Abstract
The vital role of thyroid hormones, insulin, glucagon, corticosteroids, and catecholamines in regulating metabolism is a key for an animal’s optimal growth and survival. The thyroid hormones (THs) are derived from tyrosine and have a permissive effect on growth hormone (GH) to produce growth, reproduction, and lactation in domestic animals. Apart from the THs, optimal secretion of insulin from the pancreas is responsible to mediate the growth-promoting effects of GH. As the only hypoglycemic hormone present in animals, the optimal secretion of insulin is a prerequisite for increasing glucose uptake in skeletal muscles and adipose tissue. Furthermore, the secretion of glucocorticoids and catecholamines from the adrenal gland alters the metabolic status of an animal to combat various stressors effectively. Whereas, the mineralocorticoids regulate the standard concentration of sodium and potassium ions in the blood, which is essential for the establishment of resting membrane potential in almost all cells. In addition to the aforementioned cations, the tight regulation of extracellular fluid (ECF) Ca2+ levels by hormones such as parathormone (PTH), calcitriol, and calcitonin is crucial for the excitation of neurons, skeletal muscles, and smooth muscles. Overall, the endocrine system warrants proper metabolic health by controlling energy homeostasis and mineral balance in animals.
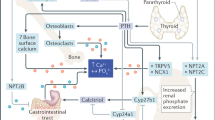
Graphical Abstract

Description of the graphic: Role of the endocrine system in regulating metabolic health of animals (1). Hormones secreted from the thyroid gland, parathyroid gland, pancreas, and adrenal gland modulate a variety of metabolic processes to maintain optimal energy and mineral balance in animals (2). Hormonal regulation of blood glucose levels. [MCs mineralocorticoids; GCs glucocorticoids; T3 triiodothyronine; T4 tetraiodothyronine.]
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
Biosynthesis and target effects of thyroid hormones
-
Endocrine functions of the pancreas
-
Role of insulin and glucagon in regulating intermediary metabolism
-
Regulation of Na+ and K+ in ECF by aldosterone
-
Biological effects of cortisol on metabolism
-
Calcium homeostasis by parathormone, calcitriol, and calcitonin
1 Thyroid Gland
Intermediary metabolism includes all the biochemical reactions that involve in the conversion of dietary nutrients into energy or cellular components. The energy derived through metabolism will be utilized to maintain homeostasis, growth, production, and reproduction in animals. It drives crucial processes such as cell proliferation, sustenance, and the synthesis of cellular components. The rate of metabolism varies according to different physiological states or stages in an animal’s life. Hormones of the thyroid gland, pancreas, and adrenal gland play a major role in defining the plane of metabolism to support a specific physiological function in an animal and are hence known as metabolic hormones. They primarily affect metabolic processes such as glycogenolysis, glycogenesis, gluconeogenesis, lipogenesis, and lipolysis to meet the metabolic needs of an animal.
It is a bi-lobed gland present on either side of the trachea, derived from the foregut endoderm layer, and produces thyroid hormones (THs), i.e., triiodothyronine (T3) and tetraiodothyronine (T4). These hormones are responsible for multifaceted functions including organ development, growth, homeostasis, oxidative metabolism, reproduction, and production.
1.1 Histology
The endodermal-derived thyroid progenitor cells form thyroid follicles that constitute the thyroid gland. Thyroid follicles are often referred to as the manufacturing units of THs. Within these follicles are the specialized epithelial cells known as “thyrocytes” that serves as a site of synthesis, storage, and secretion of TH. The thyrocytes have polarized apical and basal membranes, which help in regulating specific bidirectional transport of substances back and forth from the lumen. Apart from thyrocytes, another type of neuroendocrine cell known as “parafollicular or C-cells” exists in close association with the thyroid follicles (Fig. 16.1).
1.2 Synthesis of Thyroid Hormones
1.2.1 Synthesis of Thyroglobulin
Thyroglobulin (TG) is a homodimeric (660 kDa) glycoprotein synthesized in thyrocytes and subsequently stored in the lumen. The thyroglobulin is secreted and stored in the follicular lumen and is commonly termed as “colloid”. Each TG monomer has about 70 tyrosine residues and it acts as a scaffold for the synthesis of THs. The TSH-dependent stimulation of TSHR present on the basal membrane of thyrocytes increases the rate of TG gene expression and its subsequent translation. TG undergoes post-translational modifications that favor protein folding, trafficking, iodination, and hormonogenesis during its transit into the lumen.
1.2.2 Iodine Uptake
The dietary iodide absorbed from the GIT reaches thyroid gland via systemic circulation. A specialized “Sodium (Na+)-Iodide (I-) symporter (NIS)” present on the basolateral membrane helps in the secondary active transport of iodide into the cytoplasm of thyrocytes. Another apical membrane-bound iodide transport protein known as “Pendrin” helps in the rapid efflux of cytoplasmic I− into the lumen. These carrier proteins confer the unique ability of thyrocytes to concentrate I− by 30–60 fold within their cytoplasm and this exclusive phenomenon occurring is referred to as “iodide trapping.” During the efflux of I− into the follicular lumen, it is converted into Iodine (I) by the apical membrane-bound enzyme “thyroid peroxidase (TPO)” (Fig. 16.2).
Iodide trapping in thyrocytes [The iodide in the circulation is actively transported by the NIS protein and stored inside the thyrocytes or used for thyroid hormonogenesis. TP thyroid peroxidase; TG thyroglobulin; AC adenylyl cyclase; NIS sodium-Iodide symporter; BM basal membrane; LM luminal membrane; RER rough-endoplasmic reticulum]
1.2.3 Organification
In a process known as “Organification,” the highly reactive iodine reacts with the tyrosine residues present on the TG to form monoiodotyrosine (MIT) and diiodotyrosine (DIT). While roughly half of the tyrosine residues present in each TG monomer can be iodinated depending on the availability of superficial tyrosine molecules.
1.2.4 Coupling
This refers to the combination of MIT and DIT molecules within TG to form triiodothyronine (T3), tetraiodothyronine (T4), and reverse-triiodothyronine (rT3). Although they collectively comprise the thyroid gland secretions, only T3 and T4 are active hormonal forms that can elicit biological effects in the target tissues. Only a few out of the many MIT and DIT molecules embedded in TG undergo thyroid hormonogenic coupling reactions (Fig. 16.3).
Organification and coupling reactions in thyroid hormonogenesis [Iodination of tyrosine residues in the TG is catalyzed by TPO and results in the production of MIT and DIT, which will be further to yield THs. [MIT monoiodotyrosine; DIT di-iodo tyrosine; T3 triiodothyronine; T4 tetraiodothyronine reverse; rT3 triiodothyronine]
1.2.5 Endocytosis and Lysosomal Degradation
The TSH-dependent stimulation of thyrocytes ensues endocytic reuptake of TG surrounding the apical membrane. Thus, the reinternalized TG is conveyed to lysosomes for enzymatic degradation resulting in the liberation of T3, T4, MIT, and DIT. The thyroid hormones are then released into the bloodstream through monocarboxylate transporter 8 (MCT8) present on the basal membrane. While the MIT, DIT, and TG molecules will be degraded to reutilize iodine for further synthesis of thyroid hormones. Principally, T4 is the major form of TH produced by the thyroid gland.
1.2.6 Transport in Blood
THs are lipophilic and therefore require plasma proteins for their circulatory transport. More than 99% of THs in the circulation will be bound to the carrier proteins and liberated rapidly when required. The plasma proteins, such as thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA or transthyretin), and serum albumin act as the carrier proteins of THs. The TBG is the major plasma protein that binds to THs, it has a greater affinity to T4 than T3and hence a long half-life (Table 16.1). They act mainly as an extra-thyroid pool of THs, preventing their rapid metabolism and excretion from the animal’s body.
1.3 Mechanism of Action
1.3.1 Transport Across the Cell Membrane
Only free or unbound THs are carried through the MCT 8 present on the cell membranes of target cells. Since T3 binds to the nuclear thyroid receptors (TRs) with a great affinity when compared to T4, it is considered as the more potent and active form of TH. While T4 is the major secretory product of the thyroid gland, subsequent conversion of T4 into the active T3 form by intracellular deiodinases takes place in target cells. 5′-deiodinase type 1 (D1), 5′-deiodinase type 2 (D2), and 5-deiodinase type 3 (D3) are different forms of deiodinases distributed in various tissues. The D1 and D2 enzymes are responsible for the conversion cum activation of T4 to T3. Whereas D3 catalyzes the conversion of T3 to rT3, causing its inactivation. Therefore, MCT 8 and deiodinases are essential factors in determining the magnitude of biological response in target tissues.
1.3.2 Intracellular Signaling
Produced either from the thyroid gland or by the conversion of prohormone T4 in peripheral tissues, T3 initiates a signaling cascade by binding to thyroid receptors (TRs) localized in the nucleus. TR α and TR β are the two major differentially expressed isoforms of TR found in various tissues, they determine the activation of specific regulatory pathways of metabolism. Generally, TR forms a heterodimer with retinoid X receptor (RXR) and binds to specific regions of DNA known as thyroid response element (TRE). When the heterodimeric complex is not bound to T3, it is associated with corepressors like nuclear receptor corepressor (NCoR) or silencing mediator for retinoid and thyroid receptor (SMRT). These repressors recruit histone deacetylases (HDACs) that bind to the promoter regions of various genes and help in maintaining their repression. Subsequent dissociation of corepressors and recruitment of coactivators take place when T3 binds to the TR complex, resulting in the transcription of TH regulated genes (Fig. 16.4).
Mechanism of thyroid signaling in the target cells [The thyroid hormones in the circulation are taken up by the target tissues and exert their biological effects by binding to the thyroid receptors residing in the nucleus. T3 triiodothyronine; T4 tetraiodothyronine reverse; rT3 triiodothyronine; TRE thyroid response element; D2 5′-deiodinase type 2; D3 5′-deiodinase type 3; RXR retinoid X receptor; TR thyroid receptors; MCT 8 monocarboxylate transporter 8]
1.4 Biological Effects
1.4.1 Effect on Carbohydrate Metabolism
THs stimulate intestinal absorption, glycolysis, glycogenolysis, and gluconeogenesis in various tissues. The enhanced glucose production by the aforementioned pathways is necessary to maintain basal metabolic rate (BMR), thermogenesis, and animal growth (Fig. 16.5).
1.4.1.1 Effect on Intestinal Absorption
THs increase the absorption of glucose from the small intestine by upregulating the activities of Sodium (Na+)-Glucose cotransporter 1 (SGLT1) and Na+-K+ ATPase pump.
1.4.1.2 Effect on Liver
The liver is a major organ regulating glucose homeostasis in animals. THs have direct effects on glucose uptake, production, and oxidation in hepatocytes. They increase both the uptake and secretion of glucose from hepatocytes by stimulating the expression of glucose transporter-2 (GLUT2). Simultaneously, upregulation of glycolytic enzymes enhances subsequent oxidation of glucose via the glycolytic pathway. The key enzyme encoding genes of gluconeogenesis such as pyruvate carboxylase, phosphoenolpyruvate carboxykinase (PEPCK), and glucose-6-phosphatase are positively regulated by the THs. Moreover, THs also stimulates the rate of glycogenolysis in hepatocytes due to an increased rate of oxidation of glucose. Together, THs stimulate glycolysis, gluconeogenesis, and glycogenolysis in the liver leading to a concomitant rise in blood glucose levels.
1.4.1.3 Effect on Pancreas
THs play a crucial role in the development, maturation, and functioning of cells in the islets of Langerhans. They inhibit glucose-stimulated insulin release from the β cells leading to glucose intolerance.
1.4.1.4 Effect on Glucose Uptake in Skeletal and Adipose Tissue
The insulin-dependent upregulation of GLUT4 leads to increased glucose uptake in the skeletal muscles. In the same way, THs enhance the glucose uptake in adipocytes, which help in lipogenesis.
1.4.2 Effect on Protein Metabolism
Thyroid hormones stimulate both protein catabolism and anabolism in tissues. The degradation of proteins in skeletal muscles results in the elevation of plasma amino acid concentration to support gluconeogenesis in various tissues. In the liver, THs stimulate the synthesis of intracellular and secretory proteins. Altogether, THs stimulate protein turnover in the liver and skeletal muscle cells.
1.4.3 Effect on Fat Metabolism
Thyroid hormones stimulate the hepatic production of cholesterol by upregulating the HMG-CoA reductase gene. Thus, the increased amount of cholesterol is utilized to manufacture bile acids. They stimulate lipolysis in both white adipose tissue (WAT) and brown adipose tissue (BAT) to produce free fatty acids, which are used for thermogenesis. Further, THs also enhance lipogenesis to counter the depletion of lipid stores.
1.4.4 Effect on BMR and Thermogenesis
The altered levels of intracellular Na+ and Ca2+ by THs augment the activity of Na+-K+ ATPase pump and sarcoplasmic/endoplasmic reticulum Ca2+-dependent ATPase (SERCA) in skeletal muscle, heart, and other cells. The enhanced activity of the above-mentioned ion pumps corresponds to a proportional rise in the hydrolysis of ATP with subsequent generation of heat and elevation of BMR. Furthermore, the increased ATP requirement is ensured through the catabolism of glucose, fatty acids, and amino acids. In addition, exposure to cold invokes the activity of D2 and subsequent conversion of T4 to T3 in brown adipose tissue (BAT), initiating a thermogenic response. Altogether, THs increase metabolic heat production due to enhanced oxidation of glucose and fatty acids, a phenomenon that is commonly referred to as non-shivering thermogenesis.
1.4.5 Effect on Mitochondrial Functioning and Biogenesis
THs have a direct effect on mitochondrial biogenesis by binding to TRs localized in mitochondria leading to increased mtRNA and protein synthesis. In addition, the upregulation of nuclear transcription factors like nuclear respiratory factor 1 (NRF-1) and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1a) will further activate the transcription of nuclear genes that encode mitochondrial proteins. They increase ATP synthesis by stimulating ATP synthase needed for the functioning of Na+-K+ ATPase and SERCA. Furthermore, THs lead to the uncoupling of oxidative phosphorylation by stimulating proton leak from the inner mitochondrial membrane by uncoupling proteins 1/2/3 (UCP1/2/3) or by inhibiting the movement of reducing equivalents into mitochondria. This uncoupling leads to the generation of heat with a consequent decrease in ATP synthesis.
1.4.6 Miscellaneous Effects
The increased oxygen demand at the tissue level due to elevated mitochondrial respiration is met by increasing cardiac output, systemic blood pressure, and respiratory rate. THs have stimulatory effects on neuronal activity, GIT motility, sleep, and milk production.
1.5 Hypothalamic–Pituitary–Thyroid Axis
The secretion of THs is regulated primarily by TRH and TSH (Fig. 16.6) released from the hypothalamic–pituitary axis. TRH from the hypothalamus stimulates the secretion of TSH from the pituitary gland. Then, the TSH acts on the thyroid gland and stimulates the production of THs. Increased circulatory levels of THs exert negative feedback signals on the secretion of both TRH and TSH. Other hormones such as leptin, somatostatin, dopamine, and cortisol also can modulate their secretion.
Regulation of thyroid hormone secretion. [The increased circulatory levels of THs exert a feedback inhibition on the secretion of TRH and TSH from the hypothalamus and anterior pituitary, respectively. [↑ increase; BMR basal metabolic rate; PVN para-ventricular nucleus, GIT gastrointestinal tract; TRH thyrotropin releasing hormone; TSH thyroid-stimulating hormone]
Know More…
-
Thyroid hormones have permissive effect on GH and absence of which leads to stunting of animal’s growth.
-
Iodinated casein: Resembles thyroid hormones and used in dairy cows to increase milk production.
-
Hypothyroidism: Decreased circulatory levels of thyroid hormones. Seen in panhypopituitarism, iodine deficiency, and congenital deficiency of thyroid peroxidase. It results in “cretinism”, characterized by impaired physical growth and neural development.
-
Hyperthyroidism: Increased circulatory levels of thyroid hormones, often due to hyperactivity of thyroid gland seen in grave’s disease and thyroid adenoma.
-
Goiter: The pathological condition characterized by abnormal enlargement of thyroid gland. Goiter can be caused due to iodine deficiency (endemic goiter) and in grave’s disease (toxic goiter).
2 Pancreas
The pancreas is an abdominal, mixed type of gland that significantly contributes to digestion and intermediary metabolism. The digestive enzymes secreted from exocrine pancreatic acini aid in intestinal digestion, whereas the different hormones from the endocrine pancreas are implicated in regulating various metabolic processes (Fig. 16.7). The deranged enzymatic and hormone secretory patterns of the pancreas will immediately affect animal metabolism, energy homeostasis, and production status.
2.1 Endocrine Pancreas
The endocrine function is imparted by islets of Langerhans, composed of specialized cells dispersed amidst the exocrine regions of the pancreas. They make up 2–3% of the pancreas, consists of four different endocrine cell types, i.e., α, β, γ, and δ. These distinct endocrine cells are responsible for the production of glucagon, insulin, somatostatin, and pancreatic polypeptide hormones, thereby regulating metabolic homeostasis (Table 16.2).
2.1.1 Glucagon
The islet α-cells secrete glucagon derived from the precursor proglucagon. The proglucagon is also expressed in other tissues such as the brain stem, hypothalamus, and enteroendocrine L cells. The prohormone convertases (PC1/2/3) further help in the process of conversion of proglucagon. The PC2 in α-cells converts proglucagon into glucagon, a polypeptide hormone with 29 amino acids. Whereas, the PC1 in the intestine and brain converts proglucagon to glucagon-like peptide 1 (GLP-1) and glucagon-like peptide 2 (GLP-2).
2.1.1.1 Mechanism of Secretion
Glucagon is primarily released in response to the decreased level of glucose in the blood. The presence of glucose transporter 1 (GLUT1) on the cell membrane of α-cells helps in the influx of glucose during normal conditions. Then, the glucose entered inside will be oxidized to produce ATP, which will be used to open ATP-sensitive potassium channels (KATP channels) and hence prevents depolarization by promoting the efflux of K+ ions. During hypoglycemia, the consequent reduction in ATP production due to reduced glucose influx leads to the closure of KATP channels. Subsequently, the build-up of K+ ions inside the cytoplasm triggers the opening of voltage-dependent Ca2 +channels allowing the influx of Ca2+ions. The rise in the intracellular Ca2+ levels stimulates the exocytosis of glucagon stored in the form of vesicles (Fig. 16.8).
Mechanism of glucagon secretion by α-cell. [The reduced ATP generation during hypoglycemia inhibits the outward movement of K+, resulting in the depolarization of α-cells and exocytosis of glucagon. [GLUT1 glucose transporter 1; ATP adenosine triphosphate; G glucagon; K+ potassium; Ca2+ calcium; ↓ decrease; ↑ increase]
2.1.1.2 Mechanism of Action
The glucagon exerts its biological actions by binding to the glucagon receptors (GCGR) on the target cells, with the liver having more GCGR than any other tissue. Being a G-protein coupled receptor; activated GCGR primarily activates the adenylyl cyclase (AC) system resulting in the production of cAMP and followed by the activation of protein kinase A (PKA). Thus, activated PKA migrates to the nucleus, activates transcription factors such as cAMP response element-binding protein (CREB) to promote the transcription of genes that mediate specific biological effects.
2.1.1.3 Role of Glucagon on Intermediary Metabolism
Glucagon increases hepatic glucose production by stimulating glycogenolysis, gluconeogenesis, along with concomitant inhibition of glycolysis and glycogenesis (Fig. 16.9). It also stimulates the catabolism of lipids and amino acids. In addition, it stimulates a positive effect on heart rate and contractility and inhibits gastric acid secretion and appetite. Altogether, glucagon is a catabolic hormone that profoundly affects intermediary metabolism by stimulating hyperglycemia, ketosis, and ureagenesis.
Effects of glucagon on intermediary metabolism. [Glucagon increases circulatory levels of glucose by stimulating glycogenolysis, gluconeogenesis both in the liver and skeletal muscle. In addition, it prevents the utilization of glucose by increasing the lipolysis in adipose tissue and amino acid catabolism in skeletal muscles]
2.1.1.4 Effect on Carbohydrate Metabolism
It stimulates the transcription of glucose 6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) genes that help in enhancing gluconeogenesis. In addition, activated PKA phosphorylates the pyruvate kinase (PK) to suppress glycolysis. Whereas, PKA-dependent activation of glycogen phosphorylase stimulates the release of glucose from the glycogen. The resulting increased glucose output from the liver acts to correct the hypoglycemic state. The increased glucose levels further stimulate the release of insulin from the pancreas, which will help in the mobilization of glucose in peripheral tissues. Henceforth, the hyperglycemic effect of glucagon is essential to ensure the proper functioning of absolute glucose-dependent tissues such as the brain and skeletal muscles.
2.1.1.5 Effect on Protein Metabolism
It stimulates the uptake, deamination of amino acids to generate ATP in the liver and further facilitates the conversion of ammonia to urea by inducing the enzymes involved in ureagenesis. When the hepatic glycogen stores are depleted, glucagon recruits gluconeogenic amino acids to produce glucose (gluconeogenesis). However, the protein catabolism does not take place in skeletal muscles as they lack glucagon receptors.
2.1.1.6 Effect on Lipid Metabolism
Glucagon stimulates the transcription of carnitine palmitoyltransferase I (CPT 1) in hepatocytes, thereby activating the β-oxidation of fatty acids to produce acetate. The acetate reacts with Co-enzyme A to form acetyl-CoA and is metabolized via the citric acid cycle (TCA). Moreover, glucagon-induced PKA-dependent phosphorylation of hormone-sensitive lipase (HSL) in adipocytes leads to the catabolism of triglycerides to free fatty acids and glycerol.
2.1.1.7 Regulation of Secretion
Decreased blood glucose levels (hypoglycemia) remain the primary stimulus for the secretion of glucagon, while hyperglycemia has the opposite effect. In addition, ingestion of a protein-rich diet or increased levels of glutamine or alanine, cortisol, and β-adrenergic activity stimulates the release of glucagon. Other pancreatic hormones such as insulin and somatostatin act in a paracrine manner to inhibit the secretion of glucagon.
2.1.2 Insulin
Produced by the β-cells in islets of Langerhans, insulin is a heterodimeric polypeptide hormone consisting of A and B chains held together by two disulfide bridges. The A chain consists of 21 amino acids with an intra-chain disulfide bridge, whereas the B chain has 30 amino acid residues. The precursor molecule proinsulin will be acted upon by a trypsin-like enzyme to produce the mature insulin and C-peptide. The mature hormone along with C-peptide is stored as secretory vesicles in the cytoplasm and released when the need arises.
2.1.2.1 Mechanism of Secretion
Insulin is primarily secreted due to high circulatory glucose levels. The glucose passes through the cell membrane of β-cells through GLUT2, phosphorylated by glucokinase and subsequently metabolized to generate ATP. The energy thus produced decreases the efflux of K+ by inhibiting the KATP channels. The accumulation of K+ ions stimulates the ion gated Ca2+ channels, thereby depolarizing the cell and facilitating the exocytosis of insulin. The concomitant fall of ATP levels during hypoglycemia leads to the hyperpolarization of cells, thereby inhibiting the secretion of insulin (Fig. 16.10).
Mechanism of insulin secretion by β-cells [Increased glucose entry during hyperglycemia prevents the conductance of K+ ions, resulting in the opening of voltage-dependent Ca2+ channels and subsequent exteriorization of insulin from the storage vesicles. [GLUT2 glucose transporter 2; ATP adenosine triphosphate; I insulin; C C-peptide; K+ potassium; Ca2+ calcium; ↓ decrease; ↑ increase]
2.1.2.2 Mechanism of Action
The transmembrane insulin receptor (IR) is a disulfide-linked dimer that belongs to the receptor tyrosine kinase (RTK) family and plays a pivotal role in eliciting the downstream signaling pathways. The intracellular domain of IR has a tyrosine kinase activity, which will be activated when insulin binds to the extracellular region. The activated tyrosine kinase phosphorylates the tyrosine residues outside the kinase domain and these in turn act as sites for various docking proteins such as insulin receptor substrate 1-6 (IRS 1-6) and Shc (Src homology 2 domain containing). They further mediate the activation of the PI3K/AKT and Raf/Ras/MEK/MAPK pathways that are responsible for various biological effects.
2.1.2.3 Biological Effects
Insulin is the only hypoglycemic hormone acting against all other hyperglycemic hormones (GH, THs, cortisol, and catecholamines). Hence, it is widely considered as an important regulator of metabolism in animals. Principally, insulin regulates carbohydrate metabolism by affecting glycogenesis, glycogenolysis, gluconeogenesis, and glycolysis. In addition, it also regulates protein and lipid metabolism. With liver, skeletal muscles, adipose tissue, and endothelium as major target tissues, insulin has an overall anabolic effect on intermediary metabolism (Fig. 16.11). Moreover, the control on a wide range of metabolic pathways confers the permissive effect of insulin on the actions of GH.
Anabolic effects of Insulin on intermediary metabolism. [Insulin establishes normoglycemia by stimulating the glucose uptake, glycogenesis, and lipogenesis in liver, skeletal muscles, and adipose tissues. [GLUT4 glucose transporter 4; PEPCK phosphoenolpyruvate carboxykinase; G6Pase glucose 6-phosphatase; FBP fructose-1, 6-bisphosphatase; GP glycogen phosphorylase; GP glycogen synthase; HSL hormone-sensitive lipase; CPT1 carnitine O-palmitoyltransferase-1; FAS fatty acid synthase; PDH pyruvate dehydrogenase; ACC acetyl-CoA carboxylase (ACC); ↓ decrease; ↑ increase]
2.1.2.3.1 Effect on Glucose Metabolism
Insulin-dependent regulation of glucose metabolism is mainly due to the activation of the PI3K/AKT pathway in target tissues. Activation of the PI3K/AKT pathway in skeletal muscles and adipose tissue enhances cellular uptake of glucose by stimulating the integration of GLUT4 on their cell membrane. GLUT4 mediated facilitated diffusion of glucose into these cells with its consequent entry into various metabolic pathways remains as the archetype effect of insulin. Thus, the increased glucose uptake increases glycolysis in both skeletal muscle and adipose tissue, while increased glycogen synthesis happens only in skeletal muscle. In the liver, insulin inhibits glycogen phosphorylase and side by side activates glycogen synthase resulting in decreased glycogenolysis with a simultaneous increase in glycogenesis, respectively. In addition, the insulin-dependent downregulation of PEPCK, G6Pase, and fructose-1, 6-bisphosphatase (FBP) genes inhibits gluconeogenesis in hepatocytes. Overall, insulin produces hypoglycemia by increasing glucose uptake in the liver and other peripheral tissues. Together with increased glycogenesis and decreased glycogenolysis, gluconeogenesis results in imparting the anabolic effect of insulin on glucose metabolism.
2.1.2.3.2 Effect on Amino Acid Metabolism
Insulin increases the skeletal muscle uptake of amino acids such as isoleucine, leucine, tyrosine, phenylalanine, and valine. Thus, the increased amino acid uptake facilitates the formation of new proteins along with a reduction in amino acid catabolism. Moreover, increased uptake of amino acids and inhibition of gluconeogenesis facilitate protein synthesis in the liver.
2.1.2.3.3 Effect on Lipid Metabolism
The insulin-mediated inhibition of hormone-sensitive lipase (HSL), carnitine O-palmitoyltransferase-1 (CPT1) reduces lipolysis and β-oxidation in adipocytes. Furthermore, increased glucose uptake along with upregulation of fatty acid synthase (FAS), pyruvate dehydrogenase (PDH), and acetyl-CoA carboxylase (ACC) genes helps in lipogenesis. Therefore, the formation of lipid stores along with a reduction in their breakdown results in the anabolism of lipids.
2.1.2.4 Regulation of Secretion
The rise in blood glucose levels (hyperglycemia) is the major metabolic stimulus for the secretion of insulin. However, an increase in circulatory levels of fatty acids, amino acids, GH, cortisol, gastrin, secretin, and cholecystokinin (CCK) positively regulates the secretion of insulin. However, hypoglycemia, somatostatin, and leptin inhibit the secretion of insulin.
2.1.3 Somatostatin
Produced from δ-cells in the pancreas, enteroendocrine D-cells, and in the brain (GHIH from the hypothalamus). Somatostatin secreted from the pancreas and intestine has 28 amino acids (SS-28) while the hypothalamic type has 14 amino acids (SS-14). SS-14, SS-28 were first isolated in the ovine brain and porcine gut, respectively.
2.1.3.1 Mechanism of Action
It binds to the somatostatin receptors (SSTRs) belonging to the GPCRs family. Activation of SSTR inhibits adenylyl cyclase (AC), leading to reduced intracellular cAMP and Ca2+ levels that further inhibit hormone secretion from target tissues.
Know More…
-
Insulin is the first peptide hormone/protein to be sequenced by Fredrick Sanger in 1955.
-
β-cell is the major cell type present in the islets of Langerhans.
-
Diabetes mellitus (DM): A pathological condition due to a reduction in insulin production (Type-I DM) or in the number of insulin receptors (Type-II DM), characterized by hyperglycemia, ketosis, and skeletal muscle depletion.
-
Bovine insulin and porcine insulin differ from human insulin only by 3 and 1 amino acids, respectively. Hence, they were used in treating diabetes mellitus in humans in the twentieth century.
-
Although glucagon and insulin have antagonistic effects on various metabolic pathways, they both increase the cellular uptake of amino acids.
2.1.3.2 Biological Effects
Generally, it is a negative regulator of neuroendocrine, pancreatic, and GIT hormones. It inhibits the secretion of growth hormone (GH), thyroid-stimulating hormone (TSH), and prolactin (PRL) in the brain. It inhibits the secretion of insulin and glucagon in the pancreas in a paracrine manner by stimulating the efflux of K+ with subsequent inhibition of Ca2+ influx. In addition, it inhibits the secretion of bile acids, gastric acid, pancreatic enzymes mainly by inhibiting the secretion of GIT hormones such as CCK, VIP, and gastrin.
2.1.3.3 Regulation of Secretion
GH, GHRH, and glucose regulate the secretion of the hypothalamic SS-14. The gastric SS-28 is primarily stimulated by the autonomic nervous system (ANS), CCK, and gastrin. In addition, Substance P produced in the intestine has a negative effect on the secretion of SS-28.
2.1.4 Pancreatic Polypeptide (PP)
Secreted from F-cells, PP is a peptide hormone with 36 amino acids and belongs to the neuropeptide Y (NPY) family of proteins. It binds to the Y4 receptor, a GPCR belonging to the NPY receptor family. When activated, it inhibits the AC system resulting in a reduction of cAMP levels. PP inhibits gastric motility, gall bladder contraction, and exocrine pancreatic secretion. In addition, it stimulates the secretion of gastric juice and suppresses anxiety. Its secretion is increased by a protein-rich diet, exercise, and fasting.
3 Adrenal Gland
3.1 Introduction
The adrenal gland located cranially on each kidney consists of an outer cortex and inner medulla, functions as two discrete endocrine glands with distinct embryological origins and endocrine activities. With a mesodermal origin, the adrenal cortex secretes cholesterol-derived hormones that are collectively known as corticosteroids. Whereas, the adrenal medulla derived from neural ectoderm is involved in the production of tyrosine-derived catecholamines. Together, corticosteroids and catecholamines help in regulating glucose metabolism, electrolyte balance, and antagonize stressors. Hence, appropriate functioning of the adrenal gland is essential for an animal’s survival.
3.2 Adrenal Cortex: Histology
The adrenal cortex has three different histological zones, namely: the outer zona glomerulosa, the central zona fasciculata, and the inner zona reticularis (Fig. 16.12). The presence of zone-specific hydroxylases in adreno-cortical cells helps in converting cholesterol to different classes of zone-specific steroid hormones. The corticosteroids are not stored in the cortical cells but their synthesis is rapidly stimulated in response to specific stimuli (Table 16.3).
3.3 Mechanism of Synthesis of Corticosteroids
The cholesterol required for the synthesis of corticosteroids is primarily derived from the circulation although a smaller proportion is derived from the de novo synthesis. The abundant lipid stores, mitochondria, and smooth endoplasmic reticulum are the major characteristics of cortical cells. The cholesterol influx or de novo synthesis from the cellular lipid stores depends on various stimuli like the adreno-corticotropin hormone (ACTH), altered ionic concentrations (K+), etc. The cholesterol is transported into the inner mitochondrial membrane from the outer mitochondrial membrane by steroidogenic acute regulatory protein (StAR). Subsequently, the cholesterol is converted to pregnenolone by CYP11A1 (p450scc/Cholesterol desmolase). The formation of pregnenolone from cholesterol is a rate-limiting step that is primarily stimulated by ACTH. The pregnenolone will be further acted upon by zone-specific hydroxylases to be converted into mineralocorticoids in zona glomerulosa or glucocorticoids in zona fasciculata or sex steroids in zona reticularis.
3.4 Zona Glomerulosa: Site of Synthesis for Mineralocorticoids
The zona glomerulosa is a thin outermost layer with columnar cells arranged in irregular cords. They are responsible for the secretion of a class of hormones known as mineralocorticoids, which are implicated in regulating major electrolytes (Na+, K+) present in the blood. Aldosterone is the potent and major mineralocorticoid secreted across different species of animals. In addition, corticosterone and 11-deoxycorticosterone have slight mineralocorticoid activity. Within these cortical cells, pregnenolone is converted to progesterone by 3β-hydroxysteroid dehydrogenase (3βHSD). Then the subsequent conversion of progesterone by 21β-hydroxylase results in the production of 11-deoxycorticosterone. Further, the 11-deoxycorticosterone is converted to corticosterone by 11 β-hydroxylase. Finally, the aldosterone synthase that is present exclusively in the zona glomerulosa converts corticosterone to aldosterone (Fig. 16.13). The enzymatic activity of aldosterone synthase is regulated by angiotensin-II. Furthermore, the inability of zona glomerulosa cells to secrete cortisol or other androgens is due to the absence of 17 α-hydroxylase.
Biosynthesis of aldosterone in zona glomerulosa [Produced from cholesterol, the corticosterone is converted exclusively in the zona glomerulosa by the enzyme aldosterone synthase to yield aldosterone. [ACTH adrenocorticotropic hormone; StAR steroidogenic acute regulatory protein; OMM outer mitochondrial membrane; IMM inner mitochondrial membrane; CYP11A1 cholesterol side-chain cleavage enzyme; 3βHSD 3β-hydroxysteroid dehydrogenase]
3.4.1 Mechanism of Action
Aldosterone exerts its biological effects mainly by binding to intracytoplasmic mineralocorticoid receptors (MR). Once the hormone-receptor complex is formed, it migrates to the nucleus and stimulates the transcription of Na+-K+ ATPase and epithelial sodium channels (ENaC) genes. Hence, the effects of aldosterone are not evident soon after its release and require a brief period. Most importantly, the principal cells (PC) and intercalated cells (IC) are recognized as the major cellular targets of aldosterone.
3.4.2 Biological Effects
The restoration of normal circulatory levels of Na+ and K+ by inhibiting natriuresis with a concomitant rise in potassium secretion from kidneys is regarded as the crucial biological effects. In addition, the rise in systemic circulatory volume and arterial blood pressure are secondary effects due to increased reabsorption of water from the renal tubules.
3.4.3 Effect on Principal Cells
The principal cells are present in the late distal tubule and effectively contribute to the reabsorption of Na+ and secretion of K+. The Na+-K+ ATPase located on the basolateral membrane pumps Na+ ions in exchange for K+ ions from blood, resulting in the establishment of low Na+ and high K+ concentrations inside. This resultant decrease in the intracellular Na+ concentration facilitates its influx from the tubular filtrate through ENaC. During the reabsorption of Na+, K+ ions are secreted down the concentration gradient to maintain electrical neutrality. Furthermore, the reabsorption of Na+ leads to the simultaneous movement of water and leads to a minor or no increase in the circulatory Na+ levels. Whereas, the increased secretion of K+ ions leads to decreased circulatory levels of K+ (hypokalemia) (Fig. 16.14).
Biological effects of aldosterone [Aldosterone acts on the principal and intercalated cells in the distal convoluted tubules to increase the tubular reabsorption of Na+ along with water and tubular excretion of K+ ions. [A aldosterone; MR mineralocorticoid receptor; RAS renin-Angiotensin system; Na+ sodium ion; K+ potassium ion; HCO3 bicarbonate ion; ENaC epithelial sodium channels; H2CO3 carbonic acid; CA carbonic anhydrase; BM basal membrane; LM luminal membrane; ↓ decrease; ↑ increase]
3.4.4 Effect on Intercalated Cells
Intercalated cells (IC) are the other type of distal tubular cells affected by aldosterone. It stimulates the H+ ATPase/H+-K+ ATPase pumps present on the apical membrane to secrete H+ ions and reabsorb K+ ions. Thus, this particular activity of IC is critical for the excretion of H+ ions and imparting a regulatory role in acid-base balance. In addition, there is an interdependency between the functioning of IC and PC.
3.4.5 Regulation of Secretion
Although ACTH is necessary for the production of aldosterone, the circulatory concentration of K+ ions is by far the most potent stimulator for its secretion. The angiotensin-II is the second most potent stimulator of aldosterone production. It increases the secretion of aldosterone by directly acting on the zona glomerulosa cells and by stimulating the production of ACTH from the anterior pituitary. The initiation of the renin–angiotensin–aldosterone system (RAAS) plays an important role in regulating the circulatory volume and arterial pressure. Moreover, an increase in the circulatory concentration of Na+ ions suppresses the secretion of aldosterone.
3.5 Zona Fasciculata: Site of Synthesis for Glucocorticoids
The zona fasciculata is the middle and broadest layer of adrenal cortex comprising polyhedral cells. They secrete a group of hormones known as glucocorticoids, which are implicated in regulating metabolic pathways that enable the animals to endure various stressors. The presence of 17 α-hydroxylase in fascicular cells converts pregnenolone to 17-hydroxypregnenolone. Then, it is further converted to 17-hydroxyprogesterone by the enzyme 3βHSD. Consequent action of 21β-hydroxylase results in the conversion of 17 hydroxy-progesterone to 11-deoxycortisol. Finally, the 11-deoxycortisol is converted by 11 β-hydroxylase to produce cortisol, the predominant glucocorticoid in animals. Additionally, pregnenolone is converted to progesterone and further to 11-deoxycorticosterone and corticosterone, both of which are found in the circulation as minor glucocorticoids (Fig. 16.15). Cortisol then secreted will be bound to transcortin (corticosteroid-binding globulin) present in the circulation.
Biosynthesis of glucocorticoids in zona fasciculata. [The cholesterol taken up by the fascicular cells is enzymatically converted to produce glucocorticoids such as corticosterone and cortisol. [ACTH adrenocorticotropic hormone; StAR steroidogenic acute regulatory protein; OMM outer mitochondrial membrane; IMM inner mitochondrial membrane; CYP11A1 cholesterol side-chain cleavage enzyme; 3βHSD 3β-hydroxysteroid dehydrogenase]
3.5.1 Mechanism of Action
Glucocorticoids exert their effects on the target tissues by binding to glucocorticoid receptors (GCR) localized in the cytoplasm. Generally, the GCRs in their inactive state are bound with heat shock protein 90 (Hsp90). Upon their interaction with glucocorticoids will result in the dissociation of Hsp90 with a simultaneous translocation of the hormone-receptor complex to the nucleus. In the nucleus, hormone-receptor complexes dimerize and bind to DNA (glucocorticoid response element) to regulate the transcription of genes.
3.5.2 Biological Effects of Glucocorticoids
The glucocorticoids have a prominent role in maintaining energy homeostasis by regulating metabolic pathways in the liver, adipose tissue, and skeletal muscles. Glucocorticoids increase gluconeogenesis, lipolysis, and glycogenolysis in various tissues resulting in hyperglycemia, mobilization of fat stores, and depletion of protein reserves. Moreover, it has a potent suppressive effect on the animal’s immune system. In total, the secretion of glucocorticoids is obligatory for animal survival by regulating their metabolism to drive the functioning of various vital organs, especially the brain (Fig. 16.16).
Effects of cortisol on intermediary metabolism and its regulation of secretion [Glucocorticoids increase the catabolism of lipids and amino acids for cellular metabolism, thereby maintain a constant elevated blood glucose levels to combat stressful conditions. [CRH corticotropin-releasing hormone; ACTH adrenocorticotropic hormone; PEPCK phosphoenolpyruvate carboxykinase; G6Pase glucose 6-phosphatase; GLUT4 glucose transporter 4; FAS fatty acid synthase; ACC acetyl-CoA carboxylase (ACC); FBP fructose-1, 6-bisphosphatase; GP glycogen phosphorylase; GP glycogen synthase; HSL hormone-sensitive lipase, (−) negative feedback inhibition; IL-2 interleukin 2; IL-3 interleukin 3; IL-5 interleukin 5; GM-CSF granulocyte-macrophage colony-stimulating factor; ↓ decrease; ↑ increase]
3.5.2.1 Effect on Hepatic Metabolism
Glucocorticoids stimulate gluconeogenesis and glycogenolysis, respectively, by stimulating the transcription of key genes such as PEPCK and G6Pase. This helps in increased hepatic glucose production and released into the circulation. They also upregulate FAS and ACC genes, which leads to increased lipogenesis. Along with lipogenesis, simultaneous inhibition of the β-oxidation of fatty acids leads to an increase in hepatic lipid accumulation (hepatic steatosis).
3.5.2.2 Effect on Skeletal Muscle
Glucocorticoids inhibit the insulin secretion from β-cells, insulin-dependent uptake of glucose and amino acids by inhibiting the insulin-mediated PI3K/Akt signaling pathway. Thus, the inhibition of the PI3K/Akt pathway results in reduced glucose uptake and glycogenesis due to declined translocation of GLUT4 and downregulation of the glycogen synthase gene respectively. Furthermore, glucocorticoids increase the protein degradation by the proteasome, cathepsin-L, and ubiquitin C to produce free amino acids required for generating energy and glucose. Altogether, glucocorticoids produce a catabolic effect on skeletal muscles characterized by the exhaustion of glycogen and protein reserves.
3.5.2.3 Effect on Adipose Tissue
They activate hormone-sensitive lipase (HSL) in peripheral adipocytes, this leads to increased lipolysis and FFA production. In addition, they inhibit PEPCK and insulin-dependent glucose uptake to decrease triglyceride formation. Whereas, the same glucocorticoids stimulate the differentiation and hypertrophy of central adipocytes. Altogether, glucocorticoids redistribute lipids from peripherally located adipose tissues to central adipose depots, especially in the abdomen.
3.5.2.4 Effect on the Immune System
Glucocorticoids inhibit the production of cytokines like interleukin 3(IL-3), interleukin 5 (IL-5), GM-CSF which regulate maturation, differentiation, and survival of eosinophils. Their effect on inhibiting IL-2 and T-cell growth factor results in the inhibition of T cell proliferation and with a concurrent increase in T-cell apoptosis. Furthermore, glucocorticoids inhibit the migration of leucocytes by downregulating the expression of adhesion molecules and chemokines from the inflammatory site.
3.5.2.5 Hypothalamo–Pituitary–Adrenal (HPA Axis)
The secretion of glucocorticoids is primarily regulated through CRH and ACTH released from the hypothalamic–pituitary axis. The release of CRH is stimulated during physical stress, physiological stress, or behavioral stress. Further, the increased circulatory levels of glucocorticoids have negative feedback on the secretion of CRH and ACTH. The circadian rhythm also affects the secretion of cortisol, attaining peak secretion during the early morning.
3.6 Zona Reticularis and Adrenal Androgens
The dehydroepiandrosterone (DHEA) and androstenedione are the two adrenal androgens secreted from the innermost zona reticularis in response to ACTH (Fig. 16.17). The 17-hydroxypregnenolone produced from cholesterol is converted by the action of 17, 20 lyase to DHEA. Further, DHEA is converted to androstenedione by 3βHSD. They bind to albumin and sex hormone-binding globulin (SHBG) in circulation. These weak adrenal androgens cannot bind to androgen receptors and require transformation to potent forms such as testosterone and dihydrotestosterone to elicit target effects. Although the amount of adrenal androgens produced in male animals is negligible, they might play a role in producing an anabolic effect on muscle mass, bone density, and estrous behavior in female animals.
Biosynthesis of adrenal androgens. [Zona reticularis is bestowed with the production of androgens from cholesterol, which are further converted in to active or more potent forms in the gonads. [DHEA dehydroepiandrosterone; ACTH adrenocorticotropic hormone; StAR steroidogenic acute regulatory protein; OMM outer mitochondrial membrane; IMM inner mitochondrial membrane; CYP11A1 cholesterol side-chain cleavage enzyme; 3βHSD 3β-hydroxysteroid dehydrogenase; ↑ increase]
Know More…
-
Addison’s disease: Also known as hypoadrenocorticism, characterized by reduced secretion of corticosteroids.
-
Cushing’s disease: Pathological condition due to the hyperactivity of adrenal cortex.
-
Fetal cortisol is the hormone that initiates the parturition reflex in animals.
-
Circulatory cortisol levels are often used as a stress marker and useful assess well-being of animals.
-
In birds, mice, and rats, corticosterone is the major glucocorticoid secreted from adrenal cortex.
-
Aldosterone escape: The expansion of circulatory volume due to aldosterone triggers the release of atrial natriuretic peptide (ANP) from heart to induce natriuresis and diuresis.
3.7 Adrenal Medulla: Histology
The adrenal medulla is the innermost part of the adrenal gland, it forms one-fifth of the adrenal mass. Generally considered an extension of the sympathetic system, the adrenal medulla comprises postganglionic sympathetic cells that can secrete hormones. When stimulated by pre-ganglionic sympathetic neurons, they synthesize and secrete epinephrine (adrenaline) and norepinephrine (noradrenaline). Since these neuroendocrine cells display a high affinity toward chromium salts, they are also known as chromaffin cells.
3.7.1 Mechanism of Synthesis
Derived from the amino acid tyrosine, both epinephrine and norepinephrine are known as adrenal catecholamines. The tyrosine required for their synthesis is derived either from the diet or through the enzymatic conversion of phenylalanine. The further conversion of tyrosine to dihydroxy-phenylalanine (DOPA) by tyrosine hydroxylase (TH) is the rate-limiting step in the biosynthesis of adrenal catecholamines. Subsequently, DOPA is formed from dopamine due to the enzymatic action of L-aromatic amino acid decarboxylase (AAAC). Later, norepinephrine is produced from dopamine under the influence of dopamine-β-hydroxylase (DBH). Additionally, 80% of chromaffin cells possess phenylethanolamine N-methyltransferase (PNMT) enzyme, which converts norepinephrine to form epinephrine. Therefore, epinephrine is the major catecholamine to be secreted from the adrenal medulla (Fig. 16.18).
Synthesis of adrenal catecholamines [Produced from tyrosine, the catecholamines are released into the circulation from the adrenal medulla in response to adverse situations such as fear, stress, cold, and apnea. [SNS sympathetic nervous system; PH phenylalanine hydroxylase; TH tyrosine hydroxylase; AAAD L-aromatic amino acid decarboxylase; DBH dopamine-β-hydroxylase; PNMT phenylethanolamine N-methyltransferase; E epinephrine; NE norepinephrine; Na+ sodium ion; Ca2+ calcium ion; ↑ increase]
3.7.2 Mechanism of Action
Epinephrine and norepinephrine bind to specific adrenergic receptors (AR), which are further classified into two major subtypes α and β. Epinephrine has an equal affinity toward both the receptor types, whereas norepinephrine predominantly excites β type of adrenergic receptors. The adrenergic receptors belong to the GPCRs family, upon activation they either stimulate or inhibit adenylyl cyclase (AC) and phospholipase-C (PLC) systems to produce biological effects in the target organs. Hence, the target effects of adrenal catecholamines depend on the type of receptor expressed on the target tissues.
3.7.3 Biological Effects
Even though the adrenal medulla is not essential for life, hormones secreted from it activate physiological and behavioral responses collectively known as “fight or flight” to overcome acute stress. The biological effects are predominant in the cardiovascular system, skeletal muscles, energy metabolism, GI tract, and kidneys.
3.7.3.1 Effects on the Cardiovascular System
Both epinephrine and norepinephrine directly stimulate the SA node, AV node, and Purkinje conduction system leading to an increased heart rate. In addition, they also increase the strength of myocardial contractions. Both these effects are mediated by the activation of β-ARs and downstream AC system. Furthermore, catecholamines produce α-AR-mediate vasoconstriction in the lungs, kidneys, and GIT. The concurrent vasodilation occurring in skeletal muscles due to the activation of β-ARs will lead to the redistribution of blood to them. The redistribution and vasoconstrictor effects of catecholamines lead to an increase in the systemic arterial pressure to maintain adequate blood supply to vital organs.
3.7.3.2 Effects on the Smooth Muscle System
Adrenal catecholamines have profound effects on smooth muscles present in various organs. The vasoconstrictor effect in different visceral organs is due to the contraction of vascular smooth muscles present in small arterioles and pre-capillary sphincter. They also act on smooth muscles present in bronchioles, GIT, and urinary bladder resulting in bronchodilation, inhibition of GIT motility, and urine retention, respectively.
3.7.3.3 Effects on Metabolism
The circulating catecholamines inhibit insulin and stimulate glucagon secretion by activating α-ARs and β-ARs, respectively. In addition, they stimulate glucose production through glycogenolysis in the liver and skeletal muscles. They also augment lipolysis by activating triglyceride lipase in adipose tissue to release free fatty acids. The aforementioned effects of adrenal medullary hormones will lead to an increased concentration of glucose and free fatty acids in the blood. The resultant changes in metabolism will help the skeletal muscles, heart, and brain to function normally even during adverse conditions.
3.7.3.4 Effect on Skeletal Muscles
The redistribution of blood to skeletal muscles from visceral organs will help in meeting the nutrients required for increased muscular activity seen during flight or fight response. They stimulate glycolysis and β-oxidation of fatty acids for deriving the energy required for muscular contraction (Fig. 16.19).
3.7.3.5 Miscellaneous Effects
Other effects of catecholamines include natriuresis, activation of the renin–angiotensin system, mydriasis, and inhibition of micturition. They also play a crucial in learning and memory consolidation.
3.7.4 Regulation of Secretion
The secretion of adrenal catecholamines is mainly due to the stimulation of the sympathetic nervous system (SNS) by cold, apnea, physical or environmental stress, and fear.
4 Calcium Homeostasis
4.1 Introduction
Nearly 99% of the total calcium is present in bones and teeth, with 1% inside cells and 0.1% in circulation. The ionized calcium (Ca2+) present in circulation plays an indispensable role in blood coagulation, excitation of neurons, and contraction of smooth muscle/cardiac cells/skeletal muscles. Furthermore, a rise in the intracellular Ca2+ ions is a prerequisite for the exocytosis of enzymes and hormones. Hence, hormone-mediated calcium homeostasis of circulatory Ca2+ is critical for growth, reproduction, and production in animals. Parathormone (PTH), calcitriol, and calcitonin constitute the triad of hormones implicated in calcium homeostasis (Table 16.4).
4.2 Parathormone (PTH)
PTH is a single-chain polypeptide hormone isolated initially in the bovine parathyroid gland. It is synthesized and secreted from chief cells residing in the parathyroid gland. Oxyphil cells are an additional type of inactive cells present in the parathyroid gland. PTH is initially synthesized as a precursor polypeptide with 115 amino acids. Successive modifications in the precursor molecule yield an active form of PTH with 88 amino acid residues. The initial 34 amino acids confer biological activity of PTH on its target tissues.
4.2.1 Mechanism of Action
PTH binds to at least three specific types of GPCRs known as parathormone receptors 1/2/3 (PTH 1/2/3R). When bound to PTH, they trigger the production of second messengers such as cAMP and calcium to activate PKA and PKC, respectively. PTH activates PTH1R present on osteoblasts and tubular epithelial cells making them major target cells.
4.2.2 Biological Effects
The target effects of PTH on kidney and bone are aimed principally at increasing circulatory Ca2+ levels (hypercalcemia). Further, PTH facilitates the activation of Vitamin D3 (Vit.D) in kidneys, thereby indirectly stimulating the intestinal absorption of dietary Ca2+.
4.2.2.1 Effect on Bone
PTH acts on osteoblasts and stimulates the release of bone degrading proteases and cytokines that activate osteoclasts. Two such molecules that mediate activation and differentiation of osteoclasts are the macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL). The production of monocyte chemoattractant protein-1 (MCP-1) from osteoblasts will stimulate the formation of new osteoclasts. The increased production, activation, and differentiation of osteoclasts result in the demineralization of bones. In addition, PTH stimulates osteocytes to redistribute Ca2+ from bone fluid to circulation, termed osteocytic osteolysis. Together, osteolysis and osteoclast-mediated bone resorption increase the mobilization of Ca2+ and Pi (phosphate) from bone into the blood.
4.2.2.2 Effect on Kidney
PTH stimulates the tubular reabsorption of Ca2+ by upregulating the Ca2+-ATPase and Na+-Ca2+ antiporter genes in ascending loop of Henle and distal convoluted tubule (DCT). It also inhibits the reabsorption of Pi in the proximal tubule, thus useful in excreting excess Pi (phosphaturic effect) accumulated during bone resorption. In addition, it stimulates the production of calcitriol (1, 25-dihydroxy cholecalciferol) by upregulating the 1α-Hydroxylase gene in the kidney and increases the absorption of Ca2+ from GIT. Overall, increased mobilization of Ca2+ from bone with concurrent inhibition of its excretion produces the hypercalcemic effects of PTH (Fig. 16.20).
Regulation of calcium by PTH [Parathormone increases the resorption of Ca2+ from the bones and kidneys to restore the circulatory Ca2+ levels to normalcy. CaSR calcium-sensing receptors; Ca2+ calcium ion; Pi inorganic phosphorus; RANKL receptor activator of nuclear factor kappa B ligand; M-CSF macrophage colony-stimulating factor; MCP-1 monocyte chemoattractant protein-1; ↑ increase]
4.2.3 Regulation of Secretion
The calcium-sensing receptors (CaSR) present on chief cells will help to detect the minute-to-minute variations in circulatory Ca2+ levels. Increased binding of Ca2+ to CaSR results in the inhibition of PTH release from the chief cells, whereas vice versa is true for PTH release. Since the parathyroid is bestowed with an exceptional sensitivity toward circulatory concentration of Ca2+, it is the foremost regulator of Ca2+ homeostasis.
4.3 Calcitriol (1, 25-Dihydorxy Cholecalciferol)
Vit.D3 (Cholecalciferol) is a secosteroid derived from the precursor 7-dehydrocholesterol in the skin or from dietary supplements. Because of its lipophilic nature, Vit.D3 binds to Vit.D binding protein (DBP) in circulation. Then, 25 α-hydroxylase present in the hepatocytes mediates the hydroxylation of Vit.D3 to produce 25-hydroxycholecalciferol (25-hydroxy Vit.D3). Finally, 25-hydroxycholecalciferol (Calcidiol) is further hydroxylated by 1α-hydroxylase in proximal tubular cells to produce the active form of Vit.D3, i.e., 1, 25-dihydroxycholecalciferol, also known as calcitriol. The upregulation of 1α-hydroxylase is independently stimulated by a fall in Ca2+ and increased PTH in the blood (Fig. 16.21).
Biosynthesis and mechanism of action of calcitriol [The cholecalciferol produced in the skin is sequentially hydroxylated in liver and kidneys to yield the active form calcitriol. The calcitriol produced binds to the VDR present in the nucleus to produce the target effects. [PTH parathormone; VD calcitriol; VDR Vit.D receptor; RXR retinoid X receptor; ↑ Increase]
4.3.1 Mechanism of Action
Calcitriol binds to vitamin D receptor (VDR) localized in the nucleus, fundamentally a type of ligand-dependent transcriptional factor. Expressed in various tissues, VDR is abundant in bones, intestinal epithelium, parathyroid gland, skin, and even in germ tissues. Once activated, VDR forms a hetero dimer with RXR and binds to a specific DNA region known as vitamin D response element (VDRE). The binding of heterodimer results in the transcriptional activation of different genes that contribute to producing the biological effects.
4.3.2 Biological Effects
Calcitriol acts in concert with PTH to elevate the blood Ca2+ levels back to normal. Its major targets tissues are bones and the intestine epithelium. Apart from the hypercalcemic effect, calcitriol also regulates cellular proliferation, differentiation, and immune response. Together, increased absorption of calcium from the intestine and bones helps in increasing the blood calcium concentration.
4.3.2.1 Effects on the Intestinal Epithelium
Calcitriol stimulates enterocytes to produce more calcium-binding protein (CaBP), Na+-Ca2+ pumps and increases the brush border permeability to Ca2+. The Ca2+ forms a complex with CaBP and is subsequently absorbed into the blood. The presence of a Na+-Ca2+ pump on the basolateral membrane helps in pumping out the Ca2+ accumulated in the enterocytes to the blood. The Na+-K+ ATPase pump is very much essential to maintain the activity of the Na+-Ca2+ pump and hence this method of Ca2+absorption is energy-dependent and considered an active process. Additionally, the Ca2+-CaBP complex can be translocated from the gap junctions between the enterocytes or by fusing with lysosomes and exocytosed into the blood. Furthermore, it also increases the intestinal absorption of phosphate, thus increasing its concentration in blood.
4.3.2.2 Effects on the Bone
Calcitriol has a synergistic effect on the PTH-mediated resorption of Ca2+ from the bone. It activates osteoclasts by stimulating the paracrine signals from osteoclasts. In the absence of calcitriol, the effect of PTH on osteoclast activation is negligible.
4.3.3 Regulation of Secretion
The activation of Vit.D3 depends on the circulatory levels of PTH and Ca2+. Elevated PTH in the blood increases the calcitriol formation, whereas increased levels of Ca2+ result in the conversion of 25-hydroxycholecalciferol to inactive 24, 25-dihydroxy cholecalciferol.
4.4 Calcitonin
Calcitonin is a polypeptide hormone secreted from C-cells (also known as parafollicular cells) in the thyroid gland. The mature hormone consisting of 32 amino acids is derived from the precursor prohormone molecule with 136 amino acids.
4.4.1 Mechanism of Action
Calcitonin binds to calcitonin receptors (CTR) that belong to the GPCR superfamily. The activation of CTR initiates both AC and PLC systems to initiate downstream signaling pathways in the target cells, especially in renal tubular epithelial cells and osteoclasts.
4.4.2 Biological Effects
Released in response to hypercalcemia, calcitonin acts to bring the circulatory Ca2+levels back to normal. It acts on the osteoclasts to suppress their release of acid phosphatase, motility, and differentiation resulting in the inhibition of bone resorption. In addition, calcitonin inhibits the renal tubular reabsorption of Ca2+ promoting its excretion. Together, a concurrent decrease in Ca2+ release from bone and its simultaneous excretion from kidneys produce a hypercalcemic effect.
4.4.3 Regulation of Secretion
Secretion of calcitonin is chiefly triggered by the rise of Ca2+ concentration in blood.
Know More…
Hyperparathyroidism: A pathological condition characterized by increased secretion of PTH.
Hypoparathyroidism: Condition characterized by decreased secretion of PTH.
Milk fever: A metabolic disease in post-parturient cows due to the decreased calcium levels in blood.
Rickets: Deficiency of Vit.D3 resulting in the abnormal bending of bones in young animals.
Osteomalacia: Deficiency of Vit.D3 in adult animals leading to an abnormal softening of bones.
Renal rickets: A pathological condition characterized by the absence of 1α-hydroxylase in kidneys and subsequent deficiency of calcitriol.
Learning Outcomes
-
Thyroid hormones: Thyrocytes present in the thyroid follicles synthesize triiodothyronine (T3) and tetraiodothyronine (T4) from the amino acid tyrosine. While T4 is the major secretory form (90%), biological effects are due to T3. Hence, T4 is converted to T3 in the target cells by the action of deiodinases. It stimulates glycogenolysis, gluconeogenesis, and glycolysis in the liver resulting in the increased secretion of glucose into the circulation. T3 stimulates glucose uptake, glycolysis, and protein degradation in skeletal muscle to generate more nutrients. Moreover, increased nutrients along with an increased mitochondrial number and activity to augment oxidative phosphorylation and subsequent thermogenesis.
-
Glucagon: It is a polypeptide hormone released from the α-cells in response to hypoglycemia. Glucagon stimulates the rate of production of glucose from the liver by stimulating glycogenolysis, gluconeogenesis, and β-oxidation of fatty acids with concomitant inhibition of glycolysis. It also produces a marked degradation of skeletal muscle protein and lipolysis in adipose tissue to produce glucose via gluconeogenesis. The resultant increase in blood glucose levels supports the functioning of brain and other glucose-dependent cells present in the body.
-
Insulin: Insulin produced from the β-cells is the only hypoglycemic hormone produced in animals. It is a heterodimeric polypeptide hormone released during hyperglycemia. It stimulates the glucose uptake in skeletal muscles and adipocytes to produce glycogen and triglycerides, respectively. It inhibits glucose production by inhibiting gluconeogenesis and stimulates protein synthesis in both the liver and skeletal muscles. The anabolic effects thus produced by insulin make it as one of the crucial mediator of growth due to GH.
-
Mineralocorticoids: Mineralocorticoids are implicated in regulating the electrolyte balance and circulatory fluid volume. Aldosterone is the major mineralocorticoid synthesized exclusively in zona glomerulosa due to the presence of aldosterone synthase. Hyperkalemia is the most potent stimulator for aldosterone secretion, followed by angiotensin-II. Primarily, it affects principal cells and intercalated cells in distal tubules to increase Na+ reabsorption, K+ excretion, H+ secretion, and water reabsorption. These biological effects result in bringing down the K+ ion levels and restoring circulatory volume.
-
Glucocorticoids: Released from zona fasciculata, they are responsible for regulating glucose levels in circulation. Cortisol is the major glucocorticoid present in most animals, whereas corticosterone is the primary glucocorticoid in birds, mice, and rats. The glucocorticoids have a catabolic effect on glycogen, protein, and adipose tissue stores present in an animal’s body. Thus, the activation of various catabolic pathways results in hyperglycemia to support the functioning of glucose-dependent vital organs such as the brain during stress. In addition to its effects on metabolism, it causes potent inhibition of immune responses.
-
Ca2+ homeostasis: Parathormone, calcitriol, and calcitonin constitute the triad of hormones that regulates calcium homeostasis. Parathormone and calcitriol are released from the parathyroid gland and kidneys, respectively, to elevate blood Ca2+ levels. During the period of hypocalcemia, they stimulate the reabsorption of Ca2+ from bone and kidney, increase the intestinal absorption of Ca2+ and renal excretion of phosphorus (Pi). Calcitonin released from the thyroid gland during hypercalcemia increases the renal excretion of Ca2+, thereby decreasing the Ca2+levels back to normal.
Exercises
Objective Questions
-
Q1.
The glycoprotein secreted by the thyrocytes is known as _____________
-
Q2.
The iodide trapping seen in the thyroid gland is an example of ___________ type of active transport
-
Q3.
______________ is the enzyme required for the conversion of iodide into iodine in the thyroid gland
-
Q4.
What is the major form of thyroid hormone produced by the thyroid gland?
-
Q5.
What is the major plasma protein to which the thyroid hormones bind in the circulation?
-
Q6.
The group of enzymes that metabolism of the thyroid hormones in the target tissue are known as ____________
-
Q7.
The increase in the metabolic heat production by the thyroid hormones in the target tissues is an example for the _______________ type of thermogenesis
-
Q8.
What is the major endocrine cell type present in the islets of Langerhans?
-
Q9.
What is the pancreatic hormone released during hypoglycemia?
-
Q10.
The insulin receptor belongs to the _____________ family
-
Q11.
______________ is the widest layer in adrenal cortex
-
Q12.
The embryological origin of the adrenal medulla is ____________
-
Q13.
The adrenal catecholamines are derived from _____________ amino acid
-
Q14.
What is the major mineralocorticoid secreted from the adrenal cortex?
-
Q15.
The major type of corticosteroid in birds and reptiles is _____________
-
Q16.
Which hormone is responsible for the aldosterone escape?
-
Q17.
Which catecholamine preferentially binds to the β type of adrenergic receptors?
-
Q18.
The hypercalcemic hormone is produced by ____________ cells present in the parathyroid gland
-
Q19.
The last step in the activation of calcidiol to form calcitriol is catalyzed by the enzyme _____________
-
Q20.
The parafollicular cells in the thyroid gland are responsible for the production of ______________ hormone
Subjective Questions
-
Q1.
Describe the process of iodide trapping in thyroid follicles.
-
Q2.
Explain the sequential steps in thyroid hormone synthesis
-
Q3.
How the thyroid hormones are transported in blood?
-
Q4.
Describe the mechanism of action of thyroid hormones.
-
Q5.
What are the biological effects of T3?
-
Q6.
Explain the regulation of the secretion of thyroid hormones.
-
Q7.
What are the different cell types present in islets of Langerhans? Explain their endocrine function?
-
Q8.
Describe the effects of glucagon on intermediary metabolism.
-
Q9.
Describe the molecular mechanisms involved in the secretion of insulin
-
Q10.
Briefly explain the intracellular signaling of insulin in target tissues.
-
Q11.
Substantiate why insulin is known as an anabolic hormone.
-
Q12.
Explain the histology of the adrenal gland.
-
Q13.
Write a short note on the synthesis of mineralocorticoids
-
Q14.
Describe the biological effects of aldosterone.
-
Q15.
List out different glucocorticoids and their biosynthesis.
-
Q16.
Why the secretion of glucocorticoids is considered essential for life?
-
Q17.
What is the hypothalamus–pituitary–adrenal (HPA) axis?
-
Q18.
Explain the steps involved in the synthesis of adrenal catecholamines.
-
Q19.
Explain the events in the fight or flight response.
-
Q20.
Explain the hormonal regulation of Ca2+ homeostasis
Answer to the Objective Questions
-
A1.
Thyroglobulin
-
A2.
Secondary
-
A3.
Thyroid peroxidase (TPO)
-
A4.
T4(Thyroxine)
-
A5.
Thyroxine-binding globulin (TBG)
-
A6.
Deiodinases
-
A7.
Non-shivering thermogenesis
-
A8.
β-cells
-
A9.
Glucagon
-
A10.
Tyrosine kinase
-
A11.
Zona fasciculata
-
A12.
Neural ectoderm
-
A13.
Tyrosine
-
A14.
Aldosterone
-
A15.
Corticosterone
-
A16.
Atrial natriuretic peptide (ANP)
-
A17.
Norepinephrine
-
A18.
Chief cells
-
A19.
1α-hydroxylase
-
A20.
Parathormone
Keywords for the Answer to Subjective Questions
-
A1.
Sodium (Na+)-Iodide (I−) symporter (NIS), secondary active transport, pendrin
-
A2.
Iodine uptake, organification¸ endocytosis
-
A3.
Thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA or Transthyretin), serum albumin
-
A4.
MCT 8, 5′-deiodinase type 1 (D1), 5′-deiodinase type 2 (D2), thyroid receptors (TRs), retinoid X receptor (RXR), thyroid response element (TRE)
-
A5.
Increases glucose production, basal metabolic rate (BMR), glycolysis, gluconeogenesis, glycogenolysis, lipolysis, basal metabolic rate (BMR).
-
A6.
Hypothalamic–Pituitary–Thyroid axis, TRH, and TSH
-
A7.
α cells-glucagon, β cells-Insulin, δ cells-somatostatin, and F/γ cells-pancreatic polypeptide
-
A8.
Inhibition of glycolysis and glycogenesis, catabolism of lipids and amino acids, hyperglycemia, ureagenesis
-
A9.
GLUT2, KATP channels, Ca2+ channels, and exocytosis
-
A10.
Insulin receptor (IR), tyrosine kinase, insulin receptor substrate 1-6 (IRS 1-6), PI3K/AKT pathway, Raf/Ras/MEK/MAPK pathway
-
A11.
Decreased glycogenolysis, increase in glycogenesis, inhibit gluconeogenesis, protein synthesis in the liver, decrease lipolysis, increase lipogenesis
-
A12.
Zona glomerulosa, zona fasciculata, zona reticularis, and adrenal medulla
-
A13.
Pregnenolone, progesterone, 11-deoxycorticosterone, corticosterone, aldosterone synthase, aldosterone
-
A14.
Principal cells (PC), epithelial sodium channels (ENaC) genes, Na+-K+ ATPase, intercalated cells (IC), H+ ATPase, H+ ion secretion, normovolemia
-
A15.
Cholesterol, pregnenolone, 11-deoxycorticosterone, corticosterone, cortisol
-
A16.
Gluconeogenesis, glycogenolysis, lipolysis, hyperglycemia
-
A17.
Hypothalamus-CRH, anterior pituitary gland-ACTH, adrenal-cortisol
-
A18.
Phenylalanine, tyrosine, dihydroxy-phenylalanine (DOPA), dopamine, norepinephrine, epinephrine
-
A19.
Increased cardia output, blood flow to skeletal muscle, increased muscular activity, and glycogenolysis
-
A20.
Parathormone, calcitriol, and calcitonin
Further Reading
Textbooks
Feingold KR, Anawalt B, Boyce A, et al (eds) (2000) Endotext. South Dartmouth, MA: MDText.com, Inc. https://www.ncbi.nlm.nih.gov/books/NBK278943/
Hall JE, Hall ME (2020) Guyton and Hall textbook of medical physiology e-Book. Elsevier Health Sciences
McDonald LE, Pineda MH, Dooley MP (2003) McDonald’s veterinary endocrinology and reproduction. 5th ed./edited by M.H. Pineda, with the editorial assistance of Michael P. Dooley. Ames, Iowa: Iowa State Press. Print
Nussey SS, Whitehead SA (2001) Endocrinology: an integrated approach. https://www.ncbi.nlm.nih.gov/books/NBK22
Reece WO, Erickson HH, Goff JP, Uemura EE (eds) (2015) Dukes’ physiology of domestic animals. Wiley
Takei Y, Ando H, Tsutsui K (eds) (2015) Handbook of hormones: comparative endocrinology for basic and clinical research. Academic Press. https://doi.org/10.1016/C2013-0-15395-0
Articles
Braun D, Schweizer U (2018) Thyroid hormone transport and transporters. Vitam Horm 106:19–44. https://doi.org/10.1016/bs.vh.2017.04.005
Citterio CE, Targovnik HM, Arvan P (2019) The role of thyroglobulin in thyroid hormonogenesis. Nat Rev. Endocrinol 15(6):323–338. https://doi.org/10.1038/s41574-019-0184-8
Feldt-Rasmussen U, Rasmussen AK (2007) Thyroid hormone transport and actions. Pediatr Adolesc Med 11(R):80
Fleet JC (2017) The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol 453:36–45. https://doi.org/10.1016/j.mce.2017.04.008
Goltzman D (2018) Physiology of parathyroid hormone. Endocrinol Metab Clin 47(4):743–758. https://doi.org/10.1016/j.ecl.2018.07.003
Janah L, Kjeldsen S, Galsgaard KD, Winther-Sørensen M, Stojanovska E, Pedersen J, Wewer Albrechtsen NJ (2019) Glucagon receptor signaling and glucagon resistance. Int J Mol Sci 20(13):3314. https://doi.org/10.3390/ijms20133314
Kim K, Kopylov M, Bobe D, Kelley K, Eng ET, Arvan P, Clarke OB (2021) The structure of natively iodinated bovine thyroglobulin. Acta Crystallogr D Struct Biol 77(11):1451–1459. https://doi.org/10.1107/S2059798321010056
Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94(2):355–382. https://doi.org/10.1152/physrev.00030.2013
Newton R (2000) Molecular mechanisms of glucocorticoid action: what is important? Thorax 55(7):603–613. https://doi.org/10.1136/thorax.55.7.603
Vegiopoulos A, Herzig S (2007) Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275(1–2):43–61. https://doi.org/10.1016/j.mce.2007.05.015
Veerappa S, McClure J (2020) Intermediary metabolism. Anaesth Intensive Care Med 21(3):162–167. https://doi.org/10.1016/j.mpaic.2020.01.003
William Tank A, Lee Wong D (2011) Peripheral and central effects of circulating catecholamines. Compr Physiol 5(1):1–15. https://doi.org/10.1002/cphy.c140007
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sai Kumar, B.A.A. (2023). Hormonal Regulation of Metabolism, Water, and Minerals. In: Das, P.K., Sejian, V., Mukherjee, J., Banerjee, D. (eds) Textbook of Veterinary Physiology. Springer, Singapore. https://doi.org/10.1007/978-981-19-9410-4_16
Download citation
DOI: https://doi.org/10.1007/978-981-19-9410-4_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9409-8
Online ISBN: 978-981-19-9410-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)