Abstract
Close causative relationship between psychological stresses and cardiovascular morbidity is now well documented. Research on humans has been attempting to unravel the significance of this association by investigating psychological and social characteristics in relation to cardiovascular health. However, this research is limited by the difficulty to control and standardize for the individual social history, the impossibility to apply psychosocial stress stimuli for mere experimental purposes, as well as the long time span of cardiovascular pathogenesis in humans. Animal studies controlling for social environment and adverse social episodes allow for partially overcoming these limitations. The aim of this review is to provide an up-to-date reference of the experimental evidence so far collected on the link between psychosocial factors and cardiovascular dysfunction in rodents, with special emphasis on modeling stress-induced sudden cardiac death, cardiac arrhythmias, stress cardiomyopathy, and psychogenic hypertension and with focusing on acute and chronic psychological and social stresses, aggressiveness, and negative mood states as causative factors.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
The variety of psychosocial risk factors can be classified into three major categories, namely, the social environment, personality traits, and negative affect (von Kanel 2012). Numerous studies, both clinical/epidemiological and experimental in humans and animals, provide compelling evidence of a tight link between psychosocial factors and cardiovascular morbidity (Costoli et al. 2004; Krantz and McCeney 2002; Rozanski et al. 1999; Sgoifo et al. 2009; Verrier and Lown 1984). Factors such as anxiety and mood states, personality traits such as anger and hostility, coping strategies, socioeconomic status, acute and chronic psychological or social stressors, as well as the absence of significant social support have all been shown to modulate and interfere with cardiovascular health (Albus 2010; De Vogli et al. 2007; Steptoe et al. 2010; Van der Kooy et al. 2007). These psychosocial variables appear to be independent risk factors, as important as traditional ones (smoking, cholesterol levels, waist fat, body mass index, and poor physical activity), for the onset and progression of coronary artery disease, hypertension, myocardial stunning, stroke, and cardiac arrhythmias (Hemingway et al. 2001; Strike and Steptoe 2004; Wittstein et al. 2005).
An intrinsic limitation of research in humans lies in the difficulty to control and standardize for the individual social history preceding laboratory or clinical assessment. In addition, the application of psychosocial stress stimuli for experimental purposes is obviously limited by ethical concerns and regulations. It is here that animal studies become indispensible. Normal cardiovascular consequences of psychological stressors have been extensively studied in animals. Physiological mechanisms mediating such reactions are relatively well understood; this chapter focuses on psychogenic cardiovascular disturbances that cross the physiological homeostatic boundaries and lead to the state of disease. More specifically, it addresses animal models that provide insight into the mechanisms of stress-induced cardiovascular dysfunction. Based on the time scale of their development, psychogenic cardiovascular disturbances could be divided in two types – acute and chronic. Acute, immediate effects occur during or shortly (minutes/hours) after stressful events; stress-induced ventricular arrhythmias and stress (takotsubo) cardiomyopathy belong to this class. Sustained or enduring effects are provoked by chronic stressors and last for many days, months, or years; in humans, cardiovascular disturbances of this type are represented by psychogenic hypertension and depression-related cardiac disturbances.
Cardiac Disturbances Provoked by Acute Psychophysical Stressors
Causes of Sudden Cardiac Death
Psychological distress can provoke sudden death in humans. There is now solid clinical evidence that the principal cause of sudden death is ventricular arrhythmia, which is often preceded by acute psychological disturbances (Lampert et al. 2000; Reich et al. 1981; Rozanski et al. 1999). Other studies reinforce the importance of the link between psychological stress and cardiac arrhythmias (see Rozanski et al. (1999) for comprehensive review). Well-known examples are a sixfold increase in the sudden cardiac death rate on the day of Los Angeles (Northridge) earthquake in 1994 (Leor et al. 1996) and tripling of the incidence of arrhythmias in patients with implanted cardioverting defibrillators following the World Trade Center terrorist attack (Steinberg et al. 2004). An important question in the field of stress-induced cardiac arrhythmias is why some individuals are more susceptible to them than others. The causative role of myocardial electrical instability in the genesis of these arrhythmias is now firmly established, mainly due to extensive studies of patients with long QT syndrome. In these patients, sudden alerting stimuli may trigger polymorphic ventricular tachycardia, with a latency of just a few seconds (Wilde et al. 1999), indicating the neurogenic nature of these arrhythmias.
Heart Rate Is Not an Adequate Biomarker for Ventricular Changes
Animal studies of cardiac effects of acute stress employed various experimental paradigms. Similarly to laboratory or natural stressors in humans, psychological stressors (restraint, fear conditioning, loud noise, airjet, social defeat) consistently elevate heart rate in rats and mice. This tachycardia is a normal physiological response; it has been extensively studied, and its mechanisms are described elsewhere in great detail (see reviews by Bandler et al. 1991, Dampney et al. 2008, and Fontes et al. 2014). It was however rarely acknowledged that tachycardia (an index of autonomic influences at the cardiac pacemaker region) does not adequately reflect pro-arrhythmic status of the ventricular myocardium; in fact, it could be quite misleading. The sinoatrial node, the cardiac conducting system, and the ventricular myocardium may be controlled independently of each other (Nalivaiko et al. 2003, 2007). Consequently, an increase in HR does not necessarily predict neural influences in the myocardium. Furthermore, tachycardia by itself is cardioprotective as it reduces the duration of the ventricular diastolic period with enhanced electrical excitability (also called “ventricular vulnerable period”). These considerations emphasize that in animal studies of stress-induced cardiac dysfunction, heart rate alone is not an adequate index of pro-arrhythmic effects and that assessment of ventricular indices should be employed. Of the variety of such indices, we will focus on the following: (i) direct ECG signs of cardiac arrhythmia, (ii) invasive measurements of cardiac electrical stability, and (iii) radiographic assessment of ventricular wall motion.
Ventricular Arrhythmias in Stressed Animals with Predisposed Hearts
Several studies combined psychological stressors with mechanical or pharmacological intervention that altered the electrical stability of the myocardium prior to subjecting animals to stressors. In an early work performed in pigs (Skinner et al. 1975), an occluder was implanted in the left anterior descending coronary artery during preliminary surgery. After recovery, animals were stressed simply by immobilization and exposure to an unfamiliar environment. When coronary occlusion was performed during this stressor, it precipitated ventricular fibrillation (VF). In a subsequent study, authors demonstrated that crioblockade of the dorsomedial hypothalamus (or areas located just caudal to it) either prevented or markedly delayed the onset of VF after coronary occlusion (Skinner and Reed 1981). In a canine study conducted by Lown et al. (Corbalan et al. 1974), it was demonstrated that psychological stress reliably precipitated ventricular tachyarrhythmias in animals that had just recovered from myocardial infarction. The results of these two studies are complimentary as they both modeled situations in which stress is associated with the acute phase of myocardial infarction (Skinner and Reed 1981) or with the early post-infarct period (Corbalan et al. 1974).
Another way of unmasking arrhythmogenic effects of stressful interventions is presenting them to animals whose myocardial electrical stability is affected by pharmacological means. This approach was employed in early works of Natelson and colleagues who administered digitalis (a cardiac glycoside with well-known cardiotoxic properties) to their fear-conditioned guinea pigs. When a conditioned stimulus was presented to these animals, they developed more malignant arrhythmias and with higher incidence compared to digitalis-injected controls that were not subjected to fear conditioning (Natelson et al. 1978).
A major advance in understanding the mechanisms of sudden cardiac death in humans was the discovery and detailed characterization of the so-called long QT syndrome (LQTS) – a group of inherited (genetic) or acquired (drug-induced) cardiac channelopathies. A large part of these disorders is related to abnormal function of potassium channels expressed in cardiac myocytes; this results in a prolonged repolarization phase (hence “long QT” on the ECG) and elevated myocardial excitability. Patients with LQTS are prone to malignant ventricular tachyarrhythmias (“torsade de pointes”) that may be triggered by either physical or psychological stressors. In rabbits, administration of dofetilide (a selective blocker of the rapid component of the delayed rectifier potassium current) mimics LQT2 type of the syndrome, and presentation of sudden alerting stimuli to these animals consistently triggered ventricular arrhythmias, including torsade-like events (Nalivaiko et al. 2004). The short latencies of these events suggested that they were neurally mediated, and sensitivity to beta-adrenergic blockade confirmed that they occurred due to increased cardiac sympathetic activity. These findings are in good accord with human data indicating that elevated cardiac sympathetic activity could be a principal cause of malignant ventricular tachyarrhythmias (Meredith et al. 1991).
The data presented above emphasize the importance of conducting animal stress studies in models that include provocations of electrical instability in the myocardium as animals with healthy hearts rarely exhibit overt ECG abnormalities. One of the first studies describing stress-induced arrhythmia in animals with intact hearts was conducted in guinea pigs (Natelson and Cagin 1979). While in resting conditions ectopic ventricular beats were only occasionally observed in some animals during 24-h ECG monitoring, restraint stress resulted in frequent occurrence of ectopics in all animals; additionally, in some of them, short periods of ventricular tachycardia were noted. In rat arrhythmia, vulnerability is even larger during social defeat compared to restraint because social defeat causes larger neuroendocrine activation compared to nonsocial stressors (Sgoifo et al. 1997).
Ventricular Electrical Events in Stressed Animals with Intact Hearts
Several cardiac electrophysiological protocols allow assessment of “silent” myocardial electrical stability, i.e., without obvious ECG signs of ventricular arrhythmias. These methods are invasive and require either implantation of cardiac pacing electrodes during preliminary surgery or, in larger animals, inserting them via the femoral artery in acute studies. One way of cardiac electrophysiological assessment is to measure the threshold for ventricular fibrillation (minimal intensity of current that triggers VF). This technique, however, requires subsequent resuscitation of the animal, and it is preferable to measure the threshold for a repetitive ventricular response (repetitive extrasystole) (Lown et al. 1973). Lown and colleagues used this approach in a number of studies in conscious dogs. They found that psychological stress (anticipation of footshocks) substantially reduced the threshold for the repetitive ventricular response. Providing close correlation between this index and ventricular fibrillation threshold, the authors concluded that psychological stress is a potent predisposing/triggering factor for sudden cardiac death. They further demonstrated that sympathetic overactivity is the major mechanism affecting myocardial electrical stability (Matta et al. 1976) and that vagal stimulation counteracts this effect (Kolman et al. 1976).
Using repetitive extrasystole threshold as an index of ventricular vulnerability, in several follow-up studies, Lown and colleagues demonstrated cardioprotective properties of systemically administered serotonin (5-HT) precursors, agonists, or agents affecting central 5-HT levels (Blatt et al. 1979; Rabinowitz and Lown 1978). These cardioprotective effects were associated with an increase of 5-HT content in the cerebrospinal liquid and were largely mediated by reduced cardiac sympathetic nerve activity, unequivocally confirming that the effects were central (Lehnert et al. 1987). The authors thus concluded that altering central serotonergic neurotransmission is a promising therapeutic target for the prevention and treatment of ventricular tachyarrhythmias precipitated by stress. Only recently, this idea has been experimentally confirmed by Nalivaiko et al. (2009) who demonstrated that activation of 5-HT1A receptors with a selective agonist (8-OH-DPAT) virtually abolished stress-induced arrhythmias in rats subjected to social defeat. It is likely that the effect of the drug was due to the inhibition of cardiomotor presympathetic neurons in the medullary raphe, as local microinjection of 8-OH-DPAT into this area efficiently suppressed stress-induced tachycardia in rats and rabbits (Nalivaiko et al. 2005; Ngampramuan et al. 2008). Involvement of central serotonergic neurotransmission has been extensively studied during recent decades (see McCall and Clement 1994, Nalivaiko and Sgoifo 2009, Ramage 2001, and Ramage and Villalon 2008 for reviews), and the above-described experiments are in good agreement with the generally established view that 5-HT1A receptors possess central sympatholytic and vagomimetic properties.
Animal Models of Stress Cardiomyopathy
Stress cardiomyopathy (also known as “apical ballooning,” “takotsubo cardiomyopathy,” or “broken heart syndrome”) was first described by Japanese cardiologists nearly 10 years ago (Satoda et al. 1995); see also relevant chapter in this book. Clinically, the dysfunction mimics myocardial infarction, with symptoms such as chest pain and with ECG abnormalities, but without concomitant coronary spasm or ischemia-induced enzymatic release from the myocardium. Stress cardiomyopathy prevails in postmenopausal women and is usually triggered by potent emotional stressors. The most distinctive feature of this disorder is ventricular wall dysfunction, predominantly in the apical region (Akashi et al. 2010).
A naturalistic animal model of stress cardiomyopathy was developed by Ueyama and colleagues (2002). They found that rats subjected to immobilization stress developed transient left ventricular dysfunction very similar to that observed in humans. The effect was mediated by catecholamines as it was sensitive to pharmacological blockade of adrenoreceptors. This is an interesting example of animal study preceding similar findings in humans: in 2005 Wittstein and colleagues (2005) reported that in patients with stress cardiomyopathy, the levels of plasma catecholamines were dramatically elevated. Using their rat model, Ueyama and colleagues substantially contributed to the understanding of the pathophysiology of the stress cardiomyopathy. They demonstrated that during immobilization stress, activation of adrenoreceptors causes upregulation of immediate early genes in cardiac tissues (Ueyama 2004); potential molecular consequences of this effect are discussed in detail by Akashi et al. (2010).
Rat studies also provided potential explanation of why stress cardiomyopathy occurs more frequently in postmenopausal women. During menopause, a lack of estrogen resulted in cardiac vulnerability to stress; a relevant model approach for this situation is ovariectomized rats. In such animals, estrogen supplementation attenuated immobilization-induced cardiomyopathy (Ueyama et al. 2007). Additionally, it was found that the cardioprotective properties of estrogens could be mediated both at the level of the brain and at the level of end-organs heart and adrenal medulla (Ueyama et al. 2006, 2007).
Importance of Social Factor and Cardiac Disturbances Provoked by Acute Social Stressors
In humans, various forms of inadequate social interaction could be the most relevant stress factor for the onset and progression of stress-related disorders, both psychosomatic (such as cardiovascular pathologies) and psychological (e.g., anxiety and depression) (Bjorkqvist 2001). Social stress episodes do not necessarily imply overt aggressive acts; rather, they usually follow verbal and psychological pathways (Pico-Alfonso et al. 2007). In animals, disputes for territory control, food resources, and sexual partners can be harsh and frequent and may produce severe physical and psychological damage. For many years, stress biologists implemented a variety of experimental paradigms with the aim of understanding the physiological and behavioral features of stress responses and the mechanisms underlying stress-related disturbances. Paradigms that are traditionally used in rodent psychophysical stress procedures (e.g., immobilization or electric shock) bear limited ethological relevance (Sgoifo et al. 2005). More recent research benefited from the use of highly translational, naturalistic stress models such as social conflict, subordination, and long-term social isolation.
Acute and Chronic Effects of Social Defeat
In rodents, a single episode of social defeat has a strong physiological and behavioral impact. Social defeat is obtained by means of the resident-intruder test, which consists in introducing the experimental animal in the territory of a conspecific dominant male. Defeat is the outcome of repeated attacks by the resident rat, and the declaration of subordination by the intruder is clearly detectable on the basis of characteristic behavioral patterns (Koolhaas et al. 2013). In the short term (minutes to hours), defeat produces tachycardia, vagal withdrawal, cardiac arrhythmias, hypertension, hyperthermia, and elevated plasma levels of glucocorticoids and catecholamines (Sgoifo et al. 1999). Interestingly, 8-OH-DPAT (a 5-HT1A receptor agonist) attenuates defeat-induced tachycardia and arrhythmogenesis, due to suppression of both cardiac sympathetic drive and vagal withdrawal (Nalivaiko et al. 2009).
In the long term (days/weeks), social defeat dramatically affects animals’ behavior (McGrady 1984; Meerlo et al. 1996a; Ruis et al. 1999), memory (Von Frijtag et al. 2000), and neuroendocrine and immune function (Buwalda et al. 1999; Engler et al. 2004; Meerlo et al. 1996b; Stefanski and Engler 1998). In particular, social defeat may result in severe perturbations of circadian rhythms of heart rate (Meerlo et al. 1999), which consist mainly in a dampening of the rhythm amplitude that may be due to an increase in the resting phase values and/or a decrease in the active phase ones (Meerlo et al. 2002). These alterations could persist for up to 2 weeks after challenge termination (Meerlo et al. 1999; Tornatzky and Miczek 1993).
When social subordination becomes a chronic state, consequences can be irreversible. For instance, repeated defeat episodes associated with the threat represented by the constant vicinity of the aggressor were shown to produce permanent cardiac anatomical alterations (Costoli et al. 2004). Specifically, when a male mouse was exposed for 2 weeks to a daily episode of aggression and was permanently maintained under the threat of further attacks, a structural damage at the level of the heart was observed, with substantial accumulation of fibrotic tissue – a well-documented substrate for the development of cardiac arrhythmias (Costoli et al. 2004). The outcome of this experiment supports the hypothesis that the physiological responses to an intermittent homotypic stressor, although allowing short-term adaptation and gradually fading in intensity across days, can bring about an overload for the organism in the long term and contribute to permanent pathological alterations (McEwen 1998). The precise mechanisms underlying these alterations are currently unknown; they may include massive release of catecholamines in response to the persistent social threat condition.
When a similar chronic psychosocial stress protocol was applied to mice lacking 1A serotonin receptors (5-HT1A KO mice), a quarter of the animals died from cardiac arrest (Carnevali et al. 2012a). Indeed, genetic lack of 5-HT1A receptors appears to be detrimental for cardiovascular health, by increasing the risk of fatal cardiac events in mice undergoing chronic stress. This evidence corroborates the protective role of these receptors for cardiovascular stress homeostasis, as previously described for acute anti-arrhythmogenic and anti-tachycardic effects of serotonin 1A agonists in a social defeat context (Nalivaiko et al. 2009; Nalivaiko and Sgoifo 2009). Similar cardioprotective role of central 5-HT1A receptors has been reported in cynomolgus monkeys exposed to social stress (Shively et al. 2006).
Another contribution to the elucidation of the neurobiological mechanisms underlying social stress-induced autonomic imbalance came from a recent study by Sevoz-Couche and colleagues (2013). They found that rats exposed to four consecutive days of social defeat sessions developed reduced heart rate variability (HRV), cardiac sympathetic prevalence, and reduced baroreflex gain. The inhibition of the dorsomedial hypothalamus with muscimol and the blockade of the nucleus tractus solitarii 5-HT3 receptors with granisetron reversed these cardiovascular alterations and highlighted the role of these regions in cardiovascular autonomic adaptation following repeated social challenge.
Effects of Social Isolation
Deleterious effect of another social challenge – social isolation – has been extensively studied by Grippo and colleagues in prairie voles (Microtus ochrogaster) (see for instance Grippo 2009; Grippo et al. 2011). The advantage of this animal species is in their stable monogamous social relationships and engagement in the surrounding social context (Carter et al. 2008; Young and Wang 2004) These unique features of sociality make this animal model extremely interesting in translational terms. Female and male prairie voles are sensitive to long-term deprivation of social contact from a sexual partner or a family member, with significant behavioral and physiological consequences including anxious and depressive behavior, as well as autonomic, neuroendocrine, and cardiac dysfunctions (Grippo et al. 2011, 2012; McNeal et al. 2014).
Chronic Social Stressors and Interaction Between Depression and Cardiac Disorders
Assessment of Depression-Like State in Experimental Animals
Both experimental and clinical evidence points to a robust bidirectional link between depressive disorders and cardiovascular dysfunction. In particular, depressed patients have a markedly higher risk of cardiac morbidity (Khawaja et al. 2009; Lippi et al. 2009), independently from traditional risk factors such as hypertension, smoking, elevated cholesterol, physical inactivity, and elevated body mass index (Sowden and Huffman 2009; Whooley 2006). Although many studies highlighted the association between depression and heart disease, common underlying substrates are only partially understood. The major difficulty in conducting animal studies related to depression is the extreme paucity of objective pro-depressive indices; the two most commonly used are the sucrose preference and the immobility in the forced swimming test. The former is based on reduced animals’ normal preference for sugar-sweetened water; this is believed to reflect anhedonia, a classical symptom of depression. In the latter test, reduced swimming activity is believed to reflect despair, another feature of depression in humans. Cardiovascular measures thus are of substantial value here as they can be objectively measured. One possible pathophysiological mechanistic link between depression and cardiac health appears to be an alteration of cardiac sympathovagal balance, due to elevated cardiac sympathetic and/or reduced cardiac vagal tone (Barton et al. 2007; Carney et al. 1988; Kemp et al. 2010; Pitzalis et al. 2001; Rechlin et al. 1994). Perturbations of the autonomic nervous system may result in ventricular tachyarrhythmias and sudden cardiac death, the latter being one of the leading causes of cardiovascular mortality (Zipes and Wellens 1998).
Mechanistic Links Between Depression-Like State and Cardiac Disorders
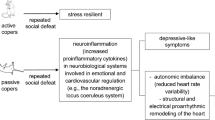
Social stress is a critical environmental factor for the development of both clinical depression (Kendler et al. 1999) and cardiovascular disease (Steptoe and Brydon 2009). Major social life events, such as job strain, the loss of a family member, and social isolation, may sensitize individuals to subsequent stress and thereby increase the risk of developing such disorders (Post 1992). To understand the associations among social stress, depression, and cardiovascular dysfunction, various experimental approaches have been used (Nestler and Hyman 2010). Among them, social challenge based on a single episode of social defeat followed by a period of isolation is an experimental paradigm that relies on robust theoretical prerequisites to meet construct and etiological validity. As previously summarized, social defeat and social isolation separately induce acute and long-lasting behavioral and physiological changes that well resemble core aspects of mood disorders in humans. Using this combined social defeat /social isolation paradigm in rats, Carnevali and colleagues (2012b) recently found that this challenge induced behavioral signs of depression, functional and structural changes of the hypothalamic-pituitary-adrenocortical axis, and increased anxiety levels. The cardiovascular consequences consisted of transitory heart rate circadian rhythm alterations and moderate cardiac hypertrophy.
Only a few studies assessed mechanistic links between chronic social stress and cardiac malfunction. In one of them, Carnevali and colleagues (2013) demonstrated that subjecting rats to repetitive social defeats over 3 weeks results not only in behavioral (reduced sucrose preference) and biological (loss of body weight and alterations in circadian rhythm amplitudes) pro-depression signs but also caused pro-arrhythmic effect on cardiac electrophysiological variables. The high-density epicardial mapping analysis revealed a significant decrease in transversal conduction velocity of the electrical wavefront, a shortening of the effective refractory period and an increase in myocardial excitability in the ventricles of stressed animals compared to controls, conditions that are generally considered as important determinants of arrhythmogenesis (Carnevali et al. 2013).
Behavioral Coping Style and Cardiac Vulnerability
In humans it is now well established that certain patterns of behavior are associated with elevated cardiovascular risk. Specifically, individuals with so-called “type A” behavior (aggressive, hostile, impatient, competitive, achievement striving) are more vulnerable to heart disease than type B counterparts (relative absence of type A characteristics) (Betensky and Contrada 2010; Friedman and Rosenman 1959; Kop 1999; Rozanski et al. 1999; Smith 1992; Smith et al. 2004). Putative pathophysiological mechanisms that mediate this link may include an impairment of autonomic nervous system control over cardiac function. Abundant evidence demonstrates that reduced autonomic modulation of the heart, as shown by HRV measurements, predicts the development of heart disease in initially healthy subjects (Liao et al. 1997; Tsuji et al. 1996), as well as poorer survival rate in patients with myocardial infarction or heart failure (Bigger et al. 1992; La Rovere et al. 1998, 2003). A deeper insight into the underlying mechanisms might be gained by the use of animal models, since most described behavioral traits in humans are identifiable in animals as well. In rats, the extent of the trait level of aggressiveness could be assessed on the basis of the latency time to attack a male intruder in a classical resident-intruder test (Koolhaas et al. 2013). In line with the characterization of personality in many other animal species (Bell and Sih 2007; Groothuis and Carere 2005; Reale et al. 2007; Sih et al. 2004), high levels of aggression in rodents are considered an important indicator and component of a more general proactive coping style, whereas low levels of aggression are believed to be a reflection of a reactive coping style (Koolhaas 2008; Koolhaas et al. 1999). These divergent behavioral coping styles have frequently been associated with different patterns of both autonomic nervous and endocrine (re)activity (de Boer et al. 2003; Koolhaas et al. 1999). However, the investigation of the cardiac autonomic control of these distinct behavioral and physiological coping styles has been conducted only sporadically and provided inconclusive evidence (Sgoifo et al. 2005, 1996). In a recent rat study specifically addressing this issue, Catnevali and colleagues (2013) found that high-aggressive rats had reduced resting heart rate variability, mostly in terms of lower vagal modulation, compared to nonaggressive animals. Most importantly, high-aggressive rats had higher incidence of ventricular tachyarrhythmias during stress or during pharmacological beta-adrenergic stimulation. These findings are consistent with the view that high levels of aggressive behavior in rats are associated with signs of cardiac autonomic impairment and increased arrhythmogenic susceptibility that may predict vulnerability to cardiac morbidity and mortality.
Psychogenic Hypertension
Epidemiological Evidence of Psychogenic Hypertension
Numerous epidemiological studies provide compelling evidence for the causative role of psychosocial factors in developing essential hypertension in humans (Henry and Cassel 1969; Steptoe 2000). One of the best known is a longitudinal study of more than a hundred Italian nuns living monastic life: in the course of this study (20 years), there were virtually no changes in their values of systolic and diastolic arterial pressure (AP). Conversely, in age-matched laywomen, AP substantially and significantly increased over the same period (Timio et al. 1988). In a later paper, the authors confirmed their initial finding by describing another 12 years of the follow-up and presented arguments suggesting that other lifestyle differences between nuns and laywomen did not contribute to the AP differences found between the two groups (Timio et al. 2001). “Job strain” (professional activities associated with high demand and low perceived control) is now a recognized lifestyle risk factor for developing hypertension, as demonstrated by many carefully designed studies (e.g., Pickering 2004; Schnall et al. 1990).
Early Animal Models of Psychogenic Hypertension
Not surprisingly, numerous attempts were made to establish an animal model of stress-induced hypertension, and the aim of this section is to provide a critical review of the most relevant studies in this area. “Stress-induced hypertension” here is defined as long-term, sustained elevation of the AP that lasts well beyond the duration of stressors; the acute pressor effects of psychological stressors are very well documented, extensively studied, and broadly described elsewhere. One major conclusion that could be made on the basis of existing studies is that their results are often conflicting and contradictory. Various factors that potentially contributed to this controversy may include different kind of stress protocols, duration of their presentation, animals’ age and their strain, and, most importantly, the method used to assess AP.
In two early rat studies, animals were subjected to unpredictable noxious sensory stimuli (flashing lights, loud sounds, shaking cages) three times a week for 12–20 weeks (Cox et al. 1985; Smookler and Buckley 1969). Substantial hypertension was found starting from the second week of stress and lasted throughout the entire stress period. In one of these studies, animals were monitored for 20 weeks after the termination of the stress protocol, and during all this period, AP remained high.
Role of Genetic Makeup in Psychogenic Hypertension
Some researchers were particularly interested in the potential genetic predisposition in the rats’ sensitivity to stressors. To address this issue, Lawler (Lawler et al. 1981) set up a novel animal model called borderline hypertensive rats (BHR, a first-generation offspring of SHR and Wistar-Kyoto rats) that had mildly elevated AP, without frank hypertension. It was suggested that such prehypertensive animals would be more susceptible to psychological stressors in terms of developing sustained hypertension. When BHR were subjected to a complex stress paradigm (conditioned footshock avoidance) on a daily basis, they developed elevated blood pressure within 2–3 weeks; this hypertension persisted during the entire stress period (3–4 months) as well as during the post-stress phase (Lawler et al. 1981, 1984). Interestingly, substantial inconsistencies were reported even in studies performed in the same laboratory and using the same animal strain and stress paradigm: in the first study (Lawler et al. 1981), AP steadily increased in the course of the experiment reaching a difference of 40 mmHg, while in the second study, the pressor effect was smaller (20–25 mmHg), reached plateau during 2–3 weeks, and did not increase further (Lawler et al. 1984). It is important to note that in all studies mentioned so far, AP was determined indirectly, using the tail-cuff method.
BHR rats were used in several later experiments employing less complex stressors such as inescapable footshocks (Cox et al. 1985), restraint with airjet (Hatton et al. 1993), or social crowding (Muller et al. 2001), all producing variable pressor effects (10–30 mmHg). Tail-cuff method was used in all of these studies, and in two of them, AP was additionally measured at the end of stress period directly, using catheters implanted into the aorta (Cox et al. 1985; Hatton et al. 1993). Later, Mansi and Drolet (1997) reported mild hypertension in BHR subjected to 8-week restraint/airjet stress. Finally, in two other studies where AP in BHR was assessed using biotelemetry, no signs of stress-induced hypertension were found (Mansi and Drolet 1997; Muller et al. 2001).
Another approach for assessing the effect of genetic predisposition on psychogenic hypertension was based on breeding two rat lines based on their AP response to salt ingestion. These lines are known as Dahl-sensitive and Dahl-resistant rats. Aversive Pavlovian conditioning did not provoke hypertension in these rats (Dahl et al. 1968), but when they were subjected to a highly noxious footshock/food conflict (in order to obtain food pellet, rats had to press a lever that triggered a footshock), Dahl-sensitive rats had developed hypertension that persisted for the entire 25-week stress period (Friedman and Iwai 1976). The hypertensive effect of stress was also present in Dahl-resistant animals, but developed slower and was less prominent.
Controversy in the Research on Psychogenic Hypertension in Animals and Potential Causes of This Controversy
A good example of unexplained controversy in chronic stress/hypertension animal studies could be illustrated by the following sequence of experimental works.
Initially, Henry and Cassel (1969) presented evidence that various stressors (social crowding, exposure to a cat odor, and territorial fights) provoke considerable sustained elevation in the AP in mice (65). This finding was reproduced in rats by Wexler and Greenberg (1978) who also found that maintaining an unstable social hierarchy, by frequently moving males from one communal cage to another, provokes dramatic hypertension (+80 mmHg), even in normotensive Sprague-Dawley rats. Several years after, however, Harrap et al. (1984) put these findings under serious doubt by demonstrating that a similar chronic stress paradigm had absolutely no effect on AP, not only in normotensive SD and WKY but also in BHR rats. In response, Henry conducted a very detailed and prolonged (6-month) study using exactly the same stress protocol as Wexler and found that, indeed, AP remains unaffected in WKY rats, is slightly elevated (~10 mmHg) during some measuring monthly points in SD rats, and slowly raises in Long-Evans rats (Henry et al. 1993). Authors explain this interstrain difference by the higher levels of aggression and more frequent fights displayed by Long-Evans rats. It remained however entirely unexplained why identical stress procedures applied to the same strain (Sprague-Dawley) provoked rapid and dramatic hypertensive effects within 1 month in the first study (Wexler and Greenberg 1978), but had only very mild and delayed action in the later one (Henry et al. 1993).
It must be noted that in most of the above-cited studies, AP was assessed using the tail-cuff method – a simple noninvasive technique based on the same principle as indirect measurement of arterial pressure in humans. However, tail-cuff measurement has some serious limitations. Firstly, the tail vascular bed is thermoregulatory in rats and mice, and at normal laboratory ambient temperatures of 20–25 °C, tail blood flow varies very significantly, sometimes falling near zero (Blessing 2005; Garcia et al. 2001). Thus, in order to maintain it at a constant and measurable level, animals should be kept in a warm environment (33–38 °C) before and during the measurements. Secondly, animals should be restrained, and restrainers used for this purpose – plastic cylinders – are not dissimilar to those used for provoking restraint stress. Thirdly, it is not widely recognized that the tail vascular bed is controlled separately from other vasculatures, and even mild stressors can provoke dramatic selective vasoconstriction in the tail vascular bed (Blessing 2005). Thus, it is questionable whether AP measures taken by means of tail cuff represent true resting values. Indeed, several recent studies where AP was assessed telemetrically during tail-cuff procedure support this doubts: Van Acker et al. (2001) found that tail-cuff measurement provokes substantial increases in both AP and heart rate. In another carefully designed study, it was convincingly demonstrated that even after 3 weeks (nine sessions) of habituation, restraint and heating still provoked dramatic pressor and tachycardic responses, as well as elevation in plasma noradrenaline and angiotensin II concentrations (Grundt et al. 2009). Finally, handling alone, as well as handling and restraint, provoke substantial rises in AP and heart rate, and these responses do not habituate even after ten sessions (McDougall et al. 2005).
Biotelemetry is a “gold standard” for assessing cardiovascular parameters in conscious unrestrained animals. So far there are four published studies that used this method in order to determine whether chronic stress has any sustained effects on AP (Bobrovskaya et al. 2013; Gelsema et al. 1994; Lemaire and Mormede 1995; Muller et al. 2001). Together, these studies involved four rat strains, including BHR and SHR, and employed the same stress paradigms that caused hypertension in previous studies using tail-cuff measurements. Marginal elevation of AP was found in only one of them (Bobrovskaya et al. 2013); in three other instances, AP remained consistently stable.
In summary, it appears that experimenters using tail-cuff measurements succeeded in demonstrating that only some stressors evoke rise in AP in only some rat strains, whereas most biotelemetric studies failed to demonstrate any effect on the blood pressure of any stressor in any strain. It is thus most likely that the major cause of such dramatic discrepancies is the intrusiveness of the tail-cuff method, with associated restraint: values measured in this case may reflect the animal’s reaction to the measurement procedure rather than the basal levels of AP in the undisturbed state. This most probably happened at least in those cases where these measurements were performed only once a week (Lawler et al. 1984; Wexler and Greenberg 1978) or even once a month (Henry et al. 1993), without any preliminary habituation. It could be that previously stressed animals responded more vigorously to the tail-cuff procedure compared to unstressed controls. While manufacturers recommend that prior to the tail-cuff measurements, the animals should be habituated to measurement conditions, it is currently not clear how many habituation sessions are sufficient.
Tail cuff is still widely used, and several more recent studies employed it to document the hypertensive effects of chronic stress in normotensive rat strains (Andrews et al. 2003; Blake et al. 1995); in one instance, this was confirmed by direct measurements as well (Alkadhi et al. 2005). The dynamics of developing hypertension in this later study (plateau at +40 mmHg in just 4 days) is in sharp contrast with several other previous reports where pressor changes required 2–3 weeks of stress exposure. Presented above considerations suggest that positive pressor effects of prolonged stressors must be taken with some caution.
It may be that factors other than the method of AP assessment contributed to discrepancies in results from different studies as well as to inconsistencies between animal models of psychogenic hypertension and the actual condition in humans. In his seminal review, when discussing psycho-emotional causes of hypertension, Folkow (1982) suggested that if pressor-inducing stress episodes are repeated frequently enough, this may lead to sustained elevation of AP. In most works reviewed here, stressful procedures were either performed daily or were lasting permanently, and further increasing the frequency of stressful interventions does not seem to be a promising idea. Another issue that could contribute to the conflicting results in animal models of psychogenic hypertension is the type of stressor that is employed. It is now accepted in the field of stress research that a critical determinant for a stressor to induce adverse consequences is the ability of the animal to exert control over these events (Maier 1984). Having actual or perceived control over a stressful situation powerfully reduces its negative physiological consequences. It is likely that animals exposed to chronic or repetitive aversive stressors develop effective coping strategies to these putative adverse events that somehow combat the deleterious effects of these stressors. It thus follows that future animal studies targeting psychogenic hypertension must develop and employ new stress protocols specifically focusing on uncontrollability and unpredictability of stressors.
Conclusions and Clinical Implications
From the data summarized in this chapter, it is evident that animal models of acute and chronic stress-induced cardiac disturbances are extremely useful. They allowed researchers to reproduce human clinical conditions, provided insight into pathogenesis, and also suggested new therapeutic strategies. In some instances, they preceded and predicted clinical findings made in humans. It is likely that by following this research direction with rodent models, it will be possible to obtain not only clearer insights into the physiological/neural/behavioral substrates of psychological and social stress-related psychosomatic disorders but also the biological evidence of the beneficial effects of social support and, more generally speaking, of positive social relationships.
References
Akashi, Y. J., Nef, H. M., Mollmann, H., & Ueyama, T. (2010). Stress cardiomyopathy. Annual Review of Medicine, 61, 271–286.
Albus, C. (2010). Psychological and social factors in coronary heart disease. Annals of Medicine, 42(7), 487–494. doi:10.3109/07853890.2010.515605.
Alkadhi, K. A., Alzoubi, K. H., Aleisa, A. M., Tanner, F. L., & Nimer, A. S. (2005). Psychosocial stress-induced hypertension results from in vivo expression of long-term potentiation in rat sympathetic ganglia. Neurobiology of Disease, 20(3), 849–857.
Andrews, E., Jenkins, C., Seachrist, D., Dunphy, G., & Ely, D. (2003). Social stress increases blood pressure and cardiovascular pathology in a normotensive rat model. Clinical and Experimental Hypertension, 25(2), 85–101.
Bandler, R., Carrive, P., & Zhang, S. P. (1991). Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: Viscerotopic, somatotopic and functional organization. Progress in Brain Research, 87, 269–305.
Barton, D. A., Dawood, T., Lambert, E. A., Esler, M. D., Haikerwal, D., Brenchley, C.,... Lambert, G. W. (2007). Sympathetic activity in major depressive disorder: Identifying those at increased cardiac risk? Journal of Hypertension, 25(10), 2117–2124.
Bell, A. M., & Sih, A. (2007). Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecology Letters, 10(9), 828–834. doi:10.1111/j.1461-0248.2007.01081.x.
Betensky, J. D., & Contrada, R. J. (2010). Depressive symptoms, trait aggression, and cardiovascular reactivity to a laboratory stressor. Annals of Behavioral Medicine, 39(2), 184–191. doi:10.1007/s12160-010-9176-6.
Bigger, J. T., Jr., Fleiss, J. L., Steinman, R. C., Rolnitzky, L. M., Kleiger, R. E., & Rottman, J. N. (1992). Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation, 85(1), 164–171.
Bjorkqvist, K. (2001). Social defeat as a stressor in humans. Physiology and Behavior, 73(3), 435–442.
Blake, M. J., Klevay, L. M., Halas, E. S., & Bode, A. M. (1995). Blood pressure and heat shock protein expression in response to acute and chronic stress. Hypertension, 25(4 Pt 1), 539–544.
Blatt, C. M., Rabinowitz, S. H., & Lown, B. (1979). Central serotonergic agents raise the repetitive extrasystole threshold of the vulnerable period of the canine ventricular myocardium. Congressional Record, 44(5), 723–730.
Blessing, W. W. (2005). Clozapine increases cutaneous blood flow and reduces sympathetic cutaneous vasomotor alerting responses (SCVARs) in rats: Comparison with effects of haloperidol. Psychopharmacology, 181(3), 518–528.
Bobrovskaya, L., Beard, D., Bondarenko, E., Beig, M. I., Jobling, P., Walker, F. R.,... Nalivaiko, E. (2013). Does exposure to chronic stress influence blood pressure in rats? Autonomic Neuroscience, 177(2), 217–223. doi:10.1016/j.autneu.2013.05.001.
Buwalda, B., de Boer, S., ED, S., Felszeghy, K., Nyakas, C., A, S.,... Koolhaas, J. (1999). Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. Journal of Neuroendocrinology, 11(7), 513–520.
Carnevali, L., Mastorci, F., Audero, E., Graiani, G., Rossi, S., Macchi, E.,... Sgoifo, A. (2012a). Stress-induced susceptibility to sudden cardiac death in mice with altered serotonin homeostasis. PLoS One, 7(7), e41184. doi:10.1371/journal.pone.0041184, [pii] PONE-D-12-09604.
Carnevali, L., Mastorci, F., Graiani, G., Razzoli, M., Trombini, M., Pico-Alfonso, M. A.,... Sgoifo, A. (2012b). Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiology and Behavior, 106(2), 142–150. doi:10.1016/j.physbeh.2012.01.022.
Carnevali, L., Trombini, M., Porta, A., Montano, N., de Boer, S. F., & Sgoifo, A. (2013a). Vagal withdrawal and susceptibility to cardiac arrhythmias in rats with high trait aggressiveness. PLoS One, 8(7), e68316. doi:10.1371/journal.pone.0068316.
Carnevali, L., Trombini, M., Rossi, S., Graiani, G., Manghi, M., Koolhaas, J. M.,... Sgoifo, A. (2013). Structural and electrical myocardial remodeling in a rodent model of depression. Psychosomatic Medicine, 75(1), 42–51. doi:10.1097/PSY.0b013e318276cb0d, [pii] PSY.0b013e318276cb0d.
Carney, R. M., Rich, M. W., teVelde, A., Saini, J., Clark, K., & Freedland, K. E. (1988). The relationship between heart rate, heart rate variability and depression in patients with coronary artery disease. Journal of Psychosomatic Research, 32(2), 159–164.
Carter, C. S., Grippo, A., Pournajafi-Nazarloo, H., Ruscio, M. G., & Porges, S. W. (2008). Oxytocin, vasopressin and sociality. Prog. in brain res (Vol. 170, pp. 331–336).
Corbalan, R., Verrier, R., & Lown, B. (1974). Psychological stress and ventricular arrhythmias during myocardial infarction in the conscious dog. Congressional Record, 34(6), 692–696.
Costoli, T., Bartolomucci, A., Graiani, G., Stilli, D., Laviola, G., & Sgoifo, A. (2004). Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. American Journal of Physiology – Heart and Circulatory Physiology, 286(6), H2133–H2140. doi:10.1152/ajpheart.00869.2003.
Cox, R. H., Hubbard, J. W., Lawler, J. E., Sanders, B. J., & Mitchell, V. P. (1985). Exercise training attenuates stress-induced hypertension in the rat. Congressional Record, 7(5), 747–751.
Dahl, L. K., Knudsen, K. D., Heine, M., & Leitl, G. (1968). Hypertension and stress. Nature, 219(5155), 735–736.
Dampney, R., Horiuchi, J., & McDowall, L. (2008). Hypothalamic mechanisms coordinating cardiorespiratory function during exercise and defensive behaviour. Autonomic Neuroscience, 142, 3–10.
de Boer, S. F., van der Vegt, B. J., & Koolhaas, J. M. (2003). Individual variation in aggression of feral rodent strains: A standard for the genetics of aggression and violence? Behavior Genetics, 33(5), 485–501.
De Vogli, R., Chandola, T., & Marmot, M. G. (2007). Negative aspects of close relationships and heart disease. Archives of Internal Medicine, 167(18), 1951–1957. doi:10.1001/archinte.167.18.1951.
Engler, H., Dawils, L., Hoves, S., Kurth, S., Stevenson, J. R., Schauenstein, K., & Stefanski, V. (2004). Effects of social stress on blood leukocyte distribution: The role of alpha- and beta-adrenergic mechanisms. Journal of Neuroimmunology, 156(1–2), 153–162. doi:10.1016/j.jneuroim.2004.08.005.
Folkow, B. (1982). Physiological aspects of primary hypertension. Congressional Record, 62(2), 347–504.
Fontes, M. A., Xavier, C. H., Marins, F. R., Limborco-Filho, M., Vaz, G. C., Muller-Ribeiro, F. C., & Nalivaiko, E. (2014). Emotional stress and sympathetic activity: Contribution of dorsomedial hypothalamus to cardiac arrhythmias. Brain Research, 1554, 49–58. doi:10.1016/j.brainres.2014.01.043.
Friedman, R., & Iwai, J. (1976). Genetic predisposition and stress-induced hypertension. Congressional Record, 193(4248), 161–162.
Friedman, M., & Rosenman, R. H. (1959). Association of specific overt behavior pattern with blood and cardiovascular findings; blood cholesterol level, blood clotting time, incidence of arcus senilis, and clinical coronary artery disease. Journal of the American Medical Association, 169(12), 1286–1296.
Garcia, J. N., Pedersen, N. P., Nalivaiko, E., & Blessing, W. W. (2001). Tail artery blood flow measured by chronically implanted Doppler ultrasonic probes in unrestrained conscious rats. Journal of Neuroscience Methods, 104(2), 209–213.
Gelsema, A. J., Schoemaker, R. G., Ruzicka, M., & Copeland, N. E. (1994). Cardiovascular effects of social stress in borderline hypertensive rats. Journal of Hypertension, 12(9), 1019–1028.
Grippo, A. J. (2009). Mechanisms underlying altered mood and cardiovascular dysfunction: The value of neurobiological and behavioral research with animal models. Neuroscience and Biobehavioral Reviews, 33(2), 171–180. doi:10.1016/j.neubiorev.2008.07.004.
Grippo, A. J., Carter, C. S., McNeal, N., Chandler, D. L., Larocca, M. A., Bates, S. L., & Porges, S. W. (2011). 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosomatic Medicine, 73(1), 59–66. doi:10.1097/PSY.0b013e31820019e4.
Grippo, A. J., Moffitt, J. A., Sgoifo, A., Jepson, A. J., Bates, S. L., Chandler, D. L.,... Preihs, K. (2012). The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: Investigating the role of negative social experiences in an animal model. Psychosomatic Medicine, 74(6), 612–619. doi:10.1097/PSY.0b013e31825ca8e5.
Groothuis, T. G., & Carere, C. (2005). Avian personalities: Characterization and epigenesis. Neuroscience and Biobehavioral Reviews, 29(1), 137–150. doi:10.1016/j.neubiorev.2004.06.010.
Grundt, A., Grundt, C., Gorbey, S., Thomas, M. A., & Lemmer, B. (2009). Strain-dependent differences of restraint stress-induced hypertension in WKY and SHR. Physiology and Behavior, 97(3–4), 341–346.
Harrap, S. B., Louis, W. J., & Doyle, A. E. (1984). Failure of psychosocial stress to induce chronic hypertension in the rat. Journal of Hypertension, 2(6), 653–62.
Hatton, D. C., DeMerritt, J., Coste, S. C., & McCarron, D. A. (1993). Stress-induced hypertension in the borderline hypertensive rat: Stimulus duration. Congressional Record, 53(4), 635–641.
Hemingway, H., Malik, M., & Marmot, M. (2001). Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. European Heart Journal, 22(13), 1082–1101. doi:10.1053/euhj.2000.2534.
Henry, J. P., & Cassel, J. C. (1969). Psychosocial factors in essential hypertension. Recent epidemiologic and animal experimental evidence. Congressional Record, 90(3), 171–200.
Henry, J. P., Liu, Y. Y., Nadra, W. E., Qian, C. G., Mormede, P., Lemaire, V.,... Hendley, E. D. (1993). Psychosocial stress can induce chronic hypertension in normotensive strains of rats. Hypertension, 21(5), 714–723.
Kemp, A. H., Quintana, D. S., Gray, M. A., Felmingham, K. L., Brown, K., & Gatt, J. M. (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074. doi:10.1016/j.biopsych.2009.12.012.
Kendler, K. S., Karkowski, L. M., & Prescott, C. A. (1999). Causal relationship between stressful life events and the onset of major depression. The American Journal of Psychiatry, 156(6), 837–841.
Khawaja, I. S., Westermeyer, J. J., Gajwani, P., & Feinstein, R. E. (2009). Depression and coronary artery disease: The association, mechanisms, and therapeutic implications. Psychiatry (Edgmont), 6(1), 38–51.
Kolman, B. S., Verrier, R. L., & Lown, B. (1976). Effect of vagus nerve stimulation upon excitability of the canine ventricle. Role of sympathetic-parasympathetic interactions. American Journal of Cardiology, 37(7), 1041–1045.
Koolhaas, J. M. (2008). Coping style and immunity in animals: Making sense of individual variation. Brain, Behavior, and Immunity, 22(5), 662–667. doi:10.1016/j.bbi.2007.11.006.
Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H.,... Blokhuis, H. J. (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews, 23(7), 925–935.
Koolhaas, J. M., Coppens, C. M., de Boer, S. F., Buwalda, B., Meerlo, P., & Timmermans, P. J. (2013). The resident-intruder paradigm: A standardized test for aggression, violence and social stress. Journal of Visualized Experiments, 77, e4367. doi:10.3791/4367.
Kop, W. J. (1999). Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosomatic Medicine, 61(4), 476–487.
Krantz, D. S., & McCeney, M. K. (2002). Effects of psychological and social factors on organic disease: A critical assessment of research on coronary heart disease. Annual Review of Psychology, 53, 341–369. doi:10.1146/annurev.psych.53.100901.135208.
La Rovere, M. T., Bigger, J. T., Jr., Marcus, F. I., Mortara, A., & Schwartz, P. J. (1998). Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet, 351(9101), 478–484.
La Rovere, M. T., Pinna, G. D., Maestri, R., Mortara, A., Capomolla, S., Febo, O.,... Cobelli, F. (2003). Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation, 107(4), 565–570.
Lampert, R., Jain, D., Burg, M. M., Batsford, W. P., & McPherson, C. A. (2000). Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation, 101(2), 158–164.
Lawler, J. E., Barker, G. F., Hubbard, J. W., & Schaub, R. G. (1981). Effects of stress on blood pressure and cardiac pathology in rats with borderline hypertension. Congressional Record, 3(4), 496–505.
Lawler, J. E., Barker, G. F., Hubbard, J. W., Cox, R. H., & Randall, G. W. (1984). Blood-pressure and plasma-renin activity responses to chronic stress in the borderline hypertensive rat. Physiology & Behavior, 32(1), 101–105.
Lehnert, H., Lombardi, F., Raeder, E. A., Lorenzo, A. V., Verrier, R. L., Lown, B., & Wurtman, R. J. (1987). Increased release of brain serotonin reduces vulnerability to ventricular fibrillation in the cat. Journal of Cardiovascular Pharmacology, 10(4), 389–397.
Lemaire, V., & Mormede, P. (1995). Telemetered recording of blood pressure and heart rate in different strains of rats during chronic social stress. Physiology and Behavior, 58(6), 1181–1188.
Leor, J., Poole, W. K., & Kloner, R. A. (1996). Sudden cardiac death triggered by an earthquake. New England Journal of Medicine, 334(7), 413–419.
Liao, D., Cai, J., Rosamond, W. D., Barnes, R. W., Hutchinson, R. G., Whitsel, E. A.,... Heiss, G. (1997). Cardiac autonomic function and incident coronary heart disease: A population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 145(8), 696–706.
Lippi, G., Montagnana, M., Favaloro, E. J., & Franchini, M. (2009). Mental depression and cardiovascular disease: A multifaceted, bidirectional association. Seminars in Thrombosis and Hemostasis, 35(3), 325–336. doi:10.1055/s-0029-1222611.
Lown, B., Corbalan, R., & Verrier, R. L. (1973). Psychologic stress and threshold for repetitive ventricular response. Congressional Record, 182(114), 834–836.
Maier, S. F. (1984). Learned helplessness and animal models of depression. Progress in Neuropsychopharmacology and Biological Psychiatry, 8(3), 435–446.
Mansi, J. A., & Drolet, G. (1997). Chronic stress induces sensitization in sympathoadrenal responses to stress in borderline hypertensive rats. Congressional Record, 272(3 Pt 2), R813–820.
Matta, R. J., Lawler, J. E., & Lown, B. (1976). Ventricular electrical instability in the conscious dog: Effects of psychologic stress and beta adrenergic blockade. American Journal of Cardiology, 38(5), 594–598.
McCall, R. B., & Clement, M. E. (1994). Role of serotonin1A and serotonin2 receptors in the central regulation of the cardiovascular system. Pharmacological Reviews, 46(3), 231–243.
McDougall, S. J., Lawrence, A. J., & Widdop, R. E. (2005). Differential cardiovascular responses to stressors in hypertensive and normotensive rats. Experimental Physiology, 90(1), 141–150.
McEwen, B. S. (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338(3), 171–179.
McGrady, A. V. (1984). Effects of psychological stress on male reproduction: A review. Archives of Andrology, 13(1), 1–7.
Meerlo, P., De Boer, S. F., Koolhaas, J. M., Daan, S., & Van den Hoofdakker, R. H. (1996a). Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiology and Behavior, 59(4–5), 735–739.
Meerlo, P., Overkamp, G. J., Benning, M. A., Koolhaas, J. M., & Van den Hoofdakker, R. H. (1996b). Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiology and Behavior, 60(1), 115–119.
Meerlo, P., Sgoifo, A., De Boer, S. F., & Koolhaas, J. M. (1999). Long-lasting consequences of a social conflict in rats: Behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behavioral Neuroscience, 113(6), 1283–1290.
Meerlo, P., Sgoifo, A., & Turek, F. W. (2002). The effects of social defeat and other stressors on the expression of circadian rhythms. Stress, 5(1), 15–22. doi:10.1080/102538902900012323.
Meredith, I. T., Broughton, A., Jennings, G. L., & Esler, M. D. (1991). Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. Congressional Record, 325(9), 618–624.
Muller, J. R., Le, K. M., Haines, W. R., Gan, Q., & Knuepfer, M. M. (2001). Hemodynamic response pattern predicts susceptibility to stress-induced elevation in arterial pressure in the rat. Congressional Record, 281(1), R31–37.
Nalivaiko, E., & Sgoifo, A. (2009). Central 5-HT receptors in cardiovascular control during stress. Neuroscience and Biobehavioral Reviews, 33(2), 95–106. doi:10.1016/j.neubiorev.2008.05.026. [pii] S0149-7634(08)00094-8.
Nalivaiko, E., De Pasquale, C. G., & Blessing, W. W. (2003). Electrocardiographic changes associated with the nasopharyngeal reflex in conscious rabbits: Vago-sympathetic co-activation. Autonomic Neuroscience, 105(2), 101–104. doi:10.1016/S1566-0702(03)00048-1. [pii] S1566-0702(03)00048-1.
Nalivaiko, E., De Pasquale, C. G., & Blessing, W. W. (2004). Ventricular arrhythmias triggered by alerting stimuli in conscious rabbits pre-treated with dofetilide. Basic Research in Cardiology, 99(2), 142–151. doi:10.1007/s00395-003-0448-1.
Nalivaiko, E., Ootsuka, Y., & Blessing, W. W. (2005). Activation of 5-HT1A receptors in the medullary raphe reduces cardiovascular changes elicited by acute psychological and inflammatory stresses in rabbits. American Journal of Physiology – Regulatory Integrative & Comparative Physiology, 289(2), R596–R604.
Nalivaiko, E., Catcheside, P. G., Adams, A., Jordan, A. S., Eckert, D. J., & McEvoy, R. D. (2007). Cardiac changes during arousals from non-REM sleep in healthy volunteers. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 292(3), R1320–R1327. doi:10.1152/ajpregu.00642.2006. [pii] 00642.2006.
Nalivaiko, E., Mastorci, F., & Sgoifo, A. (2009). 8-OH-DPAT prevents cardiac arrhythmias and attenuates tachycardia during social stress in rats. Physiology and Behavior, 96(2), 320–327.
Natelson, B. H., & Cagin, N. A. (1979). Stress-induced ventricular arrhythmias. Congressional Record, 41(3), 259–262.
Natelson, B. H., Cagin, N. A., Donner, K., & Hamilton, B. E. (1978). Psychosomatic digitalis-toxic arrhythmias in guinea pigs. Congressional Record, 22(24), 2245–2250.
Nestler, E. J., & Hyman, S. E. (2010). Animal models of neuropsychiatric disorders. Nature Neuroscience, 13(10), 1161–1169. doi:10.1038/nn.2647.
Ngampramuan, S., Baumert, M., Beig, M. I., Kotchabhakdi, N., & Nalivaiko, E. (2008). Activation of 5-HT(1A) receptors attenuates tachycardia induced by restraint stress in rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 294(1), R132–R141. doi:10.1152/ajpregu.00464.2007. [pii] 00464.2007.
Pickering, T. G. (2004). Reflections in hypertension: Work and blood pressure. Congressional Record, 6(7), 403–405.
Pico-Alfonso, M. A., Mastorci, F., Ceresini, G., Ceda, G. P., Manghi, M., Pino, O.,... Sgoifo, A. (2007). Acute psychosocial challenge and cardiac autonomic response in women: The role of estrogens, corticosteroids, and behavioral coping styles. Psychoneuroendocrinology, 32(5), 451–463. doi:10.1016/j.psyneuen.2007.02.009.
Pitzalis, M. V., Iacoviello, M., Todarello, O., Fioretti, A., Guida, P., Massari, F.,... Rizzon, P. (2001). Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. American Heart Journal, 141(5), 765–771. doi:10.1067/mhj.2001.114806.
Post, R. M. (1992). Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. The American Journal of Psychiatry, 149(8), 999–1010.
Rabinowitz, S. H., & Lown, B. (1978). Central neurochemical factors related to serotonin metabolism and cardiac ventricular vulnerability for repetitive electrical activity. American Journal of Cardiology, 41(3), 516–522.
Ramage, A. G. (2001). Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Research Bulletin, 56(5), 425–439.
Ramage, A. G., & Villalon, C. M. (2008). 5-Hydroxytryptamine and cardiovascular regulation. Trends in Pharmacological Sciences, 29, 472–481.
Reale, D., Reader, S. M., Sol, D., McDougall, P. T., & Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biological Reviews of the Cambridge Philosophical Society, 82(2), 291–318. doi:10.1111/j.1469-185X.2007.00010.x.
Rechlin, T., Weis, M., Spitzer, A., & Kaschka, W. P. (1994). Are affective disorders associated with alterations of heart rate variability? Journal of Affective Disorders, 32(4), 271–275.
Reich, P., DeSilva, R. A., Lown, B., & Murawski, B. J. (1981). Acute psychological disturbances preceding life-threatening ventricular arrhythmias. JAMA, 246, 233–235.
Rozanski, A., Blumenthal, J. A., & Kaplan, J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation, 99(16), 2192–2217.
Ruis, M. A., te Brake, J. H., Buwalda, B., De Boer, S. F., Meerlo, P., Korte, S. M.,... Koolhaas, J. M. (1999). Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology, 24(3), 285–300.
Satoda, T., Takahashi, O., Uchida, T., & Mizuno, N. (1995). An anterograde-retrograde labeling study of the carotid sinus nerve of the Japanese monkey (Macaca fuscata). Neuroscience Research, 22, 381–387.
Schnall, P. L., Pieper, C., Schwartz, J. E., Karasek, R. A., Schlussel, Y., Devereux, R. B.,... Pickering, T. G. (1990). The relationship between ‘job strain,’ workplace diastolic blood pressure, and left ventricular mass index. Results of a case-control study. JAMA, 263(14), 1929–1935.
Sevoz-Couche, C., Brouillard, C., Camus, F., Laude, D., De Boer, S. F., Becker, C., & Benoliel, J. J. (2013). Involvement of the dorsomedial hypothalamus and the nucleus tractus solitarii in chronic cardiovascular changes associated with anxiety in rats. Journal of Physiology, 591(Pt 7), 1871–1887. doi:10.1113/jphysiol.2012.247791. [pii] jphysiol.2012.247791.
Sgoifo, A., de Boer, S. F., Haller, J., & Koolhaas, J. M. (1996). Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: Relationship with aggression. Physiology and Behavior, 60(6), 1403–1407.
Sgoifo, A., de Boer, S. F., Westenbroek, C., Maes, F. W., Beldhuis, H., Suzuki, T., & Koolhaas, J. M. (1997). Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. American Journal of Physiology, 273(4 Pt 2), H1754–H1760.
Sgoifo, A., Koolhaas, J. M., De Boer, S. F., Musso, E., Stilli, D., Buwalda, B., & Meerlo, P. (1999). Social stress, autonomic neural activation, and cardiac activity in rats. Neuroscience and Biobehavioral Reviews, 23(7), 915–923.
Sgoifo, A., Costoli, T., Meerlo, P., Buwalda, B., Pico’-Alfonso, M. A., De Boer, S.,... Koolhaas, J. (2005). Individual differences in cardiovascular response to social challenge. Neuroscience and Biobehavioral Reviews, 29(1), 59–66. doi:10.1016/j.neubiorev.2004.07.001.
Sgoifo, A., Montano, N., Shively, C., Thayer, J., & Steptoe, A. (2009). The inevitable link between heart and behavior: New insights from biomedical research and implications for clinical practice. Neuroscience and Biobehavioral Reviews, 33(2), 61–62. doi:10.1016/j.neubiorev.2008.10.007.
Shively, C. A., Friedman, D. P., Gage, H. D., Bounds, M. C., Brown-Proctor, C., Blair, J. B.,... Buchheimer, N. (2006). Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of General Psychiatry, 63(4), 396–403.
Sih, A., Bell, A. M., Johnson, J. C., & Ziemba, R. E. (2004). Behavioral syndromes: An integrative overview. Quarterly Review of Biology, 79(3), 241–277.
Skinner, J. E., & Reed, J. C. (1981). Blockade of frontocortical-brain stem pathway prevents ventricular fibrillation of ischemic heart. American Journal of Physiology, 240(2), H156–H163.
Skinner, J. E., Lie, J. T., & Entman, M. L. (1975). Modification of ventricular fibrillation latency following coronary artery occlusion in the conscious pig. Circulation, 51(4), 656–667.
Smith, T. W. (1992). Hostility and health: Current status of a psychosomatic hypothesis. Health Psychology, 11(3), 139–150.
Smith, T. W., Glazer, K., Ruiz, J. M., & Gallo, L. C. (2004). Hostility, anger, aggressiveness, and coronary heart disease: An interpersonal perspective on personality, emotion, and health. Journal of Personality, 72(6), 1217–1270. doi:10.1111/j.1467-6494.2004.00296.x
Smookler, H. H., & Buckley, J. P. (1969). Relationships between brain catecholamine synthesis, pituitary adrenal function and the production of hypertension during prolonged exposure to environmental stress. Congressional Record, 8(1), 33–41.
Sowden, G. L., & Huffman, J. C. (2009). The impact of mental illness on cardiac outcomes: A review for the cardiologist. International Journal of Cardiology, 132(1), 30–37. doi:10.1016/j.ijcard.2008.10.002.
Stefanski, V., & Engler, H. (1998). Effects of acute and chronic social stress on blood cellular immunity in rats. Physiology and Behavior, 64(5), 733–741.
Steinberg, J. S., Arshad, A., Kowalski, M., Kukar, A., Suma, V., Vloka, M.,... Rozanski, A. (2004). Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. Journal of the American College of Cardiology, 44(6), 1261–1264.
Steptoe, A. (2000). Psychosocial factors in the development of hypertension. Annals of Medicine, 32, 371–375.
Steptoe, A., & Brydon, L. (2009). Emotional triggering of cardiac events. Neuroscience and Biobehavioral Reviews, 33(2), 63–70. doi:10.1016/j.neubiorev.2008.04.010.
Steptoe, A., Hamer, M., O'Donnell, K., Venuraju, S., Marmot, M. G., & Lahiri, A. (2010). Socioeconomic status and subclinical coronary disease in the Whitehall II epidemiological study. PLoS One, 5(1), e8874. doi:10.1371/journal.pone.0008874.
Strike, P. C., & Steptoe, A. (2004). Psychosocial factors in the development of coronary artery disease. Progress in Cardiovascular Diseases, 46(4), 337–347.
Timio, M., Verdecchia, P., Venanzi, S., Gentili, S., Ronconi, M., Francucci, B., Montanari, M., & Bichisao, E. (1988). Age and blood pressure changes. A 20-year follow-up study in nuns in a secluded order. Congressional Record, 12(4), 457–461.
Timio, M., Saronio, P., Verdura, C., Schiaroli, M., Timio, F., & Monarca, C. (2001). A link between psychosocial factors and blood pressure trend in women. Congressional Record, 73(3), 359–363.
Tornatzky, W., & Miczek, K. A. (1993). Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiology and Behavior, 53(5), 983–993.
Tsuji, H., Larson, M. G., Venditti, F. J., Jr., Manders, E. S., Evans, J. C., Feldman, C. L., & Levy, D. (1996). Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation, 94(11), 2850–2855.
Ueyama, T. (2004). Emotional stress-induced Tako-tsubo cardiomyopathy: Animal model and molecular mechanism. Annals of the New York Academy of Sciences, 1018, 437–444.
Ueyama, T., Kasamatsu, K., Hano, T., Yamamoto, K., Tsuruo, Y., & Nishio, I. (2002). Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: A possible animal model of ‘tako-tsubo’ cardiomyopathy. Circulation Journal, 66(7), 712–713.
Ueyama, T., Tanioku, T., Nuta, J., Kujira, K., Ito, T., Nakai, S., & Tsuruo, Y. (2006). Estrogen alters c-Fos response to immobilization stress in the brain of ovariectomized rats. Brain Research, 1084(1), 67–79.
Ueyama, T., Ishikura, F., Matsuda, A., Asanuma, T., Ueda, K., Ichinose, M.,... Beppu, S. (2007). Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circulation Journals, 71(4), 565–573.
Van Acker, S. A., Fluttert, M. F., Sibug, R. M., & De Kloet, E. R. (2001). Intracerebroventricular administration of a glucocorticoid receptor antagonist enhances the cardiovascular responses to brief restraint stress. Congressional Record, 430(1), 87–91.
Van der Kooy, K., van Hout, H., Marwijk, H., Marten, H., Stehouwer, C., & Beekman, A. (2007). Depression and the risk for cardiovascular diseases: Systematic review and meta analysis. International Journal of Geriatric Psychiatry, 22(7), 613–626. doi:10.1002/gps.1723.
Verrier, R. L., & Lown, B. (1984). Behavioral stress and cardiac arrhythmias. Congressional Record, 46, 155–176.
Von Frijtag, J. C., Reijmers, L. G., Van der Harst, J. E., Leus, I. E., Van den Bos, R., & Spruijt, B. M. (2000). Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behavioural Brain Research, 117(1–2), 137–146.
von Kanel, R. (2012). Psychosocial stress and cardiovascular risk: Current opinion. Swiss Medical Weekly, 142, w13502. doi:10.4414/smw.2012.13502.
Wexler, B. C., & Greenberg, B. P. (1978). Pathophysiological differences between paired and communal breeding of male and female Sprague-Dawley rats. Congressional Record, 42(1), 126–135.
Whooley, M. A. (2006). Depression and cardiovascular disease: Healing the broken-hearted. JAMA, 295(24), 2874–2881. doi:10.1001/jama.295.24.2874.
Wilde, A., Jongbloed, R., Doevendans, P., Duren, D., Hauer, R., van Langen, I.,... Geelen, J. (1999). Auditory stimuli as a trigger for arrhythmic events differentiate HERG-related (LQTS2) patients from KVLQT1-related patients (LQTS1). Journal of the American College of Cardiology, 33(2), 327–332.
Wittstein, I. S., Thiemann, D. R., Lima, J. A., Baughman, K. L., Schulman, S. P., Gerstenblith, G.,... Champion, H. C. (2005). Neurohumoral features of myocardial stunning due to sudden emotional stress. New England Journal of Medicine, 352(6), 539–548.
Young, L. J., & Wang, Z. (2004). The neurobiology of pair bonding. Nature Neuroscience, 7(10), 1048–1054. doi:10.1038/nn1327.
Zipes, D. P., & Wellens, H. J. (1998). Sudden cardiac death. Circulation, 98(21), 2334–2351.
McNeal, N., Scotti, M. A., Wardwell, J., Chandler, D. L., Bates, S. L., Larocca, M.,... Grippo, A. J. (2014). Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic Neuroscience, 180, 9–16. doi:10.1016/j.autneu.2013.10.001.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this entry
Cite this entry
Nalivaiko, E., Carnevali, L., Grippo, A.J., Sgoifo, A. (2016). Animal Models of Psychogenic Cardiovascular Disorders. In: Alvarenga, M., Byrne, D. (eds) Handbook of Psychocardiology. Springer, Singapore. https://doi.org/10.1007/978-981-287-206-7_45
Download citation
DOI: https://doi.org/10.1007/978-981-287-206-7_45
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-205-0
Online ISBN: 978-981-287-206-7
eBook Packages: Behavioral Science and PsychologyReference Module Humanities and Social SciencesReference Module Business, Economics and Social Sciences