Abstract
The generation of environmental changes, as well as the implementation of agricultural and cultural practices, induces a large number of problems related to abiotic or biotic stress in plants, which produce not only negative, also positive effects, since the plant uses its engineering mechanical, biochemical and molecular to defend its integrity. In this sense, various primary and secondary metabolites are produced under these conditions of stress, and among the secondary ones, phenolic compounds are the most synthesized. These compounds are considered one of the most important and diverse groups in plants, mainly due to their structural characteristics that provide important antioxidant activity. Antioxidants have the ability to neutralize reactive oxygen species; likewise, this defense mechanism can be used to consider plants as biofactories of phenolic compounds with a possible potential in health. In addition, the increase in the content of phenolic compounds could be manipulated depending on the plant, through its exposure to stress conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Phenolic compounds are obtained mainly from plants, which are characterized by presenting in their structure the phenyl ring and a C3 side chain; they mainly derive from the phenylalanine. Within those compounds, there is a large number of phenolic derivates such as flavonoids, phenolics acids coumarins, lignins, and proanthocyanidins; they all represent the main antioxidant system of defense in plants. Also, they are considered as antibiotics, natural pesticides, attractants for pollinators, protectors against abiotic conditions, and structural material in plants (Heldt and Piechulla 2011). Climate changes like extreme temperature, drought, light, deforestation, and rain contribute to stress in plants as well as that produced by infestations that can also be derived from the same environmental change (Karuppanapandian et al. 2011). Plants use different mechanisms to counteract stress, and one considered important is the production of phenolic compounds (Koyro and Huchzermeyer 2018). The production of these compounds is from a stimulus that generates the activation of different transcription factors (TF), which regulate various signals for the activation of genes that intervene in the metabolic pathways where phenolic compounds are synthesized (Lamers et al. 2020).
Therefore, due to these changes constant in natural conditions as well as in the management of some plants, the synthesis of different metabolites that are generated in the plant is promoted to establish a balance between antioxidant systems and reactive oxygen species (ROS), by the increase of phenolic compounds, this not only generates a positive effect on the plant but could also be exploited as a biofactory of phenolic compounds as they can be isolated or used in synergy for the development of products of agronomic interest and possible drugs with potential benefits on health.
Currently, the interest in the use of products of natural origin has come to revolutionize science and technology, and plants being a source of a large number of metabolites can be considered as a biofactory, since different exogenous and endogenous factors promote the generation of various compounds. Mainly, phenolic compounds are synthesized, due to their property of donating an electron to free radicals to stabilize them and, in turn, create species less damaging to the integrity of the cell (Madrigal-Santillán et al. 2013). Phenolics can be overproduced under stress and are of pharmaceutical interest, which makes them an excellent natural resource for the treatment of diseases (Koyro and Huchzermeyer 2018).
10.2 Stress in Plants
Due to their inability to mobilize, plants have developed defensive strategies to protect themselves to the continuous attacks from abiotic (environmental conditions) and biotic (bacteria, viruses, oomycetes, fungi, among others) factors that may affect their growth, development, or productivity (Hammond-Kosack and Jones 2015; Shinozaki et al. 2015). Under conditions of abiotics stress, the plants may trigger a defense called pathogen- and effector-triggered immunity, which are activated as a result of pathogen-associated molecular patterns. Moreover, under abiotic stress, plant signaling is activated by complex cellular and subcellular receptors (Meraj et al. 2020). The molecular signaling pathway triggered in response to these stresses involves diverse and intricate mechanisms such as ROS, mitogen-activated protein kinase, ethylene, salicylic acid, and jasmonic acid (Meraj et al. 2020; Camejo et al. 2016; Creelman and Mullet 1995; Fujita et al. 2006; Pitzschke et al. 2009; Yang et al. 2004).
As a defense mechanism, plants synthesize a set of a rich diversity of secondary metabolites as alkaloids, terpenoids, flavonoids, and phenolic acids. These molecules are normally produced at a low and steady rate to protect plants; however, during stress conditions, the biosynthesis of these molecules can be modulated positively or negatively (Meraj et al. 2020; Croteau et al. 2015). However, abiotic plant stresses have been the focus of many studies because of cultivation levels. Phenolic accumulation is a trait that has been exploited in agricultural research. Nonetheless, abiotic stresses might cause plants to accumulate bioactive compounds like phenolic compounds. However, this might also have a detrimental effect on the yield and organoleptic quality of fruits and vegetables (Toscano et al. 2019).
10.2.1 Abiotic Stress
Some authors define abiotic stress as any factor that might affect the ideal functions of an organism. Some of the most common abiotic factors are salinity, drought, nutrient depletion, temperature, and altitude; these can cause alterations in plants such as their growth and even might even cause death (Ali et al. 2020). Abiotic stresses elicit a response in plants, some of them are non-favorable; for instance, abiotic stress can overproduce ROS like superoxide (O2−), singlet oxygen (1O2), hydroxyl radical (OH−), and hydrogen peroxide (H2O2 a precursor of ROS), among others (Broad et al. 2020). As a result, abiotic stress triggers a signaling cascade to activate a plant response mechanism to try to recover homeostasis and protect damaged membranes or proteins (Ali et al. 2020; Ahmad et al. 2010). Within the action mechanisms of the plant in response to abiotic stress, is the activation of the enzymatic antioxidant machinery. Short-term exposure to abiotic stresses might enhance the phenolic content of plants (Zhou et al. 2017). However, long-term exposure to these conditions might cause damage to plant tissues and slow down the secondary plant metabolism, thus, decreasing the phenolic content (Król et al. 2015).
10.2.1.1 UV Light Stress
Within solar radiation, there is the ultraviolet wave (UV), visible, and infrared light, which have an impact on the development and growth of plants, because they are implicated in different physiological processes such as photosynthesis where they can also activate photoreceptors causing modifications in macromolecules (Verdaguer et al. 2017). UV radiation is divided into three regions UV-A (315–400 nm), UV-B (280–320 nm), and UV-C (200–280 nm). UV-C radiation being short wavelength is all but complete absorbed by the ozone layer, likewise also most solar UV-B is absorbed, and just a few passes thought the atmosphere while UV-A radiation passes almost unaltered to the earth’s surface (Morales et al. 2010; Solovchenko and Merzlyak 2008). UV-B light has the highest energy in daylight and could cause cell damage in plants, causing DNA mutations and raising levels of ROS. In this case, the plant cell responds by accumulating phenolic compounds in the epidermal cells (Sharma et al. 2019; León-Chan et al. 2017; Isah 2019). Light is an important factor that can affect plant production of phytochemicals (Naikoo et al. 2019). Phenolic compounds like anthocyanins and other flavonoids can absorb visible and UV radiations, granting protection against free radical damage and oxidative stress (Lattanzio 2013; Landi et al. 2015; Sharma et al. 2019).
10.2.1.2 Temperature Stress
High or low temperatures are the most common environmental abiotic factors that affect plant growth, as well as the production of primary and secondary metabolites (Hounsome et al. 2008; Son et al. 2020). Temperature strongly influences metabolic activity, and species have to adjust their metabolism to survive variations in both climate change and different seasons of the year (Akula and Ravishankar 2011). The plant response will depend on the plant species, and sometimes its effect on the phenolic accumulation can be detrimental and, in other instances, it favors their accumulation. It might often be used in combination with other types of abiotic stress to enhance the content of these molecules to improve its potentially beneficial effect on human health (Son et al. 2020; León-Chan et al. 2020; Sharma et al. 2019; Sakamoto and Suzuki 2015).
10.2.1.3 Water Stress
One of the most common water stresses plants suffer drought, which causes an impact in plant development and growth, because under this condition there is a lack or insufficient water in the soil, to carry out the different physiological processes of the plant as photosynthesis and homeostasis general of the plant (Impa et al. 2012). High temperatures and solar radiation generally accompany this drought. It has been become a problem in different regions all the world, mainly in the arid and semi-arid areas (Xu et al. 2010). However, this stressful condition can enhance the increase of phenolic compounds in response to the generation of ROS. Some studies report that drought causes overexpression of MYB12/PFG1 or MYB75/PAP1 TFs, increasing the biosynthesis of flavonols and anthocyanins (Nakabayashi et al. 2014; Kirakosyan et al. 2003).
10.2.1.4 Salinity
Salinity in soil or water causes of abiotic stress in plants, especially those growing or cultivated in semi-arid and arid climates. Salinity has been related to harmful effects produced in the plant such as nutritional imbalance, reduction in osmotic potential, as well as an effect on specific ions, and/or a combination of the aforementioned factors (Acosta-Motos et al. 2017; Ashraf and Harris 2004). The severity of the effects of soil or water salinity on plants also be determined by factors as environmental conditions and the capacity of plants to survive to saline conditions. In this sense, plants can be classified as glycophytes or euhalophythes. Most crop plants belong to the classification of glycophytes and are highly sensitive to increased salinity levels. On the other hand, euhalophythes are plants that have adaptative saline systems (Ashraf and Harris 2004). Also, this type of stress could increase the phytochemical content of plants due to the ionic and osmotic stress caused by salinity (Akula and Ravishankar 2011).
10.2.2 Biotic Stress
Plants can suffer from biotic stress from the attack of viruses, bacteria, fungi, insects, and herbivores, affecting plant germination, growth, and plant production (ul Haq et al. 2019). Biotic stress triggers the production of ROS and increases the risk of oxidative stress and cell death. In addition, biotic stress causes losses before and after harvest. Fortunately, plants have an adaptive complex defense system, as a reaction to the different types of biotic stresses (ul Haq et al. 2019; Dodds and Rathjen 2010). Plants have two main mechanisms to respond to pathogens, one is known as the pathogen-associated molecular patterns, which are elicitors that trigger a reaction recognized by pattern recognition receptors (Dodds and Rathjen 2010; Koeck et al. 2011). The intricate signaling molecular network is complex, and there are currently ongoing studies trying to understand the precise mechanisms of action of biotic stress plant response. However, biotic stress is less explored than abiotic stress. For instance, our literature research using the keywords “biotic stress” and “phenolic compounds” from 2010 to 2020 showed only 112 studies, of which 103 were original research papers and 9 reviews. On the other hand, abiotic stress studies on the Web of Science database showed 416 original research papers and 42 reviews. The high number may be explained because abiotic stress has been extensively studied to take advantage of the plant response in the phytochemical enhancement (Jacobo-Velazquez et al. 2011, 2015; Villarreal-Garcia et al. 2016; Heredia and Cisneros-Zevallos 2009).
10.3 Plant Response Mechanism to Stresses
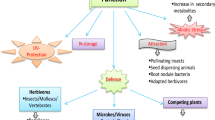
Plants have many reactions to different stresses, basically depending on the type of abiotic or biotic stress. For example, it could reduce their growth and photosynthesis, close stomata, generate ROS, and increase their production of secondary metabolites under abiotic stress. (Mahajan et al. 2020). Plants can change or modify their morphological structure or produce toxic chemicals as alkaloids, terpenoids, anthocyanins, phenolic compounds, and quinones; also, when herbivores attack them, insects, pathogens or to protect themselves of environmental factors negatives (drought, extremes temperatures, salinity, and radiation) (War et al. 2012). In stress situations, the plant activates a cascade of signals, which activate ion channels, kinases, TFs, phytohormones (salicylic acid, SA; abscisic acid, ABA; ethylene, ET; and jasmonic acid, JA) all of these signals lead to activate defense mechanisms. However, the response to the stress may be subject to the type of plant and stress conditions (Fraire-Velázquez et al. 2011; Cass et al. 2015).
Under biotic and abiotic stress situations, the plant uses defense mechanisms different, alone, or combined (Fig. 10.1). The first response is about morphological or structural changes, which includes the reduction of the cycle of life or the growing season, transpiration control by the stomatal closure, the change of the roots architecture system, and the shedding of leaves to reduce the surface area (Farooq et al. 2009; Das and Bhattacharya 2017). In this mechanism, there are structural deterrents of various types such as spines, hairs (trichomes), sclerophylly, leaf surface wax, cell wall thickness, lignification, and deposit of granular minerals; this mechanism is considered as “direct defense” of the plant (Hanley et al. 2007; War et al. 2012).
Likewise, under stress conditions, other mechanisms can be initiated, the molecular where some genes are expressed, and can affect the synthesis of some proteins to their protection and depend on the stress type duration and severity (Das and Bhattacharya 2017; Dos Reis et al. 2012; Moreno 2009). The different types of stresses (biotic and abiotic) generate a response in the activation of genes for the expression of enzymes that allow the biosynthesis of secondary metabolites (Suárez-Medina and Coy-Barrera 2016). In this sense, under a stress condition, specific TFs (essential proteins in the signaling network) are activated and regulate the stress-induced gene expression, through the binding to specific DNA sequence in the promoters of respective target genes (Verma et al. 2013; Woodrow et al. 2012). WRKY (tryptophan, W; arginine, R; lysine, K; tyrosine, Y) is an important TFs, which perform regulatory functions in defense of the plant against different types of stresses as mechanical damage, salinity, drought, cold, pest, and diseases (Vinod 2012). Other TFs such as MYB and bHLH (basic helix-loop-helix) are important for the regulation of phenylpropanoid biosynthesis pathway (Zhao et al. 2005).
On the other hand, in each stress type, different classes of TFs participate. For example, TFs that are involved in drought are called dehydration element-binding proteins (DREB), which induce genes to promote the adapting to the stress (Woodrow et al. 2012). Likewise, several TFs are mediators in phytohormone signaling pathways, which are widely recognized for their role in signaling abiotic and biotic stress. The hormones involved in biotic stress are JA, ET (necrotrophic pathogens and herbivorous insects), and SA (biotrophic and hemibiotrophic pathogens), while in abiotic is ABA (Fraire-Velázquez et al. 2011; Moreno 2009; Gómez and Rodríguez 2012; Verma et al. 2013).
Interestingly, the response of plants can vary activating different TFs depending on stress, as expressed in Arabidopsis thaliana, where they were subjected to different temperatures and nitrogen depletion, finding that TFs PAP1 and PAP2 increase strongly at low temperatures (5–10 °C) and depletion of nitrogen, as well as TFs GL3 and MYB12, which coincides with an increase in flavonols but not in kaempferols and quercetin (Olsen et al. 2009).
Another mechanism is biochemical, which involves an osmotic adjustment, an antioxidant defense system, and a purifier. For which the plant uses enzymatic and non-enzymatic compounds (Das and Bhattacharya 2017). In the osmotic adjustment, some compounds like amino acids, carbohydrates, and organics acids have an osmolytic function and can provide plant protection against some abiotic stresses mainly (Mantri et al. 2012; Farooq et al. 2009).
The enzymatic system refers to the reaction of the cell to control the ROS excess, which is produced by stress. This reaction involves the oxidative enzymes catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), ascorbate peroxidase (APX), and glutathione reductase (GR). These enzymes are contained in chloroplast, mitochondria, and peroxisome (Impa et al. 2012). Each enzyme has a significant role in the reduction of ROS (hydrogen peroxide, superoxide anion, singlet oxygen, hydroxyl radical, nitric oxide, and peroxynitrite). The SOD enzyme, act in the superoxide decomposition, is the first line of defense and converts •OH to H2O2; then H2O2 (a new ROS formed) is dealt with CAT and APX obtaining water and dioxygen as byproducts (Huang et al. 2019; Cruz de Carvalho 2008; Smirnoff 2008). The isozyme GPX reduces both H2O2 and organic peroxides. Finally, GR regulates the levels of reduced glutathione because an excessive accumulation of oxidized glutathione is harmful to the plant (Huang et al. 2019; Ahmad et al. 2010).
The non-enzymatic compounds are indispensable to protect plants against environmental stresses as well as pathogens, insects, and herbivores (Akula and Ravishankar 2011). The non-enzymatic compounds with antioxidant activity are glutathione, α-tocopherol, ascorbic acid, and phenolic compounds, which eliminate hydroxyl radicals and singlet oxygen mainly (Choudhury et al. 2013; Huang et al. 2019). The compounds derived of secondary metabolism are considered non-essential. However, they participate in the survival of the plant, based on their attributed effects against ROS, which are produced during a systematic response to stress conditions. They are located in a majority of the plant cells and can act in different ways against stress, such as ascorbic acid, which it is used as a substrate for the reduction of H2O2 by the activity of the enzyme ascorbate oxidase; this antioxidant compound plays a role minimizing damage caused by the oxidative process and can react with superoxide, hydrogen peroxide, and singlet oxygen (Suárez-Medina and Coy-Barrera 2016; Choudhury et al. 2013).
The α-tocopherol is a lipophilic antioxidant and an efficient singlet oxygen radical scavenger. Due to its nature, it has the ability to interact with polyunsaturated acyl groups and it eliminates and quenches ROS. Glutathione is a tripeptide with functions as an antioxidant, scavenges H2O2, and also reacts with the radicals singlet oxygen, superoxides, and hydroxyls (Shao et al. 2007; Choudhury et al. 2013). Carotenoids are lipophilic compounds with a positive effect against the stress through the stabilization of the lipid phase of the thylakoid membrane, besides participating in the biosynthesis of ABA (Arbona et al. 2013). The phenolic compounds are a diverse group of secondary metabolites in plants that act as a mechanism of defense against stress biotic and abiotic. Under adverse conditions, those compounds are synthesized by the phenylpropanoid or shikimate pathway (Arbona et al. 2013; Smirnoff 2008).
10.4 Biosynthesis of Phenolic Compounds Under Stress Conditions
Phenolic compounds constitute a wide group of metabolites derivate of the secondary metabolism of the plants (Das and Bhattacharya 2017); they are synthesized from phenylpropanoids/shikimate pathway, starting with the amino acids l-phenylalanine or l-tyrosine as their primary precursors. The molecular structure of the phenolic compounds contains at least one aromatic ring joined with one or more hydroxyl groups (Lattanzio 2013; Del Rio et al. 2013; Peñarrieta et al. 2014). This group comprises simple to very complex molecules, which exist more than 10,000 compounds, and is classified in phenolic acids, stilbenes, flavonoids, and lignans (Cheynier 2012; Li et al. 2014).
The phenolic compound production is one of the mechanism defense developed by the plant immune system (Isah 2019). Under stress conditions, there is an increase of phenylalanine ammonia-lyase (PAL) activity and other enzymes implicated in the phenylpropanoid pathway (Arbona et al. 2013).
The mechanism cellular for the biosynthesis of phenolic compounds begins when the plant receptors receive an extracellular or intracellular signal in the plasma membrane or endomembrane, which triggers a signal transduction network; this allows de novo activation or biosynthesis of TFs that promote the expression of genes implicated in the synthesis of secondary metabolites (Isah 2019; Zhao et al. 2005).
The genes encoding PAL proteins differ depending on the plant species, and a single species can present from two to ten or more copies. Likewise, the isoenzymes of this family of genes are expressed in different tissues. That is to say, in the same species, we can find different isoenzymes expressed in different parts (stem, leaves, roots, and flowers) of the plant (Kong 2015). PAL is an important enzyme in the phenylpropanoid pathway; however, other enzymes are also relevant in this pathway, such as the isoforms of the ligase coumarate-4-hydroxylase and 4-coumaroyl CoA, which are also activated under stress conditions (Bartwal et al. 2013).
The biosynthesis of phenolic compounds in plants is increased under stress conditions, which starts from the formation of aromatic amino acids and of the shikimic acid in the pathway of the shikimic acid. Subsequently, the aromatic amino acid l-phenylalanine derived from the before-mentioned pathway is catalyzed by PAL (which is increased under stress conditions) and loses an ammonium molecule to form trans-cinnamic acid, which is hydroxylated by the trans-cinnamate-4-hydroxylase and forms p-coumaric acid (Heldt and Piechulla 2011; Singh et al. 2010).
On the one hand, this p-coumaric acid can be oxidized to caffeic acid, then methylated in the meta position producing ferulic acid. Then it is oxidized and methylated again, resulting in synaptic acid. Likewise, p-cumaric acid is also transformed by the enzyme 4-coumarate/CoA ligase into an activated CoA-ester, the coumaroyl-CoA. The later compound plus three molecules of malonyl CoA catalyzed by the enzyme chalcone synthase produces chalcone from which all flavonoids are generated and also condensed tannins (flavonoid polymers). While, the hydrolyzable tannins (from gallic acid linked to hexoses molecules) are formed from products of the shikimate pathway (Fig. 10.2) (Sakihama et al. 2002; Barros and Dixon 2020; Martin 2018; Heldt and Piechulla 2011; Grace 2008; Deng and Lu 2017).
Diverse studies have been shown the increase in the content of phenolic compounds in different plants when they are in stress conditions. In this sense, Nag and Kumaria (2018) found in the plant Vande coerulea Griff. Ex Lindl. from the family Orchidaceae that the enzyme VcPAL (Vande coerulea Phenylalanine Ammonia Lyase) maintained a proportional relationship with the expression of the PAL gene, in function to the stress condition to which the seedling was subjected. Treatments were salinity (5–200 μM NaCl), UV-B (1500 μJ/m2), cold (4 °C), dark, and wounding (cutting leaves in pieces of 1 cm), all the treatments were collected after 0, 24, 48, 72, 96, and 120 h. Likewise, the content of phenolic compounds and flavonoids was evaluated concerning the different stresses. Results showed that there is an increase in the content in these phytochemicals of up to 3.54-fold more in flavonoids and 2.76-fold more in phenolic compounds, compared to the groups control. In the end, a relationship with the enzymatic activity as observed, indicating that the exposure to abiotic stress allows an increase in phytochemicals by stimulating the activity of the PAL enzyme.
For their part, Koyama et al. (2012) studied the effect of exposure to visible light and UV light of grapes (Vitis vinifera) cv. Cabernet Sauvignon, where they found that proanthocyanidin biosynthesis is induced under visible light and flavonols by UV light effect. In this sense, Agati et al. (2011) exposed Ligustrum vulgare plant outdoors under 100% sunlight irradiance without UV waveband, also to UV light and salinity (125 mM NaCl in the root zone); they found a dramatic increase in flavonoids (quercetin 3-O-glycoside, luteolin 7-O-glycoside, and cyanidin 3-O-glycoside) in leaves, concluding that flavonoids have an antioxidant function in photoprotection. Similarly, Takshak and Agrawal (2015) exposed the Coleus forskhlii plant to stress by UV-B radiation and found that the content of the different phenolic compounds in the leaves and roots increased. In this sense, the anthocyanin content increased in the roots, more than in the leaves under stress. However, flavonoids, phenols, lignin, and tannins were higher in the leaves than in the roots. Likewise, the enzymatic activity related to the biosynthesis of those metabolites presented an increase.
Fini et al. (2012) experimented with Flaxanis ornus plants which were subjected to solar radiation and different humidity conditions (100%, 40%, and 20% irrigated daily); they found that quercetin 3-O-glycoside content increased as the sun exposure time elapsed as well as at each humidity level, presenting a high content of this flavonoid in severe drought. The phytochemical esculetin showed the same behavior. The compounds acted as filters by accumulating in the vacuoles of the mesophyll cells, Likewise, the authors mention that these compounds exert a reducing power on H2O2 produced during stress due to exposure to excessive leaf light.
Leaves of Mikania glomerata Spreng. and Mikania leavigata Sch. Bip. Ex Baker were treated with full light, 25% shade, and 50% shade, also were exposed at 10, 17, and 22 °C of temperatures and water availability (excess and deficiency). The results showed that M. leavigata and M. glomerata have different responses in the biosynthesis of metabolites as phenolic compounds depending of the type of stress. However, in both species under sunlight and low temperatures, there was an increase of chlorogenic and dicaffeoylquinic acid.
On the other hand, in water availability, M. glomerata presented lower chlorogenic and dicaffeolyquinic acid content under drought, likewise M. leavigata had more coumarins content, with more shade (50% shade) (de Lazzari Almeida et al. 2017). In this sense, Caser et al. (2019) reported the drought stress (irrigation at 100% as control, 50% as moderate and 0% as severe) during 34 days in Salvia dolomitica Codd. leaves, and they evaluated phenolic compounds and flavonoids; it was found a decrease in those metabolites, contrary to what it was reported in other plants, which corroborates that the response in the synthesis of phenolic compounds is based on the species. The response may be through the activation of another biosynthetic pathway since, in this study, genes that enabled terpenoid biosynthesis were activated. For their part, Cheng et al. (2018) found in Scutellaria baicalensis Georgi roots that the mild drought stress increases the baicalin (flavone) and decrease in severe drought and was associated with the enzymes (PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate-CoA ligase; CHS, chalcone synthase) involved in the biosynthesis of flavonoids.
Two species Larrea divaricata and Lycium chilense (leaves and roots) under drought stress due to seasonal changes (autumn, winter, spring, and summer) showed drought effects in the biosynthesis of phenolic compounds. The specie L. divaricate presented a higher accumulation of flavonols in leaves in autumn and spring seasons than that of L. chilense. Also, a higher concentration of proanthocyanidins in leaves and roots of both species was observed, which could be related to the structural role of these compounds. The phenolic acids were higher in L. divaricata leaves than in L. chilense leaves in autumn. The most evident effect was in the autumn because, at that time, the water is limited by the scarce rain and the low humidity of the soil (Varela et al. 2016).
Salinity is an important stress condition, especially in crops. In a studio developed by Elsheery et al. (2020), cucumber plants grafted in four rootstocks of pumpkin (Cucurbita moschata L.), bottle gourd (Lagenaria siceraria L.), Nubian watermelon (Citrullus lanatus L. var. colocynthoide), and winter squash (Cucurbita maxima L., commercial cultivar Flexil). The phenolic compounds showed an increase in all rootstocks when different doses of NaCl (0 control, 50, and 100 mM) were applied. Likewise, the authors associated the increase in the polyphenol oxidase activity with the phenolic content and suggested that those are positive factors against salinity stress.
Similarly, Arman et al. (2019) germinated hulled barley to 60 mM NaCl to induce saline stress; they found an increase in the content of free and bound phenolic compounds (including gallic acid, p-hydroxybenzoic acid, protocatechuic acid, sinapic acid, vanillic acid, p-coumaric acid, ferulic acid, and syringic acid) during the 6 days of evaluation. The ferulic acid increased 1.21 times more than the control treatment. Mentha pulegium, an important medicinal plant, was exposed to salt treatments (25, 50, 75, and 100 mM NaCl) for 2 weeks. The results showed a diminished growth at 75 mM NaCl in all organs; however, the accumulation of salt as higher in leaves compared with roots, which allowed the leaf to present a greater synthesis of phenolic compounds in response to saline stress (Oueslati et al. 2010).
The extreme temperature is another abiotic stress factor, by which plants can synthesize different phenolic compounds, as reported by Schmidt et al. (2010). They studied the effect of temperature and radiation conditions on kale of different genotypes. Results showed variations in flavonoid content depending on the genotype, also as temperatures decrease and radiation intensifies, quercetin and isorhamnetin increase its content, while kaempferol decreases. In this sense, two varieties (pink and violet) of Lantana camara were found an increase of lignin under extreme heat more than cold stress. Likewise, the content of phenolic compounds and tannins is increased in exposition moderate stress conditions in both the varieties (Nischal and Sharma 2020).
Another study carried out by Oh et al. (2009) on lettuce subjected to conditions of heat (40 °C for 10 min), cold (4 °C for 1 day), and high-intensity light (800 μmol/m2/s for 1 day) showed similar total phenolic content both in the cold as in heat at day 3 of exposure, while exposure to light from day 1 showed an increase. Within the analyzed phenolic compounds profile, they obtained an increase in chicoric acid (ester of caffeic acid) and chlorogenic acid, mainly due to the effect of light and cold. The same behavior was found in luteolin-7-O-glucoside and quercetin-3-O-glucoside; these increases were four to six times higher than the control plants.
During the biotic stress, the phenolic compounds also are synthesized. They can act as feeding deterrents, where plants respond with an increase in lignin, a compound that can be a non-degradable barrier for most microorganisms (Moura et al. 2010). In this matter, phytoalexins are not detected before the attack of pathogens in plants; this phytochemical is synthesized from signaling molecules called elicitors such as fungal carbohydrates, fragments of the cell wall of the plant, and microbial enzymes that can also be activated by abiotic stress (Lattanzio et al. 2006). Phytoalexins act limiting the sporulation, spore germination, and hyphal growth in fungi, and has bacteriostatic properties (Kubalt 2016). Likewise, tannins contribute resistance to infestation, which can act in different ways: provide an astringent flavor affecting palatability, reduce feed consumption and digestibility, and act as enzyme inactivator (Lattanzio et al. 2006).
Some investigations have shown the synthesis of phenolic compounds in the presence of an infestation, such as that reported by Ballester et al. (2013). They found the presence of ten phenylpropanoid genes in flavedo of Citrus sinensis inoculated with Penicillium digitatum. This response is reflected in the presence of chlorogenic acid, acid caffeic, eriocitrin, narirutun, hesperidin, didymin, isohoifolin, diosmin, isosinensetin, hexamethyl-O-gossypetin, sinensetin, hexamethyl-O-quercetagetin, nobelin, tetramethyl-O-scutellarein, heptamethoxyflavone, and tangeretin, mostly in flavedo than albedo. Likewise, Chinese cabbage (Brassica rapa L.), under Erwinia carotovora subsp. carotovora infection, produces a mechanism of defense mognolignins (coniferyl and sinapyl) (Zhang et al. 2007).
Trichoderma spp. are fungi used as a biocontrol to protect plants, which suppress pathogens by different mechanisms but also induce the synthesis of phenolic compounds. In this sense, Pascale et al. (2017) evaluated two Trichoderma strains on Vitis vinifera, to induce polyphenols and resistance to Uncinula necator; they found that the fungi suppressed the disease caused by U. necator and increased the total phenolics. Due to these, fungi established a relation with host plants and induced several changes like the regulation of PAL and the expression of proteins to regulate pathogenesis.
De Ascensao and Dubery (2003) reported a similar effect in Musa acuminate roots exposed to cell wall elicitor from Fusarium oxisporum, showing an increase in the synthesis of phenolic compounds (conjugated and not-conjugated). The total phenolics presented an increase of 4.5-folds in 36 h compared with an initial time of exposition. In contrast, bound-phenolics, ester-bound phenolics, glycoside-bound phenolics, and free phenolics increased 6.3-, 4.2-, 3.0-, and 2.3-fold, respectively. The increase of bound phenolics was related to deposition of lignin, since it increased its content by elicitor effect, compared with the control treatment.
Likewise, Rani and Jyothsna (2010) found that rice plant increases the phenolic acids content as vanillic acid, syringic acid, cinnamic acid, and p-coumaric under infestation with Scirpophaga incertulas (Lepidoptera: Pyralidae), Cnaphalocrosis medinalis (Guenée), and Nilaparvata lugens (Homoptera: Delphacidae). On the other hand, the enzymatic activity of β-1,3-glucanase, SOD, and PAL decreased while peroxidase, CAT, and chitinase have been enhanced. The biochemical changes were in response to the pest attack and depended on the type of insect feeding.
10.5 Conclusions
The ability of plants to produce their metabolites as a defense mechanism or as the first line of defense against different types of stress, both biotic and abiotic, gives them the characteristic of being considered as biofactories of a wide variety of compounds derived from primary and secondary metabolism. Within these metabolites, phenolic compounds represent a large part of the secondary metabolites produced by the plant itself, which have a wide spectrum of activity against different types of stress. The presence of metabolites represents a great benefit in plants and an opportunity for the food, cosmetics, and mainly pharmaceutical industry, by producing a natural source of beneficial compounds in human health.
References
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7(1). https://doi.org/10.3390/agronomy7010018
Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M (2011) The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol 168(3):204–212. https://doi.org/10.1016/j.jplph.2010.07.016
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175. https://doi.org/10.3109/07388550903524243
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731. https://doi.org/10.4161/psb.6.11.17613
Ali M, Javaid A, Naqvi SH, Batcho A, Kayani WK, Lal A, Sajid IA, Nwogwugwu JO (2020) Biotic stress triggered small RNA and RNAi defense response in plants. Mol Biol Rep. https://doi.org/10.1007/s11033-020-05583-4
Arbona V, Manzi M, Ollas CD, Gómez-Cadenas A (2013) Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci 14(3):4885–4911. https://doi.org/10.3390/ijms14034885
Arman MSI, Al Mahmud A, Mahmud HR, Reza AA (2019) Free radical, oxidative stress and diabetes mellitus: a mini review. J Discov Phytomed 6(3):99–101
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166(1):3–16. https://doi.org/10.1016/j.plantsci.2003.10.024
Ballester A-R, Teresa Lafuente M, González-Candelas L (2013) Citrus phenylpropanoids and defence against pathogens. Part II: gene expression and metabolite accumulation in the response of fruits to Penicillium digitatum infection. Food Chem 136(1):285–291. https://doi.org/10.1016/j.foodchem.2012.08.006
Barros J, Dixon RA (2020) Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci 25(1):66–79. https://doi.org/10.1016/j.tplants.2019.09.011
Bartwal A, Mall R, Lohani P, Guru SK, Arora S (2013) Role of secondary metabolites and Brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32(1):216–232. https://doi.org/10.1007/s00344-012-9272-x
Broad RC, Bonneau JP, Hellens RP, Johnson AAT (2020) Manipulation of ascorbate biosynthetic, recycling, and regulatory pathways for improved abiotic stress tolerance in plants. Int J Mol Sci 21(5). https://doi.org/10.3390/ijms21051790
Camejo D, Guzmán-Cedeño Á, Moreno A (2016) Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant Physiol Biochem 103:10–23. https://doi.org/10.1016/j.plaphy.2016.02.035
Caser M, Chitarra W, D’Angiolillo F, Perrone I, Demasi S, Lovisolo C, Pistelli L, Pistelli L, Scariot V (2019) Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind Crop Prod 129:85–96. https://doi.org/10.1016/j.indcrop.2018.11.068
Cass CL, Peraldi A, Dowd PF, Mottiar Y, Santoro N, Karlen SD, Bukhman YV, Foster CE, Thrower N, Bruno LC, Moskvin OV, Johnson ET, Willhoit ME, Phutane M, Ralph J, Mansfield SD, Nicholson P, Sedbrook JC (2015) Effects of phenylalanine ammonia lyase (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J Exp Bot 66(14):4317–4335. https://doi.org/10.1093/jxb/erv269
Cheng L, Han M, Yang L-m, Li Y, Sun Z, Zhang T (2018) Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind Crop Prod 122:473–482. https://doi.org/10.1016/j.indcrop.2018.06.030
Cheynier V (2012) Phenolic compounds: from plants to foods. Phytochem Rev 11(2–3):153–177. https://doi.org/10.1007/s11101-012-9242-8
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8(4):e23681. https://doi.org/10.4161/psb.23681
Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci 92(10):4114. https://doi.org/10.1073/pnas.92.10.4114
Croteau R, Kutchan TM, Lewis NG (2015) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry & molecular biology of plants. American Society of Plants, Rockville, MD, pp 1250–1318
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species. Plant Signal Behav 3(3):156–165. https://doi.org/10.4161/psb.3.3.5536
Das S, Bhattacharya SS (2017) Environmental stress and stress biology in plants. In: Siddiqui MW, Bansal V (eds) Plant secondary metabolites, vol 3. Apple Academic Press, Oakville, ON, pp 21–58. https://doi.org/10.1201/9781315366302-7
de Ascensao ARFDC, Dubery IA (2003) Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 63(6):679–686. https://doi.org/10.1016/S0031-9422(03)00286-3
de Lazzari Almeida C, Xavier RM, Borghi AA, dos Santos VF, Sawaya ACHF (2017) Effect of seasonality and growth conditions on the content of coumarin, chlorogenic acid and dicaffeoylquinic acids in Mikania laevigata Schultz and Mikania glomerata Sprengel (Asteraceae) by UHPLC–MS/MS. Int J Mass Spectrom 418:162–172. https://doi.org/10.1016/j.ijms.2016.09.016
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A (2013) Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18(14):1818–1892. https://doi.org/10.1089/ars.2012.4581
Deng Y, Lu S (2017) Biosynthesis and regulation of phenylpropanoids in plants. Crit Rev Plant Sci 36(4):257–290. https://doi.org/10.1080/07352689.2017.1402852
Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat Rev Genet 11(8):539–548. https://doi.org/10.1038/nrg2812
Dos Reis SP, Lima AM, De Souza CRB (2012) Recent molecular advances on downstream plant responses to abiotic stress. Int J Mol Sci 13(7):8628–8647. https://doi.org/10.3390/ijms13078628
Elsheery NI, Helaly MN, Omar SA, John SVS, Zabochnicka-Swiątek M, Kalaji HM, Rastogi A (2020) Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. S Afr J Bot 130:90–102. https://doi.org/10.1016/j.sajb.2019.12.014
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Agron Sust Dev 29:153–188. https://doi.org/10.1051/agro:2008021
Fini A, Guidi L, Ferrini F, Brunetti C, Di Ferdinando M, Biricolti S, Pollastri S, Calamai L, Tattini M (2012) Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: an excess light stress affair? J Plant Physiol 169(10):929–939. https://doi.org/10.1016/j.jplph.2012.02.014
Fraire-Velázquez S, Rodríguez-Guerra R, Sánchez-Calderón L (2011) Abiotic and biotic stress response crosstalk in plants. In: Shanker AK, Venkateswarlu B (eds) Abiotic stress response in plants—physiological, biochemical genetic perspectives. InTech, Rijeka, pp 3–26
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9(4):436–442. https://doi.org/10.1016/j.pbi.2006.05.014
Gómez MR, Rodríguez A (2012) Mecanismos de defensa y respuestas de las plantas en la interacción micorrícica: una revisión. Rev Colomb Biotecnol 14(1):271–284. https://doi.org/10.15446/rev.colomb.biote
Grace S (2008) Phenolic as antioxidants. In: Smirnoff N (ed) Antioxidants and Reactive Oxygen Species in Plant. Wiley-Blackwell, India, p 320. https://doi.org/10.1002/9780470988565.ch6
Hammond-Kosack KE, Jones JDG (2015) Responses to plant pathogens. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants, 2nd edn. Wiley Blackwell, New York
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8(4):157–178. https://doi.org/10.1016/j.ppees.2007.01.001
Heldt H-W, Piechulla B (2011) Phenylpropanoids comprise a multitude of plant secondary metabolites and cell wall components. In: Heldt H-W, Piechulla B (eds) Plant biochemistry, 4th edn. Academic Press, San Diego, pp 431–449. https://doi.org/10.1016/B978-0-12-384986-1.00018-1
Heredia JB, Cisneros-Zevallos L (2009) The effects of exogenous ethylene and methyl jasmonate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce. Food Chem 115(4):1500–1508. https://doi.org/10.1016/j.foodchem.2009.01.078
Hounsome N, Hounsome B, Tomos D, Edwards-Jones G (2008) Plant metabolites and nutritional quality of vegetables. J Food Sci 73(4):R48–R65. https://doi.org/10.1111/j.1750-3841.2008.00716.x
Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800. https://doi.org/10.3389/fpls.2019.00800
Impa S, Nadaradjan S, Jagadish S (2012) Drought stress induced reactive oxygen species and antioxidants in plants. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants. Springer, New York, pp 131–147. https://doi.org/10.1007/978-1-4614-0634-1
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52. https://doi.org/10.1186/s40659-019-0246-3
Jacobo-Velazquez DA, Martinez-Hernandez GB, Rodriguez SD, Cao CM, Cisneros-Zevallos L (2011) Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J Agric Food Chem 59(12):6583–6593. https://doi.org/10.1021/jf2006529
Jacobo-Velazquez DA, Gonzalez-Aguero M, Cisneros-Zevallos L (2015) Cross-talk between signaling pathways: the link between plant secondary metabolite production and wounding stress response. Sci Rep 5:10. https://doi.org/10.1038/srep08608
Karuppanapandian T, Moon J-C, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725
Kirakosyan A, Seymour E, Kaufman PB, Warber S, Bolling S, Chang SC (2003) Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J Agric Food Chem 51(14):3973–3976. https://doi.org/10.1021/jf030096r
Koeck M, Hardham AR, Dodds PN (2011) The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol 13(12):1849–1857. https://doi.org/10.1111/j.1462-5822.2011.01665.x
Kong J-Q (2015) Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv 5(77):62587–62603. https://doi.org/10.1039/c5ra08196c
Koyama K, Ikeda H, Poudel PR, Goto-Yamamoto N (2012) Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 78:54–64. https://doi.org/10.1016/j.phytochem.2012.02.026
Koyro H-W, Huchzermeyer B (2018) Coordinated regulation of photosynthesis in plants increases yield and resistance to different types of environmental stress. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN (eds) Plant metabolites and regulation under environmental stress. Elsevier, London, pp 281–309. https://doi.org/10.1016/B978-0-12-812689-9.00014-5
Król A, Amarowicz R, Weidner S (2015) The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J Plant Physiol 189:97–104. https://doi.org/10.1016/j.jplph.2015.10.002
Kubalt K (2016) The role of phenolic compounds in plant resistance. Biotechnol Food Sci 80(2):97–108
Lamers J, van der Meer T, Testerink C (2020) How plants sense and respond to stressful environments. Plant Physiol 182(4):1624–1635. https://doi.org/10.1104/pp.19.01464
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17. https://doi.org/10.1016/j.envexpbot.2015.05.012
Lattanzio V (2013) Phenolic compounds: introduction. In: Ramawat KG, Mérillon J-M (eds) Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Springer, Berlin, pp 1543–1580. https://doi.org/10.1007/978-3-642-22144-6_57
Lattanzio V, Lattanzio VMT, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F (ed) Phytochemistry: advances in research, vol 2. Research Signpost, Trivandrum, pp 23–67
León-Chan RG, López-Meyer M, Osuna-Enciso T, Sañudo-Barajas JA, Heredia JB, León-Félix J (2017) Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ Exp Bot 139:143–151. https://doi.org/10.1016/j.envexpbot.2017.05.006
León-Chan RG, Lightbourn-Rojas LA, López-Meyer M, Amarillas L, Heredia JB, Martínez-Bastidas TF, Villicaña C, León-Félix J (2020) Differential gene expression of anthocyanin biosynthetic genes under low temperature and ultraviolet-B radiation in bell pepper (Capsicum annuum). Int J Agric Biol 23(3):501–508. https://doi.org/10.17957/IJAB/15.1315
Li A-N, Li S, Zhang Y-J, Xu X-R, Chen Y-M, Li H-B (2014) Resources and biological activities of natural polyphenols. Nutrients 6(12):6020–6047. https://doi.org/10.3390/nu612020
Madrigal-Santillán E, Madrigal-Bujaidar E, Cruz-Jaime S, Valadez-Vega M, Sumaya-Martínez MT, Pérez-Ávila KG, Morales-González JA (2013) The chemoprevention of chronic degenerative disease through dietary antioxidants: progress, promise and evidences. In: Morales-González JA (ed) Oxidative stress chronic degenerative diseases—a role for antioxidants. InTech, Rijeka, pp 155–185. https://doi.org/10.5772/52162
Mahajan M, Kuiry R, Pal PK (2020) Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J Appl Res Med Aromatic Plants 18:100255. https://doi.org/10.1016/j.jarmap.2020.100255
Mantri N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: present and future. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants. Springer, New York, pp. 1–19. https://doi.org/10.1007/978-1-4614-0634-1
Martin DA (2018) Los compuestos fenólicos: un acercamiento a su biosíntesis, síntesis y actividad biológica The phenolic compounds: an approach to your biosynthesis, synthesis and biological activity. Rev Invest Agraria Ambient 9(1):81–104. https://doi.org/10.22490/21456453.1968
Meraj TA, Fu J, Raza MA, Zhu C, Shen Q, Xu D, Wang Q (2020) Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 11(4). https://doi.org/10.3390/genes11040346
Morales LO, Tegelberg R, Brosché M, Keinänen M, Lindfors A, Aphalo PJ (2010) Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol 30(7):923–934. https://doi.org/10.1093/treephys/tpq051
Moreno LP (2009) Respuesta de las plantas al estrés por déficit hídrico. Una revisión. Agronomía Colombiana 27(2):179–191
Moura JCMS, Bonine CAV, De Oliveira Fernandes Viana J, Dornelas MC, Mazzafera P (2010) Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol 52(4):360–376. https://doi.org/10.1111/j.1744-7909.2010.00892.x
Nag S, Kumaria S (2018) In silico characterization and transcriptional modulation of phenylalanine ammonia lyase (PAL) by abiotic stresses in the medicinal orchid Vanda coerulea Griff. ex Lindl. Phytochemistry 156:176–183. https://doi.org/10.1016/j.phytochem.2018.09.012
Naikoo MI, Dar MI, Raghib F, Jaleel H, Ahmad B, Raina A, Khan FA, Naushin F (2019) Role and regulation of plants phenolics in abiotic stress tolerance: an overview. In: Khan MIR, Reddy PS, Ferrante A, Khan NA (eds) Plant signaling molecules. Woodhead Publishing, Duxford, pp. 157–168. https://doi.org/10.1016/B978-0-12-816451-8.00009-5
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77(3):367–379. https://doi.org/10.1111/tpj.12388
Nischal P, Sharma AD (2020) Stomatal and pollen dependant metabolic changes as a metric of stress tolerance and invasive potential of invasive plant—Lantana camara (L.) growing under abiotic stress like conditions. S Afr J Bot 131:406–420. https://doi.org/10.1016/j.sajb.2020.03.028
Oh M-M, Carey EE, Rajashekar CB (2009) Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol Biochem 47(7):578–583. https://doi.org/10.1016/j.plaphy.2009.02.008
Olsen KM, Slimestad R, Lea US, Brede C, Lovdal T, Ruoff P, Verheul M, Lillo C (2009) Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ Exp Bot 32(3):286–299. https://doi.org/10.1111/j.1365-3040.2008.01920.x
Oueslati S, Karray-Bouraoui N, Attia H, Rabhi M, Ksouri R, Lachaal M (2010) Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol Plant 32(2):289–296. https://doi.org/10.1007/s11738-009-0406-0
Pascale A, Vinale F, Manganiello G, Nigro M, Lanzuise S, Ruocco M, Marra R, Lombardi N, Woo SL, Lorito M (2017) Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot 92:176–181. https://doi.org/10.1016/j.cropro.2016.11.010
Peñarrieta JM, Tejeda L, Mollinedo P, Vila JL, Bravo J (2014) Phenolic compounds in food. Rev Boliv Quím 31(2):68–81
Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12(4):421–426. https://doi.org/10.1016/j.pbi.2009.06.008
Rani PU, Jyothsna YJAPP (2010) Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol Planta 32(4):695–701
Sakamoto M, Suzuki T (2015) Effect of root-zone temperature on growth and quality of hydroponically grown red leaf lettuce (Lactuca sativa L. cv. Red Wave). Am J Plant Sci 6(14):2350
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177(1):67–80. https://doi.org/10.1016/S0300-483X(02)00196-8
Schmidt S, Zietz M, Schreiner M, Rohn S, Kroh LW, Krumbein A (2010) Genotypic and climatic influences on the concentration and composition of flavonoids in kale (Brassica oleracea var. sabellica). Food Chem 119(4):1293–1299. https://doi.org/10.1016/j.foodchem.2009.09.004
Shao H-B, Chu L-Y, Lu Z-H, Kang C-M (2007) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4(1):8–14. https://doi.org/10.7150/ijbs.4.8
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng BS (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24(13):22. https://doi.org/10.3390/molecules24132452
Shinozaki K, Uemura M, Bailey-Serres J, Bray EA, Weretilnyk E (2015) Responses to abiotic stress. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants, 2nd edn. Wiley Blackwell, New York
Singh R, Rastogi S, Dwivedi UN (2010) Phenylpropanoid metabolism in ripening fruits. Compr Rev Food Sci Food Saf 9(4):398–416. https://doi.org/10.1111/j.1541-4337.2010.00116.x
Smirnoff N (2008) Antioxidants and reactive oxygen species in plants. Biological science series. Wiley-Blackwell, New Delhi. https://doi.org/10.1002/9780470988565
Solovchenko AE, Merzlyak MN (2008) Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ J Plant Physiol 55(6):719. https://doi.org/10.1134/S1021443708060010
Son K-H, Ide M, Goto E (2020) Growth characteristics and phytochemicals of canola (Brassica napus) grown under UV radiation and low root zone temperature in a controlled environment. Hortic Environ Biotechnol 61(2):267–277. https://doi.org/10.1007/s13580-019-00219-4
Suárez-Medina K, Coy-Barrera E (2016) Diversidad de los compuestos orgánicos bioactivos de origen natural: Una singularidad manifestada por la plasticidad en el metabolismo secundario. Rev Facult Ciencias Básicas 12(2):252–269. https://doi.org/10.18359/rfcb.2031
Takshak S, Agrawal SB (2015) Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: augmentation of secondary metabolites and antioxidants. Plant Physiol Biochem 97:124–138. https://doi.org/10.1016/j.plaphy.2015.09.018
Toscano S, Trivellini A, Cocetta G, Bulgari R, Francini A, Romano D, Ferrante A (2019) Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front Plant Sci 10:1212. https://doi.org/10.3389/fpls.2019.01212
ul Haq S, Khan A, Ali M, Khattak AM, Gai W-X, Zhang H-X, Wei A-M, Gong Z-H (2019) Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int J Mol Sci 20(21). https://doi.org/10.3390/ijms20215321
Varela MC, Arslan I, Reginato MA, Cenzano AM, Luna MV (2016) Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol Biochem 104:81–91. https://doi.org/10.1016/j.plaphy.2016.03.014
Verdaguer D, Jansen MAK, Llorens L, Morales LO, Neugart S (2017) UV-A radiation effects on higher plants: exploring the known unknown. Plant Sci 255:72–81. https://doi.org/10.1016/j.plantsci.2016.11.014
Verma S, Nizam S, Verma PK (2013) Biotic and abiotic stress signaling in plants. In: Sarwat M, Ahmad A, Abdin M (eds) Stress signaling in plants: genomics and proteomics perspective, vol 1. Springer, New York, pp 25–49. https://doi.org/10.1007/978-1-4614-6372-6
Villarreal-Garcia D, Nair V, Cisneros-Zevallos L, Jacobo-Velazquez DA (2016) Plants as biofactories: postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front Plant Sci 7:11. https://doi.org/10.3389/fpls.2016.00045
Vinod KK (2012) Stress in plantation crops: adaptation and management. In: Venkateswarlu B, Shanker AK, Shanker C, Maheswari M (eds) Crop stress and its management: perspectives and strategies. Springer, Dordrecht, pp 45–137. https://doi.org/10.1007/978-94-007-2220-0
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7(10):1306–1320. https://doi.org/10.4161/psb.21663
Woodrow P, Pontecorvo G, Ciarmiello LF, Annunziata MG, Fuggi A, Carillo P (2012) Transcription factors and genes in abiotic stress. In: Venkateswarlu B, Shanker AK, Shanker C, Maheswari M (eds) Crop stress and its management: perspectives and strategies. Springer, Dordrecht, pp 317–357. https://doi.org/10.1007/978-94-007-2220-0
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5(6):649–654. https://doi.org/10.4161/psb.5.6.11398
Yang Y, Qi M, Mei C (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40(6):909–919. https://doi.org/10.1111/j.1365-313X.2004.02267.x
Zhang S-H, Yang Q, Ma R-C (2007) Erwinia carotovora ssp. carotovora infection induced “defense lignin” accumulation and lignin biosynthetic gene expression in chinese cabbage (Brassica rapa L. ssp. pekinensis). J Interact Plant Biol 49(7):993–1002. https://doi.org/10.1111/j.1672-9072.2007.00478.x
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23(4):283–333. https://doi.org/10.1016/j.biotechadv.2005.01.003
Zhou Z, Schenke D, Miao Y, Cai D (2017) Investigation of the crosstalk between the flg22 and the UV-B-induced flavonol pathway in Arabidopsis thaliana seedlings. Plant Cell Environ 40(3):453–458. https://doi.org/10.1111/pce.12869
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Contreras-Angulo, L.A., Emus-Medina, A., Gutierrez-Grijalva, E.P., Heredia, J.B. (2023). Stressed Plants: An Improved Source for Bioactive Phenolics. In: Lone, R., Khan, S., Mohammed Al-Sadi, A. (eds) Plant Phenolics in Abiotic Stress Management. Springer, Singapore. https://doi.org/10.1007/978-981-19-6426-8_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-6426-8_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6425-1
Online ISBN: 978-981-19-6426-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)