Abstract
Rauwolfia serpentina is a valuable medicinal plant belonging to Apocynaceae family. The plant is rich with various phytochemicals particularly indole alkaloids like reserpine. Root extracts of plant have been used from centuries for the ailment of neurological disorders. Successful clinical studies have unravelled the properties like antihypertensive, antidiabetic nature, etc. However, the plant which was available widely in southern western ghats of India is now under threat of extinction. Unrestrained human exploitation of medicinal plants in anthropocene epoch has led to the reduction of plants like R.serpentina. An elaborate literature survey of phytochemicals and the so far proven medicinal properties of R. serpentina was performed. Advent of in vitro propagational strategies and the accomplishment of Rhizogenes-induced roots were also presented as strategies to conserve the plant in vitro. Moreover, the other biotechnological approaches used to raise the production of secondary metabolites as well for conservation were discoursed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Medicinal plants are gaining attention of pharmaceutical industry at global level because of their immense medicinal value and reliability. As a matter of fact, these are the driving factors causing the extensive exploitation and endangering of these treasures. Rauwolfia serpentina (Linn.) Benth. Ex Kurz in Apocynaceae family is one such plant which was exploited indiscriminately due to their unparalleled effects on nervous system. Some other plants belonging to this genus are R. tetraphylla, R. vomitoria, R. hookeri, R. micrantha, and R. verticillate. Rauwolfia serpentina was named in honour of Leonard Rauwolf, a German physician (Goenka 2007). The plant is popular in Sanskrit name Sarpagandha (Snake root) which was first mentioned in historic works of Charak (1000–800 bc). The medicinal properties were also deliberated in other Ayurvedic literatures of Sushruta, Vrindamadha, and Bhavprakasha (Chauhan et al. 2017a).There are a lot of controversies regarding this naming. But the most accepted argument behind the naming is the antivenom property of this plant. Moreover, some assumptions are regarding the resemblance of roots with snake (sarpa). Another unlikely belief is that snakes run away because of the smell of the plant. Some other vernacular names are Chandra, Naakooli, Amalapori, Patalagaruda, etc. The plant is widely observed and cultivated in India, China, Japan, Sri Lanka, and Bangladesh. Appreciable vegetative growth is observed in acidic sandy soil and at an annual rainfall of 200–250 cm. It is found to be indigenous in Himalayas, Punjab, Assam, Meghalaya, Uttarakhand, Sikkim, Andaman, and Western Ghats (Agrawal 2019; Bhanwaria et al. 2021; Dey and De 2010).
This tropical shrub is 6 in. to 2 ft. long and their thick, lengthy roots possess a characteristic lens-shaped structure. These greyish brown clustered roots with uneven cervices generally penetrate deep into the soil. Leaves in whorls are ecliptic or lanceolate. Another unique feature is the clump of pink as well as white flowers. The fleshy, tiny drupe type green fruits become black purple on maturing. The flowering period is usually between November and December (Chauhan et al. 2017a; Agrawal 2019).
The therapeutic significance of plant was dated centuries ago. Advent of use of dried root powder as ‘Pagal ki dawa’ for treatment of mentally violent patients was a milestone. The beginning of twentieth century witnessed several attempts to determine phytochemical constituents of the plant (Chatterjee 1953; Monachino 1954). A revolutionary step in the saga of Rauwolfia serpentina was put forward by Rustom Jal Vakil. This renowned scientist in cardiology carried out tremendous clinical trials for studying the antihypertensive property of dried root powder of the plant. His unparalleled efforts led to the worldwide recognition of the plant as an antihypertensive drug and he was later entitled as ‘Father of Rauwolfia serpentina’ (Goenka 2007). Unfortunately, the overexploitation of R. serpentina had led to a considerable reduction in its overall number and was later categorized as an endangered plant by International Union for the Conservation of Nature and Natural Resources (IUCN) and also in CITES (Convention on International Trade in Endangered Species) (Mehrotra et al. 2015). R. serpentina is a victim of unconstrained human exploitation during the anthropocene epoch, characterized by escalating anthropogenic activities amidst climate anomalies, aggravating climate change impact on the environment, biodiversity, and society as a whole. Tremendous and swift measures are necessary for the safeguarding of these plants.
This chapter emphasizes on the significance of conservation of Rauwolfia serpentina through highlighting renown pharmacological properties and its phytochemicals rendering those activities. Besides, various in vitro propagation and biotechnological approaches utilized in the R. serpentina research have also been discussed.
2 Phytochemicals
Phytochemicals are naturally available chemical compounds which attribute beneficial properties to plants such as defence against pathogen attack and abiotic stresses. Interestingly, these phytochemicals also have therapeutic properties like antimicrobial, anticarcinogenic activity, etc. (Njerua et al. 2013). An array of diverse extraction techniques have been employed for qualitative and quantitative phytochemical profiling of extracts of R. serpentina (Hussain et al. 2015; Kumar et al. 2016b; Namita and Yadav 2016; Das et al. 2019). The concentration of phytochemicals estimated via various detection strategies is depicted in Table 4.1.
2.1 Alkaloids
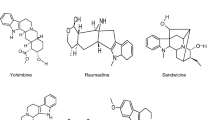
Rauwolfia serpentina is rich with monoterpenoid indole alkaloids which are the key factors that attribute the antihypertensive properties (Geissler et al. 2016). For instance, reserpine, serpentine, reserpiline, ajmalicine, ajmaline, ajmalimine, aricine, rescinnamidine, rescinnamine, deserpidine, corynanthine, isoreserpiline, isoreserpine, indobinine, indobine, yohimbine, isorauhimbinic acid, yohimbinic acid, 3 hydroxysarpagine, N(b)-methylisoajmaline, and N(b)-methylajmaline. Alkaloids are basically classified as reserpine-like and yohimbine-like. They considerably vary in their chemical structure (Fig. 4.1). The alkaloid profiling of different tissue extracts of wild plant, cultivated plant, and in vitro regenerated plant has been performed (Panwar et al. 2011). Furthermore, they have been successfully isolated and crude forms were used for various experimental studies (Sagi et al. 2016; Singh et al. 2017b). On the other hand, large bunches of root are necessary for the isolation of minor alkaloids (Falkenhagen et al. 1993). Ammoniacal chloroform was elucidated to be the best solvent for alkaloid extraction (Bindu et al. 2014). The techniques employed for the alkaloid examination were thin layer chromatography (Le Xuan et al. 1980), High-performance liquid chromatography (Wachsmuth and Matusch 2002), High-performance thin layer chromatography (Klyushnichenko et al. 1995; Panwar and Guru 2011), spectrophotometry (Singh et al. 2004), and GC-MS (Hong et al. 2013; Hussain et al. 2015).The advance techniques with more productivity like liquid chromatography with quadrupole time-of-flight mass spectrometry (Kumar et al. 2016a), UHPLC with Hybrid Triple Quadrupole Linear Ion Trap Mass Spectrometry (Kumar et al. 2016b), UPHLC-photo diode array-mass spectrometry (Sagi et al. 2016), and high-performance liquid chromatography coupled with electrospray ionization quadrapole time of flight tandem mass spectrometry (HPLC–ESI-QToF-MS/MS) (Bindu et al. 2014) were also later employed. The pharmacological properties of these alkaloids, particularly remedies on mental discomforts, have been established in the mid-1900s itself (Banerjee and Lewis 1954). Even though the concentration of alkaloids varies with plant parts, the roots are considered to be most therapeutically significant part of the plant (Lucas 1963). Later, studies were conducted to evaluate the dependency of alkaloid content on geographical conditions. Large variations were attained underlining the necessity of genetic breeding programmes for developing high yield variety (Usmani et al. 2015).
2.1.1 Reserpine
Reserpine is considered as the most potent and therapeutically efficient indole alkaloid derived from the root extracts of R. serpentina. Chemically, the compound is 11, 17 α-Dimethoxy-18 β-[(3,4,5-Trimethoxybenzoyl) Oxy]-3 β, 20 α-yohimban-16 β-carboxylic acid methyl ester (C33H40N2O9) (Singh et al. 2017b). It is whitish yellow powder, insoluble in water. The compound was extracted and used by Robert Wallace Wiggins (Lobay 2015). The bioavailability of the compound ranges from 50 to 70%. The ingested reserpine distributed throughout the body even in breast milk. Tissue-specific biosynthesis of reserpine in roots was spotted in many investigations (Panwar et al. 2011). In addition to this, reserpine has been successfully isolated and quantified (Negi et al. 2014). The mass production of reserpine from the plant at industrial scale later began (Zafar et al. 2020a).
Reserpine is now a commercially valuable pharmaceutical drug approved by FDA in 1953 for hypertensive patients (McQueen et al. 1954). Reserpine can act on sympathetic-parasympathetic equilibrium of central nervous system and evokes a sedative sensation. Along with this, some studies pointed out the effect of reserpine on level of glycogen, acetylcholine, and anti-diuretic hormones (Goel et al. 2009). Interestingly, it drastically reduced the blood pressure and exhibited vasodilating effect on comparison with synthetic drugs. The action on blood vessels was found to be persistent (Mcqueen and Blackman 1955; Shamon and Perez 2016). It was also perceived that frequent administration of minimum doses of reserpine induced variations in motor signals in pharmacological models of Parkinson’s disease (Fernandes et al. 2012). Moreover, apoptosis inducing ability of reserpine has been noted in many studies. It inhibited DNA synthesis as well as destabilized the mitochondrial membrane potential. Recently, it had been proven that reserpine is an efficient therapeutic agent in cancer treatment. This alkaloid supressed cell proliferation and DNA repair and even induced apoptosis by regulating TGF-β signalling pathway (Ramu et al. 2020). However, certain assumptions circulated regarding the chances of genetic mutation on regular administration of reserpine. But in early 1990s itself, these controversies were proved to be wrong (von Poser et al. 1990). Various drug formulations with distinct trade names like Regroton, Demi-Regroton, Salutensin, Hydroserpine, and Hydropres-50 are now available in market (Singh et al. 2017b).
2.1.2 Rescinnamine
Rescinnamine (C35H42N2O9), the 3,4,5-trimethoxycinnamic acid ester of methyl reserpate, is another bioactive compound with antihypertensive as well as cytotoxic property (AbdelHafez et al. 2013). It was reported that reserpine could easily dissolve and exert vasodilating effect on blood vessels (Mcqueen and Blackman 1955). The underlying factor behind this result was the ability of rescinnamine to block the conversion of angiotensin I to angiotensin II. Further studies proved that it also reduced aldosterone secretion. The earlier studies evidenced the hypotensive, bradycardic, and depressive nature of this compound (Klohs et al. 1954). Rescinnamine, its similar compounds, and the synthetic production procedure were officially patented in 1975. Commercially, now it is available in the name ‘Tsuruselpi S’ (Singh et al. 2017b).
2.1.3 Ajmaline
Ajmaline is another therapeutically consumed indole alkaloid for antiarrhythmic effect. Ajmaline (C20H26N2O2) is chemically a monoterpenoid alkaloid with ajmalan backbone in which hydroxyl groups are substituted at 17 and 21 positions. This alkaloid was isolated in 1931 and named ajmaline in honour of Hakim Ajmal, a legendary person in Unani medical system (Agrawal 2019).
Ajmaline is a class I antiarrhythmic agent which can alter the electrocardiogram characteristics (Roten et al. 2012). It decreases J wave and extends P-Q interval, Q-T interval, QRS complex, and R wave. It is a potent sodium channel blocker and employed in the diagnosis of Brugada syndrome and its subtypes (Rolf et al. 2003). Moreover, it is also administrated in the treatment of tolerated monomorphic ventricular tachycardias and atrial fibrillation cases in Wolff-Parkinson-White syndrome (Singh et al. 2017b). Cardenolide and bufadienolide are two important synthetic derivatives of ajmaline with antiarrhythmic and cardiotonic property. These compounds as well as the protocol for production was patented in 1975 (Makarevich et al. 1979). Now it is commercially available in brand names like Ajmalinum, Gilurytmal, Ajmalina, etc. (Singh et al. 2017b).
2.1.4 Yohumbine
Yohumbine (C21H26N2O3), the α-adrenergic blocker, is widely used in erectile dysfunction disorders as it could increase blood flow towards penis (Ernst and Pittler 1998). It specifically blocks presynaptic neuron and is experimentally validated in rabbits (Starke et al. 1975). It was reported that administration of yohumbine stimulated the fight or flight responses like anxiety, shivering, palpitations, hot, cold flashes, pupil dilation, etc. in experimental subjects (Charney et al. 1984). Yohumbine is also often considered as a sexual desire-inducing agent. It is also well-known for its hypnotic activity (Mehrotra et al. 2015).
2.1.5 Ajmalicine
Ajmalicine (C21H24N2O) is an indoline alkaloid extracted from R. serpentina as well as Catharanthus species (Zenk et al. 1977). The concentration of ajmalicine was reported to be more in stem extract when compared to that of roots (Deshmukh et al. 2012). It was reported that the amount of ajmalicine in roots could be raised up by optimizing growth media conditions like utilizing sucrose, phosphate, and 6.5 pH (Bhagat et al. 2020). The commercial production has also been enhanced by use of CdCl2 (Zafar et al. 2020a) Interestingly, similar to other phytochemicals, ajmalicine maintains cerebral blood pressure and also possesses psychotic effects (Agrawal 2019). Ajmalicine also possesses renal vasodilation properties and it selectively inhibits postganglionic functioning of sympathetic system (Mehrotra et al. 2015).
2.1.6 Other Alkaloids
Reserpiline is a yohimban alkaloid with antipsychotic property (Gupta et al. 2012). Serpentine is an anhydronium alkaloid with sedative effect. However, it also excites heart rate. Interestingly, serpentine also exhibited antihistaminase activity and was testified in guinea pigs (Sachdev et al. 1961). It also inhibits succinate dehydrogenase in liver and brain tissues. Isoajmaline, neoajmaline, and rauwolfinine are some other alkaloids with apparent actions on nervous system (Singh et al. 2017b). 3-hydroxysarpagine, yohimbinic acid, isorauhimbinic acid, Nb-methylajmaline, and Nb-methylisoajmaline are some other indole alkaloids. Remarkably, yohimbinic acid exhibited significant inhibition on topoisomerase which could be further researched for anticancer therapy (Itoh et al. 2005). It was noted that alkaloids like vomilenine and perakine were produced from cell suspension cultures in contrast to wild plant (Nikolaeva and Alterman 1993).
2.2 Other Phytochemicals
R. serpentina is also rich in phenols, flavonoids, and tannins. Phenols are intensely valuable because of its antioxidant, antimicrobial, anticancer, antidiabetic, anti-inflammatory, hepatoprotective, neuroprotective, and cardioprotective nature (Saibabu et al. 2015). Flavonoids are free radical scavengers and widely studied phytochemical in fields of cancer, inflammation, etc. (Panche et al. 2016). The presence of radical scavenging potential of phenols and flavonoids of R. serpentina was assessed and found to be higher (Keshavkant et al. 2008). Later, the use of salicylic acid (elicitor) and AcCN/H2O (solvent) was suggested with the intention of elevating the production of phenolic acids and flavonoid rutin at commercial scale (Nair et al. 2013). Quercetin, a most potent flavonoid antioxidant, was also isolated from the leaves of R. serpentina. It exhibits anti-inflammatory, anticancer properties and is used in the treatment of arthritis, bladder infections, asthma, eczema, and diabetes (Gupta et al. 2015). Similarly, rutin was also detected. Rutin prevents DNA alteration, lipoprotein peroxidation, and thus mutations (Gupta and Gupta 2015). Tannins are peculiar with its antiseptic property (Vieira Pereira et al. 2015). In addition to this, the presence of saponins and fat was identified in n-hexane extract of roots of R. serpentina (Hussain et al. 2015). The presence of carbohydrates, glycosides, starch, coumarins, emodins, and phlobatanins was also later revealed. Anthraquinones were also ascertained in stem extracts of the plant (Deshmukh et al. 2012; Das et al. 2020; Vaishnav and Sahoo 2020).
The plant is also nutritionally copious with vitamins like riboflavin, ascorbic acid, thiamine, and niacin. The macronutrients like calcium, phosphorous, magnesium, and sodium were detected in the plant extract. However, zinc and iron are major microelements. (Singh et al. 2017b). All these elements are essential for the proper growth and development of our body. Unfortunately, presence of some heavy metals like arsenic, chromium, and cadmium was also detected in trace amounts via atomic absorption spectroscopy (Gupta and Gupta 2016). Recently, the presence of titanium, tin, vanadium, molybdenum, chromium, aluminium, silicon, mercury, strontium, nickel, cadmium, lead, and bismuth in limited amounts was identified in leaves and seeds by means of direct current arc optical emission spectroscopy (Bharti et al. 2020). Above all, antioxidant enzyme superoxide dismutase has been identified in the extracts (Kirillova et al. 2001).
3 Pharmacological Properties
Curative properties of R. serpentina have been efficiently utilized by tribal people in different parts of the world for the treatment of various ailments. According to literature surveys, tribal groups in Similipal Biosphere Reserve are using the root extract of R. serpentina for curing malaria (Panda 2014). As reported in Indian Ayurvedic literature, the root extracts of the plant were used in doctoring mental illness, sleeplessness, cardiovascular diseases, erotic violent behaviours, snake bites, diarrhoea, and severe pain (Lobay 2015; Bunkar 2017a). It was also administrated to ease uterine contraction during delivery (Singh and Singh 2009). It is now available in the market in the form of herbal drugs (Rungsung et al. 2014). When compared to the synthetic drug, extracts of R. serpentina have the following advantages like fewer side effects, no digestive issues, increased bioavailability, and reduced chances of drug resistance development (Bunkar 2017a). Figure 4.2 represents the phytochemical properties of R. serpentina.
3.1 Antioxidant Nature
The formation of oxidative stress in our body leads to various diseases like cancer and diabetes. Medicinal plants are chemically rich in antioxidants. In this context, extracts of the R. serpentina could be efficiently utilized to prevent and treat oxidative damages. Various studies have assessed the antioxidant potential of extracts of R. serpentina via 2, 2′-diphenyl-1-picryl Hydrazyl Radical (DPPH) assay (Fazal et al. 2011). Interestingly, methanolic extracts of roots showed higher free radical scavenging activity than leaves and buds (Dey et al. 2016). In addition to this, further studies exposed that wild varieties showed more activity than cultivated ones (Chauhan et al. 2017b).
3.2 Antidiabetic Activity
The aqueous extracts of R. serpentina were used as an antidiabetic drug for periods by the local clan in northern Thailand (Manosroi et al. 2011). Ample clinical studies revealed a decrease in blood glucose level on the administration of Rauwolfia therapy. The antidiabetic activity of R. serpentina was primarily investigated via alpha-amylase assay. The hypoglycaemic efficacy of wide and cultivated varieties of the plant was explored and it was observed that the plant is a good candidate drug for diabetic treatments (Chauhan et al. 2017b). Interestingly, the methanolic root extracts showed the antidiabetic effect on alloxan-induced diabetic rats (Qureshi et al. 2009). Furthermore, 10–60 mg/kg was found to be the appropriate dose of an extract with hypoglycaemic property based on trials in Wistar mice (Azmi and Qureshi 2012b). The level of glycosylated haemoglobin in blood was used as a parameter to investigate the effects of methanolic extract of R. serpentina (Azmi and Qureshi 2012a). Later, the glucose suppressing activity of hydromethanolic extracts was also experimentally perceived. In contrast to previous studies, higher doses of the extract were effective and with less side effects (Azmi and Qureshi, 2014). The action of extract over pancreatic cells helped in maintaining blood glucose homeostasis (Azmi et al. 2018). Similarly, investigation in fructose-induced type 2 diabetic mice was also performed. The findings suggested that the hypoglycaemic effect was either because of reduced intake of fructose into the intestine or of low insulin resistance (Azmi and Qureshi 2013). These results were further corroborated by docking studies. The phytochemicals like ajmaline, serpentine, rauwolfine, yohimbine, and sarpagine in R. serpentina were found to be activators of insulin and thus mediated the diabetic effect (Ganugapati et al. 2012). The dry lab studies using aldose reductase as a target led to the identification of potent phytochemicals like indobine in attributing antidiabetic nature (Pathania et al. 2013).
3.3 Hypolipidemic Property and Role in Cardiovascular Diseases
The hypolipidemic effect of methanolic extracts of R. serpentina was experimentally proven in Wistar albino mice. Remarkably, a significant reduction in the level of total cholesterol, triglyceride, and low density lipoprotein was observed underlining the role of the herbal drug in maintaining lipid equilibrium (Azmi and Qureshi 2013). Parallel studies were performed to evaluate the antilipidemic activity of hydromethanolic extract. These actions were endorsed most likely probably because of inhibition of HMG-CoA reductase and AcetlyCoA carboxylase or by promoting lipase (Azmi et al. 2018). More studies were conducted on albino mice and alloxan-induced mice, validating the antihyperlipidemic activity with less side effects (Qureshi et al. 2009; Shah et al. 2020). Additionally, MolDock studies revealed the contribution of phytochemicals like reserpine, ajmalicine, yohimbine, and indobine in inhibiting the 3-hydroxy-3-methyl-glutaryl-CoA reductase and thus the cholesterol biosynthetic pathway (Azmi et al. 2021).
Herbal medicines are good alternatives for the expensive pharmaceuticals for healing cardiovascular diseases (Rastogi et al. 2016). The hypolipidemic property of R. serpentina root extract is the core for its healing role in cardiovascular ailments. Clinical trials using human subjects in the 1950s proved the efficacy of Rauwolfia therapy in treating angina pectoris and coronary artery disease (Lewis et al. 1956). In vivo studies were performed by inducing oxidative stress on left anterior coronary artery ligation in dogs, followed by administration of the herbal formulation of R. serpentina with other medicinal plants. The plant formulation succeeded in maintaining the biochemical, haemodynamic parameters, and thus the cardiac health (Afsheen et al. 2018). The blood pressure moderating ability of the reserpine was also reported (Bunkar 2017a). Increased blood pressure leads to ailments like hypertension. The action of the reserpine on the vaso-motor centre leads to generalized vasodilation and subsequently reduced blood pressure. Several clinical trials have been carried out to appraise the antihypertensive efficacy of root extracts of R. serpentina. In mid of 1900s, Vakil conducted a notable work on 50 hypertensive patients. The uptake of rauwolfia resulted in a fall of blood pressure for around 80% of patients (Vakil 1949). Alseroxylon, a drug derived from Rauwolfia, was administrated to a group of 84 patients with varying intensity of hypertension. Most of them showed positive results with harmless side effects (Livesay and Moyer 1954). Correspondingly, studies on patients with hypertensive disorder for almost 11 months by giving R. serpentina resulted in a decrease in blood pressure (Markvotiz et al. 1955). A series of studies succeeding Vakil’s findings were performed during 1950s. All of them unequivocally pointed out the impact of R. serpentina, specifically reserpine on hypertension. Moderate side effects like sedation and drowsiness were observed in most cases. But it was considered as blessing in disguise for patients (Lobay 2015). A review work was performed to expound the dose-dependent action of reserpine by exploring all the available clinical trials. They reported the effectiveness of reserpine similar to the available drugs, but couldn’t make a conclusion regarding exact dosage of reserpine. However, it was suggested that low dosage of drug is acceptable with least side effects. (Shamon and Perez 2016) Recently, a major fall in diastolic BP, systolic BP, and level of angiotensin converting enzyme was observed as a result of experimental tests using a combination of R. serpentina and Curcuma longa on hypertension-induced dogs (Farieha et al. 2019). Besides, the cardioprotective indices were elevated and the risk of coronary attacks was reduced on the use of methanolic extracts of R. serpentina in alloxan-induced mice (Azmi and Qureshi 2012a).
3.4 Antimicrobial Activity
Medicinal plants have been extensively used for microbial infections for centuries ago. Being an important medicinal plant, the antimicrobial potential of R. serpentina was also investigated extensively. The bactericidal activity of the root extract was first validated via MIC and MBC against S. typhi. It was also observed that indole alkaloids like reserpine confer this property (Deshmukh et al. 2012). Later, methanol, chloroform, and aqueous extracts of the plant exhibited inhibition against a number of pathogenic microbes. Among them, methanol extract had shown the highest MIC against S. aureus. The root extract was found to be effective against bacteria like Saccharomyces cerevisiae, Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Streptococcus pneumonia and Proteus vulgaris as well as against fungi like Aspergillus niger and Candida albicans (Owk and Lagudu 2016). Interestingly, one of the bactericidal activity-bestowing factors was identified to be stigmasterol in n-hexane fraction of methanolic extract of the plant (Dey et al. 2016). The ethanolic extract of the plants has also showed similar repressing effect on bacteria (Bunkar 2017a, b). Analogously, the methanolic, ethanolic, and acetonic extracts of the plant were considered for investigation for fungi like Trichoderma viride, Fusarium oxysporum, and Penicillium notatum. The antifungal and antibacterial nature of R. serpentina was once again substantiated (Singh et al. 2017a, b).
3.5 Role in Mental Health
R. serpentina is legendary in Ayurvedic literature for its extraordinary effects on the nervous system and subsequent curing of various mental disorders. This antipsychotic herb was widely employed in the treatment of insomnia, tremor, and frenzy conditions. Studies on schizophrenic patients revealed the impact of rauwolfia in reducing aggressiveness. Further studies on neurotic patients induced dreams (Azima et al. 1957). Similarly, raudixin, a drug derived from the roots of R. serpentina, was proven to be a probable dream-inducing agent. But more elaborate studies are required to overcome the objections on the efficiency of the drug as well as to explain the underlying mechanism (Azima 1958). Clinical studies on 118 patients suffering from mental illness revealed the sedative potential of reserpine. Substantial reduction in nervousness and the comforting effect was produced on administration (Glynn 1955). The herbal drug was also found to be effective in reducing overanxiety in psychiatric patients. Unfortunately, no impact was observed in depression (Lowinger 1957). Interestingly, studies on autistic children with low doses showed hopeful changes like reduced hyperactivity, calmness, and better sleep (Lehman et al. 1957). Another study proved that reserpine in the form of drug serpasil had lessen the insane treatments of drug and alcohol addicts (Avol and Vogel 1955). The mitigating effect of phytochemicals like ajmaline and reserpine on Alzheimer’s disease was evaluated via in vitro and in silico studies. Both of the compounds exhibited β-site amyloid cleaving enzyme (BACE-1) suppression, anti-cholinesterase, and monoamine oxidase-B (MAO-B) inhibition. The neuroprotective ability of these bioactive compounds promises a new treatment strategy for alleviating Alzheimer’s disease (Kashyap et al. 2020).
3.6 Anticancer Property
Anticancer potential of reserpine, an important phytochemical extracted from roots of R. serpentina, was an experimental subject among researchers in the nineteenth centuries. But was not prized because of the toxic nature. At the same time, lot of rumours were circulated regarding the incidence of breast cancer on use of R. serpentina. Later, proper clinical studies contradicted these false assumptions (Lobay 2015). Fortunately, exceptional role of reserpine in killing resistant tumour cells was later identified and is now a hope in the anticancer studies. Resazurin reduction assay, doxorubicin uptake assay, was employed to study cytotoxicity and further confirmed by molecular docking studies (Abdelfatah and Efferth 2015). Moreover, it was evidenced that reserpine suppressed cell proliferation and also induced apoptosis in oral cancer cells via TGF-β signalling (Ramu et al. 2020). It was also found that anticancer activity of leaves extract was comparatively higher through studies on HeLa cell lines (Deshmukh et al. 2012). The anticancer property of alstonine, serpentine, was experimentally proven on groups of mice bearing either Ehrlich ascites carcinoma cells or lymphoma cells. These are bioactive compounds present in R. serpentina (Beljanski and Beljanski 1986). More elaborate studies are demanded to explore the anticancer potential of this wonder drug.
3.7 Antivenom Property
The Indian ethnic folks have been reported to use the paste of roots and leaves of R. serpentina as an herbal antidote (Upasani et al. 2017, 2018). It was found to be effective with combination of Tylophora indica paste (Ignacimuthu et al. 2006). This traditional knowledge was later proven to be true by identifying lead compounds in the plant extract against the venom of cobra. The docking studies demonstrated that phytochemicals have a suppressing effect on phospholipase A2 like potential factors in venom (Sreekumar et al. 2014). Stigmasterol is an important phytochemical attributing this antivenom nature. It was also noted that the concentration of stigmasterol in tropical plants varies considerably with the altitudinal location of growth. The factors like wind, rainfall, temperature, and humidity have influential roles in the content of stigmasterol and thus antidote nature (Dey and Pandey 2014).
3.8 Miscellaneous Properties
Investigation on guinea pigs with extracts of R. serpentina unwrapped the antihistaminase nature. Serpentine was the potent phytochemical that hindered histaminase in vivo (Sachdev et al. 1961). Fascinatingly, studies on HIV antigens uncovered the anti-HIV activity of the root extracts of the plant (Sabde et al. 2011). Aqueous and ethanolic extracts were considered for testing the antimalarial activity in mice diseased with malaria. The results indicated substantial suppression in the growth of plasmoids. Topoisomerase II inhibiting the nature of serpentine was the crucial factor that leads to the death of parasites. This promises R. serpentina as a candidate herbal for developing an antimalarial drug (Omoya et al. 2019). The presence of symbiotic actinomycetes has also been discovered within R. serpentina. These actinomycetes are efficient in the production of bioactive chemicals (Gohain et al. 2015). Correspondingly, Cladosporium sp., an antibactericidal fungus, has been isolated from the leaves of R. serpentina. Additionally, potential metabolites like naphthoquinones were also found to be synthesized from this fungi (Khan et al. 2016). A unique strain of rhizobacterium named Delftia tsuruhatensis was identified from R. serpentina with plant promoting properties. Above all, these bacterium also produced a novel antibiotic(amino(5-(4-methoxyphenyl)-2-methyl-2-(thiophen-2-yl)-2,3-dihydrofuran-3-yl)methanol) with profound fungicidal activity (Prasannakumar et al. 2015). Twenty fungal batches belonging to Fusarium sp., Aspergillus sp., Xylaria sp., Phomopsis sp., Cladosporium sp., Alterneria sp., Gleomastix sp., and Colletotrichum sp. have been isolated and four of them showed signs of noticeable antibacterial activity (Singh et al. 2016). Lately, another fusarium sp. with antistaphylococcal action was observed in leaves of R. serpentina. Sixteen more potent antibacterial fungal strains were also identified in this category. The potential of these fungal groups was not only limited to antibacterial activity, but they also mimicked the antioxidant and hypolipidemic effect. These studies also pointed out that C. gloeosporioides is the most colonized fungi within R. serpentina (Nath et al 2013) Another noteworthy property is the action of the plant against the genotoxic impact produced on the exposure of carbofuran in freshwater fishes. The antioxidant nature of R. serpentina attributes to this mechanism of protection (Tiwari and Trivedi 2019). Recently, the hepatoprotective and renoprotective nature of methanolic extracts of R. serpentina was revealed through investigations on albino mice. These studies shed new light on the pharmacological properties of R. serpentina and cancelled the proposed side effects of the use of these drugs (Shah et al. 2020). One more striking property of R. serpentina was corrosion inhibition activity on aluminum alloys. The inhibitory activity was greatly increased by intensifying the concentration of plant extracts and temperature (Chaubey et al. 2017). Likewise, the alkaloid extracts of the plant demonstrated the formation of protective layer over steel supressing the corrosion. Reserpine was also proposed as the possible candidate involved in this biological protection (Raja and Sethuraman 2010).
4 Growth and Conventional Propagation Methods
Generally, the plant grows in wide geographical area with an annual rainfall of 1500–4000 mm and a temperature range of 10–38 °C (Agrawal 2019). It grows well in humus-rich loamy soil. Seeds are usually used for propagation. Seeds are collected around the beginning of June and can be maintained for 6 months in proper storage conditions. Tap roots with filiform secondary roots are chosen sometimes to propagate. Soft stem cuttings could also be employed. Sproutings are seen within a month. Comparatively, low germination rates were observed on the use of these plant parts. Moreover, generated plants are susceptible to pathogen attacks and climate fluctuations. Most importantly, each generation may show genetic variability and which will subsequently affect the alkaloid content within the plant (Mukherjee et al. 2019).
5 Biotechnological Approaches
The various disadvantages of conventional propagation strategies, increased economic value of phytochemicals like reserpine, and the categorization of R. serpentina as an endangered plant attracted the biotechnologists more towards the vast realm of this medicinal plant.
5.1 In Vitro Propagational Strategies
Growing cells, tissues, and organs of plants under artificial synthetic media and appropriate environmental conditions is termed as tissue culture (Van Eck and Smith 1997). Tissue culture is a better option for the mass propagation of endangered plants like R. serpentina. Shoot tips, hairy root, leaf, callus, axillary meristems, nodal and internodal segments, and seeds could be used as explant to develop the plant. Even inflorescence was efficaciously exercised to develop callus (Kaur 2018). Young leaves have been reported as an assuring explant in many studies (Nurcahyani 2008). Nodal, internodal segments, and leaves were compared to develop callus and it was observed that leaf is a better choice for callus induction and plant regeneration (Panwar et al. 2011). Apposite sterilization is a major step ensuring the success of tissue culture. A proper sterilization pattern is followed for R. serpentina cultures; Bavistin (0.1–0.2%) followed by Tween-20 (10%) for 5 min, Sodium hypochlorite for 5 min, cetrimide solution (1%) for 5 min, ethanol for 30 s, and HgCl2 (0.1%)for 5 min (Mukherjee et al. 2019). Several other variations in terms of concentration and exposure of chemicals have been practiced (Bahuguna et al. 2011; Mallick et al. 2012; Pan et al. 2013). Murashige and Skoog (MS) medium is generally used for organogenesis, embryogenesis, and callus induction. Half concentration of MS media (pH—5.8) was employed for root induction as well as for maintaining shoot and leaves (Benjamin et al. 1993; Goel et al. 2009). MS medium with BAP (0.5–1.5 mg/L) with KIN (0.5–1.0 mg/L) or NAA (0.5–1.0 mg/L) was reported to be best medium for callus induction. The use of other media like Gambrog’s B5 medium (Pan et al. 2013), Linsmaier Skoog medium (Yamamoto and Yamada 1986), and Woody plant medium (Alatar 2015) were also successfully established. (Yamamoto and Yamada 1986) They used LS media for 13 years and successfully alleviated the reserpine production via persuading stress conditions by amending hormone concentration. One of the studies proved that woody plant medium supplemented with 5.0 μM BA and1.0 μM NAA was found to be the best choice to generate shoot artificially (Abdurahman 2012). Sucrose (3%) is the major carbon source for nourishment of growing tissues. However, MS media with1.5% sucrose was also to be effective in inducing root (Bhadra et al. 2009). Additionally, fruitful use of MS with 4% sucrose for producing roots was also reported (Pandey et al. 2007). Optimum temperature and light intensity were reported to be 20–25 ± 1–2 °C and 3000 lux, respectively. Moreover, 50–70% of relative humidity and 16 h exposure to light were enough for the growth of callus in synthetic media. Auxins, cytokinins, and gibberellic acid were the common growth regulators used by most investigators and variations in the concentration and combination ratios were also attuned to favour root and shoot initiation (Mukherjee et al. 2019). The impact of concentrations of growth regulators is depicted in Table 4.2. Various other addictives like coconut milk (Aryal and Joshi 2009), mixture of proteins, ascorbic acid, citric acid (Kataria and Shekhawat 2005), copper sulfate (Ahmad et al. 2001), and antibiotics (Benjamin et al. 1993) have also been utilized to induce and enhance the growth of in-vitro plants. Use of thidiazuron in media caused profused shooting in explants (Pandey et al. 2007). Moreover, prior treatment of thidiazuron before culturing explants in liquid MS media resulted in amplified number of shoots (Alatar 2015). Total alkaloid quantity was also elevated by adequate supply of nitrogen (Akram and Ilahi 1986). Interestingly, the shoot tips were treated with colchicine to induce autotetraploidy with the intention of ameliorating alkaloid content (Mathur et al. 1987). Direct and indirect regeneration systems were compared to study the augmentation in reserpine production. In vitro regeneration was proved to be more effective (Mukherjee et al. 2020). These direct and indirect organogeneses have been employed in many studies to explore the ways of micropropagation (Bahuguna et al. 2011). Somatic embryos were also successfully established from roots of R. serpentina in liquid MS media. Additionally, flow cytometry studies evidenced the stability of regenerated plants from these embryos (Zafar et al. 2019). Proper acclimatization must be followed after all these for the generation of healthy plants. Commonly used are soil, sand, and leaf manure or farmyard wastes (Mukherjee et al. 2019). Different combinations of vermiculite, soil, and sand were other good alternatives to generate plantlets (Kad et al. 2017).
5.1.1 Use of Elicitors
The massive production of phytochemicals like reserpine could be achieved by use of elicitors. Elicitors can be either biotic or abiotic (David Paul Raj et al. 2020). Use of elicitors like copper and zinc imparted the increase in number of shoots generated (Nurcahyani 2008; Ahmad et al. 2014). Use of other chemicals like melaphene exhibited solid impact on alkaloid content (Kozlova et al. 2005). Studies conducted on different concentrations of methyl jasmonate showed that higher concentrations caused a 7.3 times rise in total reserpine content (Harisaranraj et al. 2009). Similarly, acetyl salicylic acid (ASA), salicylic acid (SA), and methylsalicylic acid were applied directly onto the plants in form of sprays. This caused tremendous increase in the phenolic acid-derived metabolites like cichoric acid (Nair et al. 2013). The eliciting property of salicylic acid (SA), dimethyl sulphoxide (DMSO), and abscisic acid (ABA) was assessed. A positive effect was imparted by all except DMSO. An association between the action of SA and trypamine (precursor) was also uncovered. About 57.64 mg g−1 of reserpine was produced on 36 days of elicitation (Panwar and Guru 2013). Similarly, the use of CdCl2 caused considerable increase in reserpine and ajmaline content by inducing stress (Zafar et al. 2020b). An ascent in reserpine synthesis was also reported on the use of AlCl3 (0.15 mM). The antioxidant assays evidenced the stress induced by AlCl3. However, it became the source of a positive effect (Zafar et al. 2017). Outstandingly, the 2.9-fold of rise in amount of ajmaline was recorded on use of mannan from Saccharomyces cerevisiae (100 mg/L). Meanwhile, use of sodium chloride elevated the production of ajmalicine (Srivastava et al. 2016). All the reports underline the fact that induction of stress via chemicals caused a considerable advance in phytochemical production.
5.1.2 Synthetic Seed Development
The artificial seed technology was a breakthrough in the field of in vitro propagation. It can be defined as encapsulated somatic embryo, any meristematic tissue like shoot tips that can be cultivated as seeds and which are capable to regenerate the whole plant (Chandrasekhara 2012). Meristematic nodal segments, shoot tips are most often used for producing synthetic seeds. The explants were encapsulated using 3% sodium alginate and calcium chloride. The efficacy of different media in generating artificial seeds was also considered in the above study. Woody plant medium supplemented with plant hormones like NAA and BA showed sproutings within 2 weeks of growth (Faisal et al. 2012). Later, studies testified the optimum concentration of Sodium alginate as 3% and of Calcium chloride as 75 mM. In contrast to the above study, comparatively higher germination rates were observed in ½ MS liquid and semisolid media (Gantait et al. 2017). Storage at 4 °C was evaluated as a means for maintenance of alginated shoot ends up to 18 weeks (Ray and Bhattacharya 2008). It was found that proper storage at 8 °C will retain the reserpine content and viability of seeds (Gantait and Kundu 2017).
5.2 Use of Genetic Markers
Variation in genetic stability was a common consequence of in vitro propagation studies. Therefore, fidelity of the clone produced must be assessed via various gene markers. RAPD (Random Amplified Polymorphic DNA) and ISSR (Inter Simple Sequence Repeats) markers were used to study the genetic stability of artificial seeds. Genomic DNA was extracted from young leaves using CTAB method followed by PCR. Out of 20 RAPD primers, 19 generated distinct, consistent bands. In case of ISSR, all the seven primers produced bands. All the clones produced bands monomorphic to mother, endorsing the efficacy of in vitro propagation methodology (Faisal et al. 2012). Similarly, it was reported that 10 RAPD primers produced 97 reproducible bands, while ISSR produced 68 consistent maps. This study once again cemented the reliability of synthetic seed approach for micropropagation (Gantait et al. 2017). Finger printing bands generated via RAPD markers confirmed the uniformity between mother and clones produced by direct organogenesis (Goel et al. 2009; Senapati et al. 2014). The ISSR markers were fruitfully employed to investigate the genetic diversity within R. serpentina collected from different geographical locations of Southern Western Ghats. Gene flow (Nm), Nei’s gene diversity (h), Degree of genetic differentiation (Gst), and Shannon index of genetic diversity (I) were also considered. The high polymorphism observed signified the need of proper conservation strategies for this vital plant. Moreover, it also strengthened the reliability of ISSR markers in genetic studies (Pillai et al. 2012). They have been used to evaluate the clonality of indirect organogenesis-regenerated plants. Out of 18 primers used, one that was reported to be not monomorphic band was further characterized (Saravanan et al. 2011). By the same token, genetic diversity was assessed via RAPD markers in R. serpentina variants of Western ghats. Large variation in the quantitative traits within R. serpentina varieties was observed. Along with RAPD, researchers also analysed the horticultural and chemical profiling. Implementation of proper conservation strategies localizing each small area is mandatory for protecting this natural treasure (Nair et al. 2014). In contrast to above all, DNA barcoding of different tissues and time periods of R. serpentina was performed. They detected species unique indels in the rps16 intron for R. serpentina (Eurlings et al. 2013).
5.3 Rhizogenes-Induced Hairy Roots
It is a declared fact that roots are the major source of alkaloids in R. serpentina. Transformation strategies were mainly focussed to produce bulk amount of roots via Agrobacterium rhizogenes. Ri-induced cultures are a better conservational strategy to guard and propagate this endangered plant (Srivastava and Misra 2017). The first attempted was reported in 1983 where shoot cultures were infected with AT 15834. Remarkably, callus with hairy roots was produced and later fully developed into plants. Unfortunately, much variation in alkaloid content was not reported. However, the advent of transformation techniques gave an economic preface to the studies of R. serpentina (Benjamin et al. 1993). Relatedly, the strain A4 was used to produce roots in wounded leaf explants. This study also led to the discovery of a new alkaloid called 12-hydroxyajmaline (Falkenhagen et al. 1993). Analogous studies were reported in the other species of Rauwolfia (Sudha et al. 2003). Another, Agrobacterium strain LBA 9402, was used to transform shoots and leaves of R. serpentina. The presence of rolA and rolB genes was detected by PCR to mark the transformation. Variations in the alkaloid content were observed in transformed root lines. Physiological condition of resultant cell, copy number of T-DNA, insertion site, and expression of rol genes were most possible reasons (Ray et al. 2014a). Genetic variability in root lines was also reported on studies using strains of LBA 9402 and A4. Various cytogenic studies showed the difference in TL and TR DNA integration pattern and in ajmalicine content too. It was recommended to choose the variant with more alkaloid content for scale-up studies (Ray et al. 2014b). Relatively, transformation efficiency of A4 was reported to be more. Plants generated from Ri-induced roots were found to be normal and produced higher amount of secondary metabolites compared to cultivated plants. It unfolds the possibility of using Ri-induced plants for reserpine production commercially (Mehrotra et al. 2013a). Successful in vitro studies were later upgraded to industrial level by means of Erlenmeyer flasks and bioreactors. The use of 5 L bioreactor with provisions for air supply and all other necessities was reported for the scale up of root cultures of R. serpentina. The growth rate of cultures was considerably high in bioreactor than flasks (Mehrotra et al. 2015). A standardized protocol by the above-mentioned authors for mass production of root cultures via AT 5 strain in B 5 medium in a 5 L bench top bioreactor was later published (Mehrotra et al. 2016). Interestingly, a ANN-based combinatorial model was predicted by them standardizing the culture conditions to obtain maximum metabolite yield from root cultures of R. serpentina (Mehrotra et al. 2013b). In between, the idea of using table sugar instead of sucrose was auspicious which will reduce the heavy expenses of industrial manufacturing. They also reported the upturn in the ajmaline, yohimbine, and reserpine contents on a long-term culture of 6 years and also suggested that this longevity elevated the genetic stability of strain (Pandey et al. 2014). Since the inoculants are highly susceptible to contamination, a better cryopreservation storage strategy was established. Root tips were first encapsulated using sodium alginate and were priorly cultured in sucrose. Gel beads were stored in cryovials and kept in liquid N2 for storage. It was successfully revived later (Mehrotra et al. 2015).
5.4 Metabolic Engineering
Increased demand for bioactives like reserpine for pharmacological needs caused a considerable upsurge in metabolic engineering approaches. Metabolic engineering is defined as the manipulation of metabolic pathway in a selected organism with the intention of producing economically valuable metabolites. The decade of 1900s witnessed early attempts of researchers to alleviate the alkaloid content by incorporating microbes as bioreactors. Interestingly, strictosidine synthase, a key catalytic agent in alkaloid biosynthesis, was isolated from R. serpentina and was expressed in E. coli. According to the report, this is the first enzyme from plant secondary metabolism to be expressed in an microorganism (Kutchan 1989). The enzyme was structurally and chemically similar to the plant-derived enzyme. Later, microsomes were artificially synthesized using cell cultures of R. serpentina and guinea pig liver. Vomeline, an intermediate in ajmaline biosynthetic pathway, was successfully produced via vinorine hydroxylase (Cytochrome P 450-Dependent) at 40 °C and pH 8.3 (Falkenhagen and Stöckigt 1995). Interestingly, phytoene desaturase (PDS) was silenced via biolistic-mediated VIGS in R. serpentina. The construct (pTRV2-RsPDS) was introduced in eight plantlets and bleaching of leaves was perceived indicating the transformation (Corbin et al. 2017). In addition to this, Catharanthus tryptophan decarboxylase (tdc) was overexpressed in roots of R. serpentina via AT 4 strain. tdc is elicitor responsive gene in TIA pathway. An intensification in reserpine and ajmalicine biosynthesis was observed (Mehrotra et al. 2013c). Sadly, very few metabolic engineering works are reported. Elaborate researches are recommended to study the possible ways to ameloriate the bioactives in R. serpentina.
5.5 Novel Approaches
Nanotechnology has been efficaciously employed in the research of R. serpentina. Green synthesized Copper oxide nanoparticles (CuO Nps) were subjected to antibacterial study. These highly porous, spongy nanoparticles were found to be active against both gram positive and gram negative bacteria. Interestingly, these particles decayed trypan blue dye on exposure to ultraviolet rays (Lingaraju et al. 2015). Ample studies resulted in the development of transcriptomic assembilies for R. serpentina(Góngora-Castillo et al. 2012). In silico approaches carried out in R. serpentina resulted in the identification of functional MicroRNAs. MicroRNAs are short non-coding RNAs with specific functional roles. Remarkably, 15 conserved miRNAs were detected and most of them are proposed to be involved in developmental metabolic roles of the plant. These studies can be considered as benchmark in recognizing the possibilties of genetic pathways involved in metabolite production. Interestingly, the investigators also performed the phylognetic study of the evoluntionary relationships between rse-miRNA precursors and stem-loop sequence found in some other plants (Prakash et al. 2016).
5.6 Conclusion
R. serpentina plants are well-known for their medicinal properties and were found easily in many regions of India. However, the colossal exploitation of these valuable medicinal plants during the Anthropocene epoch has led to the considerable reduction in their number. Rampant effective strategies are mandatory to sustain a considerable population needed by society. Effectual in vitro propagation and biotechnological approaches should be utilized to curtail the endangering of plant in the wild. Unfortunately, a very few metabolic engineering approaches have only been reported. More intricate and effective studies are indispensable to get insights of the unexplored possibilities of this plant. Ample computational studies will be revoluntionary in determining the genes involved in secondary metabolite production and subsequent engineering. Besides, stringent regulations are required for the efficacious protection of plants without exploiting them.
References
Abdelfatah SAA, Efferth T (2015) Cytotoxicity of the indole alkaloid reserpine from Rauwolfia serpentina against drug-resistant tumor cells. Phytomedicine 22:308–318. https://doi.org/10.1016/j.phymed.2015.01.002
AbdelHafez EMN, Diamanduros A, Negureanu L et al (2013) Computational and synthetic studies towards improving rescinnamine as an inducer of MSH2-dependent apoptosis in cancer treatment. Mol Cancer Biol 1:44. https://doi.org/10.9777/mcb.v1i1.42
Abdurahman AA (2012) High frequency shoot regeneration and plant establishment of Rauvolfia serpentina: an endangered medicinal plant. J Med Plants Res 6:3324. https://doi.org/10.5897/jmpr12.111
Afsheen N, Khalil-Ur-Rehman JN et al (2018) Cardioprotective and metabolomic profiling of selected medicinal plants against oxidative stress. Oxidative Med Cell Longev 2018:1–17. https://doi.org/10.1155/2018/9819360
Agrawal S (2019) Rauvolfia serpentina: a medicinal plant of exceptional qualities. Altern Med Chiropr Open Access J 2:1–6
Ahmad S, Amin MN, Azad MAK, Mosaddik MA (2001) Micropropagation and plant regeneration of Rauvolfia serpentina by tissue culture technique. Pak J Biol Sci 5:75–79. https://doi.org/10.3923/pjbs.2002.75.79
Ahmad N, Alatar AA, Faisal M et al (2014) Effect of copper and zinc on the in vitro regeneration of Rauvolfia serpentina. Biol Plant 59:11–17. https://doi.org/10.1007/s10535-014-0479-5
Akram M, Ilahi I (1986) Plantlet formation in root callus of Rauwolfia serpentina. Pak J Bot 18:15–19
Alatar AA (2015) Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentina - a potent antihypertensive drug producing plant. Biotechnol Biotechnol Equip 29:489–497. https://doi.org/10.1080/13102818.2015.1017535
Aryal S, Joshi SD (2009) Callus induction and plant regeneration in Rauvolfia Serpentina (L.) Benth Ex. Kurz. J Nat Hist Mus 24:82–88. https://doi.org/10.3126/jnhm.v24i1.2245
Avol M, Vogel PJ (1955) Treatment of delirium tremens with reserpine (serpasil): a preliminary report. J Am Med Assoc 159:1516–1520. https://doi.org/10.1001/jama.1955.02960330016005
Azima H (1958) The possible dream inducing capacity of the whole root of Rauwolfia serpentina. Can Psychiatr Assoc J 3:47–51. https://doi.org/10.1177/070674375800300110
Azima H, Cramer-Azima FJ, De Verteuil R (1957) Effects of Rauwolfia derivatives on psychodynamic structure. In: 113th annual meeting of the American Psychiatric Association, Chicago
Azmi MB, Qureshi SA (2012a) Methanolic root extract of Rauwolfia serpentina benth improves the glycemic, antiatherogenic, and cardioprotective indices in alloxan-induced diabetic mice. Adv Pharmacol Sci 2012:376429. https://doi.org/10.1155/2012/376429
Azmi MB, Qureshi SA (2012b) Methanolic root extract of Rauwolfia serpentina improves the glucose tolerance in Wister mice. J Food Drug Anal 20:484–488. https://doi.org/10.6227/jfda.2012200208
Azmi MB, Qureshi SA (2013) Rauwolfia serpentina improves altered glucose and lipid homeostasis in fructose-induced type 2 diabetic mice. Pak J Pharm Sci 29:1619–1624
Azmi MB, Qureshi SA (2014) Glucose lowering potential of hydromethanolic extract of Rauwolfia. World J Pharm Sci 2:219–223
Azmi MB, Qureshi SA, Ahmed SDH et al (2018) Ameliorative effect of hydro-methanolic extract from roots of Rauwolfia serpentina on some biochemical parameters of type 1 diabetic mice. Pak J Pharm Sci 31:663–668
Azmi MB, Sultana S, Naeem S, Qureshi SA (2021) In silico investigation on alkaloids of Rauwolfia serpentina as potential inhibitors of 3-hydroxy-3-methyl-glutaryl-CoA reductase. Saudi J Biol Sci 28:731–737. https://doi.org/10.1016/j.sjbs.2020.10.066
Bahuguna RN, Joshi R, Singh G et al (2011) Micropropagation and total alkaloid extraction of Indian snake root (Rauwolfia serpentina). Indian J Agric Sci 81:1124–1129
Baksha R, Akhter A, Khatun R, Liton J (2007) In vitro rapid clonal propagation of Rauvolfia serpentina (Linn.) Benth. Bangladesh J Sci Ind Res 42:37–44
Banerjee JN, Lewis JJ (1954) A note on the pharmacology of the alkaloids of Rauwolfia Serpentina Benth. J Pharm Pharmacol 8:50–54. https://doi.org/10.1111/j.2042-7158.1956.tb12127.x
Beljanski M, Beljanski MS (1986) Three alkaloids as selective destroyers of cancer cells in mice. Synergy with classic anticancer drugs. Oncology 43:198–203. https://doi.org/10.1159/000226363
Benjamin BD, Roja G, Heble MR (1993) Agrobacterium rhizogens mediated transformation of Rauvolfia serpentina: regeneration and alkaloid synthesis. Plant Cell Tissue Organ Cult 35:253–257. https://doi.org/10.1007/BF00037278
Bhadra SK, Akhter T, Hossain MM (2009) In vitro micropropagation of Rauvolfia serpentina L. through induction of direct and indirect organogenesis. Plant Tissue Cult Biotechnol 19:169–175. https://doi.org/10.3329/ptcb.v19i2.5434
Bhagat P, Verma SK, Yadav S et al (2020) Optimization of nutritive factors in culture medium for the growth of hairy root and ajmalicine content in sarpgandha, Rauwolfia serpentina. J Environ Biol 41:1018–1025. https://doi.org/10.22438/JEB/41/5/MRN-1316
Bhanwaria R, Singh B, Gochar R (2021) Indian snake root and devil root as distinctive medicinal plant for curing human disease: biology, chemistry and cultivation practices of Rauwolfia serpentina and Rauwolfia tetraphylla. In: Plants for novel drug molecules: ethnobotany to ethnopharmacology. New India Publishing Agency, New Delhi, pp 445–465
Bharti AS, Sharma S, Uttam KN (2020) Elemental assessment of the leaf and seed of Rauwolfia serpentina (Sarpagandha) by direct current arc optical emission spectroscopy. Natl Acad Sci Lett 43:361–365. https://doi.org/10.1007/s40009-019-00872-4
Bhatt R, Arif M, Gaur AK, Rao PB (2008) Rauwolfia serpentina: protocol optimization for in vitro propagation. African J Biotechnol 7:4265–4268. https://doi.org/10.5897/AJB08.718
Bindu S, Rameshkumar KB, Kumar B et al (2014) Distribution of reserpine in rauvolfia species from India - HPTLC and LC-MS studies. Ind Crop Prod 62:430–436. https://doi.org/10.1016/j.indcrop.2014.09.018
Bunkar AR (2017a) Phytochemical and antimicrobial study of Rauwolfia serpentina. Int J Biol Res 2:13–16
Bunkar RA (2017b) Therapeutic uses of Rauwolfia serpentina. Int J Adv Sci Res 2:23–26
Chandrasekhara M (2012) Synthetic seeds: a review in agriculture and forestry. African J Biotechnol 11:14254–14275. https://doi.org/10.5897/ajb12.770
Charney DS, Heninger GR, Breier A (1984) Noradrenergic function in panic anxiety: effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch Gen Psychiatry 41:751–763. https://doi.org/10.1001/archpsyc.1984.01790190025003
Chatterjee A (1953) Rauwolfia alkaloids. In: Progress in the chemistry of organic natural products. Springer, Vienna, pp 390–470
Chaubey N, Yadav DK, Singh VK, Quraishi MA (2017) A comparative study of leaves extracts for corrosion inhibition effect on aluminium alloy in alkaline medium. Ain Shams Eng J 8:673–682. https://doi.org/10.1016/j.asej.2015.08.020
Chauhan S, Kaur A, Pareek RK (2017a) Pharmacobotanical and pharmacological evaluation of ayurvedic crude drug: Rauwolfia serpentina (Apocynaceae). Int J Green Pharm 11:S686–S693
Chauhan S, Kaur A, Vyas M, Khatik GL (2017b) Comparison of antidiabetic and antioxidant activity of wild and cultivated variety of Rauwolfia serpentina. Asian J Pharm Clin Res 10:404–406. https://doi.org/10.22159/ajpcr.2017.v10i12.21287
Chikte SS, Dhakolkar P, Chikhale NJ, Burghate SK (2013) In vitro evaluation and comparison of differentiated roots for alkaloids of Rauwolfia serpentine. Sch J Agric Sci 3:573–575
Corbin C, Lafontaine F, Sepúlveda LJ et al (2017) Virus-induced gene silencing in Rauwolfia species. Protoplasma 254:1813–1818. https://doi.org/10.1007/s00709-017-1079-y
Das S, Saraf A, Sharma D et al (2019) Qualitative screening of bioactive secondary metabolites present in Withania somnifera and Rauwolfia serpentina root and stem extracts. Int J Res Anal Rev 6:69–74
Das S, Saraf A, Sharma D et al (2020) Study of phytochemical analysis of Withania somnifera and Rauwolfia serpentina plant extract Study of phytochemical analysis of Withania somnifera and Rauwolfia serpentina plant extract 8:1–4
David Paul Raj RS, Regi R, Mathew AA, Beena Kanimozhi R (2020) In vitro production of secondary metabolites from Andrographis paniculata and Rawvolfia serpentina against snake venom. Drug Invent Today 14:179–182
Deshmukh SR, Ashrit DS, Patil BA (2012) Extraction and evaluation of indole alkaloids from Rauwolfia serpentina for their antimicrobial and antiproliferative activities. Int J Pharm Pharm Sci 4:329–334
Dey A, De JN (2010) Rauvolfia serpentina (L).Benth. ex Kurz.-a review. Asian J Plant Sci 9:285–298
Dey A, Pandey DK (2014) HPTLC detection of altitudinal variation of the potential antivenin stigmasterol in different populations of the tropical ethnic antidote Rauvolfia serpentina. Asian Pac J Trop Med 7:S540–S545. https://doi.org/10.1016/S1995-7645(14)60287-X
Dey A, Mukherjee S, De A, Pandey DK (2016) A Stigmasterol containing n-hexane fraction of Rauvolfia serpentina methanolic extract shows tissue-specific variation of biocidal and antioxidant activities. Int J Geogr Inf Syst 22:81–91. https://doi.org/10.1080/10496475.2015.1042617
Ernst E, Pittler MH (1998) Yohimbine for erectile dysfunction: a systematic review and meta- analysis of randomized clinical trials. J Urol 159:433–436. https://doi.org/10.1016/S0022-5347(01)63942-9
Eurlings MCM, Lens F, Pakusza C et al (2013) Forensic identification of Indian snakeroot (Rauvolfia serpentina Benth. ex Kurz) using DNA barcoding. J Forensic Sci 58:822–830. https://doi.org/10.1111/1556-4029.12072
Faisal M, Alatar AA, Ahmad N et al (2012) Assessment of genetic fidelity in rauvolfia serpentina plantlets grown from synthetic (encapsulated) seeds following in vitro storage at 4 °C. Molecules 17:5050–5061. https://doi.org/10.3390/molecules17055050
Falkenhagen H, Stöckigt J (1995) Enzymatic biosynthesis of vomilenine, a key intermediate of the ajmaline pathway, catalyzed by a novel cytochrome P 450-dependent enzyme from plant cell cultures of Rauwolfia serpentina. Zeitschrift fur Naturforsch Sect C J Biosci 50:45–53. https://doi.org/10.1515/znc-1995-1-208
Falkenhagen H, Stockigt J, Kuzovkina IN et al (1993) Indole alkaloids from “hairy roots” of Rauwolfia serpentina. Can J Chem 71:2201–2203. https://doi.org/10.1139/v93-276
Farieha, Jahan N, Khalil-Ur-Rahman, Ali S (2019) Effect of herbal mixture as angiotensin converting enzyme inhibitor in angiotensin-II dependent hypertension. Pak Vet J 39:25–30. https://doi.org/10.29261/pakvetj/2018.103
Fazal H, Ahmad N, Khan MA (2011) Physico-chemical, phytochemical evaluation and dpph-scavenging antioxidant potential in medicinal plants used for herbal formulation in Pakistan. Pak J Bot 43:63–67
Fernandes VS, Santos JR, Leão AHFF et al (2012) Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behav Brain Res 231:154–163. https://doi.org/10.1016/j.bbr.2012.03.008
Gantait S, Kundu S (2017) Does synthetic seed storage at higher temperature reduce reserpine content of Rauvolfia serpentina (L.) Benth. ex Kurz.? Rend Lincei 28:679–686. https://doi.org/10.1007/s12210-017-0637-8
Gantait S, Kundu S, Yeasmin L, Ali MN (2017) Impact of differential levels of sodium alginate, calcium chloride and basal media on germination frequency of genetically true artificial seeds of Rauvolfia serpentina (L.) Benth. ex Kurz. J Appl Res Med Aromat Plants 4:75–81. https://doi.org/10.1016/j.jarmap.2017.01.005
Ganugapati J, Baldwa A, Lalani S (2012) Docking studies of Rauwolfia serpentina alkaloids as insulin receptor activators. Int J Comput Appl 43:32–37. https://doi.org/10.5120/6173-8600
Geissler M, Burghard M, Volk J et al (2016) A novel cinnamyl alcohol dehydrogenase (CAD)-like reductase contributes to the structural diversity of monoterpenoid indole alkaloids in Rauvolfia. Planta 243:813–824. https://doi.org/10.1007/s00425-015-2446-6
Glynn JD (1955) Rauwolfia serpentina (serpasil) in psychiatry. J Neurol Neurosurg Psychiatry 18:225–227. https://doi.org/10.1136/jnnp.18.3.225
Goel MK, Mehrotra S, Kukreja AK et al (2009) In vitro propagation of Rauwolfia serpentina using liquid medium, assessment of genetic fidelity of micropropagated plants, and simultaneous quantitation of reserpine, ajmaline, and ajmalicine. Methods Mol Biol 547:17–33
Goenka AH (2007) Rustom Jal Vakil and the saga of Rauwolfia serpentina. J Med Biogr 15:195–200. https://doi.org/10.1258/j.jmb.2007.06-53
Gohain A, Gogoi A, Debnath R et al (2015) Antimicrobial biosynthetic potential and genetic diversity of endophytic actinomycetes associated with medicinal plants. FEMS Microbiol Lett 362:1–10. https://doi.org/10.1093/femsle/fnv158
Góngora-Castillo E, Childs KL, Fedewa G et al (2012) Development of transcriptomic resources for interrogating the biosynthesis of monoterpene indole alkaloids in medicinal plant species. PLoS One 7:e52506. https://doi.org/10.1371/journal.pone.0052506
Gupta J, Gupta A (2015) Isolation and identification of flavonoid rutin from Rauwolfia serpentina. Int J Chem Stud 3:113–115. https://doi.org/10.13140/RG.2.2.22346.88003
Gupta J, Gupta A (2016) Determination of trace metals in the leaves of Rauwolfia serpentina by using atomic absorption spectroscopy. Int J Chem Stud 4:94–96
Gupta S, Khanna VK, Maurya A et al (2012) Bioactivity guided isolation of antipsychotic constituents from the leaves of Rauwolfia tetraphylla L. Fitoterapia 83:1092–1099. https://doi.org/10.1016/j.fitote.2012.04.029
Gupta J, Gupta A, Gupta AK (2015) Extraction and identification of flavonoid natural antioxidant in the leaves of Rauwolfia serpentina. Int J Chem Stud 3:35–37
Harisaranraj R, Suresh K, Saravana Babu S (2009) Production of reserpine in somatic embryos of Rauwolfia serpentina cultured in bioreactors by the induction of elicitor (methyl jasmonate). Glob J Biotechnol Biochem 4:143–147
Hong B, Li WJ, Song AH, Zhao CJ (2013) Determination of indole alkaloids and highly volatile compounds in Rauvolfia verticillata by HPLC-UV and GC-MS. J Chromatogr Sci 51:926–930. https://doi.org/10.1093/chromsci/bms191
Hussain A, Neupane P, Jha R (2015) Phytochemical and GC-MS analysis of n-hexane extract of Rauvolfia serpentina L. Benth. ex Kurz. Chem Sci Rev Lett 4:223–229
Ignacimuthu S, Ayyanar M, Sivaraman KS (2006) Ethnobotanical investigations among tribes in Madurai District of Tamil Nadu (India). J Ethnobiol Ethnomed 2:1–7. https://doi.org/10.1186/1746-4269-2-25
Itoh A, Kumashiro T, Yamaguchi M et al (2005) Indole alkaloids and other constituents of Rauwolfia serpentina. J Nat Prod 68:848–852. https://doi.org/10.1021/np058007n
Jain V, Singh D, Saraf S, Saraf S (2003) In-vitro micropropagation of Rauwolfia serpentina through multiple shoot generation. Anc Sci Life 23:44–49
Kad A, Pundir A, Sharma S, Sood H (2017) Ex-vitro rooting in rauwolfia serpentina for rapid and large scale multiplication. Int J Innov Res Sci Eng 3:135–143
Kashyap P, Kalaiselvan V, Kumar R, Kumar S (2020) Ajmalicine and reserpine: indole alkaloids as multi-target directed ligands towards factors implicated in Alzheimer’s disease. Molecules 25:1–26. https://doi.org/10.3390/molecules25071609
Kataria V, Shekhawat NS (2005) Cloning of Rauvolfia serpentina - an endangered medicinal plant. J Sustain For 20:53–65. https://doi.org/10.1300/J091v20n01_04
Kaur S (2018) In vitro callus induction in inflorescence segments of medicinally important endangered plant Rauwolfia serpentina (L.) Benth. ex Kurz – a step towards ex situ conservation. Ann Plant Sci 7:1986–1991. https://doi.org/10.21746/aps.2018.7.2.1
Keshavkant S, Sukhdev T, Srinivasarao C, Naithani SC (2008) Antioxidant activities, phenols and flavonoid contents of Withania somnifera and Rauwolfia serpentina. Indian J Plant Physiol 13:394–399
Khan MIH, Sohrab MH, Rony SR et al (2016) Cytotoxic and antibacterial naphthoquinones from an endophytic fungus, Cladosporium sp. Toxicol Reports 3:861–865. https://doi.org/10.1016/j.toxrep.2016.10.005
Khan S, Banu T, Akter S et al (2018) In vitro regeneration protocol of Rauvolfia serpentina L. Bangladesh J Sci Ind Res 53:133–138. https://doi.org/10.3329/bjsir.v53i2.36674
Kirillova NV, Smirnova MG, Komov VP (2001) Sequential isolation of superoxide dismutase and ajmaline from tissue cultures of Rauwolfia serpentina Benth. Appl Biochem Microbiol 37:181–185. https://doi.org/10.1023/A:1002875714260
Klohs MW, Draper MD, Keller F (1954) Alkaloids of Rauwolfia serpentina benth. 111.1 rescinnamine, a new hypotensive and sedative principle. J Am Chem Soc 76:2843. https://doi.org/10.1021/ja01639a079
Klyushnichenko VE, Yakimov SA, Tuzova TP et al (1995) Determination of indole alkaloids from R. serpentina and R. vomitoria by high-performance liquid chromatography and high-performance thin-layer chromatography. J Chromatogr A 704:357–362. https://doi.org/10.1016/0021-9673(95)00082-X
Kozlova RY, Vinter VG, Fattakhov SG et al (2005) Melamine salt of bis(methanol)phosphinic acids (melaphene) as a regulator of Rauwolfia serpentine specialized metabolism. Dokl Akad Nauk 401:556–559
Kumar S, Singh A, Bajpai V et al (2016a) Structural characterization of monoterpene indole alkaloids in ethanolic extracts of Rauwolfia species by liquid chromatography with quadrupole time-of-flight mass spectrometry. J Pharm Anal 6:363–373. https://doi.org/10.1016/j.jpha.2016.04.008
Kumar S, Singh A, Bajpai V, Srivastava M (2016b) Simultaneous determination of bioactive monoterpene indole alkaloids in ethanolic extract of seven Rauvolfia species using UHPLC with hybrid triple quadrupole linear ion trap mass spectrometry. Phytochem Anal 27:296. https://doi.org/10.1002/pca.2631
Kumari A, Kumar S, Anam A et al (2015) In vitro cloning of an endangered medicinal plant, Rauwolfia serpentina (L.). J Plant Dev Sci 7:555–561
Kutchan TM (1989) Expression of enzymatically active cloned strictosidine synthase from the higher plant Rauvolfia serpentina in Escherichia coli. FEBS Lett 257:127–130. https://doi.org/10.1016/0014-5793(89)81802-2
Le Xuan P, Munier RL, Meunier S (1980) Two-dimensional separations and behaviour of Rauwolfia, Corynanthe and Pseudocinchona alkaloids on unmodified silica gel. Chromatographia 13:693–697. https://doi.org/10.1007/BF02303439
Lehman E, Haber J, Stanley R (1957) The use of reserpine in autistic children. J Nerv Ment Dis 125:351–356
Lewis BI, Wild JB, Material C (1956) Rauwolfia serpentina in the treatment of angina pectoris. In: 28th annual scientific sessions of the American Heart Association, New Orleans, pp 227–232
Lingaraju K, Raja Naika H, Manjunath K et al (2015) Rauvolfia serpentina-mediated green synthesis of CuO nanoparticles and its multidisciplinary studies. Acta Metall Sin 28:1134–1140. https://doi.org/10.1007/s40195-015-0304-y
Livesay WR, Moyer JH (1954) Treatment of hypertension with Rauwolfia serpentina alone. J Am Med Assoc 155:1027–1035
Lobay D (2015) Rauwolfia in the treatment of hypertension. Integr Med 14:40–46
Lowinger P (1957) Rauwolfia serpentina in the control of anxiety. Psychiatry Q 31:445–453. https://doi.org/10.1007/BF01568739
Lucas RA (1963) The chemistry and pharmacology of the Rauwolfia alkaloids. Prog Med Chem 3:146–186. https://doi.org/10.1016/S0079-6468(08)70118-8
Makarevich IF, Khadzhai YI, Nikolaeva AV, Pavlova VV (1979) Cardenolide and bufadienolide derivatives of ajmaline. Chem Nat Compd 15:466–468. https://doi.org/10.1007/BF00565048
Mallick SR, Jena RC, Samal KC (2012) Rapid in vitro multiplication of an endangered medicinal plant Sarpgandha (Rauwolfia serpentina). Am J Plant Sci 03:437–442. https://doi.org/10.4236/ajps.2012.34053
Manosroi J, Moses ZZ, Manosroi W, Manosroi A (2011) Hypoglycemic activity of Thai medicinal plants selected from the Thai/Lanna Medicinal Recipe Database MANOSROI II. J Ethnopharmacol 138:92–98. https://doi.org/10.1016/j.jep.2011.08.049
Markvotiz M, Koik VJ, Hick KF, Grissom JR (1955) Hexamethonium, hydralazine and Rauwolfia serpentina therapy in hypertension. Am J Med Sci 229:486–489
Mathur A, Mathur AK, Kukreja AK et al (1987) Establishment and multiplication of colchi-autotetraploids of Rauvolfia serpentina L. Benth. ex Kurz. through tissue culture. Plant Cell Tissue Organ Cult 10:129–134. https://doi.org/10.1007/BF00035910
Mcqueen EG, Blackman JG (1955) Direct effects of reserpine, rescinnamine and canescine on blood vessels. Australas Ann Med 4:250–254. https://doi.org/10.1111/imj.1955.4.4.250
McQueen EG, Doyle AE, Smirk FH (1954) Mechanism of hypotensive action of reserpine, an alkaloid of Rauwolfia serpentina. Nature 174:1015. https://doi.org/10.1038/1741015b0
Mehrotra S, Goel MK, Rahman LU, Kukreja AK (2013a) Molecular and chemical characterization of plants regenerated from Ri-mediated hairy root cultures of Rauwolfia serpentina. Plant Cell Tissue Organ Cult 114:31–38. https://doi.org/10.1007/s11240-013-0302-6
Mehrotra S, Prakash O, Khan F, Kukreja AK (2013b) Efficiency of neural network-based combinatorial model predicting optimal culture conditions for maximum biomass yields in hairy root cultures. Plant Cell Rep 32:309–317. https://doi.org/10.1007/s00299-012-1364-3
Mehrotra S, Srivastava V, Rahman LU, Kukreja AK (2013c) Overexpression of a Catharanthus tryptophan decarboxylase (tdc) gene leads to enhanced terpenoid indole alkaloid (TIA) production in transgenic hairy root lines of Rauwolfia serpen … Overexpression of a Catharanthus tryptophan decarboxylase (tdc). Plant Cell Tissue Organ Cult 115:377–384. https://doi.org/10.1007/s11240-013-0369-0
Mehrotra S, Goel MK, Srivastava V, Rahman LU (2015) Hairy root biotechnology of Rauwolfia serpentina: a potent approach for the production of pharmaceutically important terpenoid indole alkaloids. Biotechnol Lett 37:253–263. https://doi.org/10.1007/s10529-014-1695-y
Mehrotra S, Srivastava V, Goel MK, Kukreja AK (2016) Scale - up of Agrobacterium rhizogenes-mediated hairy root cultures of Rauwolfia serpentina: a persuasive approach for stable reserpine production. In: Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants, 2nd edn. pp 241–257
Monachino J (1954) Rauvolfia serpentina-its history, botany and medical use. Econ Bot 8:349–365. https://doi.org/10.1007/BF02908608
Mondal S, Silva J, Ghosh P (2011) In vitro flowering in Rauvolfia serpentina (L.) Benth. ex. Kurz. Int J Plant Dev Biol 5:75–77
Mukherjee E, Gantait S, Kundu S et al (2019) Biotechnological interventions on the genus Rauvolfia: recent trends and imminent prospects. Appl Microbiol Biotechnol 103:7325–7354. https://doi.org/10.1007/s00253-019-10035-6
Mukherjee E, Sarkar S, Bhattacharyya S, Gantait S (2020) Ameliorated reserpine production via in vitro direct and indirect regeneration system in Rauvolfia serpentina (L.) Benth. ex Kurz. 3 Biotech 10:294. https://doi.org/10.1007/s13205-020-02285-3
Nair VD, Panneerselvam R, Gopi R, Hong-bo S (2013) Elicitation of pharmacologically active phenolic compounds from Rauvolfia serpentina Benth. Ex. Kurtz. Ind Crops Prod 45:406–4415. https://doi.org/10.1016/j.indcrop.2013.01.008
Nair VD, Raj RPD, Panneerselvam R, Gopi R (2014) Assessment of diversity among populations of Rauvolfia serpentina Benth. Ex. Kurtz. from Southern Western Ghats of India, based on chemical profiling, horticultural traits and RAPD analysis. Fitoterapia 92:46–60. https://doi.org/10.1016/j.fitote.2013.09.017
Namita B, Yadav M (2016) Evaluation of the chemical composition of Rauwolfia serpentina and Leucas Aspera - a comparative study. World J Pharm Pharm Sci 5:914–920. https://doi.org/10.20959/wjpps201610-7812
Nath A, Chattopadhyay A, Joshi SR (2013) Biological activity of endophytic fungi of Rauwolfia serpentina Benth: an ethnomedicinal plant used in folk medicines in Northeast India. Proc Natl Acad Sci-Sect B Biol Sci 85. https://doi.org/10.1007/s40011-013-0184-8
Negi JS, Bisht VK, Bhandari AK et al (2014) Quantification of reserpine content and antibacterial activity of Rauvolfia serpentina (L.) Benth. ex Kurz. African J Microbiol Res 8:162–166. https://doi.org/10.5897/ajmr2013.5847
Nikolaeva L, Alterman I (1993) Alkaloid formation in hairy roots and cell suspensions of rauwolfm serpentina benth. Nat Prod Lett 3:107–112. https://doi.org/10.1080/10575639308043846
Njerua SN, Matasyoh J, Mwaniki CG et al (2013) A review of some phytochemicals commonly found in medicinal plants
Nurcahyani N (2008) The reserpine production and callus growth of Indian Snake root (Rauvo/vla serpentlna (L.) Benth. Ex Kurz) culture by addition of Cu2+. Biodiversitas 9:177–179. https://doi.org/10.13057/biodiv/d090305
Omoya FO, Ade- O, Falusi M, Ogundare AO (2019) In vivo antimalarial activity and toxicological effects of ethanolic and hot water extracts of Rauwolfia serpentina leaf in mice infected with chloroquine- sensitive Plasmodium berghei. J Microbiol Antimicrob Agents 3:3–12
Owk AK, Lagudu MN (2016) In-vitro antimicrobial activity of roots Rauvolfia serpentina L. Benth Kurz. Natulac Sci Biol 8:312–316. https://doi.org/10.15835/nsb.8.3.9831
Pan S, Neeraj A, Srivastava K et al (2013) Effects of growth regulators on in vitro response and multiple shoot induction in some endangered medicinal plants. OA Biotechnol 2:1–7. https://doi.org/10.13172/2052-0069-2-1-428
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41
Panda SK (2014) Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J Ethnopharmacol 151:158–175. https://doi.org/10.1016/j.jep.2013.10.004
Pandey VP, Kudakasseril J, Cherian E, Patani G (2007) Comparison of two methods for in vitro propagation of Rauwolfia serpentina from nodal explants. Indian Drugs 44:514–519. https://doi.org/10.5281/zenodo.3597204
Pandey VP, Cherian E, Patani G (2010) EffEct of growth regulators and culture conditions on direct root induction of Rauwolfia serpentina L. (apocynaceae) benth by leaf explants. Trop J Pharm Res 9:27–34. https://doi.org/10.4314/tjpr.v9i1.52031
Pandey P, Kaur R, Singh S et al (2014) Long-term stability in biomass and production of terpene indole alkaloids by hairy root culture of Rauvolfia serpentina and cost approximation to endorse commercial realism. Biotechnol Lett 36:1523–1528. https://doi.org/10.1007/s10529-014-1495-4
Panwar GS, Guru SK (2011) Alkaloid profiling and estimation of reserpine in rauwolfia serpentina plant by TLC, HP-TLC and HPLC. Asian J Plant Sci 10:393–400. https://doi.org/10.3923/ajps.2011.393.400
Panwar GS, Guru SK (2013) Stimulation of reserpine production in the whole plant culture of Rauwolfia serpentina L. by elicitors and precursor feeding. J Plant Biochem Biotechnol 24:49–55. https://doi.org/10.1007/s13562-013-0235-5
Panwar G, Attitalla I, Guru S (2011) An efficient in vitro clonal propagation and estimation of reserpine content in different plant parts of Rauwolfia serpentina L. Am Eurasian J Sci Res 6:217–222
Pathania S, Randhawa V, Bagler G (2013) Prospecting for novel plant-derived molecules of Rauvolfia serpentina as inhibitors of aldose reductase, a potent drug target for diabetes and its complications. PLoS One 8:1–16. https://doi.org/10.1371/journal.pone.0061327
Pillai PP, Sajan JS, Menon KM et al (2012) ISSR analysis reveals high intraspecific variation in Rauvolfia serpentina L. - a high value medicinal plant. Biochem Syst Ecol 40:192–197. https://doi.org/10.1016/j.bse.2011.10.019
von Poser G, Andrade HHR, da Silva KVCL et al (1990) Genotoxic, mutagenic and recombinogenic effects of rauwolfia alkaloids. Mutat Res 232:37–43. https://doi.org/10.1016/0027-5107(90)90107-F
Prakash P, Rajakani R, Gupta V (2016) Transcriptome-wide identification of Rauvolfia serpentina microRNAs and prediction of their potential targets. Comput Biol Chem 61:62–74. https://doi.org/10.1016/j.compbiolchem.2015.12.002
Prasannakumar SP, Gowtham HG, Hariprasad P et al (2015) Delftia tsuruhatensis WGR-UOM-BT1, a novel rhizobacterium with PGPR properties from Rauwolfia serpentina (L.) Benth. ex Kurz also suppresses fungal phytopathogens by producing a new antibiotic-AMTM. Lett Appl Microbiol 61:460–468. https://doi.org/10.1111/lam.12479
Qureshi SA, Nawaz A, Udani SK, Azmi B (2009) Hypoglycaemic and hypolipidemic activities of Rauwolfia serpentina in alloxan-induced diabetic rats. Int J Pharmacol 5:323–326. https://doi.org/10.3923/ijp.2009.323.326
Raja PB, Sethuraman MG (2010) Studies on the inhibition of mild steel corrosion by Rauvolfia serpentina in acid media. J Mater Eng Perform 19:761–766. https://doi.org/10.1007/s11665-009-9541-4
Ramu AK, Ali D, Alarifi S et al (2020) Reserpine inhibits DNA repair, cell proliferation, invasion and induces apoptosis in oral carcinogenesis via modulation of TGF-β signaling. Life Sci 264:1–10. https://doi.org/10.1016/j.lfs.2020.118730
Rani A, Kumar K, Sanjeev K (2014) Effect of growth regulators on micropropagation of Rauvolfia serpentina (L.) Benth. J Appl Nat Sci 6:507–511. https://doi.org/10.31018/jans.v6i2.490
Rastogi S, Pandey MM, Rawat AKS (2016) Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine 23:1082–1089. https://doi.org/10.1016/j.phymed.2015.10.012
Ray A, Bhattacharya S (2008) Storage and plant regeneration from encapsulated shoot tips of Rauvolfia serpentina - an effective way of conservation and mass propagation. S Afr J Bot 74:776–779. https://doi.org/10.1016/j.sajb.2008.06.002
Ray S, Majumder A, Bandyopadhyay M, Jha S (2014a) Genetic transformation of sarpagandha (Rauvolfia serpentina) with Agrobacterium rhizogenes for identification of high alkaloid yielding lines. Acta Physiol Plant 36:1599–1605. https://doi.org/10.1007/s11738-014-1536-6
Ray S, Samanta T, Majumder A et al (2014b) Cytogenetic characterization of Agrobacterium rhizogenes transformed root lines of Rauvolfia serpentina. Nucleus 57:105–112. https://doi.org/10.1007/s13237-014-0112-1
Roja PC, Slpahimalani AT, Heble MR, Chadha MS (1987) Multiple shoot cultures of Rauvolfia Serpentina: growth and alkaloid production. J Nat Prod 50:872–875. https://doi.org/10.1021/np50053a016
Rolf S, Bruns HJ, Wichter T et al (2003) The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol. Eur Heart J 24:1104–1112. https://doi.org/10.1016/S0195-668X(03)00195-7
Roten L, Derval N, Sacher F et al (2012) Ajmaline attenuates electrocardiogram characteristics of inferolateral early repolarization. Hear Rhythm 2:232–239. https://doi.org/10.1016/j.hrthm.2011.09.013
Rungsung W, Dutta S, Mondal DN et al (2014) Pharmacognostical profiling on the root of Rauwolfia serpentina. Int J Pharmacogn Phytochem Res 6:612–616
Sabde S, Bodiwala HS, Karmase A et al (2011) Anti-HIV activity of Indian medicinal plants. J Nat Med 65:662–669. https://doi.org/10.1007/s11418-011-0513-2
Sachdev KS, Aiman R, Rajapurkar MV (1961) Antihistaminase activity of serpentine. Br J Pharmacol Chemother 16:146–152. https://doi.org/10.1111/j.1476-5381.1961.tb00307.x
Sagi S, Avula B, Wang YH, Khan IA (2016) Quantification and characterization of alkaloids from roots of Rauwolfia serpentina using ultra-high performance liquid chromatography-photo diode array-mass spectrometry. Anal Bioanal Chem 408:177–190. https://doi.org/10.1007/s00216-015-9093-4
Saibabu V, Fatima Z, Khan LA, Hameed S (2015) Therapeutic potential of dietary phenolic acids. Adv Pharmacol Sci 2015:1–10
Salma U, Rahman MS, Islam S et al (2008a) Mass propagation of Rauwolfia serpentina L.Benth. Pakistan J Biol Sci 11:1273–1277
Salma U, Rahman MS, Islam S et al (2008b) The influence of different hormone concentration and combination on callus induction and regeneration of Rauwolfia serpemtina L. Benth. Pakistan J Biol Sci Biol 11:1638–1641
Saravanan S, Sarvesan R, Vinod MS (2011) Identification of DNA elements involved in somaclonal variants of Rauvolfia Serpentina (L.) arising from indirect organogenesis as evaluated by ISSR analysis. Indian J Sci Technol 4:1241–1245. https://doi.org/10.17485/ijst/2011/v4i10/30164
Sarker KP, Islam A, Islam R et al (1996) In vitro propagation of Rauvolfia serpentina through tissue culture. Planta Med 62:358–359. https://doi.org/10.1055/s-2006-957903
Senapati SK, Lahere N, Tiwary BN (2014) Improved in vitro clonal propagation of Rauwolfia serpentina L. Benth – an endangered medicinal plant. Plant Biosyst 148:885–888. https://doi.org/10.1080/11263504.2013.845264
Shah SMA, Naqvi SAR, Munir N et al (2020) Antihypertensive and antihyperlipidemic activity of aqueous methanolic extract of Rauwolfia serpentina in albino rats. Dose Resp 18:1–7. https://doi.org/10.1177/1559325820942077
Shamon SD, Perez MI (2016) Blood pressure-lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst Rev. Rev 21:CD007655
Singh A, Singh PK (2009) An ethnobotanical study of medicinal plants in Chandauli District of Uttar Pradesh, India. J Ethnopharmacol 121:324–329. https://doi.org/10.1016/j.jep.2008.10.018
Singh DK, Srivastava B, Sahu A (2004) Spectrophotometric determination of Rauwolfia alkaloids: estimation of reserpine in pharmaceuticals. Anal Sci 20:571–573. https://doi.org/10.2116/analsci.20.571
Singh P, Singh A, Shukla AK et al (2009) Somatic embryogenesis and in vitro regeneration of an endangered medicinal plant sarpgandha (Rauvolfia serpentina L.). Life Sci J 6:57–62
Singh SK, Verma M, Ranjan A, Singh RK (2016) Antibacterial activity and preliminary phytochemical screening of endophytic fugal extract of. Open Conf Proc J 7:104–113. https://doi.org/10.2174/2210289201607010104
Singh HK, Charan AA, Charan AI, Prasad SM (2017a) Antifungal and antibacterial activity of methanolic, ethanolic and acetonic leaf extracts of sarpagandha (Rauwolfia serpentina). J Pharmacogn Phytochem 6:152–156
Singh M, Kaur R, Rajput R, Mathur G (2017b) Evaluating the therapeutic efficiency and drug targeting ability of alkaloids present in Rauwolfia serpentina. Int J Green Pharm 11:132–142
Sinha NK, Kumar G (2020) Rauwolfia serpentina: protocol optimization for callus induction from leaf explants. Int J Innov Sci Res Technol 5:289–292
Sreekumar S, Nisha NC, Biju CK, Krishnan PN (2014) Identification of potential lead compounds against cobra venom in Rauvolfia serpentina (L.) Benth. Ex Kurz. through molecular docking. Int J Pharm Res Dev 6:32–43
Srivastava M, Misra P (2017) Enhancement of medicinally important bioactive compounds in hairy root cultures of Glycyrrhiza, Rauwolfia, and Solanum through in vitro stress application. In: Production of plant derived natural compounds through hairy root culture. Springer, Cham, pp 117–132. https://doi.org/10.1007/978-3-319-69769-7_6
Srivastava M, Sharma S, Misra P (2016) Elicitation based enhancement of secondary metabolites in Rauwolfia serpentina and Solanum khasianum hairy root cultures. Pharmacogn Mag 12:S315–S320. https://doi.org/10.4103/0973-1296.185726
Starke K, Borowski E, Endo T (1975) Preferential blockade of presynaptic alpha-adrenoceptors by yohimbine. Eur J Pharmacol 34:385–388
Subandi M, Dikayani D, Firmansyah E (2018) Production of reserpine of rauwolfia serpentina [L] kurz ex benth through in vitro culture enriched with plant growth regulators of NAA and kinetin. Int J Eng Technol 7:274–278. https://doi.org/10.14419/ijet.v7i2.29.13331
Sudha CG, Reddy BO, Ravishankar GA, Seeni S (2003) Production of ajmalicine and ajmaline in hairy root cultures of Rauvolfia micrantha Hook f., a rare and endemic medicinal plant. Biotechnol Lett 25:631–636. https://doi.org/10.1023/A:1023012114628
Susila T, Satyanarayana Reddy G, Jyothsna D (2013) Standardization of protocol for in vitro propagation of an endangered medicinal plant Rauwolfia serpentina Benth. J Med Plants Res 7:2150–2153. https://doi.org/10.5897/jmpr11.066
Tiwari V, Trivedi SP (2019) Investigations on remedial role of Rauwolfia serpentina root extract against carbofuran formulation induced genotoxicity in Channa punctatus. J Exp Biol 40:1023–1028
Tiwari S, Shah P, Singh K (2003) Efficient in vitro clonal propagation of Rauwolfia serpentina L.: an important medicinal plant. Plant Cell Biotechnol Mol Biol 4:157–162
Upasani SV, Beldar VG, Tatiya AU et al (2017) Ethnomedicinal plants used for snakebite in India: a brief overview. Integr Med Res 6:114–130. https://doi.org/10.1016/j.imr.2017.03.001
Upasani MS, Upasani SV, Beldar VG et al (2018) Infrequent use of medicinal plants from India in snakebite treatment. Integr Med Res 7:9–26. https://doi.org/10.1016/j.imr.2017.10.003
Usmani G, Chawhaan PH, Mishra Y et al (2015) Geographical variation of total alkaloid and reserpine content in Rauwolfia serpentina (L.) Benth. ex. Kurz. Euphytica 202:427–434. https://doi.org/10.1007/s10681-014-1311-1
Vaishnav V, Sahoo D (2020) Comparative phytochemical analysis of stem and root extracts from Rauwolfia serpentina. Int J Adv Sci Res Manag 5:5–7. https://doi.org/10.36282/ijasrm/5.1.2020.1681
Vakil RJ (1949) A clinical trial of Rauwolfia serpentina in essential hypertension. Br Heart J 11:350–355. https://doi.org/10.1136/hrt.11.4.350
Van Eck JM, Smith FD (1997) Tissue culture. In: Environmentally safe approaches to crop disease control, 1st edn, pp 317–331
Vieira Pereira A, Santana GM, Góis MB, Sant’ana DMG (2015) Tannins obtained from medicinal plants extracts against pathogens: antimicrobial potential. In: The battle against microbial pathogens: basic science, technological advances and educational programs, pp 228–235
Wachsmuth O, Matusch R (2002) Anhydronium bases from Rauvolfia serpentina. Phytochemistry 61:705–709
Yahya AF, Hyun J-O, Lee J-H, Jung M-S (2007) Effect of explant types, auxin concentration and light condition on root production and alkaloid content of (L.) Benth. ex Kurz. J Korean For Soc 96:178–182
Yamamoto O, Yamada Y (1986) Production of reserpine and its optimization in cultured Rauwolfia serpentina Benth. cells. Plant Cell Rep 5:50–53. https://doi.org/10.1007/BF00269717
Zafar N, Mujib A, Ali M et al (2017) Aluminum chloride elicitation (amendment) improves callus biomass growth and reserpine yield in Rauvolfia serpentina leaf callus. Plant Cell Tissue Organ Cult 130:357–368. https://doi.org/10.1007/s11240-017-1230-7
Zafar N, Mujib A, Ali M et al (2019) Genome size analysis of field grown and tissue culture regenerated Rauvolfia serpentina (L) by flow cytometry: histology and scanning electron microscopic study for in vitro morphogenesis. Ind Crop Prod 128:545–555. https://doi.org/10.1016/j.indcrop.2018.11.049
Zafar N, Mujib A, Ali M et al (2020a) Cadmium chloride (CdCl2) elicitation improves reserpine and ajmalicine yield in Rauvolfia serpentina as revealed by high-performance thin-layer chromatography (HPTLC). 3 Biotech 10:1–14. https://doi.org/10.1007/s13205-020-02339-6
Zafar N, Muzamil A, Mujib A et al (2020b) Cadmium chloride (CdCl2) elicitation improves reserpine and ajmalicine yield in Rauvolfia serpentina as revealed by high-performance thin-layer chromatography (HPTLC). 3 Biotech 10:344. https://doi.org/10.1007/s13205-020-02339-6
Zenk MH, El-Shagi H, Arens H et al (1977) Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharanthus roseus, pp 27–43
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Varghese, R., Gothandam, K.M., Buot, I.E., Chandrasekaran, R., Ramamoorthy, S. (2023). Extinction of Medicinal Plants in Anthropocene Epoch: Special Reference to Rauwolfia serpentina. In: Ramamoorthy, S., Buot Jr., I.E., Rajasekaran, C. (eds) Plant Diversity in Biocultural Landscapes. Springer, Singapore. https://doi.org/10.1007/978-981-19-8649-9_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-8649-9_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8648-2
Online ISBN: 978-981-19-8649-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)