Abstract
Micro-biological geotechnics is a relatively young and dynamic field where microbiological methods are employed to address geotechnical issues. Microbially induced carbonate precipitation (MICP) is one such sustainable method, which enables cementation in loose sandy mass through calcium carbonate precipitation. Among a series of possible mechanisms (i.e. photosynthesis, sulphate reduction, de-nitrification, iron reduction and urea hydrolysis) to attain MICP, urea hydrolysis associates with greater efficacy and ease of practice. In the present study, widely accepted urease positive microorganism was employed as a source of urease enzyme which helps in biocementation process. Additionally, the effectiveness of MICP technique on sand stabilization and the role of particle sizes on the development of cementation bonds were investigated. Unconfined compressive strength (UCS) and hydraulic conductivity (k) tests were performed on samples treated with 1 M urea-calcium chloride cementation solution. To further endorse cementation of sand particles, microstructure analysis such as scanning electron microscopy (SEM) was performed. The detailed analysis showed that MICP has the potential to bind the particles through bio-mineralization which was further warranted by microstructure analysis. SEM images clearly disclosed mesoscopic and microscopic semblance of calcium carbonate precipitation on sand particles, resulting in the stabilization process.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The entire load of a structure is ultimately taken by subgrade, whose properties may vary considerably depending upon its origin. In some cases, the properties might not change spatially, while in others, they may significantly vary from one point to another within a short distance [1,2,3]. It is obvious that where ever the local soil lacks desired properties, which is deemed necessary for engineering application, the best remedy is to stabilize or alter the soil with a suitable foreign high strength materials, which are compatible [4,5,6]. However, such modifications are associated with cost and time [7].
Some of the ground improvement techniques that commonly employed are soil replacement, mechanical stabilization by compaction, drainage by soil consolidation, chemical treatment (using cement, lime, calcium chloride etc.), vacuum-assisted pre-consolidation, thermal treatment, stone/sand columns, dynamic compaction by heavy tamping, vibro-flotation and deep mixing. Among many afore-mentioned methods, cement and lime due to their ease in availability, application and cost effectiveness are ubiquitously used worldwide. However, their harmful impact on environment cannot be turned down. Researchers have documented that one metric ton production of ordinary Portland cement liberates around one metric ton of carbon dioxide and similarly one metric ton of lime releases around 0.86 metric ton of carbon dioxide [8, 9]. The obvious consequences of CO2 release into the atmosphere are well known in the form problems like global warming, while other negative aspects include ground water contamination, particulate particle emissions, etc. [10].
On the flip side, high consumption of natural resource material causing excessive exploitation of nature, leading to depletion of natural resources, environmental degradation and enhanced cost of cementing materials [11]. The non-availability of quality natural materials and incurrence of exorbitant prices on procurement of cementing materials have led the research fraternity to devise novel techniques for the soil stabilization, which are both economical as well as environmentally benign [12].
There has been a significant increase in the research to explore the possible biological technologies that can be applied in the construction industry and can work as a carbon sink. MICP is one such technology, which when applied in civil engineering projects enables the confluence of microbiology, chemistry and civil engineering disciplines. Out of the various possible mechanisms (i.e. photosynthesis, sulphate reduction, nitrogen reduction, iron reduction and urea hydrolysis) to attain MICP, urea hydrolysis associates highest efficacy and ease [13]. MICP has shown promising results in the stabilization of sand, wastewater treatment, strengthening of concrete and enhanced oil recovery [14,15,16]. MICP is sometimes also referred as “Bio-Grouting” where bio-based suspension is injected in a granular material in order to catalyze biochemical reactions and further facilitate calcium carbonate precipitation [17, 18]. In the present study, authors aim to cater some of the geotechnical issues using MICP technique. Effect of void space and efficiency of MICP along the sample depth is also evaluated.
2 Mechanism of Calcite Precipitation Through Urea Hydrolysis
Formation of calcite by urease positive bacteria is highly substrate dependent. These bacteria release urease enzyme which hydrolyze urea (the substrate) by utilizing two moles of water (H2O) and leads to formation of carbonate (CO32−) and ammonium ions (NH4+) as per Eqs. 1, 2:
The above process tends to increase pH of the solution and as soon as the pH value increases 8.5, precipitation of calcite begins in the presence of a calcium source. This precipitation can be delayed by the use of different buffer at different concentration [19]. The progress of the above process is governed based on various chemical, environmental and geotechnical parameters. The scope of the present study is limited to geotechnical parameters.
3 Materials
3.1 MICP Recipe
A gram-positive bacteria strain was used in this study and BHI solution mixed with 20 gm/l of sterile filtered urea solution was used as an inoculation medium. The prepared mixture was kept in an incubator cum orbital shaker (at 37 °C) for 24 h at 160–180 rpm. Bacterial cell harvesting was done through centrifugation at 4600 × g for 10 min (Hermile Centrifuge Z 326 K) and an OD600 of 0.8–1.2 was adjusted. The selection of time was made after different trials, ensuring stable cell pellet. After harvesting, the supernatant was removed and cell pellets were resuspended in buffer.
3.2 Sand
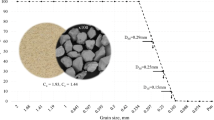
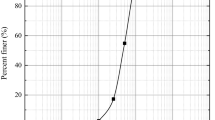
For the present study, a sand sample was collected from the banks of river Mahanadi in the state of Odisha, India. This sample is designated as Mahanadi River Sand (MRSM). The same sand was further sieved and subcategorized as coarse MRS (MRS1), medium MRS (MRS2) and fine MRS (MRS3). The gradational characteristics of the sand samples are shown in Fig. 1. The sand used in the study does not exhibit requisite engineering properties for most of the pavement and well as geotechnical applications. A summary of particle size characteristics is provided in the Table 1.
4 Experimental Methodology
4.1 Sample Preparation
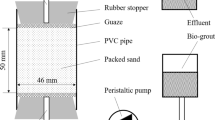
Several identical test specimens of approximately 38 mm diameter and 76 mm height were prepared by pouring the sand adopting to rainfall technique. Sand was filled in five separate layers to achieve desired relative density of 82%. Grouting was done in two different phases. First phase involved injection of 0.5 void volume (vv) bacterial suspension (a mixture of 50 mM Tris buffer, Bacterial suspension, 3 g/L BHI broth), while the second phase, which begun after 6 h, involved injection of cementation solution (a mixture of 1 M Urea-Calcium Chloride) through the top port of triaxial chamber with the help of peristaltic pump (10 mL/min) (see Fig. 2). The rate was decided after calibrating flow with soil permeability and to ensure that no impounding occurs. Prior to the above phases, 1 vv autoclaved/de-ionized water was down flushed to remove excess ions, entrapped air and to saturate sample. Moreover, to increase bacterial retention 0.3 M calcium chloride solution was injected immediately after first phase. The intermediate time provided between the two phase was to give microorganism chance to adhere to the soil grains and to avoid instantaneous flush out of bacteria solution. About 5–8 times cementation solutions were injected to ensure dense precipitation at about 12 h interval.
4.2 CaCO3 Content (CCC)
Acid wash method was adopted to determine calcium carbonate content. The calcium carbonate percentage is expressed as mass of CaCO3 divided by mass of soil as given in Eq. 3 [20].
where W1 is the mass of treated soil and W2 is the mass of the soil recorded after acid wash.
In the present study, change in bio-mineralization (i.e. CCC) as a function of depth of sample was evaluated. Samples for the same were carefully prepared by dissecting the specimen into four layers as shown in Fig. 3 (DL1-DL4).
4.3 Permeability
Permeability is one of the prime factors governing the behaviour of a material. A highly porous material with high permeability restricts the occurrence of excess pore water pressure and vice versa. In the present study, permeability test on the untreated and bio-mineralized sample was conducted in a laboratory using constant head method in accordance with the Indian standard code of practice [21]. All the treated and untreated samples were completely saturated prior to permeability test with 1L water to remove the entrapped air.
4.4 Morphological Characteristics
Micro-level analysis was performed to evaluate the morphological, mineralogical, characteristics of precipitation and degree of bonding between the soil grains and were ascertained using MERLIN compact field emission scanning electron microscope (FE-SEM) with EDS unit attached. Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) images were captured of both treated and untreated samples. The change in precipitation at different depths was also analyzed. For the preparation of powder sample, 2–3 g of over dry sample passing sieve 75 microns was used. Prior to sample evaluation in the FESEM, samples were coated (sputtered) with gold using Q150R ES Sputter Coater (make, Quorum, UK).
5 Results and Discussion
5.1 Unconfined Compressive Strength
After 5 to 8 injections, the bacterial treated samples were left for 7 days curing. UCS tests conducted on different MRS treated samples revealed that a maximum strength of about 544 kPa can be attained with 1 M urea-calcium chloride concentration on well graded sand samples (see Fig. 4). Results obtained from the study are similar to the findings of [22,23,24,25].
5.2 Calcium Carbonate Content (CCC)
In the present study, four different graded sands, fine, medium, coarse and a mixture of the trio, were used. All soils are quite distinct in their permeation characteristics, which is one of the key for ensuing the uniform calcite precipitations across as well as along its length. To affirm the fact that the precipitated compound is calcium carbonate and that it is uniform from top to the bottom of sample, acid wash technique to determine CCC was performed. An average CCC precipitated at different dissection locations was also estimated to correlate it to the degree of precipitation and contribution to strength development. Figure 5 shows the variation of precipitation intensity estimated at different dissection locations and the same values are also listed in Table 3. A simple observation of results reveals relatively low CCC precipitations (2.87%) in the case of MRS1 vis-à-vis with that of MRSW soil (6.26%). One of the reasons for low precipitation in MRS1 may be low bacterial cell density or retention in the sample as the major bacterial suspension was found to get discharged with the cementing injections. It can also be discerned from Fig. 5 that the pattern of CCC precipitations in all the four grades of sand are almost similar at different dissection locations, though the values differ significantly. It was observed that CCC decreased with an increase in depth, except for the last layer (Table 2). The increase in CCC at DL4 may be due to the placing of filter paper at the outlet end, which might have facilitated the retention of more bacterial cells resulting in higher CCC precipitation (refer to Fig. 4). As such, results in Fig. 5 clearly demonstrate a fact that permeability, void space and bacterial retention play an important role in accentuating the cementation effect within the soil sample.
5.3 Permeability
It is interesting to note from the above results that there is a contrasting effect between permeability and precipitation. Understandably, these two properties are highly interdependent. While the former property is crucial in ensuing the latter one up to a certain limit (till it does not lead to the free flow of bacterial suspension), which at the later stage of testing plays a decisive role of making the medium an impervious. This highlights a fact that resorting to MICP technique fetches multiple advantages such as strength enhancement concurrently diminishing permeability characteristics of the media. With this in mind, permeability tests were also conducted on bio-cemented MRS soils. The process of MICP can offer significant increase in strength, maintaining the permeability of the material to such an extent that the negligible pore water pressure is generated. The values of permeability measured on various bio-cemented samples are presented in Table 3. The results indicate a significant decrease in permeability with bio-treatment. It was found that permeability reduced by 48–85% based on the pore space or void space. Similar findings have been reported by Van Paassen et al. [26] and Harkes et al. [27], who reported a reduction in permeability within the same range.
6 Morphological Analysis
SEM analysis was performed to examine the microscopic semblance and portray the presence of calcium carbonate precipitation within the materials. From Fig. 6, a distinct layer of precipitation can vividly be seen, as untreated particles (Fig. 6a) have smooth surface and that of treated ones coated with minerals.
Based on the images presented in Fig. 7, it can be substantiated that the improvement in compressive strength in MRS soil occurred mainly because of bridging action together with the precipitation and cementation between the particles, in particular at grain contacts.
Therefore, in order the soil to exhibit greater binding action or compressive strength, precipitations should happen maximum at the contact junction of particles or on the grain surface, apart from the pore space, which might associate with an increase in pore water pressure leading to decrease in effective stress. Such precipitation, importantly also, widens the applicability of MICP technique to develop the impervious barriers.
SEM images in Fig. 7 exhibits a progressive calcite precipitation in the MRS soil based upon the number of injections. It can be inferred from the images that precipitations might be happening concurrently on the grain surface and particle contact junctions.
7 Conclusions
Based on the extensive experimental investigations carried out in the present work, the following salient conclusions can be drawn:
-
The various results demonstrate that MICP is an effective means of stabilizing poor soils by calcite mineralization.
-
It has been observed that the UCS value of MRSM sample increased from 0 to 0.544 MPa when treated by resorting to MICP technique.
-
The morphological analysis clearly revealed that bacterium is efficient in cementation by bridging mechanism. It also portrayed that not only voids are the place of precipitation, rather binding also occurred on the grain surface.
-
The results also highlight that particles size and pore spaces are major factors governing the efficiency and applicability of the process.
-
Results pertinent to dissection analysis disclose the variable intensity of carbonate precipitations along the depth of the sample.
References
Bhattarai, P., Tiwari, B., Marui, H.: Variation of soil properties in mudstone with depth and its effect on slope stability. In: Embankments, Dams, and Slopes: Lessons From the New Orleans Levee Failures and Other Current Issues, pp. 1–10 (2007)
Bilgin, Ö., Mansour, E.: Variability of soil properties and reliability of empirical equations on soil settlement predictions. In: Foundation Engineering in the Face of Uncertainty: Honoring Fred H. Kulhawy, pp. 298–307 (2013)
Tahasildar, J., Rao, B.H., Shukla, S.K.: Mineralogical compositions of some indian expansive soils and their influence on swelling properties. Int. J. Geosynth. Ground Eng. 3, 5 (2017). https://doi.org/10.1007/s40891-016-0081-3
Turner, J.P.: Soil stabilization using oil-shale solid waste. J. Geotech. Eng. 120(4), 646–660 (1994)
Poh, H.Y., Ghataora, G.S., Ghazireh, N.: Soil stabilization using basic oxygen steel slag fines. J. Mater. Civ. Eng. 18(2), 229–240 (2006)
Biswal, D.R., Sahoo, U.C., Dash, S.R. Durability and shrinkage studies of cement stabilsed granular lateritic soils. Int. J. Pavement Eng. 1–12 (2018)
Doré, G., Ficheur, A., Guimond, A., Boucher, M.: Performance and cost-effectiveness of thermal stabilization techniques used at the Tasiujaq airstrip. In: Cold Regions Engineering 2012: Sustainable Infrastructure Development in a Changing Cold Environment, pp. 32–41
Pavithra, P., Reddy, M.S., Dinakar, P., Rao, B.H., Satpathy, B.K., Mohanty, A.N.: A mix design procedure for geopolymer concrete with fly ash. J. Clean. Prod. 133, 117–125 (2016)
Reddy, M.S., Dinakar, P., Rao, B.H.: A review of the influence of source materials oxide composition on the compressive strength of geopolymer concrete. J. Microporous Mesoporous Mater. 234, 12–23 (2016)
Joel, M., Agbede, I.O.: Mechanical-cement stabilization of laterite for use as flexible pavement material. J. Mater. Civ. Eng. 23(2), 146–152 (2010)
Mishra, M.C., Babu, K.S., Reddy, N.G., Dey, P.P., Rao, B.H.: Performance of lime stabilization on extremely alkaline red mud waste under acidic environment. J. Hazardous Toxic Radioactive Waste 23(4)
Barnett, H.J., Morse, C.: Scarcity and Growth: The Economics of Natural Resource Availability. RFF Press (2013)
Shashank, B.S., Sharma, S., Sowmya, S., Latha, R.A., Meenu, P.S., Singh, D.N.: State-of-the-art on geotechnical engineering perspective on bio-mediated processes. Environ. Earth Sci. 75(3), 270 (2016)
DeJong, J.T., Soga, K., Banwart, S.A., Whalley, W.R., Ginn, T.R., Nelson, D.C., Barkouki, T., et al.: Soil engineering in vivo: harnessing natural biogeochemical systems for sustainable, multi-functional engineering solutions. J. Royal Soc. Interface 8(54), 1–15 (2010)
Weaver, T.J., Burbank, M., Lewis, A., Lewis, R., Crawford, R., Williams, B.: Bio-induced calcite, iron, and manganese precipitation for geotechnical engineering applications. In Geo-Frontiers 2011: Advances in Geotechnical Engineering, pp. 3975–3983 (2011)
Cheng, L., Cord-Ruwisch, R., Shahin, M.A.: Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 50(1), 81–90 (2013)
Hamed Khodadadi, T., Kavazanjian, E., van Paassen, L., DeJong, J.: Bio-grout materials: a review. In: Grouting, pp. 1–12 (2017)
Yang, Z., Cheng, X., Li, M.: Engineering properties of MICP-bonded sandstones used for historical masonry building restoration. In: Geo-Frontiers: Advances in Geotechnical Engineering, pp. 4031–4040 (2011)
Knapp, C.W., El Mountassir, G., Singh, D.N., Minto, J.M., Shashank, B.S.: Guidance for investigating calcite precipitation by urea hydrolysis for geomaterials. J. Test. Eval. 46(4) (2018)
Mortensen, B.M., Haber, M.J., DeJong, J.T., Caslake, L.F., Nelson, D.C.: Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 111(2), 338–349 (2011)
IS 2720-17: Methods of test for soils, Part 17: Laboratory determination of permeability by Bureau of Indian Standards, New Delhi, India
Whiffin, V.S., van Paassen, L.A., Harkes, M.P.: Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 24(5), 417–423 (2007)
Cheng, L., Cord-Ruwisch, R.: In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 42, 64–72 (2012)
Zhao, Q., Li, L., Li, C., Zhang, H., Amini, F.: A full contact flexible mold for preparing samples based on microbial-induced calcite precipitation technology. Geotech. Test. J. 37(5), 917–921 (2014)
Cheng, L., Shahin, M.A., Mujah, D.: Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotech. Geoenviron. Eng. 143(1), 04016083 (2016)
Van Paassen, L.A., Ghose, R., van der Linden, T.J., van der Star, W.R., van Loosdrecht, M.C.: Quantifying biomediated ground improvement by ureolysis: large-scale biogrout experiment. J. Geotech. Geoenviron. Eng. 136(12), 1721–1728 (2010)
Harkes, M.P., Van Paassen, L.A., Booster, J.L., Whiffin, V.S., van Loosdrecht, M.C.: Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 36(2), 112–117 (2010)
Acknowledgements
Authors place their sincere gratitude’s to Dr. D. N. Singh, Professor and Dr. B. S. Shashank, Research Scholar, in the Department of Civil Engineering at IIT Bombay for their immense support in providing bacteria for the research work. Authors are also thankful to Dr. Anasuya Roychowdhury and members of bioscience laboratory at IIT Bhubaneswar for their constant support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Khanna, V., Sahoo, U.C., Hanumantha Rao, B. (2021). Strength Improvement of Sand by State-of-the-Art Microbially Induced Carbonate Precipitation (MICP) Technique. In: Patel, S., Solanki, C.H., Reddy, K.R., Shukla, S.K. (eds) Proceedings of the Indian Geotechnical Conference 2019 . Lecture Notes in Civil Engineering, vol 136. Springer, Singapore. https://doi.org/10.1007/978-981-33-6444-8_36
Download citation
DOI: https://doi.org/10.1007/978-981-33-6444-8_36
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6443-1
Online ISBN: 978-981-33-6444-8
eBook Packages: EngineeringEngineering (R0)