Abstract
The biosensors are seemed as a potential device to monitor and hit upon ecological pollutants, contaminants, and, more commonly, chemical or organic markers of potential intimidations to human well-being. They are essentially composed of a sensor element made up of either biologically active molecules or whole cells (live) coupled to a reporter/transducer technological element. The live cell biosensors may be based on animal tissues, unicellular microorganisms, or eukaryotic microorganisms, e.g., microalgae and yeasts. Various biosensors based on live cells have been disclosed in the prior arts for the past many years and these studies have revealed the prodigious prospective of their usage in the environmental pollution detection areas and in biomedical diagnostics. The particular characteristics of live cell biosensors lie in their potential to detect stress, toxicity, and bioavailability in situ, in addition to the benefits of easy use, quick response, sensitivity, portability, and low cost. All these factors make live cell biosensors an attractive device for health-associated applications. This chapter centers around bacterial live cell biosensors for diagnosis and treatment of cancer, innovation and identification of antibiotics, and assessment of fitness hazards. This chapter additionally considers the future insights and challenges of biosensors in clinical practices.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
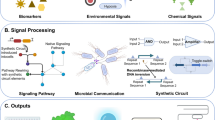
For detection and identification of a component present inside a cell, tissue, or organ of the body, a device called “biosensor” can be used. It is made up of various forms of chemical or physical transducers and biomolecule recognition elements [1, 2]. Based on their interdisciplinary construction nature, their developmental studies have been published in different fields of information science, chemistry, biology, and physics [3,4,5,6]. On the basis of variations in their cellular, tissue, and molecular sensing elements, the biosensors can be categorized into three different classes, i.e., molecular, cellular, and tissue [7]. The utilization of various biologically active molecules, e.g., antibodies, antigens, enzymes, DNA, and biofilms as reporter elements have been reported in molecular-based biosensors [8]. The foremost advantage of the molecular-based biosensor is its high selectivity [1], but the shortcomings like the short functional lifetime of the recognized molecules, expensive isolation costs of macromolecule, and restricted detection capability have limited applicability of this kind of biosensor [9]. The live cell biosensors that are produced from cells or intact tissue, in comparison to molecular-based biosensors, have seen rapid growth in novel immobilization and microfabrication methods, and these very recent vicissitudes have given specific and unexploited benefits to these types of biosensors [10]. The query that arises is then: why is it desirable to move deep into live cell (whole) based biosensors? A living cell is considered as a natural bio-catalyst factory and the process for obtaining these bio-catalysts (e.g., enzymes) involves a process of separation and purification of required bio-catalysts from the microbial strain or tissue, which is resource as well as time-consuming process [11]. The live cells produce a metabolic aggregate of enzymes, coenzymes, and cofactors, constituting a precise mechanism to guarantee chemical reactions which might be essential for their function, further, they self-control the recycling procedures for such materials; analog methods may be observed in tissues, but the necessities associated with preservation and price for microorganisms culturing are underneath from those of tissue cultures [12]. One benefit of utilizing live cells is that it is possible to achieve very complicated reactions by coupling several enzymes in one single step [13]. Therefore, the choice of an appropriate live cell for a bio-sensing application, basically, could obey the features rendered by means of the chosen microorganism on perceived surroundings, to set an instance, the organisms that live in harsh surroundings result in metabolic activities that involve the performance of specific compounds and are profusely available on the targeted environs. The prior example is not a limitation, inherent live cell is not confined to organisms that are harvested beneath extreme environments, and organisms present in friendlier surroundings can react to very precise stimuli, such circumstances allow the screening of various organisms as prospect candidates for a preferred bio-sensing application [14]. Even though the live cell biosensors are not susceptible to different ecological vicissitudes as molecular-based biosensors, they are possibly altered through simple recombinant techniques to facilitate their utilization to perceive a sequence of composite responses inside a live cell [1]. It is feasible to modify the genetic configuration of live cells consequently modifying a given organism’s enzymatic expression, transmitting the opportunity to respond to various substrates or to encompass responses that may be effortlessly examined, the induced reaction is arbitrated for what is called a bio-reporter gene [15, 16].
Further, the overall performance of a live cell biosensor involves the choice of reporter gene and the selectivity and sensitivity of the molecular recognition that occurs whereas target analytes are bind to their respective regulator proteins [17]. The detection of specific analyte species and amplification of this identification into an electrical and optical signal through a processor is the main mechanism of a typical live cell biosensor (Fig. 1). By the usage and immobilization of live cells or microbes as the component that offers the elements of molecular recognition, this readout process is detectable. The live cell-based biosensors, unlike a conventional biosensor, can perceive a broad variety of materials and therefore are more susceptible to modification in the tissue sample’s electrochemical state, other cells or in the surroundings, for the reason that they can genetically alter and can function in a wider range of conditions that involve different pH and temperature values [18,19,20]. Due to the evident benefits of live cell biosensors like their high selectivity, good sensitivity, and their ability for in situ detection (high-throughput), they have been implemented efficiently in arenas including pharmacology, food analysis, environmental monitoring, and drug screening [17].
Diagrammatic representation of a typical live cell-based biosensor [1]
In this chapter, the summarization is done of what is befallen recently within the development and design of live cell biosensors by highlighting their utility for biomedical diagnostics and environmental pollution monitoring, two regions where these biosensors are most usually applied. The consecutive segment of this chapter relates to the association between chemical transduction and live cell (whole) transduction, benefiting from the suggested grouping of the latter segment, it aids a bridge among various disciplines, and it is here clarified the criteria to be mindful of both interactions. The discrepancies between electrochemical effects in live cells (amperometric, potentiometric conductometric, and impedance sensors) and physiological effects are overcome, either because of respirometry, external stimuli, or the findings associated with bio-reporters. This chapter delivers a literature precis on the choice of host cells, regulatory proteins, reporter genes, and multi-functionalization for detecting various pollutants present in surroundings such as organic waste and heavy metals. It also provides an overview of current advances in the utilization of biosensors based on live cell in the area of micronutrient detection, precision medicine, and diseases diagnosis. In addition, this work offers an overview of the current problems and possible opportunities for the functional implementation of live cell-based biosensors. The last segment presents the latest innovations and the commercially available alternatives using live cell biosensors, further closing this chapter with the confab of the possible prospects and obstacles.
2 Live Cell-Based Biosensors: General Principles
The live cells have the capability to make critical vicissitudes on various substrates via a very well-described sequence of reactions; such modifications are benefitted as energy or as indispensable elements for the cell’s dynamic processes. An individual species can interact at the same time with numerous substrates, each of which is driven in every instance by a very specific sequence of chemical reactions that compose a metabolic pathway. The initiation of certain chemical reactions is mediated by the enzymes and they are programmed to complete a chain of events that ensure that metabolic and physiological responses are accomplished by the live cells to ensure survival.
A naturally occurring event that presents a modified energetic pathway would be an enzyme-catalyzed reaction [21]. The production of an enzyme is simultaneously controlled by the living cell’s genetic code, a distinctive imprint for every one-of-a-kind strain. The genetic integrity information is stored in what may be equated to the DNA (storage unit), i.e., the genome. Although, every subject has a different genome but with same basic functional unit, which will eventually suggest the uniqueness of the given strain. As a complete programming code, the resulting sequential amino acid arrangement constitutes the enzymatic structure [21, 22]. Through the coiling and folding of amino acids, the enzymes’ very complex 3D structures form fragments that serve as pockets prepared for the substrate’s coupling, known as active sites, which are fitted with a particular geometric shape and an atomic arrangement complementary to those of the substrate. Via the “lock-and-key theory” [23], a general method for enzyme-substrate binding mechanisms can be envisaged: Emil Fischer in 1894, proposed the rigid structures, enzymes would act as a lock with a particular shape; on the other hand, the substrate resembles a key: if an active site with correct shape is presented, it will act as an adequate key hole, so it will “unlock” the consequent enzymatic reaction ([24]; Fig. 2).
Schematic representation of the lock-and-key hypothesis (https://socratic.org/questions/58f64d5c11ef6b44e4d659b6)
David Koshland presented an alternative to Fischer’s postulate in 1958, by stating that the enzymes, rather than sturdy, are flexible; therefore the active site is actively altering its shape to adjust to the substrate and if there are some chemical bonds formed among the substrate and the active site in the enzyme, and are aligned to the catalytic groups, the reaction would only take place. The substrates can bind via covalent and hydrogen-bonds; ionic- or van der Waals forces, these interactions are usually weak, but it forms a solid binding with many of these weak interactions taking place at the same time. The postulate given by Koshland is acknowledged as the “induced-fit theory” [21, 23]. In order to understand selectivity, the necessary conditions are enclosed by the distinctive binding mechanism and the central of the enzymatic technique depends on the acceleration of the chemical reaction rate. Both mechanisms rely on the development of molecular interactions with the substrate, as is inferred from the induced-fit theory. Although the enzyme-substrate interaction at initial stage is weak, the growing number of bindings produced among the enzyme’s active site and the substrate, which is merely possible if there is a suitable substrate, will prompt structural vicissitudes on it until the firm attachment of the substrate to the enzyme is there; further, multiple substrates, coenzymes, and cofactors will be arranged by analogous mechanisms. Any of the four acceleration mechanisms could be caused by the correct alignment, to wit, covalent catalysis, approximation of the reactants, the introduction of distortion or strain in the substrate, or general acid-base catalysis [25, 26]. In terms of the thermodynamic characteristics of the system, a reaction can be understood, based on the first and second thermodynamics laws, which are energy conservation and increasing entropy. It was proposed by Willard Gibbs in 1873, a model, similar to the potential energy in classical mechanics, taking into account that to produce work under constant conditions of temperature and volume in a given closed system, would have a thermodynamic potential, namely Gibbs (G) free energy, originally referred as available energy [21, 27, 28].
In any chemical reaction, the free energy (maximum amount) derived, is determined by the difference between the free energy of the products and the reactants (ΔG), as soon as the energy is consumed by the reaction, i.e., the ΔG of reactants is less than that of the products, it is believed to be an endergonic reaction, i.e., it needs an external energy source, e.g., heat. But it is said to be exergonic when the energy is released from the reaction; it is thus named a thermodynamically favorable reaction, implicating that it may occur spontaneously. Then, when a spontaneous (exergonic) reaction occurred, if the energy is not delivered to the system (ΔS > 0), the entropy (S) will increase. Then again, if there is no change in entropy, the energy release can best be related to the system’s internal energy, a feature termed as enthalpy (H), as it is generally calculated as heat, the difference among the resulting and the initial reaction state (ΔH) is acknowledged as the heat of a reaction. Consequently, it is termed as exothermic (ΔH < 0), when the resulting process of the system releases energy, in contrast, it is named endothermic (ΔH > 0); it is required to pressurized that not every exergonic reaction is inevitably exothermic, i.e., the released energy is not always as heat, so a reaction with ΔG < 0 may have ΔH < 0, ΔH = 0 or ΔH > 0. In Gibbs fundamental equation, these features were correctly explained as follows:
The equation (Gibbs) describes a functional state, which shows that it relies exclusively on the equilibrium state of the system, irrespective of how it reached that state. Catalytic processes however pass within a tighter boundary of the chemical reaction procedure, not influencing the initial or final reaction state, but affecting the direction from one end to the other. The enzymatic process decreases the required free energy for the reaction to happen (Fig. 2). The frequency of unpremeditated chemical reactions is hindered by the influence of energetic barrier, designated as activation energy; in order for any reaction to happen, sufficient energy must be supplied to the system to out-perform it in a non-catalyzed reaction into a transition state; this can be done by heating up the system. This perilous moment signifies a state where the products and the reactants concurrently exist, due to the bonds concurrence, both from the product state and the reactant state. Such molecular form is exceptionally unsteady and hence associated with a huge quantity of free energy. The enzyme-catalyzed reaction would represent an alternative way for the reaction to happen [21, 25, 29].
It is easier to explain a predictable outcome through analytical applications devoid of much regard for the specifics of a particular metabolic pathway by considering the catalytic process as an energy-state transformation. Under constant temperature conditions, let us consider the Gibbs fundamental free energy equation again, as given in Eq. (1). Also consider, an exergonic reaction, such that there is neither energy delivered to the system, nor is it delivered by external means either because of a preceding enzymatic reaction to the metabolic pathway. In the simplest case, it should be concluded that from the system, there is no energy release, the merely probable result would be an upsurge in the system’s entropy. Dissociation of the elements can be estimated as soon as the substrate is consisting of molecules created by numerous chemical elements, and in certain situations, one or more of the molecules can be easily identified by an acknowledged technique of chemical sensing. If this is the situation, it might be believed that the sensing strategy would not be aimed at the substrate, but at a related reaction or a by-product. This situation will be referred to as a biotransformation-based strategy. A distinctive circumstance would be considered when the outcome of such biotransformation produces a readily detected bioluminescent signal, often considered as bio-reporter [16, 21, 30].
If no entropy change is considered, an additional case can be elucidated therefore the mere effect on the resulting free energy equation will be caused by an enthalpy modification. If the initial conditions agreed on those formerly suggested, the exothermic reaction would happen and the outcome would be an energy release that can be calculated by appropriate means. This method is classified as a stimuli response-based strategy. The actual situations of the reaction that are catalyzed by enzyme are a lot more composite than those considered above, the metabolic pathway creates a complicated reaction network that may be interconnected with different pathways, some of which are active during the course of the cell vital routines, others associated to the precise social behavior of various species, in both inter and intra species responses [31, 32]. Such situations combine diverse features on the enthalpy and entropic properties of the reaction, and the reaction’s exergonic-endergonic nature. But such combinations would result in a comparable analysis, favoring either the expression of the by-products (biotransformation-based strategy) or by energetic measurable changes approached (stimuli response-based strategy). Table 1 lists some benefits and drawbacks of using both strategies.
3 Live Cell Sensing Technique: Transduction
In the preceding section, two bio-sensing strategies based on the specific contributory thermodynamic effects of each term were introduced, namely Biotransformation-based strategy (BtB strategy), which is based on entropy-related intake (molecular-related response); and Stimuli response-based strategy (SRB strategy), which is based on the concept of enthalpy (energy-related response). In this segment, transduction is defined as the process by which the presence of a specific substrate causes an appropriate reaction to an information-translated measurable unit. For both BtB and SRB techniques, the notion presented in this part is closer to that posed by the analytical procedure of the physical context; nonetheless, few insights into the physics and thermodynamic foundation of some concepts are provided, which are frequently overlooked in the literature.
3.1 Biotransformation-Based Strategy
In the current scope, the biotransformation-based strategy is considered as a collection of by-product-mediated sensing methods. The dissociation and consumption of various compounds can be stringently considered in the chemical realm of many reaction outcomes. Different methods developed for specific substances can easily detect the transformation occurring within the living cell and not the generated energy straight inside the live cell. Table 2 lists several examples of the electrodes used.
The chemical bond conformation, from the viewpoint of the molecular interaction, resulting from specific pathways of the metabolism, functions as an additional choice for substrate targeting. The approaches provided by the living cell might be only possible if they are complemented with a different substance, e.g., dependence of aerobic organisms on the consumption of oxygen for completion of the processes within their complete metabolic network, the volume of oxygen on a contained environment can be utilized as a measurement unit of the living cell activity. Besides the optical, colorimetric, and electrochemical microbial biosensors, baroxymeter microbial sensor, based on manometric bacterial respirometry, is used to detect the pressure change, and the infrared analyzer-based microbial biosensor is used for the detection of the microbial respiration product CO2 [34].
Further steps beyond strain-specific reactions will be needed for the transduction of a biotransformation-based strategy; the biological assignment is restricted to the intermediate fabrication of agents to release additional chemical reactions that could, for example, be converted into signals (electrical), chemically similar to the approaches proposed for the transduction of the stimuli response-based strategy. The quantification and recognition of the by-product created must conform with simple procedures. The use of bio-reporters, defined by means of the unique reporter gene responsible for controlling the substance production on a particular metabolic pathway, could address the targeting of the production and intake of substances for reporting the existence of a substrate given [9]. The expression “bio-reporter” is commonly referred to in terms of very particular products that can be recognized, e.g., by fluorescence and phosphorescence-based optical instruments [21].
Various approaches leveraged from genetic engineering can insert bio-reporter genes into the cell genome [16, 21], they are involved in precise metabolic pathways because of the sequential nature of the deployment of the reactions within various pathways of the metabolism. The activation of the bio-reporter is happening only when the corresponding pathway and specific reporter gene have reached the same stage thus reporting on the sought substrate’s presence. The molecules released become the focus of different chemical sensing approaches, connecting the translation for substrate quantification, in an identical way, the electrical response to the stimuli response-based technique is proportional to the quantity of substrate detected, as there is less/more substrate concentration, this molecule will be consumed/released.
3.2 Stimuli Response-Based Strategy
The stimuli response-based strategy is supposed underneath the energy intake/release principle, the promising example of an exothermic reaction. In view of this primary glance, when acting together with a substrate, such an approach may be considered to be based on the living cell (whole) heat production as a responsive event. But, while the principle involves a direct situation to the thermal characteristics of the reaction, the energy intake/release is not inherently of thermal nature, it is also important to consider the effects on the electric domain. For the consideration of the stimuli response-based strategy, the reactions powered by the enzymatic behavior of the live cell are based on ionic effects, such as ionic transport occurrences [35], that cause alterations over an adequate substrate, namely electrode, freely measurable on the electric domain.
The most common reaction involved in biological methods is named as redox (reduction oxidation) reaction. When it is deployed, the reactants undergo an electron transfer process, that produces Gibbs’ free energy (fractional quintets, [36, 37]), which is released in the form of heat. The breaking, forming, and reconfiguring of the atomic bonds often contribute to the heat production, both effects imitate the metabolic activity of the specific strain when interacting with particular substrates. Thermal vicissitudes react straight to these chemical reactions [28]. The released energy in these reactions is effortlessly benefitted by using the temperature detection approaches relevant to biological interactions through micro-calorimetric techniques, defined initially by Rubner [38], considering the heat flow measurement of biological process, that advances correspondingly as chemical interactions happen [39]. Akin interaction takes place on the interfaces of the electrode with live cell and this electrode acts as an effective redox reaction electron acceptor/donor set up on the substrate’s existence. The ionic clusters released transport to a lower concentration region from a higher concentration region; production of a diffusion transport effect due to the concentration gradient [28]; the potential of the cell was first reported by Nernst, who interrelated such potential to the free energy state (Gibbs) of the reactants [40]. There is a free energy-dependent alteration for every chemical reaction [41]; the changes prompted on the electrode transform its electric structure and produce electrical units (readily measurable); usually, such reaction attains either a potential or charge accumulation (potentometric), a difference in current (amperometric), modifies the conductive characteristics among surfaces (conductometric), creates vicissitudes on the impedance (impedimetric) or potentiometric vicissitudes on a gate electrode (field-effect, [35, 42, 43]).
The microbial fuel cells (MFC) biosensors provide an additional interaction for the considered reaction [21, 44]. The ensuing ions that are required by the products of live cell enzymatic reactions or which are disconnected from them may be swapped across the cell membrane, and the ionic concentration gradient creates a quantifiable electromotive force as an electric energy difference. On the other hand, not all cell has the capability to transfer the ion cluster to the electrode directly because of the membrane’s non-conductive nature; a membrane is usually utilized that will assist as a selectively transfer protons/electrons mediator to the electrode. Hitherto, certain electrochemically active species [45], such as Rhodofoferax ferrireducens [46], Aeromonas hydrophilia [47], Enterococcus gallinarum [21], Geobacter sulfurreducens [48], Desulfoblus propionicus [49], Clostridium butyricum [50], and Shewanella putrefaciens [21], would be capable to provide a microbial fuel cell (mediator less). Since the reaction is driven by the substrate transformation, the resulting electrical gradient is proportional to the concentration of the substrate; therefore, the output signal is a quantitative indicator that depends precisely on the quantity of the substrate that interacts with the cell surface [21]. Under the current concept, the detection proposed for the utilization of the stimuli response-based strategy is arbitrated only by an electrode usage, interfaced directly with the live cell or arbitrated through an electron/proton exchange membrane. The stimuli response-based strategy is considered for some of the exposed circumstances where the given substrate interaction creates a measurable change over an electrode, interfacing the live cell; these strategies generally include the cell immobilization on the electrode surface, cell trapping techniques for immobilization of polyvinyl alcohol (PVA, [51]), immobilization crosslinking method [52], hydrogel immobilization [53], and physical confinement [54], among others.
The two characteristics shared by the approaches set out in this strategy are the direct use of electrodes for the evaluation of proportional electrical measurement and the prevalence of the contribution of the system’s enthalpy to the total reaction. The consideration of using a stimuli response-based strategy with an acceptable live species is beneficial if as a non-mediated direct response, a one-reaction one-response measure is essential, resulting in quicker quantity acquisition, less composite data transduction systems, and less possibility of side-effect reactions to enhance noise to the measure.
4 Live Cell Biosensors: Recent Advances
The selection of an appropriate approach for biosensor development reacts to different features hindered by the selection of the strain utilized. One of the key obstacles is to identify the particular substrate that can be directed by different species, or which species is ideally suited to a particular target. A thorough analysis of some cases is performed in Table 3, compiling different whole-cell strains, differentiating the recorded genetically modified strains, and documenting different targets alleged to be recognized for those strains.
The cell (whole) biosensors show a potential alternative to utilize in early warning screening because of their quick reaction to toxins according to the US Environmental Protection Agency [89]; some technologies are also disclosed in the same report, under the recent commercial application, but in accordance with the definition of biological test, including BioTox™ [90], ToxScreen-II (currently III) [90], POLYTOX™ [91], DeltaTox® [92], and microMAX-TOX, the latter is a potential device proclaimed by the Italian company Systea S.p.a. [93] that would shield the probable features of a biosensor based on the whole cell for continuous and online monitoring. Some of the commercially available biosensors based on whole cell are given in Table 4.
For commercial use, the recent preference for the use of the biotransformation-based strategy is clearly observed, while researchers have made efforts to build biosensors on the fringes of the stimuli response-based strategy. A novel generation of biosensors focused on the possibilities of different strains to change the given electrical structures of the electrode will lead to the trend posed by the current developments within the framework of the stimuli response-based strategy. In addition, the benefits of the excessive use of chemicals and the quick response will undoubtedly function significantly in favor of the inclusion of the stimuli response-based strategy biosensors development. The imminent innovations will involve the use of Archaea as a promising means for the highly efficient stimuli response-based strategy biosensors development, an important area to explore is the affinity of such domain-type live cells with different substrates and the potential it provides for strong electrode reactions.
4.1 Live Cell-Based Biosensors in Medical Diagnostics
The ion channels, enzymes, and receptors are a few of the molecular recognition components that are available and expressed by whole cells. These compounds are frequently vulnerable to their respective analytes due to their inherent biological mechanism [105]. Biosensors based on living cells can thus be utilized to monitor and analyze a range of physiological indicators in real time. As a result, these whole cell-based sensors can be utilized at the cellular level to understand biological metabolic states and other disorders, resulting in their widespread use in biomedicine, such as cellular physiological analysis, pharmacological evaluation, and medical diagnosis.
4.2 Precision Medicine
The latest investigation goal of precision medicine is to understand the genomic alteration of the biological target of a pharmacologically active medicinal compound, e.g., G-protein-coupled receptors and enhancements in their response towards drug. In order to characterize drug responses mediated by G-protein-coupled receptors in lymphoblastoid cell lines, a new biosensor based on the live cell was designed (label-free, [106]). This suggests that they could be used as a cellular model system to investigate the pharmacology of G-protein-coupled receptors in vitro in precision medicine. By means of a bullfrog fibroblast cell line, Feng et al. [107] invented a cell-based biosensor expressing G-protein-coupled receptors as its basis, to assess these receptors’ activity and determine the adrenaline quantity they secreted. At this point, a dominant downstream target gene p21 (tumor suppressor gene) of the activated p53 protein was utilized because it is vulnerable to carcinogens and can thus serve as a sensor. In another study, a human hepatoma cell-based biosensor was tested by Zager et al. [108] that under a p21 promoter regulation, utilized a plasmid encoding enhanced green fluorescent protein (EGFP) to detect genotoxic agents easily and quickly.
4.3 Detection of Micronutrients
The live cell-based biosensors have another area of application in the arena of micronutrients. The lack of a crucial vitamin for human well-being, i.e., riboflavin can lead to serious ailments, e.g., cataracts, metabolism disorders, and some cancers [1, 109]. It is also harmful to excessively intake riboflavin which further contributes to oxidative damage in light-exposed tissue [110]. To resolve this, Si et al. [111] described a biosensor based on a whole-cell (bio-electrochemical) system for the amperometric detection of riboflavin. A bio-electrochemical wire was developed, consisting of cytochrome C strung and riboflavin between S. oneidensis MR-1 as shown in Fig. 3. The addition of riboflavin to the bio-electrochemical wire system resulted in an electrochemical response. A 200-fold increase in electrochemical signal output was observed compared to traditional chemical biosensors. There was a wide linear range (5 nM–10 μM, 3 orders of magnitude), a high sensitivity (2.2 nM, S/N = 3), and a high resistance to signal interference in the cell-based biosensor.
Diagrammatic representation of an electron transfer pathway utilized in biosensors based bio-electrochemically on live cell. (OM outer cell membrane, Cyto C cytochrome C proteins, and FccA fumarate reductase, [111])
4.4 Diseases Diagnosis
A significant objective in the disease diagnosis is the fast and reliable identification of pathogens. As microorganisms in patient urine and blood samples must initially be pre-cultured to adequate quantities for their detection, traditional approaches of microbiology may take a number of days to weeks. Thus, for the precise detection of microorganisms that did not involve this lengthy culture stage, a new method based on the biosensors (whole cell) was developed [112]. As seen in Fig. 4, through impedimetric detection of E. coli, the cell-based biosensor, which comprised bacteriophages involved as recognition receptors, was immobilized to a functionalized carbon electrode (screen-printed) covalently.
Diagrammatic representation of the assay for immobilizing phages onto electrochemical electrodes [112]
In the diagnosis of diseases, biosensors based on cells may offer high-content screening and analysis. For instance, the microbial genome (4–10%) and its proteome (more than 20%) were affected by microbe quorum-sensing molecules, suggesting that quorum-sensing was correlated with the basic metabolic processes as well as the production of the modulate virulence factor [1]. The biosensors based on the cells have also been utilized as noninvasive procedures to assess quorum-sensing molecules in physiological samples collected from patients suffering from bacterial gastrointestinal disorders [113]. Detection limits of quorum-sensing molecules in biological matrices have been improved to the nanomolar level [114]. This was significant because the key reason for human morbidity and mortality observed in sub-Saharan African at-risk adults and young children is invasive non-typhoid Salmonella [115]. A cell-based electrochemical immune-sensor, hosted in yeast, was also documented by Venkatesh et al. [116] to detect invasive non-typhoid Salmonella antigens. The yeast cells were genetically engineered in that study to display on their surfaces, gold-binding peptide as well as single-chain variable fragment (scFv) antibodies. A wide dynamic range with high nanomolar sensitivity was shown by the resulting cell-based biosensor and was capable of detecting invasive non-typhoid Salmonella OmpD antigens [116]. The advantages of cell-based biosensors have evolved in part due to their ease of use and quick deployment for the diagnosis of a variety of illnesses. As a result, biomedical diagnosis techniques based on live cell-based biosensors showed great promise in the field of biomedical engineering.
5 Application of Cells as Biosensors Against Environmental Analytes
5.1 Bioavailability Detection
A substantial research subject is the rapid identification of environmental pollutants and the assessment of their health impacts. To determine the exact content and composition of pollutants in a given sample, traditional chemical and physical-based analytical approaches may be highly reliable and sensitive, but only limited varieties of pollutants may be examined for their bioavailability, genotoxicity, and toxicity [117]. In many circumstances, when using living cells, it is only possible to measure some important parameters [118]. Two distinctive benefits of the biosensors based on the cells include: (1) the effortlessness with which they can be field trialed and (2) the effortlessness with which the samples containing a bioavailable pollutant can be detected.
An example of this is given in Fig. 2, where a live cell-based biosensor Pseudomonas putida and a high-performance liquid chromatography were investigated and compared for their capability to detect phenanthrene, added to red soil samples [119]. At variable ranges from 10 to 60 mg/kg, the initial concentration of phenanthrene was measured. It was found that high-performance liquid chromatography detected approximately 80% phenanthrene. The application of cell-based biosensor Pseudomonas putida, on the other hand, has been able to bioavailable fraction detection at levels much lesser than those detected with the total phenanthrene material. During high-performance liquid chromatography measurement, this was largely due to the sample extraction process. Peltola et al. [120] have checked the copper and lead concentrations bioavailability in natural soil using an analogous bioluminescent live cell-based biosensor in addition to the identification of organics. Again, this finding was consistent with the findings shown by the phenanthrene study; the cell-based biosensor was used to achieve a much higher selectivity. The inorganic study was also reported to be costlier, tedious, and allowed the procedure to be performed in a specialized laboratory. Finally, it has been shown that the cell-based biosensors can continuously monitor, in real time and in the sample (in situ), the bioavailability and concentration of toxic compounds [121].
5.2 Reporter Genes
Usually, to detect environmental pollutants, the efficiency of live cell-based biosensors depends on the reporter genes selected for transcriptional contaminant regulation and the nature of regulatory protein allied with these promoters. A reporter gene present in the living cells utilized as a sensor may translate its biotic response into a physicochemically detectable signal. The live cell-based biosensors’ selectivity and sensitivity are essential for this process. There are several commonly operated reporter genes that have been revealed to integrate effectively into biosensors, e.g., firefly Luciferase (luc), bacterial luciferase (lux), β-galactosidase (lacZ), and green fluorescent protein (gfp). It can be difficult to pick which reporter gene to use because there are a large number of the same to choose from [1]. A few of the benefits and drawbacks of widely described reporter genes employed to generate a biosensor based on cells are listed in Table 5.
The reporter gene (Gfp) codes for the green fluorescent protein (GFP) and it does not need an ATP or a substrate to emit as it autofluorescence [127]. But the intrinsic fluorescence of some cells (host) usually upsurges the background fluorescence besides this reporter, and this may result in signal interference. Therefore, biosensors based on gfp are typically unable to investigate with as much sensitivity as other lacZ and lux-based biosensors [128]. Moreover, in order to emit a stable fluorescence, GFP takes a longer period, decreasing its maximal detection activity [129].
Thus, live cell biosensors based on gfp are usually incompatible with rapid contaminants detection. Similarly, the bacterial luciferases (lux) largely suffer from dimeric protein interference and thermal lability and also restrict its application as a mammalian cell’s reporter gene [1]. The luc (firefly luciferase) reporter gene was often fused into mammalian cells to avoid these limitations in view of its wide linear range (up to 7–8 orders of magnitude) and high sensitivity [130]. A well-characterized bacterial enzyme, β-Galactosidase (lacZ), another reporter, similarly was widely utilized in molecular biology as it provides an outstanding transfection efficiency monitor. For identification using either fluorescent or colorimetric methods, the lacZ has some specific advantages as its usage with a sample is easy and quick [169]. The broad availability of lacZ electrochemical and chemiluminescent substrates also offers the benefits of ultra-high sensitivity, an extensive dynamic detection range, and low detection limit (as low as 2 fg, [1]).
CrtA, a unique type of reporter gene created by Fujimoto et al. [131], is responsible for carotenoid production in another reporter system. When applied to a sample, without the addition of a supporting substrate, the crtA-based live cell biosensors shift the culture media’s color from yellow to red and are therefore appraised as a good choice for quick detection in emergency circumstances [132]. For the identification of environmental contaminants, e.g., organic and waste heavy metals, Table 6 compares the bio-sensitivity of developed biosensors based on cells in recent times. In these systems, a number of cell lines and reporter genes have been used. Sharma et al. [138], for example, utilized E. Coli and luxCDABE to construct biosensors based on live cells which were demonstrated to have a detection sensitivity of 0.74 g/L when arsenic was introduced to water (10 g/L), which is well below the EU and US minimum safety criteria for arsenic. Furthermore, the sensitivity of live cell biosensors for detecting organic waste was shown to be exceptional, with enormous potential.
5.3 Regulatory Proteins
The complex interactions between regulatory protein and the target analytes of the contaminants of interest are important for the sensitivity and specificity of the biosensors based on live cell. There are several research studies elsewhere with the metallo-regulatory protein’s discovery in recent years that have used these biosensors in soil and water samples to detect heavy metals. Compared to traditional biosensors, they have revealed improved sensitivity, increased detection ranges, and greater selectivity, e.g., a MerR family’s regulatory protein (GolS protein), was described to have a great discernment for Au ions [144]. It was also observed that the detection ranges of GolS-based live cell biosensors were enhanced by including in the GolS protein, a single amino acid at the position 77 [145] and became ideal for the detection of cadmium, lead, mercury, and/or gold ions (Fig. 5).
(a) GolS77C-based biosensor platform, the sensor protein GolSS77C is expressed utilizing its chromosomally encoded gene with an operon with golT encoding the P1B-type Au (I) transporter (b) Genetic organization of the site selected for the golTSS77C-cat locus insertion in the E. coli chromosome [145]
A cysteine-rich peptide (Metallothionein, MT) was disclosed to possess a great affinity towards numerous heavy metals and contained five isoforms encoded by genes of T. thermophila [146, 147]. Further, a biosensor based on a live cell with MTT1 and MTT5 promoters (parted from MTs) was fabricated by Amaro et al. [118] and reacted sturdily and quickly to the heavy metal contaminants’ existence. The Sinorhizobium meliloti chpA promoter was stably stimulated by the pesticide chlorpyrifos, according to Whangsuk et al. [148], who used the transcriptional activator ChpR. The biosensor based on a promoter (chpA) was introduced in E. coli in another study utilized for the chlorpyrifos detection of chlorpyrifos, over a linear response range of 25–500 nM [149]. Elsewhere, an extremely sensitive biosensor based on cells containing the ars operon’s promoter region and a reporter gene, the crtA gene was developed by Fujimoto et al. [131] and was found to detect arsenite efficiently. And when the arsenite concentration was present at 5 μg/L, the color change was clearly recognized by the naked eye. Ars operon has also been reported to be associated with resistance towards arsenite, whereas the crtA gene, that regulates the Rhodovulum sulfidophilum’ carotenoids synthesis, was responsible for shifting the color of the culture from yellow to red as the arsenite concentration changed [132].
DNA microarrays technology can be used as a high-throughput process for positive regulatory genes selection for biosensors as these arrays comprise several proteins, one of which usually reacts to the existence of the contaminant of interest in a sample, e.g., the use of DNA microarray information to choose suitable biomarker genes that were strongly induced following the paraquat toxin’s exposure [150]. An alkane-inducible (AlkSp protein) biosensor, for short-chain alkanes detection was fabricated via two rounds of directed evolution of the transcriptional regulator, and the inventors were capable to demonstrate a fivefold increase in the emission in its reporter signal [151].
5.4 Host Cells
The host cell type choice is also vital as the sensitivity, specificity, and time-response of a biosensor can be greatly affected by the host cell type utilized as the sensing vehicle. In the meantime, there is a great resemblance between a host organism and eukaryotic-based biosensors in terms of genome, metabolism, and cellular organization, about 85% of live cell (eukaryotes) based biosensors are presently utilized for metal detection [152]. For the perception of monocyclic aromatic compounds in various ecological samples, Hernández-Sánchez et al. [153] constructed many cell-based biosensors utilizing different host cells, on the other hand, the same recombinant regulatory framework. The Alcanivorax borkumensis SK2 biosensor was reported to have a higher salinity tolerance but a lower pollutant tolerance and showed the best performance for the detection of contaminants at low concentrations in seawater samples. Because of its highest solvent resistance at high concentrations, the biosensor based on P. putida DOT-T1E was established as the best alternative for highly polluted conditions before it became saturated [151]. Because of the emulsifying capacity and low accessibility of the E. coli-oil mixture to oil droplets, obtaining tiny oil droplets in the E. coli-oil mixture was difficult. However, A. baylyi ADP1 and its derivative ADPWH-alk (the circles in Fig. 4) were definitely an oil-water interface adhesive and could emulsify both crude and mineral oils into 10–80 m diameter oil droplets [154]. These characteristics make ADP1 an exceptional microbial substrate for the live cell-based biosensors’ production to detect a wide variety of carbon chain length alkanes and alkenes (C7–C36) contained in water, seawater, and soils samples [154,155,156].
The Alcanivorax borkumensis-based biosensor, specializing in assimilation of linear alkanes, revealed a fourfold lesser fuel octane detection sensitivity (0.5 μM), in comparison with the biosensors that used E. coli as a vehicle. When evaluating the very low concentrations of petrol or pure alkanes in the samples, this performance improvement was most apparent [157]. Brutesco et al. [158] prepared Deinococcus deseri (a radiation and desiccation-tolerant environmental bacterium)-based functional biosensor and stated that after 7 days of storage, these sensors were able to detect arsenite. In potable water, for nickel detection, numerous cell-based biosensors have also been prepared from different E. coli strains. The E. coli (TD2158 wild-type) exhibited a ten times greater sensitivity and activity than E. coli K12 (W3110) equivalent, even supposing the identical mechanism was utilized with a natural target promoter (rcnA) fused to the luc reporter genes and RcnR Ni/Co metallo-regulator was used [159]. The limit of detection for nickel was stated to be very low (80 nM) in this analysis and was therefore accounted to meet the necessary quality requirements for most potable water. These analyses have shown that the host bacteria selection has a major effect on the efficiency of a fully fabricated live cell-based biosensor.
5.5 Multi-functionalization
Despite the fact that live cell-based biosensors generally exhibit enhanced performance of sensing than traditionally available biosensors based on chemicals, the subject prior art has focussed on enhancing the sensors’ accuracy, sensitivity, and applicability. This concern probably reveals an understandable response to the extensive and enhanced pollution levels [160]. Diverse types of functional cell-based biosensors have recently been mixed together and it was found that it showed improved identification and measurement of contaminants than a single type of biosensor, e.g., for the monitoring of water samples, a combination of a yeast-based estrogenic activity assay and bioluminescent bacteria-based toxicity screening was applied [161]. Via a regulatory proteins mixture, such as ZntR, CadC, and ArsR, numerous biosensors were prepared, that then reacted concurrently when numerous different metals were added [1, 162]. In another example, a tailored biosensor set based on live cells was produced by examining an E. coli sensor set utilizing binary linear and regression equations for the refining purpose of the accurate and specific bioavailablity recognition of Pb, As, and Cd in co-polluted surroundings, to reduce the signal interference generated when diverse metals were presented (e.g., Pb, Cd, and As, [163]). In that study, the sets of sensors were categorized into two different groups in accordance with their particular response to Pb, Cd, and As. Group 1 had pzntRluc and pcadCluc sensors to detect the bioavailability of Pb and Cd, while Group 2 had a parsRluc sensor to detect the particular bioavailability of As. The higher concentration ranges of mixes resulted in a linear increase in the relative light unit. To evaluate the bioavailability concentrations of Pb, Cd, and As in samples of soils from a polluted mine site, two sensor groups with three binary liner equations were used. With Group 1, using a linearly improved relative light unit, the coinciding ranges of concentration (0.1–1.0 μM) of mixed Cd-Pb were calculated. The overlapping concentration ranges for Group 2 were found to be 5.0–10.0 μM for Cd and 0.1–1.0 μM for As. These findings showed in this study that the bioavailability of the detected heavy metals appeared to be overestimated by a traditional single target cell-based sensor device. When a multiple cell-based biosensor was applied, more detailed bioavailability data was obtained.
This chapter, in some way, stems from growing trepidations about the alleged “antibiotics crisis” involving too much unnecessary pesticides, antimicrobials utilization, and their unregulated release into the environment [164]. There is a crucial need for biosensors based on live cell fabrication for the steady and quick detection of various antibiotics in samples obtained from tremendously contaminated environment, e.g., the P. putida DOT-T1E-based microbial biosensors have presented a detection capability for a broad range of structurally different antibiotics because of its environmental adaptability and its tolerance to numerous toxic organic compounds [165]. Camanzi et al. [166], on the other hand, found that following rehydration many months after freeze-drying, bacteria transmitted a steady light signal. Prévéral et al. [167] also report the fabrication of a cell biosensor based on arsenite that kept its performance for the sensitive detection of arsenite 7 months after lyophilization.
While compared to their traditional chemical equivalents, the live cell-based biosensors have usually shown superior efficiency, their commercialization realization is still a major challenge. Stability and reproducibility are still not sufficiently effective during long-term storage and transport to meet the requirements for large-scale production. However, Camanzi et al. [166] discovered that several months later, after their rehydration, freeze-dried microorganisms released a stable luminous signal. Another study proposed a flow-through biosensor based on disposable modular biochips including agar-immobilized bioluminescent recombinant reporter bacteria for online and continuous monitoring of water toxicity [168]. This cell-based biosensor was revealed to function properly in continuous flowing water over several days.
6 Future Perspectives and Conclusion
Due to the speedy advances seen in cell immobilization and in microfabrication, the biosensors fabricated from active cells have become an attractive arena for investigators. By translating signals that produce their homeostasis part into outputs (optical and electrical) that may then be detected, the biosensors based on live cell can quantitatively recognize data related to the position of live microbial or animal cells. These live cells can offer rapid, unique, and real-time information streams of the cell’s homeostatic position and, by implication, and its microenvironment, with very high selectivity and sensitivity compared to traditional chemically based biosensors. This study explored recent progress in the production, use, and implementation of live cell-based biosensors across numerous arenas, with a specific emphasis on the arenas of medical diagnostics and ecological pollution monitoring. The biosensors based on live cells have numerous benefits, but their use is also limited. A huge number of contaminants and other naturally occurring molecules are present in most environmental samples examined and together they cover the signal from the analyst of interest. The toxic nature of the samples is another issue, as they may comprise organic contaminants or heavy metals and their presence may restrict the option of cells used to resist the action of those microbes. Finally, as these cells experience diffusion or leakage, emission from sensors based on live cell employed for prolonged time periods may become unstable over time. The potential prospects of biosensors based on live cells can prove exciting. Another possible goal for live cell-based biosensors is the development of precise and multi-functional biosensors for speedy and real-time detection in extremely unfriendly environments with extreme acidity, alkalinity, severe temperature, high salinity, and poisonous substrates. For intense environmental research, it could be envisaged that several strains (cells), e.g., halophiles, thermophiles, and alkaliphiles, etc., could be utilized as host cells for their application as live cell-based biosensors.
References
Gui Q, Lawson T, Shan S, Yan L, Liu Y. The application of whole cell-based biosensors for use in environmental analysis and in medical diagnostics. Sensors. 2017;17(7):1623.
Nikhil B, Pawan J, Nello F, Pedro E. Introduction to biosensors. Essays Biochem. 2016;60(1):1–8.
Cheng R, Liu Y, Ou S, Pan Y, Zhang S, Chen H, Dai L, Qu J. Optical turn-on sensor based on graphene oxide for selective detection of D-glucosamine. Anal Chem. 2012;84(13):5641–4.
Wen W, Chen W, Ren QQ, Hu XY, Xiong HY, Zhang XH, Wang SF, Zhao YD. A highly sensitive nitric oxide biosensor based on hemoglobin–chitosan/graphene–hexadecyltrimethylammonium bromide nanomatrix. Sensors Actuators B Chem. 2012;166:444–50.
Zhang W, Chen C, Yang D, Dong G, Jia S, Zhao B, Yan L, Yao Q, Sunna A, Liu Y. Optical biosensors based on nitrogen-doped graphene functionalized with magnetic nanoparticles. Adv Mater Interfaces. 2016;3(20):1600590.
Zhang W, Li X, Zou R, Wu H, Shi H, Yu S, Liu Y. Multifunctional glucose biosensors from Fe 3 O 4 nanoparticles modified chitosan/graphene nanocomposites. Sci Rep. 2015;5:11129.
Pancrazio JJ, Whelan JP, Borkholder DA, Ma W, Stenger DA. Development and application of cell-based biosensors. Ann Biomed Eng. 1999;27(6):697–711.
Baldassarre A, Mucci N, Lecca LI, Tomasini E, Parcias-do-Rosario MJ, Pereira CT, Arcangeli G, Oliveira PA. Biosensors in occupational safety and health management: a narrative review. Int J Environ Res Public Health. 2020;17(7):2461.
Daunert S, Barrett G, Feliciano JS, Shetty RS, Shrestha S, Smith-Spencer W. Genetically engineered whole-cell sensing systems: coupling biological recognition with reporter genes. Chem Rev. 2000;100(7):2705–38.
Liu Q, Wu C, Cai H, Hu N, Zhou J, Wang P. Cell-based biosensors and their application in biomedicine. Chem Rev. 2014;114(12):6423–61.
Robinson PK. Enzymes: principles and biotechnological applications. Essays Biochem. 2015;59:1–41.
Richter M. Functional diversity of organic molecule enzyme cofactors. Nat Prod Rep. 2013;30(10):1324–45.
Lin B, Tao Y. Whole-cell biocatalysts by design. Microb Cell Factories. 2017;16(1):106.
Vasudevan N, Jayshree A. Extremozymes and extremoproteins in biosensor applications. Encycl Mar Biotechnol. 2020;3:1711–36.
Salis H, Tamsir A, Voigt C. Engineering bacterial signals and sensors. In: Bacterial sensing and signaling. Vol. 16. Karger Publishers; 2009. p. 194–225.
Van Der Meer JR, Belkin S. Where microbiology meets microengineering: design and applications of reporter bacteria. Nat Rev Microbiol. 2010;8(7):511–22.
Raut N, O’Connor G, Pasini P, Daunert S. Engineered cells as biosensing systems in biomedical analysis. Anal Bioanal Chem. 2012;402(10):3147–59.
Behzadian F, Barjeste H, Hosseinkhani S, Zarei AR. Construction and characterization of Escherichia coli whole-cell biosensors for toluene and related compounds. Curr Microbiol. 2011;62(2):690–6.
Ben-Yoav H, Biran A, Pedahzur R, Belkin S, Buchinger S, Reifferscheid G, Shacham-Diamand Y. A whole cell electrochemical biosensor for water genotoxicity bio-detection. Electrochim Acta. 2009;54(25):6113–8.
Tian Y, Lu Y, Xu X, Wang C, Zhou T, Li X. Construction and comparison of yeast whole-cell biosensors regulated by two RAD54 promoters capable of detecting genotoxic compounds. Toxicol Mech Methods. 2017;27(2):115–20.
Hernández CA, Osma JF. Whole cell biosensors. Biosensors: recent advances and mathematical challenges. 2014. p. 51–96.
Siegfried E. Genes code for proteins. In: Siegfried E, editor. Lewin’s genes X, vol. 136. Jones and Bartlett Publishers; 2011. p. 26–41.
Koshland DE Jr. The key–lock theory and the induced fit theory. Angew Chem Int Ed Engl. 1995;33(23–24):2375–8.
Tripathi A, Bankaitis VA. Molecular docking: from lock and key to combination lock. J Mol Med Clin Appl. 2017;2(1).
Jencks WP. Catalysis in chemistry and enzymology. Dover Publicatons; 1987. p. 7–322.
Johnson KA. Role of induced fit in enzyme specificity: a molecular forward/reverse switch. J Biol Chem. 2008;283(39):26297–301.
Gibbs JW. A method of geometrical representation of the thermodynamic properties by means of surfaces. Trans Conn Acad Arts Sci. 1873;2:382–404.
Newman J, Thomas-Alyea KE. Electrochemical systems. Wiley; 2012.
Copeland RA. Enzymes: a practical introduction to structure, mechanism, and data analysis. Wiley; 2004.
Leveau JH, Lindow SE. Bioreporters in microbial ecology. Curr Opin Microbiol. 2002;5(3):259–65.
Shank EA, Kolter R. New developments in microbial interspecies signaling. Curr Opin Microbiol. 2009;12(2):205–14.
Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46.
Aston WJ, Turner AP. Biosensors and biofuel cells. Biotechnol Genet Eng Rev. 1984;1(1):89–120.
Lei Y, Chen W, Mulchandani A. Microbial biosensors. Anal Chim Acta. 2006;568:200–10.
Ikeda T, Kano K. An electrochemical approach to the studies of biological redox reactions and their applications to biosensors, bioreactors, and biofuel cells. J Biosci Bioeng. 2001;92(1):9–18.
Marcus R. On the theory of oxidation-reduction reactions involving electron transfer. J Chem Phys. 1956;24(5):966–978 (b).
Marcus RA. Electrostatic free energy and other properties of states having nonequilibrium polarization. I. J Chem Phys. 1956;24(5):979–89 (a).
Rubner M. Über den Eiweißansatz. Arch Physiol. 1911:67–84.
Braissant O, Wirz D, Göpfert B, Daniels AU. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol Lett. 2010;303(1):1–8.
Nernst W. Barr G. The new heat theorem. 1926. p. 78–86.
Ulstrup J, Jortner J. The effect of intramolecular quantum modes on free energy relationships for electron transfer reactions. J Chem Phys. 1975;63(10):4358–68.
Grieshaber D, MacKenzie R, Vörös J, Reimhult E. Electrochemical biosensors-sensor principles and architectures. Sensors. 2008;8(3):1400–58.
Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron. 2001;16(1–2):121–31.
Stein NE, Keesman KJ, Hamelers HV, van Straten G. Kinetic models for detection of toxicity in a microbial fuel cell-based biosensor. Biosens Bioelectron. 2011;26(7):3115–20.
Chang IS, Moon HS, Bretschger O, Jang JK, Park HI, Nealson KH, Kim BH. Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J Microbiol Biotechnol. 2006;16(2):163–77.
Chaudhuri SK, Lovley DR. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol. 2003;21(10):1229–32.
Pham CA, Jung SJ, Phung NT, Lee J, Chang IS, Kim BH, Yi H, Chun J. A novel electrochemically active and Fe (III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol Lett. 2003;223(1):129–34.
Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol. 2003;69(3):1548–55.
Holmes DE, Bond DR, Lovley DR. Electron transfer by Desulfobulbus propionicus to Fe (III) and graphite electrodes. Appl Environ Microbiol. 2004;70(2):1234–7.
Park HS, Kim BH, Kim HS, Kim HJ, Kim GT, Kim M, Chang IS, Park YK, Chang HI. A novel electrochemically active and Fe (III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe. 2001;7(6):297–306.
Rouillon R, Sole M, Carpentier R, Marty JL. Immobilization of thylakoids in polyvinylalcohol for the detection of herbicides. Sensors Actuators B Chem. 1995;27(1–3):477–9.
Babu VS, Patra S, Karanth NG, Kumar MA, Thakur MS. Development of a biosensor for caffeine. Anal Chim Acta. 2007;582(2):329–34.
Gäberlein S, Spener F, Zaborosch C. Microbial and cytoplasmic membrane-based potentiometric biosensors for direct determination of organophosphorus insecticides. Appl Microbiol Biotechnol. 2000;54(5):652–8.
Hernandez CA, Gaviria LN, Segura SM, Osma JF. Concept design for a novel confined-bacterial-based biosensor for water quality control. In: 2013 Pan American Health Care Exchanges (PAHCE) 2013 Apr 29. IEEE; 2013. p. 1–3.
Ikeda T, Kato K, Maeda M, Tatsumi H, Kano K, Matsushita K. Electrocatalytic properties of Acetobacter aceti cells immobilized on electrodes for the quinone-mediated oxidation of ethanol. J Electroanal Chem. 1997;430(1–2):197–204.
Stoytcheva M, Zlatev R, Valdez B, Magnin JP, Velkova Z. Electrochemical sensor based on Arthrobacter globiformis for cholinesterase activity determination. Biosens Bioelectron. 2006;22(1):1–9.
Lei Y, Mulchandani P, Chen W, Wang J, Mulchandani A. A microbial biosensor for p-nitrophenol using Arthrobacter Sp. Electroanalysis. 2003;15(14):1160–4.
Lei Y, Mulchandani P, Chen W, Wang J, Mulchandani A. Whole cell–enzyme hybrid amperometric biosensor for direct determination of organophosphorous nerve agents with p-nitrophenyl substituent. Biotechnol Bioeng. 2004;85(7):706–13.
Sumathi R, Rajasekar R, Narasimham KC. Acetobacter peroxydans based electrochemical biosensor for hydrogen peroxide. Bull Electrochem. 2000;16(1):25–8.
Liu J, Björnsson L, Mattiasson B. Immobilised activated sludge based biosensor for biochemical oxygen demand measurement. Biosens Bioelectron. 2000;14(12):883–93.
Jha SK, Kanungo M, Nath A, D’Souza SF. Entrapment of live microbial cells in electropolymerized polyaniline and their use as urea biosensor. Biosens Bioelectron. 2009;24(8):2637–42.
Tan TC, Qian Z. Dead Bacillus subtilis cells for sensing biochemical oxygen demand of waters and wastewaters. Sensors Actuators B Chem. 1997;40(1):65–70.
Verma N, Singh M. A disposable microbial based biosensor for quality control in milk. Biosens Bioelectron. 2003;18:1219–24.
König A, Reul T, Harmeling C, Spener F, Knoll M, Zaborosch C. Multimicrobial sensor using microstructured three-dimensional electrodes based on silicon technology. Anal Chem. 2000;72(9):2022–8.
Mascini M, Memoli A, Olana F. Microbial sensor for alcohol. Enzym Microb Technol. 1989;11(5):297–301.
Taranova LA, Fesay AP, Ivashchenko GV, Reshetilov AN, Winther-Nielsen M, Emnéus J. Comamonas testosteroni strain TI as a potential base for a microbial sensor detecting surfactants. Appl Biochem Microbiol. 2004;40(4):404–8.
Bechor O, Smulski DR, Van Dyk TK, LaRossa RA, Belkin S. Recombinant microorganisms as environmental biosensors: pollutants detection by Escherichia coli bearing fabA′: lux fusions. J Biotechnol. 2002;94(1):125–32.
Horsburgh AM, Mardlin DP, Turner NL, Henkler R, Strachan N, Glover LA, Paton GI, Killham K. On-line microbial biosensing and fingerprinting of water pollutants. Biosens Bioelectron. 2002;17(6–7):495–501.
Rasmussen LD, Sørensen SJ, Turner RR, Barkay T. Application of a mer-lux biosensor for estimating bioavailable mercury in soil. Soil Biol Biochem. 2000;32(5):639–46.
Held M, Schuhmann W, Jahreis K, Schmidt HL. Microbial biosensor array with transport mutants of Escherichia coli K12 for the simultaneous determination of mono-and disaccharides. Biosens Bioelectron. 2002;17(11–12):1089–94.
Tront JM, Fortner JD, Plötze M, Hughes JB, Puzrin AM. Microbial fuel cell biosensor for in situ assessment of microbial activity. Biosens Bioelectron. 2008;24(4):586–90.
Kitagawa Y, Ameyama M, Nakashima K, Tamiya E, Karube I. Amperometric alcohol sensor based on an immobilised bacteria cell membrane. Analyst. 1987;112:1747–9.
Smutok O, Dmytruk K, Gonchar M, Sibirny A, Schuhmann W. Permeabilized cells of flavocytochrome b2 over-producing recombinant yeast Hansenula polymorpha as biological recognition element in amperometric lactate biosensors. Biosens Bioelectron. 2007;23(5):599–605.
Ohki A, Shinohara K, Ito O, Naka K, Maeda S, Sato T, Akano H, Kato N, Kawamura Y. A BOD sensor using Klebsiella oxytoca AS1. Int J Environ Anal Chem. 1994;56(4):261–9.
Nomura Y, Ikebukuro K, Yokoyama K, Takeuchi T, Arikawa Y, Ohno S, Karube I. A novel microbial sensor for anionic surfactant determination. Anal Lett. 1994;27(15):3095–108.
Rastogi S, Kumar A, Mehra NK, Makhijani SD, Manoharan A, Gangal V, Kumar R. Development and characterization of a novel immobilized microbial membrane for rapid determination of biochemical oxygen demand load in industrial waste-waters. Biosens Bioelectron. 2003;18(1):23–9.
Liu J, Olsson G, Mattiasson B. Short-term BOD (BODst) as a parameter for on-line monitoring of biological treatment process: part I. A novel design of BOD biosensor for easy renewal of bio-receptor. Biosens Bioelectron. 2004;20(3):562–70.
Liu J, Olsson G, Mattiasson B. Short-term BOD (BODst) as a parameter for on-line monitoring of biological treatment process: part II: instrumentation of integrated flow injection analysis (FIA) system for BODst estimation. Biosens Bioelectron. 2004;20(3):571–8.
Dhall P, Kumar A, Joshi A, Saxsena TK, Manoharan A, Makhijani SD, Kumar R. Quick and reliable estimation of BOD load of beverage industrial wastewater by developing BOD biosensor. Sensors Actuators B Chem. 2008;133(2):478–83.
Chee GJ, Nomura Y, Ikebukuro K, Karube I. Development of photocatalytic biosensor for the evaluation of biochemical oxygen demand. Biosens Bioelectron. 2005;21(1):67–73.
Emelyanova EV, Reshetilov AN. Rhodococcus erythropolis as the receptor of cell-based sensor for 2, 4-dinitrophenol detection: effect of ‘co-oxidation’. Process Biochem. 2002;37(7):683–92.
Peter J, Hutter W, Stöllnberger W, Hampel W. Detection of chlorinated and brominated hydrocarbons by an ion sensitive whole cell biosensor. Biosens Bioelectron. 1996;11(12):1215–9.
Tag K, Riedel K, Bauer HJ, Hanke G, Baronian KH, Kunze G. Amperometric detection of Cu2+ by yeast biosensors using flow injection analysis (FIA). Sensors Actuators B Chem. 2007;122(2):403–9.
Kim MN, Kwon HS. Biochemical oxygen demand sensor using Serratia marcescens LSY 4. Biosens Bioelectron. 1999;14(1):1–7.
Karube I, Yokoyama K, Sode K, Tamiya E. Microbial BOD sensor utilizing thermophilic bacteria. Anal Lett. 1989;22(4):791–801.
Jia J, Tang M, Chen X, Qi L, Dong S. Co-immobilized microbial biosensor for BOD estimation based on sol–gel derived composite material. Biosens Bioelectron. 2003;18(8):1023–9.
Chen C, Zhao J, Zhang P, Chai Z. Speciation and subcellular location of Se-containing proteins in human liver studied by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and hydride generation-atomic fluorescence spectrometric detection. Anal Bioanal Chem. 2002;372(3):426–30.
Trosok S, Driscoll B, Luong J. Mediated microbial biosensor using a novel yeast strain for wastewater BOD measurement. Appl Microbiol Biotechnol. 2001;56(3–4):550–4.
EPA. Technologies and techniques for early warning systems to monitor and evaluate drinking water quality: a state-of-the-art review, vol. 165. Washington: Office of Research and Development; 2005.
EPA. Verification Report Checklight Ltd. Toxscreen-II Test Kit. 2006.
EPA. Verification Statement Interlab Supply, Ltd. Polytox Rapid Toxicity Testing Systems. 2003.
EPA. Verification Report Strategic Diagnostics Inc. Deltatox® Rapid Toxicity Testing System. 2003.
SYSTEA S.p.A. 2013. http://www.systea.it/. Accessed 30 Aug.
Aqua Sentnel. 2013. http://www.envirotechinstruments.com/aquasentnel.html.
Upton J, Pickin SR. AmtoxTM—a new concept for rapid nitrification inhibition testing applicable to the laboratory and on-line at treatment works. Spec Publ R Soc Chem. 1996;193:54–63.
Baroxymeter. 2013. http://www.baroxymeter.com/.
Aboatox Environmental Analysis. 2013. http://www.aboatox.com/environmental_analysis.html#flash.
Fluotox. 2013. http://www.ifetura.com/es/actvites-departements/arnatronic/produits/fluotox.html.
Stfey AV, Nicolaids TG. US Patent No. 5,580,785. Washington, DC: US. Patent and Trademark Office. Chicago. 1995. Issued December 3, 1996.
Farré M, Pasini O, Alonso MC, Castillo M, Barceló D. Toxicity assessment of organic pollution in wastewaters using a bacterial biosensor. Anal Chim Acta. 2001;426(2):155–65.
Hach-Lange UK-LUMIStox. 2013. http://www.hach-lange.co.uk/view/product/EU-LPV384/LUMIStox?productCode=EU-LPV384.
MetPLATETM. 2013. http://www.ees.essie.ufl.edu/homepp/bitton//metplate.asp.
van der Schalie WH, James RR, Gargan TP II. Selection of a battery of rapid toxicity sensors for drinking water evaluation. Biosens Bioelectron. 2006;22(1):18–27.
Keenan PO, Knight AW, Billinton N, Cahill PA, Dalrymple IM, Hawkyard CJ, Stratton-Campbell D, Walmsley RM. Clear and present danger? The use of a yeast biosensor to monitor changes in the toxicity of industrial effluents subjected to oxidative colour removal treatments. J Environ Monit. 2007;9(12):1394–401.
Wolf D, Mascher T. The applied side of antimicrobial peptide-inducible promoters from Firmicutes bacteria: expression systems and whole-cell biosensors. Appl Microbiol Biotechnol. 2016;100(11):4817–29.
Hillger JM, Schoop J, Boomsma DI, Slagboom PE, IJzerman AP, Heitman LH. Whole-cell biosensor for label-free detection of GPCR-mediated drug responses in personal cell lines. Biosens Bioelectron. 2015;74:233–42.
Feng X, Castracane J, Tokranova N, Gracias A, Lnenicka G, Szaro BG. A living cell-based biosensor utilizing G-protein coupled receptors: principles and detection methods. Biosens Bioelectron. 2007;22(12):3230–7.
Zager V, Cemazar M, Hreljac I, Lah T, Sersa G, Filipic M. Development of human cell biosensor system for genotoxicity detection based on DNA damage-induced gene expression. Radiol Oncol. 2010;44(1):42–51.
Hilary HJP. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77:1352–60.
Besaratinia A, Kim SI, Bates SE, Pfeifer GP. Riboflavin activated by ultraviolet A1 irradiation induces oxidative DNA damage-mediated mutations inhibited by vitamin C. Proc Natl Acad Sci. 2007;104(14):5953–8.
Si RW, Yang Y, Yu YY, Han S, Zhang CL, Sun DZ, Zhai DD, Liu X, Yong YC. Wiring bacterial electron flow for sensitive whole-cell amperometric detection of riboflavin. Anal Chem. 2016;88(22):11222–8.
Shabani A, Zourob M, Allain B, Marquette CA, Lawrence MF, Mandeville R. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal Chem. 2008;80(24):9475–82.
Kumari A, Pasini P, Deo SK, Flomenhoft D, Shashidhar H, Daunert S. Biosensing systems for the detection of bacterial quorum signaling molecules. Anal Chem. 2006;78(22):7603–9.
Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci. 1995;92(5):1490–4.
Morpeth SC, Ramadhani HO, Crump JA. Invasive non-typhi Salmonella disease in Africa. Clin Infect Dis. 2009;49(4):606–11.
Venkatesh AG, Sun A, Brickner H, Looney D, Hall DA, Aronoff-Spencer E. Yeast dual-affinity biobricks: progress towards renewable whole-cell biosensors. Biosens Bioelectron. 2015;70:462–8.
Belkin S. Microbial whole-cell sensing systems of environmental pollutants. Curr Opin Microbiol. 2003;6(3):206–12.
Amaro F, Turkewitz AP, Martín-González A, Gutiérrez JC. Whole-cell biosensors for detection of heavy metal ions in environmental samples based on metallothionein promoters from Tetrahymena thermophila. Microb Biotechnol. 2011;4(4):513–22.
Wei H, Ze-Ling S, Le-Le C, Wen-Hui Z, Chuan-Chao D. Specific detection of bioavailable phenanthrene and mercury by bacterium reporters in the red soil. Int J Environ Sci Technol. 2014;11(3):685–94.
Peltola P, Ivask A, Åström M, Virta M. Lead and Cu in contaminated urban soils: extraction with chemical reagents and bioluminescent bacteria and yeast. Sci Total Environ. 2005;350(1–3):194–203.
Ecken H, Ingebrandt S, Krause M, Richter D, Hara M, Offenhäusser A. 64-Channel extended gate electrode arrays for extracellular signal recording. Electrochim Acta. 2003;48(20–22):3355–62.
Hakkila K, Maksimow M, Karp M, Virta M. Reporter genes lucFF, luxCDABE, gfp, and dsred have different characteristics in whole-cell bacterial sensors. Anal Biochem. 2002;301(2):235–42.
Sagi E, Hever N, Rosen R, Bartolome AJ, Premkumar JR, Ulber R, Lev O, Scheper T, Belkin S. Fluorescence and bioluminescence reporter functions in genetically modified bacterial sensor strains. Sensors Actuators B Chem. 2003;90(1–3):2–8.
Mascher T, Zimmer SL, Smith TA, Helmann JD. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis. Antimicrob Agents Chemother. 2004;48(8):2888–96.
Gutiérrez JC, Amaro F, Martín-González A. Heavy metal whole-cell biosensors using eukaryotic microorganisms: an updated critical review. Front Microbiol. 2015;6:48.
Chong H, Ching CB. Development of colorimetric-based whole-cell biosensor for organophosphorus compounds by engineering transcription regulator DmpR. ACS Synth Biol. 2016;5(11):1290–8.
Singh SK, Grimaud R, Hoskins JR, Wickner S, Maurizi MR. Unfolding and internalization of proteins by the ATP-dependent proteases ClpXP and ClpAP. Proc Natl Acad Sci. 2000;97(16):8898–903.
Stocker J, Balluch D, Gsell M, Harms H, Feliciano J, Daunert S, Malik KA, Van der Meer JR. Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ Sci Technol. 2003;37(20):4743–50.
Yagi K. Applications of whole-cell bacterial sensors in biotechnology and environmental science. Appl Microbiol Biotechnol. 2007;73(6):1251–8.
Joyeux A, Balaguer P, Germain P, Boussioux AM, Pons M, Nicolas JC. Engineered cell lines as a tool for monitoring biological activity of hormone analogs. Anal Biochem. 1997;249(2):119–30.
Fujimoto H, Wakabayashi M, Yamashiro H, Maeda I, Isoda K, Kondoh M, Kawase M, Miyasaka H, Yagi K. Whole-cell arsenite biosensor using photosynthetic bacterium Rhodovulum sulfidophilum. Appl Microbiol Biotechnol. 2006;73(2):332–8.
Yeliseev AA, Eraso JM, Kaplan S. Differential carotenoid composition of the B875 and B800-850 photosynthetic antenna complexes in Rhodobacter sphaeroides 2.4.1: involvement of spheroidene and spheroidenone in adaptation to changes in light intensity and oxygen availability. J Bacteriol. 1996;178(20):5877–83.
Joe MH, Lee KH, Lim SY, Im SH, Song HP, Lee IS, Kim DH. Pigment-based whole-cell biosensor system for cadmium detection using genetically engineered Deinococcus radiodurans. Bioprocess Biosyst Eng. 2012;35(1–2):265–72.
Tecon R, Beggah S, Czechowska K, Sentchilo V, Chronopoulou PM, McGenity TJ, van der Meer JR. Development of a multistrain bacterial bioreporter platform for the monitoring of hydrocarbon contaminants in marine environments. Environ Sci Technol. 2010;44(3):1049–55.
Sticher P, Jaspers MC, Stemmler K, Harms H, Zehnder AJ, Van Der Meer JR. Development and characterization of a whole-cell bioluminescent sensor for bioavailable middle-chain alkanes in contaminated groundwater samples. Appl Environ Microbiol. 1997;63(10):4053–60.
Korpela MT, Kurittu JS, Karvinen JT, Karp MT. A recombinant Escherichia coli sensor strain for the detection of tetracyclines. Anal Chem. 1998;70(21):4457–62.
Willardson BM, Wilkins JF, Rand TA, Schupp JM, Hill KK, Keim P, Jackson PJ. Development and testing of a bacterial biosensor for toluene-based environmental contaminants. Appl Environ Microbiol. 1998;64(3):1006–12.
Sharma P, Asad S, Ali A. Bioluminescent bioreporter for assessment of arsenic contamination in water samples of India. J Biosci. 2013;38(2):251–8.
De Mora K, Joshi N, Balint BL, Ward FB, Elfick A, French CE. A pH-based biosensor for detection of arsenic in drinking water. Anal Bioanal Chem. 2011;400(4):1031–9.
Branco R, Cristóvão A, Morais PV. Highly sensitive, highly specific whole-cell bioreporters for the detection of chromate in environmental samples. PLoS One. 2013;8(1):e54005.
Ravikumar S, Ganesh I, Yoo IK, Hong SH. Construction of a bacterial biosensor for zinc and copper and its application to the development of multifunctional heavy metal adsorption bacteria. Process Biochem. 2012;47(5):758–65.
Shingler V, Moore T. Sensing of aromatic compounds by the DmpR transcriptional activator of phenol-catabolizing Pseudomonas sp. strain CF600. J Bacteriol. 1994;176(6):1555–60.
Nakamura SI, Oda Y, Shimada T, Oki I, Sugimoto K. SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat Res Lett. 1987;192(4):239–46.
Pontel LB, Audero ME, Espariz M, Checa SK, Soncini FC. GolS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol Microbiol. 2007;66(3):814–25.
Cerminati S, Soncini FC, Checa SK. A sensitive whole-cell biosensor for the simultaneous detection of a broad-spectrum of toxic heavy metal ions. Chem Commun. 2015;51(27):5917–20.
Díaz S, Amaro F, Rico D, Campos V, Benítez L, Martín-González A, Hamilton EP, Orias E, Gutiérrez JC. Tetrahymena metallothioneins fall into two discrete subfamilies. PLoS One. 2007;2(3):e291.
Shang Y, Song X, Bowen J, Corstanje R, Gao Y, Gaertig J, Gorovsky MA. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc Natl Acad Sci. 2002;99(6):3734–9.
Whangsuk W, Dubbs JM, Sallabhan R, Somsongkul K, Mongkolsuk S, Loprasert S. ChpR is a chlorpyrifos-responsive transcription regulator in Sinorhizobium meliloti. J Mol Microbiol Biotechnol. 2010;18(3):141–7.
Whangsuk W, Thiengmag S, Dubbs J, Mongkolsuk S, Loprasert S. Specific detection of the pesticide chlorpyrifos by a sensitive genetic-based whole cell biosensor. Anal Biochem. 2016;493:11–3.
Gross M. Antibiotics in crisis. Curr Biol. 2013;23:R1063.
Espinosa-Urgel M, Serrano L, Ramos JL, Fernández-Escamilla AM. Engineering biological approaches for detection of toxic compounds: a new microbial biosensor based on the Pseudomonas putida TtgR repressor. Mol Biotechnol. 2015;57(6):558–64.
Magrisso S, Erel Y, Belkin S. Microbial reporters of metal bioavailability. Microb Biotechnol. 2008;1(4):320–30.
Hernández-Sánchez V, Molina L, Ramos JL, Segura A. New family of biosensors for monitoring BTX in aquatic and edaphic environments. Microb Biotechnol. 2016;9(6):858–67.
Zhang D, He Y, Wang Y, Wang H, Wu L, Aries E, Huang WE. Whole-cell bacterial bioreporter for actively searching and sensing of alkanes and oil spills. Microb Biotechnol. 2012;5(1):87–97.
Ratajczak A, Geißdörfer W, Hillen W. Expression of alkane hydroxylase from Acinetobacter sp. strain ADP1 is induced by a broad range of n-alkanes and requires the transcriptional activator AlkR. J Bacteriol. 1998;180(22):5822–7.
Throne-Holst M, Wentzel A, Ellingsen TE, Kotlar HK, Zotchev SB. Identification of novel genes involved in long-chain n-alkane degradation by Acinetobacter sp. strain DSM 17874. Appl Environ Microbiol. 2007;73(10):3327–32.
Sevilla E, Yuste L, Rojo F. Marine hydrocarbonoclastic bacteria as whole-cell biosensors for n-alkanes. Microb Biotechnol. 2015;8(4):693–706.
Brutesco C, Prévéral S, Escoffier C, Descamps EC, Prudent E, Cayron J, Dumas L, Ricquebourg M, Adryanczyk-Perrier G, de Groot A, Garcia D. Bacterial host and reporter gene optimization for genetically encoded whole cell biosensors. Environ Sci Pollut Res. 2017;24(1):52–65.
Cayron J, Prudent E, Escoffier C, Gueguen E, Mandrand-Berthelot MA, Pignol D, Garcia D, Rodrigue A. Pushing the limits of nickel detection to nanomolar range using a set of engineered bioluminescent Escherichia coli. Environ Sci Pollut Res. 2017;24(1):4–14.
Wan NA, Wan J, Wong LS. Exploring the potential of whole cell biosensor: a review in environmental applications. Int J Chem Environ Biol Sci. 2014;2(1).
Bazin I, Seo HB, Suehs CM, Ramuz M, De Waard M, Gu MB. Profiling the biological effects of wastewater samples via bioluminescent bacterial biosensors combined with estrogenic assays. Environ Sci Pollut Res. 2017;24(1):33–41.
Tauriainen S, Karp M, Chang W, Virta M. Recombinant luminescent bacteria for measuring bioavailable arsenite and antimonite. Appl Environ Microbiol. 1997;63(11):4456–61.
Hou Q, Ma A, Wang T, Lin J, Wang H, Du B, Zhuang X, Zhuang G. Detection of bioavailable cadmium, lead, and arsenic in polluted soil by tailored multiple Escherichia coli whole-cell sensor set. Anal Bioanal Chem. 2015;407(22):6865–71.
Kim BC, Youn CH, Ahn JM, Gu MB. Screening of target-specific stress-responsive genes for the development of cell-based biosensors using a DNA microarray. Anal Chem. 2005;77(24):8020–6.
Reed B, Blazeck J, Alper H. Evolution of an alkane-inducible biosensor for increased responsiveness to short-chain alkanes. J Biotechnol. 2012;158(3):75–9.
Camanzi L, Bolelli L, Maiolini E, Girotti S, Matteuzzi D. Optimal conditions for stability of photoemission and freeze drying of two luminescent bacteria for use in a biosensor. Environ Toxicol Chem. 2011;30(4):801–5.
Prévéral S, Brutesco C, Descamps EC, Escoffier C, Pignol D, Ginet N, Garcia D. A bioluminescent arsenite biosensor designed for inline water analyzer. Environ Sci Pollut Res. 2017;24(1):25–32.
Elad T, Almog R, Yagur-Kroll S, Levkov K, Melamed S, Shacham-Diamand Y, Belkin S. Online monitoring of water toxicity by use of bioluminescent reporter bacterial biochips. Environ Sci Technol. 2011;45(19):8536–44.
Jain VK, Magrath IT. A chemiluminescent assay for quantitation of β-galactosidase in the femtogram range: application to quantitation of β-galactosidase in lacZ-transfected cells. Anal Biochem. 1991;199(1):119–24.
Kumar S, Kundu S, Pakshirajan K, Dasu VV. Cephalosporins determination with a novel microbial biosensor based on permeabilized Pseudomonas aeruginosa whole cells. Appl Biochem Biotechnol. 2008;151(2):653–64.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The author gratefully acknowledges The Director, CSIR, Institute of Himalayan Bioresource Technology, Palampur, Himachal Pradesh, and the Council of Scientific and Industrial Research (CSIR), New Delhi, for providing basic computational facilities for carrying out the present study. The manuscript represents the IHBT Publication No. 5228.
Funding
No external funding was received for writing this chapter.
Conflicts of Interest
The author declares that there is no conflict of interest.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Devi, S. (2023). Live Cells as Biosensors. In: Kumar, P., Dash, S.K., Ray, S., Parween, S. (eds) Biomaterials-Based Sensors. Springer, Singapore. https://doi.org/10.1007/978-981-19-8501-0_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-8501-0_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8500-3
Online ISBN: 978-981-19-8501-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)