Abstract
This chapter lists current evidence (from molecular level, cellular level, animal level to patient level) and some potential mechanisms for the effects of static magnetic field on cancer inhibition. The direct impacts of static magnetic fields on cancer cells are summarised, including cancer cell proliferation, division, migration and invasion, as well as cancer cell stemness. Moreover, static magnetic field s can also affect microcirculation and angiogenesis, and regulate immune system to inhibit cancer in vivo. Furthermore, the prospective applications of static magnetic field alone or in combination with chemotherapy drugs, time-varying magnetic fields as well as radiotherapy in cancer treatment are reviewed. The potential mechanisms and factors that contributed to the inconsistencies are also discussed. These evidences demonstrate that static magnetic fields have a great potential to be used as a physical tool to inhibit cancer, but further investigations are still needed to optimize the static magnetic field parameters and exposure procedures, as well as combinational therapy modalities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
The advances in tumor treating fields (TTFs) electric therapy, which has been approved by the Food and Drug Administration (FDA) to be used on recurrent and newly diagnosed glioblastoma in 2011 and 2015, respectively, provided a great example to illustrate the advantages of physical modality in cancer treatment. However, although magnetic therapy using SMF has been used by some people as alternative treatment on multiple chronic diseases for years, the scientific foundation is still lacking. As we have introduced in previous chapters, many studies have investigated the biological effects of static magnetic fields (SMFs), with results that depended on multiple factors including SMF parameters, biological sample and experimental procedure differences. In particular, the difference in cell types made a significant impact. A large number of reports show that cancer cells and some specific cell types, including stem cells, embryonic or neuronal cells, are more susceptible to SMFs, while most other non-cancer cells are much less affected.
Here we would like to focus on the impacts of SMFs on cancer. It is well known that cancer cells are different from normal cells in various aspects. For example, multiple types of cancers proliferate in response to signalling from oncoproteins such as EGFR (epidermal growth factor receptor) and we found that SMF can affect EGFR orientation to reduce its activity as well as related pathways to inhibit some cancer cell proliferation (Zhang et al. 2015, 2016). Moreover, most cancer cells are at a more active dividing state compared to normal cells. We found that moderate and strong SMFs can interfere with microtubules so that the cell division can be affected (Zhang et al. 2017a). The metastatic behaviours and stemness of cancer cells are also drastically different from non-cancer cells. We recently found that moderate SMF could inhibit ovarian cancer cell migration, invasion and stemness, while having a negligible effect on the non-cancer ovarian cells (Song et al. 2021). However, Zhao et al. reported that the osteosarcoma stem cells metastasis in mice was promoted by moderate SMFs of tilted and gradient direction (Zhao et al. 2021). In addition, the cancer microcirculation/angiogenesis and immune responses in vivo are also different from normal tissues. Here I summarize the SMFs effects on cancer in mice studies (Table 9.1), which indicates that higher field SMF, longer treatment time, and vertically upward direction seem to be positively correlated with the anti-cancer efficacy. For example, Zhu et al. found that 0.6 T SMF treatment for 2–3 months efficiently inhibited cancer growth in transgenic polyoma middle T oncoprotein (PyMT) mice by ~60–70%, but 0.3 T did not have this effect (Zhu et al. 2020). Our group found that for the same SMF flux density, the upward SMFs could inhibit cancer growth in mice while the downward SMFs could not (Tian et al. 2018; Yang et al. 2021). Moreover, for the upward direction 9.4 T SMF treatment, a 200-h treatment can inhibit cancer growth by 62.88% (Tian et al. 2022) while 88-h can inhibit cancer growth by 44.7% (Yang et al. 2021), although we did not compare the same types of cancer side-by-side.
In this chapter, I will first introduce the studies about the direct SMF effects on cancer cells in vitro and in vivo, including cancer cell proliferation, division, migration and invasion, as well as stemness. Then from the in vivo point of view, the contributions of SMF effect on microcirculation/angiogenesis and immune regulation are also discussed, which is followed by the combination of SMFs with other treatments, including chemodrugs, time-varying magnetic fields, etc.
9.2 Direct Effects of Static Magnetic Fields on Cancer Cells In Vitro and In Vivo
9.2.1 Static Magnetic Fields Could Inhibit Some Cancer Cell Proliferation
As introduced in previous chapters, the exact cellular effects of SMFs on cells are largely dependent on cell types so that there is no consensus effect of SMF on various kinds of cells. For example, Sullivan et al. examined the effect of 35–120 mT SMFs on four different types of cells and found that the effects varied greatly among them (Sullivan et al. 2011). However, among different cell types, the cell growth/proliferation inhibition effects of SMF on cancer cells are much more consistent compared to other cell types.
Multiple studies have shown that SMFs could inhibit cancer cell growth while having a minimal effect on non-cancer cells. Although in each individual study, the cell types examined were very limited, we can see a clear trend that SMFs tend to inhibit cancer cells but not non-cancer cells. For example, in 1996, Rayman et al. showed that cell growth of a few cancer cell lines could be inhibited by 7 T SMF (Raylman et al. 1996). Later, a few studies used both cancer and non-cancer cells and found that they respond to the SMFs differentially. For example, in 2003, Aldinucci et al. found that 4.75 T SMF did not affect human peripheral blood mononuclear cells (PBMC) but inhibited Jurkat leukemia cell proliferation (Aldinucci et al. 2003b). In 2006, Ghibelli et al. showed that 1 T SMF could increase the chemotherapy-induced apoptosis in human tumor U937 monocytes but not mononuclear white blood cells (Ghibelli et al. 2006). In 2011, Tatarov et al. tested the effect of 100 mT SMF on mice bearing metastatic mouse breast tumor EpH4-MEK-Bcl2 cells. They found that exposure of the mice to magnetic fields for 3 or 6 h, but not 1 h, daily for as long as 4 weeks suppressed tumor growth (Tatarov et al. 2011). Their study not only indicated that the moderate SMF could inhibit mouse breast cancer growth, but also showed that the inhibition was directly correlated to the SMF exposure time (Tatarov et al. 2011). In 2015, Zafari et al. investigated the effects of SMF (5, 10, 20 and 30 mT) for 24–96 h on the viability of the human cervical cancer HeLa cells and fibroblast cells. They found that the increase of SMF intensity and incubation time increased cell death percent and proliferation rate in HeLa cells more obviously compared to fibroblast cells (Zafari et al. 2015).
There are some mechanistic studies that have explored the differential effects of SMFs on cancer vs. non-cancer cell proliferation. For example, many types of cancer cells proliferate in response to signalling from Receptor Tyrosine Kinases (RTKs), and the effect of magnetic fields (MFs) on EGFR phosphorylation has been investigated in several studies (Jia et al. 2007; Sun et al. 2008, 2013). It was shown that both 0.4 mT 50 Hz low frequency and 2 μT 1.8 GHz radiofrequency time-varying MFs increased EGFR phosphorylation. However, it was very interesting that this effect could be reversed by incoherent (“noise”) MFs of the same MF intensities (Sun et al. 2008, 2013). These results not only demonstrate that EGFR is a molecular target for MFs, but also show that the different types of MFs have differential effects on EGFR activities. In 2016, our group tested SMF effects on EGFR and found that moderate and strong SMFs could actually inhibit EGFR activity both in vitro and in cells in a MF flux density-dependent way (Zhang et al. 2016) (Fig. 9.1a). We further explored the underlying mechanism using scanning tunnelling microscopy (STM) (Fig. 9.1b) and molecular dynamics (MD) simulation (Fig. 9.1c). We found that SMF could affect the orientation of EGFR kinase domain, which interfered with the normal interaction between EGFR monomers to inhibit their activation. In addition, although the CHO (Chinese hamster ovary) cell number was not affected by 0.05 T, 1 T or 9 T SMFs, EGFR transfected CHO cells became responsive to SMFs and were effectively inhibited by 1 T and 9 T SMFs (Fig. 9.1d). This indicates that EGFR is at least one of the key factors that contribute to SMF-induced cancer cell inhibition.
Static magnetic fields inhibit EGFR activity by changing its orientation to inhibit cell proliferation. (a) In vitro kinase assays show that moderate SMFs could inhibit EGFR kinase domain autophosphorylation. Western blot of phosphor-EGFR was shown. SMFs of 0.005–1 T were tested. Incubation time was 10 min. (b) Liquid-phase scanning tunnelling microscopy (STM) shows that a 0.4 T SMF could change EGFR kinase domain orientation. (c) Computer-based calculation shows that the probability of the EGFR kinase domain net dipole moment aligns with SMF field direction in a MF flux density-dependent manner. (d) The cell number of CHO cells was not affected by 0.05, 1, or 9 T SMF while the cell number of CHO cells overexpressing EGFR-Flag was significantly reduced by 1 T and 9 T SMFs. Incubation time was 3 days. *p < 0.05; **p < 0.01. [Figures were adapted from reference (Zhang et al. 2016). Copyright © 2016 Impact Journals, LLC. Open access]
As mentioned above, most studies have only tested one or very few cell types, which prevented people from getting a comprehensive view of the cellular effects of SMF on different kinds of cells. Therefore, our group side-by-side compared 15 different cell lines, including 12 human (7 cancer cell lines and 5 non-cancer cell lines) and 3 rodent cell lines for their responses to 1 T inhomogeneous SMF provided by a permanent magnet. We found that SMF not only affect cell proliferation in a cell type-dependent manner, the cell density also played indispensable roles (Table 9.2) (Zhang et al. 2017b). For example, the growth of A549 lung cancer cells was inhibited by 1 T SMF when they were seeded at a high density but the growth of normal lung cells was promoted (Table 9.2).
We further analysed their EGFR-mTOR-Akt pathway and found that the A549 lung cancer and HSAEC2-KT non-cancer lung cells have dramatically different EGFR-mTOR-Akt pathway expression and activation (Fig. 9.2) (Zhang et al. 2017b). The EGFR expression and phosphorylation levels are much higher in A549 lung cancer cells than in HSAEC2-KT normal lung cells. The mTOR and AKT expression and phosphorylation levels are also significantly higher in A549 lung cancer cells. These results, combined with the EGFR studies mentioned above, demonstrate that EGFR-mTOR-Akt pathway is likely to be one of the key factors that contribute to the cell type differences in SMF-induced cell proliferation changes. In addition, it should be mentioned that the cell density also affected the A549 lung cancer cells and normal lung cells HSAEC2-KT in different pattern (Fig. 9.2). For example, the EGFR and 4EBP1 expression and phosphorylation level were increased in higher cell density compared to lower cell density in A549 lung cancer cells but not in HSAEC2-KT normal lung cells. These results indicate that EGFR-mTOR-Akt pathway may be a key factor that contributes to both cell type- and cell density-dependent SMF effects.
Human lung cancer A549 and normal lung HSAEC2-KT cells have differential EGFR-Akt-mTOR pathway expression and phosphorylation. Human lung cancer A549 and normal lung cells HSAEC2-KT cells were plated at four different cell densities 1 day ahead before they were harvested for Western Blot. “1” indicates the lowest cell density. “4” indicates the highest cell density. [Reprinted from Ref. (Zhang et al. 2017b). Copyright © 2016 Impact Journals, LLC. Open access]
Besides RTK pathway, the SMF effect on DNA synthesis is also an important step in cell proliferation, which has been introduced in Chap. 6. Using BrdU incorporation assay to measure DNA synthesis rates, we first found that 1 T moderate SMF could inhibit DNA synthesis in colon cancer HCT116 and LoVo, and lung cancer PC9 and A549 cells (Yang et al. 2020), but 0.5 T SMF has no effects on DNA synthesis (Yang et al. 2021). Then we used higher field SMF provided by a superconducting magnet, and found that DNA synthesis was significantly decreased by both upward (14.3%, p < 0.01) and downward (18.6%, p < 0.01) 9.4 T SMFs after 24 h (Fig. 9.3a). We also used Western blot analysis to examine the level of TOP2α (DNA topoisomerase II Alpha), which functions to bring the higher order compaction of chromatin to form condensed mitotic chromosomes during G2-M transition. Our results show that TOP2α was decreased in both upward and downward 9.4 T SMF-treated cells (Fig. 9.3b). The DNA synthesis inhibition by SMFs is likely due to the DNA supercoil changes through Lorenz forces on the negatively charged DNA in motion. More specifically, we have previously proposed that the upward SMF could cause tightened DNA supercoils while the downward SMF causes loosen supercoils (Yang et al. 2020). Interestingly, we found that the upward 9.4 T SMF significantly increased reactive oxygen species (ROS) level (Fig. 9.3c), while the downward 9.4 T SMF did not (Fig. 9.3c). It is well known that ROS play central roles in multiple cellular processes, including triggering P53 activation, a key tumor suppressor. In fact, our data showed that the upward 9.4 T SMF could activate and upregulate P53 (Fig. 9.3d), but the downward 9.4 T SMF had no such effect (Fig. 9.3e), which is consistent with the ROS level changes. It is possible that the tightened DNA supercoils caused by Lorenz forces in upward 9.4 T SMF is a key step to boost ROS level, which consequently activates P53 and further inhibits DNA replication and cell proliferation.
High field 9.4 T SMFs inhibit DNA synthesis and regulate p53 in a SMF direction-dependent manner. (a) 9.4 T SMFs obviously inhibited the DNA replication of cells. (b) The level of TOP2α treated with 9.4 T SMFs analyzed by Western blots and quantified by ImageJ software. (c) Upward 9.4 T SMF significantly increased the ROS levels of A549, but the downward SMF did not. (d) Representative Western blots shows the level of phosphorylated P53 (S15) and P53 in the cells exposed with upward 9.4 T SMF were dramatically increased. (e) Representative Western blots shows the level of phosphorylated P53 (S15) and P53 in the cells exposed with downward 9.4 T SMF and statistical analysis for P53. [Reprinted from reference (Yang et al. 2021). Open access]
To further confirm the results we got in vitro, we examined the tumor tissues of the mice treated with or without 9.4 T SMF for the tumor suppressor P53 and the proliferation marker Ki-67. It is obvious that the P53 level was significantly increased by the upward 9.4 T SMF, but not downward 9.4 T SMF (Fig. 9.4a, b). Moreover, the Ki-67 level was significantly decreased by the upward 9.4 T SMF, but not much by the downward 9.4 T SMF. These are consistent with our findings that 9.4 T upward SMF could inhibit A549 lung cancer cell growth both in vitro and in vivo. Therefore, although both the upward and downward 9.4 T SMF could inhibit DNA synthesis in vitro, only the upward 9.4 T SMF significantly increased ROS and P53 levels, decreased mitotic index and caused G2 cell arrest, which collectively lead to tumor growth inhibition in tumor bearing mice (Fig. 9.4c).
9.4 T SMF increased P53 level and decreased Ki-67 level in mice tumor tissues. Representative images of P53 and Ki-67 immunohistochemistry staining or HE staining of sham, (a) upward 9.4 T SMF or (b) downward 9.4 T SMF treated mice tumor tissues. Scale bar: 50 μm. (c) The model of 9.4 T magnetic fields influence the cell number of A549 lung cancer cells. [Reprinted from reference (Yang et al. 2021). Open access]
However, it should be mentioned that there are also a few studies showing that SMFs could promote cancer cell proliferation. For example, we previously found that moderate SMFs can inhibit cancer cell proliferation when they are plated at high density, but can also increase some cancer cell numbers when they are plated at low density (Table 9.2) (Zhang et al. 2017b). It is a pity that we were not aware of the importance in SMF direction in this study at that time, so the SMF direction information was missing. In addition, Fan et al. show that ~150 mT SMF treatment accelerated 4 T1 breast cancer cell proliferation. However, they also showed that SMF treatment shortened the telomere length, decreased telomerase activity, and inhibited the expression of the cancer-specific marker telomerase reverse transcriptase (TERT) (Fan et al. 2020). However, the SMF direction and cell density information are both missing, so we cannot exclude the possibility of the direction- and cell plating density-induced effects. More research is needed to test various SMF conditions and cancer cells to get more complete information.
9.2.2 Static Magnetic Fields and Cancer Cell Division
Besides cell proliferation, there are other cellular components that play indispensable roles in SMF-induced cancer inhibition, such as cell division. Since cell division is a key step that leads to tumor growth, perturbations that disrupt or interfere with cell division could inhibit tumor growth. In fact, there are multiple chemodrugs that target cell division, such as Taxol. In addition, the most well studied electromagnetic therapy in cancer treatment, the TTF, also target cell division.
The key structure that controls the whole cell division process is the mitotic spindle, which is mainly composed of microtubules. It is well known that microtubules can be affected by SMFs and recent evidences showed that cell division could also be affected by SMFs, which was discussed in Chap. 6. In 2017, we reported that the SMF-induced spindle orientation and morphology changes are due to the combined alignment effects of both microtubules and chromosomes in the magnetic field (Fig. 9.5). Application of the magnetic field parallel to the coverslip allowed us to discriminate torques on chromatin vs. microtubules, and in this case, it appears that torques on well aligned chromatin dominated, aligning spindles preferentially with their microtubules normal to the field, and their metaphase plate parallel to the field. More importantly, although high-field SMFs can change the spindle orientation and morphology in both cancer and non-cancer cells, we found that the non-cancer cells can recover after the cells were taken out of SMFs. However, the cancer cells do not have a recovery ability, and their growth will be halted even after they are taken out of the SMF.
Models show that ultra-high static magnetic fields align microtubules and chromosomes to change spindle orientation and morphology. Blue upward arrows show the magnetic field direction. Cells were plated on coverslips, which were placed in the ultra-high magnetic field either normal to or in parallel with the field direction. ‘1’ measures the pole angle of metaphase spindles in parallel to the magnetic field/gravity direction and ‘2’ measures the pole angle of metaphase spindles normal to the magnetic field/gravity direction. [Reprinted reference (Zhang et al. 2017a). Open access]
9.2.3 Static Magnetic Fields and Cancer Metastasis
Metastasis is the leading cause of cancer patient death, which involves cancer cell migration and invasion and is regulated by multiple factors. As far as we know, there are only three studies that have investigated on the SMF effects on cancer cell migration/invasion and/or cancer metastasis. In 2020, Fan et al. reported that a moderate SMF of ~150 mT can inhibit 4 T1 breast cancer cell migration (Fan et al. 2020), but they did not perform animal experiments. In 2021, our group found that gradient moderate SMFs (~0.5 T) provided by a superconducting magnet or permanent magnet can increase ROS level and inhibit ovarian cancer cell migration, invasion (Fig. 9.6), and inhibit ovarian cancer metastasis in mice (Fig. 9.7) (Song et al. 2021). However, also in 2021, using a titled direction gradient SMF provided by a superconducting magnet (Fig. 9.8), Shang’s group reported a metastasis promoting effects on osteosarcoma (Zhao et al. 2021).
Moderate SMFs inhibit ovarian cancer invasion in a ROS-dependent manner. (a) Cells were placed in the upper part of the superconducting magnet, where the SMF is about 0.5 T. (b) Transwell invasion assays and (c) migration assays of SKOV3 and HO8910 ovarian cancer cells in the absence or presence of SMF and/or NAC. *p < 0.05. [Reprinted from reference (Song et al. 2021). Open access]
Moderate SMFs inhibit ovarian cancer metastasis in mice. Mice bearing ovarian cancer were exposed to moderate SMFs using a superconducting magnet (10 h/day and 7 days/week) or a permanent magnet plate (continuously 6 weeks). Mice were examined for metastasis at the end of the experiment. *p < 0.05; ns not significant. [Reprinted from reference (Song et al. 2021). Open access]
Scheme of the exposure system that provides a 0.2–0.4 T SMF by a 16 T superconducting magnet. [Illustration courtesy of Ding Joe Wang, based on reference (Zhao et al. 2021)]
9.2.4 Static Magnetic Fields and Cancer Cell Stemness
There have been multiple studies that have investigated the effects of SMFs on stem cells, such as dental pulp stem cells (DPSCs), bone marrow stromal cells (BMSCs), human adipose-derived stem cells (hASCs), etc., which have been introduced in Chap. 6 of this book, and in some reviews (Sadri et al. 2017; Marycz et al. 2018; Ho et al. 2019). However, the effect of SMFs on cancer cell stemness was not reported until recently, which reported opposite effects of moderate SMF on cancer stemness and metastasis (Song et al. 2021; Zhao et al. 2021).
The report from our group using vertically upward direction SMFs of ~0.5 T provided by either a permanent magnet, or a superconducting magnet (Figs. 9.6a and 9.7), showed that these SMFs can increase ROS levels in ovarian cancer cells and inhibited their stemness and metastasis (Song et al. 2021). It is known that ROS could affect the epithelial-mesenchymal transition (EMT), promote the transition of mesenchymal cancer stem cells (CSCs) into epithelial CSCs and then bulk cells (Fig. 9.9a). We exposed SKOV3 cells to inhomogeneous moderate SMFs provided by permanent magnets (0.1–0.5 T) for 24 h and used real-time PCR to find out that the stemness-related genes were significantly downregulated by SMF treatment, including SRY-box transcription factor 2 (Sox2), Nanog, cell myc proto-oncogene protein (C-myc), hyaluronan receptor (CD44), and CD133 (Fig. 9.9b). Moreover, the cell morphology of SKOV3 cells changed from mesenchymal-like states to epithelial-like states after SMF exposure (Fig. 9.9c). Furthermore, we exposed the HO8910 and SKOV3 cells to SMF for 12 days and detected their sphere-forming ability. The number and size of OC cell spheres were obviously decreased by SMF (Fig. 9.9d) (Song et al. 2021). These data suggested that ovarian cancer stemness was significantly reduced by this moderate SMF treatment.
Moderate SMFs reduce ovarian cancer stemness. (a) Illustration of the effects of ROS level on CSCs. (b) The relative mRNA expressions of stemness genes were measured by qPCR. (c) Representative bright-field images of SKOV3 cells exposed to Sham or moderate SMF for 24 h. (d) The sphere number and size were measured in SKOV3 and HO8910 cells treated with SMF for 12 days. All comparisons were made between the experimental group and the Sham control group by Student’s t test. *p < 0.05 and **p < 0.01. [Reprinted from reference (Song et al. 2021). Open access]
In contrast, another study from the Shang’s group reported that a tilted gradient SMF provided by a superconducting magnet can also increase the ROS levels in osteosarcoma stem cell, but promoted their stemness (Zhao et al. 2021). It is interesting that two independent studies both performed cellular and animal experiments about moderate SMFs on cancer cell stemness, but got opposite effects. There are multiple possible reasons: (1) They used different cell lines, Song et al. used SKOV3 and HO8910 ovarian cancer cells while Zhao et al. used osteosarcoma stem cells. The cells in the bone system are very susceptible to SMF treatment, which will be discussed in Chap. 11. The magnetic directions were different. Song et al. used vertically upward SMFs while Zhao et al. used a tilted SMF. Although the mechanisms for SMF direction-induced bioeffects are still unclear, the differences have been extensively discussed previously discussed in Chap. 2. Obviously, we cannot get any conclusions about SMF and their effects on cancer cell stemness yet at this time point. However, given the importance of cancer stem cells in cancer development, people should perform more studies to get a better understanding on this topic.
9.3 Static Magnetic Fields and Tumor Microcirculation and Angiogenesis
The above-mentioned effects of SMFs are directly on cancer cells, including cancer cell proliferation, division, migration and invasion, as well as cancer cell stemness. In fact, there are a few studies indicating that moderate SMFs could inhibit angiogenesis and tumor microcirculation to inhibit cancer growth in vivo. For example, in 2008, Strieth et al. examined the effects of SMF (<600 mT) on A-Mel-3 tumors growing in dorsal skinfold chamber preparations of Syrian Golden hamsters. They found that short-time exposure to SMF (~150 mT) resulted in a significant reduction of red blood cell velocity (vRBC) and segmental blood flow in tumor microvessels (Strieth et al. 2008). At 587 mT, a reversible reduction of vRBC and a reduction of functional vessel density were observed. In addition, they found that prolongation of the exposure time from 1 min to up to 3 h had a more significant result. Moreover, SMFs not only reduced blood flow in tumor vessels but also activated and increased the adherence of platelets (Strieth et al. 2008). In 2009, Strelczyk et al. further evaluated the effects of prolonged exposure to SMFs on tumor angiogenesis and growth. They found that 586 mT SMF exposure for 3 h could inhibit both tumor angiogenesis and growth (Strelczyk et al. 2009). Detailed analysis revealed that the functional vessel density, vessel diameters and vRBC in tumors were all reduced by SMFs. In addition, they also observed increased edema after SMF exposure, which indicated that SMFs might increase tumor microvessel leakiness. In 2014, their group did some further analysis and found that the 587 mT SMF did increase the tumor microvessel permeability significantly in A-Mel-3-tumor-bearing hamsters (Gellrich et al. 2014) (Fig. 9.10). It was interesting but not surprising that the functional tumor microvessels, labeled by FITC-dextran, were much decreased after SMF exposure, especially after the repeated SMF exposure, which was likely due to the inhibited tumor angiogenesis. Nevertheless, it was obvious that both SMF single exposure and repeated exposure increased the blood vessel leakiness and the repeated SMF exposure had stronger effects. In addition, the authors propose that the increased microvessel permeability was likely the reason for the improved anti-tumor efficacy of SMFs in combination with paclitaxel (Fig. 9.10) (Gellrich et al. 2014).
A 587 mT SMF exposure induces intratumoral microvascular leakiness in A-Mel-3-tumor-bearing hamsters. On day 10 after tumor cell implantation representative ROIs (regions of interest) were chosen after FITC-dextran administration, highlighting functional tumor microvessels, before rhodamine-labeled albumin was given intravenously. In control groups, there was a continuous slight increase of fluorescent albumin in the extravascular compartment but the increase was stronger after SMF exposure. (a) In vivo fluorescence microscopy for analysis of microvascular leakiness during SMF-exposure. Animals were exposed to the sham control or the SMF of 587 mT during the whole in vivo assessment of microvascular permeability on day 10. (b) In vivo fluorescence microscopy of animals that have been repeatedly exposed to SMF of 587 mT for 3 h on day 5, 7, 9 after tumor implantation. The intratumoral microvascular leakiness was stronger in animals after repeated exposure to SMF even with regard to the obviously rather low functional vessel density. [Reprinted with permission from reference (Gellrich et al. 2014). Copyright © 2013 Elsevier Ireland Ltd.]
An independent group also reported the effects of SMF on angiogenesis. In 2009, Wang et al. investigated the effects of the gradient SMF (0.2–0.4 T, 2.09 T/m, exposure time 1–11 days) on angiogenesis in the human umbilical veins endothelial cells (HUVECs) as well as two in vivo models, a chick chorioallantoic membrane (CAM) and a Matrigel plug (Wang et al. 2009). Their results showed that the HUVECs proliferation was significantly inhibited after 24-h exposure. In addition, the two in vivo models both showed decreased angiogenesis after 7 or 11 days of exposure (Wang et al. 2009). Although this study was not carried out in a tumor-related model, it showed the inhibition effect of moderate SMFs on angiogenesis, which was consistent with the results reported by Strieth and co-workers (Strieth et al. 2008; Strelczyk et al. 2009). Moreover, our group analyzed the lung cancer A549 cell formed tumor tissue in mice that were exposed to 9.4 T SMFs for 88 h. We stained them with CD31, a blood vessel marker, and counted the vessel numbers in each group of mice. We found that the vessel numbers are reduced in both upward and downward SMF groups (Fig. 9.11) (unpublished data), which indicates that the inhibition effects of SMF on angiogenesis is not MF direction-dependent.
Both upward and downward 9.4 T homogeneous SMFs reduce the vessel number in lung cancer tissues. (a) The tumor tissues were stained for a blood vessel marker, CD31. (b, c) The vessel numbers were counted from 6 independent views from the tissues. Data represent means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Our group unpublished work
Taken together, these studies showed that moderate to high SMFs have the ability to reduce angiogenesis in some animal models, which implied their potential for tumor growth inhibition in vivo. Additional research is needed to ascertain this effect, such as the effects of other magnetic field intensities as well as more types of tumor models.
9.4 Static Magnetic Fields Inhibit Cancer Through Immune Regulation
It has been demonstrated that immune status in humans and in mouse models affects the risk of cancer development in an etiology-dependent manner (Reiche et al. 2004; de Visser et al. 2006). Genetic elimination or depletion of immune cells alters cancer progression in experimental models. Activation of anti-tumour adaptive immune responses can suppress tumour growth. There are some reports about time-varying MFs regulating immune system to inhibit cancer. However, although several studies have shown that SMFs can affect immune systems, which are summarized in Chap. 12 of this book, there are currently only two studies that have addressed the effect of SMF on immune system and their regulation on cancer (Lin et al. 2019; Zhu et al. 2020).
In 2020, Zhu et al. reported a comprehensive study showing that exposure to moderate SMFs (Max magnetic flux density at the surface of the magnetic cubes is at 0.6 T) led to increased granule and cytokine secretion as well as ATP production and mitochondrial respiration from CD8+ T cells (Zhu et al. 2020). These effects were inhibited by knocking down the Uqcrb and Ndufs6 genes of the mitochondrial respiratory chain, whose transcriptions were regulated by candidate magnetoreceptor genes Isca1 and Cry1/Cry2. SMF exposure also promoted CD8+ T cell granule and cytokine secretion and repressed tumor growth in vivo. SMFs enhanced CD8+ T cell cytotoxicity, and the adoptive transfer into tumor-bearing mice resulted in significantly enhanced antitumor effects (Fig. 9.12). Their study suggests that moderate SMFs enhance CD8+ T cell cytotoxicity by promoting mitochondrial respiration and promoted the antitumor function of CD8+ T cells.
Moderate SMFs promote the antitumor response of CD8+ T cells in vivo. PyMT mice were exposed to magnetic plates made of small magnetic cubes (surface Max 0.6 T), N pole upward facing the mice. Tumor onset (a) and tumor growth (b) of PyMT mice were monitored. (c) HE-stained mammary tumor sections from PyMT mice (scale bars 200 μm). (d) % Statistics for CD4+, CD8+ T cells, and the CD8+/CD4+ T cell ratio among tumor-infiltrating T cells in PyMT mice, (e) % Statistics for the expression of CD69, CD44 and CD25 in tumor-infiltrating CD8+ T cells in PyMT mice, and (f) cytokine/granule production of tumor-infiltrating CD8+ T cells in PyMT mice as analyzed by flow cytometry. (g, h) Percentage (g) and MFI (h) statistics for the expression of GzmB, IFNγ and TNFα in tumor-infiltrating CD8+ T cells in PyMT mice as analyzed by flow cytometry. Data were analyzed by log-rank test (a), two-way ANOVA (b), or Student’s t test (d, e, g) (NS no significance, **p < 0.01, ***p < 0.001, ****p < 0.0001). Error bars indicate the SEM. [Figure and legend are adapted from reference (Zhu et al. 2020). Open access]
In fact, in 2019, Lin et al. have explored the potential for enhancing the killing ability of NK cells by co-culturing the NK cells with K562 leukemia cells under a 0.4 T SMF (Lin et al. 2019). They found that the viability and killing activity of the NK92-MI cells were significantly increased by the 0.4 T SMF. Although their study was only performed at the cellular level, and they did not test them in animals, these results indicate the great potential of moderate SMFs to boost NK cells to inhibit cancer. Moreover, it should be mentioned that in Zhu et al’s study, the 0.3 T SMF did not generate such effects (Zhu et al. 2020), which is consistent with our previously mentioned point that SMF strength is a critical factor in the SMF effects on cancer inhibition.
9.5 Static Magnetic Fields in Combination with Other Treatments
9.5.1 Static Magnetic Fields in Combination with Chemodrugs
There are a large number of researches studied the combinational effects of SMF with chemotherapy drugs, and most of them used moderate SMFs (Table 9.3). Multiple studies have achieved enhanced anti-tumor efficacy compared to SMF or chemodrugs alone. For example, in 2014, Gellrich et al. found that a 587 mT SMF could significantly increase the anti-tumor efficiency of paclitaxel chemotherapy in A-Mel-3-tumor-bearing hamsters because the 587 mT SMF inhibited tumor angiogenesis and inceased tumor microvessel permeability significantly (Gellrich et al. 2014). Our group also found that 1 T moderate intensity SMF could increase the antitumor efficacy of mTOR inhibitors, EGFR inhibitors, Akt inhibitors, as well as Taxol and 5-Fu (Zhang et al. 2015; Luo et al. 2016). In addition, chemotherapy drug adriamycin had an enhanced inhibition effect on the growth of leukemic cells K562 and transplanted mammary tumors in mice when it was combined with moderate intensity SMFs of 110 mT or 8.8 mT, respectively (Gray et al. 2000; Hao et al. 2011). In 2006, Ghibelli et al. showed that 1 T SMF increased apoptosis induced by anti-tumor drugs in human tumor U937 monocytes but not mononuclear white blood cells (Ghibelli et al. 2006).
It was proposed that the cell membrane permeability can be increased by SMFs to allow more drugs to enter cells (Tofani et al. 2003; Liu et al. 2011; Gellrich et al. 2014). This is an appealing explanation because it can explain the combined effects of SMFs and chemodrugs. It is also explainable because SMFs were shown to affect lipids. However, it is puzzling that SMFs have variable effects when combined with chemotherapy drugs (Table 9.3), which indicates that the combinational effects of SMFs with chemodrugs may be drug-specific and/or cell type-specific.
However, it should be mentioned that the current experimental results about combination of SMFs with Cisplatin are not completely consistent. Although we and Vergallo et al. found that SMFs did not increase the efficacy of Cisplatin, there are also some other evidences showing opposite results. For example, it was shown that SMFs could increase the antitumor effects of Cisplatin in mice bearing Lewis lung carcinoma (Tofani et al. 2003) and leukemic cells K562 (Chen et al. 2010). This is probably due to the different magnetic intensities in independent studies or cell type differences. Both of these factors could directly influence the magnetic effects as we have discussed earlier. More specifically, studies reported that SMFs of 1–10 mT could increase the antitumor efficacy of Cisplatin (Tofani et al. 2003; Chen et al. 2010) but in ours (Luo et al. 2016) and Vergallo et al.’s studies (Vergallo et al. 2014), we both used stronger magnetic fields (31.7–232 mT in Vergallo et al.’s study and 1 T in our study). Maybe lower magnetic field intensity could increase the Cisplatin efficacy while higher magnetic field intensity has the opposite functions. The exact effects and mechanisms of combining SMFs with Cisplatin in different cells need to be further investigated.
In fact, there are some studies indicated that both MF intensity and cell type could influence the effect of SMF in combination with drugs. For example, in 1999, Fanelli et al. found that SMFs with different intensities starting from 6 gauss could decrease the extent of cell death by apoptosis induced by several agents in different human cell systems via modulation of Ca2+ influx, and this effect was MF intensity-dependent (Fanelli et al. 1999). This directly showed that the MF intensity could influence the effect of SMFs with drugs. For cell type induced difference, in 2003, Aldinucci et al. tested a few different cell types for the effects of combining a 4.75 T SMF and a pulsed EMF of 0.7 mT generated by an NMR apparatus for 1 h. They found that in T cell leukemia Jurkat cells the calcium level was reduced significantly after exposure (Aldinucci et al. 2003b) but in normal or in PHA challenged lymphocytes the calcium level was increased (Aldinucci et al. 2003a). Moreover, in 2006, Ghibelli et al. compared two different MF intensities (1 T vs. 6 mT), four different cell lines (two cancer cell lines, human leukemic monocyte lymphoma U937 cells and T cell leukemia Jurkat cells as well as two types of normal cells, human monocytes and lymphocytes) (Ghibelli et al. 2006). It was not surprising that neither the 1 T nor the 6 mT SMF induced apoptosis in all four types of cells. However, it is interesting that 1 T SMF increased puromycin (PMC)-induced apoptosis in U937 cells, but not in other three cell types (Ghibelli et al. 2006). In addition, unlike 1 T SMF, the 6 mT SMF did not increase the PMC-induced apoptosis in any of the cells. In contrast, it reduced the PMC-induced apoptosis in U937 cells (Ghibelli et al. 2006). Moreover, Tenuzzo et al. used 6 mT SMF and apoptosis-inducing agents to compare their effects on multiple types of cells and found that SMF interfered with apoptosis in a cell type- and exposure time-dependent manner (Tenuzzo et al. 2006). In addition, we have reported that 1 T SMF could increase the efficacy of some chemodrugs (5-Fu, Taxol) in multiple human solid cancer cell lines, such as breast cancer MCF-7, colon cancer HCT116, nasophageal cancer CNE-2Z cells but only at some drug concentrations (Luo et al. 2016). Therefore, MF intensity, cell type, drug concentration, and even exposure time, could all influence the combinational effect of SMF with drugs.
Moreover, although most studies have used moderate SMFs, we recently reported that 9.4 T high-field SMF can also increase the efficacy of the chemotherapy drug imatinib mesylate. More importantly, it also ameliorates chemodrug-induced toxicity and depression in mice (Fig. 9.13) (Tian et al. 2022). We compared the anti-tumor effects of 9.4 T SMF with or without imatinib mesylate on BALB/c (Nu/Nu) mice bearing gastrointestinal stromal tumor (GIST-T1) cells. We found that the tumor growth was inhibited up to 62.88% when treated with 9.4 T SMF alone for 200 h. More importantly, 9.4 T SMF combined with 20 mg/kg imatinib mesylate can result in 92.75% tumor suppression, which is close to the anti-tumor effect of high dose (80 mg/kg) imatinib. However, 80 mg/kg imatinib caused severe side effects, including significantly reduced gain of body weight, abnormal liver function and depressive behaviors in mice. In contrast, 9.4 T SMF treatment significantly reduced these side effects, especially for the depressive behaviors. Thus, our results demonstrate that 9.4 T SMF not only has anti-tumor effects on its own, but also could improve the anti-tumor effect of imatinib mesylate, reduce its toxicity and improve the mice mental health, which unraveled the great clinical potentials of high SMF in future applications.
9.4 T SMF inhibits GIST-T1 tumor growth and increases the efficacy of imatinib mesylate. Food (a) and water (b) consumption, the relative body weight (c) and tumor volume (d) were measured every 2 days. Tumor (e) and their weight (f) were measured at the end of the experiment. (g) HE and Ki67 staining of the tumor tissues. Scale bar: 50 μm. Data are presented as the mean ± SEM. For those that have statistical significance, we label them as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. [Reprinted with permission from (Tian et al. 2022)]
Therefore, it is clear that although in most cases, SMFs could increase the efficacy of chemodrugs, there are also some studies showed different results (Table 9.3). These differential effects could be caused by cell type, field intensity as well as drug differences, etc. Consequently, the strategy of combining SMFs of different intensities with various chemodrugs in different cancer cells also needs to be further investigated.
9.5.2 Static Magnetic Fields in Combination with Time-Varying Magnetic Fields
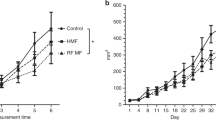
There are multiple studies showing that SMFs combined with time-varying magnetic fields could inhibit cancer cell growth (Tofani 2015) (Table 9.4). For example, Tofani et al. have made series progresses on the combination of SMF and 50 Hz time-varying MF. In 2001, Tofani et al. showed that 3 mT SMF combined with 50 Hz time varying MF could induce more apoptosis in cells compared to SMF or the 50 Hz time varying MF alone (Tofani et al. 2001). In addition, it was interesting that apoptosis only occurred in the two transformed cell lines (WiDr human colon adenocarcinoma and MCF-7 human breast adenocarcinoma) but not the nontransformed cell line (MRC-5 embryonal lung fibroblast). They also tested them in nude mice xenografted with WiDr cells and exposed them for 70 min/day, 5 days/week, to ≤5 mT SMF in combination with time-varying MF for 4 weeks and found that the tumor was significantly inhibited (up to 50%) (Tofani et al. 2001). In 2002, they further tested the effects of 5.5 mT SMF in combination with 50 Hz time varying MF and found that the survival time of nude mice with WiDr cells was increased by 31% when the mice was exposed to magnetic fields for 70 min/day for 4 weeks (Tofani et al. 2002). When the mice were exposed to the magnetic fields for 4 consecutive weeks, significant inhibition of tumor growth (40%) together with a decrement in tumor cell mitotic index and proliferative activity were observed. In addition, they also found a significant increase in apoptosis together with a reduction in immunoreactive p53 expression (Tofani et al. 2002). These works indicate that SMF + 50 Hz time-varying MF of above 3 mT may have anti-cancer potentials. In contrast, lower MF intensity, such as 1 mT SMF did not induce cell apoptosis as 3, 10 or 30 mT SMFs did (Tofani et al. 2001). Actually, their results could potentially explain why Bodega et al. did not observe any changes when they exposed cultured astroglial cells to a combined 1 mT SMF with sinusoidal 50 Hz time-varying MF for 11 days (Bodega et al. 2005), which might due to the low magnetic field strength.
To our knowledge, all reported studies used the combination of milli-Tesla SMFs (1–10 mT) with 50 Hz time-varying MF of similar MF intensity (Table 9.4). The combination effects of SMFs with higher magnetic field intensity and/or in combination with time-varying MFs of other frequencies besides 50 Hz have not been reported. Whether the currently reported cancer inhibition effects of milli-Tesla SMFs with 50 Hz time-varying MF can also be applied to other magnetic field parameters, such as different magnetic field intensity or frequency, is still unknown. In addition, since the three cell lines Tofani et al. tested showed different responses to the combinational treatment of SMF+ time-varying MF (increased apoptosis in two cancer cells lines WiDr and MCF-7 but not non-cancer cell line MRC-5), it is likely that the effects are also cell type-dependent. Whether other cancer cell types can also be inhibited by SMF+ time-varying MF still need more investigations.
9.5.3 Static Magnetic Fields in Combination with Radiotherapy
Radiation therapy (radiotherapy) is commonly used in cancer treatment. It uses high-energy radiation to kill cancer cells and reduce tumor size. Currently, the most commonly used types of radiation are X-rays. In some cases, gamma rays and charged particles are also used for cancer treatment. In recent years, image-guided radiotherapy (IGRT) has greatly improved the precision and accuracy of radiotherapy, which takes advantage of modern imaging techniques such as ultrasound, X-ray and CT (computed tomography) scan. The information provided by these imaging techniques before and during radiotherapy treatment not only shows the size, shape and position of the tumor itself, the surrounding tissues and bones, but also allows instant correction for positioning deviations and thereby improves the precision of daily radiotherapy fractions. Although CT scan is mostly used in current IGRT, MRI-guided radiotherapy is attracting increasing attention. It is well known that MRI gives superior soft tissue contrast and more importantly, MRI could offer the advantage of providing IGRT without delivering an additional radiation dose to the patients compared to CT or X-ray imaging. Currently, multiple groups are building or starting to test MRI-guided radiotherapy.
Along with the introduction of MRI-guided radiotherapy, the potential effects of SMFs on ionizing radiation have become increasingly important. However, the accompanied lab studies about the combinational effects of SMF and radiation is lacking. Although there are some evidences showing that the effects of ionizing radiation on cells could be strengthened by Time-varying MFs, such as 50 Hz magnetic fields (Francisco et al. 2013), the studies about SMFs in combination with radiotherapy are much less. So far there are only a few studies that have investigated the combinational effects of SMFs with ionizing radiation and most of these studies indicated that SMFs might be able to increase the effectiveness of radiotherapy (Table 9.5). For example, in 2002, Nakahara found that although 10 T SMF itself had no effect on CHO-K1 cell growth, cell cycle distribution, or micronucleus frequency, they could cause an increase in the micronucleus formation induced by 4 Gy X-rays (Nakahara et al. 2002). In 2010, Sarvestani et al. investigated the effects of a 15 mT SMF alone for 5 h or 0.5 Gy X-ray +15 mT SMF sequential exposures (first X-ray and then SMF for 5 h) on cell cycle progression in rat bone marrow stem cells (BMSC). They did not find any cell cycle changes in SMF alone treated cells but found that 15 mT SMF exposure could further increase the G2/M cell percentage induced by 0.5 Gy X-ray (Sarvestani et al. 2010). In 2014, Teodori et al. investigated the genotoxic effect of 80 mT SMF, both alone and in combination with X-ray irradiation, on primary glioblastoma cells. Their results showed that exposure of cells to 5 Gy of X-ray irradiation alone led to extensive DNA damage, which was significantly reduced by 80 mT SMF (Teodori et al. 2014). The DNA damage promotion effect of 10 T SMF in CHO-K1 cells (Nakahara et al. 2002) and the DNA damage reduction effect of 80 mT SMF in primary glioblastoma cells (Teodori et al. 2014) seem to be controversial. However, this difference could be due to the cell type or magnetic field intensity difference. In 2013, Politanski et al. investigated the combined effect of X-ray radiation and SMFs on ROS in lymphocytes from male albino Wistar rats. Their results indicated that 5 mT SMF increased the ROS changes induced by 3 Gy X-ray radiation while “0 mT” (50 μT magnetic field induction opposite to the geomagnetic field) always showed opposite effects compared to 5 mT SMF (Politanski et al. 2013). This indicated that different magnetic field intensity could directly influence its effect on radiation-induced effects. More researches are needed to get a complete understanding about different magnetic field intensities, especially around the range of MRI scanners, and their effects on radiation-induced effects on different cell types. Other types of radiation, such as gamma radiation, should also be investigated.
9.6 Patient Studies
It is interesting and promising that time-varying electromagnetic fields have been shown to be effective in multiple studies at the patient level and were introduced as a novel cancer treatment modality. The most famous example was the tumor treating fields (TTF, or TTFields) therapy, which delivers low-intensity, intermediate-frequency (100–300 kHz), alternating electric fields that cause apoptosis or cell death by inducing mitotic catastrophe and can effectively inhibit the growth of a variety of human and rodent tumor cell lines, with no significant damage to normal non-dividing cells (Kirson et al. 2004; Pless and Weinberg 2011; Davies et al. 2013). In addition, Barbault et al. examined patients with various types of cancer using a noninvasive biofeedback method to identify “tumor-specific frequencies” (Barbault et al. 2009). They implied that cancer-related frequencies appeared to be tumor-specific and treatment with tumor-specific frequencies was feasible, well tolerated and may have biological efficacy in patients with advanced cancer (Barbault et al. 2009). Recently, Kim et al. used TTF to study the metastatic potential of U87 and U373 glioblastoma cell lines and found that TTF affected NF-κB, MAPK and PI3K/AKT signalling pathways as well as downregulated VEGF, HIF1α and matrix metalloproteinases 2 and 9, which indicated that TTF could be a promising novel anti-invasion and anti-angiogenesis therapeutic strategy for glioblastoma patients (Kim et al. 2016). More importantly, studies reported that treating recurrent glioblastoma patients with TTF improved overall survival (OS) and there was no unexpected adverse effects (De Bonis et al. 2012; Rulseh et al. 2012). Due to these clinical outcomes, TTF was approved by the FDA as an alternative to the standard treatment for patients with recurrent and newly diagnosed glioblastoma.
In contrast, although a large number of in vitro and in vivo studies indicated the anticancer potentials of SMFs, there is only a very small amount of data concerning their application in clinical cancer treatment so far. In 2003, Salvatore et al. found that there was no increase in the severity of chemotherapy toxicity as measured by white blood cell count and platelet count in the participants exposed to SMF (Salvatore et al. 2003). In 2004, Ronchetto et al. examined 11 patients with “heavily pretreated” advanced cancer in a pilot study with different SMF exposure and found that the magnetic fields can be safely administrated according to their exposure schedules (Ronchetto et al. 2004). Although these studies indicated the safety of SMFs at patient level, the effectiveness of these SMFs on cancer inhibition is still lacking, which still needs to be proved. In fact, there are some clinical studies reported in some Chinese journals about the successful application of SMFs on some cancer treatments, which have been reviewed by Dr. Zhou, although also written in Chinese (Zhou 2000). In these studies, it seems that applying permanent magnets either alone or in combination with time-varying MF or radiotherapy could have positive effects in cancer inhibition, and the effects are correlated with the magnetic field intensities. More specifically, it was shown that the SMF of 0.2 T and above had anti-cancer effects but SMFs below 0.1 T did not. To my point of view, although these studies do not really meet the criteria of scientific investigations, they appear promising. However, more double blinded, well controlled clinical investigations are needed to confirm their claims.
In the meantime, it is interesting and promising that there are also some positive findings for magnetic devices that use permanent magnets, but spin them at low speed, called extremely low-frequency magnetic fields (Wang et al. 2011; Sun et al. 2012a; Nie et al. 2013a, b). For example, in 2012, Sun et al. investigated the effects of 420 r/min, 0.4 T magnetic fields on the survival and palliation of general symptoms in 13 advanced non-small cell lung cancer (NSCLC) patients (Sun et al. 2012a). The patients were treated for 2 h/day, 5 days/week for 6–10 weeks. While the median survival of the advanced NSCLC patients receiving supportive care was 4 months, their “spinning magnetic device” could prolong the median survival to 6 months, which was increased by 50%. Although 6 months median survival was still shorter than that of patients receiving chemotherapy (Cisplatin, 9.1 months; Carboplatin, 8.4 months), the magnetic field-treated patients had no severe toxicity or side-effects. More importantly, the 1-year survival rate was 31.7%, which was much higher than patients only receiving supportive care (15%) and comparable to patients receiving chemotherapy (Cisplatin, 37%; Carboplatin, 34%). In the meantime, the magnetic fields treated patients had improved physical conditions and alleviated symptoms in general (Sun et al. 2012a). In fact, the effect of this type of machine has also been proved to be effective on advanced cancer patients (Yang et al. 2018) as well as in cancer cells and mice models (Wang et al. 2011; Nie et al. 2013a, b). Meanwhile, there are also other unofficial reports claiming that spinning magnets could be used as alternative treatments for patients. Therefore, it is a promising field to explore but apparently these reported studies are still at a very preliminary stage. In fact, an important criticism of these human case reports is the lack of control subjects. Therefore, more rigorous, well controlled and double-blinded clinical trials are strongly needed to prove the effectiveness of SMFs in cancer treatment. The magnetic field parameters, such as the field strength, fixed or spinning, exposure schedule and cancer types should all be tested.
9.7 Discussion
The mechanisms of the differential responses of cancer vs. non-cancer cells to SMFs still remain partially understood. However, SMF-induced microtubule interference is a broad impact on most dividing cells. Moreover, cancer and non-cancer cells have been shown to respond differentially to cell cycle perturbations. For example, it has been reported that the human non-transformed cells and cancer cells have significant survival difference in response to the microtubule drugs treatment (Brito and Rieder 2009). Brito and Rieder found that both nocodazole and Taxol, two microtubule poisons, could kill much more HeLa and U2OS cancer cells than the non-cancer RPE1 cells. Specifically, 5 nM of Taxol, which is approximately the clinical concentration for chemotherapy, could kill 93% of HeLa cells and 46% of U2OS cells but only killed 1% of RPE1 cells (Brito and Rieder 2009). In addition, different types of cancer cells also have differential responses to microtubule drugs (Tang et al. 2013). Moreover, the depletion of plk1 (polo-like kinase), which is a vital regulator in multiple cellular processes, especially in cell cycle progression, caused significant cell proliferation and cell cycle abnormalities in human cervical cancer HeLa cells, but not the non-cancer RPE1 or MCF10A breast cells (Liu et al. 2006). Therefore, targeting microtubules or cell cycle could generate different effects on cancer vs. non-cancer cells or in different types of cancer cells.
Meanwhile, we should keep in mind that although EGFR and cell division are important, they are definitely not the only reasons that can explain the differences between SMF-induced differential effects among various cell types. Other factors are also likely involved. For example, Short et al. showed that 4.7 T SMF could alter the ability of human malignant melanoma cells attachment onto the tissue culture plate, but had no effect on normal human fibroblasts (Short et al. 1992), which indicated that the cell attachment was differentially affected by SMF in cancer vs. non-cancer cells. Moreover, other aspects should also be carefully investigated, such as cell metabolism, mitochondria functions, ROS (reactive oxygen species) responses and ATP level, which could all be affected differentially in cancer vs. normal cells. Our group is currently working on these topics and we expect to have a much better understanding on this issue in the near future.
9.8 Conclusion
Cancer is a heterogeneous disease and its complexity has hindered the development of effective and safe treatments. The studies listed in this chapter greatly helped us to understand some of the mechanisms that SMFs affect cancer cells and their potential applications in cancer treatment in the future. We only discussed about membrane receptor EGFR, cell division and microcirculation here, but it is likely that other aspects are also involved in SMF-induced cancer inhibition, such as ion channels, ROS, the immune system as well as metabolism. Moreover, current cellular studies and animal models of SMF effects on cancers are variable in reproducibility, and further systematic studies of different treatment parameters would be definitely beneficial. In the meantime, while some mechanisms of action have been proposed, their substantiation is needed. Although more research should be conducted to demonstrate its safety and efficacy, current experimental results indicate that SMF is relatively safe. Understanding and exploiting the potential application of SMFs would be an essential aspect of adjuvant therapies targeting conventional treatment-resistant tumor in the future.
References
Aldinucci C, Garcia JB, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP (2003a) The effect of exposure to high flux density static and pulsed magnetic fields on lymphocyte function. Bioelectromagnetics 24(6):373–379
Aldinucci C, Garcia JB, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP (2003b) The effect of strong static magnetic field on lymphocytes. Bioelectromagnetics 24(2):109–117
Barbault A, Costa FP, Bottger B, Munden RF, Bomholt F, Kuster N, Pasche B (2009) Amplitude-modulated electromagnetic fields for the treatment of cancer: discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J Exp Clin Cancer Res 28:51
Bodega G, Forcada I, Suarez I, Fernandez B (2005) Acute and chronic effects of exposure to a 1-mT magnetic field on the cytoskeleton, stress proteins, and proliferation of astroglial cells in culture. Environ Res 98(3):355–362
Brito DA, Rieder CL (2009) The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells. Cell Motil Cytoskeleton 66(8):437–447
Chen WF, Qi H, Sun RG, Liu Y, Zhang K, Liu JQ (2010) Static magnetic fields enhanced the potency of cisplatin on K562 cells. Cancer Biother Radiopharm 25(4):401–408
Davies AM, Weinberg U, Palti Y (2013) Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci 1291:86–95
De Bonis P, Doglietto F, Anile C, Pompucci A, Mangiola A (2012) Electric fields for the treatment of glioblastoma. Expert Rev Neurother 12(10):1181–1184
de Visser KE, Eichten A, Coussens LM (2006) Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6(1):24–37
Fan Z, Hu P, Xiang L, Liu Y, He R, Lu T (2020) A static magnetic field inhibits the migration and telomerase function of mouse breast cancer cells. Biomed Res Int 2020:7472618
Fanelli C, Coppola S, Barone R, Colussi C, Gualandi G, Volpe P, Ghibelli L (1999) Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J 13(1):95–102
Francisco AC, del Mar SAM, Irene C, Sandra RA, Josefa L, Elisa RM, Nicolas O, Isabel NM (2013) Could radiotherapy effectiveness be enhanced by electromagnetic field treatment? Int J Mol Sci 14(7):14974–14995
Gellrich D, Becker S, Strieth S (2014) Static magnetic fields increase tumor microvessel leakiness and improve antitumoral efficacy in combination with paclitaxel. Cancer Lett 343(1):107–114
Ghibelli L, Cerella C, Cordisco S, Clavarino G, Marazzi S, De Nicola M, Nuccitelli S, D'Alessio M, Magrini A, Bergamaschi A, Guerrisi V, Porfiri LM (2006) NMR exposure sensitizes tumor cells to apoptosis. Apoptosis 11(3):359–365
Gray JR, Frith CH, Parker JD (2000) In vivo enhancement of chemotherapy with static electric or magnetic fields. Bioelectromagnetics 21(8):575–583
Hao Q, Wenfang C, Xia A, Qiang W, Ying L, Kun Z, Runguang S (2011) Effects of a moderate-intensity static magnetic field and adriamycin on K562 cells. Bioelectromagnetics 32(3):191–199
Ho SY, Chen IC, Chen YJ, Lee CH, Fu CM, Liu FC, Liou HH (2019) Static magnetic field induced neural stem/progenitor cell early differentiation and promotes maturation. Stem Cells Int 2019:8790176
Jia C, Zhou Z, Liu R, Chen S, Xia R (2007) EGF receptor clustering is induced by a 0.4 mT power frequency magnetic field and blocked by the EGF receptor tyrosine kinase inhibitor pd153035. Bioelectromagnetics 28(3):197–207
Kim EH, Song HS, Yoo SH, Yoon M (2016) Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget 7(40):65125–65136
Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y (2004) Disruption of cancer cell replication by alternating electric fields. Cancer Res 64(9):3288–3295
Kubinyi G, Zeitler Z, Thuroczy G, Juhasz P, Bakos J, Sinay H, Laszlo J (2010) Effects of homogeneous and inhomogeneous static magnetic fields combined with gamma radiation on DNA and DNA repair. Bioelectromagnetics 31(6):488–494
Lin SL, Su YT, Feng SW, Chang WJ, Fan KH, Huang HM (2019) Enhancement of natural killer cell cytotoxicity by using static magnetic field to increase their viability. Electromagn Biol Med 38(2):131–142
Liu X, Lei M, Erikson RL (2006) Normal cells, but not cancer cells, survive severe PLK1 depletion. Mol Cell Biol 26(6):2093–2108
Liu Y, Qi H, Sun RG, Chen WF (2011) An investigation into the combined effect of static magnetic fields and different anticancer drugs on K562 cell membranes. Tumori 97(3):386–392
Luo Y, Ji XM, Liu JJ, Li ZY, Wang WC, Chen W, Wang JF, Liu QS, Zhang X (2016) Moderate intensity static magnetic fields affect mitotic spindles and increase the antitumor efficacy of 5-FU and Taxol. Bioelectrochemistry 109:31–40
Marycz K, Kornicka K, Rocken M (2018) Static magnetic field (SMF) as a regulator of stem cell fate—new perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev Rep 14(6):785–792
Nakahara T, Yaguchi H, Yoshida M, Miyakoshi J (2002) Effects of exposure of Cho-K1 cells to a 10-T static magnetic field. Radiology 224(3):817–822
Nie Y, Chen Y, Mou Y, Weng L, Xu Z, Du Y, Wang W, Hou Y, Wang T (2013a) Low frequency magnetic fields enhance antitumor immune response against mouse h22 hepatocellular carcinoma. PLoS One 8(11):e72411
Nie Y, Du L, Mou Y, Xu Z, Weng L, Du Y, Zhu Y, Hou Y, Wang T (2013b) Effect of low frequency magnetic fields on melanoma: tumor inhibition and immune modulation. BMC Cancer 13:582
Pless M, Weinberg U (2011) Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs 20(8):1099–1106
Politanski P, Rajkowska E, Brodecki M, Bednarek A, Zmyslony M (2013) Combined effect of X-ray radiation and static magnetic fields on reactive oxygen species in rat lymphocytes in vitro. Bioelectromagnetics 34(4):333–336
Raylman RR, Clavo AC, Wahl RL (1996) Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics 17(5):358–363
Reiche EM, Nunes SO, Morimoto HK (2004) Stress, depression, the immune system, and cancer. Lancet Oncol 5(10):617–625
Ronchetto F, Barone D, Cintorino M, Berardelli M, Lissolo S, Orlassino R, Ossola P, Tofani S (2004) Extremely low frequency-modulated static magnetic fields to treat cancer: a pilot study on patients with advanced neoplasm to assess safety and acute toxicity. Bioelectromagnetics 25(8):563–571
Rulseh AM, Keller J, Klener J, Sroubek J, Dbaly V, Syrucek M, Tovarys F, Vymazal J (2012) Long-term survival of patients suffering from glioblastoma multiforme treated with tumor-treating fields. World J Surg Oncol 10:220
Sabo J, Mirossay L, Horovcak L, Sarissky M, Mirossay A, Mojzis J (2002) Effects of static magnetic field on human leukemic cell line HL-60. Bioelectrochemistry 56(1–2):227–231
Sadri M, Abdolmaleki P, Abrun S, Beiki B, Samani FS (2017) Static magnetic field effect on cell alignment, growth, and differentiation in human cord-derived mesenchymal stem cells. Cell Mol Bioeng 10(3):249–262
Salvatore JR, Harrington J, Kummet T (2003) Phase I clinical study of a static magnetic field combined with anti-neoplastic chemotherapy in the treatment of human malignancy: initial safety and toxicity data. Bioelectromagnetics 24(7):524–527
Sarvestani AS, Abdolmaleki P, Mowla SJ, Ghanati F, Heshmati E, Tavasoli Z, Jahromi AM (2010) Static magnetic fields aggravate the effects of ionizing radiation on cell cycle progression in bone marrow stem cells. Micron 41(2):101–104
Short WO, Goodwill L, Taylor CW, Job C, Arthur ME, Cress AE (1992) Alteration of human tumor cell adhesion by high-strength static magnetic fields. Invest Radiol 27(10):836–840
Song C, Yu B, Wang J, Ji X, Zhang L, Tian X, Yu X, Feng C, Wang X, Zhang X (2021) Moderate static magnet fields suppress ovarian cancer metastasis via ROS-mediated oxidative stress. Oxid Med Cell Longev 2021:7103345
Strelczyk D, Eichhorn ME, Luedemann S, Brix G, Dellian M, Berghaus A, Strieth S (2009) Static magnetic fields impair angiogenesis and growth of solid tumors in vivo. Cancer Biol Ther 8(18):1756–1762
Strieth S, Strelczyk D, Eichhorn ME, Dellian M, Luedemann S, Griebel J, Bellemann M, Berghaus A, Brix G (2008) Static magnetic fields induce blood flow decrease and platelet adherence in tumor microvessels. Cancer Biol Ther 7(6):814–819
Sullivan K, Balin AK, Allen RG (2011) Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 32(2):140–147
Sun W, Gan Y, Fu Y, Lu D, Chiang H (2008) An incoherent magnetic field inhibited EGF receptor clustering and phosphorylation induced by a 50-Hz magnetic field in cultured FL cells. Cell Physiol Biochem 22(5–6):507–514
Sun CT, Yu HM, Wang XW, Han JQ (2012a) A pilot study of extremely low-frequency magnetic fields in advanced non-small cell lung cancer: effects on survival and palliation of general symptoms. Oncol Lett 4(5):1130–1134
Sun RG, Chen WF, Qi H, Zhang K, Bu T, Liu Y, Wang SR (2012b) Biologic effects of SMF and paclitaxel on K562 human leukemia cells. Gen Physiol Biophys 31(1):1–10
Sun W, Shen X, Lu D, Lu D, Chiang H (2013) Superposition of an incoherent magnetic field inhibited EGF receptor clustering and phosphorylation induced by a 1.8 GHz pulse-modulated radiofrequency radiation. Int J Radiat Biol 89(5):378–383
Tang Y, Xie T, Florian S, Moerke N, Shamu C, Benes C, Mitchison TJ (2013) Differential determinants of cancer cell insensitivity to antimitotic drugs discriminated by a one-step cell imaging assay. J Biomol Screen 18(9):1062–1071
Tatarov I, Panda A, Petkov D, Kolappaswamy K, Thompson K, Kavirayani A, Lipsky MM, Elson E, Davis CC, Martin SS, DeTolla LJ (2011) Effect of magnetic fields on tumor growth and viability. Comp Med 61(4):339–345
Tenuzzo B, Chionna A, Panzarini E, Lanubile R, Tarantino P, Di Jeso B, Dwikat M, Dini L (2006) Biological effects of 6 mT static magnetic fields: a comparative study in different cell types. Bioelectromagnetics 27(7):560–577
Teodori L, Giovanetti A, Albertini MC, Rocchi M, Perniconi B, Valente MG, Coletti D (2014) Static magnetic fields modulate X-ray-induced DNA damage in human glioblastoma primary cells. J Radiat Res 55(2):218–227
Tian X, Wang D, Zha M, Yang X, Ji X, Zhang L, Zhang X (2018) Magnetic field direction differentially impacts the growth of different cell types. Electromagn Biol Med 37(2):114–125
Tian X, Wang C, Yu B, Fan Y, Zhang L, Zhang X (2022) 9.4 T static magnetic field ameliorates imatinib mesylate-induced toxicity and depression in mice. Eur J Nucl Med Mol Imaging 2022:05976
Tofani S (2015) Electromagnetic energy as a bridge between atomic and cellular levels in the genetics approach to cancer treatment. Curr Top Med Chem 15(6):572–578
Tofani S, Barone D, Cintorino M, de Santi MM, Ferrara A, Orlassino R, Ossola P, Peroglio F, Rolfo K, Ronchetto F (2001) Static and elf magnetic fields induce tumor growth inhibition and apoptosis. Bioelectromagnetics 22(6):419–428
Tofani S, Cintorino M, Barone D, Berardelli M, De Santi MM, Ferrara A, Orlassino R, Ossola P, Rolfo K, Ronchetto F, Tripodi SA, Tosi P (2002) Increased mouse survival, tumor growth inhibition and decreased immunoreactive p53 after exposure to magnetic fields. Bioelectromagnetics 23(3):230–238
Tofani S, Barone D, Berardelli M, Berno E, Cintorino M, Foglia L, Ossola P, Ronchetto F, Toso E, Eandi M (2003) Static and elf magnetic fields enhance the in vivo anti-tumor efficacy of cis-platin against Lewis lung carcinoma, but not of cyclophosphamide against B16 melanotic melanoma. Pharmacol Res 48(1):83–90
Vergallo C, Ahmadi M, Mobasheri H, Dini L (2014) Impact of inhomogeneous static magnetic field (31.7–232.0 mT) exposure on human neuroblastoma SH-SY5Y cells during cisplatin administration. PLoS One 9(11):e113530
Wang H, Zhang X (2019) ROS reduction does not decrease the anticancer efficacy of X-ray in two breast cancer cell lines. Oxid Med Cell Longev 2019:3782074
Wang Z, Yang P, Xu H, Qian A, Hu L, Shang P (2009) Inhibitory effects of a gradient static magnetic field on normal angiogenesis. Bioelectromagnetics 30(6):446–453
Wang T, Nie Y, Zhao S, Han Y, Du Y, Hou Y (2011) Involvement of midkine expression in the inhibitory effects of low-frequency magnetic fields on cancer cells. Bioelectromagnetics 32(6):443–452
Wang L, Hoogcarspel SJ, Wen Z, van Vulpen M, Molkentine DP, Kok J, Lin SH, Broekhuizen R, Ang KK, Bovenschen N, Raaymakers BW, Frank SJ (2016) Biological responses of human solid tumor cells to X-ray irradiation within a 1.5-Tesla magnetic field generated by a magnetic resonance imaging-linear accelerator. Bioelectromagnetics 37(7):471–480
Yang J, Yu M, Guo Z, Zhang X (2018) Moderate intensity rotating low frequency magnetic fields and their effects on human bodies. In: Zhang X, Junfeng W (eds) Interdisciplinary research of magnetic fields and life sciences. Science Press, Beijing, pp 210–223
Yang X, Li Z, Polyakova T, Dejneka A, Zablotskii V, Zhang X (2020) Effect of static magnetic field on DNA synthesis: the interplay between DNA chirality and magnetic field left-right asymmetry. FASEB Bioadv 2(4):254–263
Yang X, Song C, Zhang L, Wang J, Yu X, Yu B, Zablotskii V, Zhang X (2021) An upward 9.4 T static magnetic field inhibits DNA synthesis and increases ROS-p53 to suppress lung cancer growth. Transl Oncol 14(7):101103
Yuan LQ, Wang C, Zhu K, Li HM, Gu WZ, Zhou DM, Lai JQ, Zhou D, Lv Y, Tofani S, Chen X (2018) The antitumor effect of static and extremely low frequency magnetic fields against nephroblastoma and neuroblastoma. Bioelectromagnetics 39(5):375–385
Yudhistiara B, Zwicker F, Weber KJ, Huber PE, Ruehle A, Brons S, Haering P, Debus J, Hauswald SH (2019) The influence of a magnetic field on photon beam radiotherapy in a normal human TK6 lymphoblastoid cell line. Radiat Oncol 14(1):11
Zafari J, Javani Jouni F, Abdolmaleki P, Jalali A, Khodayar MJ (2015) Investigation on the effect of static magnetic field up to 30 mT on viability percent, proliferation rate and IC50 of hela and fibroblast cells. Electromagn Biol Med 34(3):216–220
Zhang L, Yang XX, Liu JJ, Luo Y, Li ZY, Ji XM, Wang WC, Zhang X (2015) 1 T moderate intensity static magnetic field affects Akt/mTOR pathway and increases the antitumor efficacy of mTOR inhibitors in CNE-2Z cells. Sci Bull 60(24):2120–2128
Zhang L, Wang J, Wang H, Wang W, Li Z, Liu J, Yang X, Ji X, Luo Y, Hu C, Hou Y, He Q, Fang J, Wang J, Liu Q, Li G, Lu Q, Zhang X (2016) Moderate and strong static magnetic fields directly affect EGFR kinase domain orientation to inhibit cancer cell proliferation. Oncotarget 7(27):41527–41539
Zhang L, Hou Y, Li Z, Ji X, Wang Z, Wang H, Tian X, Yu F, Yang Z, Pi L, Mitchison TJ, Lu Q, Zhang X (2017a) 27 T ultra-high static magnetic field changes orientation and morphology of mitotic spindles in human cells. Elife 6:e22911
Zhang L, Ji X, Yang X, Zhang X (2017b) Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 8(8):13126–13141
Zhao B, Yu T, Wang S, Che J, Zhou L, Shang P (2021) Static magnetic field (0.2–0.4 T) stimulates the self-renewal ability of osteosarcoma stem cells through autophagic degradation of ferritin. Bioelectromagnetics 42(5):371–383
Zhou W (2000) Application and review of magnetic field treatment for cancer. J Magn Mater Devices 31(4):32–34
Zhu X, Liu Y, Cao X, Liu H, Sun A, Shen H, Zhao J, Li R, Wu L, Fang Z, Wang H, Zhai Q (2020) Moderate static magnetic fields enhance antitumor CD8+ T cell function by promoting mitochondrial respiration. Sci Rep 10(1):14519
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhang, X. (2023). Potential Applications of Static Magnetic Fields in Cancer Treatment. In: Zhang, X. (eds) Biological Effects of Static Magnetic Fields. Springer, Singapore. https://doi.org/10.1007/978-981-19-8869-1_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-8869-1_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8868-4
Online ISBN: 978-981-19-8869-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)