Abstract
Phosphate is a multivalent ion critical for a variety of physiological functions including bone formation, which occurs rapidly in the developing infant. In order to ensure maximal bone mineralization, young animals must maintain a positive phosphate balance. To accomplish this, intestinal absorption and renal phosphate reabsorption are greater in suckling and young animals relative to adults. This review discusses the known intestinal and renal adaptations that occur in young animals in order to achieve a positive phosphate balance. Additionally, we discuss the ontogenic changes in phosphotropic endocrine signalling as it pertains to intestinal and renal phosphate handling, including several endocrine factors not always considered in the traditional dogma of phosphotropic endocrine signalling, such as growth hormone, triiodothyronine, and glucocorticoids. Finally, a proposed model of how these factors may contribute to achieving a positive phosphate balance during development is proposed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phosphate (Pi) is critical for a myriad of physiological functions including cell signalling, cell metabolism, as a constituent of the phospholipid bilayer as well as nucleic acids and in the maintenance of bone integrity (Penido and Alon 2012; Peacock 2020). In bone, Pi and calcium form a hydroxyapatite salt ((Ca)10(PO4)6(OH)2) which is interspersed in the extracellular matrix of bone tissue (Bhadada and Rao 2020). In blood, the concentration of phosphate is maintained within a narrow range which varies with age, and in adults is approximately 0.9–1.5 mM. The highest serum Pi concentration is observed in infants and it decreases with age to typical adult values (Greenberg et al. 1960). Females tend to display higher serum Pi than males (Zhang et al. 2014). This difference is particularly pronounced approaching and following menopause, which may be in part due to the decrease in oestrogen which has a phosphaturic effect (Zhang et al. 2014; Faroqui et al. 2008). The concentration of intracellular Pi greatly exceeds that of serum by approximately 100-fold (Penido and Alon 2012). However, this does not reflect total intracellular inorganic Pi content as inorganic Pi is found in intracellular molecules such as nucleoside triphosphates and cyclic nucleoside monophosphates. Phosphate-dependent bone formation is particularly important early in life, as neonates and infants experience rapid bone formation with growth (Sanders et al. 2017). Phosphate homeostasis is maintained by a delicate balance between intestinal absorption, deposition to and resorption from bone, renal reabsorption and urinary excretion. Perturbations in one or more of these sites of regulation can result in disease including hypophosphatemic rickets, osteoporosis and osteomalacia (Bitzan and Goodyer 2019; Francis and Selby 1997; Lee and Cho 2015; Imel 2020; Marcucci and Brandi 2020; Ito and Fukumoto 2020).
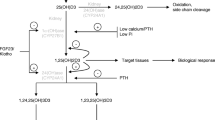
Phosphate homeostasis is subject to hormonal control at all of the aforementioned regulatory points (Fig. 1). For instance, in response to an increase in serum Pi, parathyroid hormone (PTH) is released from the parathyroid gland into the blood (Almaden et al. 1996; Almaden et al. 1998; Centeno et al. 2019; Kritmetapak and Kumar 2019). Circulating PTH increases bone resorption by liberating Pi from hydroxyapatite. It also has a direct phosphaturic effect via inhibiting Pi reabsorption from the renal proximal tubule (Lee et al. 2017). In addition, PTH indirectly enhances intestinal Pi absorption by increasing the expression of 1α-hydroxylase, an enzyme that catalyses the production of 1,25 (OH)2 Vitamin D3 (also known as calcitriol). 1,25 (OH)2 Vitamin D3 increases the expression and activity of the predominant intestinal Na+-dependent Pi cotransporter and consequently intestinal Pi absorption (Xu et al. 2002; Yagci et al. 1992; Katai et al. 1999). Despite enhanced intestinal Pi absorption, the net effect of PTH secretion is a slight decrease in serum Pi levels secondary to renal loss of Pi. Parathyroid hormone also has an indirect but noteworthy phosphaturic effect, both directly and indirectly. PTH stimulates the production of 1,25 (OH)2 Vitamin D3 which is a stimulus for secretion of FGF23 from osteocytes and osteoblasts (Kaneko et al. 2015). Fibroblast growth factor 23 (FGF23) has a pronounced phosphaturic effect by reducing Pi reabsorption from the proximal tubule (Gattineni et al. 2009). FGF23 acts by binding to the FGF receptor and its coreceptor alpha-klotho which enhances its binding (Urakawa et al. 2006). Interestingly, a cleaved soluble form of klotho also induces phosphaturia independent of FGF23 (Hu et al. 2010). Importantly FGF23 increases in response to prolonged (days) high Pi consumption but does not respond to transient increases in serum Pi (Ferrari et al. 2005; Layunta et al. 2019).
Endocrine modulation of phosphate homeostasis. Phosphate is absorbed from the small intestine into the systemic circulation. An increase in serum Pi elicits secretion of PTH from the parathyroid gland. PTH increases the synthesis of 1,25 (OH)2 Vitamin D3 in the kidney. Increased serum 1,25 (OH)2 Vitamin D3 stimulates secretion of FGF23 above baseline levels from osteoblasts and osteocytes in the bone. An elevation in serum 1,25 (OH)2 Vitamin D3 also elicits increased Pi absorption from the intestine. The aforementioned increases in serum [PTH] and [FGF23] decreases renal Pi reabsorption; increasing urinary Pi excretion. Consequently, serum [Pi] decreases towards the normal physiological range
1.1 Transcellular Intestinal Phosphate Absorption/Renal Phosphate Reabsorption
Dietary free Pi, hereafter referred to simply as Pi, is a common food additive and a common constituent of the Western diet (Calvo et al. 2014). Pi, as it is not covalently linked to amino acids in protein or another organic molecule, does not require enzymatic cleavage to be liberated and absorbed. Inorganic Pi can thus be freely absorbed from the small intestine. In contrast, phosphorus bound to carbon-containing molecules such as polypeptides, lipids, and nucleic acids, henceforth referred to as P, must be enzymatically cleaved prior to absorption. This is typically accomplished by alkaline phosphatase (ALP), a hydrolase that catalyses the nonspecific cleavage of phosphomonoester bonds. Once liberated from an organic molecule, Pi flux across intestinal and renal epithelia can occur via either the transcellular or paracellular pathway (Fig. 2) (Saurette and Alexander 2019). The transcellular pathway is characterized by entry of Pi into the epithelial cell via movement across the apical membrane, followed by exit across the opposing basolateral membrane (N.B. there is no evidence of transcellular phosphate secretion, i.e. basolateral to apical movement). Given that transcellular Pi flux in both the small intestine and the kidney depends on the activity of secondary active transporters, the transcellular pathway is saturable. In the small intestine, transcellular Pi absorption is mediated at the apical membrane by Na+-dependent secondary active transport (Fig. 3). Specifically, the type-II Na+-Pi cotransporter NaPiIIb (gene name human/mouse – SLC34A2/Slc34a2) transports Na+ and Pi in a 3:1 stoichiometric ratio and is recognized as the principal mediator contributing apical Pi uptake in the small intestine (Hilfiker et al. 1998; Sabbagh et al. 2011). However, the overall contribution of NaPiIIb to the maintenance of phosphate homeostasis in humans is questionable, as patients with biallelic mutations in this gene have pulmonary alveolar microlithiasis and do not display a phosphate phenotype (Corut et al. 2006; Huqun et al. 2007). Moreover, clinical trials employing a NaPiIIb inhibitor were unable to lower plasma Pi in humans (Larsson et al. 2018). Also expressed in the small intestine are the type III Na+-Pi cotransporters Pit1 (gene name human/mouse – SLC20A1/Slc20a1) and Pit2 (gene name human/mouse – SLC20A2/Slc20a2) that transport Na+ and Pi in a 2:1 stoichiometric ratio. However, the contribution of Pit1/2 to apical Pi uptake in the intestine is likely minimal given negligible measurable sodium coupled Pi uptake in the NaPiIIb intestinal knockout mice (Sabbagh et al. 2011; Pastor-Arroyo et al. 2020). Further, Pit2 is certainly dispensable to maintaining global Pi homeostasis under normal dietary Pi conditions as Pit2 null mice have unaltered Pit homeostasis on this diet (Pastor-Arroyo et al. 2020). Pit1 and 2 have also been proposed to act as extracellular Pi sensors (Bon et al. 2018). The basolateral mechanism of Pi extrusion in the intestine has not been identified at the molecular level (Cross et al. 1990).
The transcellular and paracellular routes of epithelial ion flux. In contrast to the inherently saturable nature of transcellular ion movement, paracellular flux is governed by the transepithelial electrochemical gradient. Paracellular ion flux is largely regulated by claudins (indicated by blue circles) expressed in the epithelial tight junction. With respect to transcellular Pi absorption and reabsorption, both processes begin with apical secondary active transport (2° AT), coupled to sodium influx. The identity of the basolateral mechanism(s) of Pi extrusion in the intestine and kidney have not been clearly delineated (indicated by red question mark on basolateral membrane)
Intestinal phosphate absorption. Alkaline phosphatase (ALP) liberates P from organic molecules as Pi. This is accomplished by the hydrolysis reaction depicted above. Phosphate attached to a generic organic ‘R’ group is cleaved off by the hydrolase ALP, resulting in R-OH and H-Pi. The free Pi can then be absorbed via the Na+-dependent secondary active transporters NaPiIIb, Pit1 or Pit2. NaPiIIb preferentially transports the divalent Pi species (HPO42−), whereas Pit1/2 preferentially transport the monovalent Pi species (H2PO4−); conferring versatility of intestinal Pi transport with respect to luminal pH. NHE3 plays a role in paracellular Pi absorption. Little is known regarding the basolateral mechanism of Pi extrusion. PD = potential difference, which is approximately −5 mV in the small intestine
In the kidney, the vast majority, if not all of the Pi reabsorption occurs in the proximal tubule (Fig. 4) (Pastoriza-Muñoz et al. 1978; Biber et al. 2013). Chiefly responsible for Pi reabsorption in this nephron segment are the apically expressed type-II Na+-Pi cotransporters NaPiIIa (SLC34A1/Slc34a1) and NaPiIIc (SLC34A3/Slc34a3). NaPiIIc is not electrogenic, co-transporting 2 Na+:1 Pi (Segawa et al. 2002). Individuals with mutations in SLC34A3 present with hereditary hypophosphatemic rickets with hypercalciuria during childhood (Lorenz-Depiereux et al. 2006), which appears to be a lifelong disease suggesting an important role for NaPiIIc across all developmental stages. In contrast NaPiIIa takes up Na+ and Pi in a 3:1 ratio and is therefore electrogenic (Forster et al. 1999). Both isoforms contribute significantly to Pi homeostasis in humans. Consistent with this, loss of function mutations in both transporters can cause hypophosphatemia due to renal Pi wasting (Bergwitz et al. 2006; Schlingmann et al. 2016; Schönauer et al. 2019). Patients with SLC34A1 mutations, however, can display a range of phenotypes including a renal Pi wasting syndrome similar to SLC34A3 mutations, infantile neonatal hypercalcemia or simply kidney stones (Schlingmann et al. 2016; Rajagopal et al. 2014; Braun et al. 2016). However, the clinical manifestations tend to improve with age highlighting the importance of NaPiIIa early in life. Also expressed in the proximal tubule is Pit2 which, akin to its role in the small intestine, contributes less to apical Pi uptake, in adults at least (Villa-Bellosta et al. 2009; Bon et al. 2018). Xenotropic and polytropic retrovirus receptor 1 (XPR1) is expressed in the renal proximal tubule, where recent findings are consistent with a role in basolateral Pi efflux (Ansermet et al. 2017). This finding, however, should be interpreted tentatively as XPR1 has not been localized to the basolateral membrane in the proximal tubule, nor have heterozygous expression studies demonstrated the ability for XPR1 to mediate Pi flux. Recent efforts identified NaPiIIb in the thick ascending limb, but its role in renal Pi homeostasis is unclear at present (Motta et al. 2020). For a recent review of phosphate transporters please see (Levi et al. 2019).
1.2 Paracellular Phosphate Flux
Paracellular flux is the movement of an ion across an epithelium between epithelial cells (Fig. 1). The paracellular pathway is unsaturable and is driven by the transepithelial electrochemical gradient (King et al. 2018; Knöpfel et al. 2019). Thus, depending on the electrochemical gradient Pi could either be absorbed (i.e. apical to basolateral) or secreted (i.e. basolateral to apical) via the paracellular pathway. The concentration of Pi in the intestinal lumen of rodents has been reported to be in the millimolar range (Ikuta et al. 2018; Marks et al. 2015). Assuming similar luminal Pi concentrations exist in humans, a chemical gradient would exist between the lumen and the blood favouring apical-to-basolateral paracellular Pi movement. Further, the mammalian small intestine displays a negative transepithelial potential difference (lumen negative), which would also favour apical-to-basolateral flux of negatively charged ions such as Pi, especially at an alkaline pH that favours the divalent species (Gustke et al. 1981). Taken together, the electrochemical gradient for Pi across the intestine strongly favours apical-to-basolateral absorption of inorganic Pi via the paracellular pathway, which may be more pronounced when consuming a western diet high in inorganic Pi. In contrast, there does not appear to be significant paracellular flux contributing to renal Pi reabsorption (Kaufman and Hamburger 1987; Edwards and Bonny 2018).
The paracellular movement of an ion requires a permeable pore. Claudins are a family of four pass transmembrane proteins expressed in the tight junctions of epithelia including the small intestine and the renal epithelium (Günzel and Yu 2013). As hetero- or homo-tetramers, claudins form pores which confer ion selectivity to epithelia (Piontek et al. 2020). The sodium-hydrogen-exchanger isoform III (NHE3) is apically expressed in the small bowel (Fig. 3) and appears to regulate intestinal Pi absorption as its pharmacological inhibition reduces paracellular Pi permeability and intestinal Pi absorption (King et al. 2018). While the roles of the Na+-Pi cotransporters in Pi homeostasis have been largely established (Biber et al. 2013), the contribution of paracellular Pi movement to whole-body Pi status remains largely unexplored, as does the contribution of paracellular Pi absorption to maintaining a positive Pi balance throughout development. This article therefore focuses largely on transcellular mechanisms by which Pi homeostasis is maintained. Specifically, we cover the current body of literature characterizing the changes in intestinal and renal Pi handling throughout mammalian postnatal development. Included in the discussion is a review of the developmental hormonal changes influencing Pi homeostasis. Data from a number of animal models are included in this review. The reader should consider that physiological differences exist between humans and the models discussed, especially rodents which are multiparous. Thus, findings from nonhuman mammalian models should be interpreted with these considerations. We have chosen to include results from nonhuman models as these studies are often not feasible to conduct on humans for technical and/or ethical reasons and we believe they contribute insight that is likely translatable.
2 Ontogeny of Intestinal Phosphate Absorption
Given the need to rapidly mineralize bone as a neonate, it is not surprising that functional adaptations exist to optimize Pi absorption from the small intestinal lumen. These adaptive mechanisms diminish as the neonate matures into adulthood and peak bone mineralization is achieved. Recent work revealed greater 33P absorption from the jejunum, ex vivo, of 10-week-old (i.e. not fully grown) Sprague-Dawley rats relative to 20- or 30-week-old (fully grown) animals (Vorland et al. 2018). Corroborating this, Slc34a2 mRNA abundance was significantly greater in the duodenum and jejunum at 10 weeks of age relative to 20 weeks of age (Vorland et al. 2018). Further, Borowitz and Granrud found that in New Zealand White Rabbits, duodenal brush-border membrane vesicles (BBMV) from 2- to 4-week-old rabbits (preweaning age) displayed higher Na+-dependent Pi uptake than 6- or 12-week-old animals, consistent with greater transcellular Pi uptake in vivo at a younger age (Borowitz and Granrud 1992). This study also demonstrated that at any given Pi concentration, Na+-dependent Pi uptake was higher in duodenal BBMV vesicles derived from 2-week old rather than older animals (Borowitz and Granrud 1992). Finally, they observed the highest ALP activity in small intestine BBMV of young rabbits, which decreased with age. The in situ and ex vivo Pi flux data is consistent with enhanced transcellular intestinal Pi uptake in the neonate, which decreases with age. The high intestinal ALP activity in the infant described by Borowitz and Granrud also supports this. Increased ALP enzyme activity would enable the liberation and subsequent absorption of Pi found in organic sources, such as casein in milk. It is noteworthy that enterocyte turnover in the small intestine is much lower in young animals in comparison to adults (Smith and Jarvis 1978). Thus, increased intestinal ALP activity observed in younger animals may reflect longer enterocyte presence in the small intestine accommodating increased ALP expression and subsequent activity. Taken together the data is consistent with young animals having an increased capacity for transcellular Pi absorption from the small intestine relative to adults.
The relative importance of paracellular versus transcellular intestinal Pi absorption, particularly in the young, is unclear. Pi permeability in the jejunum and ileum is significantly greater at pH = 6 than pH = 8.4 (Knöpfel et al. 2019). The human duodenal pH is approximately 6.6, which increases to 7.5 at the terminal ileum, consistent with Pi permeability being highest in the most acidic segment, i.e. the duodenum (Evans et al. 1988). However, the brief sojourn time of dietary Pi in the duodenum compounded with the rate-limiting step of ALP liberation of dietary P from organic sources likely limits the contribution of the duodenum to overall Pi absorption (Duflos et al. 1995). This phenomenon may be particularly relevant in the infant, given that most, if not all of the Pi in breastmilk is organic; i.e. found in whey, casein and phospholipids (Gridneva et al. 2018; Suzuki et al. 1991; Lönnerdal et al. 2017). In the case of breastmilk, the protein-bound P component would need to be enzymatically liberated prior to absorption. By the time this occurs the previously bound Pi may be in a more distal segment given the brief sojourn time of the duodenum. The conceivably low luminal [Pi] contributed by enzymatic cleavage may necessitate secondary active transport and thus may be absorbed in a transcellular fashion. Unfortunately, the precise percentage breakdown between free Pi and bound P in mammalian breastmilk has not been established. By the stated rationale, however, if the majority of phosphorus content in breastmilk exists as bound P, the duodenum may not be a site of high Pi absorption during suckling. Duodenal Pi absorption may become more important after weaning from breastmilk to a diet containing free ionized Pi, which is freely absorbed. Furthermore, a post-weaning diet higher in free Pi likely contributes a large electrochemical gradient for Pi across the duodenum, which combined with an acidic luminal pH, would favour paracellular Pi absorption from the proximal small bowel. As the pH progressively increases in the distal small intestine and as the luminal sojourn time increases, Pi absorption in the jejunum and ileum may become a balance between transcellular and paracellular flux.
3 Ontogeny of Renal Phosphate Handling
Renal tubular Pi reabsorption is greater early in life compared to older animals (Bistarakis et al. 1986; Kaskel et al. 1988; Neiberger et al. 1989). A 1988 in situ study demonstrated functional differences between juvenile and adult guinea pigs with respect to renal Pi handling (Kaskel et al. 1988). Proximal tubular micropuncture experiments found that Pi reabsorption (normalized to GFR) and fractional Pi reabsorption (i.e. the percentage of filtered load) significantly decreases from 1 week (preweaning) to 7 weeks (post-weaning) of age. Similarly, in vitro studies employing renal cortical BBMVs from juvenile (3–14 days old, i.e. suckling) guinea pigs had a significantly higher rate of Na+-dependent Pi uptake than BBMVs derived from older (>57 days old, i.e. post-weaning) animals (Neiberger et al. 1989). This work also revealed that the Vmax of Na+-Pi uptake in juvenile-derived kidney BBMV was significantly greater than that of adults. These findings are consistent with increased reabsorption of Pi in the neonatal proximal tubule relative to the adult. Of note, the Vmax of Na+-Pi cotransport in BBMVs isolated from adult kidneys is inversely correlated to dietary Pi content, whereas juvenile animals did not show the same capacity to regulate Na+-Pi cotransport in response to changes in dietary Pi (Neiberger et al. 1989). Consistent with this, serum Pi significantly increases in young animals when given an oral gavage of inorganic Pi (Neiberger et al. 1989). A failure to attenuate tubular Pi reabsorption in response to increased dietary Pi in juvenile animals likely also contributes to maintaining a positive Pi balance while suckling. Evidence supporting enhanced tubular Pi reabsorption in neonates has also been found in humans. The urinary fractional excretion of Pi increases between 3 and 6 months of age (Bistarakis et al. 1986). However, the mechanism conferring increased renal Pi reabsorption in suckling mammals is unclear (Segawa et al. 2002). Western Blot analysis of kidney BBMV from suckling, weaning and adult rats found the lowest expression of NaPiIIc in animals that were suckling, with significantly higher NaPiIIc expression in weaning rats relative to the adult. Increased renal Pi reabsorption in suckling animals, despite lower NaPiIIc expression, may be secondary to greater abundance or apical membrane expression of NaPiIIa or because of increased Pit1 or 2 activity. Regardless of the mechanism, the data strongly supports increased proximal tubule Pi reabsorption in young mammals compared to adults. This increased renal Pi reabsorption likely contributes to a positive Pi balance necessary for optimal growth.
4 Ontogeny of Hormonal Factors Directing Intestinal and Renal Phosphate Handling
PTH
Developmental alterations in hormonal signalling likely contribute to the observed differences with respect to renal and intestinal Pi handling in younger animals. PTH is a central hormone fundamental to Pi homeostasis. The ability for PTH to inhibit proximal tubular Pi reabsorption in the kidney changes with mammalian development (Johnson and Spitzer 1986). In contrast to the well-recognized effect of PTH to enhance renal Ca2+-reabsorption, which is present in the neonate, PTH-mediated phosphaturia is not present in the newborn and appears to develop in older animals (Johnson and Spitzer 1986). A potential mechanism for this phenomenon is reduced phospholipase A2 (PLA2) activity in the neonatal proximal tubule. Of note, PLA2 is a downstream signalling molecule after activation of the PTH receptor, which produces arachidonic acid (AA) via hydrolysis of phospholipid substrates (Friedlander and Amiel 1994). Sheu et al. (1997) found that 14C-AA production by liposomes purified from the adult rabbit kidney significantly exceeded that of the juvenile (Sheu et al. 1997). The authors interpreted increased 14C-AA production as a hallmark of elevated PLA2 activity in adult proximal tubule. Inhibition of Na+/Pi transport by AA metabolites has been demonstrated in opossum kidney (OK) cells, a common proximal tubular cell model (Silverstein et al. 1999). The absence of PTH-induced phosphaturia in the neonate could therefore be secondary to reduced PLA2 activity in the neonatal proximal tubule, resulting in lower intracellular [AA], and thus reduced or absent inhibition of apical Na+/Pi cotransport, thereby limiting phosphaturia. Parathyroid hormone-mediated Ca2+ reabsorption combined with the absence of PTH-induced phosphaturia in the neonate likely enhances the retention of Ca2+ and Pi so as to optimize skeletal mineralization during early development (Linarelli 1972).
FGF23
Another hormone central to Pi homeostasis is the phosphatonin, FGF23. This hormone also inhibits proximal tubule phosphate reabsorption but in contrast to PTH, inhibits rather than stimulates calcitriol synthesis. FGF23 levels nearly double between 5 days and 3 months of age. This rapid increase is followed by a gradual decline to levels at 1 year of age which are comparable to adult values (Braithwaite et al. 2016; Schoppet et al. 2012). Low serum FGF23 levels in the immediate postnatal period likely also contribute to an overall positive Pi balance early in life by minimizing phosphaturia.
Calcitriol
The production and handling of 1,25 (OH)2 vitamin D3/calcitriol changes throughout mammalian development. Serum calcitriol increases rapidly following birth, followed by a marked decline into adulthood (Ross and Dorsey 1991). Consistent with this, the production rate of calcitriol declines with age. We are only aware that the ontogeny of 1,25 (OH)2 Vitamin D3 production has been studied in sheep, however, it is plausible that heightened neonatal calcitriol production occurs ubiquitously in mammals. Elevated calcitriol levels in the neonate may contribute to the increased intestinal Pi absorption observed. In addition to altered 1,25 (OH)2 Vitamin D3 levels across development, calcitriol signalling in the small intestine undergoes developmental change. The administration of 1,25 (OH)2 Vitamin D3 induces similar changes in magnitude of Na+-dependent Pi uptake in small intestine BBMV in both juvenile and adult rats. However, the fold-increase in Slc34a2 (gene encoding NaPiIIb) mRNA abundance in young animals is significantly greater than that of adults, which may reflect greater NaPiIIb abundance if protein expression similarly increases (Xu et al. 2002). Post-transcriptional regulation of NaPiIIb by calcitriol is possible given its regulation of other target genes at this level (Moor et al. 2018). While functional response to 1,25 (OH)2 Vitamin D3 administration with respect to Na+-dependent Pi absorption is similar between juvenile and adult mammals, the mechanisms underpinning this response differ between age groups. Since the BBMV technique examines predominately the transcellular pathway, the results of the Xu et al. suggest that 1,25 (OH)2 Vitamin D3 may increase Slc20a1 and/or Slc20a2 expression in the small intestine in adults (Keasey et al. 2016). However, since vitamin D is a transcriptional regulator, it is also possible that calcitriol regulates the transcription of claudins mediating paracellular Pi flux in addition to regulating NaPiIIb. Consistent with this possibility, claudin-2 and -12, two calcium permeable claudins are upregulated in the intestine in response to vitamin D (Fujita et al. 2008; Zhang et al. 2015). Thus, the functional distinction in Pi absorption between young and adult animals could be due to a combination of increased capacity for paracellular Pi absorption and increased NaPiIIb expression in the neonate.
GH
In addition to the roles of PTH and calcitriol in contributing to a positive Pi balance in young animals, growth hormone (GH) also displays phosphotropic properties that may contribute to a positive Pi balance early in life. The administration of an antagonist to GH-releasing factor in juvenile (aged 4–5 weeks) rats significantly increased renal fractional Pi excretion (Mulroney et al. 1989). As GH levels in rodents peak in the first week of life, high serum GH in the neonate may contribute to the elevated Pi reabsorption observed in the kidneys of neonates (Toriz et al. 2019).
Glucocorticoids
May also contribute to renal mediated regulation of Pi homeostasis. The incubation of renal BBMVs with dexamethasone is associated with a significant decrease in the Vmax of Na+-Pi uptake (Levi et al. 1995). Additionally, dexamethasone also decreases mRNA and protein abundance of a NaPiII transporter (N.B. this work was performed prior to the differentiation between the two renal NaPiII isoforms; NaPiIIa and c). Corticosterone, the predominant rodent glucocorticoid, has low serum levels until postnatal day 12 in rats, when it increases dramatically to a maximum at 24 hrs of life (Henning 1978). Given data supporting a role for glucocorticoids mediating phosphaturia, low glucocorticoid levels early in life likely also limit renal phosphate excretion thereby contributing to a positive Pi balance.
Triiodothyronine (T3)
Triiodothyronine (T3) is another phosphotropic factor that is not commonly thought of in this regard. Exogenous administration of T3 to suckling Sprague-Dawley rats was associated with several physiological alterations consistent with enhanced renal Pi reabsorption (Euzet et al. 1995). The T3-treated animals displayed a significant decrease in fractional renal excretion of Pi compared to controls. Animals receiving T3 also had significantly higher NaPiII (NB – this work was performed pre-distinction between renal NaPiII isoforms) protein abundance and elevated Pi uptake into renal cortical BBMVs relative to vehicle-administered rats. Given the marked postnatal increase in T3 observed in Sprague-Dawley rats (Chanoine et al. 1993), elevated serum T3 levels may contribute to a positive Pi balance in young animals. Of note, the increase in renal BBMV Pi uptake as well as the increase in NaPiII expression in response to exogenous T3 are blunted in old (24 month) Wistar rats compared to young (3 month) rats (Alcalde et al. 1999). While a 3-month old rat is certainly not neonatal, this finding nevertheless supports the possibility that T3 may contribute to greater Pi-retention in younger animals.
5 A Proposed General Model: Intestinal and Renal Ontogeny of Phosphate Handling
Given the abundance of evidence, it is reasonable to conclude that increased intestinal NaPiIIb expression in the neonate likely contributes to a positive Pi balance. Increased neonatal 1,25 (OH)2 Vitamin D3 production in the neonate, which would result in enhanced intestinal Pi absorption, also likely contributes to a positive Pi balance. In the young mammal, the mechanism for 1,25 (OH)2 Vitamin D3-mediated increase in Pi absorption appears to be secondary to an increase in intestinal NaPiIIb expression whereas the mechanism in the adult is less clear. While certainly increased NaPiIIb expression contributes, the possibility that 1,25 (OH)2 Vitamin D3 also increases Pit1 and/or Pit2 expression in the adult which in turn would confer increased Na+-Pi transport cannot be dismissed. The route of intestinal Pi absorption may change from pre- to post-weaning as the dietary Pi source changes from largely bound P to a combination of P and Pi. The rate-limiting step of intestinal ALP liberating Pi from organic molecules may generate a low luminal concentration of free Pi, necessitating the use of secondary active transport (i.e. NaPiIIb or Pit1,2) to absorb Pi derived from organic sources. The consumption of free ionized Pi post-weaning, however, will generate a high luminal Pi concentration resulting in an electrochemical gradient sufficient to drive paracellular Pi absorption. With the exception of the role of ALP, this mechanism has been previously proposed (Knöpfel et al. 2019). Thus, transcellular absorption may predominate in the preweaning period, whereas paracellular absorption may take on a larger role post-weaning for persons consuming a western diet. Furthermore, the relative importance of different intestinal segments to Pi absorption may change across development. As children begin to consume ionized dietary Pi, the duodenum may take on a more significant role in intestinal Pi absorption; whereas the net contribution of the jejunum and ileum to Pi absorption may decrease.
In addition to the intestine, the neonatal kidney also contributes to a positive Pi balance through functional adaptations resulting in enhanced Pi reabsorption. Renal Pi excretion is lowest in the neonate. Given that NaPiIIc expression is at a lifetime low in suckling animals, increased abundance of other Na+-Pi cotransporters in the kidney may contribute the high Pi reabsorption observed in the neonate. Increased basolateral XPR1 expression in the proximal tubule may play an additional role in conferring elevated Pi reabsorption in the neonate (Ansermet et al. 2017). Finally, the absence of PTH-directed phosphaturia in the neonate likely also contributes to an overall positive Pi balance.
6 Inorganic Phosphate: Its High Consumption in the Western World and Potential Ramifications for the Infant
Phosphate is a common constituent of the Western diet and it is much more completely absorbed from the intestinal lumen than its organic counterpart (Noori et al. 2010). The high bioavailability of free Pi presents a potential public health issue, as elevated serum Pi is associated with renal and vascular damage as well as with increased all-cause mortality (Hong et al. 2015; Tonelli et al. 2005). It has been noted that this latter phenomenon occurs even within the normal physiological serum Pi range (Tonelli et al. 2005). The exact mechanism leading to cardiovascular complications in persons with elevated serum Pi is unclear. However, one possibility is increased FGF23 secretion in order to lower serum Pi by inducing phosphaturia. FGF23 induces Pi-dependent vascular calcification in vitro and is independently associated with aortic calcification in humans (Jimbo et al. 2014; Schoppet et al. 2012). This phenomenon is of concern when consuming a ‘Western Diet’ given the high consumption of preservative-containing foods of which free Pi is a component. It is thus not unreasonable to postulate that the consumption of ionized Pi is associated with chronically elevated FGF23, which in turn increases the risk of developing cardiovascular disease (CVD). If this association, i.e. increased CVD risk in persons with higher plasma Pi, can be extrapolated to infants it suggests formulae is a risk for CVD, since infants consuming baby formula display elevated serum Pi compared to breast fed infants (Greer 1989). For a pictorial representation of the general adaptations of the juvenile mammal to establish a high Pi balance, the reader is referred to Fig. 5.
Proposed general adaptations of the juvenile mammal to establish positive Pi balance. The juvenile mammal possesses increased absorption and reabsorption of Pi in the small bowel and kidney, respectively. This establishes a sufficiently positive Pi balance such that rapid bone deposition during growth may occur
7 Future Research
Studies Examining the Expression and Function of Mediators of Pi Transport Across Development
Much remains to study with respect to the physiological mechanisms underlying the maintenance of a positive Pi balance in young animals. The detailed characterization of the normal physiological state will enable the identification of pathophysiological mechanisms and inform their treatment. To this end, the ontogeny of Pit1/2 expression across the lifespan remains unknown and should be delineated. In light of the postulated Pi-sensing role of Pit1/2 heterodimers (Bon et al. 2018), this information may suggest the extent to which this mechanism is active in the small bowel throughout the life span. Additionally, investigation is warranted into whether Pi sensing by Pit1/2 has a downstream effect on NaPiIIb or other NaPi’s analogous to the regulation of the Trpv6 calcium channel by the calcium-sensing receptor (Lee et al. 2019). Further, the effect of 1,25 (OH)2 Vitamin D3 on Pit1/2 expression in the adult should be determined. Recently, it was demonstrated in Caco-2 cells that calcitriol upregulates the mRNA abundance of several intestinal ALP splice variants (Noda et al. 2017). Given the role of ALP in intestinal Pi absorption, future studies are warranted to examine the change in ALP expression in response to 1,25 (OH)2 Vitamin D3 administration in animal models at different ages. This information would be informative regarding if and how calcitriol contributes Pi liberation from macromolecules in milk in the suckling infant. Since studying transcript abundance is not sufficient to analyse calcitriol regulation of NaPIIb at different ages, changes in NaPiIIb protein abundance in response to calcitriol in juvenile and adult animals should be studied.
Studies Aimed at Understanding the Mechanisms Mediating Increased Renal Tubular Pi Absorption in Young Animals
As the ontogeny of NaPiIIa expression in the kidney has not been examined at all, future studies should delineate the potential role of NaPiIIa in maintaining a positive Pi balance in young animals. Other experiments aimed at determining the possibility of another transporter such as Pit2 contributing a compensatory role in Pi reabsorption in the suckling animal should also be considered. To enhance our understanding of the parathyroid-kidney interaction in development of renal Pi handling, the ontogeny of PTH receptor expression in the proximal tubule should be elucidated. Given the absence of PTH-induced phosphaturia in the newborn, low PTH receptor abundance in the proximal tubule early in life might explain this phenomenon. Additionally, studies examining the developmental changes in expression of downstream factors in PTH-mediated phosphaturia such as the Sodium-Hydrogen Exchanger Regulating Factor 1 should also be conducted (Lee et al. 2017).
Studies Aimed to Understand the Mechanisms Leading to Developmental Changes in Phosphotropic Hormone Levels
In light of the multifaceted role of 1,25 (OH)2 Vitamin D3 in intestinal Pi handling, future research should seek to determine how the synthesis and degradation of this endocrine factor is altered across mammalian development. To this end, the assessment of transcript levels for, and overall abundance of, 25 hydroxylase (CYP2R1) and 1α hydroxylase (CYP27B1) in the liver and kidney, respectively, should be ascertained. Studies examining the ontogeny of calcitriol deactivation by measuring 24 hydroxylase (CYP24A1) abundance in target tissues would also be informative. Future studies examining intestinal vitamin D absorption at different ages will further our understanding of the developmental changes in the absorption of dietary cholecalciferol prerequisite to the synthesis and action of active calcitriol. Further the abundance of VDR and RXR should be examined across ages and consideration made to the effects of calcitriol on post-transcriptional modification of phosphate transporters such as NaPi2b examined.
It is surprising that there is a paucity of literature surrounding how the production/secretion of FGF23 and PTH change across development given their instrumental role in Pi homeostasis. Serum [FGF23] changes across development have been documented, the same should be done for PTH to better understand its endocrine role during growth. Researchers may additionally examine the ontogeny of PTH and FGF23 transcript abundance in the parathyroid gland and osteocyte/osteoblast, respectively. This information may prove useful in understanding developmental changes in a mammal’s capacity to generate a robust endocrine response to an increase in serum Pi.
Examination of the Effects of Inorganic Pi on Infants and Children
The evidence linking increased serum Pi levels to all-cause mortality and CVD, even when in the normal range in adults begs further inquiry in children. Does the increased plasma Pi level observed in bottle fed infants translate into increased CVD or overall mortality later in life? The effect of breast vs bottle feeding on Pi homeostasis, FGF23 and PTH levels should also be considered. This information would be valuable to families choosing between formula and breast milk.
8 Conclusions
Given the fundamental role of Pi in skeletal development we know surprisingly little about Pi absorption in young animals. It is clear that there is increased intestinal and renal Pi absorption/reabsorption in suckling animals. While it is likely that increased NaPiIIb mediated intestinal absorption accounts for the increased Pi absorption from the small bowel, the mechanisms mediating increased renal Pi reabsorptions remains unknown and is an area in need of further study.
Abbreviations
- AA:
-
Arachidonic acid
- ALP:
-
Alkaline phosphatase
- BBMV:
-
Brush-border membrane vesicles
- CVD:
-
Cardiovascular disease
- FGF23:
-
Fibroblast growth factor 23
- GH:
-
Growth hormone
- NaPiIIa:
-
Sodium-phosphate cotransporter isoform IIa
- NaPiIIb:
-
Sodium-phosphate cotransporter isoform IIb
- NaPiIIc:
-
Sodium-phosphate cotransporter isoform IIc
- NHE3:
-
Sodium hydrogen exchanger isoform III
- Pi:
-
Phosphate
- PLA2:
-
Phospholipase A2
- PTH:
-
Parathyroid hormone
- T3:
-
Triiodothyronine
References
Alcalde AI, Sarasa M, Raldúa D, Aramayona J, Morales R, Biber J, Murer H, Levi M, Sorribas V (1999) Role of thyroid hormone in regulation of renal phosphate transport in young and aged rats. Endocrinology 140(4):1544–1551
Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, Rodriguez M (1996) Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res 11(7):970–976
Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L, Campistol JM, Torres A, Rodriguez M (1998) High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9(10):1845–1852
Ansermet C, Moor MB, Centeno G, Auberson M, Hu DZ, Baron R, Nikolaeva S, Haenzi B, Katanaeva N, Gautschi I, Katanaev V, Rotman S, Koesters R, Schild L, Pradervand S, Bonny O, Firsov D (2017) Renal Fanconi syndrome and Hypophosphatemic rickets in the absence of Xenotropic and Polytropic retroviral receptor in the nephron. J Am Soc Nephrol 28(4):1073–1078
Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H (2006) SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78(2):179–192
Bhadada SK, Rao SD (2020) Role of phosphate in biomineralization. Calcif Tissue Int:1–9. https://doi.org/10.1007/s00223-020-00729-9
Biber J, Hernando N, Forster I (2013) Phosphate transporters and their function. Annu Rev Physiol 75:535–550
Bistarakis L, Voskaki I, Lambadaridis J, Sereti H, Sbyrakis S (1986) Renal handling of phosphate in the first six months of life. Arch Dis Child 61(7):677–681
Bitzan M, Goodyer PR (2019) Hypophosphatemic rickets. Pediatr Clin N Am 66(1):179–207
Bon N, Couasnay G, Bourgine A, Sourice S, Beck-Cormier S, Guicheux J, Beck L (2018) Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J Biol Chem 293(6):2102–2114
Borowitz SM, Granrud GS (1992) Ontogeny of intestinal phosphate absorption in rabbits. Am J Phys 262(5 Pt 1):847
Braithwaite VS, Prentice A, Darboe MK, Prentice AM, Moore SE (2016) The effects of maternal iron deficiency on infant fibroblast growth factor-23 and mineral metabolism. Bone 83:1–8
Braun DA, Lawson JA, Gee HY, Halbritter J, Shril S, Tan W, Stein D, Wassner AJ, Ferguson MA, Gucev Z, Fisher B, Spaneas L, Varner J, Sayer JA, Milosevic D, Baum M, Tasic V, Hildebrandt F (2016) Prevalence of monogenic causes in pediatric patients with nephrolithiasis or nephrocalcinosis. Clin J Am Soc Nephrol 11(4):664–672
Calvo MS, Moshfegh AJ, Tucker KL (2014) Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv Nutr 5(1):104–113
Centeno PP, Herberger A, Mun H, Tu C, Nemeth EF, Chang W, Conigrave AD, Ward DT (2019) Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun 10:1–12
Chanoine JP, Veronikis I, Alex S, Stone S, Fang SL, Leonard JL, Braverman LE (1993) The postnatal serum 3,5,3′-triiodothyronine (T3) surge in the rat is largely independent of extrathyroidal 5′-deiodination of thyroxine to T3. Endocrinology 133(6):2604–2609
Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, Yildirim Z, Gocmen A, Tolun A (2006) Mutations in SLC34A2 cause pulmonary alveolar Microlithiasis and are possibly associated with testicular Microlithiasis. Am J Hum Genet 79(4):650–656
Cross HS, Debiec H, Peterlik M (1990) Mechanism and regulation of intestinal phosphate absorption. Miner Electrolyte Metab 16(2–3):115–124
Duflos C, Bellaton C, Pansu D, Bronner F (1995) Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr 125(9):2348–2355
Edwards A, Bonny O (2018) A model of calcium transport and regulation in the proximal tubule. Am J Physiol Renal Physiol 315(4):F942–F953
Euzet S, Lelièvre-Pégorier M, Merlet-Bénichou C (1995) Maturation of rat renal phosphate transport: effect of triiodothyronine. J Physiol 488(Pt 2):449–457
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD (1988) Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29(8):1035–1041
Faroqui S, Levi M, Soleimani M, Amlal H (2008) Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int 73(10):1141–1150
Ferrari SL, Bonjour J, Rizzoli R (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90(3):1519–1524
Forster IC, Loo DD, Eskandari S (1999) Stoichiometry and Na+ binding cooperativity of rat and flounder renal type II Na+-Pi cotransporters. Am J Phys 276(4):644
Francis RM, Selby PL (1997) Osteomalacia. Bailliere Clin Endocrinol Metab 11(1):145–163
Friedlander G, Amiel C (1994) Cellular mode of action of parathyroid hormone. Adv Nephrol Necker Hosp 23:265–279
Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H (2008) Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19(5):1912–1921
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M (2009) FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 297(2):F282–F291
Greenberg BG, Winters RW, Graham JB (1960) The normal range of serum inorganic phosphorus and its utility as a discriminant in the diagnosis of congenital hypophosphatemia. J Clin Endocrinol Metab 20:364–379
Greer FR (1989) Calcium, phosphorus, and magnesium: how much is too much for infant formulas? J Nutr 119(12 Suppl):1846–1851
Gridneva Z, Tie WJ, Rea A, Lai CT, Ward LC, Murray K, Hartmann PE, Geddes DT (2018) Human milk casein and whey protein and infant body composition over the first 12 months of lactation. Nutrients 10:9
Günzel D, Yu ASL (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93(2):525–569
Gustke RF, McCormick P, Ruppin H, Soergel KH, Whalen GE, Wood CM (1981) Human intestinal potential difference: recording method and biophysical implications. J Physiol Lond 321:571–582
Henning SJ (1978) Plasma concentrations of total and free corticosterone during development in the rat. Am J Phys 235(5):451
Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J (1998) Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci U S A 95(24):14564–14569
Hong S, Park S, Lee S, Kim S, Cho M (2015) Biological effects of inorganic phosphate: potential signal of toxicity. J Toxicol Sci 40(1):55–69
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24(9):3438–3450
Huqun N, Izumi S, Miyazawa H, Ishii K, Uchiyama B, Ishida T, Tanaka S, Tazawa R, Fukuyama S, Tanaka T, Nagai Y, Yokote A, Takahashi H, Fukushima T, Kobayashi K, Chiba H, Nagata M, Sakamoto S, Nakata K, Takebayashi Y, Shimizu Y, Kaneko K, Shimizu M, Kanazawa M, Abe S, Inoue Y, Takenoshita S, Yoshimura K, Kudo K, Tachibana T, Nukiwa T, Hagiwara K (2007) Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am J Respir Crit Care Med 175(3):263–268
Ikuta K, Segawa H, Sasaki S, Hanazaki A, Fujii T, Kushi A, Kawabata Y, Kirino R, Sasaki S, Noguchi M, Kaneko I, Tatsumi S, Ueda O, Wada NA, Tateishi H, Kakefuda M, Kawase Y, Ohtomo S, Ichida Y, Maeda A, Jishage K, Horiba N, Miyamoto K (2018) Effect of Npt2b deletion on intestinal and renal inorganic phosphate (Pi) handling. Clin Exp Nephrol 22(3):517–528
Imel EA (2020) Congenital conditions of hypophosphatemia in children. Calcif Tissue Int:1–7. https://doi.org/10.1007/s00223-020-00692-5
Ito N, Fukumoto S (2020) Congenital hyperphosphatemic conditions caused by the deficient activity of FGF23. Calcif Tissue Int:1–12. https://doi.org/10.1007/s00223-020-00659-6
Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T (2014) Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int 85(5):1103–1111
Johnson V, Spitzer A (1986) Renal reabsorption of phosphate during development: whole-kidney events. Am J Phys 251(2 Pt 2):251
Kaneko I, Saini RK, Griffin KP, Whitfield GK, Haussler MR, Jurutka PW (2015) FGF23 gene regulation by 1,25-dihydroxyvitamin D: opposing effects in adipocytes and osteocytes. J Endocrinol 226(3):155–166
Kaskel FJ, Kumar AM, Feld LG, Spitzer A (1988) Renal reabsorption of phosphate during development: tubular events. Pediatr Nephrol 2(1):129–134
Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, Taketani Y, Takeda E (1999) Regulation of intestinal Na+−dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J 343(Pt 3):705–712
Kaufman JS, Hamburger RJ (1987) Lack of influence of volume flux on phosphate reabsorption in the proximal tubule. Miner Electrolyte Metab 13(3):158–164
Keasey MP, Lemos RR, Hagg T, Oliveira JRM (2016) Vitamin-D receptor agonist calcitriol reduces calcification in vitro through selective upregulation of SLC20A2 but not SLC20A1 or XPR1. Sci Rep 6(1):25802
King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, Minassian NA, Jafri Q, Pan D, Kohler J, Kumaraswamy P, Kozuka K, Lewis JG, Dragoli D, Rosenbaum DP, O’Neill D, Plain A, Greasley PJ, Jönsson-Rylander A, Karlsson D, Behrendt M, Strömstedt M, Ryden-Bergsten T, Knöpfel T, Pastor Arroyo EM, Hernando N, Marks J, Donowitz M, Wagner CA, Alexander RT, Caldwell JS (2018) Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10:456
Knöpfel T, Himmerkus N, Günzel D, Bleich M, Hernando N, Wagner CA (2019) Paracellular transport of phosphate along the intestine. Am J Physiol Gastrointest Liver Physiol 317(2):G233–G241
Kritmetapak K, Kumar R (2019) Phosphate as a signaling molecule. Calcif Tissue Int. https://doi.org/10.1007/s00223-019-00636-8
Larsson TE, Kameoka C, Nakajo I, Taniuchi Y, Yoshida S, Akizawa T, Smulders RA (2018) NPT-IIb inhibition does not improve hyperphosphatemia in CKD. Kidney Int Rep 3(1):73–80
Layunta E, Pastor Arroyo EM, Kägi L, Thomas L, Levi M, Hernando N, Wagner CA (2019) Intestinal response to acute intragastric and intravenous administration of phosphate in rats. Cell Physiol Biochem 52(4):838–849
Lee AW, Cho SS (2015) Association between phosphorus intake and bone health in the NHANES population. Nutr J 14:28
Lee JJ, Plain A, Beggs MR, Dimke H, Alexander RT (2017) Effects of phospho- and calciotropic hormones on electrolyte transport in the proximal tubule. F1000Res 6:1797
Lee JJ, Liu X, O’Neil D, Beggs MR, Weissgerber P, Flockerzi V, Chen X, Dimke H, Alexander RT (2019) Activation of the calcium sensing receptor attenuates TRPV6-dependent intestinal calcium absorption. JCI insight 4:11
Levi M, Shayman JA, Abe A, Gross SK, McCluer RH, Biber J, Murer H, Lötscher M, Cronin RE (1995) Dexamethasone modulates rat renal brush border membrane phosphate transporter mRNA and protein abundance and glycosphingolipid composition. J Clin Investig 96(1):207–216
Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J, Sorribas V, Murer H (2019) Mechanisms of phosphate transport. Nat Rev Nephrol 15(8):482–500
Linarelli LG (1972) Newborn urinary cyclic AMP and developmental renal responsiveness to parathyroid hormone. Pediatrics 50(1):14–23
Lönnerdal B, Erdmann P, Thakkar SK, Sauser J, Destaillats F (2017) Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: a developmental perspective. J Nutr Biochem 41:1–11
Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM (2006) Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78(2):193–201
Marcucci G, Brandi ML (2020) Congenital conditions of hypophosphatemia expressed in adults. Calcif Tissue Int. https://doi.org/10.1007/s00223-020-00695-2
Marks J, Lee GJ, Nadaraja SP, Debnam ES, Unwin RJ (2015) Experimental and regional variations in Na+-dependent and Na+-independent phosphate transport along the rat small intestine and colon. Physiol Rep 3:1
Moor MB, Haenzi B, Legrand F, Koesters R, Hynes NE, Bonny O (2018) Renal Memo1 differentially regulates the expression of vitamin D-dependent distal renal tubular calcium transporters. Front Physiol 9:874
Motta SE, Imenez Silva PH, Daryadel A, Haykir B, Pastor-Arroyo EM, Bettoni C, Hernando N, Wagner CA (2020) Expression of NaPi-IIb in rodent and human kidney and upregulation in a model of chronic kidney disease. Pflugers Arch 472(4):449–460
Mulroney SE, Lumpkin MD, Haramati A (1989) Antagonist to GH-releasing factor inhibits growth and renal Pi reabsorption in immature rats. Am J Phys 257(1 Pt 2):29
Neiberger RE, Barac-Nieto M, Spitzer A (1989) Renal reabsorption of phosphate during development: transport kinetics in BBMV. Am J Phys 257(2 Pt 2):268
Noda S, Yamada A, Nakaoka K, Goseki-Sone M (2017) 1-alpha,25-Dihydroxyvitamin D3 up-regulates the expression of 2 types of human intestinal alkaline phosphatase alternative splicing variants in Caco-2 cells and may be an important regulator of their expression in gut homeostasis. Nutr Res 46:59–67
Noori N, Sims JJ, Kopple JD, Shah A, Colman S, Shinaberger CS, Bross R, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K (2010) Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran J Kidney Dis 4(2):89–100
Pastor-Arroyo EM, Knöpfel T, Imenez Silva PH, Schnitzbauer U, Poncet N, Biber J, Wagner CA, Hernando N (2020) Intestinal epithelial ablation of pit-2/Slc20a2 in mice leads to sustained elevation of vitamin D3 upon dietary restriction of phosphate. Acta Physiol (Oxf) 230(2):e13526
Pastoriza-Muñoz E, Colindres RE, Lassiter WE, Lechene C (1978) Effect of parathyroid hormone on phosphate reabsorption in rat distal convolution. Am J Phys 235(4):321
Peacock M (2020) Phosphate metabolism in health and disease. Calcif Tissue Int:1–13. https://doi.org/10.1007/s00223-020-00686-3
Penido M, Alon U (2012) Phosphate homeostasis and its role in bone health. Pediatr Nephrol 27(11):2039–2048
Piontek J, Krug SM, Protze J, Krause G, Fromm M (2020) Molecular architecture and assembly of the tight junction backbone. Biochim Biophys Acta Biomembr 1862(7):183279
Rajagopal A, Braslavsky D, Lu JT, Kleppe S, Clément F, Cassinelli H, Liu DS, Liern JM, Vallejo G, Bergadá I, Gibbs RA, Campeau PM, Lee BH (2014) Exome sequencing identifies a novel homozygous mutation in the phosphate transporter SLC34A1 in hypophosphatemia and nephrocalcinosis. J Clin Endocrinol Metab 99(11):2451
Ross R, Dorsey J (1991) Postnatal changes in plasma 1,25-dihydroxyvitamin D3 in sheep: role of altered clearance. Am J Phys 261(5 Pt 1):635
Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC (2011) Intestinal phosphate transport. Adv Chronic Kidney Dis 18(2):85–90
Sanders JO, Qiu X, Lu X, Duren DL, Liu RW, Dang D, Menendez ME, Hans SD, Weber DR, Cooperman DR (2017) The uniform pattern of growth and skeletal maturation during the human adolescent growth spurt. Sci Rep 7:1–9
Saurette M, Alexander RT (2019) Intestinal phosphate absorption: the paracellular pathway predominates? Exp Biol Med 244:646–654
Schlingmann KP, Ruminska J, Kaufmann M, Dursun I, Patti M, Kranz B, Pronicka E, Ciara E, Akcay T, Bulus D, Cornelissen EAM, Gawlik A, Sikora P, Patzer L, Galiano M, Boyadzhiev V, Dumic M, Vivante A, Kleta R, Dekel B, Levtchenko E, Bindels RJ, Rust S, Forster IC, Hernando N, Jones G, Wagner CA, Konrad M (2016) Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J Am Soc Nephrol 27(2):604–614
Schönauer R, Petzold F, Lucinescu W, Seidel A, Müller L, Neuber S, Bergmann C, Sayer JA, Werner A, Halbritter J (2019) Evaluating pathogenicity of SLC34A3-Ser192Leu, a frequent European missense variant in disorders of renal phosphate wasting. Urolithiasis 47(6):511–519
Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, Hawa G, Chapurlat R, Szulc P (2012) Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab 97(4):575
Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K (2002) Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277(22):19665–19672
Sheu JN, Baum M, Harkins EW, Quigley R (1997) Maturational changes in rabbit renal cortical phospholipase A2 activity. Kidney Int 52(1):71–78
Silverstein DM, Barac-Nieto M, Spitzer A (1999) Multiple arachidonic acid metabolites inhibit sodium-dependent phosphate transport in OK cells. Prostaglandins Leukot Essent Fatty Acids 61(3):165–169
Smith MW, Jarvis LG (1978) Growth and cell replacement in the new-born pig intestine. Proc R Soc Lond B Biol Sci 203(1150):69–89
Suzuki KT, Tamagawa H, Hirano S, Kobayashi E, Takahashi K, Shimojo N (1991) Changes in element concentration and distribution in breast-milk fractions of a healthy lactating mother. Biol Trace Elem Res 28(2):109–121
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G (2005) Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112(17):2627–2633
Toriz CG, Melo AI, Solano-Agama C, Gómez-Domínguez EG, Martínez-Muñoz MDLA, Castañeda-Obeso J, Vera-Aguilar E, Aguirre-Benítez EL, Romero-Aguilar L, González-del Pliego M, Jiménez-Estrada I, Luna M, Pardo JP, Camacho J, Mendoza-Garrido ME (2019) Physiological changes of growth hormone during lactation in pup rats artificially reared. PLoS One 14:8
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444(7120):770–774
Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC (2009) The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol 296(4):F691–F699
Vorland CJ, Lachcik PJ, Aromeh LO, Moe SM, Chen NX, Hill Gallant KM (2018) Effect of dietary phosphorus intake and age on intestinal phosphorus absorption efficiency and phosphorus balance in male rats. PLoS One 13(11):e0207601
Xu H, Bai L, Collins JF, Ghishan FK (2002) Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D(3). Am J Physiol Cell Physiol 282(3):487
Yagci A, Werner A, Murer H, Biber J (1992) Effect of rabbit duodenal mRNA on phosphate transport in Xenopus laevis oocytes: dependence on 1,25-dihydroxy-vitamin-D3. Pflugers Arch 422(3):211–216
Zhang D, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K (2014) Effects of sex and postmenopausal estrogen use on serum phosphorus levels: a cross-sectional study of the National Health and nutrition examination survey (NHANES) 2003-2006. Am J Kidney Dis 63(2):198–205
Zhang Y, Wu S, Lu R, Zhou D, Zhou J, Carmeliet G, Petrof E, Claud EC, Sun J (2015) Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep 5:10642
Acknowledgments
Research in the Alexander laboratory is funded by grants from the Women and Children’s Health Research Institute, which is supported by the Stollery Children’s Hospital Foundation, the Canadian Institutes of Health Research, the Kidney Foundation of Canada and the National Sciences and Engineering Research Council of Canada. Dr. Alexander is a Stollery Science laboratory Distinguished Researcher and the Canada Research Chair in Renal Epithelial Transport Physiology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
MacDonald, T., Saurette, M., Beggs, M.R., Todd Alexander, R. (2020). Developmental Changes in Phosphate Homeostasis. In: Pedersen, S.H.F. (eds) Reviews of Physiology, Biochemistry and Pharmacology. Reviews of Physiology, Biochemistry and Pharmacology, vol 179. Springer, Cham. https://doi.org/10.1007/112_2020_52
Download citation
DOI: https://doi.org/10.1007/112_2020_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74288-1
Online ISBN: 978-3-030-74289-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)