Abstract
We determined the effects of UV radiation and low root zone temperature on growth characteristics and phytochemicals of Brassica napus (canola) cultivated in a controlled environment (25/20 °C 16/8 [light/dark]; 70% relative humidity; 1000 µmol mol−1 CO2 with photosynthetic photon flux density of 100 and 200 µmol m−2 s−1 for 7 and 18 days, respectively). The 18-day-old B. napus plants were treated for 5 days with two nutrient solution–temperature regimes (normal temperature [NT] and low-temperature [10 °C; LT]) and three levels of UV radiation (0, 0.3, and 0.6 W m−2). Treatment with 0.6 W m−2 UV decreased quantum efficiency of photosystem II the most. Most growth characteristics decreased under LT + UV treatments. Treatments with 0.6 W m−2 UV, compared to those without UV, significantly inhibited shoot height. LT + 0.3 and +0.6 UV decreased shoot height the most. Temperature (T) × UV interaction did not affect most growth characteristics except leaf area, specific leaf weight, and shoot water content. Antioxidant capacity (total ORAC) resembled lipophilic ORAC and showed the highest value in the LT + 0.6 UV treatment. T × UV interaction did not affect ORAC values, although LT + 0.3 UV and LT + 0.6 UV treatments produced the highest values. Both LT and UV radiation enhanced the total phenolic content, and in the combined treatments, it was two times that of the control. UV intensities (0.3 and 0.6 W m−2) remained constant regardless of variation in root zone temperature. UV radiation enhanced total flavonoid content equally across different intensities; LT + 0.6 W m−2 UV treatment produced a relatively high value. Thus, LT and UV negatively affected plant growth parameters, except total dry weight, whereas LT, UV, or LT + UV positively affected antioxidant properties, total phenolics, and flavonoids, suggesting the potential of using an LT treatment in a hydroponic system as well as UV radiation to enhance growth performance of greenhouse and factory plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the bioactive phytochemicals in Brassicaceae vegetables are a good source of antioxidants, Brassica species are becoming popular as staple foods worldwide (Jahangir et al. 2009a), including in China, Japan, India, and European countries (Cartea et al. 2011). Among the Brassicaceae plants, Brassica napus L. (rapeseed or canola) has been generally grown as a primary source of oil, accounting for approximately 15% of global oil production (Fang et al. 2012). Meanwhile, B. napus leaves (vegetative canola tissues) also produce phytochemicals as antioxidant phenolics, including glucosinolates, phenylpropanoids, flavonoids, and phytoalexins (Font et al. 2005). In B. napus, these phytochemicals belong to a group of natural products and may be stimulated by and play important roles against biotic and abiotic stresses (Li et al. 2010).
During growth, plants may be exposed to various biotic and abiotic stresses. Abiotic factors such as water, light, temperature, and UV radiation are known to strongly affect primary and secondary metabolites in Brassica species (Jahangir et al. 2009a). Secondary metabolites, such as flavonoids, phenolics, terpenes, nitrogen-containing alkaloids, and sulfur-containing compounds, can be classified by their biosynthetic pathways (Crozier et al. 2006). Recently, environmental factors affecting edible Brassicaceae species high in secondary metabolites have been examined, and the antioxidant activity of such species has been investigated as a source of beneficial compounds in human diets (Cartea et al. 2011). Phenolics are among the most important groups of beneficial phytochemicals (Dixon and Paiva 1995), whereas flavonoids are the most widespread and diverse group of polyphenols in Brassica species (Cartea et al. 2011).
Among the various environmental factors exerting stress on the plants, low-temperature (LT) stress may induce the accumulation of secondary metabolites by reactive oxygen species (ROS) as a defense mechanism (Suzuki and Mittler 2006). Meanwhile, the effect of LT on the root zone of plants cultivated hydroponically has rarely been reported. Studies on the effects of root zone temperature (RZT) using a hydroponic system have been reported in various species, including Spinacia oleracea (spinach; Chadirin et al. 2011), Lactuca sativa (lettuce; He et al. 2009; Ilahi et al. 2017), Solanum lycopersicum (tomato; Adebooye et al. 2010), Cucumis sativus (cucumber; Yan et al. 2013), Brassica oleracea (broccoli; Díaz-Pérez 2009), and Capsicum annuum (bell pepper; Aidoo et al. 2017). However, there has been scarce research on the effects of RZT in B. napus. Previous studies that investigated the effects of RZT on B. napus have examined plant biomass, though the accumulation of phytochemicals has received little attention (Ye et al. 2003, 2006). For vegetables grown hydroponically in a controlled environment, environmental stress factors such as osmotic pressure, dissolved oxygen, and temperature can be easily regulated by manipulating the conditions of the nutrient solution in the root zone. Nonetheless, the accumulation of secondary metabolites affected by RZT remains largely unclear. Thus, the effect of RZT on the accumulation of phytochemicals should be further explored.
As an environmental stressor, UV radiation may damage plants and affect plant metabolites and nutritional qualities such as carbohydrates, organic and amino acids, vitamins, hormones, flavonoids, phenolics, and glucosinolates (Hounsome et al. 2008). In the UV range (280–400 nm), UV-B (280–315 nm) may impair plant growth and development. Enhanced levels of UV-B radiation inhibit plant physiological processes and growth (Day and Neale 2002). Meanwhile, UV-B can induce changes in the accumulation of antioxidants and secondary metabolites such as phenolic compounds, flavonoids, and hydroxycinnamic acids by increasing the levels of ROS (Jansen et al. 2008). Enhanced UV-B also affects the flavonoids and epicuticular wax as protective compounds in plants (Treutter 2005). Thus, the effects of UV radiation on plant secondary metabolites have recently been reviewed. Supplemental or full artificial lighting is used in plant production systems such as in greenhouses and plant factories. UV radiation is especially important for the production of optimal-quality crops (Goto 2003).
Applying environmental stresses during cultivation is one method for manipulating the growth and bioactive compounds in plants. This is important for achieving more environmentally friendly agronomic practices and optimization of culture conditions. Previous studies have considered the effects of one factor (e.g., UV or LT) in plants; however, few studies have considered multiple factors such as UV-B and CO2 (Qaderi and Reid 2005; Qaderi et al. 2007), temperature, and UV-B and water stress (Qaderi and Reid 2009; Qaderi et al. 2010). Meanwhile, significant interactions between the temperature and UV-B radiation have been observed, and the synergic effect on flavonoid concentration was more induced by LT and UV-B radiation (Martel and Qaderi 2016). León-Chan et al. (2017) also observed that LT with UV-B induce higher accumulation of phenolic compounds than each stress alone and detected specific flavonoids by LT and UV-B stress in bell pepper plants. On the basis of previous findings in other species, we hypothesized that both LT and UV radiation, during the cultivation of B. napus, affect the growth characteristics and accumulation of phytochemicals and that exposure to LT makes B. napus more vulnerable to the UV radiation effects on phytochemical accumulation than does exposure to normal RZT. However, there is limited information on the relationship between LT and UV radiation in B. napus. Therefore, multifactorial experiments are required to determine the plant growth responses as well as accumulation of bioactive compounds following environmental stress. The objective of this study was to investigate the interactive effects of LT and UV radiation on the growth characteristics and accumulation of phytochemicals in B. napus. Moreover, the results of the LT and UV radiation used in this study will provide information for their practical application in the greenhouse and plant factories.
2 Materials and methods
2.1 Plant materials and cultivation
One-day-old germinated B. napus seeds (Brassica napus L., ‘Kizakinonatane’; Noguchiseed, Saitama, Japan) were moved into a urethane sponge (M urethane, 2.3 × 2.3 × 2.7 cm, L × W × H; M Hydroponic Research Co., Aichi, Japan) and maintained in a plant factory under controlled environmental conditions (air temperature, average of 25/20 °C [16L/8D]; relative humidity [RH], 70%; CO2 concentration, 1000 µmol mol−1; photosynthetic photon flux density [PPFD] generated by fluorescent lamps [FL, FHF32EXNH; Panasonic Co., Osaka, Japan], 100 ± 10 µmol m−2 s−1; photoperiod, 16 h) for 7 d. B. napus seedlings were then transplanted into a cultivation panel (64-well normal type; M Hydroponic Research Co., Aichi, Japan) and cultivated using the deep flow technique (DFT) in a plastic container (32 plants per container; 55.5 × 26 × 14 cm, L × W × H) under the same conditions used before transplanting without the light condition (PPFD, 200 ± 10 μmol m−2 s−1) (Table 1). A quarter strength of commercial nutrient solution (Otsuka Chemical Co., Osaka, Japan) was maintained a 6.4 pH, electrical conductivity (EC) value of 1.8 dS m−1, and replaced every 3 d after transplanting.
2.2 LT and UV treatments
Eighteen days after sowing (DAS), B. napus plants were moved and grown on another shelf equipped with six treatments. LT and UV treatments consisted of two nutrient solution temperature regimes (normal temperature [NT] and low-temperature [10 °C; LT]) and three levels of UV radiation (0, 0.3, and 0.6 W m−2), respectively: (1) NT with 0 W m−2 UV (control); (2) NT with 0.3 W m−2; (3) NT with 0.6 W m−2; (4) LT with 0 W m−2 UV; (5) LT with 0.3 W m−2; and (6) LT with 0.6 W m−2. Inside of the shelves, environmental conditions were the same as those during the initial vegetative growth (see above). The low temperatures of the nutrient solution were maintained using a compact handy cooler (TRL-107NHF; Thomas Kagaku Co., Tokyo, Japan). The control was not kept in the cooler. During the treatment, the temperature of each nutrient solution was recorded by a data logger (GL820; Graphtec Co., Kanagawa, Japan) at 10-min intervals (Fig. 1). A specifically developed fluorescent lamp containing the UV spectrum (UV-FL) (FHF 32-EX-N–H; Panasonic Co., Osaka, Japan) was used for the UV treatments in this study. The spectral distribution and intensity were measured using a spectroradiometer (USR-45; USHIO INC., Yokohama, Japan) and UV radiometer (R203; Macam Photometrics Ltd., Livingston, UK), respectively (Fig. 2 and Table 2). At 18 DAS, the B. napus plants were subjected to each treatment for 5 d.
Relative spectral distribution of lighting systems used in this study. Each light system consisted of white light-emitting diodes (W LEDs) and/or UV fluorescent lamp (UV-FL). W LEDs (a) (Control; 0 W m−2 UV), W LEDs with 0.3 W m−2 UV-FL (b), and W LEDs with 0.6 W m−2 UV-FL (c) were used in this study. The UV-FL lighting source emits UV and visible wavelengths (d). Total photosynthetic photon flux density (PPFD) was 200 ± 10 µmol m−2 s−1 in each treatment. Spectral scans were measured using a spectroradiometer
2.3 Growth characteristics
The heights of shoots and roots, fresh and dry weights of shoots and roots, leaf number, total leaf area, and specific leaf weight (SLW) were measured after the treatments. The heights and fresh weights of shoots and roots were measured with a ruler and an electronic scale (ASP2102; AS ONE Co., Osaka, Japan), respectively. The dry weights of shoots and roots were obtained by drying the sample for 74 h at 80 °C in an oven (MOV-202F; Sanyo Electric Co., Osaka, Japan). The total leaf area was obtained from scanned leaf images using ImageJ software (Version 1.51 k; available online). Using leaf thickness, the SLW was calculated by dividing the milligrams of dry weight of shoots by the total leaf area. The water content was determined according to the equation:
where FW represents fresh weight and DW represents dry weight of shoots or roots.
2.4 Chlorophyll fluorescence
Chlorophyll (Chl) fluorescence was measured at the third leaf on the adaxial leaf surface with a portable Chl fluorescence meter (Mini-PAM; Walz, Effeltrich, Germany). Fluorescence readings were taken at 3, 4, and 5 d after treatment. The ratio of variable to maximal fluorescence (Fv/Fm) was measured in dark-adapted leaves that had been enclosed in cuvettes for 20 min. The saturating light pulse was delivered at 400 µmol m−2 s−1 for 0.8 s.
2.5 Total phenolic content
The total phenolic content of leaves was extracted from the third leaf position within each treatment. Approximately 0.1 g (fresh tissue) of each third leaf from the bottom was extracted in 1.5 mL of 80% acetone and ground using an automatic grinder (Mixer Mill MM 200; Retch, Haan, Germany) at 30 Hz for 6 min. The extract was then incubated in darkness at 4 °C overnight. Subsequently, the extract was centrifuged at 20,500×g for 2 min, and the supernatant was used to measure the total phenolic concentration. The total phenolic content was determined using the modified Folin-Ciocalteu reagent method (Ainsworth and Gillespie 2007). A mixture of 135 mL distilled water, 750 mL 1/10 dilution Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO), and 600 mL 7.5% (w/v) Na2CO3 was added to 50 mL of the supernatant and vortexed for 10 s. The mixture was incubated at 45 °C in a water bath for 15 min and allowed to cool to room temperature (25 °C). The absorbance of the samples was read at 765 nm with a blank (50 mL of 80% [v/v] acetone) using a UV–VIS spectrophotometer (V-550, JASCO Co., Tokyo, Japan). The total phenolic content was expressed as milligrams gallic acid equivalent (GAE) per gram of dry weight of B. napus leaves.
2.6 Antioxidant capacity (ORAC)
Antioxidant capacity was determined using the ORAC assay following a modified method of Zhao et al. (2007). Recently, plant-derived compounds with antioxidant capacity and their involvement in human health have received much interest. Among the methods for examining antioxidant capacity in plants, the ORAC assay provides the advantage of direct measurement in both hydrophilic and lipophilic compartments (Prior 2015). Approximately 0.1 g (fresh tissue) of each third leaf from the bottom was extracted in 1 mL of 75 mmol L−1 phosphate buffer (pH 7.4) using a Mixer Mill MM 200 at 30 Hz for 6 min. The homogenate was then centrifuged at 20,500×g and 4 °C for 15 min. The supernatant was transferred to a microtube for use as the hydrophilic extract. The pellet was resuspended in 0.22 mL of acetone, vortexed for 5 s, and sonicated for 10 min. This acetone supernatant was used as the lipophilic extract. Briefly, 150 µL of 8.16 × 10−5 mmol L−1 fluorescein solution and 25 µL of phosphate buffer blank (75 mmol L−1, pH 7.4) or 25 µL of Trolox standard (500, 250, 125, 62.5, 31.25, and 15.625 µmol L−1) or 25 µL of diluted hydrophilic and lipophilic extraction were transferred to a 96-well plate. The well plate and 153 mmol L−1 2, 2ʹ-azobis (2-amidinopropane) dihydrochloride (AAPH) were immediately incubated at 37 °C for 30 min. Next, 25 µL of AAPH was added to each well. Fluorescence was measured using a microplate reader (SH-9000Lab; Corona Electric, Ibaraki, Japan) every 120 s at an emission and excitation wavelength of 520 and 485 nm, respectively. The ORAC values were expressed as mmol Trolox equivalents per dry mass (mM Trolox equivalents g−1 DW).
2.7 Total flavonoid content
The total flavonoid concentration was determined according to the method of Dewanto et al. (2002). Approximately 0.2 g (fresh tissue) of each third leaf from the bottom of the third leaf was extracted with 3 mL of 70% (v/v) ethanol (pH 3.2, using formic acid) and incubated overnight in the dark at 4 °C. Subsequently, the extract was centrifuged at 20,500×g for 2 min, and the supernatant was used for total flavonoids. A mixture of 0.75 mL distilled water and 45 µL of 5% NaNO2 was added to 150 µL of the extract. After 5 min, 90 µL of 10% AlCl3 was added in the mixture and reacted for 6 min. Then, 300 µL of 1 M NaOH with 165 µL of distilled water was added to the mixture and reacted for 5 min. The absorbance of the mixture was measured at 510 nm after being vortexed for 10 s. The total flavonoid content was expressed as milligrams of (+)-catechin hydrate equivalents per gram of dry weight of B. napus leaves.
2.8 Statistical analyses
The experiment was repeated three times to verify the reproducibility of each experimental condition and time in a plant factory. All parameters were measured using four plants per treatment except for measurement of Chl fluorescent (three plants per treatment). Statistical analyses were conducted with Statistical Products and Service Solutions for Windows, ver. 24.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed by two-way analysis of variance (ANOVA) to determine the interaction effect between UV radiation and temperature, and the differences between the means were tested using Duncan’s multiple comparison test.
3 Results and discussion
3.1 Chlorophyll fluorescence
After dark acclimation, the maximum quantum efficiency of photosystem II (Fv/Fm) decreased in the treatments subjected to 0.6 W m−2 UV (NT or LT with +0.6UV) (Fig. 3). At 3 d after treatment, only the 0.6 W m−2 UV treatment had gradually decreased in Fv/Fm until 5 d after treatment. Although Fv/Fm in the combined treatment LT +0.6UV significantly decreased at 4 d after treatment, the Fv/Fm in the combined treatment LT +0.6UV slightly increased and had a value (0.784) similar to that of the control at 5 d after treatment.
The Fv/Fm value is a good indicator of photoinhibitory damage caused by light or other environmental stresses in plants (Maxwell and Johnson 2000). The reduction in Fv/Fm under UV radiation (0.6 W m−2) at both NT and LT indicates that the PSII reaction center might have been damaged by UV radiation, which might have resulted in a lower quantum efficiency or photosynthetic capacity (Olsson et al. 2000). In accordance with our result that Fv/Fm was not affected by RZT, low and high RZT did not affect Fv/Fm in cucumber (Haghighi et al. 2015). Qaderi et al. (2007) also observed that B. napus leaves exposed to UV-B radiation at two levels of CO2 concentration decreased Fv/Fm but had no effect on the two-way interaction between UV-B and CO2. This result is consistent with that of Moghadam et al. (2012), who observed that UV radiation had a stronger effect than water stress and CO2 on Fv/Fm. As the energy of UV increased (UV-B doses; 8.5, 34, 68, 102 kJ m−2 day−1), the Fv/Fm was decreased in Ocimum basilicum L. (sweet basil). In fact, UV radiation using high doses inhibit photosynthetic efficiency and increase in dissipation energy (Mosadegh et al. 2018). We observed that the Fv/Fm value did not decrease at 0.3 W m−2 UV radiation, suggesting that 0.3 W m−2 UV radiation did not affect PSII.
3.2 Growth characteristics
Overall, LT and UV radiation significantly influenced the growth characteristics (Tables 3, 4) of B. napus. Shoot height decreased as UV radiation increased. Meanwhile, treatments with 0.3 W m−2 UV radiation (NT +0.3UV and LT +0.3UV), regardless of nutrient solution temperature, did not significantly inhibit shoot height, relative to the treatments without UV radiation (NT −UV and LT −UV). However, treatments with 0.6 W m−2 UV radiation (NT +0.6UV and LT +0.6UV) did significantly inhibit shoot height compared with the treatments without UV radiation (NT −UV and LT −UV). LT, compared with NT, also inhibited shoot height, regardless of UV radiation. Combined treatments (LT +0.3UV and LT +0.6UV) yielded the lowest values in shoot height among all treatments. Root length was not significantly affected by nutrient solution temperature or UV radiation. The fresh weights of shoots and roots exhibited a similar trend with shoot height, which decreased as UV radiation or LT increased. The root fresh weight was more significantly affected by temperature than UV radiation, while there was no significant effect of UV radiation on root fresh weight. The dry weight of B. napus plants showed little difference between treatments except for the combined treatment with 0.6 W m−2 UV radiation (LT +0.6UV), which showed the greatest inhibition of dry matter (Table 3). Leaf number shared a similar trend with the dry weight of B. napus plants. Treatment with UV radiation or temperature as well as combined treatments significantly inhibited leaf area. Notably, leaf area was inhibited more by UV radiation than the nutrient solution temperature, which had a greater effect on shoot fresh weight. Leaf thickness (SLW) was increased by UV radiation and LT. The water content of the shoots and roots was significantly decreased by LT. Except for leaf area, SLW, and shoot water content, there was no interactive effect of T × UV on the majority of B. napus growth characteristics (Table 4).
Reduced height in plants exposed to UV radiation implied that the specific photomorphogenic response of plants could be related to a UV-B photoreceptor by UV-B radiation (Lercari et al. 1990). Reduced plant height was also observed in B. napus (Qaderi and Reid 2005) and two other Brassica species (B. rapa and B. nigra) exposed to UV-B radiation (Conner and Zangori 1997). Meanwhile, root length showed no significant difference between treatments. RZT with LT enhanced root length in lettuce (He et al. 2009) but inhibited root length in cucumber seedlings (Yan et al. 2013) and a bell pepper cultivar (Aidoo et al. 2017). These results on the root morphology of RZT may affect the rate of water and nutrient intake (Domisch et al. 2001), functional activities of the root (Yan et al. 2013), and sensitivity of cells expanding in the root growth zone (Engels 1994). The results of these studies suggest that root physiological and morphological responses to UV or RZT may depend on the plant species. Most of the results demonstrate that various growth parameters were more inhibited by LT than by UV radiation. In general, plants sensitive to the effects of LT on the root system may respond quickly by restricting growth, but very little is known in this respect. The water content of shoots and roots decreased by LT might have partially restricted water uptake, which would inhibit photosynthesis and carbohydrate production (Table 4). The RZT affects the assimilate partitioning response in both shoot and root growth (Ye et al. 2006). Meanwhile, the dry mass of B. napus plants was little affected by LT (Table 3). Ye et al. (2003) reported that tropical species are more sensitive to LT than temperate species such as B. napus (oilseed rape). Low RZT affects root cell membranes, increases water viscosity, and slows movement of water through roots (Kaufmann 1975), thus inhibiting plant growth through the reduced nutrient uptake and water content of plants, which was coincident with the current results. UV-B radiation can damage plants in many ways, impacting plant DNA (Britt et al. 1993), the photosynthetic apparatus (Greenberg et al. 1996), and plant growth (Day and Neale 2002). Qaderi et al. (2010) observed reductions in B. napus growth, such as in leaf number and area as well as plant dry mass by UV-B radiation. Gerhardt et al. (2008) also observed that UV treatment inhibited the morphology and growth of B. napus plants. The leaf area exposed to UV radiation in this study (Table 4) may be related to the UV-B-induced inhibition of cell expansion (Liu et al. 1995). The increased leaf thickness (SLW) due to UV radiation (Table 4) might act as a defense mechanism to prevent penetration into deeper tissues (Cen and Bornman 1993). Meanwhile, in some cases, UV-B radiation did not impair plant growth owing to increased tolerance or adaptation by the plants (Tevini 2000). Such findings are consistent with the present results that UV radiation did not affect the fresh and dry weight of roots, total dry weight, leaf number per plant, and water content (Tables 3, 4). This suggests that the UV radiation regimes used in this study were moderate in terms of plant dry mass production.
3.3 Antioxidant capacity (ORAC)
Hydrophilic ORAC was not significantly affected by UV radiation in both NT or LT, while LT significantly enhanced the hydrophilic ORAC up to two-fold (Table 5). Lipophilic ORAC was significantly increased by 0.6 W m−2 UV radiation and LT treatments compared with that in the control. The total ORAC was similar to that of lipophilic ORAC and showed the highest value in the LT +0.6UV combined treatment. The interaction between T × UV did not affect the ORAC values, although the combined treatments (LT +0.3 and 0.6UV) yielded the highest values.
Yan et al. (2013) observed the induced higher antioxidant enzyme activities caused by low RZT in cucumber seedlings. Moreover, LT stress in spinach roots induced antioxidation in shoots, which is applicable to the production of value-added leafy vegetables (Chadirin et al. 2011, 2012). These results on antioxidants and LT stress in the root system might influence secondary metabolism in plants. The ORAC values in this study may estimate two broad classes of antioxidants: hydrophilic compounds such as vitamin C and most polyphenolic antioxidants (phenolic acids, anthocyanins, and other flavonoids) and lipophilic antioxidants such as vitamin E and most carotenoids (Catoni et al. 2008). Interestingly, lipophilic ORAC had a significant effect on UV radiation (p < 0.05). In Brassica vegetables containing high levels of vitamins (e.g. carotenes and tocopherols), lipophilic phytochemicals such as tocopherols, sterols, and sterol esters also have important health-promoting properties (Jahangir et al. 2009b). The carotenoids exposed to UV radiation in B. napus plants presented no significant changes in previous studies (Olsson et al. 1999; Zhu and Yang 2015). Consistent with the results of the present study (data not shown), this finding implies that lipophilic phytochemicals other than carotenoids may play a more important role through UV radiation. Moreover, all ORAC values in the combined treatment with LT +0.6UV were the highest among all treatments, suggesting that the combination of LT and UV radiation may have a stronger effect on antioxidant capacity in B. napus than LT or UV radiation alone. Interactions of UV and other environmental conditions (light intensity, drought, or temperature) may stimulate the stress or affect the balance between pro-oxidant production and scavenging capacity (Apel and Hirt 2004). Thus, environmental control of combination treatment must also be considered.
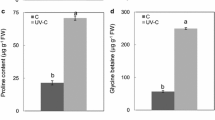
3.4 Total phenolic content
The total phenolic content was significantly affected by LT and UV radiation (Fig. 4). Both LT and UV radiation enhanced the total phenolic content, while, regardless of RZT, the different UV intensities (0.3 and 0.6 W m−2) had similar effects. Meanwhile, the total phenolic content of the combined treatments was the highest and increased up to two-fold compared with that in the control.
“Phenolic compound” is a generic term that refers to a large number of compounds (more than 8000). They are widely dispersed throughout the plant kingdom and are produced in plants as secondary metabolites via the shikimic acid pathway (Cartea et al. 2011). Enhanced phenolic contents under LT and UV radiation in this study may stimulate the key enzyme of the phenylpropanoid metabolism, speeding up the biosynthesis of phenolic compounds (Luengo Escobar et al. 2017). In total phenolic content, similar patterns with ORAC values in combined treatments suggested that the combined effects of LT and UV radiation may have a stronger effect on phenolic acid in B. napus than LT or UV radiation alone. León-Chan et al. (2017) also observed that the combination of LT and UV-B induces higher accumulation of individual phenolic compounds than each stress alone. Lee et al. (2019) observed that a combined chilling and UV-A treatment (10 °C plus UV-A LED radiation at 30.3 W m−2) induced the total phenolic contents and phenylalanine ammonia-lyase activity in kale, compared to single (chilling or UV-A) treatments. They revealed that the carbon source could readily be distributed to secondary metabolite pathways under the combined chilling and UV-A treatment; therefore, two stressors might have synergetic effects on plants when applied simultaneously. Thus, our results suggested that plants exposed to LT stress are more susceptible to the direct damage of UV radiation because the plants induced more biosynthesis of UV-related compounds with a higher antioxidant capacity by LT to reduce the ROS produced in cells by the stress.
3.5 Total flavonoid content
Total flavonoid content was significantly affected by LT or UV (Fig. 5). UV radiation enhanced the total flavonoid content, while different UV intensities did not have significantly different effects (0.3 and 0.6 W m−2). The combined treatment with LT and 0.6 W m−2 UV yielded a relatively high value. In contrast with total phenolic content and ORAC, sole LT (LT −UV) did not significantly affect the total flavonoid content compared with that in the control. The interactive effect on the total flavonoid content was observed only in the treatment with 0.6 W m−2 UV radiation (NT +0.6UV vs. LT +0.6UV).
The higher total flavonoid content in the leaf suggests a higher nutritional value of leaves, as flavonoids possess strong antioxidant activity and inhibit oxidative stress (Pourcel et al. 2007). Our results are consistent with those of Olsson et al. (1998), who reported that B. napus subjected to UV-B showed a 70–150% increase in the overall flavonoid content (quercetin and kaempferol glucosides). Although many studies have reported an increase in flavonoids due to UV radiation in B. napus, the effect of flavonoids on the RZT is limited. In contrast with the results regarding ORAC and total phenolic content, LT did not affect flavonoids. This suggested that the flavonoids are more sensitive to UV radiation than they are to LT. León-Chan et al. (2017) also found that UV-B induces higher flavonoid synthesis than LT conditions. No significant differences in flavonoid levels were detected under chilling stress in kale (10 °C), but combined chilling and UV-A treatments led to significant increases in the levels of these compounds (Lee et al. 2019). Flavonoids are predominantly found in the epidermal cell, so they mainly absorb UV radiation to quench the ROS produce by UV stress (Castañeda-Ovando et al. 2009). In general, flavonoids, biosynthesized via the phenylpropanoid pathway, have a more important role in photoprotection against UV and free-radical scavengers than in other environmental conditions (Zoratti et al. 2014). As demonstrated in B. napus, the leaf epidermal layer produces flavonoids to protect the underlying tissues against UV-B radiation (Cen and Bornman 1993; Olsson et al. 1998). Meanwhile, further studies would be required to determine the synergetic effects of combined treatments with LT and UV radiation on flavonoids in B. napus.
4 Conclusion
In conclusion, this study revealed that RZT and UV radiation affect the growth and phytochemistry of B. napus. LT and UV radiation had negative effects on plant growth except for total dry weight, whereas LT, UV radiation, or combined treatment with LT and UV had positive effects on the antioxidant properties, total phenolics, and flavonoids of the plants. Even though LT did not influence the accumulation of flavonoids, the application of LT or UV radiation might have positively affected the antioxidant phenolic compounds without inhibiting dry biomass. Moreover, the combined treatment with LT and UV provided novel information to improve the quality of B. napus; however, a detailed study on the synergetic effects under two environmental factors such as LT and UV should be considered. The results indicate the potential application of LT and UV radiation in a hydroponic system to induce antioxidant properties, phenolics, and flavonoids in the greenhouse and plant factory.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adebooye OC, Schmitz-Eiberger M, Lankes C, Noga GJ (2010) Inhibitory effects of sub-optimal root zone temperature on leaf bioactive components, photosystem II (PS II) and minerals uptake in Trichosanthes cucumerina L. Cucurbitaceae. Acta Physiol Plant 32:67–73
Aidoo MK, Sherman T, Lazarovitch N, Fait A, Rachmilevitch S (2017) A bell pepper cultivar tolerant to chilling enhanced nitrogen allocation and stress-related metabolite accumulation in the roots in response to low root-zone temperature. Physiol Plant 161:196–210
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Britt AB, Chen JJ, Wykoff D, Mitchell D (1993) A UV sensitive mutant of Arabidopsis defective in the repair of pyrimidine-pyrimidinone (6–4) dimers. Science 261:1571–1574
Cartea ME, Francisco M, Soengas P, Velasco P (2011) Phenolic compounds in Brassica vegetables. Molecules 16:251–280
Castañeda-Ovando A, de Lourdes Pacheco-Hernández M, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113:859–871
Catoni C, Peters A, Schaefer HM (2008) Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim Behav 76:1107–1119
Cen YP, Bornman JF (1993) The effect of exposure to enhanced UV-B radiation on the penetration of monochromatic and polychromatic UV-B radiation in leaves of Brassica napus. Physiol Plant 87:249–255
Chadirin Y, Hidaka K, Takahashi T, Sago Y, Wajima T, Kitano M (2011) Application of temperature stress to roots of spinach I. Effect of the low temperature stress on quality. Environ Control Biol 49:133–139
Chadirin Y, Sago Y, Hidaka K, Wajima T, Kitano M (2012) Application of temperature stress to root zone of spinach III. Effective method for short term application of low and high temperature stresses to roots. Environ Control Biol 50:199–207
Conner JK, Zangori LA (1997) A garden study of the effects of ultraviolet-B radiation on pollination success and lifetime female fitness in Brassica. Oecologia 111:388–395
Crozier A, Jaganath IB, Clifford MN (2006) Phenols, polyphenols and tannins: an overview. In: Plant secondary metabolites: occurrence, structure and role in the human diet 1. Blackwell, Oxford, pp 1–24
Day TA, Neale PJ (2002) Effects of UV-B radiation on terrestrial and aquatic primary producers. Annu Rev Ecol Syst 33:371–396
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Díaz-Pérez JC (2009) Root zone temperature, plant growth and yield of broccoli [Brassica oleracea (Plenck) var. italica] as affected by plastic film mulches. Sci Hortic 123:156–163
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Domisch T, Finer L, Lehto T (2001) Effects of soil temperature on biomass and carbohydrate allocation in Scots pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Physiol 21:465–472
Engels C (1994) Effect of root and shoot meristem temperature on shoot to root dry matter partitioning and the internal concentrations of nitrogen and carbohydrates in maize and wheat. Ann Bot 73:211–219
Fang J, Reichelt M, Hidalgo W, Agnolet S, Schneider B (2012) Tissue-specific distribution of secondary metabolites in rapeseed (Brassica napus L.). PLoS ONE 7:e48006
Font R, Rio-Celestino M, Cartea E, Haro-Bailon A (2005) Quantification of glucosinolates in leaves of leaf rape (Brassica napus ssp. pabularia) by near-infrared spectroscopy. Phytochemistry 66:175–185
Gerhardt KE, Lampi MA, Greenberg BM (2008) The Effects of far-red light on plant growth and flavonoid accumulation in Brassica napus in the presence of ultraviolet B radiation. Photochem Photobiol 84:1445–1454
Goto E (2003) Effects of light quality on growth of crop plants under artificial lighting. Environ Control Biol 41:121–132
Greenberg BM, Wilson MI, Gerhardt KE, Wilson KE (1996) Morphological and physiological responses of Brassica napus to Ultraviolet-B radiation: photomodification of ribulose-1,5-bisphosphate carboxylase/oxygenase and potential acclimation processes. J Plant Physiol 148:78–85
Haghighi M, Mozafariyan M, Abdolahipour B (2015) Effect of cucumber mycorrhiza inoculation under low and high root temperature grown on hydroponic conditions. J Crop Sci Biotechnol 18:89–96
He J, Tan LP, Lee SK (2009) Root-zone temperature effects on photosynthesis, 14C-photoassimilate partitioning and growth of temperate lettuce (Lactuca sativa cv. ‘Panama’) in the tropics. Photosynthetica 47:95–103
Hounsome N, Hounsome B, Tomos D, Edwards-Jones G (2008) Plant metabolites and nutritional quality of vegetables. J Food Sci 73:R48–R65
Ilahi WFF, Ahmad D, Husain MC (2017) Effects of root zone cooling on butterhead lettuce grown in tropical conditions in a coir-perlite mixture. Hortic Environ Biotechnol 58:1–4
Jahangir M, Abdel-Farid IB, Kim HK, Choi YH, Verpoorte R (2009a) Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ Exp Bot 67:23–33
Jahangir M, Kim HK, Choi YH, Verpoorte R (2009b) Health-affecting compounds in Brassicaceae. Compr Rev Food Sci Food Saf 8:31–43
Jansen MA, Hectors K, O’Brien NM, Guisez Y, Potters G (2008) Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci 175:449–458
Kaufmann MR (1975) Leaf water stress in Engelmann spruce: influence of root and shoot environments. Plant Physiol 56:841–844
Lee JH, Kwon MC, Jung ES, Lee CH, Oh MM (2019) Physiological and metabolomic responses of kale to combined chilling and UV-A Treatment. Int J Mol Sci 20:4950
León-Chan RG, López-Meyer M, Osuna-Enciso T, Sañudo-Barajas JA, Heredia JB, León-Félix J (2017) Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ Exp Bot 139:143–151
Lercari B, Sodi F, Lipucci di Paola M (1990) Photomorphogenic responses to UV radiation: involvement of phytochrome and UV photoreceptors in the control of hypocotyl elongation in Lycopersicon esculentum. Physiol Plant 79:668–672
Li X, Gao MJ, Pan HY, Cui DJ, Gruber MY (2010) Purple canola: Arabidopsis PAP1 increases antioxidants and phenolics in brassica napus leaves. J Agric Food Chem 58:1639–1645
Liu L, Gitz DC, McClure JW (1995) Effects of UV-B on flavonoids, ferulic acid, growth and photosynthesis in barley primary leaves. Physiol Plant 93:725–733
Luengo Escobar A, Magnum de Oliveira Silva F, Acevedo P, Nunes-Nesi A, Alberdi M, Reyes-Díaz M (2017) Different levels of UV-B resistance in Vaccinium corymbosum cultivars reveal distinct backgrounds of phenylpropanoid metabolites. Plant Physiol Biochem 118:541–550
Martel AB, Qaderi MM (2016) Does salicylic acid mitigate the adverse effects of temperature and ultraviolet-B radiation on pea (Pisum sativum) plants? Environ Exp Bot 122:39–48
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Moghadam HRT, Ghooshchi F, Zahedi H (2012) Effect of UV radiation and elevated CO2 on physiological attributes of canola (Brassica napus L.) grown under water stress. Rev Cient UDO Agricola 12:353–364
Mosadegh H, Trivellini A, Ferrante A, Lucchesini M, Vernieri P, Mensuali A (2018) Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci Hortic 229:107–116
Olsson LC, Veit M, Weissenböck G, Bornman JF (1998) Differential flavonoid response to enhanced UV-B radiation in Brassica napus. Phytochemistry 49:1021–1028
Olsson LC, Veit M, Bornman JF (1999) Epidermal transmittance and phenolic composition in leaves of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napus grown under enhanced UV-B radiation. Physiol Plant 107:259–266
Olsson LC, Fraysee L, Bornman JF (2000) Influence of high light and UVB radiation on photosynthesis and D1 turnover in atrazine-tolerant and sensitive cultivars of Brassica napus L. J Exp Bot 51:265–274
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36
Prior RL (2015) Oxygen radical absorbance capacity (ORAC): new horizons in relating dietary antioxidants/bioactives and health benefits. J Funct Foods 18:797–810
Qaderi MM, Reid DM (2005) Growth and physiological responses of canola (Brassica napus) to UV-B and CO2 under controlled environment conditions. Physiol Plant 125:247–259
Qaderi MM, Reid DM (2009) Methane emissions from six crop species exposed to three components of global climate change: temperature, ultraviolet-B radiation and water stress. Physiol Plant 137:139–147
Qaderi MM, Reid DM, Yeung EC (2007) Morphological and physiological responses of canola (Brassica napus) siliquas and seeds to UVB and CO2 under controlled environment conditions. Environ Exp Bot 60:428–437
Qaderi MM, Basraon NK, Chinnappa CC, Reid DM (2010) Combined effects of temperature, ultraviolet-B radiation, and watering regime on growth and physiological processes in canola (Brassica napus). Int J Plant Sci 171:466–481
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant 126:45–51
Tevini M (2000) Ultraviolet-B radiation affects antioxidant status and survival in the zebrafish, Brachdanio rerio. Photochem Photobiol 72:271
Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7:581–591
Yan QY, Duan ZQ, Mao JD, Xun LI, Fei DONG (2013) Low root zone temperature limits nutrient effects on cucumber seedling growth and induces adversity physiological response. J Integr Agric 12:1450–1460
Ye Z, Huang L, Bell RW, Dell B (2003) Low root zone temperature favours shoot B partitioning into young leaves of oilseed rape (Brassica napus). Physiol Plant 118:213–220
Ye Z, Bell RW, Dell B, Huang L, Xu Q (2006) Effect of root zone temperature on oilseed rape (Brassica napus) response to boron. Commun Soil Sci Plant Anal 37:2791–2803
Zhao X, Iwamoto T, Carey EE (2007) Antioxidant capacity of leafy vegetables as affected by high tunnel environment, fertilization and growth stage. J Sci Food Agric 87:2692–2699
Zhu PJ, Yang L (2015) Ambient UV-B radiation inhibits the growth and physiology of Brassica napus L. on the Qinghai-Tibetan plateau. Field Crops Res 171:79–85
Zoratti L, Karppinen K, Luengo Escobar A, Häggman H, Jaakola L (2014) Light-controlled flavonoid biosynthesis in fruits. Front Plant Sci 5:534
Acknowledgements
This work was supported by the Ministry of Economy, Trade and Industry of Japan [a Grant-in-Aid from the Development of Fundamental Technologies for the Production of High-Value Materials Using Transgenic Plant project].
Author information
Authors and Affiliations
Contributions
K-HS performed the experiments, collected the results and wrote the manuscript. MI participated in experiments and analysis the data. EG conceived made substantial contributions to the conception and design of the study. All authors have contributed significantly, and all authors are in agreement with the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Myung-Min Oh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Son, KH., Ide, M. & Goto, E. Growth characteristics and phytochemicals of canola (Brassica napus) grown under UV radiation and low root zone temperature in a controlled environment. Hortic. Environ. Biotechnol. 61, 267–277 (2020). https://doi.org/10.1007/s13580-019-00219-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00219-4