Abstract
A "phase" is an important physical identity of any material. Pure materials undergo phase transition when the heat is absorbed or released at a constant temperature known as melting or boiling point temperature. This phase transition is associated with "latent heat", which researchers are trying to exploit in multiple ways for different applications. The temperature range for these applications is such that selected materials undergo a phase change. Thus, their latent heat comes into play. There are various applications of these phase change materials (PCMs) from low-temperature passive heating/cooling and thermal management to high-temperature storage for solar thermal systems. PCM implementation requires knowledge of their types, properties, thermal characterization procedure, and property enhancement techniques, to map their suitability for a particular application. An assessment follows their implementation. There are different models for simulating the phase change process for different configurations, for assessing the impact of PCM incorporation. PCM caters to a vast arena of thermal applications and is used for either to enhance thermal cooling performance or to enhance thermal efficiency by wisely exploiting the energy storage potential. Here, we present mathematical modeling and different computational approaches for studying PCM-based systems. The general and most preferred practices in PCMs are discussed along with the different approaches of handling computational grids. Various methods based on discerning the energy equations are discussed along with phase field and volume of fluid methods. Also, the sophisticated commercial/research-based tools available for modeling the phase change materials are detailed. Such a comprehensive overview will be helpful for researchers/engineers looking to realize PCM, especially for energy and building applications.The later part of the chapter provides a comprehensive review of PCMs, followed by a detailed description of various applications and research prospects. This study discusses both heating and cooling applications of PCMs. PCM implementation in buildings can result in energy savings of up to 30%. PCMs application for thermal regulation of batteries, electronic circuits, and photovoltaic module are also discussed. Heat transfer enhancement techniques required to increase PCM dispatch ability, with suitable case studies, have been discussed in detail. The application of PCM in wearable devices to sustain extreme temperatures is still uncharted and can prove to be handy for thermal management and providing sustainable solutions. PCM implementation for solar thermal applications as high-temperature storage material has been discussed in this present study. In summary, this chapter provides a holistic review of different PCM applications and their modeling. It highlights the research, required to be carried out to overcome the shortcomings of PCM implementation to form a feasible solution for various problems. This study also highlights the importance of PCMs in energy conservation, thus contributing to a reduction in CO2 emissions and climate change.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Latent thermal energy storage
- PCM
- Passive systems
- PCM characterization
- Thermal modeling
- Temperature regulation
- Energy storage systems
- Energy conservation and management
13.1 Introduction
Energy consumption around the world is increasing, while the resources available for sustenance of life on earth are limited. For ensuring the supply of energy to our future generations, it is vital to realize the necessity of conserving energy and use it judiciously. The researchers are working on multiple solutions to this problem. One such solution can be using Phase Change Materials (PCMs). When material transforms from one phase to another, it absorbs or releases energy which is called 'latent heat". The temperature of phase change is such that it may be exploited for the application. Another significant advantage is a large amount of heat associated with the phase change, which makes the storage size compact, due to its high energy density. There are several applications that are being discussed here to compile the scattered information available in the literature. The general perception, as seen in most of the studies, is they target an application, thus, limiting the scope of the study. This chapter, however, discusses various applications of phase change materials starting from environmental management to human comfort solutions, some of which are still not discussed elsewhere. This study focuses on imparting knowledge on simulation studies, methods to simulate the phase change materials followed by the experimental studies being carried out for buildings energy conservation, and various storage systems for low, medium, and high-temperature applications. For understanding the process, it is first essential to understand the concept of phase change material and its working.
13.1.1 PCM and Its Working
Materials undergo phase transition when the heat is absorbed or released. This occurs mostly at a constant temperature, for pure substances, known as the melting or boiling point, depending it is a solid–liquid or liquid–gas phase transition. The process solid–liquid phase transition can be understood from Fig. 13.1.
In the solid phase, the material absorbs heat, and consequently, its temperature rises. This continues until melting temperature is reached. Further, the temperature stops rising, and the energy supplied is stored in the form of internal energy, which results in phase change or melting of the material. This heat, absorbed by the material to undergo a phase change, is referred to as latent heat of the material. The reason for researchers being interested in this latent heat is its magnitude which is much higher compared to the sensible heat. Another important aspect is that this energy is absorbed or released at constant or over a narrow range of temperature. This is useful for many applications which are discussed subsequently in this chapter. The following equations can represent the heat stored for sensible and latent heat storage:
where Qsensible is the amount of heat stored by sensible heat storage materials with subsequent rise/fall in temperature, denoted by ΔT as shown in Eq. 13.1. The heat stored in latent heat storage material, Qlatent, is given by the product of mass and latent heat capacity of the material at the phase change temperature (Eq. 13.2).
13.1.2 Advantages of Using a PCM
As discussed already, the advantage of using PCM is their large heat storage capacity and storing/retrieval of heat at an almost constant temperature known as the phase change temperature. Due to the enormous magnitude of this heat that can be stored, the volume of storage reduces substantially—thus saving on the mass of material required and space to store it. The constant temperature heat storage adds to the application part of PCMs, where the temperature is critical for the heat source. For example, in the case of the solar thermal power plant, the heat can be retrieved at a constant temperature from the storage, which can be transferred to the heat transfer fluid in the cycle.
Another advantage is explored in case of the building where a range of temperature variation is narrow, and these PCMs can be suitable for maintaining the comfort temperature and reduce the temperature fluctuations within the building thereby, resulting in energy savings. Similarly, advantages are observed in the case of PV panels, batteries, and electronic circuits where PCMs can be advantageous in controlling the temperature and avoiding overheating.
13.1.3 Scope of Present Study
The scope of this study is first to classify different thermal energy storage materials. Then comparing the suitability of their application, adjudge them based on their properties and highlight various research prospects that exist for PCMs to become a commercially viable solution soon. This study also highlights different approaches used to model PCMs followed by experimental analysis carried out by many researchers on various PCM applications. The potential of PCM application varies from environment conservation to various engineering solutions that may be usefully implemented in many industrial applications. This study would also discuss different commercial products available and that can be developed using PCMs.
13.2 PCM as Thermal Energy Storage Material
13.2.1 Classification
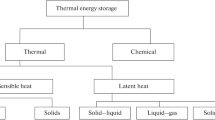
The energy storage materials can be classified, as shown in Fig. 13.2. This study, however, focuses on different phase change materials. The phase transition can be solid–liquid, solid–gas, liquid–gas, and solid–solid. It is observed that in all these cases, the energy associated with each can be tremendous. In the case of solid–gas and liquid–gas phase changes, the change in volume is quite high. Thus, solid–liquid phase transitions are of significant concern and studied worldwide. Solid–liquid PCMs are subdivided into two types organic and inorganic based on their chemical composition and chemistry. The thermal conductivity of these PCMs is observed to be low in general. Thus, researches are being carried out for PCM enhancement to increase their effective thermal conductivity. This is discussed in detail in Sect. 13.3 of this chapter. It is also observed that organic PCMs seem more promising due to their cyclic stability and non-corrosive nature compared to inorganic PCMs; however, their cost is also relatively higher. Inorganic PCMs, on the other hand, are cheap but they also have supercooling effect. It is defined as the change in melting and solidification temperature. It is observed that the material needs to be cooled below its freezing point to initiate the nucleation to take place, resulting in the starting of the solidification.
Storage material classification [1]
The research is being carried out to find suitable nucleating agents that may be added to avoid the supercooling to take place in case of inorganic PCMs. Another category of PCMs is eutectics which can be either organic or inorganic. It is the mixture of two or PCMs mixed such that they melt at ordinary melting temperature. These eutectics can be cheap and more stable. Thus, these materials are also researched for various applications.
13.2.2 Parameters for PCM Selection
Selection of PCM is of crucial importance and is dependent on the type of application for which it is to be applied. For example, PCMs with low thermal conductivity are suitable for cooling load reduction in hot climatic condition and should be used on the outer side exposed to the sun [2]. Whereas, for cold climatic conditions, higher thermal conductivities are desirable. With higher thermal conductivity, the charging rate of PCM during the day is faster, and it readily discharges during the night by releasing heat to the inside. Thus, the inside temperature can be maintained warmer for a longer duration during the off-sunshine hours, reducing the auxiliary heating load within the building [3]. Therefore, PCM properties are of prime importance. Another critical parameter for PCM is its phase change temperature and temperature of application. It is necessary to map the PCM based on the temperature of use as suitable PCM would result in better thermal characteristics [4]. Thus, mapping of the right PCM is mandatory for the desired output. Furthermore, in building perspective, if PCMs have a solidification temperature lower than the minimum temperature of the place solidification may not occur. Hence, the material would only behave as sensible heat storage on a subsequent day. Therefore, two significant parameters are the requisite properties of PCM, and the temperature of application based on which PCM should be carefully selected.
13.2.3 Properties and Characterization
PCM properties are of critical importance. The properties of PCM can fall in the following categories:
-
Thermal Properties: Phase change temperature/range, latent heat capacity, specific heat, thermal conductivity, volume change ratio (critical during phase change)
-
Chemical Properties: Cyclic stability, compatibility, corrosion characteristics, phase segregation (must not occur), sub-cooling/supercooling, flammability (should be non-inflammable), environment friendly, and non-polluting
-
Economics: Easily available, low cost, and should be recyclable.
For determining the PCM properties, it is necessary to characterize them. It plays a critical role in terms of PCM selection for design, modeling, and experimentation for various applications. The knowledge available in the literature is limited and also inaccurate in some cases [5]. Another important aspect is the procedure followed for carrying out the characterization. It is observed that the characterization must be carried out as per the application, or else the results may vary [6]. Inorganic PCMs show the tendency of sub-cooling, also referred to as supercooling, i.e., there is a difference in melting and solidification temperature. The latter is lower compared to the prior. This generally goes unreported as in many cases only melting, or solidification cycles are studied and illustrated. In contrast, in actual practice, both melting and solidification cycles must be studied simultaneously to avoid erroneous results in simulations, etc. This phenomenon must be accounted for in the modeling, which is generally neglected, and is of critical importance especially when temperature variation is small, as in the case of buildings, etc. Thus, accurate information on the thermophysical properties of PCMs is critically necessary. The latent heat, specific heat, melting and solidification temperature, and supercooling can be accurately determined by differential scanning calorimeter (DSC). For measuring the thermal conductivity, the thermal conductivity meter is used. The measurement is generally done by two methods, i.e., steady state and transient. The steady-state methods include guarded hot plate and heat flow meter techniques both based on Fourier’s law. This, however, is suitable for only solid materials (PCM, however, exits in dual-phase solid–liquid during the operation) and requires long waiting time period to establish a stable temperature gradient within the specimen. However, with technological advancement, there are other methods also available of thermal conductivity measurement for both solid as well as liquid phases, using steady-state methods, in short duration of time. The transient method measures thermal conductivity within seconds. Thus, these methods are gaining popularity. Different transient techniques used are the transient plane source (TPS), transient hot wire (THW), and laser flash.
13.2.4 PCM Enhancement
PCM enhancement may be referred to as methods to improve the workability of PCMs. Mostly, all PCMs have an inherent problem of poor thermal conductivity. Thus, studies have been carried out for useful thermal conductivity improvement by increasing the surface area with the use of fins [7,8,9] or by addition of nanoparticles [10], graphene, and carbon nanotubes [11], etc. It is necessary to identify the need for enhancement and application. For example, nano-enhanced PCMs may be a viable solution for buildings, but they may not be economically feasible. However, for cooling application in space or enhancement of dispatch, ability for storage application in spacecraft, automobiles, defense systems, water heating, etc., may be feasible as well as affordable.
Another critical aspect is inherent PCM issues such as sub-cooling, stability, their incorporation, etc. Each has a different solution to overcome these problems. For example, to avoid sub-cooling suitable additives known as surfactants can be mixed to PCMs. Similarly, for enhanced stability, stabilizing agents can be added. PCMs for incorporation are often encapsulated (micro and macro) depending on the application and cost constraints. There are several pieces of research on coating and encapsulations for PCMs [12,13,14] for various applications. Thus, it is needed to be understood that the PCM enhancement method must be selected based on the specific requirement of the application and of course the financial constraints.
13.3 Numerical Modeling of PCM
13.3.1 General Practice
Since the phase change materials (PCM) exhibit solidification and melting, which includes phase transitions, the heat conduction problem can be modeled as a Stefan problem. In such a problem, two different domains are assumed to exist, viz. solid and liquid. These phases are differentiated by a moving interface, a sharp surface front. Usually, this surface front is maintained at the specific melting temperature, a constant latent heat capacity, and has a zero thickness interface of the surface front [15, 16]. A phase change process of such a kind needs attention, and the calculations need to be performed throughout the domain. A single domain approach is very convenience in such numerical calculations since the same system of Navier–Stokes–Boussinesq equations is evaluated inside both liquid and solid phases. In the case of enthalpy porosity approach [17], an explicit treatment is not given to the melt interface, but an entity referred to as the liquid fraction is defined as the cell volume to be in liquid form. The liquid fraction is linked with each grid of the domain. It is evaluated at every iteration of the simulation by balancing enthalpy. Needless to mention that if a non-isothermal phase change is assumed (discussed in detail below), in the semisolid region, the liquid fraction values vary between 0 and 1. A more straightforward approach to handle this semisolid region is to model it as a porous region. In that case, the solid and liquid regions have porosity values of 0 and 1, respectively. It can be attained by explicitly setting the velocity to zero in finite volume methods or by applying penalty models in finite element methods based on viscosity or Carman–Kozeny relation. With these assumptions, the transport equations are given as [18].
Continuity equation:
X—momentum equation:
Y—momentum equation:
Energy equation:
The energy equation consists of sensible enthalpy, which is derived from \(H = \int_{{T_{\text{ref}} }}^{T} {c_{p} \;{\text{d}}T}\) [19, 20]. The thermal diffusivity, \(\alpha_{t} = {k \mathord{\left/ {\vphantom {k {\rho C_{p} }}} \right. \kern-0pt} {\rho C_{p} }}\) is multiplied with advection of enthalpy in the above equation. Also, u, v, x, y, p, \(\mu\), and \(\rho\) represent the horizontal component of velocity, the vertical component of velocity, horizontal direction, vertical direction, pressure, dynamic viscosity, and density, respectively. Moreover, the Boussinesq approximation holds and is introduced in the Y—momentum equation, wherein \(\beta_{t}\), \(\rho_{\text{ref}}\), and \(h_{\text{ref}}\) represent coefficient of thermal expansion, and density and enthalpy (reference values), respectively.
13.3.2 Types of Grid Techniques Used to Model PCM
There are three approaches to solve the above Stefan problem, based on the choice of grid generation [21, 22],
-
Fixed grid method: As the name suggests, the spatial nodes’ grid that is utilized for discretizing the domain stays fixed while the simulations progress. The problem is evaluated using state functions and auxiliary constitutive formulations that assists the tracing of the phase change phenomenon because of solidification or melting. The approach is most common among the studies based on the numerical technique which are available in abundance.
-
Deformed grid method: The interfaces or sharp fronts during melting/solidification are traced by moving grid points instead of being fixed. The essence of the Stefan problem is captured through this approach by the deforming grid (Stefan condition) when the solution is calculated.
-
Hybrid method: A combination of the above two methods can be applied by coupling the solutions of discretized PDEs solved using a pseudo-fixed grid, along with a local front tracking using the deformed grid method.
In this chapter, only the fixed grid method is discussed as it is most used. Further, this grid method is the simplest of the three to solve problems in the thermal engineering field with phase changes. There are primarily two types of phase changes involved: isothermal and non-isothermal. The equations above show the realistic non-isothermal process, wherein three phases exist. In the case of the isothermal process, however, only solid and fluid phases are considered.
13.3.3 Handling the Energy Equation
13.3.3.1 Overview
Based on handling of the energy equation, broadly three different methods are popular in the literature, viz. methods based on enthalpy discussed above, apparent calorific capacity method, and heat source method. In the enthalpy method, a dominant role is played by the enthalpy for distinguishing sensible and latent heat parts for both isothermal and non-isothermal phases changes. If a proper scheme is applied to handle the numerical calculations, it is faster than any other method for PCM modeling. In an apparent calorific capacity method, the sensible and latent heat energy parts are clubbed together for further simplicity. In the heat source method, the source term involves latent heat for obtaining differential equations based on temperature for a proper solution. Usually, in the cases of gradual phase changes, the apparent calorific method is chosen, while the other two methods are widely used when the phase change occurs in small temperature ranges. However, the calorific method is computationally inefficient with coarser grids and large time steps. For this method to be computationally accurate, the grids need to be closely spaced along with very small time step values. Moreover, the convergence is difficult to achieve as the latent heat may be underestimated in this method. If the phase changes occur at a fixed temperature, it is difficult to apply the calorific method. On the other hand, application of heat source method requires serious under relaxation, which calls for further investigation on finding the optimum under relaxation factor values. Further, round off errors exist in case if the melting occurs over a wide temperature range. Hence, one can see the wide applicability of the enthalpy–porosity technique and its popularity among the researchers.
In the above equations, following the heat source method, the source term involving latent heat is
When the fixed grid technique is used for modeling the melting and solidification, the major difficulty lies in considering the mass and heat transfer conditions near the phase change. An appropriate volume source term should, therefore, be introduced. In the above equation, \(\Delta H\) represents latent heat content. It should be noted that this heat content is a function of temperature and can be written in terms of latent heat of the material as \(\Delta H = sL_{\text{f}}\). The term “s” is often referred to as the liquid fraction and is derived as a temperature gradient between the solidus and liquidus temperatures.
A parameter “A” is defined in the momentum equations, which helps retaining velocity its respective value in the solid and fluid regions. As a method to do that, it is defined as [23]
It should be noted that two constants \(n\) and \(b\) are introduced in the above equation. The purpose of these constant is to maintain the mathematical feasibility of this condition. The constant \(b\) is often retained to a lower value in the range of 10−3, while the constant \(n\) is kept at a sufficiently large value of 109 in order to avoid division by zero. The aim is to transit the velocity from solid to other phases smoothly to confirm smooth convergence.
13.3.3.2 Phase Field Method
It is well known that PCMs have lower thermal conductivity values that leads to a slower rate of charging and discharging. The overall heat transfer rate is affected and, in turn, reduces efficiency. Among the different techniques to overcome this problem, a convenient method is to embed PCMs in porous metal foams with high thermal conductivity. Usually, a representative elementary volume is considered in the case of porous media modeling, wherein the fluid-saturated porous solid aggregates are held to be distributed appropriately. Further, homogenization is applied to achieve a continuum model (volume-averaged). The phase field method (PFM) contains a squishy zone, like the above case, and excludes interfacial energy balance. A free energy density function is key motivation in the case of PFM. This force is liable for the progress of the phase switch zone. Also, PFM utilizes a phase field variable that depends on position and time to ascertain whether the phase is solid or fluid. The resultant method is applicable in capturing dendritic evolution (non-equilibrium) during the supercooled melt solidification [24].
13.3.3.3 Volume of Fluid Method
Another approach to model the multiphysical phenomena is coupling volume of fluid (VoF) method with a solid–liquid phase change in the Eulerian frame. Since the introduction of the enthalpy method by Voller and Prakash [25], there have been immense efforts in handling the phase change. The VoF approach was proposed by Hirt and Nichols [26] and has been an excellent technique to capture the interface with the help of an indicator function to mark different fluids. In this approach, the PCM and gas phase are assumed to be two-phase immiscible incompressible fluid along with a fixed grid method.
Equations (13.1)–(13.4) would remain the same in this approach due to the presence of the enthalpy method. However, the VoF method includes an indicator function \(\alpha_{\text{mt}}\) to mark different fluids:
The first fluid is PCM, while the second fluid is the gas to be modeled. The volume fraction equation can be written as
In the above equation, \(\varvec{u}_{r} = \varvec{u}_{1} - \varvec{u}_{2}\), is the relative velocity between the PCM and the gas phase. Moreover, another source term has be to be included in momentum equations related to the surface tension force \((\sigma_{s} )\), given by,
The specific enthalpy can also be considered as the sum of sensible and latent heat values, to give,
Unlike the previous case, \(\gamma \left( T \right)\) is introduced here as the melt fraction, and it depends on temperature and the latent heat of fusion (Lf). It attains zero value in the solid zone, and 1 in the liquid zone, while it ranges between 0 and 1 in the mushy zone. The parameter A or Darcy-type source term is introduced in this method like the earlier approach.
If Eq. 13.12 and the energy equation, Eq. 13.6, are combined and written in a concise form, the following equation is obtained
13.3.4 Sophisticated Tools Available
The above methods discussed have a wide range of applicability. However, there are sophisticated tools available which implement these models for specific applications. The commercial packages COMSOL, FLUENT, and HEATING do provide the option to model PCMs out of the box with some level of customization to implement new models. Mostly, they implement heat source (or heat capacity) method and can be used to simulate different applications based on the finite element, finite volume, and finite difference method, respectively [27].
An abundance of pre-packaged applications is focused on the thermal management through the usage of PCM in the whole building design. Such applications help designers and manufacturing companies to focus more on inventive ideas to develop the energy and thermal performance of buildings. Aspects such as dynamic involvement among all thermal-based elements linked with ease and comfort and energy consumption are accounted that includes building envelope, air conditioning, lighting, and control devices. There are many articles about listing tools capable of handling the dynamic behaviors of a building and its systems [28].
13.3.4.1 EnergyPlus
EnergyPlus [27] is one among many of such tools. It uses the conduction transfer functions (CTFs) to approximate heat transfer in building envelope. Moreover, it has a conduction finite difference (CondFD) solution algorithm, which uses a semi-implicit finite difference scheme. The scheme is based on heat capacity method, and the latent heat evolution is considered using an auxiliary enthalpy–temperature data set. In the latest versions of the software, a fully implicit scheme can be found, providing numerical flexibility. Some limitations of this software include the compulsion to use a lower time step size, using 1/3rd of the default node space, and PCM hysteresis is left unaccounted, which may cause a discrepancy in the results.
13.3.4.2 TRNSYS
TRNSYS consists of several “TYPES” modules are linked together, making it a modular program. In such a program, the output of one model is linked with the input of another model [29]. Such a program can be useful in modeling complex building systems, wherein existing TYPES can be used, or new modules can be created, and it can be easily integrated into the TRNSYS package. Furthermore, external applications can also be linked into TRNSYS. The program has by default several models for phase change heat transfer. However, most of the times propriety research modules opt like TYPE36, TYPE58, TYPE204, TYPE1270, and so on.
13.3.4.3 ESP-r
ESP-r is a UK-based simulation tool that is of dynamic energy type [30]. It is used for modeling the thermal, visual, and acoustic performance of buildings. The effective heat capacity and additional heat source methods are used by default in ESP-r. Again, fine time steps are necessary to obtain sufficiently accurate results in this tool.
13.3.4.4 BSim
BSim is another dynamic simulation-oriented program, and it is based in Denmark. It has an attractive graphical user interface which helps the users to get oriented easily. It is based on heat capacity method using the quasi-steady state in building modeling. Overall, it gives accurate behavior of PCM but with a fine value of time step and can be used for continuous heating and cooling and heating with low initial temperature (below the melting point of PCM).
13.3.4.5 Others
There are other research-based tools as well like PCMExpress, RADCOOL, and CoDyBa. PCMExpress is a German-based planning and simulation program mainly used for thermal management of buildings. The tool assumes a building that floats freely, and one can simulate various types of building materials and weather conditions. Such a simulation helps the decision-making process during the early design stage. RADCOOL has been designed for cooling and heating system based in the USA [28].
13.4 PCM Applications
PCM application can be broadly classified based on the temperature of application. The first is for low or medium temperature applications that refer to temperature range up to 150 °C and second for high-temperature application where the temperature is above 150 °C. The application domain for both these temperature domains has been enlisted and discussed below.
13.4.1 PCM Application for Low and Medium Temperature Range
13.4.1.1 PCM Application as an Environment Management Solution
With global warming becoming a threat, people living in hilly areas are facing problems of melting glaciers and water shortage, leading to population migration. A researcher and engineer (Sonam Wagchuk) in Ladakh found a unique solution by using water in the form of ice as storage. The phase change concept was used to store the runaway water during winters in the form of huge stupas saving around 200 million liters of water in each stupa. The downstream water is made to pass through pipes, and flow is controlled using the valves. During the night at sub-zero temperature, the water is sprinkled which solidifies immediately and settles forming a stupa. The structure is grown big enough such that it could sustain and hold. This unique structure is called as the “Ice Stupa” and is shown in Fig. 13.3.

(Credit Rolex awards; https://www.rolex.org/rolex-awards/environment/sonam-wangchuk)
Ice Stupa by Sonam Wangchuk
The Ice Stupa holds the Guinness Book of the world record of the most massive human-made ice structure, which is a simple application of water as phase change material. These structures melt during April and provide water to the locals for irrigation, etc., when it is most required. This unique concept has helped to replenish the melting glaciers and helped the locals to sustain life in Ladakh, Himalayas, in India.
13.4.1.2 PCM Application in Buildings
PCM application in buildings is of interest for researchers across the globe. For maintaining a lower inside temperature, the building in olden days was built with thick walls that are they aimed at increasing the thermal mass of the buildings. To mimic that, PCM integration can be a viable solution. Due to the high storage capacity of PCMs, the thinner walls, integrated with PCM, can provide similar thermal shielding effect.
Figure 13.4 shows a schematic diagram of the working of a PCM, within a building envelope. The figure shows four different scenarios of a typical summer day (hot climatic condition), i.e., morning 8 am, evening 4 pm, night 8 pm, and early morning 4 am over a 24-hour cycle to understand the concept behind the incorporation of PCMs within a building. At 8 am, the sun is up, and solar radiation starts falling on the exterior walls/surfaces. The temperature starts rising. As the day progresses, the surface temperature tends to increase further. The surface temperature rises above the ambient temperature due to solar air effect [4]. The inside temperature is comparatively cooler. Thus, heat transfer starts taking place from outside to the inside.
As the temperature on the outer surface rises, the heat flow to the inside also increases. The temperature rises until the PCM melting temperature is reached. Now with the addition of heat, the PCM starts melting, and the temperature of the PCM layer is stagnant at its melting temperature. Now two heat flows occur one due to the temperature difference between outside layer and PCM, and other between PCM temperature and temperature on of the inside layer. The difference between the two is stored within the PCM and is utilized as phase change latent heat. This continues until complete melting of the PCM takes place. As depicted at 4 pm, the PCM is completely melted, and the temperature on the inside starts rising. However, at sunsets, the temperature on the outside goes down, as shown at 8 pm. The temperature of PCM is higher than both the ambient temperature and the inside surface temperature. Two heat flows circuits are formed, one from PCM to the inside and other from PCM to the outside. Now, since resistance is less due to lower wall thickness on the outer side, and there is a higher temperature difference, more heat is discharged outside. Only a small amount flows to the inside; thus, PCM gets discharged (releases heat) and solidifies, as shown at 4 am, in the diagram. The overall heat flowing to the inside over a 24-hour cycle can be reduced by 12–15% [31]. It is necessary to get discharged at night to be available to absorb heat the next day. This can be ensured by careful selection and benchmarking of PCMs [32]. PCMs need to be mapped to a particular climatic condition based on the melting temperature of the PCM and the climatic conditions of the location to ensure daily charging and discharging of the PCM and effective utilization.
Kaushik et al. [33] modeled PCM incorporated building in 1981. There were a series of studies that followed [34,35,36,37,38]. These studies were carried out for cold climatic conditions. In Indian perspective, studies were initially carried out by Pasupathy et al. [39] and Kant et al. [40] followed by experimental studies carried out for New Delhi by Saxena et al. [14]. These studies showed a temperature reduction up to 6 °C with PCM incorporated brick compared to the conventional ones. It is first necessary to understand the amount of solar radiation falling on to the surface. The solar radiation falling on a surface data is available for different places. However, the radiation falling on various wall surfaces is to be calculated. Typical values for the solar radiation falling on different surfaces are calculated based on the radiation data available for May first week as shown in Fig. 13.5.
Calculated values of incident solar radiation falling over different surfaces [42]
The temperature of the surface exposed to sunlight is higher than the ambient temperature. As discussed, this is due to the solar air effect [43]. This temperature generated at the surface is responsible for heat transfer to take place. The difference in this surface temperature and the inside temperature acts as the driving potential for heat transfer to take place. This has been modeled by several researchers [44,45,46,47,48] and heat flow to the inside for different scenarios, climatic conditions and wall parameters have been discussed. Further, experimental studies have been carried out for PCM incorporated bricks [41, 49,50,51] showing energy savings up to 15% over 24 h cycle and peak heat transfer reduction up to 50% during the day for both hot as well cold climatic conditions. There have been studies on PCM incorporated wall boards [52, 53]. However, leakage issues need to be addressed before the actual implementation of these PCM boards as a retrofit solution. The studies on actual PCM incorporated rooms have also been carried out by a few researchers for cold climatic conditions [54,55,56,57,58]. The results show a significant lowering of heat transfer. Still, detailed economic analysis and feasibility study need to be carried out followed by the investigation of cheaper PCM alternatives, thus, making PCM buildings as a solution for future energy-efficient buildings.
13.4.1.3 Constant Temperature Solar Drying and Space Heating Application
India is the second-largest producer of the fruits and vegetables in the world with a production of 259 million MT with wastage of nearly 4.6–15.9% in fruits and vegetables annually. As per latest estimates by the Associated Chambers of Commerce of India, our country loses approximately INR 926 B (US$ 14.33 B) after harvest every year, due to rejection at the farm gate, lack of proper storage facilities, and delays in the distribution process. Thus, food preservation can be critical for agricultural countries like India. The general meaning of food preservation is to extend their shelf life by preservatives that retard their rate of spoilage. These can be achieved by various techniques like drying, cooling, storing at a constant temperature, etc. Drying is an efficient way easy to handle and enhances crop life in such a way that its nutritional value can be retained. In the drying process of certain food products, the temperature is the crucial parameter. In the current situation, farmers are accustomed to use open-air sun drying process for drying and preserving their agricultural products. This causes browning of products, resulting in deterioration of food quality. If we use solar dryer integrated with phase change material (PCM), providing constant temperature heating of food products and simultaneously storing the excess solar energy into the form of latent heat during the day which can provide warmth during night hours as well. Thereby quality of the crop can be restored as well as browning phenomena can be avoided. The PCM incorporated solar dryer setup is shown in Fig. 13.6.
The target is a sustainable technology for the preservation of several items like vegetables, medicinal plants, fruits, etc., using constant temperature-controlled drying. Another initiative by the National Institute of Solar Research (Government of India) has developed solar dryer cum space heating system based on evacuated tube collectors and used PCMs for temperature-controlled drying and heating. These systems have been installed in Ladakh, are under testing phase, and have shown excellent results in terms of space heating as well as fruit drying.
13.4.1.4 Thermal Management of Photovoltaic (PV) Modules and Electric Circuits
There has been a significant increase in the use of PV for power generation over the past decade to harness renewable energy from solar. There have been researches carried out that shows that temperature control of the module increases their efficiency. Brano et al. [59] developed the thermal model analysis of the crystalline PV-PCM system using the finite difference method. It was found that the discharging of PV panels is essential for efficient working of the system, and there needs to be good thermal contact between PV and PCM.
Indartono et al. [60] used petroleum jelly as PCM on the backside of 10-watt-peak monocrystalline solar panel in Indonesia outdoor conditions and found that the average power and efficiency increased by 7.3 W and 6%. Abdelrazik et al. [61] did an experimental validation for a PVT/PCM system. The setup was the same old setup as everyone with PCM at the back and cooling fluid in between the PCM. The PCM used here was paraffin wax mixed with different concentrations of graphene nano-platelets along with pure water as cooling fluid. The experiment was conducted in two different conditions: one in winter and other in summer. The PVT/PCM was highly efficient in summer with an electrical efficiency enhancement of 22% versus 6.9% in the winter when compared to stand-alone PV.
Fayaz et al. [62] explored a novel design of a thermal collector made up of aluminum. The experiment was conducted for PV, PVT, and PVT-PCM systems to observe, evaluate, and analyze efficiency under different operating conditions. The experiment consisting of the PVT-PCM system was conducted using paraffin wax (commercial code name A44-PCM). For the PV-thermal hybrid systems, water flows through the serpentine thermal collector, which is passively driven by the overhead water tank. It was observed that there was a reduction in the temperature of PV cell by 8.3 and 8.1 °C in case of PVT, and 12.8 and 12 °C in the case of the PVT-PCM system from the PV module, experimentally and numerically, respectively. Similarly, the electrical efficiency obtained for the PVT-PCM, PVT, and PV systems numerically is 14, 13.85, and 13.72% when compared to experimental values of 13.87, 13.74, and 13.56%.
Waeli et al. [63] used time-varying equations to get results that are more accurate by cooling PV module with both PCM and nano-fluid.
Drawing of a mathematical model in the context of the proposed collector: (a) equivalent thermal circuit of PVT, (b)–(e) glass, PV, wax layer, and tube coil, respectively.
For glass
For cell
For PCM
13.4.1.5 Heating and Cooling Applications
Heating required for cold places is often quite large, for example, in the UK alone the heating power needed comprises one-fifth of the total power consumption. Further, hot water is required for household requirement, in hospitals,and restaurants for cleaning and sanitization purposes. One way is to use electrical power to generate heat, and another option is to use solar heat energy, store it, and use as and when required. Thus, the energy loss due to conversion can be avoided, and more of renewable power can be used. Similarly, the exhaust heat available at the end of the power cycle can be used for space heating applications. This has been implemented in some places as a pilot project in some of the cold countries. Use of PCMs as storage in these systems can enhance the heat storage capacity as latent heat storage can be 50 times more than the sensible heat storage [64]. Another important aspect is using solar energy for heating. The solar radiation is available during the day. However, the requirement of hot water/heat is more during late evening or early morning hours when the sun is not available. To bridge this gap between demand and solar availability, PCM can play a significant role in terms of storing the heat during the sunshine hours and providing it during the off-sunshine hours. Studies show that temperature stability and hold isare more for PCM solar heating system compared to any sensible heating system. The PCM utilization for heating applications has been researched where waste heat from various systems and sub-systems can be stored and used for allied applications and result in a substantial amount of energy savings. A numerical study carried out for the UK suggested that PCM water heating application can result in the lowering of CO2 emissions by 11% [65]. Another study is on nano-enhanced PCM for solar water heating application [66]. Thus, PCM application for heating application has enormous potential.
PCM can also be applied for industrial heating applications where constant controlled heat is required, for example, in the sugar industry for drying the moisture to obtain sucrose. Similarly, it can be applied in the dairy industry to produce different milk products like butter, ghee, condensed milk or khoa, etc.
Besides heating, PCMs can be used as a cooling option. One such application used since decades is desert coolers. In desert coolers, the water evaporates cooling the incoming air which is forced out after cooling through the fan. Another example is earthen pots used since ages in India, where water changes its phase from liquid to gas cooling the water inside the pots. These are used in households in India during the summer season. Further, researches are being carried out using the solid–liquid phase transition for solar-assisted cooling application in case of adsorption cooling to overcome the problem of intermittent solar radiation as a heat source during off-sunshine hours. These applications are still under research stage, and systems are being developed and tested for different ambient temperature scenarios.
13.4.1.6 Battery Thermal Management for Electric Vehicles
With the advent of electric vehicles, there have been significant researches concentrated on battery life and efficiency. Currently, lithium-ion (Li-ion) batteries are considered as the most promising technology for the storage of electrical energy, and one of the critical parameters is its working temperature range. Acceptable temperature range of EVs is between −20 and 60 °C, while the optimum temperature range is 15–35 °C. The temperatures above 60 °C possess a severe threat of causing thermal runaway in Li-ion batteries [67]. Cooling systems are therefore needed to be designed which should be lightweight and compact and should not act as a parasite on battery pack as most active cooling systems, thereby decreasing overall EV efficiency. The system should be able to perform well in adverse climatic condition and should be cost-effective and environment-friendly. For cold countries, air cooling is a viable option. However, for countries like India, the temperature of ambient air is not sufficient to cool the batteries sufficiently. Thus, there is a need for incorporating an additional cooling system. Using PCM can be a feasible solution as a passive cooling alternative used in combination with an active cooling system. Rangappa et al. [68] have reported a hybrid cooling system that uses outcomes of the computational fluid dynamics simulations. This system maintains the temperature within 313 K (40 °C) for the battery using a 9-mm-thick pure phase change material (PCM) under heat generation rate of 30,046 W/m3. The coolant flow rate can be increased beyond 2 L/min to achieve better performance of the system. Verma et al. [67] have studied prismatic and cylindrical lithium-ion batteries based on their performance in a high ambient. PCM utilization for battery thermal management is still at the research stage, and experimental setup for testing the battery characteristics is being carried out.
13.4.1.7 Wearable PCM Devices
PCM application for buildings and battery temperature control has already been discussed, and it is seen that PCM can be suitable to application for given temperature range. Similarly, in PCM incorporated fabric design solutions can be used for maintaining the comfort temperature. For instance, consider two cases of firefighter and an army officer in combat stationed in posts in extreme climate, both are exposed to extremes of temperature. Firefighters or an army officer posted in hot climatic condition of say a desert location has very few options to maintain a cold temperature. PCM fabric-based uniforms can be used for such situations as they could store extreme heat and maintain cooler/tolerable temperature for more duration. In the case of firefighters, even a few more extra minutes can be lifesaving. PCMs with enhanced storage capacity can help in sustaining the harsh environment for a longer duration. These jackets can be charged in base camps or during the night when the temperature is relatively lower. There are some products already available in the market used to relieve sprained muscle, etc., using ice packs, etc. There can be many other potential applications for these PCM fabrics which are under investigation.
13.4.2 PCM High-Temperature Applications
PCM high-temperature application has been used for ages. However, their use in the form of heat storage is a concept which was introduced later, i.e., in the last fifty years or so. Melting of substances and minerals is carried out at higher temperatures, and their solidification time defines their crystal lattice structure based on which solid–solid phase distinction occurred. Many other factors such as the inclusion of foreign particles also affected the properties such as strength. However, with precise knowledge of the amount of heat stored and its impact on the rearrangement of atoms at lattice structure level, the use of these materials for various heat storage applications and as heat transfer fluid has been studied. These materials are suitable for solar thermal power generation as they can serve as storage materials as well as heat transfer fluid at high temperature. Vignarooban et al. [69] studied various materials and compared different materials and their suitability in the application for solar thermal power generation. A typical layout of a solar thermal power plant is shown in Fig. 13.7.
Layout of parabolic trough based solar thermal power plant [70]
Figure 13.7 shows the use of storage system to absorb the excess heat during the day, which can be utilized during the off-sunshine hours. The storage materials can also act as heat transfer fluids. The typical values of heat transfer coefficient, melting temperature, and working range are shown in Table 13.1. The use of PCMs in solar thermal power has been implemented only a few plants. The major limiting factor is their cost and due to lower cost of power through solar PV. Thus, a lot of research is required for finding suitable cheaper alternatives such that power generation through solar thermal also becomes cost-effective and implementable solution rather than just a feasible solution.
13.5 Limitations and Future Recommendations
In the case of PCMs, the major challenge is the availability and choice of correct PCMs as per the desired application. Another critical parameter is currently the cost of PCMs available and researched, which is quite high. Thus, the payback period for PCM incorporated systems is very high. In modeling perspective, it must be commented that simulation of PCMs is tricky and involves many assumptions and approximations. The in-house codes developed by various research groups to model PCM for different applications have not been included for the sake of brevity. However, such an exhaustive list of codes and their merits and demerits shall benefit both novice and experts alike.
The silver lining in PCM research is that with time as the research is being carried out, the cheaper alternatives are being found, for example, PCM bricks prepared and showcased by Saxena et al. [71] the cost of brick came down from INR 2600 to just INR 90. Thus, with material research and using cheaper alternatives, followed by addressing their stability and other issues, the PCMs application can have a bright future for many applications as discussed. Concisely, the research prospects in case of PCMs are enormous but require a lot of efforts for turning it from a feasible solution to an optimal one.
13.6 Conclusions
This chapter provides a comprehensive review of PCM and its application. It includes data n different PCMs and their modeling. Numerical modeling of PCMs has several uses, especially related to the building energy optimization, which are discussed. Most popular techniques and methods, along with their merits and demerits, have been illustrated. Furthermore, details on the governing equations and the importance of various source terms variables have been discerned. It is observed that most numerical studies prefer enthalpy-based methods due to their simplicity and ease of modeling. Moreover, the models that incorporate heat capacity or source methods require a short time step value to attain the desired accuracy and, hence, results in a significant slowdown. Further, most sophisticated tools for PCM modeling related to the entire building are based on heat capacity methods. It should be noted that still a lot of work is required in this field, as most of the programs available are still computationally expensive and need substantial time step values.
About the experimental application of PCMs, it essential to state that still a lot of research is required to find suitable PCMs in affordable costs. The experiments show that the use of PCM is a feasible solution. However, due to the cost implications, the use of PCMs is still limited and restricted. PCM application in buildings, solar drying, and heating applications can be cost-effective with proper design implementation and shorter payback period. Whereas for storage applications for high temperature, it is still economically not a viable solution. The focus is also on the thermal enhancement of PCMs as per thermal conductivity and stability in case of inorganic PCMs and eutectics.
Abbreviations
- C :
-
Specific heat capacity, kJ/kg K
- Φ :
-
Nano-fluid volume fraction
- D :
-
Equivalent diameter, m
- Nu:
-
Nusselt number
- F :
-
Packing factor
- Re:
-
Reynolds number
- W :
-
Width, m
- Pe:
-
Pecklet number
- h :
-
Convective heat transfer coefficient, W/m2 K
- Pr:
-
Prandtl number
- H :
-
Sensible enthalpy, J
- k :
-
Thermal conductivity, W/m K
- I :
-
Solar radiation intensity, W/m2
- A :
-
Cross-sectional area
- L :
-
Length, m
- δ :
-
Thickness of the material
- L f :
-
Latent heat of fusion, J
- σ :
-
Stefan–Boltzman constant, W/(m2K4)
- ṁ :
-
Mass flow rate, kg/s
- ε :
-
Emissivity of glass
- p :
-
Pressure, N/m2
- t :
-
Time
- s :
-
Liquid fraction
- μ :
-
Viscosity of fluid
- B:
-
Constant parameter
- κ :
-
Boltzman constant
- T :
-
Temperature, K
- U :
-
Overall heat transfer coefficient, W/m2 K
- u :
-
Horizontal component of the velocity, m/s
- a :
-
Ambient
- v :
-
Vertical component of the velocity, m/s
- b:
-
Backplane
- x :
-
Distance in flowing direction, m
- c :
-
Solar cell
- y :
-
Distance in normal direction, m
- g:
-
Glass
- α :
-
Absorption coefficient
- p :
-
PCM layer
- α t :
-
Thermal diffusivity, m2/s
- ref:
-
Reference value at reference conditions
- α mt :
-
Indicator function to mark different fluids
- w :
-
Water
- η :
-
Photovoltaic efficiency
- f:
-
Fluid
- β :
-
Temperature coefficient of PCM
- c:
-
Coil tube through which fluid flows
- β t :
-
Thermal expansion coefficient, 1/K
- np:
-
Nano-particle
- τ :
-
Transmission coefficient
- bf:
-
Base fluid
- γ(T):
-
Melt function
- nf:
-
Nano-fluid
- F`:
-
Flat plate collector efficiency factor
- s:
-
Solid
- ρ :
-
Density
- l:
-
Liquid
- V :
-
Volume
- ref:
-
Reference value
References
Zalba B, Marı́n JM, Cabeza LF, Mehling H (2003) Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl Therm Eng 23:251–283. https://doi.org/10.1016/S1359-4311(02)00192-8
Saxena R, Agarwal N, Rakshit D, Kaushik SC (2020) Suitability assessment and experimental characterization of phase change materials for energy conservation in Indian buildings. J Sol Energy Eng 142. https://doi.org/10.1115/1.4044568
Mi X, Liu R, Cui H, Memon SA, Xing F, Lo Y (2016) Energy and economic analysis of building integrated with PCM in different cities of China. Appl Energy 175:324–336. https://doi.org/10.1016/J.APENERGY.2016.05.032
Saxena R, Rakshit D, Kaushik SC (2020) Review on PCM application for cooling load reduction in Indian buildings. In: Solar energy-system, challenges and opportunites. pp 247–275
Singh RP, Xu H, Kaushik SC, Rakshit D, Romagnoli A (2019) Charging performance evaluation of finned conical thermal storage system encapsulated with nano-enhanced phase change material. Appl Therm Eng 151:176–190. https://doi.org/10.1016/J.APPLTHERMALENG.2019.01.072
Khan A, Saikia P, Saxena R, Rakshit D, Saha S (2019) Microencapsulation of phase change material in water dispersible polymeric particles for thermoregulating rubber composites—a holistic approach. Int J Energy Res er.4925. https://doi.org/10.1002/er.4925
Hoogendoorn CJ, Bart GCJ (1992) Performance and modelling of latent heat stores. Sol Energy 48:53–58. https://doi.org/10.1016/0038-092X(92)90176-B
Singh RP, Xu H, Kaushik SC, Rakshit D, Romagnoli A (2019) Effective utilization of natural convection via novel fin design and influence of enhanced viscosity due to carbon nano-particles in a solar cooling thermal storage system. Sol Energy 183:105–119. https://doi.org/10.1016/j.solener.2019.03.005
Singh RP, Kaushik SC, Rakshit D (2018) Solidification behavior of binary eutectic phase change material in a vertical finned thermal storage system dispersed with graphene nano-plates. Energy Convers Manag 171:825–838. https://doi.org/10.1016/J.ENCONMAN.2018.06.037
Pise AT, Waghmare AV, Talandage VG (2013) Heat transfer enhancement by using nanomaterial in phase change material for latent heat thermal energy. 2:360–366
Teng TP, Cheng CM, Cheng CP (2013) Performance assessment of heat storage by phase change materials containing MWCNTs and graphite. Appl Therm Eng 50:637–644. https://doi.org/10.1016/j.applthermaleng.2012.07.002
Saxena R, Dwivedi C, Dutta V, Kaushik SC, Rakshit D (2020) Nano-enhanced PCMs for low-temperature thermal energy storage systems and passive conditioning applications. Clean Technol Environ Policy 1–8. https://doi.org/10.1007/s10098-020-01854-7
Aguayo M, Das S, Maroli A, Kabay N, Mertens JCE, Rajan SD, Sant G, Chawla N, Neithalath N (2016) The influence of microencapsulated phase change material (PCM) characteristics on the microstructure and strength of cementitious composites: experiments and finite element simulations. Cem Concr Compos 73:29–41. https://doi.org/10.1016/j.cemconcomp.2016.06.018
Chang TC, Lee S, Fuh YK, Peng YC, Lin ZY (2017) PCM based heat sinks of paraffin/nanoplatelet graphite composite for thermal management of IGBT. Appl Therm Eng 112:1129–1136. https://doi.org/10.1016/j.applthermaleng.2016.11.010
Lamé G, Clapeyron BP (1831) Mémoire sur la solidification par refroidissement d’un globe liquide. Ann. Chim. Phys. 47:1831
Stefan J (1889) Uber einige probleme der theorie der warmeletung. Sitzer. Wien. Akad. Math. Naturw. 98:473–484
Hunter LW, Kuttler JR (1989) The enthalpy method for heat conduction problems with moving boundaries. J Heat Transf 111:239–242. https://doi.org/10.1115/1.3250668
Goyal P, Dutta A, Verma V, Thangamani I, Singh RK (2013) Enthalpy porosity method for CFD simulation of natural convection phenomenon for phase change problems in the molten pool and its importance during melting of solids. In: COMSOL conference, p 10
Voller VR (1990) Numerical heat transfer, part B: fundamentals: an international journal of computation and methodology fast implicit finite-difference method for the analysis of phase change problems. Numer Heat Transf 17:155–169. https://doi.org/10.1080/10407799508928838
Brent AD, Voller VR, Reid KJ (1988) Enthalpy-porosity technique for modeling convection-diffusion phase change: application to the melting of a pure metal. Numer Heat Transf 13:297–318. https://doi.org/10.1080/10407788808913615
Augspurger M, Udaykumar HS (2016) A Cartesian grid solver for simulation of a phase-change material (PCM) solar thermal storage device. Numer Heat Transf Part B Fundam 69:179–196. https://doi.org/10.1080/10407790.2015.1097106
Sheikholeslami M (2018) Numerical modeling of nano enhanced PCM solidification in an enclosure with metallic fin. J Mol Liq 259:424–438. https://doi.org/10.1016/j.molliq.2018.03.006
Chakraborty PR (2017) Enthalpy porosity model for melting and solidification of pure-substances with large difference in phase specific heats. Int Commun Heat Mass Transf 81:183–189. https://doi.org/10.1016/j.icheatmasstransfer.2016.12.023
Sweidan AH, Heider Y, Markert B (2020) Modeling of PCM-based enhanced latent heat storage systems using a phase-field-porous media approach. Contin Mech Thermodyn 32:861–882. https://doi.org/10.1007/s00161-019-00764-4
Voller VR, Prakash C (1978) A fixed grid numerical modelling methodology for convection diffusion mushy region phase change problems. Int J Heat Mass Transf 30:1709–1719
Hirt CW, Nichols BD (1981) Volume of fluid (VOF) method for the dynamics of free boundaries. J Comput Phys 39:201–225. https://doi.org/10.1016/0021-9991(81)90145-5
Al-Saadi SN, Zhai Z (2013) Modeling phase change materials embedded in building enclosure: a review. Renew Sustain Energy Rev 21:659–673. https://doi.org/10.1016/j.rser.2013.01.024
Tabares-Velasco PC, Christensen C, Bianchi M (2012) Verification and validation of EnergyPlus phase change material model for opaque wall assemblies. Build Environ 54:186–196. https://doi.org/10.1016/j.buildenv.2012.02.019
Kuznik F, Virgone J, Johannes K (2010) Development and validation of a new TRNSYS type for the simulation of external building walls containing PCM
Heim D, Clarke JA (2004) Numerical modelling and thermal simulation of PCM-gypsum composites with ESP-r. Energy Build 36:795–805. https://doi.org/10.1016/j.enbuild.2004.01.004
Saxena R, Rakshit D, Kaushik SC (2018) Experimental assessment of characterised PCMs for thermal management of buildings in tropical composite climate. In: 4th world congress on mechanical, chemical, and material engineering (MCM’18)
Navarro L, Solé A, Martín M, Barreneche C, Olivieri L, Tenorio JA, Cabeza LF (2019) Benchmarking of useful phase change materials for a building application. Energy Build 182:45–50. https://doi.org/10.1016/j.enbuild.2018.10.005
Kaushik SC, Sodha MS, Bhardwaj SC, Kaushik ND (1981) Periodic heat transfer and load levelling of heat flux through a PCCM thermal storage wall/roof in an air-conditioned building. Build Environ 16:99–107. https://doi.org/10.1016/0360-1323(81)90026-3
Hadjieva M, Stoykov R, Filipova T (2000) Composite salt-hydrate concrete system for building energy storage. Renew Energy 19:111–115. https://doi.org/10.1016/S0960-1481(99)00024-5
Ismail KAR, Castro JNC (1997) PCM thermal insulation in buildings. Int J Energy Res 21:1281–1296. https://doi.org/10.1002/(SICI)1099-114X(199711)21:14%3c1281:AID-ER322%3e3.0.CO;2-P
Stritih U (2003) Heat transfer enhancement in latent heat thermal storage system for buildings. Energy Build 35:1097–1104. https://doi.org/10.1016/j.enbuild.2003.07.001
Mehling H, Cabeza LF (2008) Applications for heating and cooling in buildings. Heat and cold storage with PCM. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 217–295
Scalat S, Bann D, Hawes D, Paris J, Haghighata F, Feldman D (1996) Full scale thermal testing of latent heat storage in wallboard. Sol Energy Mater Sol Cells 44:49–61
Pasupathy A, Athanasius L, Velraj R, Seeniraj RV (2008) Experimental investigation and numerical simulation analysis on the thermal performance of a building roof incorporating phase change material (PCM) for thermal management. Appl Therm Eng 28:556–565. https://doi.org/10.1016/j.applthermaleng.2007.04.016
Kant K, Shukla A, Sharma A (2017) Heat transfer studies of building brick containing phase change materials. Sol Energy 155:1233–1242. https://doi.org/10.1016/j.solener.2017.07.072
Saxena R, Rakshit D, Kaushik SC (2020) Experimental assessment of Phase Change Material (PCM) embedded bricks for passive conditioning in buildings. Renew Energy 149:587–599. https://doi.org/10.1016/j.renene.2019.12.081
Saxena R, Biplab K, Rakshit D (2018) Quantitative assessment of phase change material utilization for building cooling load abatement in composite climatic condition. J Sol Energy Eng Trans ASME 140. https://doi.org/10.1115/1.4038047
Duffie JA, Beckman WA (2013) Solar engineering of thermal processes, fourth. Wiley, Hoboken
Saikia P, Azad AS, Rakshit D (2018) Thermodynamic analysis of directionally influenced phase change material embedded building walls. Int J Therm Sci 126:105–117. https://doi.org/10.1016/j.ijthermalsci.2017.12.029
Nghana B, Tariku F (2016) Phase change material’s (PCM) impacts on the energy performance and thermal comfort of buildings in a mild climate. Build Environ 99:221–238. https://doi.org/10.1016/j.buildenv.2016.01.023
Baniassadi A, Sajadi B, Amidpour M, Noori N (2016) Economic optimization of PCM and insulation layer thickness in residential buildings. Sustain Energy Technol Assessments 14:92–99. https://doi.org/10.1016/j.seta.2016.01.008
Ascione F, Bianco N, De Masi RF, De’rossi F, Vanoli GP (2014) Energy refurbishment of existing buildings through the use of phase change materials: energy savings and indoor comfort in the cooling season. https://doi.org/10.1016/j.apenergy.2013.08.045
Han Y, Taylor JE (2016) Simulating the inter-building effect on energy consumption from embedding phase change materials in building envelopes. Sustain Cities Soc 27:287–295. https://doi.org/10.1016/j.scs.2016.03.001
Castell A, Menoufi K, de Gracia A, Rincón L, Boer D, Cabeza LF (2013) Life cycle assessment of alveolar brick construction system incorporating phase change materials (PCMs). Appl Energy 101:600–608. https://doi.org/10.1016/j.apenergy.2012.06.066
Zhang C, Chen Y, Wu L, Shi M (2011) Thermal response of brick wall filled with phase change materials (PCM) under fluctuating outdoor temperatures. Energy Build 43:3514–3520. https://doi.org/10.1016/j.enbuild.2011.09.028
Li L, Yu H, Liu R (2017) Research on composite-phase change materials (PCMs)-bricks in the west wall of room-scale cubicle: mid-season and summer day cases. Build Environ 123:494–503. https://doi.org/10.1016/j.buildenv.2017.07.019
El Omari K, Le Guer Y, Bruel P (2016) Analysis of micro-dispersed PCM-composite boards behavior in a building’s wall for different seasons. doi:10.1016/j.jobe.2016.07.013
Ryms M, Klugmann-Radziemska E (2019) Possibilities and benefits of a new method of modifying conventional building materials with phase-change materials (PCMs). Constr Build Mater 211:1013–1024. https://doi.org/10.1016/j.conbuildmat.2019.03.277
Bontemps A, Ahmad M, Johannès K, Sallée H (2011) Experimental and modelling study of twin cells with latent heat storage walls. Energy Build 43:2456–2461. https://doi.org/10.1016/J.ENBUILD.2011.05.030
Guarino F, Athienitis A, Cellura M, Bastien D (2017) PCM thermal storage design in buildings: experimental studies and applications to solaria in cold climates. Appl Energy 185:95–106. https://doi.org/10.1016/J.APENERGY.2016.10.046
Dabiri S, Mehrpooya M, Nezhad EG (2018) Latent and sensible heat analysis of PCM incorporated in a brick for cold and hot climatic conditions, utilizing computational fluid dynamics. Energy 159:160–171. https://doi.org/10.1016/j.energy.2018.06.074
Navarro L, de Gracia A, Colclough S, Browne M, McCormack SJ, Griffiths P, Cabeza LF (2016) Thermal energy storage in building integrated thermal systems: a review. Part 1. active storage systems. Renew Energy 88:526–547. https://doi.org/10.1016/j.renene.2015.11.040
Xu B, Li P, Chan C (2015) Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: a review to recent developments. Appl Energy 160:286–307. https://doi.org/10.1016/j.apenergy.2015.09.016
Lo Brano V, Ciulla G, Piacentino A, Cardona F (2013) On the efficacy of PCM to shave peak temperature of crystalline photovoltaic panels: an FDM model and field validation. Energies 6:6188–6210. https://doi.org/10.3390/en6126188
Indartono YS, Suwono A, Pratama FY (2016) Improving photovoltaics performance by using yellow petroleum jelly as phase change material. Int J Low-Carbon Technol 11:333–337. https://doi.org/10.1093/ijlct/ctu033
Abdelrazik AS, Al-Sulaiman FA, Saidur R (2020) Numerical investigation of the effects of the nano-enhanced phase change materials on the thermal and electrical performance of hybrid PV/thermal systems. Energy Convers Manag 205:112449. https://doi.org/10.1016/j.enconman.2019.112449
Fayaz H, Rahim NA, Hasanuzzaman M, Rivai A, Nasrin R (2019) Numerical and outdoor real time experimental investigation of performance of PCM based PVT system. Sol Energy 179:135–150. https://doi.org/10.1016/j.solener.2018.12.057
Al-Waeli AHA, Chaichan MT, Sopian K, Kazem HA, Mahood HB, Khadom AA (2019) Modeling and experimental validation of a PVT system using nanofluid coolant and nano-PCM. Sol Energy 177:178–191. https://doi.org/10.1016/j.solener.2018.11.016
Saxena R, Rakshit D, Kaushik SC (2018) Experimental assessment of characterised PCMs for thermal management of buildings in tropical composite climate. In: MCM2018. Avestia
Pereira Da Cunha J, Eames P (2017) Phase change materials to meet domestic hot water demand in the UK—a numerical study. Avestia Publ J Fluid Flow Heat Mass Transf 4. https://doi.org/10.11159/jffhmt.2017.002
Harikrishnan S, Kalaiselvam S (2013) Experimental investigation of solidification and melting characteristics of nanofluid as PCM for solar water heating systems. 3:628–635
Verma A, Shashidhara S, Rakshit D (2019) A comparative study on battery thermal management using phase change material (PCM). Therm Sci Eng Prog 11:74–83. https://doi.org/10.1016/j.tsep.2019.03.003
Rangappa R, Rajoo S, Samin PM, Rajesha S (2020) Compactness analysis of PCM-based cooling systems for lithium battery-operated vehicles. Int J Energy Environ Eng 11:247–264. https://doi.org/10.1007/s40095-020-00339-z
Vignarooban K, Xu X, Arvay A, Hsu K, Kannan AM (2015) Heat transfer fluids for concentrating solar power systems—a review. Appl Energy 146:383–396
De Falco M Solar power concentration—oil&gas portal. In: Univ. UCBM, Rome. http://www.oil-gasportal.com/solar-power-concentration/?print=print. Accessed 24 Aug 2020
Saxena R, Rakshit D, Kaushik SC (2019) Phase change material (PCM) incorporated bricks for energy conservation in composite climate: a sustainable building solution. Sol Energy 183:276–284. https://doi.org/10.1016/j.solener.2019.03.035
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kulkarni, A., Saxena, R., Tiwari, S. (2021). Phase Change Materials and Its Applications. In: Singh, S.N., Tiwari, P., Tiwari, S. (eds) Fundamentals and Innovations in Solar Energy. Energy Systems in Electrical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-33-6456-1_13
Download citation
DOI: https://doi.org/10.1007/978-981-33-6456-1_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6455-4
Online ISBN: 978-981-33-6456-1
eBook Packages: EnergyEnergy (R0)