Abstract
Precious metals including Au, Pd, Pt, Ag and Pd and other metals such as Fe, Co, Mg and Ni as solid powder, dispersion in solution and deposition as thin films have attained wide interest in the last decades. They have induced intense research interest in nanotechnology due to their exciting properties including good conductivity, magnetic recording, localized surface plasmon resonance, antibacterial and catalytic effects [1, 2]. This chapter will introduce several main synthesis and characterization methods of metallic nanoparticles (NPs). The unique feature, key parameters and especially advantages and disadvantages of top-down (i.e. physical vapour deposition, bill milling and lithography) and bottom-up (e.g. chemical vapour deposition, sol–gel, hydrothermal/solvothermal, etc.) methodologies are discussed to trigger advances in nanotechnology advancement. Alternative green synthesis approaches are also included in this chapter. Furthermore, the basic characterization techniques for metallic NPs are pointed out for improving synthesis strategies, deciphering the topography evolution and comprehending the potential applications. Finally, emphasis has been placed on some main properties of metallic NPs for the potential of a wide range of applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Metallic nanoparticles
- Physicochemical characterization
- Nanoparticle synthesis
- Properties of metallic nanoparticles
1 Introduction
Nanoparticles (NPs) have attracted research interests for over a century and are strongly believed to be the key to future technology due to their large proportion of high-energy nanosized surface atoms compared to bulk materials, which gives rise to outstanding chemical, magnetic, physical, mechanical, catalytic and optical properties. NPs have emerged as an amazing class of materials which is defined within the dimensional range of ~1–100 nm. Depending on the overall shape, these materials can be zero-, one-, two- or three-dimensional. For decades, researchers have been paying more and more attention to different metal NPs such as Au, Ag, Pt, Pd, Fe, Co, Mg and Ni, focussing on their synthesis method, characterisations and applications in energy, magnetic imaging, drug delivery, information technology and optoelectronics. Numerous synthesis methods are being established to either enhance the properties or reduce production costs. Furthermore, synthetic techniques and fabrication tools have been continuously studied to permit the production of reproducible nanostructures. It is worth noting that various parameters should also be monitored when NPs are stored due to the changes with time in various environments. Compared to bulk materials, it is sometimes incomplete and inherently difficult to properly analyse the nanomaterials due to the small size and low quantity (i.e. laboratory-scale production). Moreover, the arrangement of particles or atoms in nanostructures brings forth unusual, sometimes exotic forms (e.g. core–shell NPs, fullerenes, nanostructured metals, dendrites, etc.). The NPs characterization is understudied and therefore demands reproducing and validating both theoretical and experimental findings for better scientific understanding, which will benefit the development of new technologies and address important issues, e.g. product lifetimes, etc. Hence, it is important to update new development in the synthesis and characterization of nanomaterials, especially the analysis of their structures, which is believed essential for further progress in nanotechnology. Here in, we explore the nature and causes of a few main NPs synthesis methods and their analytical encounters.
2 Method in Metallic Nanoparticles Synthesis
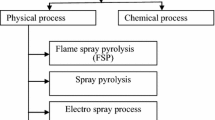
A wide range of methods have been introduced to produce metal nanoparticles which are categorized into two main types as top-down and bottom-up approaches depending on the starting materials. Hence, bulk materials are usually used as starting materials for the top-down approach while atoms or molecules are typically used for the bottom-up process (Fig. 1). Ball milling, physical vapour deposition (PVD) and lithography are typical top-down methods, while laser pyrolysis, chemical vapour deposition, hydrothermal, sol–gel and electrodeposition are classic methods for the bottom-up approach. However, these all-synthesis techniques are toxic, resulting in environmental contamination; thus, biological methods as an alternative green synthesis were found to be less toxic and environmental friendly. Different preparation methods of metallic NPs create various single or multiple metal (alloy) with different sizes, shapes and structures because of the variation of the parameters during the synthesis process (e.g. stabilizing agent for metallic NPs during the adsorption process, etc.).
2.1 Top-Down Methods
2.1.1 Mechanical Ball Milling
In this method, bulk material which is usually in the micro-dimensions is grounded down to the nanoscale by applying strong mechanical shear forces. Four types of attrition devices are generally used, namely vibration mills, planetary ball mill, tumbler ball milling and attrition mill (as shown in Fig. 2).
Different types of ball milling and its working principles: a Planetary ball milling, b Tumbler ball milling, c Attrition ball milling and d Vibration ball milling (where f is vibration frequency, A is vibration amplitude and ω is angular velocity) [3]
Among all top-down methods, ball milling has been widely used for the synthesis of various alloy nanoparticles and composites such as Al, Co, Mg, Ti, Cu and Fe [4,5,6,7,8]. By using this technique, the nanostructures are fabricated by mechanical attrition, where kinetic energy from grinding balls is used to reduce the size of material [9]. Milling conditions including the size of the milling vial, type of mills (low- or high-energy), milling speed, shape of milling media (balls or rods), milling atmosphere (e.g. inert gas or hydrogen), ball-to-powder weight ratio, milling time, milling environment (dry or wet milling) and milling temperature have a direct effect on properties, stoichiometry, particle size distribution and degree of disorder or amorphization of the final products. A more comprehensive control and monitoring of the milling conditions are suggested to improve the quality of the products.
The raw material’s temperature influences the defect concentration and diffusivity inside it, which also influences the phase transformations during milling [10]. Meanwhile, processing time is also a significant factor for this technique. Based on the literature, an increase in time of milling process improves the microhardness of as-prepared materials [11]. Generally, the rate of the internal structure refinement (e.g. crystallite size, particle size, lamellar spacing, etc.) is roughly logarithmic with processing time and thus the size of the starting materials is relatively unimportant. The lamellar spacing usually becomes small, and the grain size is refined to nanoscale within a few minutes to an hour (Fig. 1); therefore, the milled powders will exhibit increased lattice strain while decreased grain size, hence the resulting milled powders will be in a highly energetic condition [3].
Advantages: Ball milling is well known as a simple, low-production-cost and sustainable technique, and it also possesses the capability to achieve very high yields. The nanoparticle’s size ranging from 2 to 20 nm can be achieved through the different speeds of the rotation of the balls. This technique is one of the most reliable, easy-operation and reproducible process because of the speed and energy controls; moreover, it is suitable in both dry and wet conditions for a wide range of materials [4, 7, 12].
Disadvantages: Key serious issue with the milling of fine powders is the possibility of significant contamination from the milling atmosphere or media [4, 7, 9]. Iron contamination can be a problem for steel balls and containers. It has been reported that 10 atomic percentage of the iron contamination in some refractory metallic powders have been found by extended milling times in a high-energy shaker mill. On the other hand, if milling is carried out in open atmosphere, contamination with nitrogen or oxygen can occur. However, optimized milling speed and milling time may effectively help to minimize the contamination issue.
2.1.2 Nanolithography
Nanolithography is one of the most accurate and classic methods for the synthesis of nanoparticle pattern, and it provides high-resolution structures over large areas (>1 cm2) with good control of all the dimensions (length, width, height) and other features such as roughness, edge shape and inter-diffusion of the as-prepared materials [13]. This technique is widely used in integrated circuits manufacturing and nanoelectromechanical systems [14]. Lithography can be categorized into 2 main types including masked and maskless lithographies. Masked Lithography utilizes the mask or template to transfer pattern over the large area. The form of mask lithography includes soft lithography, nanoimprint lithography, [15] X-ray lithography and photolithography. On the other hand, maskless lithography technique (including electron beam lithography, Scanning Probe Lithography, etc.) fabricates subjective patterns by a series writing without the use of the mask. In general, the masked lithography has better controllability compared to maskless lithography. Masked lithography is commonly used to prepare nanomaterials that are highly dependent on the specific shape and size of the selected template. The principle of a few different types of lithography is presented in Fig. 3.
Different type of mask and maskless lithography technique [16]
Additionally, mask can be classified into 2 types, soft mark and hard mask. Surfactant molecules (e.g. CTAB, TEOS, etc.) are usually used as a soft mask, while polymeric materials (e.g. PDMS PMMA, PS, PFPE and other elastomeric material) are usually used as hard mask [17]. In general, the lithography technique is a three-step process: (1) preparation of a colloidal crystal mask made of nanospheres; (2) deposition of the desired product through the mask; and (3) etching of the mask from the layer.
Sub-10 nm Mo gratings have been successfully prepared by using photolithography [18]. However, the photolithography demands the use of a stepper, which is too expensive to be afforded by a standard research laboratory. Dot arrays can either be achieved using four-beam interference lithography or achromatic Talbot lithography (i.e. high-resolution photolithography) [19, 20]. E-beam lithography can produce features as small as 20 nm of Au NPs, [21] but is very expensive and time-consuming. Hence, it would be reasonable to use photolithography for 5 min while using e-beam lithography for approximately another 5 h to complete a lithographic process. Pros and cons of each lithography techniques are outlined in Table 1.
2.1.3 Physical Vapour Deposition (PVD)
PVD involves either high-temperature evaporation or ions bombardment of solid form materials in vacuum atmosphere; meanwhile, the reactive gas is also introduced into the system to form a compound with metal vapour and then deposited onto the substrate as a highly adherent thin film or NPs [26]. PVD can be separated into different types such as electron beam evaporation deposition (EBD), Ion plating (IP), thermal evaporation deposition (TED), pulsed laser deposition (PLD), atomic layer deposition (ALD), cathode arc deposition (CAD), dynamic ion mixing (DIM), electrophoretic deposition and sputtering technique. The type of evaporation source and deposition substrate is the essential parameter that affect the deposition quality and efficiency. The evolution of size, sharpness and phase of the product also depends on the gas pressure and deposition temperature in the deposition chamber [27]. Typically, this process takes place at the temperature in the range of 100–600 °C and is widely used for fabrication of inorganic thin films (thickness less than 5 µm). PVD coating can be used in a broad range of applications in automotive, aerospace, optical, medical, thin films (e.g. food packaging, Window tint, etc.) and textile industry.
Among all PVD techniques, PLD is carried out at low substrate temperatures with a stoichiometry of the target retained in the evaporated films; hence, it is often used for the thin film electrolytes deposition [28]. Two advantages of PLD are the simplicity in process design and the multi-choice of the target forms (e.g. sintered pellet, powder, single crystal, etc.). For the EBD technique, both the conductor and insulator can be used as a target. Meanwhile, EBD of organic materials is limited by the decomposition of the molecules. In general, a vacuum environment of 10−2 − 10−4 Pa, with deposition rates of at least 25 μm/min, is typical condition required for EBD [29]. Moreover, components are commonly preheated in vacuum at temperatures between 800 and 1100 °C, and rotation process is required during the deposition period. EBD is a more efficient choice for evaporating materials with high melting point than other PVD heating and evaporate techniques. However, it is limited for coating the inner surface of complex geometries with the thickness of thin film below 5 nm. The filament degradation in the electron gun could be a cause of non-uniform evaporating rate. The deposition rate of EBD can be obtained up to 100 μm/min depending on the materials and set up conditions [30].
TED technique involves heating a solid material (located in evaporation source, e.g. boat, coil and basket at the bottom of the chamber) to its melting point [31]. The evaporated metal constitutes vapour steam inside a vacuum chamber and then travels across the chamber to the deposition target (located at the top of the chamber), sticking to it as a coating or film. In general, TED of metals with low to moderate deposition rates at 10–100 nm/min provides a simple and reliable manner to produce nanoparticles with a wide range of size; nevertheless, this method is not applicable to substances with high cohesive energies that require high temperature for the evaporation [32]. Material holders are normally made from W, Ta, Mo or ceramic materials that capable of bearing high temperatures. Many materials can be deposited using this method, including Al, Ag, Ni, Cr, Mg, etc. The selection of the PVD technique depends on the material type and possible application. If we are looking for growing thin metallic film, EBD might be the preferred choice compared to the TED. None of PLD technique is better in all aspects. If we are looking for a good step coverage, faster deposition rate, and efficiently, EBD can be preferred, while the sputtering gives you good control on the thickness and density of the films.
Sputtering is good for the deposition of high melting point materials like refractory metals and ceramics, which are tough to be transformed to nanomaterials by evaporation technique. It has been reported that the deposition rates of sputtering technique are much lower than the EBD route (i.e. up to 50 μm/h for a magnetron system or about 10–20 μm/h for a diode system) [33]. Additionally, sputtering offers greater stoichiometric control of the thin film compared to the TED techniques. Thin films from sputtering generally have a higher density than those thin films obtained from the evaporation process while the evaporated films are likely to cause less contamination than those sputtering films because of the lower purity of the sputtering target. Inert gas condensation (IGC) is a PVD method with extra function of using the inert gas to reduce the mean value of the free path of the species. IGC technique involves two steps: evaporation of target materials in nanoscale and rapid condensation of the evaporated material. Several different techniques have been employed to evaporate inorganic or metallic materials into a vaporized form, e.g. Joule-heated refractory crucibles, laser/plasma heating or electron beam evaporation devices, etc. Typically, inert gas including He, Ne, Ar, helium, neon, argon, Kr, Xe and Ra with pressures >3 mPa is required during the process [34]. The size and shape of the as-prepared NPs can be managed by different factors including the temperature and pressure of the chamber, evaporation rate and molecular weight of the inert gas that injected into the chamber. A high pressure in the growth region could obtain large particle size due to the consecutive agglomeration and less sintering can be used to synthesize high-purity metallic/bimetallic nanoparticles. It also offers a high surface cleanliness and well-defined grain size with a narrow size distribution of the as-prepared NPs compared to other PVD method. The cost of operation for ultra-high vacuum-based deposition systems of IGC is remarkably high. IGC involves an extremely slow process and suffers from other limitations, e.g. temperature ranges, the source-precursor incompatibility, dissimilar evaporation rates in an alloy, etc.
Advantage: PVD technique can be utilised to deposit a virtually wide range of inorganic materials including metal alloys, ceramics, glass and polymer as well as some of the organic materials. Moreover, it is more environmentally and user friendly compared with other top-down process. The process demands the high vacuum condition to minimize an unwanted reaction within the free space, which helps to shape the film composition easily and causes less contamination on any substrate surface [35]. The purity of the deposited film depends on the vacuum and the quality of the source material. Coating thin film on materials via this technique will offer higher hardness value, more corrosive resistance, good impact strength and excellent abrasion.
Disadvantage: Difficult to coat undercuts and similar surface feature, high capital cost but low deposition rate. Some PVD processes are typically required to be carried out under high temperature and high vacuum, which cause a high-energy consumption and demands extra attention for operation. Water cooling systems are also needed to dissipate the large heat loads (Table 2).
2.2 Bottom-Up Methods
2.2.1 Laser Pyrolysis (LP)
LP technique involves the use of a continuous flowing of CO2, leading to molecular decomposition to form vapours to initiate nucleation, followed by the growth of NPs [48]. On the other hand, some of the other gases such ammonia (NH3), sulphur hexafluoride (SF6) and ethylene gas (C2H4) are also in use [49]. Next, NPs will be further transported to a filter by an inert gas depending on its amount. The main criterion is that either reactant or precursor should be able to absorb the energy that is supplied through the resonant vibrational mode of infrared CO2 laser radiations. Inside the system, in most of the time, gaseous precursors are used in LP synthesis; however, solid or liquid precursors can be preferable in many cases, due to safety issues or the cost and availability of the precursor; hence, volatile precursor is not abundantly available for some metals.
LP provides superfast heating and quenching of particle growth on a small area, hence resulting in rapidly nucleation within few ms. It has been acknowledged that coalescence is higher at the high temperature, resulting in spherical particles, while other shapes are obtained at low temperature. In addition, the final particle size also depends on the aggregation of the initial nuclei. Gas pressure plays a significant role in determining the particle size and their distribution. Moreover, other parameters including laser intensity, the pressure in the reactor cell, the temperature of precursor were also varied in order to obtain different crystallinity and particle size [50]. In general, LP provides more possibilities for NPs with narrow size distribution of 5–200 nm.
Advantage: LP mode provides a very rapid, in-depth heating to a steady-state temperature, offering a high and continuous production of well-dispersed NPs [51]. Moreover, it is the simplest method for producing many nanostructures even at pilot plant dimensions by balancing CO2 laser exposure and continuous flow of reactor.
Disadvantage: Elevated costs are the main constraints of this method. The requirement of a specific reagent/laser resonance with a specific installation is essential.
2.2.2 Sol–Gel
Sol–gel is a well-known wet-chemical process where a chemical solution acts as a precursor for an integrated system of discrete particles. Among bottom-up synthesis routes, sol–gel is one of the most preferred methods because of its simplicity, easy control of the particle morphology and size, as well as the ability for large volume production of metallic nanomaterial. In general, the method involves two main reactions including hydrolysis of the precursor in the acidic or base mediums (reaction 1) and polycondensation of the hydrolysed products (reactions 2 and 3) [52, 53].
In hydrolysis reaction, an alkoxy group [OR] is replaced with a hydroxyl [OH-]. The part of hydrolysed alkoxide molecules may either react with another OH–species by removing water or react with alkoxy group to produce an alcohol molecule. The hydrolysis reaction rate can be accelerated by using catalyst such as HCI and NH3. Other parameters such as reagent concentration, ageing and drying process are also important for the structure and properties of final materials network for sol–gel method.
Metal alkoxides (Mx (OR)y) and chloride compounds are usually used as starting precursors and dissolved in a suitable solvent, which is often ethanol. After completing the hydrolysis/polycondensation reaction process, wet gel product will be dried to obtain the final material (i.e. Aerogel, Xerogel or Cryogel). Ageing process could help to cushion a warping and cracking phenomenon of the gel matrix. Generally, the drying temperature is in the range of 300–500 °C to remove residual organics. Additional calcination or sintering process (temperature up to 800 °C) can also be applied to remove the organic liquid and densify the product in the final step, if required. Normally, the sintering is used for improving the density and reducing of the pore volume and surface area of as-prepared products. On the other hand, calcination often helps in term of mechanically stable of the materials. Xerogel can be obtained from the uncontrolled drying process. It is characterized by disordered porosity in the absence of structure directing agent. Meanwhile, well-controlled drying process to achieve moderate shrinkage of the gel matrix will offer the aerogel type. Hence, drying is the key process to minimize the impact on the porous structure of gel matrix. Figure 4 shows the schematic of sol–gel process from precursor to aerogel.
Schematic of sol–gel process: from precursor to aerogel [54]
Parameters such as the ratio of water to alkoxide, the nature of the R-group (e.g. inductive effects) and amount of catalysts strongly affected the reaction of sol–gel process [55]. As an example of silica preparation, the sol–gel chemical reaction normally requires either base or acid catalysts as the neutral reaction is very slow. The structure of the synthesized gel is substantially different depending on the type of additional catalyst as it can offer different in the relative rates of the hydrolysis and condensation reactions. Example of some metallic nanomaterials that synthesized via sol–gel technique is presented in Table 3.
Advantage: This method is simple, highly controllable, economical and efficient to produce high quality and high yield of nanoparticles. Moreover, it can be used for a thick coating to provide protection layer that help to against corrosion of substrate surfaces or used for thin layer coating to ensure a great bond between the top layer and substrate. This method consumes less energy compared to other techniques, especially top-down techniques. There is no need to reach the melting temperature of the precursors for low temperature reaction.
Disadvantage: The products often shrinkage and there could be crack formation during drying process, making it difficult to attain a large monolithic piece. The cost of the precursors is high. The process in general involves the use of organic solution that is toxic and harmful to environment. It is hard to avoid residual porosity and OH groups. Finally, the agglomeration issue might occur during the heating process [56].
2.2.3 Chemical Vapour Deposition (CVD)
The key difference between CVD and PVD is that the raw material/percussors for CVD is presented into the reaction chamber in the vapour phase, while the percussors are in solid form for the PVD process. The reaction of CVD process prefers to occur at the substrate rather than in the gas phase. Generally, the temperature ranges from 300 to 1200 °C (higher than PVD process) at the substrate and gas pressures are suggested to be in the range of 0.1 to 1.0 torr. Different factors can affect the deposition process and final produce quality including the precursor delivery method, carrier gas and its chemical properties, the reactor chamber pressure, deposition time and rate, substrate temperature, flow rate of precursor vapour and substrate properties [65]. CVD can be categorized into several types, such as low-pressure, atmospheric-pressure, photo-enhanced, metalorganic and thermal activated CVDs [66].
From Fig. 5, the deposition process can be summarized as three main stages (1) the volatile precursors are introduced to the reactor chamber via carrier gas; (2) the precursor vapours are adsorbed on the substrate surface and then form the intermediate products; and (3) these products are decomposed on the heated substrate, nucleated and grown as the solid layer/grains. The volatile by-products are generated and removed from the chamber by the carrier gas. Growth rate and quality of the thin film depend on the gas pressure inside the reaction chamber and the substrate temperature.
Reaction in the chemical vapour deposition [67]
Advantage: CVD offers high uniformity and good reproducibility and adhesion, with acceptable deposition rate [51]. It is not limited to a line-of-sight deposition which is a general characteristic of most of PVD processes. Complex three-dimensional configurations like recesses and holes can be relatively easily coated.
Disadvantage: The synthesis of nanostructures using CVD technique is limited by reason of the involvement of toxic precursors, trace impurities, processing time and prolonged reaction [51]. Possible chemical hazards due to toxic, corrosive and explosive precursor gases. The process typically happened at high temperatures depending on the evaporation temperature of the precursor. Size is limited to reaction chamber capacity.
2.2.4 Hydrothermal/Solvothermal
Hydrothermal/solvothermal synthesis is a solution reaction-based approach, and the reaction temperature could be in a wide range (typically between 100 to 1000 °C). Briefly, the mixed percussor solutions was transferred into lined Teflon and sealed in autoclave made of stainless steel, followed by heating in an oven at a specific temperature and reaction time [68]. Various metal NPs such as Ag, Ni, Cu and Ru [72, 75,76,77,78] have been successfully synthesized by using hydrothermal/solvothermal. An image of different NPs is shown in Fig. 6. Crystals with different morphologies (3D-sphere, 2D-rod or 1D-wire structures) are formed by varying the solvent type, concentration of precursor and kinetic control (e.g. reaction time and temperature, etc.).
SEM image of (a) Ag NPs, synthesized at 200 °C for 6 h [71], (b) Cu NPs, synthesized at 100 °C for 10 h, (c) Au NPs, synthesized at 110 °C for 12 h [72], (d) CuS nanostructure, synthesized at 150 °C for 5 h [73], TEM image of (e) Al/Zn NPs, synthesized at 200 °C for 6 h [74] and (f) Ru@C nanocomposites, synthesized at 200 °C for 24 h [75]
In general, the reaction medium of hydrothermal synthesis is aqueous solution, while the chemical reaction of solvothermal synthesis happens in various organic mediums. Water has a low boiling point which limits its ability to many reactions that require high-temperature condition. Moreover, water has a high polarity which unable to solubilize many organic and non-polar molecules; hence, capping agents/surfactant (e.g. oleic acid (OA), polyethylenimine (PEI), Ethylenediaminetetraacetic acid (EDTA) and cetyltrimethylammonium bromide (CTAB)) might be needed to stabilize inorganic nanomaterials [69, 70]. Typical precursors for hydrothermal are usually metal-nitrate, -chloride or -acetate compounds.
Advantage: The compositions of nanomaterials can be well simplified and the size, shape distribution and crystallinity in hydrothermal synthesis can be precisely controlled through varying parameters, e.g. temperatures and time of the reaction, the types of precursors, surfactants and solvents. The process can be used to prepare many geometries including bulking powders, single crystals, thin film and nanocrystals.
Disadvantage: Safety issue during the reaction process, high equipment cost (i.e. autoclave) and low products yielded could be the main drawbacks of this technique. Moreover, the reaction process during the reaction cannot be observed. Similar to sol–gel technique, hydrothermal/solvothermal require long time reaction period. This method requires soluble precursor, and large amount of solvent waste can be generated.
2.2.5 Green or Biological Synthesis
Typically, NPs can be achieved by using either microorganisms (such as bacteria, yeast, actinomycetes and fungi) or plant tissues (such as leaf, fruit, root, stem, peel and flower). The synthesis of NPs using a biological system can be categorized into 3 options: (1) using of the solvent medium, (2) using of an eco-friendly and environmentally benign reducing agent and (3) using of a non-toxic material as a capping agent [76]. Several plants and microorganisms have been successfully used for efficient extracellular synthesis of different metal NPs such as Co, Cu, Ag, Au, Pd and Pt as presented in Table 4. Factors including pH, reaction time, reactant concentration and reaction temperature can be adjusted to control the size, shape, yield and stability of as-prepared NPs from the biological synthesis [77].
NPs produced by bacteria offer a very good stability, well dispersion and activities against various pathogens. Fungi are able to produce metal nano- and meso-structures by reducing enzyme intra- or extra-cellularly with the biomimetic mineralization procedure [77]. Using different species of fungi as nano-biofactories for synthesis process is considered more straight forward and easy for stable production of NPs as compared to bacteria. Fungi offers several advantages over bacteria including (1) higher bioaccumulation of metabolites, (2) higher biomass and easy mode of culture, (3) higher wall binding capacity of metals and (4) higher tolerance and uptake capability of metals.78 Yeasts according to invention are classified into the kingdom Fungi and class Saccharomyces. One main advantage of using yeast cells as NP-carriers is simple encapsulation mechanism which implies that the synthesis process does not require stabilizers compared to other NP-carrier system.
Advantage: Green synthesis eliminates the use of chemicals and offers non-toxic products and by-products. Moreover, it can also generate a large amount of highly stable NPs with a better-size distribution than those chemical and physical methods due to non-nutrient bioactive compounds (as stabilizing agents) that are used in the reaction act [79].
Disadvantage: The large-scale production protocols of this technique require further modification to make them cost-effective and comparable to other methods. Instability and aggregation of NPs, control of morphology, crystallization and size of NPs via this technique are under the development stage. Moreover, the separation and purification process of NPs is another key issue that needs further exploration.
3 Methods Used in Metal Nanoparticles Characterization
Understanding the characteristics of NPs benefits in validating the fabrication technique, succeeding the morphology evolution, improving the method protocols and realizing the potential applications of the NPs. Characterization techniques can be classified based on the concept of the techniques used, the provided information or the types of materials that required for the technique. The main features of the techniques and their key benefit, advantages and limitations are explained and pointed out in this session. Table 5 summarizes the techniques that are suitable for different focus characterized parameter of NPs.
3.1 X-Ray-Based Techniques
-
X-ray diffraction (XRD) is a basic technique for NP characterization which provides the information of the crystalline structure, phase, lattice and grain size of NPs [105]. XRD is commonly used for powder or thin film samples. The composition of the NPs can be identified by comparing the position of the 2-theta peaks with the standard patterns (i.e. ICDD, or known as JCPDS) database [103]. In general, the intensity of X-ray source is about [108] times lower than that of the electron diffraction.
-
X-ray photoelectron spectroscopy (XPS) is a highly surface-sensitive analysis method, giving information of electronic structure of NPs and charge transfer between constituent elements in alloy and/or heterostructure NP’s surface [106]. Moreover, it is also possible to extract the element and phase composition of the NPs from surface analysis data. Binding energy presented an information of the element, whereas the peak intensity reflects the relative concentration of the elements [107]. Background subtraction and peak fitting methods are required for an analysis of the complex element that has several oxidation states.
-
X-ray absorption spectroscopy (XAS) includes both extended X-ray absorption fine structure (EXAFS) and X-ray absorption near edge structure (XANES, also known as NEXAFS). XAS is involved in the measurement of X-ray absorption coefficient of NPs as function of energy (Fig. 7.) [108]. In principle, each element has a set of characteristic absorption edges (i.e. K, L, M, etc., absorption edges) according to the different binding energies of its electrons, offering XAS element selectivity. In other words, this technique offers result of the chemical composition, unoccupied electronic states and bonding information of the NPs [109]. A highly sensitive EXAFS techniques can be used to gain the information about the interatomic distances, near neighbour coordination numbers and lattice dynamics. On the other hand, XANE gives information about the oxidation states, vacant orbitals, electronic configuration and site symmetry of the absorbing atom.
Fig. 7 XAS spectrum shows the edge XANES (within ca. 50 eV) and the edge EXAFS (>1000 eV above) of the structured absorption [110]
-
Energy-dispersive X-ray spectroscopy (EDS) and X-ray scattering (SAXS) are used for the elemental analysis or chemical characterization of NPs. Each chemical element has characteristic X-ray energy that emitted from the specimen. EDS is typically combined with SEM and TEM, allowing a clear identification of the composition of elements heavier than oxygen.
3.2 Fourier Transform Infrared Spectroscopy (FTIR)
FTIR is used for studying the vibration of the functional groups associated with NPs. FTIR records the absorption of electromagnetic radiation with wavelengths within the mid-infrared region (400–4000 cm−1) [111]. If a molecule of NPs can absorb infrared region radiation, the dipole moment somehow changed, and the molecule becomes IR active. A spectrum not only offer the information of band position related to nature and strength of bonds, but also determine the specific functional groups, which can help to obtain the information of molecular structures and interactions of NPs. In general, powder samples will be grounded with KBr (about 5% of the weight of the samples) and pressed to form the hard pellet [112].
3.3 Transmission Electron Microscopy (TEM)
The interaction between an electron beam with uniform current density and a thin sample can be observed by using TEM as the beam is transmitted through that thin film samples to form an image. In general, the energies of the electron beam are within a range of 60–150 keV. TEM is the most common technique to analyse morphologies of NPs as it can provide not only direct images of the sample but also the most accurate estimation of the NPs homogeneity due to its powerful magnification with the potential of over 1 million times compared to other SEM techniques.
High-resolution TEM (HRTEM) is an imaging mode of TEM that uses phase-contrast imaging, using the combination of transmitted and scattered electrons to produce the image of the internal structure of NPs (i.e. the arrays of atomic level in crystalline structures of NPs). It is worth mentioning that the internal structure characterization of amorphous-based structure is not always practicable by this HRTEM technique due to the random orientation of their crystals relative to the electron beam; hence, the atom directions are not well aligned, obtaining a complex image that cannot be directly used for structural analysis. Selected area electron diffraction (SAED) or an electron backscatter diffraction (EBSD) in TEM is used for the study of the crystal structure of NPs, obtaining the reverse space of the lattice planes as it can be used to determine the d-spacing value of the crystal planes of the NPs.
3.4 Scanning Electron Microscopy (SEM)
SEM is another common technique for imaging of nanomaterial surface with a resolution down to about 1 nm [113]. SEM uses electrons where the incident beam of electrons transversely scans the sample, offering data on the composition of atoms along with the physical feature of NPs [114]. The focussed beam of electrons can be able to create a magnified image with much improved magnification of 10 to 1,000,000 times. SEM usually uses three analysis modes including (1) secondary electron (SE) mode, (2) backscattered electron (BSE) mode and (3) X-ray energy dispersive spectroscopy [115]. A classic SE mode can obtain image with up to 1 nm resolution. However, SEM is limited when used to characterize non-conductive materials and coloured images (i.e. SEM only provide black and white image), or when used to acquire the height of the specimen. Coating the samples (sputter coating) with an additional conductive material layer of ~3–10 nm (such as Pt, Ag, Pd and Au) is required, especially for non-conductive sample to remove charging effects and get better quality images of the sample. However, the thin layer of conductive materials (from sputtering) may affect the atomic percentage and elemental composition analysis of the NPs.
3.5 Atomic Force Microscopy (AFM)
AFM is capable of creating 3D images of surfaces with high magnification. The measurement of this technique is based on the interacting forces between the sample surface and a probe. AFM can scan under three different modes (i.e. contact, non-contact and tapping mode) depending on the degree of proximity between the probe and the sample. Parameters including tip curvature radius and elasticity and surface energy of NPs influence the final topological values. AFM does not require any surface modification or coating prior to imaging; hence, it does not require a coating of conductive layer on the surface of non-conductive materials as SEM technique. Moreover, the topological analysis of small NPs (≤6 nm) can be obtained by AFM without requirements of the special treatment. Low density materials, which present poor contrast in electron microscopy, can also be characterized. For comparison, Fig. 8 shows the image of copper/1,4-benzenedicarboxylate (N-Cu(BDC) composite that taken by AFM, TEM and SEM.
An example of SEM, TEM and AFM of N-Cu(BDC) composite [104]
3.6 Dynamic Light Scattering (DLS)
DLS provides the measurement of the size of the NPs from the scattered light in a solution; hence, it is difficult to imaging dried samples and very sensitive to agglomeration and dynamic aggregation [116]. As the DLS directly characterizes particles in dispersion, dispersion conditions including the effect of salt or pH on colloidal stability an be monitored [117]. This method can be used to analyse in a great diameter ranging from nano- to micro-scale. However, it cannot directly observe the size of NPs from the DLS result as the mechanism of method is based on diffusion coefficient value, and the spherical shape of the particles is therefore assumed. Hence, the dimensions of a particle can only be defined by using an assumption of shape by Stokes–Einstein equation [115].
3.7 Zeta Potential Measurement
Zeta potential or an electrokinetic potential is a measurement of the “effective” electric charge at the slip plane between the bulk of base solution and the bound layer of diluent molecules that surrounds the NPs. Hence, this technique measures the charge stability of colloidal NPs, which is a key parameter that governs the electrokinetic behaviour of NPs in the solution [118]. In general, NPs with a zeta potential value between −10 and +10 mV are considered as neutral, while NPs with zeta potentials value of less than −30 mV or greater than +30 mV are considered strongly anionic or strongly cationic [119]. Zeta potential is dependent on pH and the conductivity of the dispersing medium; thus, it is important to accurately measure and report it. For example, if acid is added in nanofluid, pH will decrease, leading to the increase of positive charges on the particle surface. The minimum concentration required for analysis depends on the relative refractive index and particle size. Additionally, this technique some time can be used to predict the long-term stability of colloidal NPs. For example, NPs with zeta potentials larger than ± 60 mV have great stability, whereas when zeta values of NPs are between −10 mV and +10 mV, a rapid agglomeration can be observed unless they are sterically protected [120].
3.8 Secondary Ion Mass Spectrometry (SIMS)
SIMS is one of the key techniques for surface chemical and imaging analysis in the field of material sciences. This technique is recognized as isotopic surface analysis and the most sensitive elemental analysis technique. In the chamber, the sample is bombarded with an ion beam in vacuum atmosphere; thus, the secondary ions are sputtered from the sample and conveyed into a mass spectrometer for analysis [121]. The SIMS technique provides a unique combination of extremely high sensitivity which limits down to the ppm level for all elements from Hydrogen to Uranium and above. Moreover, it also offers high lateral resolution imaging (≥40 nm), and a very low background that allows more than 5 decades of dynamic range.
3.9 UV–VIS Spectrophotometry
It is widely known that metallic NPs possess muti-colours and, therefore, best matched for photo-related applications. Figure 9 shows characteristic colours and properties of Au NPs with the variation of sizes and shapes, which can be utilized in imaging-related applications [105]. Ultraviolet–visible (UV–Vis) and photoluminescence (PL) are the well-known techniques for the optical study of NPs materials [105]. Both techniques offer extra information about the absorption or emission capacity of the NPs and their effect on the overall excitation time of photo-excitons. Additionally, UV–Vis can quantitatively monitor the formation and provide information about the size of NPs through different responses to the electromagnetic waves, ranging from 200–700 nm [122] (Table 6).
Colour dependence of Au NPs on size and shape [105]
4 Basic Properties of Metallic Nanomaterials
4.1 Surface Plasmon Resonance Properties
Among metals, noble metals including Au, Ag, Cu, Pd and Pt gain a great attention due to their exclusive optical properties on account of their surface plasmon resonance (SPR), which can be applied in a broad range of applications such as photocatalysis, biomedicine, surface-enhanced Raman spectroscopy (SERS), plasmonic devices, sensors and photothermal therapy [136, 137]. Among these plasmonic NPs, silver and gold are most used as plasmonic metals because of their chemical stability and visual colour change to the naked eye. On the other hand, Pt or Pd exhibit only broad absorption which continuously extend throughout the near UV and visible range.
Noble metal NPs exhibit a strong UV–vis absorption band, which is not possessed by bulk metals. This absorption band is provided by the collective oscillations of electrons in the conduction band that excited by light with appropriate frequency from both visible and near-infrared ranges, which is known as localized SPR [138]. The position of the plasmon band (extinction spectrum) is best measured on a conventional UV–visible spectrophotometer, observing a band with high extinction coefficients (up to 1011/M.cm) [139]. The optical features of the localized SPR (e.g. peak absorption, peak extinction wavelength, scattering, linewidth) depend on the size, shape, composition of the metal NP, surface-adsorbed species, surface charge, interparticle interactions and the refractive index of the surrounding medium [140]. An example of optical value change of Au and Ag NPs that depend on their shape is present in Fig. 10.
A different shape of Au and Ag NPs can directly influence the change in their optical performance. Vial 0 presented the aqueous solution of 4 nm Au nanospheres and Vial 1–5 presented the higher aspect ratio gold nanorods [140]
4.2 Magnetic Properties
Magnetic properties of metal NPs are of great interest for a wide range of disciplines, such as magnetic fluids, catalysis, biomedicine, magnetic energy storage, information storage and spintronics [141]. Fine particle magnetism comes from size effects, which are based on the magnetic domain structure of ferromagnetic materials. When the size of single-domain particles is reduced below a critical diameter (<15 nm for the common materials), the coercivity is zero and such particles become superparamagnetic, which is caused by thermal effects. Each potential application of the magnetic nanoparticles requires different properties. Materials that hold ferromagnetism (e.g. Fe, Ni and Co) have aligned atomic magnetic moments of equal magnitude; thus, their crystalline structures allow for direct coupling interactions between the moments, which helps to enhance the flux density of material [142]. Many studies have confirmed low toxicity of AuNPs compared to other metal-based NPs [143], Au (3.9 ± 0.2 nm) has been used to decorate peI-Fe3O4 (Au@PEI-Fe3O4) NPs and their DC magnetization. The estimated effective magnetic anisotropy constant (Keff, at 5 K) was 2.0 × 104 j/m3, nearly 50% larger than Keff of bulk magnetite (1.1–1.3 × 104 j/m3) (Fig. 11) [144].
a DC magnetization curves gained in zero-field-cooled (ZFC, lower branch) and field-cooled (HFC = 2.39 kA/m, upper branch) modes for PEI-Fe3O4 (filled black circles) and Au@PEI-Fe3O4 (open red circles) NPs. b M vs. H curves at T = 300 K. Inset: magnification of the low-field region of the hysteresis loops. c TEM of Au@PEI-Fe3O4 NPs, inset: the histogram of particle fitted with a lognormal distribution (solid line) [145]
4.3 Mechanical Properties
The mechanical properties of NPs have gained a lot of attention over the last few years especially the tribological properties for lubricants and as reinforcements for composite coating technologies [145, 146]. In general, the mechanical properties of material involve elastic–plastic deformation, hardness, bulk modulus, Young’s modulus, scratch resistance, time-dependent creep and relaxation properties, residual stresses, fracture toughness, fatigue and yield strength. Au NPs thin films have been well studied for their high hardness, creep and strain rate effects [147]. However, the obtained results are still insufficient and some are controversial [148, 149]. There is still no conclusion whether the elastic modulus of NPs is affected by the particle size and the indentation depth. Additionally, their frictional and mechanical behaviours have not been fully understood. A study has recently reported the ultra-high compressive strength of the Ni NPs of 34 GPa (D ≈ 210 nm), as shown in Fig. 12 [150].
a Load–displacement curves for different particle sizes of NPs. b Stress–strain curves demonstrate that the strength increases with decreasing particle diameter. c Compressive strength as a function of particle diameter [150]
4.4 Thermal Properties
Water, phase change materials, oil and ethylene glycol are typical heat transfer fluids, and they are mostly used as cooling fluid in many thermal engineering applications. In general, the thermal conductivity of a standard heat transfer fluid is lower than <1 W/mK [151]. Among them, water offers the highest value of thermal conductivity (0.613 W/mK at 303 K). This is still not comparable to the value that metal NPs can offer. Ag, Al, Cu, Fe and Au NPs can offer the high thermal conductivity of 429 W/mK at 300 K, 237 W/mK at 273 K, 401 W/mK at 273–373 K, 80.4 W/mK at 273–373 K and 318 W/mK at 273–373, respectively [152, 153]. For this reason, the heat transfer fluid, which is known as nanofluid (a combination of traditional heat transfer fluids with metallic NPs that have size less than 100 nm and at very low concentration of less than 1%), has attracted huge attention to researchers in the past decade [154]. It has been reported that thermal conductivity of water can be improved by 54% through an addition of 0.1% weight of 20 nm Ag NPs [152]. Many reports agree that size, shape and volume fraction of NPs can directly affect the thermal conductivities of typical heat fluids [154, 155]. In the case of Ag, the thermal conductivity of nanofluids increases with decreasing size and increasing the concentration of nanoparticles [156].
For given volume fraction and temperature, the thermal conductivity of nanofluid containing rectangular nanoparticles was higher than those with NPs in spherical shape. Additionally, metal nanoparticles can be added into polymers, such as epoxy, to enhance thermal conductivity and to maintain electrical insulation below percolation threshold. Compared with the electrical properties in metal–polymer composites, thermal transport does not show any dramatic increase [157]. However, similar trend for metal/heat transfer fluids, it has been reported that Ni NPs with the size of 40 nm show higher thermal conductivities of 0.37 W/mK due to its wider-spread aggregation structures in epoxy compared to those larger Ni NPs with size of 70 nm (0.31 W/mK) [158].
5 Conclusion
Bottom-up techniques are more precise than top-down techniques in controlling particle size distribution. Controlling the component concentration, reaction time and temperature can be achieved with different particle sizes and shapes, or NPs. Despite the fact that these growth conditions are extremely sensitive to process variables, limiting the variation of NP morphologies and structures, top-down methods, on the other hand, have advantages over bottom-up methods, such as simple production, the use of non-organic precursors and the high potential for large-scale production with high yields. The size distribution can be controlled via the evaporation rate, system pressure or system geometry. Although many researchers have successfully prepared various types of NPs using various techniques, the precise information of the parameter conditions of each technique that allow to optimize the designed size and shape of various types of NPs is unclear and necessitates additional research for an accurate reproduction process. Mass production could be taken into consideration for further development of the metal NPs. The long-term stability of NPs and their toxicity, especially in biotechnologies, are still unclear. An intensive study must be conducted to investigate the composition, morphology, size, shape, structure and side effects of metal NPs for diverse applications in the fields of magnetic, thermal, optical, etc.
References
Haritha VS, Balan M, Hosson JTM, Krishnan G (2020) Nanoscale Adv 2:9
Krishnan G, Kooi BJ, Palasantzas G, Pivak Y, Dam B (2010) J Appl Phys 107:5
Liang L, Wang F, Rong M, Wang Z, Yang S, Wang J, Zhou H (2020) J Mater Sci Chem Eng 8:12
Munoz JE, Cervantes J, Esparza R, Rosas G (2007) J Nano Res 9:5
Zarrouk T, Nouari M, Salhi JE, Makich H, Salhi M, Atlati S, Salhi N (2022) Int J Adv Manuf Technol 119
Dercz G, Matula I, Zubko M, Liberska A (2016) Acta Physica Polonoca A 130:4.7
Pradeep NB, Rajath Hegde MM, Manjunath Patel GC, Giasin K, Pimenov DY, Wojciechowski S (2022) J Mater Res Technol 16
Hu X, Sun Z, Zhang C, Wang X, Wu K (2018) J Magnes Alloy 6:2
El-Eskandarany MS, Al-Hazza A, Al-Hajji LA, Ali N, Al-Duweesh AA, Banyan M, Al-Ajmi F (2021) Nanomaterials (Basel) 11:10
Oleszak D, Pawlyta M, Pikula T (2021) Materials (Basel) 14:24
Toozandehjani M, Matori KA, Ostovan F, Abdul Aziz S, Mamat MS (2017) Materials 10:11
Piras CC, Fernández-Prieto S, De Borggraeve WM (2019) Nanoscale Adv 1:3
Lopes WA, Jaeger HM, (2001) Nature 414.
Colson P, Henrist C, Cloots R (2013) J Nanomater 2013:948510
Kothari R, Beaulieu MR, Hendricks NR, Li S, Watkins JJ (2017) Chem Mater 29:9
Kim DE, Sung IH (2013) In: Wang QJ, Chung YW (eds) Encyclopedia of Tribology. Springer, Boston, MA
Lipomi DJ, Martinez RV, Cademartiri L, Whitesides GM In: Matyjaszewski K, Moller M (eds) Polymer science: a comprehensive reference. Elsevier BV, Amsterdam, NL
Paivanranta B, Langner A, Kirk E, David C, Ekinci Y (2011) Nanotechnology 22:37
Auzelyte V, Dais C, Farquet P, Gruetzmacher D, Heyderman L, Luo F, Olliges S, Padeste C, Sahoo P, Thomson T, Turchanin A, David C, Solak H (2009) J Micro/Nanolithogr 8:2
Vala M, Homola J (2014) Opt Express 22:15
Kim P, David E, Raboin L, Ribbe AE, Russell TP, Hoagland DA (2013) Microsc Microanal 19:6
Luo S, Hoff BH, Maier SA, De-Mello JC (2021) Adv Sci 8:24
Wu D, Rajput SN, Luo X (2016) Curr Nanosci 12:6
Leggett GJ (2011) ACS Nano 5:3
Fan P, Gao J, Mao H, Geng Y, Yan Y, Wang Y, Goel S, Luo X (2022) Micromachines 13:2
Savale PA, (2016) Arch Appl Sci Res 8
Grillo F, Van Bui H, Moulijn JA, Kreutzer MT, Van-Ommen JR (2017) J Phys Chem Lett 8:5
Kuwata N, Kawamura J, Toribami K, Hattori T, Sata N (2004) Thin-film lithium-ion battery with amorphous solid electrolyte fabricated by pulsed laser deposition. Electrochem Commun 6:4
Saunders SRJ, Nicholls JR (1996) In: Cahn RW, Haasen P (eds) Physical metallurgy, 4th edn.. North-Holland, Oxford, UK
Kerdcharoen T, Wongchoosuk C (2013) In: Jaaniso R, Tan OK (eds) Semiconductor gas sensors. Woodhead Publishing
Kozhemyakin GN, Kiiko SA, Bryl OE (2019) Crystallogr Rep 64:3
Luttge R (2011) In: Luttge R (eds) Microfabrication for industrial applications. William Andrew Publishing, Boston, US
Baptista A, Silva FJG, Porteiro J, Miguez JL, Pinto G (2018) Coatings 8:11
Suryanarayana C, Prabhu B (2007) In: Koch CC (eds) Nanostructured materials, 2nd edn. William Andrew Publishing, Norwich, NY
Shahidi S, Moazzenchi B, Ghoranneviss M (2015) Eur Phys J Appl Phys 71:3
Yin Y, Lan C, Hu S, Li C (2018) J Alloys Comp 739
Pandey PA, Bell GR, Rourke JP, Sanchez AM, Elkin MD, Hickey BJ, Wilson NR (2011) Small 7:22
Fernandez-Arias M, Zimbone M, Boutinguiza M, Del-Val J, Riveiro A, Privitera V, Grimaldi MG, Pou J (2019) Coatings 9:9
Donnelly T, O’Connell G, Lunney JG (2022) Nanomaterials 10:11
Khalily MA, Yurderi M, Haider A, Bulut A, Patil B, Zahmakiran M, Uyar T (2018) ACS Appl Mater Interfaces 10:31
Moret JLTM, Griffiths MBE, Frijns JEBM, Terpstra BE, Wolterbeek HT, Barry ST, Denkova AG, Ommen JRV (2020) J Vac Sci Technol A 38:2
Kumar G, Jagirdar Rao V (2013) Int J Nanotechnol Appl 3:1
Sun G, Ye G, Wang K, Lou M, Jia X, Xu F, Ye Z (2020) ACS Omega 5:13
Ceylan A, Rumaiz AK, Shah SI (2007) J Appl Phys 101:9
Gracia-Pinilla M, Martinez E, Vidaurri GS, Perez-Tijerina E (2009) Nanoscale Res Lett 5:1
Silva LG, Solis-Pomar F, Gutierrez-Lazos CD, Melendrez MF, Martinez E, Fundora A, Perez-Tijerina E (2014). J Nanomater 2014:643967
Ward MB, Brydson R, Cochrane RF (2006) J Physics: Conf Ser 26:296
Alexandrescu R, Morjan I, Dumitrache F, Scarisoreanu M, Soare I, Fleaca C, Birjega R, Popovici E, Gavrila L, Prodan G, Ciupina V, Filoti G, Kuncser V, Vekas L (2008) Int J Photoenergy 2008:604181
Bendre K, Bhat MP, Lee KH, Altalhi T, Ayad Alruqi M, Kurkuri M (2022) Mater Today Adv 13:100205
Spreafico C, Russo D, Degl-Innocenti R (2022) J Intell Manuf 33:2
Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG (2019) J Drug Deliv Sci Technol 53:101174
Esposito S (2019) Materials (Basel) 12:4
Modan EM, Plaiasu AG (2020) The annals of “Dunarea de Jos” University of Galati, Fascicle IX. Metall Mater Sci 43:1. Accessed 15 Mar 2020
Bokov D, Turki Jalil A, Chupradit S, Suksatan W, Javed Ansari M, Shewael IH, Valiev GH, Kianfar E (2021) Adv Mater Sci Eng 2021:5102014
Danks AE, Hall SR, Schnepp Z (2016) Mater Horiz 3:2
Mahmud NA, Habiballah AS, Affandi NSM, Osman N, Jani AMM (2018) AIP Conf Proc 2031:1
Jameel ZN (2017) Energy Procedia 119
Hamada Y, Nishi M, Shimotsuma Y, Miura K, Hirao K (2011) IOP Conf Ser: Mater Sci Eng 8:3
Ligabue ML, Terzi F, Zanardi C, Lusvardi G (2019) J Mater Sci 54:13
Gondal MA, Rashid SG, Dastageer MA, Zubair SM, Ali MA, Lienhard JH, McKinley GH, Varanasi KK (2013) IEEE Photonics J 5:3
Ingale SV, Wagh PB, Bandyopadhyay D, Singh IK, Tewari R, Gupta SC (2015) IOP Conf Ser: Mater Sci Eng 73:1
Ahlawat DS, Kumari, R, Rachna, Yadav I (2014) Int J Nanosci 13:1
Mohindru JJ, Garg UK (2017) Int J Theor Appl Sci 9:2
Panigrahi S, Kundu S, Ghosh S, Nath S, Pal T (2004) J Nanopart Res 6:4
Piszczek P, Radtke A. (2018) In: Seehra MS, Bristow AD (eds) Noble and precious metals—properties, nanoscale effects and applications. IntechOpen, London, UK. Accessed 20 Dec 2017
Xia L (2021) In: Osaka A, Narayan R (eds) Advanced ceramic materials: bioceramics. Elsevier, Osaka, JP
Chew CKT (2016) Chemical vapour deposition of gold nanoparticles and metal oxide composites. Dissertation, University College London
Ng JJ, Leong KH, Sim LC, Oh WD, Dai C, Saravanan P (2020) In: Abdeltif A, Assadi AA, Nguyen-Tri P, Nguyen TA, Rtimi S (eds) Micro and nano technol: nanomaterials for air remediation. Elsevier, Amsterdam, NL
Dunne PW, Munn AS, Starkey CL, Huddle TA, Lester EH (2015) Philos Trans A Math Phys Eng Sci 373:2057
Qiu J, Li Y, Jia Y (2021) In: Qiu J, Li Y, Jia, Y (eds) Persistent phosphors from fundamentals to applications: synthesis method. Woodhead Publishing, Oxford, UK
Tippayawat P, Phromviyo N, Boueroy P, Chompoosor A (2016) Peer J 4
Liu Y, Yang L, Shen Y (2018) J Mater Res 33:18
Murugan S, Grace A (2012) J Nano Res 18:1
Lozhkomoev AS, Kazantsev SO, Pervikov AV (2020) AIP Conf Proc 2310:1
Cored J, Garcia-Ortiz A, Iborra S, Climent MJ, Liu L, Chuang CH, Chan TS, Escudero C, Concepcion P, Corma A (2019) J Am Chem Soc 141:49
Parveen K, Banse V, Ledwani L (2016) AIP Conf Proc 1724:1
Boroumand Moghaddam A, Namvar F, Moniri M, Md Tahir P, Azizi S, Mohamad R (2015) Molecules 20:9
Roychoudhury A (2020) Indian J Pham Biol Res 8:3
Sun Y, Wang Q, Chen J, Liu L, Ding L, Shen M, Li J, Han B, Duan Y (2017) Theranostics 7:18
Ijaz I, Gilani E, Nazir A, Bukhari A (2020) Green Chem Lett Rev 13:3
Zhang D, Ma XI, Gu Y, Huang H, Zhang G (2020) Front Chem 8
AbdelRahim K, Mahmoud SY, Ali AM, Almaary KS, Mustafa AEZMA, Husseiny SM (2017) Saudi. J Biol Sci 24:1
Guilger-Casagrande M, Lima RD (2019) Front Bioeng Biotechnol 7:287
AlNadhari S, Al-Enazi NM, Alshehrei F, Ameen F (2021) Environ Res 194:110672
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Biotechnol Prog 22:2
Rautela A, Rani J, Debnath M (2019) J Anal Sci Technol 10:1
Yulizar Y, Utari T, Ariyanta HA, Maulina D (2017) J Nanomater 2017:3079636
Mali SC, Dhaka A, Githala CK, Trivedi R (2020) Biotechnol Rep 27:e00518
Amjad R, Mubeen B, Ali SS, Imam SS, Alshehri S, Ghoneim MM, Alzarea SI, Rasoo R, Ullah I, Nadeem MS, Kazmi I (2021) Polymers (Basel) 13:24
Wu S, Rajeshkumar S, Madasamy M, Mahendran V (2020) Artif Cells Nanomed B 48:1
Varaprasad T, Govindh B, Venkateswara Rao B (2017) Int J ChemTech Res 10:9
Sunkar S, Nachiyar CV (2012) Asian Pac J Trop Biomed 2:12
Du L, Jiang H, Liu X, Wang E (2007) Electro Comm 9:5
Mikheenko IP, Bennett JA, Omajali JB, Walker M, Johnson DB, Grail BM, Wong-Pascua D, Moseley JD, Macaskie LE (2022) Appl Catal B: Environ 306:121059
Ahghari MR, Soltaninejad V, Maleki A (2020) Sci Rep 10:1
Raliya R, Tarafdar JC (2014) Int Nano Lett 4:1
Li Y, Li Y, Li Q, Fan X, Gao J, Luo Y (2016) J Chem 2016:2781347
Pimprikar PS, Joshi SS, Kumar AR, Zinjarde SS, Kulkarni SK (2009) Colloids Surf B: Biointerfaces 74:1
Tian X, He W, Cui J, Zhang X, Zhou W, Yan S, Sun X, Han X, Han S, Yue Y (2010) J Colloid Interface Sci 343:1
Arya A, Gupta K, Chundawat TS, Vaya D (2018) Bioinorg Chem Appl 2018:7879403
Yılmaz Ozturk B, Yenice Gursu B, Dag I (2020) Process Biochem 89
Senapati S, Syed A, Moeez S, Kumar A, Ahmad A (2012) Mater Lett 79
Mourdikoudis S, Pallares RM, Thanh NTK (2018) Nanoscale 10:27
Tiede K, Boxall ABA, Tear SP, Lewis J, David H, Hassellov M (2008) Food Addit Contam: Part A 25:7
Khan I, Saeed K, Khan I (2019) Arabian J Chem 12:7
Lubenchenko AV, Batrakov AA, Pavolotsky AB, Lubenchenko OI, Ivanov DA (2018) Appl Surf Sci 427
Baer DR, Engelhard MH (2010) J Electron Spectrosc Relat Phenom 178–179
Yano J, Yachandra VK (2009) Photosynth Res 102:2–3
Terzano R, Denecke MA, Falkenberg G, Miller B, Paterson D, Janssens K (2019) Pure Appl Chem 91:6
Penner-Hahn JE, X-ray absorption spectroscopy. In eLS
Manjumeena R (2018) In: Tiwari A (eds) Handbook of antimicrobial coatings. Elsevier
Faghihzadeh F, Anaya NM, Schifman LA, Oyanedel-Craver V (2016) Nanotechnol Environ Eng 1:1
Venkatesh N (2018) Biomed J Sci Technol 4
Shnoudeh AJ, Hamad I, Abdo RW, Qadumii L, Jaber AY, Surchi HS, Alkelany SZ (2019) In: Tekade RK (eds) Biomaterials and bionanotechnology. Academic Press
Zavasnik J, Sestan A, Shvalya V (2021) In: Milacic R, Scancar J, Goenaga-Infante H, Vidmar J (eds) Comprehensive analytical chemistry. Elsevier, Amasterdam, NL
Eaton P, Quaresma P, Soares C, Neves C, De-Almeida MP, Pereira E, West P (2017) Ultramicroscopy 182
Malm AV, Corbett JCW (2019) Sci Rep 9:1
Griffiths D, Hole WBP, Smith J, Malloy A, Carr B (2011) NSTI-Nanotech 1
Clogston JD, Patri AK (2011) In: McNeil S (eds) Characterization of nanoparticles intended for drug delivery. Humana Press, Maryland, USA
Nanocomposix, Zeta Potential Measurements (2022) https://nanocomposix.com/pages/zeta-potential-measurements
Bonnin EA, Rizzoli SO (2020) Front Behav Neurosci 14
Wang C, Gao X, Chen Z, Chen Y, Chen H (2017) Polymers 9:12
Din M, Arshad F, Hussain Z, Mukhtar M (2017) Nanoscale Res Lett 12
Linkov P, Artemyev M, Efimov AE, Nabiev I (2013) Nanoscale 5:19
Choudhary OP, Choudhary P (2017) Int J Curr Microbiol Appl Sci 6
Smith DJ (2015) Nanocharacterisation. The Royal Society of Chemistry
Chiriaco M, Bianco M, Nigro A, Primiceri E, Ferrara F, Romano A, Quattrini A, Furlan R, Arima V, Maruccio G (2018) Sensors 18:3175
Lim J, Yeap SP, Che HX, Low SC (2013) Nanoscale Res Lett 8:1
Grunder Y, Lucas C (2016) Nano Energy 29:1
Baer DR (2022) J Vac Sci Technol 38:3
San-Miguel A (2005) Acta Crystallogr Sec A 61.
Bak SM, Lin R, Yu X, Yang XQ (2018) NPG Asia Mater 10
Thermoscientific, Advantages of a Fourier Transform Infrared Spectrometer (Technical Note). https://www.thermoscientific.com/content/dam/tfs/ATG/CAD/CAD%20Documents/Application%20%26%20Technical%20Notes/Molecular%20Spectroscopy/FTIR/FTIR%20Spectrometers/TN50674-E-0215M-FT-IR-Advantages.pdf
Baer DR, Engelhard MH, Johnson GE, Laskin J, Lai J, Mueller K, Munusamy P, Thevuthasan S, Wang H, Washton N, Elder A, Baisch BL, Karakoti A, Kuchibhatla SVNT, Moon D (2013) J Vac Sci Technol A 31:5
Khandel P, Shahi KS (2016) Int J Nanomater Biostruc 6:1
Fratoddi I, Matassa R, Fontana L, Venditti I, Familiari G, Battocchio C, Magnano E, Nappini S, Leahu G, Belardini A, Li Voti R, Sibilia C (2017) J Phys Chem C 121:33
Zhu S, Zhou W (2010) J Nanomater 2010:562035
Sau TK, Rogach AL, Jackel F, Klar TA, Feldmann J (2010) Adv Mater 22:16
Murphy CJ, Gole AM, Hunyadi SE, Stone JW, Sisco PN, Alkilany A, Kinard BE, Hankins P (2008) Chem Comm 5
Mulvaney P (1996) Optical properties of metal clusters By U. Kreibig, M. Vollmer, Springer Series in Materials Science, Advanced Materials. Wiley, Hardcover, DM
Issa B, Obaidat IM, Albiss BA, Haik Y (2013) Int J Mol Sci 14:11
Diaz C, Valenzuela ML, Laguna-Bercero MA, Orera A, Bobadilla D, Abarca S, Pena O (2017) RSC Adv 7:44
Sani A, Cao C, Cui D (2021) Biochem Biophys Rep 26
Leon Felix L, Sanz B, Sebastian V, Torres TE, Sousa MH, Coaquira JAH, Ibarra MR, Goya GF (2019) Sci Rep 9:1
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Electroanalysis 18:4
Hussain F, Hojjati M, Okamoto M, Gorga RE (2006) J Comp Mater 40:17
Maharaj D, Bhushan B (2014) Beilstein J Nanotechnol 5
Guo D, Li J, Chang L, Luo J (2013) Langmuir 29:23
Ritter C, Heyde M, Schwarz UD, Rademann K (2002) Langmuir 18:21
Sharma A, Hickman J, Gazit N, Rabkin E, Mishin Y (2018) Nat Comm 9:1
Mbambo MC, Khamlich S, Khamliche T, Moodley MK, Kaviyarasu K, Madiba IG, Madito MJ, Khenfouch M, Kennedy J, Henini M, Manikandan E, Maaza M (2020) Sci Rep 10:1
Iyahraja S, Rajadurai JS (2015) AIP Adv 5:5
Farid N, Sedigheh A (2017) J Adv Mater Proc 5:2
Liu M, Ma Y, Wu H, Wang RY (2015) ACS Nano 9:2
Navarrete N, Gimeno-Furio A, Mondragon R, Hernandez L, Cabedo L, Cordoncillo E, Julia JE (2017) Sci Rep 7:1
Warrier P, Teja A (2011) Nanoscale Res Lett 6:1
Guoqing Z, Yanping X, Hui W, Yu T, Guoliang T, Shantung T, Haiping W (2009) J Comp Mater 44:8
Li X, Park W, Chen YP, Ruan X (2013) J Heat Transfer 139:2
Acknowledgements
This work was supported by the Leverhulme Trust Early Career Fellowship (ECF-2021-657) and Nottingham Research Fellowship (A7X164).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
There are no conflicts to declare.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Thummavichai, K., Chen, Y., Wang, N.N., Zhu, Y.Q., Ola, O. (2023). Synthesis, Properties and Characterization of Metal Nanoparticles. In: Tiwari, S.K., Kumar, V., Thomas, S. (eds) Nanoparticles Reinforced Metal Nanocomposites. Springer, Singapore. https://doi.org/10.1007/978-981-19-9729-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-9729-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9728-0
Online ISBN: 978-981-19-9729-7
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)