been correlated to clinical severity and duration of asthma. Hypertrophy and hyperplasia seem to comprehend both the small and large airways. Distinctly, various molecular mechanisms, involving pro-inflammatory cytokines, chemokines and ECM proteins, can be attributed to causing hyperplasia or hypertrophy [86]. The goblet cell and mucous gland hyperplasia leads to bronchial mucous plugging [83, 84] in the airways of chronic asthma. This causes enhanced production of sputum, airway narrowing and congestion from sputum secretion followed by increase in the thickness of airway walls [86]. The interstices and folds of the airway surface are filled with liquid and mucus. These features could be conducive, leading to the narrowing of the airways by amplifying the surface tension at the air-liquid interface. Angiogenesis, a part vascular remodelling, includes increased vascularity, vasodilatation, angiogenesis and leakage in microvascular region [84]. Various cytokines, mediators released from mast cells like histamine, leukotriene B4 and prostaglandins, hypoxia-inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF) [84] are important factors contributing to angiogenesis.
Even though it was believed that inflammation-induced airway remodelling occurs via epithelial injury, the incidences of airway remodelling changes even in young children indicate the possibility of inflammation and its subsequent repair-induced airway remodelling. This further reemphasises the need to explore the possible genes involved in airway remodelling happening in asthma pathogenesis [83, 84, 87]. More research is required to explore the possible surrogate markers of future airway remodelling that could help us to segregate and treat high-risk populations at early stages.
2.6 Non-pharmacological Intervention for Asthma: Bronchial Thermoplasty
Medication is important in the treatment of asthma, to prevent and control asthma attacks. However, many people like to do something more than just taking medications. Such non-pharmacologic approaches for asthma like focused breathing techniques, pulmonary rehabilitation, avoiding triggers and bronchial thermoplasty [88] have a significant, yet underappreciated, role. In asthmatic patients who are unresponsive to the medical therapies and have evidence of airway remodelling, bronchial thermoplasty is carried out. Those patients not only exhibited long-term improvement in the quality of life but also showed reduced severity of exacerbations and health-care utilisation.
Asthma is characterised by the sub-epithelial deposition of collagen and remodelling of the bronchial architecture. In late asthma, permanent airway narrowing and airflow obstruction in asthmatic patients further leads to increase in the airway smooth muscle mass. This causes a gradual decline in lung functions [89]. Hence, a new concept of bronchial thermoplasty (BT) has been developed [88,89,90] that intents to decrease the airway smooth muscle mass and sub-epithelial fibrosis. The objective of bronchial thermoplasty is to modify the airway remodelling and diminish the bronchial constriction, thus ameliorating asthma symptoms. Thus, thermoplasty is a new therapeutic option that has to be used along with other pharmacological options in the treatment of uncontrolled severe asthma.
When thermal energy is applied to airway wall in a controlled manner, it heats the tissue that causes a reduction in the airway smooth muscle mass. A thermal energy of 65 °C temperature is delivered through bronchial thermoplasty system [90]. It comprises a sterile, single-use catheter and a radiofrequency electrical generator. BT targets for treating the distal intra-parenchymal airways up to the main stem bronchi, down to airways 3 mm in diameter. BT promotes a marked improvement in the quality of life, along with a decrease in steroid use and reduced number of patients visiting the emergency room with exacerbations. The improvement monitored is not short term; rather, it seems to be committed for a long-term positive impact. A large number of controlled human trials have shown FEV1 improvements along with improvements in the lung function [91]. However, thermoplasty-treated patients have shown adverse effects in the form of worsening and infection of the upper respiratory tract occurring during the first week of the thermoplasty session.
Although BT seems to be tedious, it is an attractive strategy that specifically targets ASM and improves the standard of asthma-related life. Therefore, future studies are called for in order to explore the possible mechanisms behind the improvements. There is also a pressing need to know the asthmatics’ phenotypes that get the maximal benefits from BT as no uniform improvement has been seen among asthmatics.
2.7 Tailoring the Patient Needs Via ‘Personalised Approach’ in Asthma
Now that we are living in an era of ‘magic bullets’, a personalised approach to asthma will undoubtedly be a fairy tale for asthmatic patients who are striving to cope up with the difficulties. Personalised medicine is an emerging approach (opposite of the approach ‘one size fits all’) to treat and prevent disease by assessing the individual’s variability in genes, environment and psychosocial characteristics.
Now we have moved from traditional gross phenotypic classification of asthma to refined molecular classification called endotypic classification [92]. After correlating with clinical and cellular phenotypes, various endotypes have been demonstrated in asthmatic patients in the form of clusters. Such endotypic classification is gradually being refined with aid from other technological advancements like analysis of genes and proteins through genomic, microbiolomic and metabolonomic strategies [92]. As a result, we can pinpoint the dominant biochemical pathways that are responsible for the appearances of symptom in a given patient. Thus, this approach can be called ‘treating the right patient with the right drug at the right time’ [93]. Biomarkers are surrogate measures that refer several indicators. They can be measured and evaluated to assess the normalcy of pathological state or any physiological state. Endotypes are thus significantly different forms of classification from phenotypes and distinctly describe the entities of the disease with a defining aetiology and/or a pathophysiological mechanism.
Asthma is considered as an umbrella condition. It comprises numerous distinct diseases (endotypes) caused by distinct pathological mechanisms. Hence, understanding the underlying heterogeneity is very vital. The observable characteristics (phenotype) in asthma are the various physiological, clinical, morphological and biochemical characteristics based upon which medications are provided [51]. All the asthma patients manifest with the similar symptoms of wheezing or squealing, chest tightness and shortness of breath and fatigue and are accompanied by variable airflow obstruction. Despite this similarity in symptoms, there is a huge variation in response to common therapy. One such example of a treatment is omalizumab that works only for IgE-dominant asthmatic patients [94]. The mechanism of action for omalizumab is focused predominantly on reducing the circulatory IgE. This further inhibits its binding to FcεRI receptors of mast cells and basophils. Sometimes, omalizumab treatment results in exacerbations of symptoms. Similar to IgE, there are many other biomarkers like sputum eosinophils, sputum neutrophils, blood eosinophil count and fractional exhaled nitric oxide. These biomarkers could help respiratory clinicians to subcategorise asthmatic patients like eosinophil-dominant airway inflammation and neutrophil-dominant airway inflammation. Such sub-classification can in turn help clinicians to choose corticosteroid for eosinophilic-dominant patients. Similarly, some biomarkers like periostin has been used as a promising surrogate marker of severe asthma [51]. Hence, it has become very crucial to look for more generic medicines instead of population-based medications. Thus, with the available huge number of biologic agents, a befitting endotypic classification with well-characterised biomarkers is essential to correlate the molecular data to phenotypes and provide tailored medicines for asthma patients.
2.8 Conclusions and Future Perspectives
Asthma is not a single entity; instead, it’s a heterogeneous, complex disorder with overlapping syndromes which makes it difficult to treat. The traditional approach of ‘blockbuster drugs’ to treat asthma is losing their edge as the drugs available in the markets are unable to alter the course of the disease. Thus, there is a pressing need to adopt the ‘personalised approach’ based on the endotype-driven classification of asthma. In this report, we have elucidated the exigency to understand the underlying disease mechanisms in different individuals with different endotypic asthma. We have also presented the idea of various cellular and molecular drivers of allergic airway inflammation that can be targeted to design novel therapies in the near future. The concept of asthma has continuously evolved, from being presumed to be an intrinsic smooth muscle abnormality of the airways to the notion accrediting the ‘inflammation theory’ for the asthmatic diathesis. Recently, the researchers are giving weightage to the concept of ‘airway remodelling’ as the primary event responsible for effectuating asthma. We have also highlighted the important facet of airway remodelling. A milieu of factors is involved in mediating the airway structural alterations including cytokines (IL-5, IL-13 and TGF-β), growth factors (VEGF) and MMPs (MMP-9, MMP-12). Targeting these genes will undoubtedly present us with a curative pharmacological intervention in the immediate future. However, caution is required in targeting MMPs as it could create havoc in lung homeostasis as they are required to act against various microbes that are entering into the airways from the environment.
Steroid-resistant asthma handicaps the situation of treating patients with corticosteroids and has emerged as a global problem for the clinicians. With the various inconclusive underlying mechanisms, like neutrophilia, obesity, bacterial or viral infection, etc., that contribute to the steroid refractory condition, it has become obligatory to understand the mechanisms intricately in order to identify novel therapeutic treatments. We have discussed about steroid-resistant asthma elaborately in the chapter entitled ‘Targeting Cellular and Molecular Mechanisms in Steroid-Resistant Asthma’. The expenses of asthma due to frequent hospitalisations and the intangible expense in terms of reduced standard of living have increased due to poor management and knowledge of the exact underlying mechanism. Future research should focus on personalisation of anti-asthmatic therapeutic strategy to satiate the individual needs based on the distinct disease endotypes.
References
World Health Organisation (2020) Asthma. https://www.who.int/news-room/q-a-detail/asthma Accessed 10 Sep 2020
Dharmage SC, Perret J, Custovic A (2019) Epidemiology of asthma in children and adults. Front Pediatr 7:246
Masoli M, Fabian D, Holt S et al (2004) The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59:469–478
Lugogo NL, Kraft M (2006) Epidemiology of asthma. Clin Chest Med 27:1–15
Smith KR (2000) Inaugural article: national burden of disease in India from indoor air pollution. Proc Natl Acad Sci U S A 97:13286–13293
Romanet-Manent S, Charpin D, Magnan A, Lanteaume A, Vervloet D, EGEA Cooperative Group (2002) Allergic vs nonallergic asthma: what makes the difference? Allergy 57:607–613
Robinson DS, Hamid Q, Ying S et al (1999) Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326:298–304
Pearce N, Pekkanen J, Beasley R (1999) How much asthma is really attributable to atopy? Thorax 54:268–272
Toskala E, Kennedy DW (2015) Asthma risk factors. Int Forum Allergy Rhinol 5:S11–S16
Boushey HA, Fahy JV (1995) Basic mechanisms of asthma. Environ Health Perspect 103:229–233
Barnes PJ (2017) Cellular and molecular mechanisms of asthma and COPD. Clin Sci 131:1541–1558
Cazzola M, Segreti A, Matera MG (2010) Novel bronchodilators in asthma. Curr Opin Pulm Med 16:6–12
Cazzola M, Rogliani P, Calzetta L, Matera MG (2019) Bronchodilators in subjects with asthma-related comorbidities. Respir Med 151:43–48
Barnes PJ (2013) Theophylline. Am J Respir Crit Care 188:901–906
Alangari AA (2014) Corticosteroids in the treatment of acute asthma. Ann Thorac Med 9:187–192
Trinh HKT, Lee SH, Cao TBT, Park HS (2019) Asthma pharmacotherapy: an update on leukotriene treatments. Expert Rev Respir Med 13:1169–1178
Frey A, Lunding LP, Ehlers JC et al (2020) More than just a barrier: the immune functions of the airway epithelium in asthma pathogenesis. Front Immunol 11:761
Gill MA (2012) The role of dendritic cells in asthma. J Allergy Clin Immunol 129:889–901
Kuipers H, Lambrecht BN (2004) The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol 16:702–708
Idzko M, Hammad H, van Nimwegen M et al (2006) Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest 116:2935–2944
Marciniak A, Camp SM, Garcia JGN, Polt R (2018) An update on sphingosine-1-phosphate receptor 1 modulators. Bioorg Med Chem Lett 28:3585–3591
Zhao ST, Wang CZ (2018) Regulatory T cells and asthma. J Zhejiang Univ Sci B 19:663–673
Nelson RK, Bush A, Stokes J et al (2020) Eosinophilic asthma. J Allergy Clin Immunol Pract 8:465–473
Moran A, Pavord ID (2020) Anti-IL-4/IL-13 for the treatment of asthma: the story so far. Expert Opin Biol Ther 20:283–294
Ingram JL, Kraft M (2012) IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol 130:829–844
Majumdar S, Ghosh A, Saha S (2018) Modulating interleukins and their receptors interactions with small chemicals using in silico approach for asthma. Curr Top Med Chem 18:1123–1134
Ridolo E, Pucciarini F, Nizi MC et al (2020) Mabs for treating asthma: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab. Hum Vaccin Immunother:1–8
Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW (2016) A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol 170:122–131
Corren J, Busse W, Meltzer EO et al (2010) A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med 181:788–796
Kasaian MT, Marquette K, Fish S et al (2013) An IL-4/IL-13 dual antagonist reduces lung inflammation, airway hyperresponsiveness, and IgE production in mice. Am J Respir Cell Mol Biol 49:37–46
Venkataramani S, Low S, Weigle B et al (2018) Design and characterization of Zweimab and Doppelmab, high affinity dual antagonistic anti-TSLP/IL13 bispecific antibodies. Biochem Biophys Res Commun 504:19–24
Hambly N, Nair P (2014) Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med 20:87–94
Pelaia G, Canonica GW, Matucci A, Paolini R, Triggiani M, Paggiaro P (2017) Targeted therapy in severe asthma today: focus on immunoglobulin E. Drug Des Devel Ther 11:1979–1987
Gould HJ, Sutton BJ (2008) IgE in allergy and asthma today. Nat Rev Immunol 8:205–217
Busse WW, Humbert M, Haselkorn T et al (2020) Effect of omalizumab on lung function and eosinophil levels in adolescents with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol 124(2):190–196
Hu J, Chen J, Ye L, Cai Z, Sun J, Ji K (2018) Anti-IgE therapy for IgE-mediated allergic diseases: from neutralizing IgE antibodies to eliminating IgE+ B cells. Clin Transl Allergy 8:27
Krystel-Whittemore M, Dileepan KN, Wood JG (2016) Mast cell: a multi-functional master cell. Front Immunol 6:620
Brightling CE, Bradding P, Symon FA et al (2002) Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 346:1699–1705
Bradding P, Walls AF, Holgate ST (2006) The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol 117:1277–1284
Zhang T, Finn DF, Barlow JW, Walsh JJ (2016) Mast cell stabilisers. Eur J Pharmacol 778:158–168
Asfour MH, Kassem AA, Salama A, Abd El-Alim SH (2020) Hydrophobic ion pair loaded self-emulsifying drug delivery system (SEDDS): a novel oral drug delivery approach of cromolyn sodium for management of bronchial asthma. Int J Pharm 585:119494
Sinniah A, Yazid S, Bena S et al (2019) Endogenous Annexin-A1 negatively regulates mast cell-mediated allergic reactions. Front Pharmacol 10:1313
Pelaia G, Vatrella A, Busceti MT et al (2015) Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediat Inflamm 2015:879783
Frigas E, Gleich GJ (1986) The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol 77:527–537
Green RH, Brightling CE, McKenna S et al (2002) Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 360:1715–1721
Kankaanranta H, Lindsay MA, Giembycz MA et al (2000) Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol 106:77–83
Rosenberg HF, Phipps S, Foster PS (2007) Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 119:1303–1310
Sven S, Scheers H, Marijsse G et al (2015) Th2-high asthma: a heterogeneous asthma population? Clin Transl Allergy 5:O1
Fahy JV (2015) Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol 15:57–65
Robinson D, Humbert M, Buhl R et al (2017) Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy 47:161–175
Kuruvilla ME, Lee FEH, Lee GB (2019) Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 56:219–233
Pavlidis S, Takahashi K, Kwong FNK et al (2019) “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J 53:1800938
Garrett JK, Jameson SC, Thomson B et al (2014) Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol 113:115–119
Maselli DJ, Velez MI, Rogers L (2016) Reslizumab in the management of poorly controlled asthma: the data so far. J Asthma Allergy 9:155–162
Castro M, Mathur S, Hargreave F et al (2011) Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 184:1125–1132
Tabatabaian F, Ledford DK, Casale TB (2017) Biologic and new therapies in asthma. Immunol Allergy Clin N Am 37:329–343
Wark PAB, Johnston SL, Moric I et al (2002) Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 19:68–75
Nakagome K, Matsushita S, Nagata M (2012) Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol 158:96–102
Moore WC, Hastie AT, Li X et al (2014) Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 133:1557–1563
Song C, Luo L, Lei Z et al (2008) IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol 181:6117–6124
Balhara J, Gounni AS (2012) The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol 5:605–609
Tliba O, Panettieri RA Jr (2019) Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol 143:1287–1294
Ray A, Oriss TB, Wenzel SE (2015) Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol 308:L130–L140
Wenzel SE (2006) Asthma: defining of the persistent adult phenotypes. Lancet 368:804–813
Licari A, Brambilla I, De Filippo M et al (2017) The role of upper airway pathology as a co-morbidity in severe asthma. Expert Rev Respir Med 11:855–865
Agrawal A, Mabalirajan U, Ahmad T, Ghosh B (2011) Emerging interface between metabolic syndrome and asthmaAm J Respir. Cell Mol Biol 44:270–275
Shore SA, Johnston RA (2006) Obesity and asthma. Pharmacol Ther 110:83–102
Beuther DA, Weiss ST, Sutherland ER (2006) Obesity and asthma. Am J Respir Crit Care 174:112–119
Holguinn F, Fitzpatrick A (2010) Obesity, asthma, and oxidative stress. J Appl Physiol 108:754–759
Droste JHJ, Wieringa MH, Weyler JJ et al (2000) Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy 30:1548–1553
Barnes PJ, Chung KF, Page CP (1998) Inflammatory mediators of asthma: an update. Pharmacol Rev 50:515–596
Nadeem A, Chhabra SK, Masood A et al (2003) Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 111:72–78
Mabalirajan U, Dinda AK, Kumar S et al (2008) Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J Immunol 181:3540–3548
Mabalirajan U, Dinda AK, Sharma SK et al (2009) Esculetin restores mitochondrial dysfunction and reduces allergic asthma features in experimental murine model. J Immunol 183:2059–2067
Mabalirajan U, Rehman R, Ahmad T et al (2013a) 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci Rep 3:1540
Mabalirajan U, Rehman R, Ahmad T et al (2013b) Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci Rep 3:1349
Ahmad T, Mukherjee S, Pattnaik B et al (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 33:994–1010
Mabalirajan U, Ahmad T, Leishangthem GD et al (2010a) Beneficial effects of high dose of L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J Allergy Clin Immunol 125:626–635
Meurs H, Zaagsma J, Maarsingh H et al (2019) Recent patents in allergy/immunology: use of arginase inhibitors in the treatment of asthma and allergic rhinitis. Allergy 74:1206–1208
Mabalirajan U, Ahmad T, Leishangthem GD et al (2010b) L-arginine reduces mitochondrial dysfunction and airway injury in murine allergic airway inflammation. Int Immunopharmacol 10:1514–1519
Tajti G, Papp C, Kardos L et al (2018) Positive correlation of airway resistance and serum asymmetric dimethylarginine (ADMA) in bronchial asthma patients lacking evidence for systemic inflammation. Allergy Asthma Clin Immunol 14:2
Xu W, Comhair S, Janocha AJ et al (2017) Arginine metabolic endotypes related to asthma severity. PLoS One 12:e0183066
Fehrenbach H, Wagner C, Wegmann M (2017) Airway remodeling in asthma: what really matters. Cell Tissue Res 367:551–569
Bergeron C, Al-Ramli W, Hamid Q (2009) Remodeling in asthma. Proc Am Thorac Soc 6:301–305
Hough KP, Curtiss ML, Blain TJ et al (2020) Airway Remodeling in asthma. Front Med 7:191
Elias JA (2000) Airway remodeling in asthma.Unanswered questions. Am J Respir Crit Care 161:S168–S171
Ergan-Arsava B, Karakaya G, Firat P et al (2011) Smooth muscle hyperplasia in an asthmatic patient: do we know it all? Respiration 81:152–156
Hall C, Nici L, Sood S et al (2017) Nonpharmacologic therapy for severe persistent asthma. J Allergy Clin Immunol Pract 5:928–935
Singh SK, Tiwari KK (2017) Bronchial thermoplasty: a non-pharmacological approach. Clin Respir J 11:13–20
Wahidi MM, Kraft M (2012) Bronchial thermoplasty for severe asthma. Am J Respir Crit Care 185:709–714
Laxmanan B, Hogarth DK (2015) Bronchial thermoplasty in asthma: current perspectives. J Asthma Allergy 8:39–49
Svenningsen S, Nair P (2017) Asthma endotypes and an overview of targeted therapy for asthma. Front Med 4:158
Weiss ST (2012) New approaches to personalized medicine for asthma: where are we? J Allergy Clin Immunol 129:327–334
Godar M, Blanchetot C, de Haard H et al (2018) Personalized medicine with biologics for severe type 2 asthma: current status and future prospects. MAbs 10:34–45
Acknowledgements
This work was supported by the projects MLP137 (MISSION LUNG) at CSIR-Indian Institute Chemical Biology, Council of Scientific and Industrial Research, Govt. of India. SS, JD, AR and AJ acknowledge the Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, for their PhD registrations.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ray, A., Singh, S., Dutta, J., Mabalirajan, U. (2021). Targeting Molecular and Cellular Mechanisms in Asthma. In: Dua, K., Löbenberg, R., Malheiros Luzo, Â.C., Shukla, S., Satija, S. (eds) Targeting Cellular Signalling Pathways in Lung Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-33-6827-9_2
Download citation
DOI: https://doi.org/10.1007/978-981-33-6827-9_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6826-2
Online ISBN: 978-981-33-6827-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)