Abstract
Aquaporins are channel proteins reported to play multiple functions in plants ranging from water, solutes, metalloids (arsenic, boron, silicon) transport, and tolerance to abiotic stresses including drought, salinity and cold. Based on their localization and sequence similarities, aquaporins have been classified into seven major subfamilies: plasma membrane intrinsic proteins (PIPs), nodulin 26-like intrinsic proteins, tonoplast intrinsic proteins, small basic intrinsic proteins, GlpF-like intrinsic protein, hybrid intrinsic proteins and the uncategorized (X) intrinsic proteins. PIP subfamily is one of the biggest subfamilies of aquaporin superfamily and they are localized to plasma membrane. Members of PIPs are involved in water and small neutral solute transport and play an important role in maintaining water homeostasis under environmental stress and are known to provide tolerance to various abiotic stresses. Recently, members of PIP subfamily have been shown to be involved in the bidirectional transport of metalloids, arsenic and boron in plants. This review highlights the involvement of various PIP homologs in plant stress responses against a variety of environmental stresses and metalloid transport and tolerance. Molecular insights and biotechnological approaches for developing climate resilient crops by modulating PIPs will be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
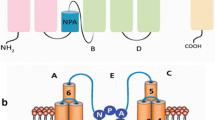
Aquaporins (AQPs), also called as Major Intrinsic Proteins (MIPs), are channel proteins that play various important roles in plants and are widely distributed in all kingdoms of life, including bacteria, plants, and mammals. The superfamily of AQPs have been classified into seven subfamilies: plasma membrane intrinsic proteins (PIPs), nodulin26-like intrinsic proteins (NIPs), tonoplast intrinsic proteins (TIPs), small basic intrinsic proteins (SIPs), GlpF-like intrinsic protein (GIPs), hybrid intrinsic proteins (HIPs) and uncategorized X intrinsic proteins (XIPs), based on their localization and sequence similarity (Kong et al. 2017). The GIPs are homologous to bacterial glycerol channel and also reported in Physcomitrella patens. The GIPs, XIPs, and HIPs were present in mosses and fern (Selaginella moellendorffii). Green plants usually have five AQP subfamilies: PIPs, TIPs, NIPs, SIPs, and XIPs (Saddhe et al. 2018). The structural features of MIPs have tetramers in which each monomer is composed of six transmembranes helices (TM1–TM6) interconnected by three extracellular (LA, LC and LE) and two intracellular (LB and LD) loops, each containing the highly conserved asparagine-proline-alanine (NPA) signature motif (Fig. 1; Hove and Bhave 2011). These NPA motifs overlapping in the middle of the membrane create a narrow hydrophilic channel and play an important role on substrate transport selectivity (Murata et al. 2000).
Schematic diagram showing the topology of a plant aquaporin. It consist of six transmembrane domain (TM1–TM6) linked by five loops (Loops A, B, C, D, E). Stars (red color) represent the amino acid residues making the a/R region. The conserved NPA motifs are represented in blue ovals.
The substrate specificity of MIP is depending on aromatic/arginine filter and two NPA motifs which can form the narrowest pore of approximately 8 Å in diameter (Forrest and Bhave 2007). MIPs are mainly involved in water homeostasis and transport of wide range of low molecular weight solutes across the membrane such as glycerol, urea, ammonia (NH3), methyl ammonium, hydrogen peroxide, formamide, acetamide, boric acid, silicic acid, lactic acid, CO2, metalloid and cations (Deshmukh et al. 2016; Byrt et al. 2017; Saddhe et al. 2018). Members of these MIP subfamilies have been well characterized in organisms ranging from bacteria, yeast, animals and plants (Liu et al. 2002; Zardoya 2005; Chaumont and Tyerman 2014). Particularly in plants, members of NIP has been studied well for their role in transport of metalloids arsenic, boron and silicon (Bienert et al. 2008; Ma et al. 2008; Pommerrenig et al. 2015) but other subfamily members such as PIPs, XIPs, SIPs and TIPs have not been fully characterized. In the last few years, some of the studies highlighted the role of PIPs in providing tolerance to abiotic stresses such as drought, salinity, cold and also for their involvement in the bidirectional transport of arsenic and boron (Mosa et al. 2012; Kumar et al. 2014; Mosa et al. 2016a).
This review emphasizes on the functional role of PIPs in abiotic stress response including salt, drought and cold stress, and transport and tolerance of metalloids such as arsenic and boron. Further, the manipulation of PIPs using modern biotechnological approaches to develop climate resilient crops is also discussed.
PIP subfamily in plants
In plants, PIPs are localized to plasma membrane and its distribution in plants ranged from three members in Selenginella to 22 each in soybean and Brassica species. PIPs are present in multiple isoforms and further classified into two subgroups PIP1 and PIP2 (Secchi et al. 2017). The distribution and classification of PIPs in various plants is represented in Table 1. In Arabidopsis and rice, there are 13 and 11 members of PIP, respectively. Multiple sequence alignment by neighbor-joining (NJ) tree from various plant species clustered PIPs into two clades PIP1 and PIP2 as shown in Fig. 2. The role of PIP1 includes water or solute transport, maintenance of root hydraulic conductance, phloem loading and unloading, and stomatal conductance (Nouri and Komatsu 2013; Kelly et al. 2014; Zhou et al. 2014). Higher water transport activity was demonstrated by PIP2 subgroup in Xenopus expression system, whereas members of PIP1 subgroup showed lower water transport activity (Chaumont et al. 2000). The difference in water permeability is due to the changes in protein sequences of PIP1 and PIP2 in the conserved amino acid residues of six membrane-spanning alpha helixes and NPA motifs (Chaumont et al. 2001). In addition to these alterations in amino acid residues of transmembrane domains, PIP2 proteins has a shorter N-terminal and longer C-terminal extension containing a putative phosphorylation site compare to PIP1 proteins (Johansson et al. 2000). Arabidopsis PIP1 subgroup represent five members of aquaporins, namely AtPIP1;1 to AtPIP1;5, and PIP2 subgroup consisting of eight members of aquaporins, namely AtPIP2;1 to AtPIP2;8 (Johanson et al. 2001). Rice PIP1 subgroup represent three members (OsPIP1;1 to OsPIP1;3) and PIP2 subgroup consist of eight members (OsPIP2;1 to OsPIP2;8). Poplar genome has 15 PIP members including five members of PIP1 subgroup and 10 members of PIP2 subgroup (Secchi and Zwieniecki 2010).
Phylogenetic analysis of PIPs from Selaginella moellendorffii, Physcomitrella patens, Brachypodium distachyon, Arabidopsis thaliana, Oryza sativa, Zea mays, Sorghum bicolor, Cajanus cajan, Glycine max, Brassica rapa and Populus trichocarpa plant species. The evolutionary history of PIPs was inferred using the Neighbor-Joining method. The bootstrap consensus tree inferred from 1000 replicates, branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method. The accessions used to construct map are provided in supplementary information
PIP regulates various plant physiological processes ranging from water and nutrient uptake and CO2 exchange in shoots (Srivastava et al. 2016). PIPs have also been shown to be involved in the transport of urea, H2O2, metalloids such as boron (B) and arsenic (As) (Mosa et al. 2012; Kumar et al. 2014; Bienert et al. 2018). In addition, the involvement of PIPs in conferring various abiotic stress tolerance including drought, salt, and cold stress are widely reported. Recently four PIPs of rice viz. OsPIP1;3, OsPIP2;4, OsPIP2;6 and OsPIP2;7, were shown to be involved in boron and arsenite (AsIII) transport and providing tolerance to AsIII and boron toxicity (Mosa et al. 2012; Kumar et al. 2014; Mosa et al. 2016a).
Role of PIPs in abiotic stress tolerance in plants
Abiotic stress including salt, drought, and cold differentially regulates the PIPs expression pattern. During water stress, aquaporin activity gets reduced to conserve water, while in long-term stress, aquaporin activity is increased for water homeostasis (Chaumont and Tyerman 2014). The differential expression of PIP genes in plants subjected to abiotic stress suggest PIP gene expression is valuable in keeping proper water status of plant shoot and roots under stress conditions. Importance of PIPs in salt, cold, and drought tolerance by differential modulation of PIPs expression was reported in various plant species (Li et al. 2016; Pou et al. 2016; Kayum et al. 2017; Pawłowicz et al. 2017). In Arabidopsis PIP2;5 was up-regulated, while most of the PIP genes were downregulated by cold stress. Rizhsky et al. (2004) showed that all AtPIP genes were down-regulated in drought stress response in leaves except AtPIP1;4 and AtPIP2;5, which were up-regulated, whereas AtPIP2;6 was constitutively expressed and was not significantly affected by the drought stress (Alexandersson et al. 2005). Expression of AtPIP2;5 was also significantly up-regulated in leaves in response to a combination of drought and heat stresses (Rizhsky et al. 2004). PIP genes were not highly modulated by salt stress, while, differential regulation of PIP expression were observed by drought stress (Jang et al. 2004). Microarray analysis revealed that Arabidopsis PIP1;1 and PIP1;2 transcripts were decreased with salinity stress (Boursiac et al. 2005). Maize PIP members, ZmPIP1 and ZmPIP2, showed down regulation under salt stress, while ZmPIP1;1, ZmPIP1;5, and ZmPIP2;4 showed transient upregulation pattern (Zhu et al. 2005). Transcript analysis of barley root tissues in salt stress underscored the involvement of PIPs with increased mRNA accumulation of HvPIP1;2, HvPIP1;3 and HvPIP2;2, while decreased transcript accumulation of HvPIP1;2, HvPIP1;3, HvPIP1;4, HvPIP2;1, HvPIP2;2 and HvPIP2;3 (Katsuhara et al. 2003). Transcriptional regulation of PIPs under salt stress depends on species, stress conditions, and plant organs studied (Zhao et al. 2015). Downregulation of some PIP isoforms plays a major role in limiting initial water loss during the early stages of salt stress and subsequently upregulation of other PIP isoforms could enhance uptake of water and maintain better cellular water homeo-stasis in plants under increased salt stress. Various molecular and cellular mechanisms are involved in regulation of PIPs under salt stress.
The exogenous application of plant growth regulator (5-aminolevulinic acid) was shown to control the expression of LePIP1 and LePIP2 in tomato seedlings under salinity stress, hence regulated water homeostasis and enhanced salt tolerance (Zhao et al. 2015). The transcript analysis of B. rapa BrPIP in response to cold, drought, salt, water logging, and ABA showed higher transcript abundance of all BrPIPs compared to other MIPs (Kayum et al. 2017). Immunoblot analysis of radish (Raphnus sativus) PIPs demonstrated that the RsPIP2-1 protein level was increased by salt stress, while expression was decreased under drought stress (Suga et al. 2002). Cucumber (Cucumis sativus) PIPs (CsPIP1;2 and CsPIP2;4) expression were highly downregulated under osmotic and salt stress exposure (Qian et al. 2015). Similarly, expression analyses of citrus mRNA under abiotic stress revealed that leaf CsPIP2;4 was highly upregulated in response to drought stress, while root CsPIP1;1 was highly upregulated under salt stress conditions (Martins et al. 2015). In grapevine, salt stress increased PIP2;1 transcript, whereas PIP2;1 transcript was downregulated with drought stress (Cramer et al. 2007). Sugarbeat (Beta vulgaris) BvPIP genes showed upregulation pattern in response to heat stress and sligh downregulation pattern were observed under salt stress (Kong et al. 2017). The differential regulation of PIP genes in shoots and roots of various plants in abiotic stress indicate that PIP genes are modulated for maintenance of water homeostasis under stress conditions.
Further, attempts were made to explain the role of PIPs in response to abiotic stress through the analysis of overexpression and loss-of-function mutant plants. In barley, the water channel activity of HvPIP1;3, HvPIP1;6 and HvPIP2;1 has been demonstrated by heterologous expression in X. laevis oocytes (Katsuhara and Shibasaka 2007; Wei et al. 2007). Overexpression of various PIPs such as wheat (Triticum aestivum) PIP2 subgroup gene (TaAQP7 and TaAQP8) resulted in improved tolerance to drought, salt and cold stress (Hu et al. 2012; Zhou et al. 2012;). Durum wheat (Triticum durum) PIPs, TdPIP1;1 or TdPIP2;1, genes showed regulation over the time under drought and salinity conditions, and it was mostly downregulated. Transgenic tobacco plants overexpressing these two genes acquired tolerant phenotypes to salt and drought compared to the WT plants in terms of root length and leaf size (Ayadi et al. 2011).
Overexpression of ZmPIP1;1 with constitutive promoter in Arabidopsis exhibited increased drought and salt stress tolerance (Zhou et al. 2018). Hickory (Carya cathayensis) CcPIP1;2, when heterogously expressed in Xenopus and Arabidopsis showed better drought stress tolerance (Kumar et al. 2018). Overexpression of MfPIP2-7 (Medicago falcate) in tobacco resulted in increased freezing and chilling stress tolerance (Zhou et al. 2016). Overexpression of banana MaPIP1;1 in Arabidopsis also conferred tolerance to drought and salt stress (Xu et al. 2014). Overexpression of MaPIP1;2 in banana exhibited enhanced tolerance to drought, cold and salt stress (Sreedharan et al. 2013), while overexpression of MaPIP2;6 enhanced tolerance to salt stress (Sreedharan et al. 2015). Purple feathergrass (Stipa purpurea) PIP1 gene when overexpressed in Arabidopsis under 35S promoter showed enhanced tolerance to drought stress (Chen et al. 2018). Soybean (Glycine max) GmPIP2;9 showed increased water transport capacity. Moreover, under induced drought condition using polyethylene glycol (PEG), the expression level of the GmPIP2;9 has been increased. Seedling overexpressing the GmPIP2;9 showed recovering ability after water withholding while the WT plants failed to recover (Lu et al. 2018).
Expression of potato (Solanum tuberosum) StPIP1 under a constitutive promoter showed improved tolerance to drought stress (Wang et al. 2017). Constitutive expression of tomato SlPIP2;1, SlPIP2;7 and SlPIP2;5 in Arabidopsis improved water content and provided drought stress tolerance (Li et al. 2016). Transgenic rice overexpressing OsPIP1 showed an enhanced tolerance to chilling stress (Matsumoto et al. 2008). Further, knockout study of AtPIP1;2 and AtPIP2;2, showed more sensitive phenotype under drought stress (Javot et al. 2003). The role of various PIPs, which has been shown to alleviate abiotic stress tolerance, is summarized in Table 2.
On the other hand, overexpression of AtPIP1;4 and AtPIP2;5 in Arabidopsis and tobacco showed more sensitive phenotype in dehydration stress (Jang et al. 2007). Overexpression studies of Arabidopsis PIP1b in tobacco showed improvement of plant vigor, but no beneficial response were observed in drought and salt stress conditions (Aharon et al. 2003). The mechanism for salt stress tolerance by PIPs was explored in Arabidopsis. Salt stress downregulated the mRNA levels of PIPs, resulting in decreased root water permeability. PIP2;1 is highly expressed in the root and salt stress disturb the transportation of PIP2;1 from endoplasmic reticulum to the plasma membrane and reposit PIP2;1 in intracellular compartments. Under normal conditions, PIP2;1 mainly localizes to the plasma membrane, and recycles between the plasma membrane and trans-Golgi network (Ueda et al. 2016). Similarly, Pou et al. (2016) reported that salt stress transcriptionally repress PIP2;7 internalization in Arabidopsis plants and negatively regulate plant hydraulics.
Regulation of PIPs activity
Different molecular mechanisms have been investigated for their involvement in regulating aquaporin functioning under abiotic stress, including post translational modifications, heteromerization, and membrane trafficking. Post-translational modifications (PTM) of aquaporin proteins play a critical role for regulating protein structure and cellular functions. Major PTM identified in plants include phosphorylation, acetylation, methylation, carbonylation, deamination, glycosylation, oxidation, glutathionylation, ubiquitination, and sumoylation (Friso and van Wijk 2015). Although plant aquaporins have been reported to carry these mentioned PTM, phosphorylation was the most studied PTM for its role in PIP trafficking and gaiting compared with other PTM (Maurel et al. 2015). For example, it has been reported that trafficking of AtPIP2;1 was regulated under NaCl treatment by a specific phosphorylated site in its C-terminus (Prak et al. 2008). A proteomic and phosphoproteomic analyses of rice shoot and root revealed that different PIPs were phosphorylated in shoot but not in root (Whiteman et al. 2008). Heteromerization of plant PIPs (PIP1 and PIP2 group) has been proposed as a regulatory mechanism for their transport activity (Jozefkowicz et al. 2017). For instance, co-expression of ZmPIP1;1 (a nonfunctional water transporter) and ZmPIP2;5 (a functional water transporter) did not result in a higher increase in osmotic water permeability coefficient (Pf) compared to ZmPIP2;5 alone. However, co-expression of the ZmPIP1;1 and ZmPIP1;2 (a nonfunctional water transporter) isoforms exhibited a Pf increase, indicating that PIP1 isoform heteromerization is required for both of them to act as functional water transporters (Fetter et al. 2004). Furthermore, PIP1 (FaPIP1;1) showed increased water transport activity when co-expressed with PIP2 (FaPIP2;1), demonstrating that this PIP1–PIP2 interaction resulted in the formation of heterotetramers, which enhanced the water permeability (Yaneff et al. 2014). A central mechanism for regulating membrane water permeability in response to abiotic stress is through membrane trafficking. For example, salt stress decreased the intracellular localization of PIP2;1 in Arabidopsis thaliana root at the plasma membrane and increased in the vacuolar lumen (Ueda et al. 2016).
Role of aquaporins in metalloids transport and tolerance in plants
Metalloids are the elements with physical and chemical characteristics that are intermediate between metals and non-metals. Generally boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At) are considered metalloids. The metalloids Ge, Te, Po and At are normally present in the environment at very low levels, trace or ultratrace levels, and are not considered of relevance in terms of environmental and biological health (Lombi and Holm 2010). Metalloids in the biological system differ in their role from essential B to beneficial Si and highly toxic As and Sb. Considerable efforts have focused in the recent years on the identification and characterization of metalloids channel proteins that facilitate metalloids uptake from soil to plant roots and its transportation through the plant tissues to be translocated into plant shoots and grains/seeds. Aquaporins have gained more attention in the last decade as major players on metalloids transport in plants (Mosa et al. 2016b). Meharg and Jardine (2003) reported that AsIII competed with glycerol on its uptake in rice roots, suggesting that AsIII might be also transported through aquaporin channel proteins. Eventually, few important studies have been published in 2008 demonstrating the ability of different members of NIPs on AsIII transport and uptake in plants. Bienert et al. (2008) reported the first evidence that different members of plant NIPs namely: Oryza sativa NIP2;1 and NIP3;2, A. thaliana NIP5;1 and NIP6;1, and Lotus japonicus NIP5;1 and NIP6;1 exhibited the bidirectional transport of AsIII when expressed in yeast strain lacking the AsIII transport system. Ma et al. (2008) demonstrated the first in planta proof confirming the vital role of OsNIP2;1 (silicon transporter Lsi1) in AsIII transport. Lsi1 mutant significantly decreased the concentration of As in shoots and roots when compared to wild type rice plants. Subsequently, many more studies were published showing the Arabidopsis and rice NIP members involvement in AsIII transport and translocations (Isayenkov and Maathuis 2008; Lindsay and Maathuis 2016; Kamiya and Fujiwara 2009; Katsuhara et al. 2014).
So far, the majority of the identified AsIII transporters in rice belong to the NIP subfamily of aquaporins. Many of these have primary roles in the transport of important metalloid nutrients such as Si (Ma & Yamaji 2015). In contrast, the role of isoforms from other aquaporin subfamilies remains to be revealed. Thus, engineering food crops and particularly rice to minimize As accumulation by loss of function of NIPs is also likely to reduce the level of Si required for rice growth, affecting mechanical strength, and disease tolerance. Indeed, the silicon uptake-deficient mutant lsi1 was more susceptible to blast disease caused by Magnaporthe grisea (Nakata et al. 2008).
Metalloids transport through PIP members of aquaporins
Apart from the NIP subfamily members, PIP members have been evident to provide plants a sensitivity/tolerance to metalloids through mediating the uptake/influx and efflux routes of metalloids through plants. Because of their unique structures, PIPs act as a pump that entangled with metalloids transport. This channel-like structure can be adopted in different manner through different plants under different environmental conditions. Such important role of PIPs for the plant to survive under severe B deficiency allow B uptake to help plants to withstand B limitation. Many PIPs members have been demonstrated as B transporters. B transport in barely (Hordeum vulgare) through HvPIP1;3 and HvPIP1;4 has been confirmed through yeast complementation (Fitzpatrick and Reid 2009). ZmPIP, HvPIP1;3, HvPIP1;4 are boron influx and overexpression of these genes in yeast shows a channel activity for B (Zangi and Filella 2012).
Plant has developed many strategies to cope with metalloids toxicity, such as metalloid exclusion, pumping out by efflux channel proteins, also through downregulation of the specialized transporters, and binding the metals with thiol compounds to be sequestered into the vacuoles. Plant PIPs play a prominent role in metalloids tolerance. In rice, plant can handle the metalloid stress through PIPs as a bidirectional pump, to avoid the accumulation of metalloids inside the plant cells. Majority of the identified AsIII transporters are belonging to NIP subfamily of aquaporins, but recent studies showed the involvement of PIP members in metalloids tolerance in rice and Arabidopsis. Recently, it was reported that members of rice PIPs are also involved in AsIII and B uptake and transport pathway (Mosa et al. 2012, Kumar et al. 2014; Mosa et al. 2016a). OsPIP2;4, OsPIP2;6, and OPIP2;7 increased AsIII uptake when expressed heterologously in Xenopus oocytes. Moreover, their transcript levels were strongly down-regulated under AsIII exposure. Transgenic Arabidopsis plants overexpressing OsPIP2;4, OsPIP2;6, and OsPIP2;7 showed tolerant phenotype to AsIII compared with wild type plants. Interestingly, roots of these transgenic Arabidopsis plants showed active influx and efflux of AsIII, supporting that these PIPs are bidirectional AsIII transporters (Mosa et al. 2012). Our recent studies also showed that OsPIP2;4, OsPIP2;6, OPIP2;7, and OsPIP1;3 are permeable to the metalloid boron (B) and having influx and efflux activity (Kumar et al. 2014; Mosa et al. 2016a). Heterlogous expression of five OsPIPs- OsPIP1;2, OsPIP1;3, OsPIP2;4, OsPIP2;7 and OsPIP2;8 in HD9 yeast mutant strain (Δfps1Δacr3Δycf1) showed higher levels of B transport activity and B accumulation which caused B sensitivity in yeast cells (Mosa et al. 2016a; Dhankher unpublished data). Transgenic Arabidopsis lines overexpressing OsPIP1;3 and OsPIP2;6 exhibited enhanced tolerance to B toxicity (Mosa et al. 2016a). Characterization of the remaining members of rice PIPs will be helpful to identify their exact in planta roles and their manipulation in developing crops with controlled metalloids transport including limiting toxic arsenic accumulation.
Molecular insights into PIPs role for enhancing crop productivity and quality
Members of the aqauporins family including the PIP members are not fully characterized and interests in the plant community is growing with regard to the role of AQPs and their importance in improving multiple abiotic stresses tolerance in crops. As evident from several studies described in this review, the members of PIP subfamily are differentially regulated in response to various stresses and metalloids exposure. Further, the overexpression of some of the PIP members have improved tolerance to various abiotic stresses as well as metalloids tolerance in several plant species. Further molecular insights using modern genomics, transcriptomic, proteomics and metabolomics approaches will be highly useful to fully characterize the members of PIP subfamily and other aquaporins for their role in multiple abiotic stress tolerance and developing climate resilient crops.
Also, the manipulation of the expression of aquaporin members using genome editing approaches including CRISPR-Cas9 and RNAi approaches will be ideal to prevent the transport for toxic metalloids arsenic from soil to roots and subsequent transport to above ground tissues. For example, expression of NIPs and PIPs known to transport arsenic can be optimized and develop arsenic free rice as safer food for human and animal consumption. Additionally, the modulation of the aromatic/arginine filter and pore channel of aquaporins to loss or gain of transport of particular metalloids in the active transport loop will be highly desirable.
Conclusion and future perspective
The role of PIPs in plant responses to environmental stresses mainly cold, drought and salt stress is well documented in past and recent years, but the role of each PIP isoforms and their cross talks is still unrevealed. The mechanism of regulating PIP function including post-translational modification such as phophosphorylation, methylation and acetylation is not completely understood. Further research on the mechanism of PIPs regulation on providing plant abiotic stress tolerance by functional genomics will improve better understanding and help to develop abiotic stress tolerant crop plants. Additionally, investigations are needed to sufficiently explain the underlying mechanisms of PIPs role in abiotic stress tolerance in plants. Detailed investigations on cross talk regulation among various PIPs and other MIP members, plant growth regulators, signaling molecules and their role in abiotic stress are required. Omics approach such as transcriptomics, proteomics and metabolomics of abiotic stress tolerant plants in relation to crop plants can be exploited to unravel various mechanisms of PIPs and their relationship with environmental stress tolerance. Knowing the exact mechanism adopted by plants to alleviate the negative effect of metalloids would have a great impact in both health and food security. The underlying mechanisms through regulating the expression of PIPs members will help to decrease the invade of metalloids, especially As to be in the food chain. Moreover, getting deep knowledge about aquaporins, particularly PIPs will help us to enhance the plants and crops ability to grow in the contaminated agriculture lands, which will increase the overall crop production, helping in the food security. As we believe that the PIPs are potential metalloids transporters in the main economic crops, therefore, targeting them in designated research and breeding programs will be beneficial to control the fate of these toxic metalloids in plants and environment. In conclusion, genetic engineering of crop plants using PIPs with improved tolerance to abiotic stress will provide a framework for sustainable agriculture and safe healthy food sufficient for the ever-growing human population.
References
Aharon, R., Shahak, Y., Wininger, S., Bendov, R., Kapulink, Y., & Galili, G. (2003). Overexpression of plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought and salt stress. The Plant Cell, 15(2), 439–447.
Alexandersson, E., Fraysse, L., Sjovall-Larsen, S., Gustavsson, S., Fellert, M., Karlsson, M., et al. (2005). Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology, 59(3), 469–484.
Anderberg, H. I., Kjellbom, P., & Johanson, U. (2012). Annotation of Selaginella moellendorffii major intrinsic proteins and the evolution of the protein family in terrestrial plants. Frontiers in Plant Science, 3, 33.
Ayadi, M., Cavez, D., Miled, N., Chaumont, F., & Masmoudi, K. (2011). Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. subsp. durum) and their role in abiotic stress tolerance. Plant Physiology and Biochemistry, 49(9), 1029–1039.
Bienert, M. D., Diehn, T. A., Richet, N., Chaumont, F., & Bienert, G. P. (2018). Heterotetramerization of plant PIP1 and PIP2 aquaporins is an evolutionary ancient feature to guide PIP1 plasma membrane localization and function. Frontiers in Plant Science, 9, 382.
Bienert, G. P., Thorsen, M., Schüssler, M. D., Nilsson, H. R., Wagner, A., Tamás, M. J., et al. (2008). A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biology, 6(1), 26.
Boursiac, Y., Chen, S., Luu, D. T., Sorieul, M., van den Dries, N., & Maurel, C. (2005). Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiology, 139(2), 790–805.
Byrt, C. S., Zhao, M., Kourghi, M., Bose, J., Henderson, S. W., Qiu, J., et al. (2017). Non-selective cation channel activity of aquaporin AtPIP2; 1 regulated by Ca2 + and pH. Plant, Cell and Environment, 40(6), 802–815.
Chaumont, F., Barrieu, F., Jung, R., & Chrispeels, M. J. (2000). Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiology, 122(4), 1025–1034.
Chaumont, F., Barrieu, F., Wojcik, E., Chrispeels, M. J., & Jung, R. (2001). Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiology, 125(3), 1206–1215.
Chaumont, F., & Tyerman, S. F. (2014). Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiology, 164(4), 1600–1618.
Chen, Q., Yang, S., Kong, X., Wang, C., Xiang, N., Yang, Y., et al. (2018). Molecular cloning of plasma membrane aquaporin in Stipa purpurea, and exploration of its role in drought stress tolerance. Gene, 665, 41–48.
Cramer, G. R., Ergül, A., Grimplet, J., Tillett, R. L., Tattersall, E. A., Bohlman, M. C., et al. (2007). Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Functional & Integrative Genomics, 7(2), 111–134.
Danielson, J. Å., & Johanson, U. (2008). Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biology, 8(1), 45.
Deshmukh, R. K., Sonah, H., & Bélanger, R. R. (2016). Plant Aquaporins: Genome-wide identification, transcriptomics, proteomics, and advanced analytical tools. Frontiers in Plant Science, 7, 1896.
Deshmukh, R. K., Vivancos, J., Ramakrishnan, G., Guérin, V., Carpentier, G., Sonah, H., et al. (2015). A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. The Plant Journal, 83(3), 489–500.
Fetter, K., Van Wilder, V., Moshelion, M., & Chaumont, F. (2004). Interactions between plasma membrane aquaporins modulate their water channel activity. The Plant Cell, 16(1), 215–228.
Fitzpatrick, K. L., & Reid, R. J. (2009). The involvement of aquaglyceroporins in transport of boron in barley roots. Plant, Cell and Environment, 32(10), 1357–1365.
Forrest, K. L., & Bhave, M. (2007). Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Functional & Integrative Genomics, 7(4), 263.
Friso, G., & van Wijk, K. J. (2015). Posttranslational protein modifications in plant metabolism. Plant Physiology, 169(3), 1469–1487.
Gupta, A. B., & Sankararamakrishnan, R. (2009). Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biology, 9(1), 134.
Hove, R. M., & Bhave, M. (2011). Plant aquaporins with non-aqua functions: Deciphering the signature sequences. Plant Molecular Biology, 75(4–5), 413–430.
Hu, W., Yuan, Q., Wang, Y., Cai, R., Deng, X., Wang, J., et al. (2012). Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant and Cell Physiology, 53(12), 2127–2141.
Isayenkov, S. V., & Maathuis, F. J. (2008). The Arabidopsis thaliana aquaglyceroporin AtNIP7; 1 is a pathway for arsenite uptake. FEBS Letters, 582(11), 1625–1628.
Jang, J. Y., Kim, D. G., Kim, Y. O., Kim, J. S., & Kang, H. (2004). An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology, 54(5), 713–725.
Jang, J. Y., Lee, S. H., Rhee, J. Y., Chung, G. C., Ahn, S. J., & Kang, H. (2007). Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Molecular Biology, 64(6), 621–632.
Javot, H., Lauvergeat, V., Santoni, V., Martin-Laurent, F., Güçlü, J., Vinh, J., et al. (2003). Role of a single aquaporin isoform in root water uptake. The Plant Cell, 15(2), 509–522.
Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjövall, S., Fraysse, L., et al. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiology, 126(4), 1358–1369.
Johansson, I., Karlsson, M., Johanson, U., Larsson, C., & Kjellbom, P. (2000). The role of aquaporins in cellular and whole plant water balance. Biochimica et Biophysica Acta, 1465(1–2), 324–342.
Jozefkowicz, C., Berny, M. C., Chaumont, F., & Alleva, K. (2017). Heteromerization of plant aquaporins. In Plant Aquaporins (pp. 29–46). Cham: Springer.
Kaldenhoff, R., Grote, K., Zhu, J. J., & Zimmermann, U. (1998). Significance of plasmalemma aquaporins for water- transport in Arabidopsis thaliana. The Plant Journal, 14(1), 121–128.
Kamiya, T., & Fujiwara, T. (2009). Arabidopsis NIP1; 1 transports antimonite and determines antimonite sensitivity. Plant and Cell Physiology, 50(11), 1977–1981.
Katsuhara, M., Koshio, K., Shibasaka, M., Hayashi, Y., Hayakawa, T., & Kasamo, K. (2003). Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant and Cell Physiology, 44(12), 1378–1383.
Katsuhara, M., Sasano, S., Horie, T., Matsumoto, T., Rhee, J., & Shibasaka, M. (2014). Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnology, 31(3), 213–219.
Katsuhara, M., & Shibasaka, M. (2007). Barley root hydraulic conductivity and aquaporins expression in relation to salt tolerance. Soil Science and Plant Nutrition, 53(4), 466–470.
Kayum, M. A., Park, J. I., Nath, U. K., Biswas, M. K., Kim, H. T., & Nou, I. S. (2017). Genome-wide expression profiling of aquaporin genes confer responses to abiotic and biotic stresses in Brassica rapa. BMC Plant Biology, 17(1), 23.
Kelly, G., Sade, N., Attia, Z., Secchi, F., Zwieniecki, M., Holbrook, N. M., et al. (2014). Relationship between hexokinase and the aquaporin PIP1 in the regulation of photosynthesis and plant growth. PLoS ONE, 9(2), e87888.
Kong, W., Yang, S., Wang, Y., Bendahmane, M., & Fu, X. (2017). Genome-wide identification and characterization of aquaporin gene family in Beta vulgaris. PeerJ, 5, e3747.
Kumar, R. S., Ji, G., Guo, H., Zhao, L., & Zheng, B. (2018). Over-expression of a grafting-responsive gene from hickory increases abiotic stress tolerance in Arabidopsis. The Plant Cell Reports, 37(3), 541–552.
Kumar, K., Mosa, K. A., Chhikara, S., Musante, C., White, J. C., & Dhankher, O. P. (2014). Two rice plasma membrane intrinsic proteins, OsPIP2; 4 and OsPIP2; 7, are involved in transport and providing tolerance to boron toxicity. Planta, 239(1), 187–198.
Li, R., Wang, J., Li, S., Zhang, L., Qi, C., Weeda, S., et al. (2016). Plasma membrane intrinsic proteins SlPIP2; 1, SlPIP2; 7 and SlPIP2; 5 conferring enhanced drought stress tolerance in tomato. Scientific Reports, 6, 31814.
Lindsay, E. R., & Maathuis, F. J. (2016). Arabidopsis thaliana NIP 7; 1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Letters, 590(6), 779–786.
Liu, Z., Shen, J., Carbrey, J. M., Mukhopadhyay, R., Agre, P., & Rosen, B. (2002). Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proceedings of the National Academy of Sciences, 99(9), 6053–6058.
Lombi, E., & Holm, P. E. (2010). Metalloids, soil chemistry and the environment. In MIPs and Their Role in the Exchange of Metalloids (pp. 33–44). New York: Springer.
Lu, L., Dong, C., Liu, R., Zhou, B., Wang, C., & Shou, H. (2018). Roles of soybean plasma membrane intrinsic protein GmPIP2; 9 in drought tolerance and seed development. Frontiers in Plant Science, 9, 530.
Ma, J. F., & Yamaji, N. (2015). A cooperative system of silicon transport in plants. Trends in Plant Science, 20(7), 435–442.
Ma, J. F., Yamaji, N., Mitani, N., Xu, X. Y., Su, Y. H., McGrath, S. P., et al. (2008). Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proceedings of the National Academy of Sciences, 105(29), 9931–9935.
Martins, C. D. P. S., Pedrosa, A. M., Du, D., Gonçalves, L. P., Yu, Q., Gmitter, F. G., Jr., et al. (2015). Genome-wide characterization and expression analysis of major intrinsic proteins during abiotic and biotic stresses in sweet orange (Citrus sinensis L. Osb.). PLoS one, 10(9), e0138786.
Matsumoto, T., Lian, H. L., Su, W. A., Tanaka, D., Liu, C. W., Iwasaki, I., et al. (2008). Role of the aquaporin PIP1 subfamily in the chilling tolerance of rice. Plant and Cell Physiology, 50(2), 216–229.
Maurel, C., Boursiac, Y., Luu, D. T., Santoni, V., Shahzad, Z., & Verdoucq, L. (2015). Aquaporins in plants. Physiological Reviews, 95(4), 1321–1358.
Meharg, A. A., & Jardine, L. (2003). Arsenite transport into paddy rice (Oryza sativa) roots. New Phytologist, 157(1), 39–44.
Mosa, K. A., Kumar, K., Chhikara, S., McDermott, J., Liu, Z., Musante, C., et al. (2012). Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Research, 21, 1265–1277.
Mosa, K. A., Kumar, K., Chhikara, S., Musante, C., White, J. C., & Dhankher, O. P. (2016a). Enhanced boron tolerance in plants mediated by bidirectional transport through plasma membrane intrinsic proteins. Scientific Reports, 6, 21640.
Mosa, K. A., Saadoun, I., Kumar, K., Helmy, M., & Dhankher, O. P. (2016b). Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Frontiers in Plant Science, 7, 303.
Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, J. B., et al. (2000). Structural determinants of water permeation through aquaporin-1. Nature, 407(6804), 599.
Nakata, Y., Ueno, M., Kihara, J., Ichii, M., Taketa, S., & Arase, S. (2008). Rice blast disease and susceptibility to pests in a silicon uptake-deficient mutant lsi1 of rice. Crop Protection, 27(3–5), 865–868.
Nouri, M. Z., & Komatsu, S. (2013). Subcellular protein overexpression to develop abiotic stress tolerant plants. Frontiers in Plant Science, 4, 2.
Pawłowicz, I., Rapacz, M., Perlikowski, D., Gondek, K., & Kosmala, A. (2017). Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species. Journal of Applied Genetics, 58(4), 421–435.
Pommerrenig, B., Diehn, T. A., & Bienert, G. P. (2015). Metalloido-porins: Essentiality of nodulin 26-like intrinsic proteins in metalloid transport. Plant Science, 238(2015), 212–227.
Pou, A., Jeanguenin, L., Milhiet, T., Batoko, H., Chaumont, F., & Hachez, C. (2016). Salinity-mediated transcriptional and post-translational regulation of the Arabidopsis aquaporin PIP2;7. Plant Molecular Biology, 92(6), 731–744.
Prak, S., Hem, S., Boudet, J., Viennois, G., Sommerer, N., Rossignol, M., et al. (2008). Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins role in subcellular trafficking of AtPIP2; 1 in response to salt stress. Molecular and Cellular Proteomics, 7(6), 1019–1030.
Qian, Z. J., Song, J. J., Chaumont, F., & Ye, Q. (2015). Differential responses of plasma membrane aquaporins in mediating water transport of cucumber seedlings under osmotic and salt stresses. Plant, Cell and Environment, 38(3), 461–473.
Reddy, P. S., Rao, T. S. R. B., Sharma, K. K., & Vadez, V. (2015). Genome-wide identification and characterization of the aquaporin gene family in Sorghum bicolor (L.). Plant Gene, 1, 18–28.
Rizhsky, L., Liang, H., Shuman, J., Shulaev, V., Davletova, S., & Mittler, R. (2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology, 134(4), 1683–1696.
Saddhe, A. A., Shweta, S., Mosa, K. A., Kumar, K., Prasad, M., & Dhankher, O. P. (2018). Genome-wide characterization of major intrinsic protein (MIP) gene family in Brachypodium distachyon. Current Bioinformatics, 13(5), 536–552.
Secchi, F., Pagliarani, C., & Zwieniecki, M. A. (2017). The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant, Cell and Environment, 40(6), 858–871.
Secchi, F., & Zwieniecki, M. A. (2010). Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant, Cell and Environment, 33(8), 1285–1297.
Sreedharan, S., Shekhawat, U. K., & Ganapathi, T. R. (2013). Transgenic banana plants overexpressing a native plasma membrane aquaporin M usa PIP 1; 2 display high tolerance levels to different abiotic stresses. Plant Biotechnology Journal, 11(8), 942–952.
Sreedharan, S., Shekhawat, U. K. S., & Ganapathi, T. R. (2015). Constitutive and stress-inducible overexpression of a native aquaporin gene (MusaPIP2; 6) in transgenic banana plants signals its pivotal role in salt tolerance. Plant Molecular Biology, 88(1–2), 41–52.
Srivastava, A. K., Penna, S., Nguyen, D. V., & Tran, L. S. P. (2016). Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Critical Reviews in Biotechnology, 36(3), 389–398.
Suga, S., Komatsu, S., & Maeshima, M. (2002). Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant and Cell Physiology, 43(10), 1229–1237.
Tao, P., Zhong, X., Li, B., Wang, W., Yue, Z., Lei, J., et al. (2014). Genome-wide identification and characterization of aquaporin genes (AQPs) in Chinese cabbage (Brassica rapa ssp. pekinensis). Molecular Genetics and Genomics, 289(6), 1131–1145.
Ueda, M., Tsutsumi, N., & Fujimoto, M. (2016). Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochemical and Biophysical Research Communications, 474(4), 742–746.
Wang, L., Liu, Y., Feng, S., Yang, J., Li, D., & Zhang, J. (2017). Roles of plasmalemma aquaporin gene StPIP1 in enhancing drought tolerance in potato. Frontiers in Plant Science, 8, 616.
Wei, W., Alexandersson, E., Golldack, D., Miller, A. J., Kjellbom, P. O., & Fricke, W. (2007). HvPIP1; 6, a barley (Hordeum vulgare L.) plasma membrane water channel particularly expressed in growing compared with non-growing leaf tissues. Plant and Cell Physiology, 48(8), 1132–1147.
Whiteman, S. A., Nühse, T. S., Ashford, D. A., Sanders, D., & Maathuis, F. J. (2008). A proteomic and phosphoproteomic analysis of Oryza sativa plasma membrane and vacuolar membrane. The Plant Journal, 56(1), 146–156.
Xu, Y., Hu, W., Liu, J., Zhang, J., Jia, C., Miao, H., et al. (2014). A banana aquaporin gene, MaPIP1; 1, is involved in tolerance to drought and salt stresses. BMC Plant Biology, 14(1), 59.
Yaneff, A., Sigaut, L., Marquez, M., Alleva, K., Pietrasanta, L. I., & Amodeo, G. (2014). Heteromerization of PIP aquaporins affects their intrinsic permeability. Proceedings of the National Academy of Sciences, 111(1), 231–236.
Zangi, R., & Filella, M. (2012). Transport routes of metalloids into and out of the cell: A review of the current knowledge. Chemico-Biology Interaction, 197(1), 47–57.
Zardoya, R. (2005). Phylogeny and evolution of the major intrinsic protein family. Biology of the Cell, 97(6), 397–414.
Zhang, D. Y., Ali, Z., Wang, C. B., Xu, L., Yi, J. X., Xu, Z. L., et al. (2013). Genome-wide sequence characterization and expression analysis of major intrinsic proteins in soybean (Glycine max L.). PLoS ONE, 8(2), e56312.
Zhao, Y. Y., Yan, F., Hu, L. P., Zhou, X. T., Zou, Z. R., & Cui, L. R. (2015). Effects of exogenous 5-aminolevulinic acid on photosynthesis, stomatal conductance, transpiration rate, and PIP gene expression of tomato seedlings subject to salinity stress. Genetics and Molecular Research, 14(2), 6401–6412.
Zhou, S., Hu, W., Deng, X., Ma, Z., Chen, L., Huang, C., et al. (2012). Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS ONE, 7(12), e52439.
Zhou, C., Wang, T., Guo, Z., & Lu, S. (2016). Overexpression of MfPIP2-7 from Medicago falcata promotes cold tolerance and growth under NO3− deficiency in transgenic tobacco plants. BMC Plant Biology, 16(1), 138.
Zhou, L., Wang, C., Liu, R., Han, Q., Vandeleur, R. K., Du, J., et al. (2014). Constitutive overexpression of soybean plasma membrane intrinsic protein GmPIP1; 6 confers salt tolerance. BMC Plant Biology, 14(1), 181.
Zhou, L., Zhou, J., Xiong, Y., Liu, C., Wang, J., Wang, G., et al. (2018). Overexpression of a maize plasma membrane intrinsic protein ZmPIP1; 1 confers drought and salt tolerance in Arabidopsis. PLoS ONE, 13(6), e0198639.
Zhu, C., Schraut, D., Hartung, W., & Schaffner, A. R. (2005). Differential responses of maize MIP genes to salt stress and ABA. Journal of Experimental Botany, 56(421), 2971–2981.
Acknowledgements
OPD acknowledge the funding support from the USDA NIFA (#2017-67013-26165) and funding support from the grant #S16000000000036 from the Ministry of Higher Education and Scientific Research in Egypt through the Egyptian Cultural and Educational Bureau, Washington, DC to OPD and AGM (GM # 1054). KK acknowledge the financial assistance from Board of Research in Nuclear Sciences (37(1)/14/28/2016-BRNS), India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, K., Mosa, K.A., Meselhy, A.G. et al. Molecular insights into the plasma membrane intrinsic proteins roles for abiotic stress and metalloids tolerance and transport in plants. Ind J Plant Physiol. 23, 721–730 (2018). https://doi.org/10.1007/s40502-018-0425-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0425-1