Abstract
Metalloids are elements with intermediate chemical properties between metals and nonmetals. The metalloids are biologically important elements, ranging from essential to extremely toxic elements with contrasting effects on organisms. Plants deal with a considerable imbalance of metalloids in the environment. Plants must acquire adequate amounts of essential metalloids for metabolism or contrarily exclude toxic metalloids to avoid cellular toxicity. The process of uptake and exclusion is guided by channel proteins, which transport metalloids across cellular membranes. Major intrinsic proteins (MIPs) are a family of selective channels that includes aquaporins (water channels) and aquaglyceroporins (glycerol and other solute channels). Aquaglyceroporin facilitates the transport of small solutes, including glycerol, small uncharged solutes, and gasses across biological membranes. Plant MIPs are grouped into five subfamilies based on sequence similarity and subcellular localization. Plant MIPs are mainly categorized into five subfamilies – plasma membrane intrinsic proteins (PIPs), nodulin-26-like intrinsic proteins (NIPs), tonoplast intrinsic proteins (TIPs), small basic intrinsic proteins (SIPs), and uncharacterized intrinsic proteins (XIPs). The uptake of environmental metalloids by aquaglyceroporins explains how beneficial elements such as silicon are taken up in plants. Conversely, toxic elements such as arsenic and antimony also enter the food chain via these channel proteins. The present review summarizes the role of various MIP homologs for transporting metalloids into and out of plant cells. This review discusses the detailed mechanism of MIPs for acquiring essential metalloids and their role in the influx and efflux in plant cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Abiotic stress conditions such as salinity, drought, temperature extremes, nutrient deficiency, metalloid toxicity, flooding, etc. cause serious damage to plants in terms of growth, development, and productivity (Singh et al. 2020; Zulfiqar et al. 2020). For instance, tainting the soil and water bodies with metals, their unfavorable effects on plants, and retransmission to the consuming populations creates significant worries worldwide. Heavy metals and metalloid stress contribute to adverse effects on the health of plants and consumers. Metalloids are semimetals that share some common properties of metals and nonmetals. Metalloid boron (B) acts as a micronutrient. At the same time, silicon (Si) is viewed as a useful component for providing mechanical strength and protection from abiotic and biotic stresses to certain crop plants.
On the other hand, arsenic (As) is an extreme ecological danger to most living forms, including plants, while the less plentiful metalloids germanium (Ge), antimony (Sb), and tellurium (Te) show variable levels of harm (Angulo-Bejarano et al. 2021; Sharma et al. 2021). Plants in small amounts require the metalloids B, Si, and Se, while some are toxic and nonessential, namely As, Ge, Sb, and Te. Cereals like wheat, rice, maize, and barley are major sources of biomagnification of metalloid(s) in consumers (Deng et al. 2019). B is conjectured to exist as an all-important micronutrient in soil; even so, its role as an essential trace element is being questioned (Pereira et al. 2021). The boric acid (BA) form of B, which possesses no charge at acidic or neutral pH, gets transported in cells by passive transport (Hrmova et al. 2020); however, that is a half story, and there exist regulated transport mechanisms that help retain B equilibrium in plants (Miwa and Fujiwara 2010; Pereira et al. 2021). The requirement of B varies among the plant species. Some plant species like cotton, beet, and Brassica require B during the entire plant development cycle. On the other hand, soybean, pea, wheat, and barley need relatively low B levels and that too during the development of seeds and initiation of flowering (Pommerrenig et al. 2015).
Arsenic is considered a potent group I carcinogen and the leading cause of health issues related to cardiovascular, neurological, hematological, renal, and respiratory systems. Various natural and anthropogenic activities release As into the environment as a potentially toxic, ubiquitous entity in soil and water bodies (Deng et al. 2019). The inorganic form of As in the form of arsenite As(III) and arsenate As(V) predominantly exists in soil and is taken up by the plants (Panda et al. 2010). Roots usually take up the excess concentrations of metalloids by various transporters, including phosphate transporters, sulfate transporters, aquaglyceroporins, nodulin-26-like proteins, Si influx transporter, hexose transporter, etc. (Deng et al. 2019). The detoxification of such metalloids via conjugation with glutathione (GSH) and phytochelatins (PCs) and vacuolar sequestration in roots could be a primary strategy for reducing the metalloid contents in cereal grains (Deng et al. 2019). Si in the form of silicates is the second most plentiful component after oxygen in the environment. The expanding proofs have shown that this metalloid is advantageous to plants, particularly under stress conditions such as salinity, drought, and biotic stresses (Yan et al. 2018). The plant accessibility of Si in soil (as monosilicic acid, H4SiO4) differs between 0.1 and 0.6 mM (Yan et al. 2018). Si is taken up and moved through the plant to be saved as SiO2 phytoliths (Epstein 2001; Ma and Yamaji 2008; Deshmukh et al. 2017a; Luyckx et al. 2017; Pavlovic et al. 2021). The main Si species accessible to living beings is uncharged orthosilicic acid. Like Si, the most bio-accessible Ge type is the uncharged germanic acid (Pommerrenig et al. 2015).
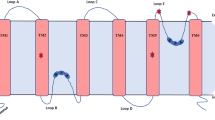
Plants have transporters that keep the required degree of metal and metalloid ion homeostasis inside the cells to perform distinctive functions. These transporters facilitate the entry, multi-organ, and subcellular distribution and exit of various metal(loids) ions. These transporters involve aquaporins, phosphate and sulfate transporters, hexose transporters, etc. (Pandey et al. 2019). Major intrinsic proteins (MIPs) are the membrane proteins that facilitate the transport of small polar molecules across membranes (Johanson et al. 2001). The plant MIP superfamily is highly conserved, with members ranging in size from 23 to 31 kDa (Gomes et al. 2009; Venkatesh et al. 2013; Maurel et al. 2015). The MIPs are divided into five main aquaporin subfamilies based on their sequence similarities and localization: plasma membrane intrinsic protein (PIPs), tonoplast membrane intrinsic proteins (TIPs), nodulin-26-like intrinsic membrane proteins (NIPs), uncharacterized intrinsic proteins (XIPs), and the small basic intrinsic proteins (SIPs) (Maurel et al. 2008; Sakurai et al. 2005; Kumar et al. 2018). Structurally, MIP tetramer is functional, and each monomer comprises six transmembrane alpha-helices (H1–H6) and five loops (LA–LE), of which loops B and E are hydrophobic. In contrast, loops A, C, and D are hydrophilic. The conserved motifs among all MIPs include two highly conserved NPA (asparagine–proline–alanine) motifs in loops B and E. The NPA motifs are known to regulate the substrate selectivity of the AQPs (Deshmukh et al. 2015). Moreover, the aromatic/arginine (ar/R) selectivity filter of MIPs is a tetrad in which each residue is located in H2 and H5 helices and loops LE1 and LE2. The ar/R filter forms a central pore and contributes to substrate selectivity (Afzal et al. 2016; Deshmukh et al. 2016, 2017b; Shivaraj et al. 2017).
The members of the TIP subfamily are mainly localized to the vacuole and facilitate the transport of water, glycerol, hydrogen peroxide, urea, and ammonia by different ar/R selectivity filters (Deshmukh et al. 2015). According to the current consensus, aquaporins have been major contributing entities in plant metalloid stress response and adaptations. The localization of MIPs and their role as potential metalloids (As, B, Si, Se, and Sb) are represented in Fig. 7.1. The possible mechanisms reported so far include changes in the activities of ROS-scavenging enzymes, the altered expression level of certain stress marker genes through the cross talk, hormone-mediated response, and so on (Sun et al. 2017). This chapter will discuss the role of different classes of MIPs in metalloid transport and stress responses.
7.2 PIP Members as Metalloid Transporters
PIP members are the largest subfamilies of plant MIPs and are localized to the plasma membrane. PIPs mainly regulate water homeostasis, and they are also known to be the transporters of urea, H2O2, CO2, and metalloids such as, B, Sb, and Si (Kumar et al. 2018). PIPs are further grouped in two subgroups, namely PIP1 and PIP2 (Chaumont et al. 2001). Plant roots absorb essential mineral nutrients such as B and Si from the soil and transport them to other parts of the plants via various transporters, including PIPs. PIPs also provide sensitivity/tolerance to metalloids, particularly As, via uptake/influx/efflux and compartmentalization of toxic metalloids in different plant tissues. PIPs form channel-like structures that act differently in plants under various environmental conditions during metalloid transport. PIPs are involved in B uptake and support the plants to survive under B limitations. In barley (Hordeum vulgare), PIP members such as HvPIP1;3 and HvPIP1;4 act as B transporters, confirmed in the heterologous system by complementation studies (Fitzpatrick and Reid 2009). The overexpression of ZmPIPs, HvPIP1;3 and HvPIP1;4, in yeast showed B influx and channel activities (Zangi and Filella 2012). Several studies in rice demonstrated that OsPIP1;3, OsPIP2;4, OsPIP2;6, and OPIP2;7 are involved in B transport and lead to influx and efflux activities in rice (Kumar et al. 2014; Mosa et al. 2016). The heterologous expression of OsPIPs genes, namely, OsPIP1;2, OsPIP1;3, OsPIP2;4, OsPIP2;7, and OsPIP2;8, in deficient strain (Δfps1Δacr3Δycf1) of yeast resulted in enhanced B transport and sensitivity (Mosa et al. 2016). Heterologous overexpression of OsPIP1;3 and OsPIP2;6 in Arabidopsis exhibited enhanced tolerance under higher B concentration (Mosa et al. 2016). Transcript accumulation of several PIP members in Arabidopsis roots was decreased under higher B concentrations (Aquea et al. 2012). The expression of PIP1;2, PIP2;1, and PIP2;2 was higher in shoots compared to roots of Arabidopsis at increased B concentration (Macho-Rivero et al. 2018). In non-AM (arbuscular mycorrhizal) plants like maize, the transcript of ZmPIP2;2 was upregulated at a higher concentration of B under drought stress, showing its role in enhancing water flow in plant roots and decreasing excessive B levels (Quiroga et al. 2020). In contrast, the AM plants under excessive B levels showed reduced ZmPIP2;2 expressions. The authors hypothesized that AM plants might have a different process to regulate excess B. Higher phosphorous (P) concentration in tissues and interplay of boron and phosphorous reduces B toxicity (Quiroga et al. 2020). These studies concluded strongly that B toxicity caused water flux reduction to the shoot and caused reduced hydraulic conductance of shoot tissue, resulting in downregulation of aquaporin genes in roots and shoots. However, further studies suggested that repression of these PIP genes in the shoot is due to the excessive B accumulation, or excess B leads to activation of signaling cascades (Macho-Rivero et al. 2018). Studies involving RNA sequencing transcriptional profiling of the Brassica napus BnaAQPs in contrasting B-resistant genotype (Qingyou10) and B-sensitive genotype (Westar10) under sufficient and deficient B conditions suggested that BnaPIPs are highly expressed in roots, old leaves, and juvenile leaves (Yuan et al. 2017). In both the cultivars, BnaPIP1;1s, BnaPIP1;2s, and BnaPIP2;2/2;3s showed elevated transcript abundance in roots under the B-sufficient condition, whereas the same PIPs transcripts were downregulated in minimal B stress (Yuan et al. 2017).
To subside the metalloid toxicity, the various plants have developed potential strategies, including downregulation of the specialized transporters, metalloid exclusion through efflux channel proteins, and metalloid complexation with thiols such as GSH and PCs, followed by vacuolar sequestration. Most of the NIP subfamilies of aquaporins are identified as As(III) transporters; however, fewer studies also revealed the role of the PIP subfamily in providing metalloids such as B tolerance in plants. To reduce the metalloid accumulation in plant cells and cope with the metalloid stress in rice, PIPs act as a bidirectional pump (Kumar et al. 2014; Mosa et al. 2016). Members of rice PIPs play a potential role in As(III) and B transport pathways (Mosa et al. 2012, 2016; Kumar et al. 2014). Several studies also demonstrated downregulation of transcripts of five rice PIPs (OsPIP1;2, OsPIP1;3, OsPIP2;4, OsPIP2;6, and OsPIP2;7) and 13 PIPs of Brassica juncea under arsenite stress (Mosa et al. 2012; Srivastava et al. 2013). In heterologously expressed rice, PIPs (OsPIP2;4, OsPIP2;6, and OsPIP2;7) in Xenopus oocytes showed enhanced As(III) transport. Studies of transgenic Arabidopsis with constitutive expression of OsPIP2;4, OsPIP2;6, and OsPIP2;7 exhibit enhanced tolerance under As(III) stress without affecting the levels of As in root and shoot tissues. To underpin the role of PIPs as bidirectional As(III) transporters, heterologous overexpression of OsPIPs in Arabidopsis roots displayed both arsenite influx and efflux processes (Mosa et al. 2012). The other members of rice PIPs need to be characterized at the plant level, and their prominent role needs to be identified. AtPIP2;2 is largely expressed with cell division-related protein (NtCyc07) and demonstrated to provide higher tolerance toward As(III) in Arabidopsis (Lee and Hwang 2012). Arabidopsis atpip2;2 exhibited decreased As(III) tolerance without difference in As(III) concentration, whereas the overexpressed AtPIP2;2 exhibited increased As(III) tolerance and decreased As(III) levels in yeast and Arabidopsis (Modareszadeh et al. 2021). The studies potentiate the role of AtPIP2;2 in bidirectional transporter of As(III). The enhanced As(III) efflux in Arabidopsis is due to the higher As(III) exporter activity compared to the importer activity indicating that AtPIP2;2 provides As(III) tolerance by reducing As(III) accumulation (Modareszadeh et al. 2021).
The various transporters present in the roots help regulate Si uptake and, therefore, act as a major element to cope with biotic and abiotic stresses in plants (Rios et al. 2017). The Si-treated roots exhibited enhanced expression of PIP genes. In salt-stressed cucumber (Cucumis sativus), Si enhances root water uptake via upregulation of aquaporin gene expression. The expression of PIPs (PIP1;2, PIP2;1, PIP2;4, PIP2;5) was upregulated on treatment with Si in cucumber (Zhu et al. 2015). Similarly, Si supplementation in leaves of bottle gourd (Lagenaria siceraria) decreased transcript accumulation of LsiPIP1-5 at 24 h, while at 72 h, LsiPIP1-5 expression was enhanced (Kumawat et al. 2021). In stem, Si treatment enhanced transcript accumulation of LsiPIP1-5, LsiPIP2-4, and LsiPIP4-1 at 24 h with a decrease at 72 h. The root Si application exhibits enhanced transcript accumulation of LsiPIP1-5 and LsiPIP2-4 (Kumawat et al. 2021). The transcript levels of Sorghum bicolor PIPs (SbPIP1;6, SbPIP2;2, and SbPIP2;6) show upregulation upon Si treatment in limited time salt stress (Liu et al. 2014). The improvement in root water uptake under dehydration stress may be due to the upregulation of PIP genes (Liu et al. 2014). These reports highlight that the manipulation of PIPs in many plant species exhibits an enhanced level of B and Si and controls and limits the toxic As concentration. However, further studies are needed to demonstrate the role of PIPs in metalloid transport and tolerance at the field level under natural environmental and soil conditions.
7.3 NIP Members as Metalloid Transporters
NIPs allow the diffusion of metalloids through membranes and facilitate their cellular transport in different parts of the plants (Wallace et al. 2006; Pommerrenig et al. 2015). Different forms of metalloids transported by NIPs include B, Si, As, Ge, selenium (Se), and Sb (Pommerrenig et al. 2015). For instance, Ma and Yamaji (2006) showed that Si transporter Lsi1 (OsNIP2;1) also transported As(III) and facilitated the entry of As(III) into rice roots. In addition to Lsi1, three other rice NIPs (OsNIP1;1, OsNIP2;2, and OsNIP3;1) were also able to mediate As(III) influx when expressed in Xenopus laevis oocytes; however, these genes were expressed at significantly low levels in rice roots (Li et al. 2009). The expression of Lsi1 in X. laevis oocytes also significantly increased the uptake of the methylated As species MMAV (monomethylarsonic acid) but not DMAV (dimethylarsonic acid) (Li et al. 2009). Lsi1 helped increase As(III) efflux in rice roots exposed to AsV (Zhao et al. 2010). Additionally, the heterologous expression of OsNIP2;1 and OsNIP3;2 in yeast increased sensitivity to As(III) and As accumulation (Bienert et al. 2008). Arabidopsis thaliana NIP1;1, NIP2;1, NIP3;1, NIP5;1, NIP6;1, and NIP7;1 were permeable to As(III) in the yeast expression system (Isayenkov and Maathuis 2008; Bienert et al. 2008), and AtNIP1;1 was capable of transporting As(III) when expressed in X. laevis oocytes (Kamiya and Fujiwara 2009). Other NIPs such as LjNIP5;1 and LjNIP6;1 from Lotus japonicas are also permeable to As(III) (Bienert et al. 2008). It is clear that members of NIP aquaporins are metalloid channels that transport Si, As, and Sb.
Although the genetic regulation of the B accumulation is less known, a recent study demonstrated that HvNIP2;2/HvLsi6 appear within quantitative trait loci (QTL) largely responsible for B-level dynamics in barley. Further, complementation studies in yeast Δatr1 mutant (B efflux transporter deficient) showed that the expression of HvNIP2;2/HvLsi6 induces growth suppression in Δatr1 yeast cells, indicating that these NIPs function as B transporters. Also, its heterologous expression in X. laevis oocytes showed a high rate of B uptake (Jia et al. 2021). As HvNIP2;2/HvLsi6 is previously reported to function as Si transporter, studies with B transport system show the versatile role of NIP aquaporins in the metalloid transport. The rapeseed’s QTL mapping and differential gene expression (DGE) analysis has identified putative NIP transporters that may transport B (Hua et al. 2016). The QTL mapping approach encouraged the identification of the B-efficient gene BnaA3.NIP5;1 which induces the root tip growth under B deficiency in Brassica napus. Additionally, the 5′-untranslated region (UTR) of BnaA3.NIP5;1 contains “CTTTC” repeats which contribute to the differential expression of the genes involved in plant growth and seed setting (He et al. 2021). Hence, not only the key functional residues but the untranslated regions of the NIPs may also function in the regulatory mechanisms involved in metalloid transport-associated mechanisms in plants. The increased expression of CiNIP5 under B-deficient conditions has been reported in trifoliate orange and Carrizo citrange; in this case, the gene’s predominant expression was found in root tissues. The function of CiNIP5 has similarities with AtNIP5;1 gene from the Arabidopsis (An et al. 2012). Thus, the tissue-specific NIP expression reflects their particular roles in plants.
Moreover, certain functions can be seen conserved among different plant species. The site-directed mutagenesis in the H2 and H5 regions of AtNIP5;1 and OsLsi1 (OsNIP2;1) shows that the amino acid residues in the H5 region of the ar/R filter are critical for the transport of B and Si (Mitani-Ueno et al. 2011). The QTL analysis revealed HvNIP2;1 marker gene associated with B and Ge toxicity in barley. Further site-directed mutagenesis in the amino acid residues of H2 and H5 regions and yeast complementation assay showed that the mutant’s B, Ge, and As transport activity was altered (Hayes et al. 2013). Thus, the conserved structural features are important in deciding the transport substrates for NIP aquaporins.
The transcriptome analysis has shown that Arabidopsis NIP5;1 gene expression increases under B-deficient conditions. Studies with the promoter-GUS fusion showed that NIP5;1 expression is upregulated in the root extension and the root hair zone under B-deficient conditions. Heterologous expression in X. laevis oocytes showed that NIP5;1 transported boric acid. Further, T-DNA mutants of NIP5;1 exhibited impaired boric acid uptake by roots, less growth of plants, and overall increased sensitivity to B inadequacy in both roots and shoots. Thus, it may be stated that under B-deficient stress conditions, NIPs could notably influence plant growth and also influence the transport processes in plants (Takano et al. 2006). Similarly, Gómez-Soto et al. (2019) proposed that the AtNIP5;1 promoter is largely regulated by phytohormones, including abscisic acid (ABA) and ethylene. When applied exogenously, the influence of ABA and ethylene results in the induced expression of the AtNIP5;1 gene. Further, the AtNIP5;1 exhibits ABA-induced B uptake and induces root growth.
Sb is toxic to all living beings, including plants, if taken up by plants in the rhizosphere system (Bienert et al. 2008; Kamiya and Fujiwara 2009). The toxicity and tolerance studies in yeast showed that the AtNIP1;1 transport antimonite (SbIII) and determine consequent sensitivity. Although selenium (Se) is not essential for plants, selenocysteine in plants is involved in the defense against oxidative stress. Vegetables and fruits are the rich sources of Se (Arnér 2010). Examples of the Se transporters in plants include sulfate transporters and OsNIP2;1 (Sors et al. 2005; Zhang et al. 2010, 2012; Zhao et al. 2010). Geranium and silica share similarities in their chemical properties. Ge has been proposed as a biomarker and has been useful for identifying OsNIP2;1 responsible for the silica accumulation (Ma and Yamaji 2006). Also, the radiolabeled Ge has been shown to trace silica accumulation in heterologous expression systems. A similar biomarker principle has been applied to assess B uptake in barley mediated by NIP aquaporins (Takahashi et al. 1976; Mitani-Ueno et al. 2011; Bárzana et al. 2014).
7.4 XIP Members as Metalloid Transporters
In the genome of different plant species, including Physcomitrella patens, Nicotiana benthamiana, grape, cotton, tomato, and poplar, various XIP subfamilies were identified (Shelden et al. 2009; Park et al. 2010; Lopez et al. 2012; Ampah-Korsah et al. 2016). The XIPs are absent in monocots and found only in diversified dicot species, except Brassicaceae (Danielson and Johanson 2008). The NPARC motif, the signature sequence for XIPs, showed cysteine residue, followed after the second NPA motif (Danielson and Johanson 2008; Gupta and Sankararamakrishnan 2009). XIPs exhibit discrepancies in amino acids of the first NPA motif and ar/R filter. XIPs are subdivided into four subclasses based on demarcating ar/R filters. In some plants, NIPs showed similarity with the two subclasses having an ar/R signature; instead, the other two subclasses had hydrophobic signature amino acids (Noronha et al. 2016). The XIP cDNA was cloned from morning glory, potato, tobacco, and tomato and showed to be localized in the plasma membrane (Bienert et al. 2011).
Bienert et al. (2011) showed that in a yeast mutant (δfps1), XIPs belonging to Solanales species transport boric acid. In the Solanales’ XIPs mutants, the expression of the various splice variants showed impaired and no growth at 10 mM and 20 mM boric acid, respectively. The role of XIPs to facilitate B transport was proved by increased sensitivity to boric acid (Bienert et al. 2011). Overexpression of NtXIP1;1 in Nicotiana tabacum with Nicotiana plumbaginifolia PMA4 promoter fused with 35S enhancer (En2pPMA4) resulted in severe B deficiency symptoms. Overexpression of NtXIP1;1 in Arabidopsis, which does not contain any XIP gene, by CaMV 35S promoter, resulted in severe B deficiency symptoms. The expression of the two splice variants NtXIP1;1α and NtXIP1;1β in Xenopus oocytes showed increased boric acid uptake (Bienert et al. 2019). However, heterologous expression of NtXIP1;1 with Arabidopsis AtNIP5;1 promoter rescued the B deficiency symptoms of Atnip5;1 mutant, indicating that NtXIP1;1 is a functional boric acid channel in N. tabacum and provides B homeostasis and its distribution in tobacco through the tissue-specific expression (Bienert et al. 2019). Some studies on the heterologous expression of grapevine Vitis vinifera VvXIP1 in yeast showed their inclusion in metalloids (B and As) and heavy metals (copper and nickel) transport and reduced yeast cell growth in the presence of 40 mM B, 0.5 mM nickel, and 5 mM copper, whereas the growth of the transformed yeast was improved in the presence of 0.45 mM As (Noronha et al. 2016). The knowledge of XIPs and their role in metals and metalloid transport in plants is very limited. Further studies are needed to decipher the exact role of the XIP subfamily in plants.
7.5 Role of TIPs in Metalloid Transport and Tolerance
TIPs are a subfamily of MIPs and are detected to be predominantly located at the tonoplast membrane (Jauh et al. 1998, 1999; Srivastava et al. 2013). However, some TIPs such as AtTIP2;1 and AtTIP1;2 are also localized in endosomal membrane compartments and plasma membrane (Liu et al. 2003). GFP fusion experiments confirmed that AtTIP5;1 is localized to mitochondria (Soto et al. 2010). PvTIP4;1 from Pteris vittata, an arsenite hyperaccumulator, has been shown to mediate As(III) transport by yeast functional complementation assay and heterologous expression in Arabidopsis (He et al. 2016). Srivastava et al. (2013), through the transcriptomic studies in B. juncea under arsenate As(V) stress, found that the TIP2 gene is responsive to different As(V) treatment periods in both root and shoot parts. The TIP function in the B transport has been investigated either in vivo by utilizing transgenic plants or in vitro by heterologous expression analysis (Bienert and Bienert 2017; Bienert et al. 2019; Pang et al. 2010). AtTIP5;1 overexpression builds the resilience to high B in the transgenic Arabidopsis plants, probably occurring through the vacuolar compartmentation (Pang et al. 2010). Porcel et al. (2018) identified a novel gene, BvCOLD1, in Beta vulgaris whose sequence shares similarities with the TIP aquaporins. However, it was localized to the endoplasmic reticulum during the subcellular localization analysis with the GFP-fused construct. In yeast cells, BvCOLD1 showed B homeostasis. When overexpressed, it exhibited enhanced tolerance against the B deficiency in Arabidopsis. However, mere sequence similarity may not determine its aquaporin type and role, and further structural studies are imperative to determine the same. Pang et al. (2017) stated that AtTIP5;1 manages elongation of hypocotyl under high convergences of B in A. thaliana by unknown mechanisms. Studies with two mutants of AtTIP5;1 showed that the elongation of cells is hampered when given high B or gibberellin (GA3) compared to the wild-type plants. Further, paclobutrazol (GA synthesis inhibitor) hindered the upregulation of AtTIP5;1 expression influenced by high B. In the case of GA3 treatment, the expression of AtTIP5;1 was upregulated compared to wild-type plants. Further, treatment with high B stimulated the transcript level increase of the GA biosynthesis genes in WT seedlings. Also, double mutant studies with the DELLA genes showed no upregulation of the AtTIP5;1 gene under high B stress. Altogether, these outcomes recommend that AtTIP5;1 function downstream to GA signaling in high B stress (Pang et al. 2017). Hence, TIP aquaporins may cross talk with phytohormones and participate in various stress regulatory mechanisms under metalloid stress. Moreover, they may stimulate growth and development under these stress conditions.
Rivera-Serrano et al. (2012) proposed that the localization of the TIP aquaporins may occur by the vesicular trafficking that involves the Golgi-dependent and Golgi-independent transport through the endoplasmic reticulum. However, the underlying mechanisms are less known; besides, the role of vesicular trafficking under metalloid stress conditions has not been elucidated. Gattolin et al. (2011) reported the localization of Arabidopsis TIP3;1 and TIP3;2 to the tonoplast and the plasma membrane; however, their significance to the metalloid stress response is not known. Some reports suggest the differential localization of the TIPs to the subcellular organelles other than the vacuole (Sudhakaran et al. 2021). Further, the localization of the TIPs to other cellular organelles has also been reported; for instance, AtTIP2;1, AtTIP1;2, and AtTIP1;1 localized to the chloroplast membrane (Ferro et al. 2010), GmTIP3;3 and AtTIP5;1 to the mitochondria, GmTIP1;1 to the endoplasmic reticulum, and GmTIP2;6, GmTIP2;7, GmTIP1;7, GmTIP2;3, and GmTIP2;1 to the plasma membrane (Deshmukh et al. 2013). Also, the cytoplasmic localization of the TIPs has been reported in the case of the flax (Shivaraj et al. 2017), nonetheless, their importance in the transport of metalloids is yet to be characterized. The representative plant MIPs’ role as metalloid transporters determined by functional studies and the studies conducted to determine functionality are listed in Table 7.1.
7.6 Future Perspectives
Metalloid contamination, its stress to plants, and accumulation in the edible plant parts have emerged as serious food production and safety problems. Cereals like wheat, rice, maize, and barley are major sources of biomagnification of metalloid(s) in consumers. For instance, rice is one of the major sources of As exposure. Compared to other crop plants, the translocation factor (TF) for As is higher in rice (0.8), indicating its ten times higher concentration in rice than in other cereals. Being a staple food in most parts of the world, As accumulation in rice grains poses a great concern to consumers. Thus major efforts are needed to reduce the As content in rice grains up to at least maximum permissible limits (safe for consumption), 200 μg/kg for white rice and 300 μg/kg for brown rice as suggested by the World Health Organization (WHO 2014). Brown rice contains 80% more inorganic As than white rice because of the germ layer in brown rice, which retains a considerable amount of As. Hence, it is imperative to reduce the deposition of this metalloid into the edible plant parts. Although notable studies have helped to understand the role of different types of transporters, including MIPs in metalloid transport in plants, many research gaps need to be identified and addressed. As stated above, certain MIPs (e.g., TIPs) have been shown to cross talk with multiple plant hormones in metalloid stress conditions. This indicates that MIPs could directly or indirectly influence plant growth and development. However, the scarcity of knowledge about the underlying signaling mechanisms may limit to employ different MIPs for the crop improvement programs. The selective transport of specific metalloid types by the aquaporins denotes their functions. However, their versatility in transporting other metalloid types directly or indirectly needs to be assessed.
As of now, only PIPs, TIPs, NIPs, and XIPs are involved in metalloid stress tolerance/sensitivity in plants. The literature shows that most of the known metalloids that influence plant growth and productivity are transported by NIP and PIP aquaporins showing their versatile role in metalloid stress response. However, some reports studied their differential regulation at the gene expression level, and functional characterization of them either by homologous or heterologous systems will certainly help uncover many unknown aspects of the NIP- and PIP-mediated metalloid stress signaling in plants. Few reports have proved the metalloid transportability of the MIPs through the heterologous expression studies, which could further be scaled up in many other model systems. When it comes to the function, the sensitivity or tolerance to the metalloid stress responses can be seen as variable among each MIP type from species to species; this discrepancy and dynamic functionality of the MIPs should be addressed at the individual level. Also, distinct MIP signaling pathways regarding the corresponding morphological and physiological modifications in plants are yet to be studied. The inconsistencies among the current data may result from the sort of plant species, the stress treatment systems, etc.
Consequently, the exploratory examinations on the model plant species could give a baseline to future research. It is well known that the MIPs contain significant key amino acid residues in certain conserved regions which take part in the metalloid transport process. Identifying such critical amino acid residues through in silico and mutant analysis approach will help understand the nature of aquaporins to show differential preference to various metalloid substrates for transport. Moreover, it may help engineer modified and improved crop plants exhibiting multimetalloid stress tolerance and biomagnification in permissible limits in the food chain (Mittler and Blumwald 2010). Additionally, studies with other biologically significant metalloids/metal-induced stress in plants will be useful to expand the spectrum of functional characterization of the MIPs.
References
Afzal Z, Howton TC, Sun Y, Mukhtar MS (2016) The roles of aquaporins in plant stress responses. J Dev Biol 4(1):9
Ampah-Korsah H, Anderberg HI, Engfors A, Kirscht A, Norden K, Kjellstrom S, Kjellbom P, Johanson U (2016) The aquaporin splice variant NbXIP1;1α is permeable to boric acid and is phosphorylated in the N-terminal domain. Front Plant Sci 7:862
An JC, Liu YZ, Yang CQ, Zhou GF, Wei QJ, Peng SA (2012) Isolation and expression analysis of CiNIP5, a citrus boron transport gene involved in tolerance to boron deficiency. Sci Hortic 142:149–154
Angulo-Bejarano PI, Puente-Rivera J, Cruz-Ortega R (2021) Metal and metalloid toxicity in plants: an overview on molecular aspects. Plants 10(4):1–28
Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff JI, Arce-Johnson PA (2012) A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant Cell Environ 35(4):719–734
Arnér ES (2010) Selenoproteins—what unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res 316(8):1296–1303
Bárzana G, Aroca R, Bienert GP, Chaumont F, Ruiz-Lozano JM (2014) New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol Plant Microbe Interact 27(4):349–363
Bienert MD, Bienert GP (2017) Plant aquaporins and metalloids. In: Plant aquaporins. Springer, Cham, pp 297–332
Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol 6(1):1–5
Bienert GP, Bienert MD, Jahn TP, Boutry M, Chaumont F (2011) Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J 66(2):306–317
Bienert MD, Muries B, Crappe D, Chaumont F, Bienert GP (2019) Overexpression of X intrinsic protein 1;1 in Nicotiana tabacum and Arabidopsis reduces boron allocation to shoot sink tissues. Plant Direct 3(6):1–16
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125:1206–1215
Danielson JÅ, Johanson U (2008) Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol 8(1):1–5
Deng F, Yu M, Martinoia E, Song WY (2019) Ideal cereals with lower arsenic and cadmium by accurately enhancing vacuolar sequestration capacity. Front Genet 10(322):1–7
Deshmukh RK, Vivancos J, Guérin V, Sonah H, Labbé C, Belzile F, Bélanger RR (2013) Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol Biol 83(4–5):303–315
Deshmukh RK, Vivancos J, Ramakrishnan G, Guérin V, Carpentier G, Sonah H, Labbé C, Isenring P, Belzile FJ, Bélanger RR (2015) A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J 83(3):489–500
Deshmukh RK, Sonah H, Bélanger RR (2016) Plant aquaporins: genome-wide identification, transcriptomics, proteomics, and advanced analytical tools. Front Plant Sci 7:1896
Deshmukh RK, Ma JF, Bélanger RR (2017a) Role of silicon in plants. Front Plant Sci 8(1858):5–7
Deshmukh RK, Nguyen HT, Belanger RR (2017b) Aquaporins: dynamic role and regulation. Front Plant Sci 8:1420
Epstein E (2001) Silicon in plants: facts vs. concepts. In: Studies in plant science, vol 8. Elsevier, Amsterdam, pp 1–15
Ferro M, Brugière S, Salvi D, Seigneurin-Berny D, Moyet L, Ramus C, Miras S, Mellal M, Le Gall S, Kieffer-Jaquinod S, Bruley C (2010) AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteome 9(6):1063–1084
Fitzpatrick KL, Reid RJ (2009) The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ 32(10):1357–1365
Gattolin S, Sorieul M, Frigerio L (2011) Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol Plant 4(1):180–189
Gomes D, Agasse A, Thiébaud P, Delrot S, Gerós H, Chaumont F (2009) Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta Biomembr BBA Biomembr 1788(6):1213–1228
Gómez-Soto D, Galván S, Rosales E, Bienert P, Abreu I, Bonilla I, Bolaños L, Reguera M (2019) Insights into the role of phytohormones regulating pAtNIP5;1 activity and boron transport in Arabidopsis thaliana. Plant Sci 287:1–13
Gupta AB, Sankararamakrishnan R (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9(1):1–28
Hayes JE, Pallotta M, Baumann U, Berger B, Langridge P, Sutton T (2013) Germanium as a tool to dissect boron toxicity effects in barley and wheat. Funct Plant Biol 40(6):618–627
He Z, Yan H, Chen Y, Shen H, Xu W, Zhang H, Shi L, Zhu YG, Ma M (2016) An aquaporin PvTIP 4;1 from Pteris vittata may mediate arsenite uptake. New Phytol 209(2):746–761
He M, Wang S, Zhang C, Liu L, Zhang J, Qiu S, Wang H, Yang G, Xue S, Shi L, Xu F (2021) Genetic variation of BnaA3. NIP5;1 expressing in the lateral root cap contributes to boron deficiency tolerance in Brassica napus. PLoS Genet 17(7):1–20
Hrmova M, Gilliham M, Tyerman SD (2020) Plant transporters involved in combating boron toxicity: beyond 3D structures. Biochem Soc Trans 48(4):1683–1696
Hua Y, Zhang D, Zhou T, He M, Ding G, Shi L, Xu F (2016) Transcriptomics-assisted quantitative trait locus fine mapping for the rapid identification of a nodulin 26-like intrinsic protein gene regulating boron efficiency in allotetraploid rapeseed. Plant Cell Environ 39(7):1601–1618
Isayenkov SV, Maathuis FJ (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett 582(11):1625–1628
Jauh GY, Fischer AM, Grimes HD, Ryan CA, Rogers JC (1998) δ-Tonoplast intrinsic protein defines unique plant vacuole functions. PNAS 95(22):12995–12999
Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11(10):1867–1882
Jia Z, Bienert MD, von Wirén N, Bienert GP (2021) Genome-wide association mapping identifies HvNIP2;2/HvLsi6 accounting for efficient boron transport in barley. Physiol Plant 171(4):809–822
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126(4):1358–1369
Kamiya T, Fujiwara T (2009) Arabidopsis NIP1;1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol 50(11):1977–1981
Kumar K, Mosa KA, Chhikara S, Musante C, White JC, Dhankher OP (2014) Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta 239(1):187–198
Kumar K, Mosa KA, Meselhy AG, Dhankher OP (2018) Molecular insights into the plasma membrane intrinsic proteins roles for abiotic stress and metalloids tolerance and transport in plants. Indian J Plant Physiol 23(4):721–730
Kumawat S, Khatri P, Ahmed A, Vats S, Kumar V, Jaswal R, Wang Y, Xu P, Mandlik R, Shivaraj SM, Deokar A (2021) Understanding aquaporin transport system, silicon and other metalloids uptake and deposition in bottle gourd (Lagenaria siceraria). J Hazard Mater 409:124598
Lee MS, Hwang S (2012) Cyc07 enhances arsenite tolerance by reducing As levels in Nicotiana tabacum and Arabidopsis thaliana. Plant Biotech Rep 6(4):391–405
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150(4):2071–2080
Liu LH, Ludewig U, Gassert B, Frommer WB, von Wirén N (2003) Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiol 133(3):1220–1228
Liu P, Yin L, Deng X, Wang S, Tanaka K, Zhang S (2014) Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J Exp Bot 65(17):4747–4756
Lopez D, Bronner G, Brunel N, Auguin D, Bourgerie S, Brignolas F, Carpin S, Tournaire-Roux C, Maurel C, Fumanal B, Martin F, Sakr S, Label P, Julien JL, Gousset-Dupont A, Venisse JS (2012) Insights into Populus XIP aquaporins: evolutionary expansion, protein functionality, and environmental regulation. J Exp Bot 63(5):2217–2230
Luyckx M, Hausman JF, Lutts S, Guerriero G (2017) Silicon and plants: current knowledge and technological perspectives. Front Plant Sci 8(411):1–8
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11(8):392–397
Ma JF, Yamaji N (2008) Functions and transport of silicon in plants. Cell Mol Life Sci 65(19):3049–3057
Macho-Rivero MA, Herrera-Rodríguez MB, Brejcha R, Schäffner AR, Tanaka N, Fujiwara T, González-Fontes A, Camacho-Cristóbal JJ (2018) Boron toxicity reduces water transport from root to shoot in Arabidopsis plants. Evidence for a reduced transpiration rate and expression of major PIP aquaporin genes. Plant Cell Physiol 59(4):841–849
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95(4):1321–1358
Mitani-Ueno N, Yamaji N, Zhao FJ, Ma JF (2011) The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot 62(12):4391–4398
Mittler R, Blumwald E (2010) Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol 61:443–462
Miwa K, Fujiwara T (2010) Boron transport in plants: coordinated regulation of transporters. Ann Bot 105(7):1103–1108
Modareszadeh M, Bahmani R, Kim D, Hwang S (2021) Decreases in arsenic accumulation by the plasma membrane intrinsic protein PIP2;2 in Arabidopsis and yeast. Environ Pollut 275:1–10
Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C, White JC, Dhankher OP (2012) Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res 21(6):1265–1277
Mosa KA, Kumar K, Chhikara S, Musante C, White JC, Dhankher OP (2016) Enhanced boron tolerance in plants mediated by bidirectional transport through plasma membrane intrinsic proteins. Sci Rep 6(1):1–4
Noronha H, Araújo D, Conde C, Martins AP, Soveral G, Chaumont F, Delrot S, Gerós H (2016) The grapevine uncharacterized intrinsic protein 1 (VvXIP1) is regulated by drought stress and transports glycerol, hydrogen peroxide, heavy metals but not water. PLoS One 11(8):1–18
Panda SK, Upadhyay RK, Nath S (2010) Arsenic stress in plants. J Agron Crop Sci 196(3):161–174
Pandey AK, Gautam A, Dubey RS (2019) Transport and detoxification of metalloids in plants in relation to plant-metalloid tolerance. Plant Gene 17:1–26
Pang Y, Li L, Ren F, Lu P, Wei P, Cai J, Xin L, Zhang J, Chen J, Wang X (2010) Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis. J Genet Genomics 37(6):389–397
Pang Y, Li J, Qi B, Tian M, Sun L, Wang X, Hao F (2017) Aquaporin AtTIP5;1 as an essential target of gibberellins promotes hypocotyl cell elongation in Arabidopsis thaliana under excess boron stress. Funct Plant Biol 45(3):305–314
Park W, Scheffler BE, Bauer PJ, Campbell BT (2010) Identification of the family of aquaporin genes and their expression in upland cotton (Gossypium hirsutum L.). BMC Plant Biol 10(1):1–7
Pavlovic J, Kostic L, Bosnic P, Kirkby EA, Nikolic M (2021) Interactions of silicon with essential and beneficial elements in plants. Front Plant Sci 12:1–19
Pereira GL, Siqueira JA, Batista-Silva W, Cardoso FB, Nunes-Nesi A, Araújo WL (2021) Boron: more than an essential element for land plants? Front Plant Sci 11:1–10
Pommerrenig B, Diehn TA, Bienert GP (2015) Metalloido-porins: essentiality of nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci 238:212–227
Porcel R, Bustamante A, Ros R, Serrano R, Mulet Salort JM (2018) BvCOLD1: a novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ 41(12):2844–2857
Quiroga G, Erice G, Aroca R, Ruiz-Lozano JM (2020) Elucidating the possible involvement of maize aquaporins in the plant boron transport and homeostasis mediated by Rhizophagus irregularis under drought stress conditions. Int J Mol Sci 21(5):1–21
Rios JJ, Martínez-Ballesta MC, Ruiz JM, Blasco B, Carvajal M (2017) Silicon-mediated improvement in plant salinity tolerance: the role of aquaporins. Front Plant Sci 8:948
Rivera-Serrano EE, Rodriguez-Welsh MF, Hicks GR, Rojas-Pierce M (2012) A small molecule inhibitor partitions two distinct pathways for trafficking of tonoplast intrinsic proteins in Arabidopsis. PLoS One 7(9):1–11
Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46(9):1568–1577
Sharma SS, Kumar V, Dietz KJ (2021) Emerging trends in metalloid-dependent signaling in plants. Trends Plant Sci 26(5):452–471
Shelden MC, Howitt SM, Kaiser BN, Tyerman SD (2009) Identification and functional characterisation of aquaporins in the grapevine, Vitis vinifera. Funct Plant Biol 36(12):1065–1078
Shivaraj SM, Deshmukh RK, Rai R, Bélanger R, Agrawal PK, Dash PK (2017) Genome-wide identification, characterization, and expression profile of aquaporin gene family in flax (Linum usitatissimum). Sci Rep 7(1):1–7
Singh S, Kumar A, Panda D, Modi MK, Sen P (2020) Identification and characterization of drought responsive miRNAs from a drought tolerant rice genotype of Assam. Plant Gene 21:1–8
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86(3):373–389
Soto G, Fox R, Ayub N, Alleva K, Guaimas F, Erijman EJ, Mazzella A, Amodeo G, Muschietti J (2010) TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J 64(6):1038–1047
Srivastava S, Srivastava AK, Suprasanna P, D’Souza SF (2013) Quantitative real-time expression profiling of aquaporins-isoforms and growth response of Brassica juncea under arsenite stress. Mol Biol Rep 40(4):2879–2886
Sudhakaran S, Thakral V, Padalkar G, Rajora N, Dhiman P, Raturi G, Sharma Y, Tripathi DK, Deshmukh R, Sharma TR, Sonah H (2021) Significance of solute specificity, expression, and gating mechanism of tonoplast intrinsic protein during development and stress response in plants. Physiol Plant 172(1):258–274
Sun H, Li L, Lou Y, Zhao H, Yang Y, Wang S, Gao Z (2017) The bamboo aquaporin gene PeTIP4;1–1 confers drought and salinity tolerance in transgenic Arabidopsis. Plant Cell Rep 36(4):597–609
Takahashi E, Syo S, Miyake Y (1976) Effect of germanium on the growth of plants with special reference to the silicon nutrition. 1. Comparative studies on the silica nutrition in plants. J Sci Soil Manure 2:191–197
Takano J, Wada M, Ludewig U, Schaaf G, Von Wirén N, Fujiwara T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18(6):1498–1509
Venkatesh J, Yu JW, Park SW (2013) Genome-wide analysis and expression profiling of the Solanum tuberosum aquaporins. Plant Physiol Biochem 73:392–404
Wallace IS, Choi WG, Roberts DM (2006) The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim Biophys Acta (BBA) Biomembr 1758(8):1165–1175
WHO (2014) Codex Alimentarius Commission, 37th Session, Geneva, 14–18 July 2014. World Health Organization, Geneva
Yan GC, Nikolic M, Ye MJ, Xiao ZX, Liang YC (2018) Silicon acquisition and accumulation in plant and its significance for agriculture. J Integr Agric 17(10):2138–2150
Yuan D, Li W, Hua Y, King GJ, Xu F, Shi L (2017) Genome-wide identification and characterization of the aquaporin gene family and transcriptional responses to boron deficiency in Brassica napus. Front Plant Sci 8:1–17
Zangi R, Filella M (2012) Transport routes of metalloids into and out of the cell: a review of the current knowledge. Chem Biol Interact 197(1):47–57
Zhang L, Yu F, Shi W, Li Y, Miao Y (2010) Physiological characteristics of selenite uptake by maize roots in response to different pH levels. J Plant Nutr Soil Sci 173(3):417–422
Zhang H, Feng X, Zhu J, Sapkota A, Meng B, Yao H, Qin H, Larssen T (2012) Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ Sci Technol 46(18):10040–10046
Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF (2010) Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol 153(4):1871–1877
Zhu YX, Xu XB, Hu YH, Han WH, Yin JL, Li HL, Gong HJ (2015) Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep 34(9):1629–1646
Zulfiqar F, Akram NA, Ashraf M (2020) Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta 251(1):1–7
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Karle, S.B., Kumar, K., Dhankher, O.P. (2022). The Versatile Role of Plant Aquaglyceroporins in Metalloid Transport. In: Kumar, K., Srivastava, S. (eds) Plant Metal and Metalloid Transporters. Springer, Singapore. https://doi.org/10.1007/978-981-19-6103-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-6103-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6102-1
Online ISBN: 978-981-19-6103-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)