Abstract
Since cDNAs of glycosyltransferase genes were isolated, and become applicable for genetic engineering of glycosylation patterns, biological functions of glycolsphingolipids have been largely elucidated via glyco-remodeling cells and animals. The progress in these glycobiology techniques has enabled us to understand the roles of “tumor-specific” carbohydrates during these 3 decades. Tumor antigens recognized by host immune systems of cancer patients were classified into three classes based on “autologous typing”, that is, class 1: individual antigens present only in the patient’s tumors, class 2: shared antigens among some group of cancers, but not in normal cells, class 3: universal antigens that are present in not only some cancers but also in normal cells. Many of class 2 antigens have been elucidated to be carbohydrate antigens, and are considered to be differentiation antigens. Many of them have been used for cancer diagnosis and treatment. Functional analyses of those cancer-associated glycosphingolipids based on the genetic engineering of glyco-genes have revealed that disialylated glycolipids generally enhance malignant properties such as cell growth, invasion, and motility. On the other hand, monosialylated glycolipids rather suppress those phenotypes. As a regulatory platform for cell signaling, cell surface microdomains, glycolipid-enriched microdomain (GEM)/rafts have been proposed. Interestingly, cancer-associated glycosphingolipids play critical roles in the composition of GEM/rafts, and in the regulation of signal transduction. Molecular complex formation of glycolipids with membrane molecules that are defined by enzyme-mediated activation of radical sources/mass spectrometry should be a key factor to regulate cell signals and to determine the cell fates. They are also expected to be targets of the cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Biological Functions of Glycosphingolipids Have Been Further Understood Due to Glycoengineering
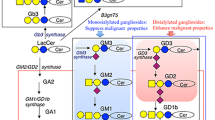
Since cDNAs of glycosyltransferases responsible for the synthesis of glycosphingolipids were isolated (Nagata et al. 1992; Haraguchi et al. 1994; Nara et al. 1994; Sasaki et al. 1994; Miyazaki et al. 1997) and became available for the genetic engineering of cultured cells and experimental animals, a number of experiments using those glyco-remodeling materials have been done, leading to the big progress in the understanding of their function and significance in the cells and mice (Furukawa et al. 2004). Main glycolipid synthetic pathways are shown in Fig. 1. Actually, many trials of genetic engineering of glycosyltransferase genes have been performed, and brought about various novel findings in the phenotypes of cultured cells and animals (Stanley 2016). Many other degradation enzymes and modifying enzymes of carbohydrates have been genetically engineered, and intriguing results have been reported (Miyagi et al. 2018; Cavdarli et al. 2020). Although the modified genes and resulting outputs are clear, mechanisms for observed abnormal phenotypes are frequently ambiguous. This is because changes of some glycosyltransferase gene generally affect all structures present at the downstream of the synthetic pathways in which the modified gene exists. Therefore, we have to clarify which glycosphingolipids in the pathway are responsible for the resulting phenotypes. In particular, remaining structures in the knockout mice/cells often compensate for the function of deleted structures, suggesting importance to carefully interpret the results (Furukawa et al. 2020).
2 Cancer-Associated Glycolipids Often Increase Malignant Properties
During a long-term search of cancer specific antigens, Old’s group proposed 3 classes of tumor antigens based on “autologous typing” (Old 1981). They are as follows: class 1: individual antigens present only in the patient’s tumor sample, class 2: shared antigens among some group of cancers in some patients, but not in normal cells, class 3: universal antigens that are present in some cancers but also in normal cells. There were many reports on carbohydrate antigens, which could be found only in cancer cells, but not in normal cells and tissues (Hakomori 1986; Lloyd 1991). Thus, these antigens have been considered as cancer-associated carbohydrates, and to be class 2 tumor antigens, being used in the diagnosis and therapy of cancers (Lloyd and Old 1989; Indellicato et al. 2020). Those carbohydrate antigens can be detected on proteins (mucine) or lipids (ceramide). Some of them are found in both proteins and lipids. Among them, sialic-acid-containing glycosphingolipids are named as gangliosides, and have been utilized as tumor markers of neuroectoderm-derived cancers. For example, ganglioside GD3 in malignant melanomas (Furukawa and Lloyd 1990; Dippold et al. 1980; Pukel et al. 1982; Portoukalian et al. 1979; Carubia et al. 1984), GD2 in neuroblastomas (Saito and Yu 1985; Schultz et al. 1984), and GD3/GD2 in gliomas (Fredman et al. 1986; Wikstrand et al. 1994; Kawai et al. 1999; Vukelić et al. 2007; Iwasawa et al. 2018). In addition to neuroectoderm-derived tumors, some other cancers also express these gangliosides. For example, T-cell leukemias (Siddiqui et al. 1984; Merritt et al. 1987; Furukawa et al. 1993; Okada et al. 1996), osteosarcomas (Shibuya et al. 2012; Azuma et al. 2005), small cell lung carcinomas (Cheresh et al. 1986; Yoshida et al. 2001), and breast cancers (Cazet et al. 2012; De Giorgi et al. 2011). Recently, many other cancers have also been reported to express these gangliosides (Sato et al. 2020; Ravindranath et al. 2004; Vantaku et al. 2017). Thus, these ganglioside antigens belong to “class 2” tumor antigens, and have been investigated on their roles in tumor cells, showing differential significances for various carbohydrate structures (Ohmi et al. 2018). On the other hand, some neutral glycolipids have also been considered to be cancer-associated antigens (Yu et al. 2020; Ragupathi 1996). Brief classification of cancer-associated antigens is shown in Table 1.
3 Membrane Microdomains GEM/Rafts as Platforms of Signal Transduction
Simons proposed a membrane microdomains, lipid rafts containing cholesterol, sphingolipids, and GPI-anchored proteins (Simons and Ikonen 1997). Majority of glycosphingolipids are enriched in this microdomain. These microdomains are resistant to nonionic detergent, being called detergent-resistant microdomain (DRM). Hakomori proposed to call this microdomain as glycolipid-enriched microdomain (GEM), and showed that GEM/rafts play crucial roles as a platform of cell signal transduction (Hakomori 2003). In particular, ganglioside GM3 is localized in GEM/rafts, and forms complexes with membrane molecules such as tetraspanins (Hakomori 2010). Although molecular basis for molecular clustering in GEM/rafts is not clear at this moment, dynamic processes of raft formation from nanoscale to microscale were proposed by Simons et al. (Simons and Toomre 2000; Simons and Gerl 2010). Kohmura et al. demonstrated that dimer formation of same ganglioside species might be an initial step for the formation of GEM/rafts using ultrahigh-resolution imaging technique (Komura et al. 2016).
Biochemical and imaging analyses of intracellular distribution of glycolipid-associated molecules demonstrated that sugar moiety of glycosphingolipids on the cell surface and cellular conditions (resting or activated) regulates their localization inside/outside of GEM/rafts. It is of great interest that fine structures of carbohydrate portion in glycolipids might control the intracellular localization of membrane molecules inside/outside of GEM/rafts as if they are regulators of the composition of GEM/rafts (Ohkawa et al. 2010; Yesmin et al. 2021), and resultant cell signals (Furukawa et al. 2014) as reported by Sonnino (Sonnino and Prinetti 2010).
4 Disialyl Glycosphingolipids Generally Enhance Malignant Phenotypes and Signals in Cancer
During analyses of phenotypic changes in cancer cells expressing cancer-associated glycosphingolipids, it has been shown that disialylated gangliosides with a tandem mode linkage 2 sialic acids often enhance malignant properties (Furukawa et al. 2012a, b) in various kinds of tumors. Namely, gangliosides GD3 and GD2 enhance malignant properties such as increased proliferation, invasion, and cell adhesion to extracellular matrices. However, these effects of gangliosides on the tumor phenotypes are not identical. The modes of action were diverse among cancer lineages (Furukawa et al. 2012a, b). This is deeply related to the expression of membrane molecules in the vicinity of cancer-associated gangliosides at membrane microdomains, GEM/rafts (Hakomori 1998; Hakomori et al. 1998). The profiles of intracytoplasmic proteins primarily expressed in the individual cells are also critical for how those gangliosides regulate the feature of cells, signals, and cell fates (Furukawa et al. 2012a).
In the study of melanomas, we clarified that various adaptor molecules such as p130Cas, paxillin, and focal adhesion kinase (FAK) were activated due to tyrosine phosphorylation, leading to the enhanced cell proliferation and invasion under GD3 expression (Hamamura et al. 2005, 2008). Then, we also revealed that integrin was involved in the regulation of cell signaling via FAK and Src family kinase Yes during cell adhesion (Ohkawa et al. 2008). Strong physical and functional association of integrins and FAK was previously shown in small cell lung cancer (SCLC) cells. Anti-GD2 monoclonal antibody induced apoptosis of SCLC cells via anoikis (Yoshida et al. 2001). During the cell detachment from culture dishes, SCLC cells showed reduced phosphorylation levels of FAK (Aixinjueluo et al. 2005). Interestingly, osteosarcoma cells showed increased cell invasion and motility under expression of GD3 and GD2 (Shibuya et al. 2012). Here, cell adhesion to extracellular matrix such as collagen 1 was markedly reduced in GD3/GD2-expressing cells compared to GD3/GD2-negative control cells. GD3/GD2-positive cells showed much lower adhesion than the controls. These findings were quite opposite compared with results observed in melanoma transfectant cells with high GD3 expression (Furukawa et al. 2012a; Hamamura et al. 2005). These differences between melanomas and osteosarcomas seemed to be due to different signaling pathways basically working in the individual cell lineages (Shibuya et al. 2012). Activated Lyn (p-Lyn) was detected in osteosarcoma cells instead of p-Yes in the case of melanoma cells (Hamamura et al. 2011).
5 Monosialyl Glycosphingolipids Often Suppress Cancer Phenotypes and Signals in Animal Cancer Cells
In contrast with disialylated gangliosides, monosialylated glycosphingolipids (or a-series gangliosides) often showed suppressive effects on the cell phenotypes. Accordingly, they also suppress cell signals in various tumor cells (Furukawa et al. 2012b). For example, a rat pheochromocytoma line PC12 showed distinct effects between GM1 and GD3 on the signaling via nerve growth factor (NGF) and Trk A receptor (Nishio et al. 2004; Fukumoto et al. 2000). NGF-TrkA signaling between GM1-expressing and GD3-expressing PC12 was quite contrastive, that is, poor phosphorylation of TrkA and subsequent lowered activation of Erk1/2 upon NGF stimulation in the former and enhanced TrkA phosphorylation and highly activated Erk1/2 in the latter. In a mouse fibroblast cell line, SWISS-3 T3, overexpression of GM1/GD1b synthase induced suppressed cell growth and lowered activation levels of PDGF receptor based on its altered localization in GEM/rafts (Mitsuda et al. 2002).
In a mouse Lewis lung cancer (LLC) cells, transfectants of GM2/GD2 synthase cDNA with high expression of GM2 showed lowered malignant properties and reduced activation levels of focal adhesion kinase (FAK) (Chen et al. 2003). On the other hand, Zhang et al. established high-metastatic and low-metastatic LLC sublines by repeated injection into mice, and compared their surface molecules. Consequently, high-metastatic sublines expressed lower levels of GM1/GD1a than the low-metastatic parent line (Zhang et al. 2006). Roles of GM1 in the suppression of metastasis were directly shown using GM1 synthase-suppressed transfectants of an RNAi-expression vector. Suppression of GM1 synthase resulted in the increased malignant phenotypes including metastatic property due to the shift of MMP-9 and integrins to GEM/rafts. The secretion and activation levels of MMP-9 were consequently elevated.
In a B16 mouse melanoma subline B78, transfectant cells of GM2/GD2 synthase cDNA showed high GM2 expression and reduced cell proliferation (Tsurifune et al. 2000).
6 GM1/GD1b Synthase and Caveolin-1 Suppressed Malignant Phenotypes of Human Cancers
In human melanoma cells, SK-MEL-73 melanoma cell line expressing GD3 and GD2 was transfected with GM1/GD1b synthase cDNA (Dong et al. 2012). GM1-high transfectant cells showed reduction of cell growth and invasion. They also showed dispersed ganglioside distribution compared with the control line. Consequently, tyrosine-phosphorylation levels of p130Cas and paxillin were reduced in the transfectant cells. Furthermore, gangliosides shifted to the non-GEM/raft fraction dominantly contained unsaturated fatty acids compared with the control cells, suggesting that glyco-remodeling affected ceramide composition of gangliosides. Interestingly, similar reduced malignant phenotypes were observed in the melanoma transfectant cells with Caveolin-1 cDNA in SK-MEL-28 (Nakashima et al. 2007). Accordingly, reduced phosphorylation of p130Cas and paxillin was also observed. Furthermore, GD3 was dispersed from GEM/rafts, losing the leading edges that could be found in the control cells with immunocytochemistry. Thus, Caveolin-1 should have regulatory functions of signaling, although it has been used as a GEM/rafts marker as well as GM1.
All these results commonly observed among various cancers indicated that the expression of monosialylated (or a-series) gangliosides results in the attenuation of malignant phenotypes and malignant signals (Furukawa et al. 2012a, b) in contrast with disialylated gangliosides as summarized in Fig. 2. These results were recently confirmed with genetically engineered mice of GD3 synthase gene (Ohkawa et al. 2021; Zhang et al. 2021).
7 A Novel Approach to Elucidate Mechanisms for Functioning of Cancer-Associated Gangliosides
To investigate molecular mechanisms for biological functions of glycosphingo- lipids on the cell surface, it seems critical to identify membrane molecules that physically associate with glycosphingolipids in the vicinity of cell membrane of living cells. This is because glycosphingolipids are embedded on the outer layer of lipid bilayer membrane, and don’t contain intracytoplasmic domains (Groux-Degroote et al. 2017).
Cluster formation of cancer-associated glycosphingolipids with various membrane molecules on the cell surface in a horizontal manner, and also in vertical connection with intracellular molecules, has been demonstrated to date. But, enzyme-mediated activation of radical sources (EMARS) combined with mass spectrometry (MS) developed by Honke and Kotani (Kotani et al. 2008), might be a markedly useful technique to identify membrane molecules that are present around cancer-associated glycosphingolipids within 100–300 nm on the cell surface without any special apparatus (Furukawa et al. 2019). From the candidate molecules detected in EMAR/MS results, we can identify physically associated molecules with cancer-associated glycolipids on the living cell membrane (Hashimoto et al. 2012). Thus, this approach enables us to identify molecular profiles of clustering molecules with particular targets (Kotani et al. 2008).
Using anti-GD3 monoclonal antibody, EMARS/MS was utilized to identify GD3-associated molecules in melanoma cells. Among molecules found both in GEM/rafts fraction and GD3-targeted EMARS-labeled molecules, Neogenin was exclusively detected only in GD3-positive cells (Hashimoto et al. 2012). This molecule was found in GEM/raft fraction of only GD3-positive melanoma cells, but not of GD3-negative cells. GD3 expression induced recruitment of Neogenin and γ-secretase to GEM/rafts, leading to the increased levels of intracytoplasmic domain (ICD) of Neogenin based on the action of γ-secretase (Kaneko et al. 2016). Consequently, Neogenin ICD promotes expression of various genes as a transcription factor (Kaneko et al. 2016), resulting in the enhanced expression of various molecules. ChIP-seq analysis revealed many target genes such as GPR126, STXBP5, MMP16, SPATA31A1, and S6K should be targets of Ne-ICD. These genes were actually upregulated by the expression of Ne-ICD. These results demonstrated, for the first time, how cancer-associated GD3 enhances malignant properties of melanomas.
In mouse glioma cells, PDGF-Rα was identified as a GD3-associated membrane molecule using EMARS-MS technique (Ohkawa et al. 2015). Physical association and clustering of GD3 and PDGF-Rα resulted in the activation of a Src family kinase, Yes and also activation of downstream paxillin (Ohkawa et al. 2015). Immunoprecipitation/immunoblotting revealed ternary complex formation among GD3, PDGFRα, and Yes, being colocalized in lamellipodia. Furthermore, in SCLC cells, ASCT2, a glutamine transporter, was identified as a GD2-associated molecule and shown to be localized in GEM/rafts. Co-operation of GD2 and ASCT2 resulted in the enhancement of cell proliferation and migration via increased phosphorylation of the mTOR complex 1 signaling axis (Esaki et al. 2018).
These results indicated that EMARS/MS technique is highly effective to elucidate the molecular complex formation around cancer-associated glycolipids on the living cell surface. It can also give us clues to reveal vertical molecular sequences originating from cancer-associated glycolipids. Thus, heterogeneity in GEM/rafts according to each “core” glycolipid might be clarified using this technique for the foresighted finding with morphological studies (Fujita et al. 2007).
8 trans-Interaction of Glycosphingolipids with Their Ligand Molecules Also Affects the Nature of Cells on Both Sides
Considering the meaning of GEM/rafts in the regulation of cell phenotypes and cell signals, their significance as a platform for the signal transduction might be the most critical and intriguing. The results elucidated by EMARS/MS approaches also support its importance in the generation of malignant signals and cancer phenotypes. Majority of molecules identified by this approach using glycolipids as the target molecules belong to cis-acting membrane molecules present around the cancer-associated glycolipids as shown in Fig. 3.
cic- and trans-interaction of glycosphingolipids and ligand molecules. As representative examples, interaction between Siglec-7 on NK cells and sialylated membrane molecules is shown. Bacterial toxins such as Cholera-toxin or Vero-toxin are also ligands of glycolipids with trans-interaction. Membrane receptors such as growth factor receptors and integrins interact with glycolipids on the same membrane with cis-interaction
On the other hand, molecular clustering on the cell membrane consisting of glycosphingolipids and associated molecules is also involved in the recognition and binding of the carbohydrate structures by carbohydrate-recognizing molecules from outside of cells (trans-acting). Binding of many bacterial toxins occurs in this manner (Fig. 3). Recently, we reported that recognition of ganglioside GD3 on the cell membrane by a sialic acid-recognizing lectin, Siglec-7, is dependent on by the clustering mode of GD3 in lipid rafts (Hashimoto et al. 2019). Here, recruitment of GD3 to lipid rafts was determined by the presence/absence of hydroxyl group in ceramide moiety. Thus, the cluster formation of GD3 in lipid rafts confers its binding with Siglec-7, and finally sensitivity to the killing with NK cells (Hashimoto et al. 2019). How Siglec-7 recognizes only clustered GD3 in GEM/rafts remains to be investigated. Consequently, trans-interaction of cancer-associated glycosphingolipids and carbohydrate-recognizing molecules (lectins) should determine the survival intensity of the cancer cells in our bodies based on the modulation of the escape of cancer cells from immune surveillance of host immune systems.
Not only Siglecs, but other glycolipid-recognizing molecules such as soluble lectins and monoclonal antibodies also interact with cancer-associated glycosphingolipids on the cell surface in a trans-binding manner. In particular, an anti-GM3 monoclonal antibody, M2590, reacted only with GM3 with “high density” or “topographical distribution” (Sakiyama et al. 1987). This binding mode might indicate that mAb M2590 can recognize only GM3 concentrated in lipid rafts, while precise mechanisms remain to be clarified.
9 Application of Signal Studies for Novel Cancer Treatment
A number of trials have been performed to apply mouse and/or human monoclonal antibodies reactive with cancer-associated gangliosides for cancer treatment (Houghton et al. 1985; Irie and Morton 1986; Yamaguchi et al. 1987). Anti-ganglioside mAbs have been rigorously studied for their effects in the suppression of mouse melanomas, human neuroblastomas, and human melanomas (Harel et al. 1990). Vaccine therapy with gangliosides or neutral glycolipids and various adjuvants have also been challenged (Yu et al. 2020; Ragupathi 1996; Livingston 1995; Sigal et al. 2022). They showed markedly nice results in some cases, but not so good in some cases. Anti-GD2 mAbs showed significant effects in extending remission intervals of neuroblastoma patients when used during remission phase (Kushner et al. 2015).
Generally, immune therapy of cancers toward cancer-associated glycosphingolipids can be successful by the definitely specific expression patterns, for example, “class 2” group in Old’s proposal (Old 1981) (Table 1). Thus, the specificity in the expression of carbohydrate structures on malignant cells has strictly been required, while those with relatively high levels in malignant cells compared to normal cells and tissues have been also accepted as Rituximab (anti-CD20) used in B-cell lymphomas (McLaughlin et al. 1998).
Various novel strategies have been constructed to eradicate malignant tumors based on the expression/function analyses of cancer-associated glycosphingolipids, and been applied in clinical. GD3 and GD2 are synthesized via the action of GD3 synthase, and specifically activate several signals that are essential for the malignant phenotypes of cancers. Therefore, in addition to these gangliosides and ganglioside synthases, signaling molecules, which are located at the downstream of signaling pathway activated by those cancer-associated gangliosides and play a crucial role in the promotion of malignant phenotypes, can be targets for cancer therapy (Furukawa et al. 2006) (Fig. 4). As fundamental studies for the substantial basis for cancer treatment toward glycosylation machinery, sh-RNA- expressing plasmids of GD3 synthase were tried (Ko et al. 2006), showing excellent effects on the gene expression and cell phenotypes. Repeated transfection of anti-GD3 synthase siRNA into lung cancer cells resulted in the suppression of GD2 expression and also reduction in the cell growth and invasion activity. Moreover, cell apoptosis could be induced by the repeated siRNA transfection. Stable introduction of RNAi expression vector (sh-RNA) in human SCLC cell lines showed slower cell growth than the control cells in severe combined immunodeficiency mice (Ko et al. 2006).
Strategies for cancer treatment by targeting molecules activated by ganglioside expression including GSL-membrane molecular complexes. In addition to cancer-associated glycolipids on the membrane, their associating molecules on the cell surface and activated signaling molecules at the downstream of the signaling pathway can be targets of therapeutics for cancers (Furukawa et al. 2006)
Small interfering RNA(s) (siRNA(s)) mixed with atello-collagen was tried to suppress human malignant melanomas grafted on nu/nu mice (Makino et al. 2016). Combined siRNAs against two adaptor molecules, that is, p130Cas and paxillin, showed most efficient suppression activity of the tumor growth in mice compared with that of either siRNA (Makino et al. 2016). In agreement with the tumor suppression effects, reduction in Ki-67-positive cell number was demonstrated, suggesting that blockade of GD3-mediated growth signaling pathway by siRNAs might be a novel and promising therapeutic strategy against malignant melanomas.
As one of important signaling molecules located at the downstream of membrane gangliosides, FAK has been also considered as a promising target of cancer treatment (van Nimwegen and van de Water 2007; Sulzmaier et al. 2014; Zimmer and Steeg 2015; Chauhan and Khan 2021; Dawson et al. 2021). FAK plays an important role in signaling pathways, which are triggered by integrin-mediated cell adhesions and by growth factor receptors (Fig. 4) (van Nimwegen and van de Water 2007). FAK is highly expressed in some solid tumors and also in stromal cells of the tumor microenvironments (Sulzmaier et al. 2014), and considered to promote tumor progression and metastasis. Therefore, small molecular weight FAK inhibitors have been developed, and been used in preclinical trials (Chauhan and Khan 2021). In particular, FAK is considered to be promising target of metastasis prevention trials (Zimmer and Steeg 2015), and targeting FAK in anticancer combination therapies is now being expected (Dawson et al. 2021). These studies well correspond with our past reports on GD3- or GD2-mediated signal activation in melanomas (Hamamura et al. 2008; Yesmin et al. 2021) or small cell lung cancers (Aixinjueluo et al. 2005).
10 Conclusion
Among 3 classes of tumor antigens listed in Table 1 (Old 1981), cancer-associated carbohydrate antigens should be included in “class 2” (shared) tumor antigens. Compared with “neoantigens” originating from gene mutations occurred in cancer cells, “class 2” antigens are much better targets for cancer immunotherapy. First of all, it is easy to construct therapeutic strategy because of common presence among similar cancers compared with class 1 (unique) antigens or neo-antigens. Secondly, it is possible to depend on relative abundance of antigens on cancers and to consider as targets of therapy, while they are expressed in some sites, some developmental stages of life with much lower levels. Finally, many successful examples have been reported so far and many trials are ongoing. Consequently, we are now challenging novel cancer treatment targeting molecules forming molecular complexes with cancer-associated glycosphingolipids on the membrane and/or in the cytoplasm (Kotani et al. 2008; Furukawa et al. 2008).
References
Aixinjueluo W, Furukawa K, Zhang Q, Hamamura K, Tokuda N, Yoshida S, Ueda R, Furukawa K (2005) Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: roles of anoikis. J Biol Chem 280:29828–29836. https://doi.org/10.1074/jbc.M414041200
Azuma K, Tanaka M, Uekita T, Inoue S, Yokota J, Ouchi Y, Sakai R (2005) Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene 24:4754–4764. https://doi.org/10.1038/sj.onc.1208654
Carubia JM, Yu RK, Macala LJ, Kirkwood JM, Varga JM (1984) Gangliosides of normal and neoplastic human melanocytes. Biochem Biophys Res Commun 120(2):500–504. https://doi.org/10.1016/0006-291x(84)91282-8
Cavdarli S, Delannoy P, Groux-Degroote S (2020) O-acetylated gangliosides as targets for cancer immunotherapy. Cell 9(3):741. https://doi.org/10.3390/cells9030741
Cazet A, Bobowski M, Rombouts Y, Lefebvre J, Steenackers A, Popa I, Guérardel Y, Le Bourhis X, Tulasne D, Delannoy P (2012) The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 22:806–816. https://doi.org/10.1093/glycob/cws049
Chauhan A, Khan T (2021) Focal adhesion kinase-An emerging viable target in cancer and development of focal adhesion kinase inhibitors. Chem Biol Drug Des 97(3):774–794. https://doi.org/10.1111/cbdd.13808
Chen HH, Fukumoto S, Furukawa K, Nakao A, Akiyama S, Urano T, Furukawa K (2003) Suppression of lung metastasis of mouse Lewis lung cancer P29 with transfection of the ganglioside GM2/GD2 synthase gene. Int J Cancer 103:169–176. https://doi.org/10.1002/ijc.10797
Cheresh DA, Rosenberg J, Mujoo K, Hirschowitz L, Reisfeld RA (1986) Biosynthesis and expression of the disialoganglioside GD2, a relevant target antigen on small cell lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer Res 46:5112–5118
Dawson JC, Serrels A, Stupack DG, Schlaepfer DD, Frame MC (2021) Targeting FAK in anticancer combination therapies. Nat Rev Cancer 21(5):313–324. https://doi.org/10.1038/s41568-021-00340-6
De Giorgi U, Cohen EN, Gao H, Mego M, Lee BN, Lodhi A, Cristofanilli M, Lucci A, Reuben JM (2011) Mesenchymal stem cells expressing GD2 and CD271 correlate with breastcancer-initiating cells in bone marrow. Cancer Biol Ther 11:812–815. https://doi.org/10.4161/cbt.11.9.15178
Dippold WG, Lloyd KO, Li LT, Ikeda H, Oettgen HF, Old LJ (1980) Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A 77:6114–6118. https://doi.org/10.1073/pnas.77.10.6114
Dong Y, Ikeda K, Hamamura K, Zhang Q, Kondo Y, Matsumoto Y, Ohmi Y, Yamauchi Y, Furukawa K, Taguchi R, Furukawa K (2012) GM1 / GD1b / GA1 synthase expression results in the reduced cancer phenotypes with modulation of composition and raft-localization of gangliosides in a melanoma cell line. Cancer Sci 101:2039–2047. https://doi.org/10.1111/j.1349-7006.2010.01613.x
Esaki N, Ohkawa Y, Hashimoto N, Tsuda Y, Ohmi Y, Bhuiyan RH, Kotani N, Honke K, Enomoto A, Takahashi M, Furukawa K, Furukawa K (2018) ASC amino acid transporter 2, defined by enzyme-mediated activation of radical sources, enhances malignancy of GD2-positive small-cell lung cancer. Cancer Sci 109:141–153. https://doi.org/10.1111/cas.13448
Fredman P, von Holst H, Collins VP, Ammar A, Dellheden B, Wahren B, Granholm L, Svennerholm L (1986) Potential ganglioside antigens associated with human gliomas. Neurol Res 8:123–126. https://doi.org/10.1080/01616412.1986.11739744
Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T (2007) Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol Biol Cell 18:2112–2122. https://doi.org/10.1091/mbc.e07-01-0071
Fukumoto S, Mutoh T, Hasegawa T, Miyazaki H, Okada M, Goto G, Furukawa K, Urano T (2000) GD3 synthase gene expression in PC12 cells results in the continuous activation of TrkA and ERK1/2 and enhanced proliferation. J Biol Chem 275:5832–5838. https://doi.org/10.1074/jbc.275.8.5832
Furukawa K, Lloyd KO (1990) In: Ferrone S (ed) Human melanoma: from basic research to clinical application. Springer, Heidelburg, pp 15–30
Furukawa K, Akagi T, Nagata Y, Yamada Y, Shimotohno K, Cheung N-K, Shiku H, Furukawa K (1993) GD2 ganglioside on human T-lymphotropic virus type I-infected T cells: possible activation of beta-1,4-N-acetylgalactosaminyltransferase gene by p40tax. Proc Natl Acad Sci U S A 90:1972–1976. https://doi.org/10.1073/pnas.90.5.1972
Furukawa K, Tokuda N, Okuda T, Tajima O, Furukawa K (2004) Glycosphingolipids in engineered mice: insights into function. Semin Cell Dev Biol 15:389–396. https://doi.org/10.1016/j.semcdb.2004.03.006
Furukawa K, Hamamura K, Aixinjueluo W, Furukawa K (2006) Biosignals modulated by tumor-associated carbohydrate antigens: novel targets for cancer therapy. Ann N Y Acad Sci 1086:185–198. https://doi.org/10.1196/annals.1377.017
Furukawa K, Hamamura K, Nakashima H, Furukawa K (2008) Molecules in the signaling pathway activated by gangliosides can be targets of therapeutics for malignant melanomas. Proteomics 8:3312–3316. https://doi.org/10.1002/pmic.200800228
Furukawa K, Hamamura K, Ohkawa Y, Ohmi Y, Furukawa K (2012a) Disialyl ganglio-sides enhance tumor phenotypes with differential modalities. Glycoconj J 29:579–584. https://doi.org/10.1007/s10719-012-9423-0
Furukawa K, Ohkawa Y, Yamauchi Y, Hamamura K, Ohmi Y, Furukawa K (2012b) Fine tuning of cell signals by glycosylation. J Biochem 151:573–578. https://doi.org/10.1093/jb/mvs043
Furukawa K, Ohmi Y, Kondo Y, Ohkawa Y, Hashimoto N, Tajima O, Furukawa K (2014) The role of glycosphingolipifds. Lessons from knockout mice. In: Sillence D (ed) Lipid rafts. Properties, controversies and roles in signal transduction. Nova Publishers, New York, pp 1–20
Furukawa K, Ohmi Y, Ohkawa Y, Bhuiyan RH, Zhang P, Tajima O, Hashimoto N, Hamamura K, Furukawa K (2019) New era of research on cancer-associated glycosphingolipids. Cancer Sci 110:1544–1551. https://doi.org/10.1111/cas.14005
Furukawa K, Ohmi Y, Yesmin F, Tajima O, Kondo Y, Pu Zhang P, Hashimoto N, Ohkawa Y, Bhuiyan RH, Furukawa K (2020) Novel molecular mechanisms for roles of gangliosides in the nervous system elucidated by genetic engineering. Int J Mol Sci 11(21):E1906. https://doi.org/10.3390/ijms21061906
Groux-Degroote S, Guérardel Y, Delannoy P (2017) Gangliosides: structures, biosynthesis, analysis, and roles in cancer. Chembiochem 18:1146–1154. https://doi.org/10.1002/cbic.201600705
Hakomori S (1986) Tumor-associated glycolipid antigens, their metabolism and organization. Chem Phys Lipids 42:209–233
Hakomori S (1998) Cancer-associated glycosphingolipid antigens: their structure, organization, and function. Acta Anat (Basel) 161(1-4):79–90. https://doi.org/10.1159/000046451
Hakomori S (2003) Structure, organization, and function of glycosphingolipids in membrane. Curr Opin Hematol 10:16–24. https://doi.org/10.1097/00062752-200301000-00004
Hakomori SI (2010) Glycosynaptic microdomains controlling tumor cell phenotype through alteration of cell growth, adhesion, and motility. FEBS Lett 584:1901–1906. https://doi.org/10.1016/j.febslet.2009.10.065
Hakomori S, Yamamura S, Handa AK (1998) Signal transduction through glyco(sphingo)lipids. Introduction and recent studies on glyco(sphingo)lipid-enriched micro-domains. Ann N Y Acad Sci 845:1–10. https://doi.org/10.1111/j.1749-6632.1998.tb09657.x
Hamamura K, Furukawa K, Hayashi T, Hattori T, Nakano J, Nakashima H, Okuda T, Mizutani H, Hattori H, Ueda M, Urano T, Lloyd KO, Furukawa K (2005) Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc Natl Acad Sci U S A 102:11041–11046. https://doi.org/10.1073/pnas.0503658102
Hamamura K, Tsuji M, Ohkawa Y, Nakashima H, Miyazaki S, Urano T, Yamamoto N, Ueda M, Furukawa K, Furukawa K (2008) Focal adhesion kinase as well as p130Cas and paxillin is crucially involved in the enhanced malignant properties under expression of ganglioside GD3 in melanoma cells. Biochim Biophys Acta 1780(3):513–519. https://doi.org/10.1016/j.bbagen.2007.11.002
Hamamura K, Tsuji M, Hotta H, Ohkawa Y, Takahashi M, Shibuya H, Nakashima H, Yamauchi Y, Hashimoto N, Hattori H, Ueda M, Furukawa K, Furukawa K (2011) Functional activation of Src family kinase yes protein is essential for the enhanced malignant properties of human melanoma cells expressing ganglioside GD3. J Biol Chem 286:18526–18537. https://doi.org/10.1074/jbc.M110.164798
Haraguchi M, Yamashiro S, Yamamoto A, Furukawa K, Takamiya K, Lloyd KO, Shiku H, Furukawa K (1994) Isolation of GD3 synthase gene by expression cloning of GM3 alpha-2,8-sialyltransferase cDNA using anti-GD2 monoclonal antibody. Proc Natl Acad Sci U S A 91:10455–10459. https://doi.org/10.1073/pnas.91.22.10455
Harel W, Shau H, Hadley CG, Morgan AC Jr, Reisfeld RA, Cheresh DA, Mitchell MS (1990) Increased lysis of melanoma by in vivo-elicited human lymphokine-activated killer cells after addition of antiganglioside antibodies in vitro. Cancer Res 50:6311–6315
Hashimoto N, Hamamura K, Kotani N, Furukawa K, Kaneko K, Honke K, Furukawa K (2012) Proteomic analysis of ganglioside-associated membrane molecules: substantial basis for molecular clustering. Proteomics 12(21):3154-3163 aa. https://doi.org/10.1002/pmic.201200279
Hashimoto N, Ito S, Tsuchida A, Bhuiyan RH, Okajima T, Yamamoto A, Furukawa K, Ohmi Y, Furukawa K (2019) The ceramide moiety of disialoganglioside (GD3) is essential for GD3 recognition by the sialic acid-binding lectin SIGLEC7 on the cell surface. J Biol Chem 294(28):10833–10845. https://doi.org/10.1074/jbc.RA118.007083
Houghton AN, Mintzer D, Cordon-Cardo C, Welt S, Fliegel B, Vadhan S, Carswell E, Melamed MR, Oettgen HF, Old LJ (1985) Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci U S A 82:1242–1246. https://doi.org/10.1073/pnas.82.4.1242
Indellicato R, Zulueta A, Caretti A, Trinchera M (2020) Complementary use of carbohydrate antigens Lewis a, Lewis b, and Sialyl-Lewis a (CA19.9 epitope) in gastrointestinal cancers: biological rationale towards a personalized clinical application. Cancers 12:1509. https://doi.org/10.3390/cancers12061509
Irie RF, Morton DL (1986) Regression of cutaneous metastatic melanoma by intralesional injection with human monoclonal antibody to ganglioside GD2. Proc Natl Acad Sci U S A 83:8694–8698. https://doi.org/10.1073/pnas.83.22.8694
Iwasawa T, Zhang P, Ohkawa Y, Momota H, Wakabayashi T, Ohmi Y, Bhuiyan RH, Furukawa K, Furukawa K (2018) Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int J Oncol 52:1255–1266. https://doi.org/10.3892/ijo.2018.4266
Kaneko K, Ohkawa Y, Hashimoto N, Ohmi Y, Kotani N, Honke K, Ogawa M, Okajima T, Furukawa K, Furukawa K (2016) Neogenin, defined as a GD3-associated molecule by enzyme-mediated activation of radical sources, confers malignant properties via intracytoplasmic domain in melanoma cells. J Biol Chem 291:16630–16643. https://doi.org/10.1074/jbc.M115.708834
Kawai K, Takahashi H, Watarai S, Ishizu H, Fukai K, Tanabe Y, Nose S, Kuroda S (1999) Occurrence of ganglioside GD3 in neoplastic astrocytes. An immunocytochemical study in humans. Virchows Arch 434:201–205. https://doi.org/10.1007/s004280050328
Ko K, Furukawa K, Takahashi T, Urano T, Sanai Y, Nagino M, Nimura Y, Furukawa K (2006) Fundamental study of small interfering RNAs for ganglioside GD3 synthase gene as a therapeutic target of lung cancers. Oncogene 25:6924–6935. https://doi.org/10.1038/sj.onc.1209683
Komura N, Suzuki KG, Ando H, Konishi M, Koikeda M, Imamura A, Chadda R, Fujiwara TK, Tsuboi H, Sheng R, Cho W, Furukawa K, Furukawa K, Yamauchi Y, Ishida H, Kusumi A, Kiso M (2016) Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat Chem Biol 12:402–410. https://doi.org/10.1038/nchembio.2059
Kotani N, Gu J, Isaji T, Udaka K, Taniguchi N, Honke K (2008) Biochemical visualization of cell surface molecular clustering in living cells. Proc Natl Acad Sci U S A 105:7405–7409. https://doi.org/10.1073/pnas.0710346105
Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Kramer K, Modak S, Yataghene K, Cheung NK (2015) Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-G(D2) immunotherapy and isotretinoin: a prospective Phase II study. Onco Targets Ther 4:e1016704. https://doi.org/10.1080/2162402X.2015.1016704
Livingston PO (1995) Augmenting the immunogenicity of carbohydrate tumor antigens. Semin Cancer Biol 6(6):357–366. https://doi.org/10.1016/1044-579x(95)90005-5
Lloyd KO (1991) Humoral immune responses to tumor-associated carbohydrate antigens. Semin Cancer Biol 2:421–431
Lloyd KO, Old LJ (1989) Human monoclonal antibodies to glycolipids and other carbohydrate antigens: dissection of the humoral immune response in cancer patients. Cancer Res 49:3445–3451
Makino Y, Hamamura K, Takei Y, Buiyan RH, Ohkawa Y, Ohmi Y, Nakashima H, Furukawa K, Furukawa K (2016) A therapeutic trial of human melanomas with combined small interfering RNAs targeting adaptor molecules p130Cas and paxillin activated under expression of ganglioside GD3. Biochim Biophys Acta 1860:1753–1763. https://doi.org/10.1016/j.bbagen.2016.04.005
McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833. https://doi.org/10.1200/JCO.1998.16.8.2825
Merritt WD, Casper JT, Lauer SJ, Reaman GH (1987) Expression of GD3 ganglioside in childhood T-cell lymphoblastic malignancies. Cancer Res 47:1724–1730
Mitsuda T, Furukawa K, Fukumoto S, Miyazaki H, Urano T, Furukawa K (2002) Overexpression of ganglioside GM1 results in the dispersion of platelet-derived growth factor receptor from glycolipid-enriched microdomains and in the suppression of cell growth signals. J Biol Chem 277:11239–11246. https://doi.org/10.1074/jbc.M107756200
Miyagi T, Takahashi K, Yamamoto K, Shiozaki K, Yamaguchi K (2018) Biological and pathological roles of ganglioside sialidases. Prog Mol Biol Transl Sci 156:121–150. https://doi.org/10.1016/bs.pmbts.2017.12.005
Miyazaki H, Fukumoto S, Okada M, Hasegawa T, Furukawa K (1997) Expression cloning of rat cDNA encoding UDP-galactose:GD2 beta1,3-galactosyl-transferase that determines the expression of GD1b/GM1/GA1. J Biol Chem 272:24794–24799. https://doi.org/10.1074/jbc.272.40.24794
Nagata Y, Nagata Y, Yamashiro S, Yodoi J, Lloyd KO, Shiku H, Furukawa K (1992) Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem 267:12082–12089
Nakashima H, Hamamura K, Houjou T, Taguchi R, Yamamoto N, Mitsudo K, Tohnai I, Ueda M, Urano T, Furukawa K, Furukawa K (2007) Overexpression of caveolin-1 in a human melanoma cell line results in dispersion of ganglioside GD3 from lipid rafts and alteration of leading edges, leading to attenuation of malignant properties. Cancer Sci 98:512–520. https://doi.org/10.1111/j.1349-7006.2007.00419.x
Nara K, Watanabe Y, Maruyama K, Kasahara K, Nagai Y, Sanai Y (1994) Expression cloning of a CMP-NeuAc:NeuAc alpha 2-3Gal beta 1-4Glc beta 1-1'Cer alpha 2,8-sialyltransferase (GD3 synthase) from human melanoma cells. Proc Natl Acad Sci U S A 91:7952–7956. https://doi.org/10.1073/pnas.91.17.7952
Nishio M, Fukumoto S, Furukawa K, Ichimura A, Miyazaki H, Kusunoki S, Urano T, Furukawa K (2004) Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J Biol Chem 279(32):33368–33378. https://doi.org/10.1074/jbc.M403816200
Ohkawa Y, Miyazaki S, Miyata M, Hamamura K, Furukawa K, Furukawa K (2008) Essential roles of integrin-mediated signaling for the enhancement of malignant properties of melanomas based on the expression of GD3. Biochem Biophys Res Commun 373:14–19. https://doi.org/10.1016/j.bbrc.2008.05.149
Ohkawa Y, Miyazaki S, Hamamura K, Kambe M, Miyata M, Tajima O, Ohmi Y, Yamauchi Y, Furukawa K, Furukawa K (2010) Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J Biol Chem 285:27213–27223. https://doi.org/10.1074/jbc.M109.087791
Ohkawa Y, Momota H, Kato A, Hashimoto N, Tsuda Y, Kotani N, Honke K, Suzumura A, Furukawa K, Ohmi Y, Natsume A, Wakabayashi T, Furukawa K (2015) Ganglioside GD3 enhances invasiveness of gliomas by forming a complex with platelet-derived growth factor receptor α and yes kinase. J Biol Chem 290:16043–16058. https://doi.org/10.1074/jbc.M114.635755
Ohkawa Y, Zhang P, Momota H, Kato A, Hashimoto N, Ohmi Y, Bhuiyan RH, Farhana Y, Natsume A, Wakabayashi T, Furukawa K, Furukawam K (2021) Lack of GD3 synthase (St8sia1) attenuates malignant properties of gliomas in genetically engineered mouse model. Cancer Sci 112:3756–3768. https://doi.org/10.1111/cas.15032
Ohmi Y, Kambe M, Ohkawa Y, Hamamura K, Tajima O, Takeuchi R, Furukawa K, Furukawa K (2018) Differential roles of gangliosides in malignant properties of melanomas. PLoS One 13:e0206881. https://doi.org/10.1371/journal.pone.0206881
Okada M, Furukawa K, Yamashiro S, Yamada Y, Haraguchi M, Horibe K, Kato K, Tsuji Y, Furukawa K (1996) High expression of ganglioside alpha-2,8-sialyltransferase (GD3 synthase) gene in adult T-cell leukemia cells unrelated to the gene expression of human T-lymphotropic virus type I. Cancer Res 56:2844–2848
Old LJ (1981) Cancer immunology: the search for specificity – G.H.A. Clows Memorial Lecture. Cancer Res 41:361–375
Portoukalian J, Zwingelstein G, Doré JF (1979) Lipid composition of human malignant melanoma tumors at various levels of malignant growth. Eur J Biochem 94:19–23. https://doi.org/10.1111/j.1432-1033.1979.tb12866.x
Pukel CS, Lloyd KO, Travassos LR, Dippold WG, Oettgen HF, Old LJ (1982) GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med 155:1133–1147. https://doi.org/10.1084/jem.155.4.1133
Ragupathi G (1996) Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother 43(3):152–157. https://doi.org/10.1007/s002620050316
Ravindranath MH, Muthugounder S, Presser N, Selvan SR, Portoukalian J, Brosman S, Morton DL (2004) Gangliosides of organ-confined versus metastatic androgen-receptor-negative prostate cancer. Biochem Biophys Res Commun 324(1):154–165. https://doi.org/10.1016/j.bbrc.2004.09.029
Saito M, Yu RK, Cheung N-K (1985) Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochem Biophys Res Commun 127:1–7. https://doi.org/10.1016/s0006-291x(85)80117-0
Sakiyama H, Takahashi T, Hirabayashi Y, Taniguchi M (1987) Change in the topographical distribution of GM3 during cell spreading and growth: immunostaining with monoclonal antibody against GM3. Cell Struct Funct 12:93–105. https://doi.org/10.1247/csf.12.93
Sasaki K, Kurata K, Kojima N, Kurosawa N, Ohta S, Hanai N, Tsuji S, Nishi T (1994) Expression cloning of a GM3-specific alpha-2,8-sialyltransferase (GD3synthase). J Biol Chem 269:15950–15956
Sato M, Shimada S, Watanabe M, Kawasaki Y, Sato T, Morozumi K, Mitsuzuka K, Ito A (2020) Expression of ganglioside disialosyl globopentaosyl ceramide in prostate biopsy specimens as a predictive marker for recurrence after radical prostatectomy. Tohoku J Exp Med 252(1):1–8. https://doi.org/10.1620/tjem.252.1
Schultz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA (1984) Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res 44:5914–5920
Shibuya H, Hamamura K, Hotta H, Matsumoto Y, Nishida Y, Hattori H, Furukawa K, Ueda M, Furukawa K (2012) Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci 103(9):1656–1664. https://doi.org/10.1111/j.1349-7006.2012.02344.x
Siddiqui B, Buehler J, DeGregorio MW, Macher BA (1984) Differential expression of ganglioside GD3 by human leukocytes and leukemia cells. Cancer Res 44:5262–5265
Sigal DS, Hermel DJ, Hsu P, Pearce T (2022) The role of Globo H and SSEA-4 in the development and progression of cancer, and their potential as therapeutic targets. Future Oncol 18(1):117–134. https://doi.org/10.2217/fon-2021-1110
Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11:688–699. https://doi.org/10.1038/nrm2977
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572. https://doi.org/10.1038/42408
Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39. https://doi.org/10.1038/35036052
Sonnino S, Prinetti A (2010) Gangliosides as regulators of cell membrane organization and functions. Adv Exp Med Biol 688:165–184. https://doi.org/10.1007/978-1-4419-6741-1_12
Stanley P (2016) What have we learned from glycosyltransferase knockouts in mice? J Mol Biol 428:3166–3182. https://doi.org/10.1016/j.jmb.2016.03.025
Sulzmaier FJ, Jean C, Schlaepfer DD (2014) FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14(9):598–610. https://doi.org/10.1038/nrc3792
Tsurifune T, Ito T, Li XJ, Yamashiro S, Okada M, Kanematsu T, Shiku H, Furukawa K (2000) Alteration of tumor phenotypes of B16 melanoma after genetic remodeling of the ganglioside profile. Int J Oncol 17:159–165
van Nimwegen MJ, van de Water B (2007) Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol 73(5):597–609. https://doi.org/10.1016/j.bcp.2006.08.011
Vantaku V, Donepudi SR, Ambati CR, Jin F, Putluri V, Nguyen K, Rajapakshe K, Coarfa C, Battula VL, Lotan Y, Putluri N (2017) Expression of ganglioside GD2, reprogram the lipid metabolism and EMT phenotype in bladder cancer. Oncotarget 8(56):95620–95631. https://doi.org/10.18632/oncotarget.21038. eCollection
Vukelić Z, Kalanj-Bognar S, Froesch M, Bîndila L, Radić B, Allen M, Peter-Katalinić J, Zamfir AD (2007) Human gliosarcoma-associated ganglioside composition is complex and distinctive as evidenced by high-performance mass spectrometric determination and structural characterization. Glycobiology 17:504–515. https://doi.org/10.1093/glycob/cwm012
Wikstrand CJ, Fredman P, Svennerholm L, Bigner DD (1994) Detection of glioma-associated gangliosides GM2, GD2, GD3, 3′-isoLM1 3′,6′-isoLD1 in central nervous system tumors in vitro and in vivo using epitope-defined monoclonal antibodies. Prog Brain Res 101:213–223. https://doi.org/10.1016/s0079-6123(08)61951-2
Yamaguchi H, Furukawa K, Fortunato SR, Livingston PO, Lloyd KO, Oettgen HF, Old LJ (1987) Cell-surface antigens of melanoma recognized by human monoclonal antibodies. Proc Natl Acad Sci U S A 84:2416–2420. https://doi.org/10.1073/pnas.84.8.2416
Yesmin F, Bhuiyan RH, Ohmi Y, Yamamoto S, Kaneko K, Ohkawa Y, Zhang P, Hamamura K, Nai-Kong CV, Kotani N, Honke K, Okajima T, Kambe M, Tajima O, Furukawa K, Furukawa K (2021) Ganglioside GD2 enhances malignant phenotypes of melanoma cells by co-operating with integrins. Int J Mol Sci 23:423. https://doi.org/10.3390/ijms23010423
Yoshida S, Fukumoto S, Kawaguchi H, Sato S, Ueda R, Furukawa K (2001) Ganglioside G(D2) in small cell lung cancer cell lines: enhancement of cell proliferation and mediation of apoptosis. Cancer Res 61:4244–4252
Yu J, Hung JT, Wang SH, Cheng JY, Yu AL (2020) Targeting glycosphingolipids for cancer immunotherapy. FEBS Lett 594(22):3602–3618. https://doi.org/10.1002/1873-3468.13917
Zhang Q, Furukawa K, Chen HH, Sakakibara T, Urano T, Furukawa K (2006) Metastatic potential of mouse Lewis lung cancer cells is regulated via ganglioside GM1 by modulating the matrix metalloprotease-9 localization in lipid rafts. J Biol Chem 281:18145–18155. https://doi.org/10.1074/jbc.M512566200
Zhang P, Ohkawa Y, Yamamoto S, Momota H, Kato A, Kaneko K, Natsume A, Yesmin F, Ohmi Y, Okajima T, Wakabayashi T, Furukawa K, Furukawa K (2021) St8sia1-deficiency in mice alters tumor environments of gliomas, leading to reduced disease severity. Nagoya J Med Sci 83(3):535–549. https://doi.org/10.18999/nagjms.83.3.535
Zimmer AS, Steeg PS (2015) Meaningful prevention of breast cancer metastasis: candidate therapeutics, preclinical validation, and clinical trial concerns. J Mol Med (Berl) 93(1):13–29. https://doi.org/10.1007/s00109-014-1226-2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Furukawa, K. et al. (2023). Opposite Functions of Mono- and Disialylated Glycosphingo-Lipids on the Membrane of Cancer Cells. In: Furukawa, K., Fukuda, M. (eds) Glycosignals in Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-7732-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-19-7732-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7731-2
Online ISBN: 978-981-19-7732-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)