Abstract
Nanotechnology has been acknowledged recently for its diversified use in the field of science including agriculture, food industry, medicine, and cosmetics. Environment and earth are being constantly exposed to nanomaterials because they are fabricated to be utilized in agribusiness, food, pharmaceuticals, personal care items as well as in biotechnology. Nanoparticle–microbe interaction performs a pivotal role in treatment of various diseases as in case of antimicrobial agents. The potential implementations of nanomaterials are being extensively researched in the field of agriculture, not only as therapeutic options to prevent phytopathogen growth in host plants, but also for early pathogenic symptoms detections and eliciting immune responses. Bacteria, fungi, virus, and other virulent pathogens through their efficient survival strategies and overcoming phyto-defenses confer to overall deterioration of food-crop produce that may sum up to 10–40%. To overcome such challenging situations, there has been constant development and application of engineered agro-nanomaterials. These may affect plant–microbe interactions in different ways. The inhibitory potential of nanoparticles against different microbial growth mainly involves release and interaction of metal ions with cell components that occur through different pathways including reactive oxygen species production, formation of pores in cell-membranes, cell wall and DNA damage, and cell-cycle arrest. The article deals with different plant pathogens, their mechanisms of phyto-pathogenesis followed by detailed responses of nanoparticle interactions with different microbes and their role in phytopathogen suppression.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phyto-diseases may evoke extensive deterioration in the plant community. It is crucial that phyto-diseases are rapidly identified and treated rationally. As per the current projections, the production demand for food globally needs to be doubled by 2050 (Tilman et al. 2011). With the anticipation that the changes in climate may contribute to disrupt the cycle of food production, such concerning prediction becomes more distressful. The plant pathogens are often greatly responsible for the annual loss of economically important plants and crops. In order to overcome this dire situation, nanotechnology extends its opportunities as a new edge weapon to improve and maintain plant health. With its diversified applications, the field of nanotechnology, specially nano-agriculture holds promise to provide us new avenues and streamline the utility of nanomaterials in crop production as well as protection of plants. Although the use of nanotechnology for phyto-disease management or diagnosis is at infancy, still it has tremendous potential in improving already existing as well as future crop production via plant protection techniques that will resist pests and diseases, help in phytopathogen monitoring, and plant diseases detection. However, there is still a lack of awareness and appropriate knowledge on how to bridge nanotechnology with agriculture and plant physiology and utilize it directly or indirectly in plant disease management. Thus, in this chapter we will discuss in detail the plant pathology and intertwined mechanisms along with the microbial organisms associated with it. Successively, new advancements and achievements acquired by utilizing nanotechnology in the field of phytopathology and agriculture will also be discussed for readers to gain insights into the role of nanomaterials in plant pathology.
2 Phytopathogens
A plant or phyto-disease can be broadly defined as any circumstance that evokes cascade of responses in plant cells and hinders the plant to perform its activity in highest potential. These diseases can be both biotic or abiotic in nature and the science of deciphering various phyto-diseases along with their causes is known as plant pathology. Plant pathology is closely related to bacteriology, virology, mycology, entomology, and weed science owing to the derogatory consequences of bacteria, virus, fungus, insects, and weeds, respectively, upon plants. Classification of plant diseases can be based on several criteria such as infected organs, disease symptoms, types of infected plants, or the causative phytopathogens. However, the phytopathogenic-based classification is considered to be more rational as it helps in easy determination of plant disease causes, related complications and probable measures of control (Cramer 1967). Following this criterion, phyto-diseases can be of two broad types: namely, biotic or infectious disease caused by microorganisms and abiotic or non-infectious disease which is the outcome of extreme environmental conditions (Horsfall and Cowling 1980). The diseases caused by abiotic factors, although being common, do not spread from plants to plants. Abiotic stresses include conditions such as excessive or deficient nutrients and moisture, soil compaction, presence of toxic chemicals in soil or air, salt injury, ice-attack, sun-scorch, etc. (Horsfall and Cowling 1980).
On the other hand, pathogenic microorganisms are the causative agents for biotic stress. The pioneering research indicating Erwinia amylovora for causing blight in pear and apple served as the foundation of plant pathology (Glawe 1992). With progress in time, several plant diseases due to bacteria, viruses, fungi, nematodes have been documented till then (Fang and Ramasamy 2015). Plant disease epidemics (epiphytotics) are also known to occur in many plants worldwide (Agrios 1997). The infectious disease occurs due to the phytopathogens’ capability to get transferred from an infected to healthy plant resulting in identical disease as well as symptoms. While the internal plant environment is preferred for inhabitation by many phytopathogens, certain other microorganisms like bacteria and fungi live on the surface of plants. Some phyto-diseases also develop due to parasitic higher plants that grow upon attachment to other plants contributing to no mutual benefit but depriving the host plant of essential nutrients. This abnormal alliance leads to fragility of the healthy host plants. Examples of such plants are dodder, mistletoe, and witchweed broomrape.
2.1 Broad Classification of Phytopathogens
As discussed earlier, pathogens affecting plant health may vary from fungi, viruses, bacteria, parasitic higher plants, mollicutes, parasitic green algae, nematodes, protozoa, and viroids. Owing to their effective penetrating ability in plant tissues, these parasites can also tolerate diverse host conditions which enable them to feed and thus proliferate in plant tissues. Such pathogens which depend on living hosts for survival are known as obligatory parasites. On the contrary, the non-obligate parasites like fungi and bacteria can survive on both living and non-living hosts and utilize different nutrient media. Among the non-obligatory parasites, those who can grow/develop on organic dead matter saprophytically are called facultative saprophytes (semi-biotrophs) (Ellingboe 1968). Another variety of facultative parasites (necrotrophs) which grow saprophytically in general can attack and cause disease in living plants but under certain circumstances. It has to be noted that severity in phyto-disease is not often dependent on the parasitism type or degree. For instance, weak parasitic pathogens are often responsible for greater derogatory outcomes in plants with respect to those caused by other obligate parasites. In most cases, the non-obligatory parasites use lysozymes for degrading plant cellular wall that allows progressive invasion as well as infection (Dollet 1984). The most common variants of pathogenic microorganisms that are responsible for diversified plant disease along with their characteristic features have been enlisted in Table 1.
The parasitic phytopathogens also possess negative impact on host metabolic processes. Vascular pathogens attack the xylem and phloem vessel tissues of host plants for their growth and multiplication processes that impede sugar and water transportation in host plant cells (Abdulkhair and Alghuthaymi 2016a). Phytopathogens (e.g., Fusarium oxysporum f. sp lycopersici, Verticillium albo-atrum, Verticillium dahliae, Xanthomonas oryzae pv. oryzae, Ralstonia solanacearum, Xylella fastidiosa, Xanthomonas campestris pv. campestris, Erwinia amylovora, Clavibacter michiganensis ssp. michiganensis) categorized as vascular wilt pathogens are responsible for overwintering soil along with plant debris (Yadeta and Thomma 2013). While most bacteria and fungi belong to groups of soil-borne microscopical pathogens, foliar pathogens are constituted of phyllosphere viruses, bacteria, and fungi. Spots, cankers, blights, overgrowth of plants, tissue rots, root branching, stunting, and leaf epinasty are some of the well-known and familiar plant disease symptoms (Martins et al. 2018).
3 Plant–Pathogen Interactions
3.1 The Disease Triangle of Host, Environment, and Pathogen
One of the most common models for studying plant pathology is represented by a triangle comprised of 3 major participants-host, environment, and pathogen (Fig. 1a). This model is based on the concept that a susceptible host can be attached by a biotic agent, that is, any virulent pathogenic microorganisms that give rise to disease. Thereby, elimination in any one of the factors may prevent disease development (Francl 2001). Such conditions can further promote the infection-inducing properties of opportunistic fungi and bacteria (Abdullah et al. 2017). As discussed earlier, phytopathogens possess the aptness for epiphytic survival or growth within tissues of host plants, soil and/or separate plants. Physical injury or weakness caused in plants by the hostile abiotic (environmental) factors further assists pathogenic entry into host cells. For instance, factors like temperature, excess or scarcity of nutrients, moisture, humidity, torrential rain, and imbalance in salinity help the pathogens to multiply as well as propagate within the host plant escalating the disease and thereby affecting the plant health adversely (Agrios 1997). It has been observed that favorable climate during the monsoon triggers disease epidemics such as pomegranate blight caused by bacteria expands exponentially under conditions of elevated humidity common in rainy season (Chikte et al. 2019). Moreover, both epiphytic and endophytic phases have been documented in life cycle of gram-negative pathogenic bacteria (Agrios 1997). The capability of phylogenetically rare species to elude disease pressure is well known and the host’s phytogenic structure also modulates pathogens’ potency to spread, thereby affecting disease severity (Gilbert and Parker 2016). Thus, knowhow of phylogenetic connection in host and also virulent pathogens serve in predicting disease risk in multi-cropping system, which may help to avoid probable economic loss. However, it is important to note that there are multiple variables within the three components of disease triangle that may alter both disease incidence and severity. Life cycle, genetic diversities, and biology of both plants and pathogens as well as environmental conditions are a few of such variables.
(a) The vicious disease triangle of host, environment and pathogen: the phytopathogenesis only develops when each factor coincided with each other. (b) the cycle of disease development: the monocyclic virulent pathovars complete their cycle following the red arrows, while the polycyclic pathogenic microorganisms normally follows the blue arrows to complete their cycle for most of the seasons but shifts to the red arrows at the end
3.2 Disease Cycle
For disease development, the tissue or cell of host plant should be successfully invaded by a virulent pathogen. Fig. 1b depicts the series of events involved in development of phyto-diseases. A disease cycle can be monocyclic or polycyclic. The various stages of the disease cycle are being briefly discussed below (Abdulkhair and Alghuthaymi 2016b):
Inoculation
This step involves the phytopathogen introduction into host. Different pathogenic microorganisms deploy different modes of inoculation and are also equipped with diversified specialized mechanisms that promote inoculation. While some pathogenic fungi use spores that are airborne for inoculation in addition to sclerotia of mycelium fragments, intact cells also represent the inoculum in cases of bacteria, protozoa, mollicutes, viruses, and viroids. The inoculum categorized as primary and secondary causes respective phyto-infections.
Penetration
While certain plant pathogens utilize wound or injury sites and naturally occurring plant openings (stomata and hydathodes) to enter the host plant tissues, other pathogens employ unique modes to penetrate directly. Under optimum temperature, moisture and other favorable environmental conditions, fungi and nematodes undergo active penetration in tissues and cells of host plant.
Infection
After invading the plant tissues, the phytopathogens develop a parasitic relation with the plant. Being unable for active penetration into the host plant tissues, phytoplasmas, bacteria, and viruses depend on alternative methods for infecting tissues and cells in plants. The virulent pathogens rely on insects as vectors that assist inoculation as well as dispersal.
Incubation
After entering the plants, the pathogenic microorganisms enter the incubation stage and stay in latent condition for a specific period of time before initiation of the phyto-diseases.
Reproduction
Depending on the type of phytopathogens, the mode of reproduction may be sexual of asexual.
Survival
The evolution process of phytopathogens has enabled them for prolonged survival by tolerating hostile environmental conditions. The dark brown colored spores produced by fungal pathogen are an example which lower the light penetration and thereby prevent cell death. Another example of survival strategy may be the habit of soybean cyst nematode laying its eggs in a cuticle case. Such casing is very rigid that prohibits penetration of harmful chemicals or microbes which kill the eggs prior to hatching.
As discussed above, disruption in one or more steps of the cycle will either curb the disease severity or may even prevent its promotion or development in host plants. Thus, the knowledge of disease cycle may help to manage various phyto-diseases.
4 Phyto-pathogenesis-Linked Molecular Mechanisms Mediated by Bacteria, Viruses, and Fungi
Phyto-pathogenesis exhibits a critical and complicated process (Fig. 2a). As a pathogenic virulent microorganism encounters a plant, it should be capable enough for adapting to the living conditions in epiphytic surfaces as well as survive for adequate time period necessary for initiating infection. Thus, throughout the process of infection, the response toward environmental conditions plays a pivotal role. The correlation of signaling pathways (both intracellular and community level) with that of environmental signals triggers responses critical for phytopathogen populations. The ultimate goal of phytopathogens is to migrate from surface of the epiphytes into the host plant tissues mediated by motility or chemotactic pathways. This migration involves introduction into plant apoplast after surpassing the physical along with chemical barriers. Following their introduction in plant tissues/cells, phytopathogens utilize an array of effectors (proteins) and phytotoxins produced by different secretory systems that co-ordinate various pathogenic functionalities (Melotto and Kunkel 2013).
(a) Basic mechanism of phytoinfection by pathogenic microbes: The common steps include (1) surface infestation and adaption of microbes along with formation of biofilm, (2) surface migration of the pathogens mediated by flagella/ pili to gain access through the apoplasts, (3) phytotoxin mediated stomatal entry, (4) damage of plant surface through ice nucleating agents (INA), (5) toxins mediated alterations of plant physiology essential functions and even immune responsiveness, (6) plant tissue degradation and disruption of cell wall through secreted enzymes. (b) Rpf conjugated QS/cdG signaling system in pseudomonas species: The sensor kinase, Rpfc upon sensing QS signal (DSF) produced by RpfF, causes phosphorylation and activation of RpfG. RpfG in turn via its phosphodiesterase activity degrades cdG thus reducing biofilm formation and Clp protein release repression. Subsequently, virulent genes undergo Clp mediated transcription. Additionally, interaction of RpfG with other GGDEF domain comprising proteins, is responsible for controlling bacterial motility. Rpf regulation of pathogenicity factor, QS/cdG quorum sensing/cyclic di-guanosine monophosphate, Clp Crp-like protein
4.1 Bacterial Phyto-pathogenesis
4.1.1 Surpassing Stress on Epiphytic Plant Surface
The surface of leaves presents a hostile environment for virulent pathogens. Bacteria have to face regular exposure to desiccation, adverse alteration in temperature, UV radiation, and mechanical abnormalities like strong wind. Despite these hindrances, epiphytic bacteria possess certain virulence strategies that promote their microbial persistence on the surface of most plants. The epiphytic survival is materially regulated by metabolic responsiveness to shock, cold, stress, and desiccation (Djonovic et al. 2013; Freeman et al. 2013). In Pseudomonas syringae (P. syringae), trehalose (osmo-protectant) has been indicated for benefiting its survival and also maintain its required population in phyllosphere (Freeman et al. 2010). It has also been suggested to potentiate nitrogen requirement and enhance proliferation in leaf apoplast thus contributing to plant disease development by P. aeruginosa (Djonovic et al. 2013). Various literatures have pointed out the pivotal contribution of exopolysaccharides (EPS) like alginate, xanthan, levan (Freeman et al. 2013; Dunger et al. 2007) in the epiphytic survival of various plant-related microbiomes such as xanthomonas species (Dunger et al. 2007) and P. syringae (Yu et al. 1999). The EPS molecules are closely correlated with epiphytic survival and contribute to enhance the pathogenic capability for resisting freeze-thaw process (Wu et al. 2012), tolerate stress due to osmosis and dryness (Freeman et al. 2013), and also maintain adequate microbial population (Dunger et al. 2007). Various phytopathogens possess the EPS molecules that mediate Ca2+ signaling quenching at the time of phyto-immune response and thereby allow the microbes to evade the immune system (Aslam et al. 2008). Relative to surface of leaves, literature points out strong upregulation of biosynthetic locus of levan in P. syringae pv. syringae (Pss) B728a in the apoplast that indicates its role in post-infection virulence (Yu et al. 1999). Moreover, the pathogenic trait of EPS molecules has also been observed in case of biofilm formation by Psa NZ V-13 (Renzi et al. 2012). On the other hand, Wss (acetylated cellulose) molecules not only foster the root colony formation of Pseudomonas fluorescens, but also facilitates P. syringae pv. tomato (Pto) DC3000 (Gal et al. 2003).
4.1.2 Signaling Cascades Regulated by Phytopathogens
The virulent phytopathogenic bacteria demonstrated evolved and efficient cell-to-cell signal transduction system which exhibits diversified and overlapping parallel input signals along with gene (non-linear) co-ordination. Moreover, the plant surface also reveals various bacterial species to be closely interlinked with various signaling systems. Among the wide variety of signaling systems QS (quorum sensing) and cdG (cyclic guanosine 3’,5’-monophosphate) signaling (second messenger pathway) are considered to perform major roles in plant pathogens.
4.1.2.1 Quorum Sensing (QS) in Phytopathogenic Bacteria
QS is utilized by ultraviolet bacteria for communicating as well as assessing cellular density via the products of autoinducers which are tiny signaling molecules. The major signals in bacteria are acyl-homoserine lactones (AHLs), DSF(diffusible signal factor), and Ax21.
QS molecules are majorly constituted by AHLs. AHL itself plays as pivotal role in positively regulating synthase gene transcription. P. syringae is known to produce 3-oxo-C6-HSL (homoserine lactone), an AHL molecule. In Pss, both synthesis of EPS and motility are controlled by ahlI/ahlR system which are essential for maintaining virulence of pathogenic bacteria as well as plant colonization (Quinones et al. 2004, 2005). While Agrobacterium tumefaciens (crown gall bacteria) control virulence by producing 3-oxo-C8-HSL that stimulates Ti plasmid copy numbers, secretion of phytotoxin and flagellum assembly coordinated motility in Burkholderia glumae (rice pathogen) is regulated by C8-HSL production (Kim et al. 2004). LasI/LasR, OscR (orphan regulator), and RhlI-RhlR are 3 AHL pathways which are employed by P. aeruginosa for producing 3-oxoC12-HSL along with C4-HSL that are known for affecting more than 300 gene expressions among which many are associated with restriction of toxins, biofilm formation, and also motility (Schuster et al. 2003). Another QS molecule called PQS (Pseudomonas quinolone signal), chemically known as 2-heptyl-3-hydroxyl-4-quinolone, is also reported to be synthesized by P. aeruginosa which control both formation of biofilm and virulence factors production (Allesen-Holm et al. 2006). Moreover, PQS, las, and rhl (complete and semi-independent) QS systems also mediate LasB expression encoding a secreted (type II) protease.
Recently, another QS signal known as Ax21 has gained quite interest. Xanthomonas oryzae pv. oryzae produces the Ax21 which is a sulfated small protein in nature and performs the role of QS molecule necessary for expressing virulence genes (Han et al. 2011). X. oryzae pv. oryzicola (Qian et al. 2013) and Stenotrophomonas maltophilia (McCarthy et al. 2011) have been documented to possess similar proteins that are interrelated with EPS and biofilm formation enhancing motility and virulence.
Another essential QS molecule termed as DSF is composed of fatty acids (unsaturated). cis11-methyl-dodecenoic acid is a DSF signal that is recognized by pathovar Xcc responsible for causing crucifer black rot (Wang et al. 2004) and is involved in modulating virulence as well as synthesis of xanthan and protease (Barber et al. 1997). DSF has also been reported to be used by Xy. Fastidiosa, X. oryzae pv. Oryzae, and other Xanthomonas species. DSF signaling pathway which is synchronized by components of the gene cluster Rpf (regulation of pathogenicity factor) present in Xcc upon mutation reduces virulence (Barber et al. 1997). DSF upon synthesis is readily detected RpfC, a component of RpfC/RpfG (hybrid sensor kinase) which is also known for negatively regulating DSF synthesis (Slater et al. 2000). Following activation of RpfC/RpfG system and signal transduction through it, cdG is degraded with subsequent repression of Clp (Crp-like protein) transcription regulator release that finally leads to activation of virulence gene expression (Fig. 2b) (Chin et al. 2010). Xcc also consists of another DSF sensor, RpfS apart from RpfC which has been proven to be essential for virulence in Chinese radish (An et al. 2014a). On the other hand, mutation of RpfF in Xylella fastidiosa increases colonization and virulence in grape xylem tissues. Xy. fastidiosa engage DSF signal transduction in a multifaceted fashion for regulating its colonization, virulency as well as adhesion potential (Chatterjee et al. 2008a, b).
4.1.2.2 cdG Signal Transduction Pathway
Cyclic adenosine monophosphate (cAMP) and cdG (cyclic-di-GMP) like nucleotides that serve as secondary messengers, function as signaling molecules with pleomorphic roles for controlling and regulating virulence of bacteria. Apart from regulating virulence, cdG the secondary messenger participates in coordinating a wide range of functions including motility, biofilm formation. PDE (Phosphodiesterase) and DCGs (diguanylate cyclase) are known for regulating intracellular levels of cdG. While DGCs utilize 2 GTP (Guanosine triphosphate) molecules for synthesizing cdG, PDEs cause degradation of the same. Often additional domains such as GAF, REC, or PAS present in DGC/PDEs are also associated with signaling. cdG interacts with a wide variety of binding domains for exerting its action, some of them being PilZ domain containing protein, DGCs, and PDEs which are enzymatically inactive, transcriptional regulators such as Clp, RNA riboswitches, and FleO (regulator of pseudomonas motility and/or EPS) (Romling et al. 2013). cdG in DSF/rpf signaling system undergoes interaction with Clp preventing its promoter binding and subsequent related target genes transcription (Fig. 2b). Clp in Xanthomonas species controls gene expressions that encode extracellular enzymes (Chin et al. 2010). Similar Clp/cdG interaction is evident in Xanthomonas axonopodis pv. Citri (Leduc and Roberts 2009). On the other hand, Xcc also exhibits a different dual component RavS/RavR system which is associated with cdG and found to be important for bacterial virulence. This RavR protein is involved in alteration of cdG levels mediated by activity of PDE via its EAL domain and also modulates expression of virulence factors through Clp, the transcriptional regulator (He et al. 2009). The regulatory effect of cdG systems in controlling multiple and essential behavioral facets of phytopathogenic Pseudomonas species along with regulating the virulence of various other pathogenic species and pathovars is well documented (Pfeilmeier et al. 2016). Previous literatures also indicate its role in managing activity of T3SS (type III secretion system) and flagellum with simultaneous regulation of proteome composition by modifying ribosomes (Trampari et al. 2015; Little et al. 2016). It has been suggested that Gac/Rsm nexus that is responsible for controlling quantum signaling, biofilm formation, secretor systems, toxin, and siderophore production, manipulates productions of PDE/DGC thereby subsequently affecting cdG levels (Moscoso et al. 2011, 2014). While expression of T6SS (type IV secretion system) is interlinked with GacA (sensor kinase) in P. syringae (Records and Gross 2010), RsmA, the post-transcriptional regulator (RNA binding), when trans-overexpressed results in repression of secretion of various virulence factors (Kong et al. 2012). The key functionalities of cdG signal transduction in Ralstonia solanacearum, Serratia, and Erwinia genera need to be elaboratively explored apart from Xanthomonas and Pseudomonas and species. Recently researches have revealed two new targets for cdG binding in XCC and P. syringae, which are XC_3703 (YajQ family protein) (An et al. 2014b) and injectisome (type III) ATPase HrcN, respectively (Trampari et al. 2015).
4.1.3 Adapting Skills of Pathogenic Bacteria to Phyto-environment
The interaction and cross talk between different components of plant microbe are known to largely affect phytopathogenic bacterial behavior in natural environment. Epiphytic endurance and phyto-infections are affected prominently by both commensal and antagonistic interactions (Delmotte et al. 2009; Ritpitakphong et al. 2016). Downstream of the signal transducing nexus, pathogenic responses are integrated to environment by transcriptional alterations related with life on phyto-surfaces. It has been documented that in Pss B728a (P. syringae strains) affecting the bean plants’ surface, there is marked upregulation of genes associated with nutrient attainment, virulence, intracellular signaling as well as membrane transport (Marco et al. 2005). On the other hand, epiphytic survival of Pss B728a on surface of the leaves is promoted by active and strong induction of osmotolerance coupled with T6SS and alginate synthesis. Another phyto-environment identifying regulatory pathway in Ag. tumefaciens involves low pH, plant-related sugars, and phenolics (acetosyringone) mediated evocation of virulent gene expression (Peng et al. 1998). In Agrobacterium, certain chemical signals-mediated induction of virulence gene at the site of plant wounds are modulated by VirAG/ChvE pathway that finally results in effective crown gall tumor formation (Peng et al. 1998).
4.1.4 Apoplastic and Plant Surface Motility of Bacteria
Motility or migration plays an important role in phyto-infections. Recently, regulation of motility as well as pili, surfactant or flagella loci expression at proper time during phyto-infection phases are being considered as one of the salient factors in pathogenicity of plant. When in contact with surface of leaves, many phytopathogenic bacteria express traits that assist in promoting bacterial persistence until apoplastic penetration is allowed by environmental conditions. Under favorable conditions, pathogens utilize different motility systems that enable their migration from surface of the leave to interior of the plants through different access sites like wounds and stomata. Flagellar motility confers epiphytic competence benefit in P. syringae and plays an important role in plant virulence and surface colony formation (Tans-Kersten et al. 2001). It has been observed that chemotaxis along with formation and utilization of surfactant molecules in bacteria are closely intertwined with expression of flagellar genes that allow bacteria for migration through leaf surfaces (Yu et al. 2013; Burch et al. 2012). Signaling genes like rpfS (in Xcc) (An et al. 2014a), rimK (in P. syringae) (Little et al. 2016), and xbmR (from X. citri ssp. Citri) (Yaryura et al. 2015) upon deletion contribute in defective bacterial virulence. Also. in P. syringae, R. solanacearum and in several other pathogens, type IV pili are found to be necessary for producing and attachment of biofilm, twitched motility, and pathogenic virulence (Nguyen et al. 2012; Kang et al. 2002).
During the plant infections, the formation as well as expression of flagella should be closely controlled for directed bacterial migration toward the apoplast and also prevent from being detected by PRRs (plasma membrane-localized pattern recognition receptors) which would then lead to initiation of pattern triggered immunity (PTI) (Macho and Zipfel 2014). The excess of flagellin monomers is known to be degraded by Apr A (alkaline protease A) (Pel et al. 2014).
4.1.5 Bacterial Invasion of Plant Tissue Mediated by Hijacking Stomatal Entry and Cell Wall Degeneration Enzymes
The plants as a part of their immune (innate) system keep their stomatal pores closed to inhibit ingression of bacteria (Melotto et al. 2006). But this defensive mechanism needs to be surpassed by phytopathogenic bacteria for gaining apoplastic access. It has been noted that Xcc (Gudesblat et al. 2009), P. syringae species (Melotto et al. 2006), and many other pathovars perturb stomatal immune system through secretion of phytotoxins. Virulent pathogens secrete varied molecules including toxins like syringolin and coronatine that enact as antistomate defense components (Melotto and Kunkel 2013) where they impede NRP1 (non-expresser of pathogenesis related 1) regulated SA (salicylic acid) signaling (Xin and He 2013). On the other hand, other pathogens secrete enzymes and specific proteins that degenerate the cell wall allowing them to enter plant tissues. Other literatures indicate another mechanism of overcoming phyto-defense systems through production of ice nucleating agents (INAs) which have been identified in P. syringae (Gaignard and Luisetti 1993), X. campestris (Gurian-Sherman and Lindow 1993), and Pantoea ananatis (Sauer et al. 2014). Water molecules are transformed by INAs into clathrin lattices identical to ice that elevate temperature allowing nucleation of ice with corresponding freezing of water at higher sub-zero temperatures (Garnham et al. 2011). Through this mechanism of ice-nucleation, the phytopathogens cause frost damage and thus gain access for entering into plants. Epiphytic bacteria also possess genes-rendering resistance to “freeze-thaw” that foster their survival irrespective of both environmental and biotic factors evoked frost conditions (Wu et al. 2012).
Additionally, phytopathogens also deploy certain secretion systems (mainly type II) through which wide arrays of enzymes are released (Korotkov et al. 2012). These contribute in degeneration of the structural molecules constituting cell walls of plants and also cause hydrolysis of the lamellae connecting individual plant cells which supply the pathogens with a source of carbon that promotes pathogens’ propagation through apoplast and get distributed throughout the host plant tissues. Xy. Fastidiosa attributes in damaging the xylem’s inter-vessel pit membranes in grapevine that facilitates pathogenic propagation (Sun et al. 2011). Extracellular enzymes like pectinases, proteases, xylanases, and cellulases are categorized as cell wall damaging enzymes that are critically related with Xanthomonas spp, Phytoplasma, and Xylella along with Erwinia and Pectobacterium genera (soft-rot pathogens) (Dejean et al. 2013; Lee et al. 2014; Toth et al. 2003).
4.1.6 Maneuvering Various Plant Protective Systems by Phytopathogenic Bacteria
The virulent microbes are also capable of synthesizing and secreting small phytotoxins that are responsible for potentiating bacterial virulency by suppressing host plant defensive mechanisms with subsequent enhancement of necrosis in tissues and chlorosis. While phytotoxins like syringopeptins and syringomycins directly deteriorate the plant cells, other toxins manipulate and interfere with different signaling pathways and metabolic activities that succor the invasion by the phytopathogens. P. syringae species synthesize toxins (modified peptides) such as mangotoxin, phaseolotoxin, and tabtoxin which trigger both tissue chlorosis as well as necrosis. The above-mentioned toxins primarily disrupt nitrogen metabolism by inhibiting the activity of targeted enzymes involved in biosynthesis of amino acids. The resultant nitrogen-rich intermediates are then successively utilized by the phytopathogens as a source of nutrition and food (Arrebola et al. 2011).
It has been observed that on release of hydrolysis-mediated toxic component of tabtoxin inside plant cell, glutamine synthetase is irreversibly inhibited and chlorophyll is degraded that consequently cause yellowing of tissues and chlorosis (Langston-Unkefer et al. 1987). On the other hand, another enzyme carbamoyl transferase is inhibited by Phaseolotoxin that also leads to similar consequences of host plant as an outcome of metabolic imbalance within plant cells (Bender et al. 1999).
The mechanisms of bacterial plant pathogens are not only restricted to these but are extensive in which they also disturb hormonal physiology in host plants and manipulate internal signal transduction cascades that exponentiate bacterial virulency and potentiate the pathogenic outcome. Structural as well as functional parallelism of most phytotoxic components with that of phytohormones like auxin has been observed. For example, coronatine which resembles the plant hormone Polyketide is involved in stimulating proliferation of apoplast, opening of stomata which finally confer to aggravated symptomatic development of phyto-diseases (Zeng and He 2010). The phyto-receptor complex COI1/JAZ (coronatine insensitive1/jasmonate ZIM-domain) senses coronatine that results in stimulation of JA (jasmonic acid) transduction in plants with simultaneous suppression of defense mechanisms arbitrated by SA (salicylic acid) signaling (Xin and He 2013).
Phytopathogens may also directly manipulate plant hormonal signaling pathways by encoding enzymes that are involved in synthesis of plant hormones. Several bacterial strains like P. syringae, Ag. tumefaciens, Pantoea agglomerans, and Pseudomonas savastanoi have been correlated with synthesis of abscisic acid (ABA), JA, indole acetic acid (auxin), cytokinins, and ethylene (Robert-Seilaniantz et al. 2011). The phytopathogens owing to their ability to produce or suppress various hormones directly exploit the critical crosstalk existing among the hormone transduction pathways that provide them the opportunity to subvert plant defensive mechanism and metabolism for their own benefit (Robert-Seilaniantz et al. 2011).
4.1.7 Effector Proteins-Mediated Bacterial Virulence
The phytopathogenic microorganisms are linked with secretion and release of miscellaneous virulent factors like phyto-toxins, enzymes, and other molecules for circumventing host plant defenses either directly into host plant’s cytosolic environment or in the extracellular locale. Xanthomonas spp., P. syringae, and other hemi-biotrophic bacteria employ amalgamation of different secretory systems for effectively exporting and delivering secreted proteins called effectors responsible for maintaining structural and functional integrity of host plant’s components to relevant locations to further potentiate the degree of infection. Co-ordinated secretory systems show prominent effect on the versatility of phytopathogens (Fig. 3a).
(a) Bacterial effectors mediated mechanisms of phytopathogenesis: (1) extracellular and intracellular stimuli coordinated the expression of virulence genes (2) different secretion systems mediated effector translocation such as by type III secretion system (T3SS, T4SS, T2SS etc.), (3) Essential functions of host plants like immunity, metabolism, cellular structure, hormonal signals, distribution of nutrients etc, are all targeted and compromised by the effectors, (4) Host–pathogen interactions exponentially increases and diversifies virulent pathogenic gene families. (b) Phytopathogens targeted defense mechanism to promote disease development: Pathogenic avirulent factors upon strong recognition activate hyperreactivity (HR) dependent programmed cell death (PCD) which is a rapid response arresting the development of pathogenic infection. On the other hand, avirulent factors upon feeble recognition as well as flagellin or chitin through FLS2 also promotes basal defenses via different pathways including MAPK. Basal defenses subsequently stimulate expression of defense genes or induces late onset of pathogenic cell death. Formation of papillae at the site of nascent colonization of bacteria or site of fungal penetration corresponds to defense mechanism associated to cell wall. Jasmonic acid (JA) signaling pathway upon activation induces suppression of salicylic acid (SA) transduction pathway leading to repressed expression of specific pathogen related gene. Reactive oxygen species triggered oxidative stress is direct bactericidal in nature. Moreover, already existing antimicrobials help in repulsion of pathogenic activity. As a counter response to pathogen attack, signaling through programmed cell death facilitates cell death. The expression of the NHO1 gene expression triggered by nonhost and/or avirulent bacterial pathogens is necessary in certain cases of non-host resistance. PR pathogen related gene, PCD programmed cell death, NHO1 non-host resistance 1
RsmA, in P. aeruginosa, functions as a molecular switch to coordinate between acute and chronic phases of infection causing translational suppression of T6SS, pel (pectate lyase), and psl mRNAs with corresponding upregulation in transcription of flagellar, T3SS, and T4SS genes (Moscoso et al. 2011). In Pss B728a, these T6SS and T3SS are negatively regulated by RetS and LadS (sensor kinases) (Records and Gross 2010). In Xanthomonas, previous reports indicate the regulatory role of QS over T2SS (Jha et al. 2005). Also, extensive researches are being conducted on modulation of hypersensitive response and pathogenicity (hrp) regulon which constitutes T3SS-related structural genes, T3SS regulators, and multiple T3ES (Buttner and Bonas 2010). During the infection phase, pathogenic bacteria keep tight control to deliver T3E hierarchically and temporally with the help of post-translational techniques (Galan et al. 2014). While salmonella enterica shows orderly recruitment and secretion of T3Es effectuated by chaperone-facilitated cytoplasmic cell sorting platform (Lara-Tejero et al. 2011), other bacterial pathogens deliver T3Es inside host cells by deploying chaperons, export control, and other hrp-associated (hpa) proteins (Lohou et al. 2013). Interaction between HpaB (global chaperone for T3E export) and HpaA in R. solanacearum causes selection of T3ES and guides them to HrcN (T3SS related ATPase) effectuating translocation (Buttner et al. 2006). Currently, the allosteric role of cdG in regulating HrcN action is also being explored that suggests its crosstalk with the dynamics of T3E translocation. In case of Pss B728a, regulated T3Es secretion is found to be essential during the phase of epiphytic growth which is marked by the prominent role of HopAA1 and HopZ3in promoting definitive leaf surface colonization (Lee et al. 2012).
Complex transportation mechanisms are required for translocating proteins and other molecules coordinately (Fig. 3a). Type I-VI transportation systems are engaged by gram-negative bacteria for delivering proteins into the extracellular milieu or inside the host cellular components (Gerlach and Hensel 2007). While T3SS plays a pivotal role in pathogenicity, T2SS is also widely used by different pathogens such as members of Ralstonia, Pseudomonas, Xanthomonas, and Erwinia genera for extracellular delivery of proteins (Jha et al. 2005; Johnson et al. 2006). T2ES mostly comprising of different virulence factors like cell-wall degeneration enzymes, toxins, and also proteases potentiate the virulency in plants. Gram-negative bacteria and members of Xanthomonas species are known to carry a lipA gene that encodes for a secreted lipase (type II) essential for imposing complete virulency by X. oryzae pv. oryzae (Aparna et al. 2009) and also X. campestris pv. vesicatoria (Tamir-Ariel et al. 2012). Additionally, it has seen discovered that T4SS which is generally associated with translocation of DNA and protein to the extracellular locale or inside host cells, mediates the transfer of T-DNA in Ag. Tumefaciens inside plant cells that confers in altered metabolic functions and morphology leading to tumor development (Bhatty et al. 2013; Gohlke and Deeken 2014).
These effectors improve microbial competence in the plant environment by interfering with essential phyto-processes. T3Es are reported to majorly suppress plant immune responses (Macho and Zipfel 2015). Xanthomonas and Ralstonia spp. members encode TAL effectors (transcription activator-like) which are phyto-transcription factors and are interlinked with promotion of plant virulence as well as providing a bacterial growth compatible environment (Bogdanove et al. 2010). X. oryzae pv. oryzae (rice pathogen) utilizes various TAL effectors to modify the gene expressions encoding sugar transporters (SWEET). The resulting sugar in apoplast, effluxed from the plant cells may be accompanied with release of water for maintenance of tissue osmotic balance that further alters the milieu of intracellular spaces thus providing advantage for bacterial infection (Streubel et al. 2013; Macho 2016). In X. campestris, AvrBs3 (TAL effector) upregulates UPA20 expression which regulates the size of plant cells and thereby causes enlargement of mesophyll cells leading to increased bacterial dissemination and growth (Kay et al. 2007). P. syringae associated T3ES like AvrPtoB, AvrRpt2, and HopX1 modulate the respective activities of ABA (Abscisic acid), auxin, and JA signal transducing pathways (Cui et al. 2013; Gimenez-Ibanez et al. 2014). Phyto-metabolic activities are also found to be directly modified by T3Es as they intrude secondary metabolites forming biosynthetic pathways. Perturbance of metabolism of phenylpropanoid by T3E belonging to the AvrF family secreted by Pantoea stewartii enhances the virulency of phytopathogens (Asselin et al. 2015). Similarly, mitochondrial activities are curbed by HopG1that impedes plant development and may also increase the virulency (Block et al. 2010).
4.1.8 Subduing Plant Defense Mechanisms by Phytopathogenic Bacteria
To circumvent the defense mechanisms of plants, pathogenic bacteria utilize diversified strategies to target and modulate core constituents of phyto-immunity including JA signal transducing pathway, HR (hypersensitive response)-dependent programmed cell death (PCD), defensive and basal gene expressions as well as cellular wall-related defense mechanisms. Table 2 and Fig. 3b represent certain host phyto-defenses that are commonly targeted by the virulent bacteria.
4.2 Viral Phyto-pathogenesis
Plant pathogenic viruses must expropriate host survival factors. The viral-encoded multifunctional proteins should strategically be involved in different phases of life cycle and elicit defensive responsiveness. Thus, most viral encoding protein usually performs the role of determinants of pathogens. The viral proteins regulating replication, encapsidation, transmission, and motility may play prominent role in directly or indirectly modulating pathogenesis.
4.2.1 RNA-replicase-Associated Viral Proteins and Their Role in Phyto-pathogenicity
Viral RNA replicase (i.e., RNA dependent RNA polymerase) through modulation of viral replication process and consequent accumulation of virus, indirectly affects phyto-pathogenesis. Reports reveal that in both Tobacco mosaic (TMV) (Lewandowski and Dawson 1993; Chen et al. 1996) and Pepper mild mottle (PMMoV) (Yoon et al. 2006) viruses, mutation of p126/p183 proteins (RNA replicase-related proteins) resulted in truncated accumulation of virus along with attenuated symptoms. Similar observations were concluded in case of 2a protein mutation in Cucumber mosaic virus (CMV). The molecular mechanism of RNA replicase in development of the phyto-disease involves auxin (Aux) responsive pathway reprogramming. It is interconnected with TMV126/183K replicase crosstalk with IAA (indole acetic acid)/Aux that confers in corresponding enhancement in viral accumulation (Padmanabhan et al. 2008). RNA replicases function as elicitors of ETI (effector triggered immunity) operated by R-gene resulting in hypersensitive response. They may influence either localized lesions due to necrosis or systemic symptoms that are viral specific. These viral polymerases may also perform the role of breaking determinants of different resistance sources thereby modulating pathogenesis. It has been seen that Tomato mosaic virus replication proteins can subvert inhibitory interplay with resistance Tm-1 gene products via point mutation (Ishibashi et al. 2007). Alteration of single amino acid in methyltransferase domain of Potato virus X replicase assists disruption of JAX1 (jacalin-type lectin required for potexvirus resistance 1)-mediated resistance in N. benthamiana (transgenic) system (Sugawara et al. 2013).
4.2.2 Viral Coat Proteins-Mediated Pathogenicity
These are prototypical and multifunctional viral proteins that are associated with multifaceted functions such as encapsidation, replication, motility, translations, and even host defense responsive system (Ni and Cheng 2013). The expression of symptoms in concerned host plants is significantly affected when the coat proteins of respective CMV (Shintaku et al. 1992), TMV (Dawson and Bubrick 1988), Turnip crinkle virus (TCV) (Heaton et al. 1991), and Brome mosaic virus (Rao and Grantham 1996) undergo point mutations. The coat proteins of Tomato mosaic virus (TMV), CMV, Potato virus X(PVX), TCV, and PMMoV (Moffett 2009; Gilardi et al. 1998) act as avirulent factors eliciting resistance coordinated by the R gene (dominant). The coat proteins of TCV promote hypersensitive response development in resistant strain of Arabidopsis ecotype Dijon. The HRT and RRT host genes as well as SA signaling control such types of responses (Kachroo et al. 2000). The transcription factor TIP belonging to the NAC family interacts with coat proteins which stimulates the action of HRT gene and also inhibits TIP localization in nucleus (Ren et al. 2000, 2005). Photosystem II electron transport is prevented by tobamovirus-mediated infection which disrupts oxygen-evolving complex (OEC) (Rahoutei et al. 2000). Infected host plants revealed that the levels of OECPsbP and PsbQ (photosystem II proteins) are decreased in comparison to healthy plants. It has been recently demonstrated that while the interaction of PspB with Alfalfa mosaic virus’s (AMV) coat protein results in inhibition of viral replication (Balasubramaniam et al. 2014), mutation of amino acid on the other hand renders virulence to the coat protein of CMV pepo strain that represses genes associated with chloroplast and photosynthesis thus causing chlorosis in tobacco plants infected with CMV (Mochizuki et al. 2014).
Similarly, interaction of coat protein of ToMV with IP-L (specific tobacco protein) causes localization of thylakoid membranes (Zhang et al. 2008). Also, the outer capsid P2 protein of phyto-reovirus upon interacting with the biosynthetic mediator of gibberellins known as entkaurene oxidase promotes dwarf symptoms (Zhu et al. 2005).
4.2.3 Viral Protein Interrelated with Movement and Their Role in Phyto-infections
The phyto-viruses are translocated into the cells of host plant with the help of movement proteins (Pallas et al. 2011; Lucas 2006) as these play an essential role in determining specificity of host (Mise et al. 1993). Various endogenous host factors are manipulated by the movement proteins facilitating the transportation of viral genome finally leading to alterations in physiology of plants with directly affecting the symptoms. The interaction of host plant factors with movement protein may facilitate or impede the viral movement as seen in case of the interplay between the crucial hydrogen peroxide decomposing enzyme tomato catalase (CAT) 1 and TGBp1 (triple gene block protein 1) of PepMV (Mathioudakis et al. 2013). This interaction leads to excessive peroxide scavenging and thus aid in development of PepMV infections by negatively regulating plant defense system. Movement protein also gives rise to hypersensitive reaction upon interaction with R gene. The avirulent determinant that potentiates Sw-5 (tomato gene)-induced resistance against tomato spotted wilt virus is suggested to be dependent on movement protein NSm (Peiro et al. 2014). The Potato virus X triple gene block protein 3 (TGBp3) promotes programmed cell death as well as unfolded protein response during the course of infections caused by PVX (Ye et al. 2013). The movement proteins also contribute in enhancing viral RNA silencing thus manipulating susceptibility of host plants mediated by stimulating silencing among cells (Amari et al. 2012).
4.2.4 Viral Suppressors of RNA Silencing
Certain viral proteins cause disruption of phyto-homeostasis counteracting with antiviral silencing and lead to development of disease symptomatology (Wang et al. 2012; Pallas and Garcıa 2011). Transgenic expression of RSSs (RNA silencing suppressors) leads to development of abnormalities that mimics disease symptoms (Chellappan et al. 2005; Dunoyer et al. 2004). These RSSs employ different mechanisms to interrupt the silencing processes, mainly at post transcriptional phase and sometimes also during transcription (Incarbone and Dunoyer 2013) that results in phyto-disease (Wang et al. 2012). miR167 upon being inactivated by RSSs like potyviral HCPro, tombusviral P19, and pecluviral P15 causes dysregulation of auxin response factor 8 which majorly contributes in development of abnormalities (Jay et al. 2011). It has also been suggested that inactivation of miRNAs regulating (negatively) NBS-LRR-R genes involved in autoimmune response induction by the RSSs may result in overexpression of these R genes conferring in lethal necrosis and other deleterious effect (Wang et al. 2012; Li et al. 2012; Shivaprasad et al. 2012). Similarly, the strategies of RSSs to inactivate AGO1 (Argonaute-1) and other effector proteins lead to inhibition of antiviral silencing and evoke developmental abnormalities as observed in cases of 2b and TGBp1proteins of CMV and PlAMV respectively (Zhang et al. 2006; Okano et al. 2014). Additionally, the ability of RSSs to induce pathogenic symptoms without blocking gene silencing directly has also been implied (Du et al. 2014). While the Cauliflower mosaic virus-associated RSS P6 interacts with ethylene signaling (Geri et al. 2004), CMV-related RSS 2b manipulates catalase of host plant (Inaba et al. 2011) and Potato virus A-associated HCPro RSS usurps microtubule-related protein (Haikonen et al. 2013). HCpro interactions are also responsible for precipitating proteasomal dysfunctions that contribute in pathogenicity (Pacheco et al. 2012). Performing the role of elicitors for ETI (effector triggered immunity), acting as second phyto-defensive layer, RSS can also cause developmental disease symptoms (Wang et al. 2012). For example, RSS P19 associated to tomato bushy stunt virus (TBSV) is known to induce hypersensitivity response (Chu et al. 2000).
4.2.5 Other Phytopathogenic Viral Factors
Together with the above-mentioned viral protein, certain addition proteins have also been researched that contribute to viral pathogenicity. P1 (Chiang et al. 2007), P3-6K1, CI (Desbiez et al. 2003), P3N-PIPO (Hisa et al. 2014), and 6K2 (Spetz and Valkonen 2004) proteins of potyviruses, p25 (Klein et al. 2007) and p31 (Rahim et al. 2007) of Beet necrotic yellow vein virus, are some of the examples of such viral determinants of pathogenicity. But the molecular mechanistic strategies of these proteins need to be thoroughly explored. Moreover, the nucleic acid of virus can also be function as direct determinants of viral pathogenicity. In case of defective interfering RNA (DI) of tombusvirus, DI-specific siRNA gets accumulated due to interference thereby saturating the capacity of silencing suppressor P19 to bind siRNA (Havelda et al. 2005).
4.3 Fungal Phyto-pathogenesis (Vadlapudi and Naidu 2011; Yang et al. 2017)
In case of fungal pathogenesis, well-conserved proteins are said to be used by fungal pathogens to induce infection. The fungal pathogens are known for deploying novel mechanisms to promote infections such as formation of appressoria, which are special infection structures that allow penetration of host plant cells.
The complete function of virulence protein is primarily facilitated by peroxisomes during this process of infection as in case of Magnaporthe oryzae (rice blast fungus). Colletotrichum higginsianum causes anthracnose disease in cruciferous plants via ChSTE7 gene that contributes in forming appressoria, vegetative and invasive growth in plant host tissue. Acetylation and other modes of histone modifications like methylation control regulation of transcription and structural chromatin organization to induce functional response. The genetic expression profiles are strategically transferred by the process of histone modification. The growth and fungal development are suggested to be controlled by SET-domain comprising proteins. It has been observed that Ash1like histone modification protein MoKMT2H essentially participates to induce pathogenesis of M. oryzae.
5 Conventional Strategic Approach for Phyto-infections and Disease Management
Epiphytotics (plants disease epidemics) transpires in crops every year and is a common phenomenon in different parts of the world. Plant pathogens negatively impact the both quality and quantity of the marketable agricultural yield that adversely affect the economy (Agrios 1997). Globally, phyto-diseases are responsible for 14% crop loss, while yield losses may account to 20–40% in the cultivates varieties (Baker et al. 1997). The pathogenic dissemination possesses a serious threat to the sustainable supply of food chain as it is responsible for enhancing the severity as well as the incidence of disease development (Savary et al. 2012). Although eradicating the phytopathogens completely still remains a challenge, extensive research is going on to explore new avenues for management of these infections in plants.
Among the various approaches that are associated in managing the plant infections, the conventional strategies include (1) implementing good and proper farming techniques that can resist infections, (2) destroying physically that is by plucking uprooting, etc. the affected or infected plant parts and tissues such as wilted roots, or stems, diseased fruits and other parts to hinder pathogen transmission from the infected to healthy parts, (3) control of pathogens through different measure which might involve insecticides or broad spectrum pesticides (e.g., copper) to check insect vectors, antibiotic to suppress infections precipitated by bacteria. These strategies mainly emphasize in preventing the plant disease to spread to healthy parts rather than on the cure of phyto-infections. Multi-integrated disease management practices are generally preferred. In cropping system and also in horticulture, use of disease-resistant plant and hybrid varieties is widely practiced. On the other hand, to minimize the use of hazardous and toxic chemical in environment, development of genetically modified plants is also being used that have the capacity of resisting development of pest and pathogens. However, such plants have limited cultivations due to their associated risk and low consumer acceptance (Hails 2000). Producers also take care and use sanitized and disinfected, certified virus/bacteria free tools and farming equipment to curb the growth of pathogens. Crop rotation techniques are followed and care is taken to prevent development of wound on plant surface that serves as an entry point for virulent pathogens. Additionally, bacteriophages are also used to control bacterial pathogenesis specifically to prevent bacterial infections. Phage-coded endolysins are also being attempted to be successfully incorporated in plants. As an alternative to use antimicrobial in agriculture, host-specific phages are being widely researched to reduce the environmental risks and concerns (Frampton et al. 2012). However, all these methods are associated with their individual shortcoming and thus require integrative approach of implementing two or more strategies to prevent crop loss to diseases. Although several countries witnessed evident abundancy in agricultural output as a consequence of green revolution (Pingali 2012), but this food and crop prosperity is gradually becoming disturbed due to pathogenic attack of food and plants, climatic changes, deterioration of soil quality, scarcity of arable lands, extensive increase in population, and many other associated factors.
In order to reinstate the food security, complementary strategies that are both efficacious and environmentally safe are need of the hour. In this context, the field of nanotechnology with its diversified applications and benefits may serve as an exciting opportunity to establish nano-weapons that may prove advantageous in phyto-disease management.
6 Nanotechnology and Its Impact on Agricultural Produce
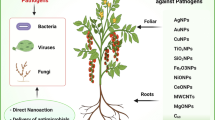
For the past decades, extensive research on nanoscience and its associated technologies are constantly evolving in the fields of agriculture and also food system (Nair et al. 2010). Development of novel nanoparticles is being implemented to improve the chain of food supply with sustainable intensification and also by managing soil and water conditions. With at least one dimension having a size range of 1–100 nm, the nanomaterials are characterized by unique size-dependent properties, which include higher surface: volume ratio, better conductance ability, optical properties. Such varied features allow these nano-systems to be used not only to protect plants but also to provide them with nutrition (Ghormade et al. 2011). Nanomaterials are also being used for biotechnological purposes (Mukhopadhyay 2014; Dapkekar et al. 2018; Silva et al. 2010) which include amelioration of complications-associated soil structure providing stability against soil erosion and maintain salinity balance, increasing availability of nutrient and mobility, for identifying moisture content, availability of macronutrients, pH of soil, etc. and controlling environmental pollution, and finally as nano-cargoes for delivery of herbicides, pesticides, siRNAs, micronutrients, DNA, etc. The utilization of nanotechnology is not only restricted to the biotechnological advancements but is also extensively implemented in agriculture (Mohmood et al. 2013; Khiyami et al. 2014; Paknikar et al. 2005) where it is used for removing water or soil contamination, antimicrobial advanced food packing, nano-barcoding, biosensors, agro-commodities shelf life indicators, nanoparticles (clay-based)-mediated water management, bioremediation, etc. Figure 4 depicts the diversified application of nanotechnology in different fields of agriculture. For the past few decades, there has been steady increase in integrating nanotechnologies with agricultural practices. However, every avenue of nanotechnology and its advantages should be explored to the fullest and implemented strategically to maintain quality, sufficiency, and security of food supply, along with prevention of phyto-infections, diagnosis of plant disease, and genetic transformations.
7 Types of Nanoparticle and Their Role in Phytopathogen Suppression
7.1 Silver Nanoparticles (AgNps)
AgNps are well known for their broad spectrum and potent antibacterial activities and based on these properties, they were first investigated for management for phyto-diseases. Numerous studies have pointed out the efficacy in plant disease management. Prior application of nano-formulation containing silver and silica with a hydrophilic polymer (0.3 mg/L) on the cucumber leaves has been reported to show protective effect against Podosphaera xanthii (Park et al. 2006). Similarly, colloidal silver nano-formulation restricted the growth of Sphaerotheca pannosa and thus prevented the occurrence of rose powdery mildew (Kim et al. 2008). Similar studies were further substantiated with more detailed investigations in which silver nanoparticles (10–100 mg/L) were sprayed on cucumber and pumpkin leaves before and after the plants were infected with powdery mildew. The results indicated that at both stages of application, highest concentration of AgNps formulation was associated with only 20% disease incidence. Such results also indicated the rationale use of AgNps for rescue treatment as the nanoparticles produced comparable outcomes with that of a few commercially available fungicides (Lamsal et al. 2011a). Another study pointed out the efficacy of NanoAg formulation (100 mg/L) in suppressing anthracnose disease outbreak when applied to peppers prior to the infection (Lamsal et al. 2011b). It was also found that the postharvest disease severity in banana caused by Colletotrichum musae was significantly reduced when applied at a concentration of 2000 mg/L (Jagana et al. 2017). Silver nano-formulations are found to be effective in controlling various phytopathogens belonging to fungal and bacterial species like Xanthomonas, Bacillus sp., Acidovorax, Pseudomonas, and Azotobacter sp., (Fayaz et al. 2009; Krishnaraj et al. 2012; Mala et al. 2012). AgNps are also found to act synergistically with other Nps of Titanium dioxide (TiO2), graphene oxide, silicon-aluminum carbide, and copper for providing protection against infections such as scabs, wilts, and molds caused to economically important agricultural crops like tomatoes, potatoes, and rice (Ocsoy et al. 2013; Boxi et al. 2016; Strayer et al. 2016; Aleksandrowicz-Trzcińska et al. 2018; Bhargava et al. 2018). Studies have pointed out AgNps stabilized by mucin derived from bovine submaxillary show potent action against seedling infections caused by both gram-negative and gram-positive bacteria such as Acidovorax, Xanthomonas, and Clavibacter, respectively (Makarovsky et al. 2018). On the other hand, bile salt sequestered AgNps were found to be effective against anthracnose disease (Shanmugam et al. 2015). While Ag-chitosan nanocomposites were found to prevent infections in strawberry caused by molds (Moussa et al. 2013), potent antifungal efficacy of amphopolycarboxyglycinate-stabilized silver nano-dispersions has been observed against phytopathogenic fungi like Phytophthora infestans isolated from pathogen infected potatoes (Krutyakov et al. 2016). AgNps are also being considered as alternative to pesticides owing to their wide range of antimicrobial potency.
7.2 Copper Nanoparticles (CuNps)
The crucial role of redox active transition element copper in plant biology is well accepted. This trace element is an integral component of most metalloenzymes that participate in different plant metabolic processes like photosynthesis respiration (Elmer and White 2018). Copper and its associated compounds comprised the first metal containing fungicides that were used to check pathogenic invasions and since then their wide-ranging antimicrobial activity has been utilized for antipathogenic management for centuries (Lamichhane et al. 2018). In order to resist bacterial blight copper hydroxide, Bordeaux mixture, copper oxychloride, etc. are still being used in pomegranate (Ruparelia et al. 2008). In the recent years, copper nanoparticles are being investigated for their anti-pathogenic activities and also being manufactured in large-scale industrial level. Various factors like of copper concentration, pH, temperature, and also pathogenic concentration affect the bioactivity of copper Nps (Ruparelia et al. 2008). Studies have pointed out CuNps treatment (0.2 mg/L) inhibited the growth of X. axonopodis pv. punicae, suppressing water-soaked lesion and thus protecting pomegranate leaves (Mondal and Mani 2012). On the other hand, CuNps formulation in conjugation with MBPF-01 (Pseudomonas fluorescens strain, antagonist strain of bacteria) conferred in 70% reduction in incidence of leaf blight infection in rice plants mediated by X. oryzae pv. Oryzae (Mondal et al. 2010). CuNps also manifest significant protective action against mungbean blight caused by X. axonopodis pv. phaseoli (Mondal and Mani 2012; Mondal et al. 2010) while wilt disease in tomato caused by Fusarium and Verticillium was markedly reduced on application of foliar nanoformulations of copper oxide, manganese oxide, and zinc oxide (Elmer and White 2016). Its destructive effects toward pathogens such as Alternaria alternata, Phoma, and Curvularia lunata are also well known (Kanhed et al. 2014). Recently, reports exhibited inhibitory efficacy of Cu-copper oxychloride (Cu-CoC) nano-formulation (50 mg/L) against Phytophthora cinnamon. Mycelial development as well as sporulation were suppressed by their synergistic effect when used against the phytopathogen Alternaria alternata. When used against Pseudomonas syringae, CuNps showed inhibitory action at 200 mg/L concentration. Studies have also pointed out the biocompatibility of these Nps as they do not adversely affect microorganisms beneficial for plants such as Rhizobium spp. and Trichoderma harzianum (Banik and Luque 2017). Novel nano-compounds such as fixed quaternary ammonium compounds, core shell copper composites, multivalent copper nanoparticles evaluated for their protective effects against tomato bacterial spot demonstrated bactericidal activity against causative agent Xanthomonas perforans (copper-resistant strain). Such copper nanoparticles have manifested evident control of phyto-diseases under greenhouse environmental conditions without negatively affecting the yield of tomatoes (Strayer-Scherer et al. 2018). They also efficiently inhibit Phytophthora infestans infections in tomatoes (Giannousi et al. 2013). When used against species belonging to fusarium genera, CuNps showed potent activity in resisting the phytopathogens Fusarium equiseti, F. oxysporum, and F. culmorum (Bramhanwade et al. 2016). Green synthesis of copper nanoparticles with leaf extract of papaya demonstrated significant inhibitory effect on soil-borne Ralstonia solanacearum, causing wilt under both normal and green house conditions (Chen et al. 2019). Additionally, CuO nanoparticles in the form foliar spray also showed bactericidal effect against Fusarium oxysporum f. sp. niveum in watermelons thereby preventing wilt. Under greenhouse surroundings also these nanoparticles evinced bactericidal actions with simultaneous increment in yield (Elmer and White 2018). Copper nano-formulations, synthesized using Streptomyces zaomyceticus Oc-5 and Streptomyces pseudogriseolus Acv-11, were found to be efficient antifungal activities against a number of phytopathogenic fungal strains like Aspergillus niger, Pythium ultimum, Alternaria alternata, and Fusarium oxysporum (Hassan et al. 2019).
7.3 Zinc Oxide (ZnO)-Based Nanoparticles
Inorganic zinc oxide possesses unique photocatalytic, optical, electrical as well as magnetic characteristics (Wang 2004). In addition to the wide-ranging usage in ceramics, pharmaceuticals, rubber industry, ZnO-Nps are also being extensively used in the agricultural industry. Apart from its function as micronutrient fertilizer, recent studies also document the antimicrobial efficacy of these Nps (Kołodziejczak-Radzimska and Jesionowski 2014; Dizaj et al. 2014). Zinkicide SG4 & 6 formulated as zinc oxide-based nano-formulations when tested against X. citri subsp. citri and C. paradisi exhibited potent bactericidal effect when applied in foliar spray and thus decreased the developmental incidence of citrus canker in sweet orange and prevented grape fruit (ruby red) rot (Graham et al. 2016). Zinkicide exhibited broad spectrum activities against disease caused by phytopathogenic fungi such as Elsinoe fawcetti and Diaporthe citri, lowering the incidence of citrus scab and melanose on grapefruit. The zinc oxide nanoparticles are also reported to resist the phytopathogenic effects of bacteria species like Xanthomonas citri subsp. citri, E. coli, and X. alfalfa subsp. citrumelonis. Moreover, their potential actions against Botrytis cinerea and Penicillium expansum causing postharvest disease are also documented. Conidiophores along with conidia development in P. expansum are restrained by the zinc-based nanoparticles that gradually lead to degeneration of the hyphae of pathogenic fungus thereby losing their ability to cause infection (He et al. 2011). In another studies ZnoNps when used in broth of mung bean broth and also in sand, resulted in significant repression of F. graminearum growth (Dimkpa et al. 2013a). It also inhibits the mycelial growth of pathogenic Sclerotinia homoeocarpa and thus prevents the appearance of dollar spots in cool season turfgrasses (Li et al. 2017). Nanocomposites of silica and zinc oxide were found to be toxic against Cercospora beticola Sacc and thus prevented sugar beet from the disease CLS (Cercospora beticola Sacc) (Derbalah et al. 2012). The inhibitory potency of these Nps against Aspergillus fumigatus and A. flavus has also been documented (Navale et al. 2015).
7.4 Titanium Dioxide (TiO2) Nanoparticles
Owing to the chemical stability and nontoxic nature of titanium dioxide, their nano-formulations are widely being explored for environmental and agricultural applications. Having a long shelf life, TiO2 has been reported to have antibacterial effect. It has been seen that titanium oxide Nps enhance the photosynthetic rate and promote growth of plants with simultaneous increment in yield and truncated disease severity (Chao and Choi 2005). In a field trial, titanium nanoparticle in the form of foliar spray manifested high protection against the phyto-diseases brown blotch as well as cercospora leaf spot in Vigna unguiculata Walp (Owolade et al. 2008). On the other hand, lesions in geranium plants and leaf spot disease in poinsettia plants caused by Xanthomonas hortorum pv. pelargonii and Xanthomonas axonopodis pv. poinsettiicola respectively were evidently reduced by the application of TiO2 nano-formulations (Norman and Chen 2011). The TiO2 nanoparticles are also known of imparting photocatalysis (Paret et al. 2013a). Studies have also revealed the potency of titanium hollow nanoparticles with or without silver doping as potent antifungal agents resisting the development of tomato or potato wilt caused by Fusarium solani and apple scab infection by Venturia inaequalis. Owing to the photocatalytic property, the significant antimicrobial efficacy was found in visible light. The titanium nano-formulations at low dose is also found to arrest the formation of fungal pathogenicity imparting naphthoquinone pigment in Fusarium solani (Boxi et al. 2016). When combined with zinc, the TiO2-Zn nanocomposites revealed reduction in severity bacterial spot in tomatoes with resisting the growth of Xanthomonas perforans without any adverse effect on the yield (Paret et al. 2013b). Colonization of Hypocrea lixii circinelloides and Mucor circinelloides is also significantly arrested by titanium oxide Nps thus preventing decay in wood (De Filpo et al. 2013). The oxidizing capability of titanium nanoparticles renders their antimicrobial efficacy. These Nps degrade the cellular membrane in bacteria leading to cellular components leakage that succumbs to impediments essential cellular activities (Frazer 2001). TiO2 is often used as nanocarriers for silver nanoparticle that allows them not to get aggregated.
7.5 Other Nano-formulations Preventing Phyto-pathogenesis
In addition to all the nanoparticles discussed individually earlier, many of them are used in combination which may impart improved antimicrobial activities. Studies have indicated the potency of silver-silicon dioxide in preventing infections caused by Phytophthora capsici, Fusarium oxysporum as well as Rhizoctonia solani (pathogenic soyabean crop fungi) mediated by generation of reactive oxygen species in association with the released silver ions from the nanoparticles’ surface. Such results imply promising role of Ag-SiO2 nanocomposites in soyabean farming (Nguyen et al. 2016). The essential functions of sulfur in plant biology is well known and it is used as one of the primary components in many formulations used for commercially managing plant infections. Recently the efficacy of sulfur nanoparticles in organic farming is also being explored to protect crops and plants like apple, tomato grapes, and potatoes (Rao and Paria 2013). Sulfur nanoformulation (1000 mg/L) has been found to significantly reduce the invasion of Erysiphe cichoracearum in okra (Gogoi et al. 2013) and requires lower concentration than that of available commercial products to prevent the powdery mildew infection. While Aspergillus niger is efficiently restricted by sulfur Nps, early blight in tomatoes and apple-scab caused by F. solani and V. inaequalis is also decreased evidently by the small-sized nanoparticles (Rao and Paria 2013). As the nanoparticles deposit on the fungal cell wall, they contribute in its digestion leading to leakage of cytoplasmic components and subsequent fungicidal activity.
Other nanoparticles possessing antibacterial potency are the graphene oxide ones. The graphene oxide (GO) Nps have shown to inactivate X. oryzae pv. oryzae strain resistant to copper, Aspergillus oryzae, A. niger, and F. oxysporum (Chen et al. 2013). GONps demonstrated 90% cell death when applied against Pseudomonas syringae, X. campestris pv. undulosa and also are being used in treating macroconidia caused by F. graminearum and oxysporum (Chen et al. 2014). Thus, they can efficiently protect against various diseases like bacterial leaf blight and leaf streak, fungal head blight. Studies have also indicated silver graphene nanocomposites to show antipathogenic efficacy against X. perforans therefore reducing the incidence bacterial spot in tomato plant (Ocsoy et al. 2013). On the other hand, silver graphene composites containing dsDNA have prominent effect in reducing severity of phyto-disease as they accumulate on the pathogenic cells destroying them (Ocsoy et al. 2013). These nanocomposites in another study were found to show potent inhibitory action against both Cu-tolerant and sensitive X. perforans strain causing tomato bacterial spots (Strayer et al. 2016). Bactericidal activity was also observed against other pathogenic strains like X. vesicatoria, and X. gardneri. Another recent study has revealed the enhanced protective effect of GO-Ag nanoparticles against the rice pathogen X. oryzae pv. oryzae in comparison to silver nanoparticles alone (Liang et al. 2017). In floriculture, and specifically during the stages of growth as well as post-harvest, the fullerene nanoparticles can render protection against rose plants infection by resisting the growth of B. cinerea (Hao et al. 2017). Zinc oxide and magnesium oxide nanoparticles and also their combination nanocomposites (ZnO-MgO) and ZnO-Mg(OH)2 are reported to possess potent bioactivity against the fungal phytopathogen Colletotrichum gloeosporioides responsible for causing anthracnose in economically important crops like Persea americana and Carica papaya. These nanoparticles cause structural degradation of conidia thereby preventing its germination and thus can prevent anthracnose disease commonly occurring in tropical fruits (De la Rosa-García et al. 2018). MgO nanoparticles when used for treating roots of tomato seedlings depicted efficacy in protecting them against the incidence of infection caused by Ralstonia solanacearum while another study revealed their efficacy in inhibiting the Cu-resistant strain of X. perforan (Imada et al. 2016; Liao et al. 2019).
7.6 Suppression of Phytopathogens by Green-synthesized Nanoparticles
In the recent years, researchers have concentrated in the field related to green synthesis and its rationale utilization to resist phytopathogens and their adverse effects. Ag, Cu, gold (Au), Zn, and other metallic nanoparticles formulated as biosynthetic preparations are known to exhibit broad spectrum antipathogenic activities with potent antibacterial efficacy against both gram-positive and -negative bacteria including Bacillus subtilis, S. aureus, and E. coli and also against certain virulent fungi like Aspergillus niger, F. oxysporum (Nisar et al. 2019). While green synthesized silver nanoparticles using Streptomyces exhibited strong antifungal activity against A. niger, Alternaria alternata, Pythium ultimum, and F. oxysporum, zinc oxide and titanium oxide nanoparticles formulated using extract of lemon fruit resisted the incidence of stem and root infection of sweet potato caused by Dickeya dadantii (Hossain et al. 2019). On the other hand, chamomile flower extract used as reducing agent in formulating magnesium oxide and manganese dioxide nanoparticles resisted the growth of Acidovorax oryzae, the bacterial strain responsible for brown stripe infection in rice.
8 The Nanoparticles and Their Mechanisms to Prevent Phytopathogenesis
8.1 Metallic Nanoparticles
The probable mechanisms of the metallic nanoparticles in imparting antipathogenic activity are documented in Table 3.
8.2 Green Synthesized Biocompatible Nanoparticles
Although metallic Nps being formulated following green synthesis exhibit potent antipathogenic effect, however, the exact mechanism by which they exert their action is still not completely known. The Nps have been documented to disrupt the normal protein functions by either oxidizing cystine in the iron binding site or degrading iron–sulfur cluster or by exchanging catalytic or structural metals. They may also cater to depletion of cell membrane potential or membrane degradation leading to impairment in cell-membrane or functions cause oxidative stress through generation of ROS. The oxidant and antioxidant balance is disturbed as the Nps deplete antioxidants and also inhibit expression of iron (III) transporter gene thus interfering with uptake of nutrients. Moreover, the nanoparticles also precipitate genotoxicity. Some or all of these mechanisms function simultaneously and contribute in the antipathogenic action (Lemire et al. 2013). The positive or very low negative charge bearing nanoparticles adheres to the microbial membranes that are negatively charged through electrostatic attraction and disrupts the morphological components and structures. These alterations depolarize the membrane and interferes with permeability of cellular membrane as well as respiration that confers in cell structures degeneration and final cell death. The degeneration of cellular structure causes the exudation of DNA, proteins, enzymes, metabolites, and other internal contents. Additionally, irregular pits produced by nanoparticles on the cell wall of the pathogens allow them to penetrate into the periplasmic tissues and intracellular spaces of the microbial cells (Gahlawat and Choudhury 2019). AgNps formulated through green synthesis technique promoted rupturing of cell wall with subsequent release of cytoplasmic as well as nuclear constituents and cellular swelling which resulted into bacterial cell death of Acidovorax oryzae strain (RS-2) (Elbeshehy et al. 2015). Similarly, according to another report, on application of another biosynthesized silver nano-formulation against Fusarium graminearum, it distorted hyphae and degenerated cell wall inducing antifungal activity (Ibrahim et al. 2020). Corresponding outcomes were also observed in case of application of such AgNps against various pathogenic microbes such as A. alternata, Botrytis cinerea, and Trichosporon asahii (Xia et al. 2016). The cytotoxicity of these Nps can also be imparted through the generation of oxidative stress mediated by accumulation of ROS (Vankar and Shukla 2012). As a consequence of the release of free radicals, microbial cell wall as well as other integral components such as DNA, proteins, and lipids are degraded. ROS may impart mutation of DNA, its deletion, double or single stranded breakage, protein crosslinking, etc. (Soenen et al. 2011) and all these have synergistic antibacterial effect.
8.3 Nanoparticles and Their Role as Immune Elicitors
The natural co-polymers, chitosan, and chitin are together available in nature. Chitosan being a biocompatible polymer has several advantages like lesser toxicity, and biodegradability and has been used as drug delivery system for years (Rodrigues et al. 2012). Studies have revealed immunomodulatory function of chitosan in plant systems. Chitosan Nps promote growth and protects plants and also have biocide actions (Sathiyabama and Parthasarathy 2016). Foliar application of chitosan Nps results in augmentation of innate immunity mediated by various mechanism such as enhancement of activities of defensive enzymes, upregulated expression of genes related to defense system, and increment in total phenolic content. Biocompatible chitosan Nps are also used as phytosanitary agents (Chandra et al. 2015). Reports have pointed out the antifungal potency of chitosan Nps to protect finger millet leaves from blast disease symptoms caused by the invasion of Pyricularia grisea. These nanoparticles trigger ROS formation and elevate peroxidase enzyme activity in the leaves thereby leading to the antifungal action (Sathiyabama and Manikandan 2016). Yet another study depicted the ability of chitosan Nps in evoking nitric oxide that induced innate defense activities and protected tea plants from symptoms of blister blight infection (Chandra et al. 2017). Chitosan nano-formulations were also found to exhibit protective effects against F. oxysporum, P. capsici, X. campestris pv. vesicatoria, and also Erwinia carotovora causing infections in tomatoes. When used in maize to protect it from Curvularia leaf spot disease, biocompatible copper-chitosan nanocomposites showed potent antimicrobial action and also promoted plant growth. These nanocomposites aggravated activities of antioxidant enzymes like superoxide dismutase, peroxidase, polyphenol oxidase, and phenylalanine ammonia-lyase thus inducing a defense response (Choudhary et al. 2017). Harpin is known for eliciting death of hypersensitive cells, incite plant to be disease as well as insect–resistant, and effectuate plant growth. Through activation of PAMP (pathogen-associated molecular pattern)-induced immunity, harpin renders plant disease-resistant. Despite such biological activities, its use as biopesticide is limited because of poor assimilation. To overcome this hurdle, chitosan is used as biocarrier of harpin and when harpinPss (an elicitor derived from P. syringae pv. syringae) incorporated chitosan Nps were used, they promoted peroxidase and phenylalanine ammonia lyase functions thereby resisting Rhizoctonia solani disease (Nadendla et al. 2018).
Selenium nanoparticles are also documented to produce resistance against root-knot nematode and thereby protect tomato plants from infection. Expression of PR-6 gene by selenium Nps results in upsurge of proteinase inhibitors activity and thus shields the roots and leaves from microbial invasion (Udalova et al. 2018). Similarly, when synthesized using Trichoderma asperellum, these selenium Nps restricted the incidence of downy mildew disease in pearl millets through truncated growth, viability of zoospore and sporulation of Sclerospora graminicola (Nandini et al. 2017). Moreover, selenium Nps are also found to arrest biofilm formation and viability of Clavibacter michiganensis subsp. sepedonicus (Perfileva et al. 2018).
9 Multifaceted Roles of Nanoparticles to Limit Plant–Pathogen Interactions
9.1 Nanomaterial-Based Diagnostic Tools for Phytopathogenic Detection
In order to efficiently manage plant disease, accurate and timely detection of the virulent pathogens with simultaneous applications of pesticides is of utmost importance. Nanoparticles may also be employed as biomarkers for detection of virulent phytopathogens (Chartuprayoon et al. 2013; Yao et al. 2009). They are either formulated for directly detecting the disease evoking pathogens or may sense certain conditions or signals that are associated with the phytopathogenic diseases. Nanochips comprise of oligoprobes (fluorescent) that are highly sensitive and specific in detection of even a single bacterial or viral nucleotide mutation. Various nanochips are being efficiently employed for pathogenic detection including antibodies conjugated silica (fluorescent) nanochips that can sense Xanthomonas axonopodis pv. vesicatoria (Yao et al. 2009) and gold nanoparticles acting as immunosensors for detecting Tilletia indica, the causative agent for karnal bunt disease in wheat (Singh et al. 2010). If utilized in certification of seed as well as in plant quarantine, these nano-sensors can provide an effective platform for detection of pathogenic diseases of plants. It is also known that stress conditions trigger various physiological changes in plants (Khan and Rizvi 2014). Stress-mediated stimulation of plant defense system coordinated majorly by salicylic and jasmonic acid and methyl jasmonate is one of such primary examples (Khan and Haque 2013). Copper (Cu) nanoparticles in combination with Au modified electrode is reported to be successfully used for identifying changes in level of ascorbic acid, salicylic acid, etc. in plants as well as seed which may help in detection of pathogen invasion (Wang et al. 2010). Advancements in such sensing technologies, which can identify alterations in physiological and/or biochemical parameters or directly monitor the pathogenic invasion may prove to be beneficial for management of phyto-diseases and protect economically important crops.
9.2 Nano-Pesticides and Efficient Carrier System
As nano-formulations act as efficient carrier systems allowing release of chemicals in controlled fashion, thus they can significantly reduce the quantity of pesticides that are generally required and its associated toxic outcome. The use of nano-pesticides will not only curb the conventional application rate of pesticides but will also exponentiate their effectivity owing to the timely and controlled release of chemicals as and when requires at the time of phyto-infection. The improved stability, lower viscosity, nanosize, and optical transparency confer nano-emulsions as better delivery cargoes for pesticides which may enhance the solubility of the active chemical ingredients and hence their bioavailability (Xu et al. 2010). Imidacloprid (1-(6 chloro-3-pyridinyl methyl)-N-nitro imidazolidin-2-ylideneamine) formulated as controlled release nano-pesticides using PEG (polyethylene glycol) and other aliphatic diacids has shown significant potency in pest control for different crops (Adak et al. 2012). While polyhydroxyl alkanate is used as a biodegradable polymer to achieves sustainable release of pesticides (Pepperman et al. 1991), Intelimer, the thermosensitive polymer on the other hand controls pesticide release based on the temperature variations in different seasons which prevent undesired leaching. The suitability of different polymers like those from natural sources like proteins and polysaccharides, or polyacrylamide, and polystyrene categorized as synthetic polymers or even inorganic materials such as zeolites, glass beads, ceramics, etc. is being extensively researched for the production of nano-pesticides (Chuan et al. 2013). SDS (sodium dodecyl sulfate) fabricated silver-titanium dioxide nano-formulation of imidacloprid have shown improved protective efficacy against phytopathogen invasion in cabbages and cucumbers at seedling phase (Guan et al. 2010). On the other hand, studies have indicated encapsulation of zineb or mancozeb, the two common pesticides inside carbon nanotubes graft poly citric acid material helped in nano-fungicide formation which significantly arrested A. alternata mediated fungal spot development (Sarlak et al. 2014).
Thus, these nano-formulations, containing active pesticide chemical, increase the efficiency of protecting the crops and plants thereby escalating productivity of food with simultaneous reduction in adverse environmental impact (Chhipa 2017). Such nano-formulations also prevent unnecessary degradation of pesticides due to evaporation and leaching. And thus, with proper eco-toxicological screening they can be used as a promising technique for harnessing plant–pathogen interactions.
10 Nanotechnology and the Threats Imposed
10.1 Environmental Impact of Metallic Nanoparticles
Although the nanoparticles play major role in controlling the plant–pathogen interactions (Elmer and White 2018), but they also impose certain adverse effect on environment (Fig. 5). The nanoparticles which resist the growth of pathogenic microbes in the crops sometimes get dispersed in the surrounding soil, water bodies, and also atmosphere through probable processes like leaching, air current-mediated transfer, and through rainwater as well as transfer trophically (Gardea-Torresdey et al. 2014). It has been indicated that the microbes existing in the roots of plants, soil and sedimental deposits may also absorb these nanoparticles which finally undergo accumulation (Kim et al. 2016). Various organisms like mollusks, arthropods, protozoa, insects, fishes, and birds when consumes or utilize plant products, microbes or even the waste products assist in trophical transferring of the NPs from one level to another (De la Torre et al. 2015). Such process lays concern that the risk and adversities posed by the nanoparticles may be inherently passed to the offspring (Shimizu et al. 2009) which is evident among the marine ecosystem and organisms and also the food chain lining plants with herbivorous and carnivorous animals (De la Torre et al. 2015; Shimizu et al. 2009; Bielmyer-Fraser et al. 2014). Keeping such scenario in mind, the nanoparticle formulations application should be standardized and rationally used to maintain their safety and sustainability in agriculture. The probable risk associated with nanoparticles should be judged carefully and proper knowledge about the nature of interaction of such Nps with the crops and plants along with the ecosystems in which they exist which includes other plants, animals and microbial organism is mandatory before they are being used in large scale field applications.
10.2 Toxic Impact of Nano-formulations on the Favorable Microbial Plant Interrelation
The epiphytic and endophytic association of various microbial organisms with the plants are well known as they exist in bulk soils surrounding rhizosphere and root. The microorganisms participate actively in producing auxins and phytohormones, N2 fixation, solubilizing phosphate, etc. (Abdallah et al. 2019). Evaluation of respiratory activities as well enzymatic functions in soil allows understanding of the alterations occurring in microbial community in soil (Simonin and Richaume 2015). Reduction in biomass, truncated enzymatic actions of urease, dehydrogenase and phosphatase along with decreased levels of phospholipid (total) fatty acid are observed in the soil microbiota on application of copper oxide and titanium dioxide Nps in flooded field of rice (Xu et al. 2015). Saline and black soils also indicate similar alterations in enzymatic activities (catalase, invertase, etc.) and diversity of soil microbiota when nano-formulations of iron, cerium, zinc, etc. are applied (You et al. 2018). Application of cerium and zinc nanoparticles have exhibited to affect soil enzyme functionalities with reduction in thermogenic metabolic action, number of bacteria enabling phosphate and potassium solubilization, azotobacter, etc. (Chai et al. 2015). Similar reports are also presented for silver and zinc oxide Nps which interferes and diminishes the number of bacteria participating in nitrogen fixation like Rhizobium leguminosarum Azotobacter chroococcum solubilization of phosphates like Arthrobacter sp. MTCC 8160, Serratia marcescens MTCC 7642 and also formation biofilm like Bacillus subtilis MTCC 441, Pseudomonas aeruginosa MTCC 7763 (Chavan and Nadanathangam 2019). While silver Nps demonstrated bactericidal consequence and modified the soil bacterial population, the zinc nanoparticles were accompanied with bacteriostatic outcome (Chavan and Nadanathangam 2019). Studies have also indicated zinc nanocomposites to possess more toxicity than that of cerium nanoparticles (Bandyopadhyay et al. 2012) against the helpful soil microbiota.
10.3 Metallic Nanoparticles and Their Adverse Impact on Plants
The nanosize dimension of the particles allows acts as a dual sword in case of nano-formulations by facilitating the plants to fight against pathogens on one hand and on the other, adversely affecting beneficial microbes and surrounding plants, animals, fishes, birds, and even humans. The nano-formulations are applied to the plant parts present both above and below the ground and even on seedling stage. These nanoparticles may induce antipathogenic effects locally or undergo translocation all through the plant system and get accumulated in different plant parts thereby contributing to toxicity in different cells or organs (Kurepa et al. 2010). Exact size, surface of particles, and even composition determine the degree of Nps uptake and distribution along with other factors like concentration of nano-formulations and species on plant to which they are applied. As they get accumulated in different plant parts, the physiology of plant gets affected which in turn adversely effects plant growth. Minimal concentration of nanoparticles is reported to have high toxicities even with rare dispersal in environment (Ali and Ali 2019). Excessive nanoparticles when used in cultivation of crops like tomato, zucchini, and wheat disrupt electron transportation chain that subsequently cause oxidative stress. This impedes the processes involved in detoxifying free radicals and causes genotoxic outcome (Dimkpa et al. 2013b; Pakrashi et al. 2014) which in turn interferes with production of plant hormones and secondary metabolites essential for plant growth (Sanzari et al. 2019). The nano-formulation restrains transportation of water in plants, and promotes chromosomal disruption, reduction in growth hormone formation, disorders of metabolic system, alterations in profiles of gene transcription, etc. which makes the plants more susceptible to naturally occurring toxins like arsenic (Morales-Díaz and Ortega-Ortíz 2017).
11 Conclusion
Nanotechnology may serve as a magic bullet for developing multimodal techniques that will contribute to improving plant health either by disease monitoring or through controlling disease or strengthening the plant immune system. Various nanometallic formulation has shown to facilitate disease suppression at much lower concentrations than their counterpart fungicides or other pesticides. However, the associated limitation of such nano-formulation on adversely affecting plant health, soil microbiota, and beneficial soil microbial interactions with plants along with other toxicities imposed on environment and ecosystems should be strategically addressed. Integrating nanotechnology and plant pathology will mark the advent of new era of the use of functionalized metallic nanoparticles as pesticides, insecticides, nutrient fertilizers, etc. and would succor to conquer the impediments of food production globally.
References
Abdallah Y, Yang M, Zhang M et al (2019) Plant growth promotion and suppression of bacterial leaf blight in rice by Paenibacillus polymyxa Sx3. Lett Appl Microbiol 68:423–429
Abdulkhair WM, Alghuthaymi MA (2016a) Plant pathogens. In: Rigobelo EC (ed) Plant growth. IntechOpen, London
Abdulkhair WM, Alghuthaymi MA (2016b) Plant pathogens. In: Plant growth. IntechOpen, London, p 49
Abdullah AS, Moffat CS, Lopez-Ruiz FJ et al (2017) Host–multi-pathogen warfare: pathogen interactions in co-infected plants. Front Plant Sci 8:1806
Abramovitch RB, Kim YJ, Chen S et al (2003) Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J 22:60–69
Adak T, Kumar J, Dey D, Shakil NA, Walia S (2012) Residue and bio-efficacy evaluation of controlled release formulations of imidacloprid against pests in soybean (Glycine max). J Environ Sci Health B 47:226–231
Agrios GN (1997) Plant pathology, 4th edn. Academic, San Diego
Aleksandrowicz-Trzcińska M, Szaniawski A, Olchowik J, Drozdowski S (2018) Effects of copper and silver nanoparticles on growth of selected species of pathogenic and wood-decay fungi in vitro. For Chron 94:109–116
Ali SH, Ali SA (2019) Nanotechnology is the potential cause of phytotoxicity. J Biomater Dent 3:1–6
Allesen-Holm M, Barken KB, Yang L et al (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128
Amari K, Vazquez F, Heinlein M (2012) Manipulation of plant host susceptibility: an emerging role for viral movement proteins? Front Plant 3:10
An SQ, Allan JH, McCarthy Y et al (2014a) The PAS domain-containing histidine kinase RpfS is a second sensor for the diffusible signal factor of Xanthomonas campestris. Mol Microbiol 92:586–597
An SQ, Caly DL, McCarthy Y et al (2014b) Novel cyclic di-GMP effectors of the YajQ protein family control bacterial virulence. PLoS Pathog 10:e1004429
Aparna G, Chatterjee A, Sonti RV et al (2009) A cell wall-degrading esterase of Xanthomonas oryzae requires a unique substrate recognition module for pathogenesis on rice. Plant Cell 21:1860–1873
Arrebola E, Cazorla FM, Perez-Garcia A et al (2011) Chemical and metabolic aspects of antimetabolite toxins produced by Pseudomonas syringae pathovars. Toxins 3:1089–1110
Aslam SN, Newman MA, Erbs G et al (2008) Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr Biol 18:1078–1083
Asselin JAE, Lin J, Perez-Quintero AL et al (2015) Perturbation of maize phenylpropanoid metabolism by an AvrE family type III effector from Pantoea stewartii. Plant Physiol 167:1117–1135
Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP (1997) Signaling in plant-microbe interactions. Science 276:726–733
Balasubramaniam M, Kim BS, Hutchens-Williams HM et al (2014) The photosystem II oxygen-evolving complex protein PsbP interacts with the coat protein of Alfalfa mosaic virus and inhibits virus replication. Mol Plant-Microbe Interact 27:1107–1118
Bandyopadhyay S, Peralta-Videa JR et al (2012) Comparative toxicity assessment of CeO2 and ZnO nanoparticles towards Sinorhizobium meliloti, a symbiotic alfalfa associated bacterium: Use of advanced microscopic and spectroscopic techniques. J Hazard Mater 241:379–386
Banik S, Luque AP (2017) In vitro effects of copper nanoparticles on plant pathogens, beneficial microbes and crop plants. Span J Agric Res 15:23
Barber CE, Tang JL, Feng JX et al (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24:555–566
Bartsev AV, Deakin WJ, Boukli NM et al (2004) NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions. Plant Physiol 134:871–879
Bender CL, Alarcon-Chaidez F, Gross DC (1999) Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63:266–292
Bhargava P, Kumar A, Kumar S, Azad CS (2018) Impact of fungicides and nanoparticles on Ustilaginoidea virens causing false smut disease of rice. J Pharmacogn Phytochem 7:1541–1544
Bhatty M, Laverde GJA, Christie PJ (2013) The expanding bacterial type IV secretion lexicon. Res Microbiol 164:620–639
Bielmyer-Fraser GK, Jarvis TA, Lenihan HS, Miller RJ (2014) Cellular partitioning of nanoparticulate versus dissolved metals in marine phytoplankton. Environ Sci Technol 48:13443–13450
Block A, Guo M, Li G et al (2010) The Pseudomonas syringae type III effector HopG1 targets mitochondria, alters plant development and suppresses plant innate immunity. Cell Microbiol 12:318–330
Bogdanove AJ, Schornack S, Lahaye T (2010) TAL effectors: finding plant genes for disease and defense. Curr Opin Plant Biol 13:394–401
Bouarab K, Melton R, Peart J et al (2002) A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418:889–892
Boxi SS, Mukherjee K, Paria S (2016) Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 27:085103
Bramhanwade K, Shende S, Bonde S, Gade A, Rai M (2016) Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ Chem Lett 14:229–235
Bretz JR, Mock NM, Charity JC et al (2003) A translocated protein tyrosine phosphatase of Pseudomonas syringae pv. tomato DC3000 modulates plant defence response to infection. Mol Microbiol 49:389–400
Brown I, Mansfield J, Bonas U (1995) hrp genes in Xanthomonas campestris pv. vesicatoria determine ability to suppress papilla deposition in pepper mesophyll cells. Mol Plant-Microbe Interact 8:825–836
Burch AY, Shimada BK, Mullin SW et al (2012) Pseudomonas syringae coordinates production of a motility enabling surfactant with flagellar assembly. J Bacteriol 194:1287–1298
Buttner D, Bonas U (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34:107–133
Buttner D, Lorenz C, Weber E et al (2006) Targeting of two effector protein classes to the type III secretion system by a HpaC- and HpaB-dependent protein complex from Xanthomonas campestris pv. vesicatoria. Mol Microbiol 59:513–527
Chai H, Yao J, Sun J et al (2015) The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull Environ Contam Toxicol 94:490–495
Chandra S, Chakraborty N, Dasgupta A et al (2015) Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep 5:15195
Chandra S, Chakraborty N, Panda K, Acharya K (2017) Chitosan induced immunity in Camellia sinensis (L.) O, Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol Biochem 115:298–307
Chao SHL, Choi HS (2005) Method for providing enhanced photosynthesis. S Korea Bull 11:1–34
Chartuprayoon N, Rheem Y, Ng JC et al (2013) Polypyrrole nanoribbon based chemiresistive immunosensors for viral plant pathogen detection. Anal Methods 5:3497–3502
Chatterjee S, Almeida RP, Lindow S (2008a) Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu Rev Phytopathol 46:243–271
Chatterjee S, Wistrom C, Lindow SE (2008b) A cell–cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc Natl Acad Sci U S A 105:2670–2675
Chavan S, Nadanathangam V (2019) Effects of nanoparticles on plant growth-promoting bacteria in Indian agricultural soil. Agronomy 9:140
Chellappan P, Vanitharani R, Fauquet CM (2005) MicroRNA-binding viral protein interferes with Arabidopsis development. Proc Natl Acad Sci U S A 102:10381–10386
Chen J, Watanabe Y, Sako N et al (1996) Mapping of host range restriction of the Rakkyo strain of tobacco mosaic virus in Nicotiana tabacum cv. Bright yellow. Virology 226:198–204
Chen J, Wang X, Han H (2013) A new function of graphene oxide emerges: inactivating phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. J Nanopart Res 15:1658
Chen J, Peng H, Wang X et al (2014) Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 6:1879–1889
Chen J, Mao S, Xu Z, Ding W (2019) Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv 9:3788–3799
Chhipa H (2017) Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett 15:15–22
Chiang CH, Lee CY, Wang CH et al (2007) Genetic analysis of an attenuated Papaya ringspot virus strain applied for cross-protection. Eur J Plant Pathol 118:333–348
Chikte RG, Paknikar KM, Rajwade JM et al (2019) Nanomaterials for the control of bacterial blight disease in pomegranate: quo vadis? Appl Microbiol Biotechnol 103:4605–4621
Chin KH, Lee YC, Tu ZL et al (2010) The cAMP receptor-like protein CLP is a novel c-di-GMP receptor linking cell–cell signaling to virulence gene expression in Xanthomonas campestris. J Mol Biol 396:646–662
Choudhary RC, Kumaraswamy RV, Kumari S et al (2017) Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci Rep. https://doi.org/10.1038/s41598-017-08571-0
Chu M, Desvoyes B, Turina M et al (2000) Genetic dissection of tomato bushy stunt virus p19-protein-mediated host-dependent symptom induction and systemic invasion. Virology 266:79–87
Chuan L, He P et al (2013) Establishing a scientific basis for fertilizer recommendations for wheat in China: yield response and agronomic efficiency. Field Crop Res 140:1–8
Cramer HH (1967) Plant protection and crop production. Pflanzenschutz-Nachr, Leverkusen, p 20
Cui F, Wu S, Sun W et al (2013) The Pseudomonas syringae type III effector AvrRpt2 promotes pathogen virulence via stimulating Arabidopsis auxin/indole acetic acid protein turnover. Plant Physiol 162:1018–1029
Dapkekar A, Deshpande P, Oak MD et al (2018) Zinc use efficiency is enhanced in wheat through nanofertilization. Sci Rep 8:6832
Dawson WO, Bubrick P (1988) Modification of the tobacco mosaic virus coat protein gene affecting replication movement and symptomatology. Phytopathology 78:783–789
De Filpo G, Palermo AM, Rachiele F, Nicoletta FP (2013) Preventing fungal growth in wood by titanium dioxide nanoparticles. Int Biodeterior Biodegradation 85:217–222
De la Rosa-García D, Susana C, Martínez-Torres P et al (2018) Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical Fruit. J Nanomater 2018:3498527
De la Torre RR, Servin A, Hawthrone J et al (2015) Terrestrial trophic transfer of bulk and nanoparticle La2O3 does not depend on particle size. Environ Sci Technol 49:11866–11874
Dean R, Van Kan JA, Pretorius ZA et al (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Dejean G, Blanvillain-Baufume S, Boulanger A et al (2013) The xylan utilization system of the plant pathogen Xanthomonas campestris pv campestris controls epiphytic life and reveals common features with oligotrophic bacteria and animal gut symbionts. New Phytol 198:899–915
Delmotte N, Knief C, Chaffron S et al (2009) Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A 106:16428–16433
Derbalah AS, Elkot GAE, Hamza AM (2012) Laboratory evaluation of botanical extracts microbial culture filtrates and silver nanoparticles against Botrytis cinerea. Ann Microbiol 62:1331–1337
Desbiez C, Gal-On A, Girard M et al (2003) Increase in Zucchini yellow mosaic virus symptom severity in tolerant zucchini cultivars is related to a point mutation in P3 protein and is associated with a loss of relative fitness on susceptible plants. Phytopathology 93:1478–1484
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2013a) Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals 26:913–924
Dimkpa CO, McLean JE, Martineau N et al (2013b) Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol 47:1082–1090
Dizaj SM, Lotfipour F, Barzegar-Jalali M et al (2014) Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C 44:278–284
Djonovic S, Urbach JM, Drenkard E et al (2013) Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog 9:e1003217
Doke N (1975) Prevention of the hypersensitive reaction of potato cells to infection with an incompatible race of Phytophthora infestans by constituents of the zoospores. Physiol Plant Pathol 7:1–7
Dollet M (1984) Plant diseases caused by flagellate protozoa (Phytomonas). Annu Rev Phytopathol 22:115–132
Du Z, Chen A, Chen W et al (2014) Nuclear-cytoplasmic partitioning of cucumber mosaic virus protein 2b determines the balance between its roles as a virulence determinant and an RNA-silencing suppressor. J Virol 88:5228–5241
Dunger G, Relling VM, Tondo ML et al (2007) Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch Microbiol 188:127–135
Dunoyer P, Lecellier CH, Parizotto EA et al (2004) Probing the microRNA and small Interfering RNA pathways with virus-encoded suppressors of RNA silencing. Plant Cell 16:1235–1250
Elbeshehy EKF, Elazzazy AM, Aggelis G (2015) Silver nanoparticles synthesis mediated by new isolates of Bacillus spp.; nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front Microbiol 6:453
Ellingboe AH (1968) Inoculum production and infection by foliage pathogens. Annu Rev Phytopathol 6:317–330
Elmer WH, White JC (2016) The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ Sci Nano 3(5):1072–1079
Elmer W, White JC (2018) The future of nanotechnology in plant pathology. Annu Rev Phytopathol 56:111–133
Espinosa A, Guo M, Tam VC et al (2003) The Pseudomonas syringae type III-secreted protein HopPtoD2 possesses protein tyrosine phosphatase activity and suppresses programmed cell death in plants. Mol Microbiol 49:377–387
Fang Y, Ramasamy RP (2015) Current and prospective methods for plant disease detection. Biosensors 5:537–561
Fayaz AM, Balaji K, Girilal M et al (2009) Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. J Agric Food Chem 57:6246–6252
Frampton RA, Pitman AR, Fineran PC (2012) Advances in bacteriophage-mediated control of plant pathogens. Int J Microbiol 2012:326452
Francl LJ (2001) The disease triangle: a plant pathological paradigm revisited. Plant Health Instruct 10:517
Frazer L (2001) Titanium dioxide: environmental white knight. Environ Health Perspect 109:174–177
Freeman BC, Chen C, Beattie GA (2010) Identification of the trehalose biosynthetic loci of Pseudomonas syringae and their contribution to fitness in the phyllosphere. Environ Microbiol 12:1486–1497
Freeman BC, Chen C, Yu X et al (2013) Physiological and transcriptional responses to osmotic stress of two Pseudomonas syringae strains that differ in epiphytic fitness and osmotolerance. J Bacteriol 195:4742–4752
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9:12944–12967
Gaignard JL, Luisetti J (1993) Pseudomonas-syringae, an epiphytic ice nucleation active and phytopathogenic bacterium. Agronomie 13:333–370
Gal M, Preston GM, Massey RC et al (2003) Genes encoding a cellulosic polymer contribute toward the ecological success of Pseudomonas fluorescens SBW25 on plant surfaces. Mol Ecol 12:3109–3121
Galan JE, Lara-Tejero M, Marlovits TC et al (2014) Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol 68:415–438
Gardea-Torresdey JL, Rico CM, White JC (2014) Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ Sci Technol 48:2526–2540
Garnham CP, Campbell RL, Walker VK et al (2011) Novel dimeric beta-helical model of an ice nucleation protein with bridged active sites. BMC Struct Biol 11:36
Geri C, Love AJ, Cecchini E et al (2004) Arabidopsis mutants that suppress the phenotype induced by transgene-mediated expression of cauliflower mosaic virus (CaMV) gene VI are less susceptible to CaMV infection and show reduced ethylene sensitivity. Plant Mol Biol 56:111–124
Gerlach RG, Hensel M (2007) Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int J Med Microbiol 297(6):401–415
Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803
Giannousi K, Avramidis I, Dendrinou-Samara C (2013) Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv 3:21743–21752
Gilardi P, Garcıa-Luque I, Serra MT (1998) Pepper mild mottle virus coat protein alone can elicit the Capsicum spp. L3 genemediated resistance. Mol Plant-Microbe Interact 11:1253–1257
Gilbert GS, Parker IM (2016) The evolutionary ecology of plant disease: a phylogenetic perspective. Annu Rev Phytopathol 54:549–578
Gimenez-Ibanez S, Boter M, Fernandez-Barbero G et al (2014) The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol 12:e1001792
Glawe DA (1992) Thomas J. Burrill, pioneer in plant pathology. Annu Rev Phytopathol 30:17–25
Gogoi R, Singh PK, Kumar R et al (2013) Suitability of nanosulphur for biorational management of powdery mildew of okra (Abelmoschus esculentus Moench) caused by Erysiphe cichoracearum. J Plant Pathol Microbiol 4:171–175
Gohlke J, Deeken R (2014) Plant responses to Agrobacterium tumefaciens and crown gall development. Front Plant Sci 5:155
Gordon T, Perlstein B, Houbara O et al (2011) Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf A Physicochem Eng Asp 374:1–8
Graham JH, Johnson EG, Myers ME et al (2016) Potential of nano-formulated zinc oxide for control of citrus canker on grapefruit trees. Plant Dis 100:2442–2447
Guan H, Chi D, Yu J, Li H (2010) Dynamics of residues from a novel nano-imidacloprid formulation in soyabean fields. Crop Prot 29:942–946
Gudesblat GE, Torres PS, Vojnov AA (2009) Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal regulated virulence factor. Plant Physiol 149:1017–1027
Gurian-Sherman D, Lindow SE (1993) Bacterial ice nucleation: significance and molecular basis. FASEB J 7:1338–1343
Haikonen T, Rajamaki ML, Tian YP, Valkonen JP (2013) Mutation of a short variable region in HCpro protein of Potato virus A affects interactions with a microtubule-associated protein and induces necrotic responses in tobacco. Mol Plant-Microbe Interact 26:721–733
Hails RS (2000) Genetically modified plants—the debate continues. Trends Ecol Evol 15:14–18
Hajipour MJ, Fromm KM, Ashkarran AA et al (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30:499–511
Han SW, Lee SW, Ronald PC (2011) Secretion, modification, and regulation of Ax21. Curr Opin Microbiol 14:62–67
Hao Y, Cao X, Ma C et al (2017) Potential applications and antifungal activities of engineered nanomaterials against gray mold disease agent Botrytis cinerea on rose petals. Front Plant Sci 8:1332
Hassan SED, Fouda A, Radwan AA et al (2019) Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J Biol Inorg Chem 24:377–393
Hauck P, Thilmony R, He SY (2003) A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci U S A 100:8577–8582
Havelda Z, Hornyik C, Valoczi A, Burgyan J (2005) Defective interfering RNA hinders the activity of a tombusvirus-encoded posttranscriptional gene silencing suppressor. J Virol 79:450–457
He P, Chintamanani S, Chen Z et al (2004) Activation of a COI1-dependent pathway in Arabidopsis by Pseudomonas syringae type III effectors and coronatine. Plant J 37:589–602
He YW, Boon C, Zhou L et al (2009) Co-regulation of Xanthomonas campestris virulence by quorum sensing and a novel two-component regulatory system RavS/RavR. Mol Microbiol 71:1464–1476
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166:207–215
Heaton LA, Lee TC, Wei N et al (1991) Point mutations in the turnip crinkle virus capsid protein affect the symptoms expressed by Nicotiana benthamiana. Virology 183:143–150
Hisa Y, Suzuki H, Atsumi G et al (2014) P3NPIPO of Clover yellow vein virus exacerbates symptoms in pea infected with White clover mosaic virus and is implicated in viral synergism. Virology 449:200–206
Horsfall JG, Cowling EB (1980) Plant disease, 3rd edn. Academic, New York
Hossain A, Abdallah Y, Ali MA et al (2019) Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeyadadantii. Biomol Ther 9:863
Ibrahim E, Zhang M, Zhang Y et al (2020) Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat fusarium head blight pathogen Fusarium graminearum. Nano 10:219
Imada K, Sakai S, Kajihara H, Tanaka S, Ito S (2016) Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol 65:551–560
Inaba J, Kim BM, Shimura H, Masuta C (2011) Virus-induced necrosis is a consequence of direct protein–protein interaction between a viral RNA-silencing suppressor and a host catalase. Plant Physiol 156:2026–2036
Incarbone M, Dunoyer P (2013) RNA silencing and its suppression: novel insights from in planta analyses. Trends Plant Sci 18:382–392
Ishibashi K, Masuda K, Naito S et al (2007) An inhibitor of viral RNA replication is encoded by a plant resistance gene. Proc Natl Acad Sci U S A 104:13833–13838
Jagana D, Hegde YR, Lella R (2017) Green nanoparticles: a novel approach for the management of banana anthracnose caused by Colletotrichum musae. Int J Curr Microbiol App Sci 6:1749–1756
Jamir Y, Guo M, Oh HS et al (2004) Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. Plant J 37:554–565
Jay F, Wang Y, Yu A et al (2011) Misregulation of auxin response factor 8 underlies the developmental abnormalities caused by three distinct viral silencing suppressors in Arabidopsis. PLoS Pathog 7:e1002035
Jha G, Rajeshwari R, Sonti RV (2005) Bacterial type two secretion system secreted proteins: double-edged swords for plant pathogens. Mol Plant-Microbe Interact 18:891–898
Jin T, Sun D, Su JY, Zhang H, Sue HJ (2009) Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7. J Food Sci 74:46–52
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043
Johnson TL, Abendroth J, Hol WGJ et al (2006) Type II secretion: from structure to function. FEMS Microbiol Lett 255:175–186
Kachroo P, Yoshioka K, Shah J et al (2000) Resistance to turnip crinkle virus in arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12:677–690
Kang Y, Liu H, Genin S et al (2002) Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol Microbiol 46:427–437
Kang L, Li J, Zhao T et al (2003) Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc Natl Acad Sci U S A 100:3519–3524
Kanhed P, Birla S, Gaikwad S et al (2014) In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater Lett 115:13–17
Kay S, Hahn S, Marois E et al (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318:648–651
Khan MR, Haque Z (2013) Morphological and biochemical responses of five tobacco cultivars to simultaneous infection with Pythium aphanidermatum and Meloidogyne incognita. Phytopathol Mediterr 52:98–109
Khan MR, Rizvi TF (2014) Nanotechnology: scope and application in plant disease management. Plant Pathol J 13:214–231
Khater M, de la Escosura-Muñiz A, Merkoçi A (2017) Biosensors for plant pathogen detection. Biosens Bioelectron 93:72–86
Khiyami MA, Almoammar H, Awad YM et al (2014) Plant pathogen nanodiagnostic techniques: forthcoming changes? Biotechnol Biotechnol Equip 28:775–785
Kim J, Kim JG, Kang Y et al (2004) Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol Microbiol 54:921–934
Kim H, Kang H, Chu G, Byun G (2008) Antifungal effectiveness of nanosilver colloid against rose powdery mildew in greenhouses. Solid State Phenom 135:15–18
Kim JI, Park HG et al (2016) Trophic transfer of nano-TiO2 in a paddy microcosm: a comparison of single-dose versus sequential multi-dose exposures. Environ Pollut 212:316–324
Klein E, Link D, Schirmer A et al (2007) Sequence variation within Beet necrotic yellow vein virus p25 protein influences its oligomerization and isolate pathogenicity on Tetragonia expansa. Virus Res 126:53–61
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Mater Ther 7:2833–2881
Kong HS, Roberts DP, Patterson CD et al (2012) Effect of overexpressing rsmA from Pseudomonas aeruginosa on virulence of select phytotoxin-producing strains of P. syringae. Phytopathology 102:575–587
Korotkov KV, Sandkvist M, Hol WGJ (2012) The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10:336–351
Krishnaraj C, Ramachandran R, Mohan K, Kalaichelvan PT (2012) Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim Acta A Mol Biomol Spectrosc 93:95–99
Krutyakov YA, Kudrinskiy AA, Zherebin PM et al (2016) Tallow amphopolycarboxyglycinate stabilized silver nanoparticles: new frontiers in development of plant protection products with a broad spectrum of action against phytopathogens. Mater Res Express 3:075403
Kurepa J, Paunesku T, Vogt S et al (2010) Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett 10:2296–2302
Lamichhane JR, Osdaghi E, Behlau F et al (2018) Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron Sustain Dev 38:1–18
Lamsal K, Kim SW, Jung JH et al (2011a) Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology 39:26–32
Lamsal K, Kim SW, Jung JH et al (2011b) Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 39:194–199
Langston-Unkefer PJ, Robinson AC, Knight TJ et al (1987) Inactivation of pea seed glutamine synthetase by the toxin, tabtoxinine-beta-lactam. J Biol Chem 262:1608–1613
Lara-Tejero M, Kato J, Wagner S et al (2011) A sorting platform determines the order of protein secretion in bacterial type III systems. Science 331:1188–1191
Leduc JL, Roberts GP (2009) Cyclic di-GMP allosterically inhibits the CRP-like protein (Clp) of Xanthomonas axonopodis pv. citri. J Bacteriol 191:7121–7122
Lee J, Teitzel GM, Munkvold K et al (2012) Type III secretion and effectors shape the survival and growth pattern of Pseudomonas syringae on leaf surfaces. Plant Physiol 158:1803–1818
Lee DH, Kim JB, Lim JA et al (2014) Genetic diversity of Pectobacterium carotovorum subsp. brasiliensis isolated in Korea. Plant Pathol J 30:117–124
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384
Lewandowski DJ, Dawson WO (1993) A single amino acid change in tobacco mosaic virus replicase prevents symptom production. Mol Plant-Microbe Interact 6:157–160
Li F, Pignatta D, Bendix C et al (2012) MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci U S A 109:1790–1795
Li J, Sang H, Guo H et al (2017) Antifungal mechanisms of ZnO and Ag nanoparticles to Sclerotinia homoeocarpa. Nanotechnology 28:155101
Liang H, Yao N, Song JT et al (2003) Ceramides modulate programmed cell death in plants. Genes Dev 17:2636–2641
Liang Y, Yang D, Cui J (2017) A graphene oxide/silver nanoparticle composite as a novel agricultural antibacterial agent against Xanthomonas oryzae pv. oryzae for crop disease management. New J Chem 41:13692–13699
Liao YY, Strayer-Scherer AL, White J et al (2019) Nano-magnesium oxide: a novel bactericide against coppertolerant Xanthomonas perforans causing tomato bacterial spot. Phytopathology 109:52–62
Lim MTS, Kunkel BN (2004) Mutations in the Pseudomonas syringae avrRpt2 gene that dissociate its virulence and avirulence activities lead to decreased efficiency of AvrRpt2-induced disappearance of RIN4. Mol Plant-Microbe Interact 17:313–332
Linsebigler AL, Lu G, Yates JT Jr (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev 95:735–758
Little RH, Grenga L, Saalbach G et al (2016) Adaptive remodeling of the bacterial proteome by specific ribosomal modification regulates Pseudomonas infection and niche colonisation. PLoS Genet 12:e1005837
Lohou D, Lonjon F, Genin S et al (2013) Type III chaperones & Co in bacterial plant pathogens: a set of specialized bodyguards mediating effector delivery. Front Plant Sci 4:435
Lucas WJ (2006) Plant viral movement proteins: agents for cell-to cell trafficking of viral genomes. Virology 344:169–184
Macho AP (2016) Subversion of plant cellular functions by bacterial type-III effectors: beyond suppression of immunity. New Phytol 210:51–57
Macho AP, Zipfel C (2014) Plant PRRs and the activation of innate immune signaling. Mol Cell 54:263–272
Macho AP, Zipfel C (2015) Targeting of plant pattern recognition receptor triggered immunity by bacterial type-III secretion system effectors. Curr Opin Microbiol 23:14–22
Makarovsky D, Fadeev L, Salam BB et al (2018) Silver nanoparticles complexed with bovine submaxillary mucin possess strong antibacterial activity and protect against seedling infection. Appl Environ Microbiol 84(4):e02212
Mala R, Arunachalam P, Sivsankari M (2012) Synergistic bactericidal activity of silver nanoparticles and ciprofloxacin against phytopathogens. J Cell Tissue Res 12:3249–3254
Marco ML, Legac J, Lindow SE (2005) Pseudomonas syringae genes induced during colonization of leaf surfaces. Environ Microbiol 7:1379–1391
Martins PM, Merfa MV, Takita MA et al (2018) Persistence in phytopathogenic bacteria: do we know enough? Front Microbiol 9:1099
Mathioudakis MM, Veiga RS, Canto T et al (2013) Pepino mosaic virus triple gene block protein 1 (TGBp1) interacts with and increases tomato catalase 1 activity to enhance virus accumulation. Mol Plant Pathol 14:589–601
McCarthy Y, Dow JM, Ryan RP (2011) The Ax21 protein is a cell–cell signal that regulates virulence in the nosocomial pathogen Stenotrophomonas maltophilia. J Bacteriol 193:6375–6378
Melotto M, Kunkel BN (2013) Virulence strategies of plant pathogenic bacteria. In: Rosenberg E (ed) The prokaryotes – prokaryotic physiology and biochemistry. Springer, Heidelberg, pp 61–75
Melotto M, Underwood W, Koczan J et al (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969–980
Mise K, Allison RF, Janda M, Ahlquist P (1993) Bromovirus movement protein genes play a crucial role in host specificity. J Virol 67:2815–2823
Mishra S, Singh BR, Singh A et al (2014) Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One 9(5):e97881
Mochizuki T, Yamazaki R, Wada T et al (2014) Coat protein mutations in an attenuated cucumber mosaic virus encoding mutant 2b protein that lacks RNA silencing suppressor activity induces chlorosis with photosynthesis gene repression and chloroplast abnormalities in infected tobacco plants. Virology 456–457:292–299
Moffett P (2009) Mechanisms of recognition in dominant R gene mediated resistance. Adv Virus Res 75:1–33
Mohmood I, Lopes CB, Lopes I et al (2013) Nanoscale materials and their use in water contaminants removal–a review. Environ Sci Pollut Res 20:1239–1260
Mondal KK, Mani C (2012) Investigation of the antibacterial properties of nanocopper against Xanthomonas axonopodis pv. punicae, the incitant of pomegranate bacterial blight. Ann Microbiol 62:889–893
Mondal KK, Bhar LM, Mani C (2010) Combined efficacy of Pseudomonas fluorescens strain MBPF-01 and nanocopper against bacterial leaf blight in rice. Indian Phytopathol 63:266–268
Morales-Díaz AB, Ortega-Ortíz H (2017) Application of nanoelements in plant nutrition and its impact in ecosystems. Adv Nat Sci Nanosci Nanotechnol 8:013001
Moscoso JA, Mikkelsen H, Heeb S et al (2011) The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via cdi-GMP signalling. Environ Microbiol 13:3128–3138
Moscoso JA, Jaeger T, Valentini M et al (2014) The diguanylate cyclase SadC is a central player in Gac/Rsm-mediated biofilm formation in Pseudomonas aeruginosa. J Bacteriol 196:4081–4088
Moussa SH, Tayel AA, Alsohim AS, Abdallah RR (2013) Botryticidal activity of nanosized silver-chitosan composite and its application for the control of gray mold in strawberry. J Food Sci 78:1589–1594
Mukhopadhyay SS (2014) Nanotechnology in agriculture: prospects and constraints. Nanotechnol Sci Appl 2014:63–71
Nadendla SR, Rani TS, Vaikuntapu PR et al (2018) HarpinPss encapsulation in chitosan nanoparticles for improved bioavailability and disease resistance in tomato. Carbohydr Polym 199:11–19
Nair R, Varghese SH, Nair BG et al (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Nandini B, Hariprasad P, Prakash HS, Shetty HS, Geetha N (2017) Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci Rep 7:2612
Navale GR, Thripuranthaka M, Late DJ, Shinde SS (2015) Antimicrobial activity of ZnO nanoparticles against pathogenic bacteria and fungi. JSM Nanotechnol Nanomed 3:1033–1041
Nguyen LC, Taguchi F, Tran QM et al (2012) Type IV pilin is glycosylated in Pseudomonas syringae pv. tabaci 6605 and is required for surface motility and virulence. Mol Plant Pathol 13:764–774
Nguyen HC, Nguyen TT, Dao TH et al (2016) Preparation of Ag/SiO2 nanocomposite and assessment of its antifungal effect on soybean plant (a Vietnamese species DT-26). Adv Nat Sci Nanosci Nanotechnol 7(4):045014
Ni P, Cheng KC (2013) Non-encapsidation activities of the capsid proteins of positive-strand RNA viruses. Virology 446:123–132
Nisar P, Ali N, Rahman L, Ali M, Shinwari ZK (2019) Antimicrobial activities of biologically synthesized metal nanoparticles: an insight into the mechanism of action. J Biol Inorg Chem 24:929–941
Norman DJ, Chen J (2011) Effect of foliar application of titanium dioxide on bacterial blight of geranium and Xanthomonas leaf spot of poinsettia. HortScience 46:426–428
Ocsoy I, Paret ML, Ocsoy MA et al (2013) Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 7:8972–8980
Okano Y, Senshu H, Hashimoto M et al (2014) In planta recognition of a double-stranded RNA synthesis protein complex by a potexviral RNA silencing suppressor. Plant Cell 26:2168–2183
Owolade OF, Ogunleti DO, Adenekan MO (2008) Titanium dioxide affects disease development and yield of edible cowpea. EJEAF Chem 7:2942–2947
Pacheco R, Garcıa-Marcos A, Manzano A et al (2012) Comparative analysis of transcriptomic and hormonal responses to compatible and incompatible plant–virus interactions that lead to cell death. Mol Plant-Microbe Interact 25:709–723
Padmanabhan MS, Kramer SR, Wang X et al (2008) Tobacco mosaic virus replicase-auxin/indole acetic acid protein interactions: reprogramming the auxin response pathway to enhance virus infection. J Virol 82:2477–2485
Paknikar KM, Nagpal V, Pethkar AV, Rajwade JM (2005) Degradation of lindane from aqueous solutions using iron sulfide nanoparticles stabilized by biopolymers. Sci Technol Adv Mater 6:370–374
Pakrashi S, Jain N, Dalai L et al (2014) In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS ONE 9:e87789
Pallas V, Garcıa JA (2011) How do plant viruses induce disease? Interactions and interference with host components. J Gen Virol 92:2691–2705
Pallas V, Genoves A, Sanchez-Pina MA, Navarro JA (2011) Systemic movement of viruses via the plant phloem. In: Caranta C, Aranda MA, Tepfer M, Lopez-Moya JJ (eds) Recent advances in plant virology. Academic, New York
Palza H (2015) Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 16:2099–2116
Paret ML, Palmateer AJ, Knox GW (2013a) Evaluation of a lightactivated nanoparticle formulation of titanium dioxide with zinc for management of bacterial leaf spot on rosa ‘Noare’. HortScience 48:189–192
Paret ML, Vallad GE, Averett DR et al (2013b) Photocatalysis: effect of light-activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology 103:228–236
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol J 22:295–302
Peiro A, Canizares MC, Rubio L et al (2014) The movement protein (NSm) of Tomato spotted wilt virus is the avirulence determinant in the tomato Sw-5 gene-based resistance. Mol Plant Pathol 15:802–813
Pel MJ, van Dijken AJ, Bardoel BW et al (2014) Pseudomonas syringae evades host immunity by degrading flagellin monomers with alkaline protease AprA. Mol Plant-Microbe Interact 27:603–610
Peng WT, Lee YW, Nester EW (1998) The phenolic recognition profiles of the Agrobacterium tumefaciens VirA protein are broadened by a high level of the sugar binding protein ChvE. J Bacteriol 180:5632–5638
Pepperman AB, Kuan CW, Mc Combs C (1991) Alginate controlled release formulations of metribuzin. J Control Release 17:105–112
Perfileva AI, Tsivileva OM, Koftin OV et al (2018) Selenium-containing nanobiocomposites of fungal origin reduce the viability and biofilm formation of the bacterial phytopathogen Clavibacter michiganensis subsp, sepedonicus. Nanotechnol Russ 13:268–276
Pfeilmeier S, Saur IM, Rathjen JP et al (2016) High levels of cyclic-di-GMP in plant-associated Pseudomonas correlate with evasion of plant immunity. Mol Plant Pathol 17:521–531
Piffanelli P, Zhou F, Casais C et al (2002) The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiol 129:1076–1085
Pingali PL (2012) Green revolution: impacts, limits, and the path ahead. Proc Natl Acad Sci 109:12302–12308
Qian G, Zhou Y, Zhao Y et al (2013) Proteomic analysis reveals novel extracellular virulence-associated proteins and functions regulated by the diffusible signal factor (DSF) in Xanthomonas oryzae pv. oryzicola. J Proteome Res 12:3327–3341
Quinones B, Pujol CJ, Lindow SE (2004) Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol Plant-Microbe Interact 17:521–531
Quinones B, Dulla G, Lindow SE (2005) Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant-Microbe Interact 18:682–693
Rahim MD, Andika IB, Han C (2007) RNA4-encoded p31 of beet necrotic yellow vein virus is involved in efficient vector transmission, symptom severity and silencing suppression in roots. J Gen Virol 88:1611–1619
Rahoutei J, Garcıa-Luque I, Baron M (2000) Inhibition of photosynthesis by viral infection: effect on PSII structure and function. Physiol Plant 110:286–292
Rao ALN, Grantham GL (1996) Molecular studies on bromovirus capsid protein. 2. Functional analysis of the amino-terminal arginine-rich motif and its role in encapsidation, movement, and pathology. Virology 226:294–305
Rao KJ, Paria S (2013) Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv 3:10471–10478
Records AR, Gross DC (2010) Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors. J Bacteriol 192:3584–3596
Ren T, Qu F, Morris TJ (2000) HRT gene function requires interaction between a NAC protein and viral capsid protein to confer resistance to turnip crinkle virus. Plant Cell 12:1917–1926
Ren T, Qu F, Morris TJ (2005) The nuclear localization of the Arabidopsis transcription factor TIP is blocked by its interaction with the coat protein of Turnip crinkle virus. Virology 331:316–324
Renzi M, Copini P, Taddei AR et al (2012) Bacterial canker on kiwifruit in Italy: anatomical changes in the wood and in the primary infection sites. Phytopathology 102:827–840
Ritpitakphong U, Falquet L, Vimoltust A et al (2016) The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol 210:1033–1043
Robert-Seilaniantz A, Grant M, Jones JD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate–salicylate antagonism. Annu Rev Phytopathol 49:317–343
Rodrigues S, Dionísio M, Lopez CR, Grenha A (2012) Biocompatibility of chitosan carriers with application in drug delivery. J Funct Biomater 3:615–641
Romling U, Galperin MY, Gomelsky M (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716
Sanzari I, Leone A, Ambrosone A (2019) Nanotechnology in plant science: to make a long story short. Biotechnology 7:120
Sarlak N, Taherifar A, Salehi F (2014) Synthesis of nanopesticides by encapsulating pesticide nanoparticles using functionalized carbon nanotubes and application of new nanocomposite for plant disease treatment. J Agric Food Chem 62:4833–4838
Sathiyabama M, Manikandan A (2016) Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr Polym 154:241–246
Sathiyabama M, Parthasarathy R (2016) Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr Polym 151:321–325
Sauer AV, Rocha KR, Pedro ED et al (2014) Ice nucleation activity in Pantoea ananatis obtained from maize white spot lesions. Semin-Cienc Agrar 35:1659–1666
Savary S, Ficke A, Aubertot JN, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Secur 4:519–537
Scholthof KB, Adkins S, Czosnek H et al (2011) Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol 12:938–954
Schuster M, Lostroh CP, Ogi T et al (2003) Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079
Shanmugam C, Gunasekaran D, Duraisamy N et al (2015) Bioactive bile salt-capped silver nanoparticles activity against destructive plant pathogenic fungi through in vitro system. RSC Adv 5:71174–71182
Shimizu M, Tainaka H et al (2009) Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part Fibre Toxicol 6:20
Shintaku MH, Zhang L, Palukaitis P (1992) A single amino acid substitution in the coat protein of cucumber mosaic virus induces chlorosis in tobacco. Plant Cell 4:751–757
Shivaprasad PV, Chen HM, Patel K et al (2012) A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24:859–874
Silva AT, Nguyen A, Ye C et al (2010) Conjugated polymer nanoparticles for effective siRNA delivery to tobacco BY2 protoplasts. BMC Plant Biol 10:1–14
Simonin M, Richaume A (2015) Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: a review. Environ Sci Pollut Res 22:13710–13723
Singh S, Singh M, Agrawal VV, Kumar A (2010) An attempt to develop surface plasmon resonance based immunosensor for Karnal bunt (Tilletia indica) diagnosis based on the experience of nano-gold based lateral flow immuno-dipstick test. Thin Solid Films 519:1156–1159
Slater H, Alvarez-Morales A, Barber CE et al (2000) A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol 38:986–1003
Soenen S, Rivera-Gil P, Montenegro JM et al (2011) Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6:446–465
Spetz C, Valkonen JPT (2004) Potyviral 6K2 protein long-distance movement and symptom-induction functions are independent and host-specific. Mol Plant-Microbe Interact 17:502–510
Strayer A, Ocsoy I, Tan W (2016) Low concentrations of a silver-based nanocomposite to manage bacterial spot of tomato in the greenhouse. Plant Dis 100:1460–1465
Strayer-Scherer AL, Liao YY, Young M et al (2018) Advanced copper composites against copper-tolerant Xanthomonas perforans and tomato bacterial spot. Phytopathology 108:196–205
Streubel J, Pesce C, Hutin M et al (2013) Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol 200:808–819
Sugawara K, Shiraishi T, Yoshida T et al (2013) A replicase of Potato virus X acts as the resistance breaking determinant for JAX1-mediated resistance. Mol Plant-Microbe Interact 26:1106–1112
Sun Q, Greve LC, Labavitch JM (2011) Polysaccharide compositions of intervessel pit membranes contribute to Pierce’s disease resistance of grapevines. Plant Physiol 155:1976–1987
Tamir-Ariel D, Rosenberg T, Navon N et al (2012) A secreted lipolytic enzyme from Xanthomonas campestris pv. vesicatoria is expressed in planta and contributes to its virulence. Mol Plant Pathol 13:556–567
Tans-Kersten J, Huang H, Allen C (2001) Ralstonia solanacearum needs motility for invasive virulence on tomato. J Bacteriol 183:3597–3605
Tilman D, Balzer C, Hill J et al (2011) Global food demand and the sustainable intensification of agriculture. PNAS 108:20260–20264
Toth IK, Bell KS, Holeva MC et al (2003) Soft rot erwiniae: from genes to genomes. Mol Plant Pathol 4:17–30
Trampari E, Stevenson CE, Little RH et al (2015) Bacterial rotary export ATPases are allosterically regulated by the nucleotide second messenger cyclic-di-GMP. J Biol Chem 290:24470–24483
Udalova ZV, Folmanis GE, Khasanov FK, Zinovieva SV (2018) Selenium nanoparticles—an inducer of tomato resistance to the root-knot nematode Meloidogyne incognita (Kofoid et White 1919) Chitwood 1949. Dokl Biochem Biophys 482:264–267
Vadlapudi V, Naidu KC (2011) Fungal pathogenicity of plants. Molecular approach. Eur J Exp Biol 1:38–42
Vankar PS, Shukla D (2012) Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl Nanosci 2:163–168
Wang ZL (2004) Nanostructures of zinc oxide. Mater Today 7:26–33
Wang LH, He Y, Gao Y et al (2004) A bacterial cell–cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51:903–912
Wang Z, Wei F, Liu SY et al (2010) Electrocatalytic oxidation of phytohormone salicylic acid at copper nanoparticles-modified gold electrode and its detection in oilseed rape infected with fungal pathogen Sclerotinia sclerotiorum. Talanta 80:1277–1281
Wang MB, Masuta C, Smith NA, Shimura H (2012) RNA silencing and plant viral diseases. Mol Plant-Microbe Interact 25:1275–1285
Wu Z, Kan FW, She YM et al (2012) Biofilm, ice recrystallization inhibition and freeze–thaw protection in an epiphyte community. Prikl Biokhim Mikrobiol 48:403–410
Xia ZK, Ma QH, Li SY et al (2016) The antifungal effect of silver nanoparticles on Trichosporon asahii. J Microbiol Immunol Infect 49:182–188
Xin XF, He SY (2013) Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol 51:473–498
Xu L, Liu Y, Bai R, Chen C (2010) Applications and toxicological issues surrounding nanotechnology in the food industry. Pure Appl Chem 82:349–372
Xu C, Peng C, Sun L et al (2015) Distinctive effects of TiO2 and CuO nanoparticles on soil microbes and their community structures in flooded paddy soil. Soil Biol Biochem 86:24–33
Yadeta K, Thomma B (2013) The xylem as battleground for plant hosts and vascular wilt pathogens. Front Plant Sci 4:97
Yang J, Hsiang T, Bhadauria V et al (2017) Plant fungal pathogenesis. Biomed Res Int 2017:9724283. https://doi.org/10.1155/2017/9724283
Yao KS, Li SJ, Tzeng KC et al (2009) Fluorescence silica nanoprobe as a biomarker for rapid detection of plant pathogens. Adv Mater Res 79:513–516
Yaryura PM, Conforte VP, Malamud F et al (2015) XbmR, a new transcription factor involved in the regulation of chemotaxis, biofilm formation and virulence in Xanthomonas citri subsp. citri. Environ Microbiol 17:4164–4176
Ye CM, Chen S, Payton M et al (2013) TGBp3 triggers the unfolded protein response and SKP1-dependent programmed cell death. Mol Plant Pathol 14:241–255
Yoon JY, Ahn HI, Kim M et al (2006) Pepper mild mottle virus pathogenicity determinants and cross protection effect of attenuated mutants in pepper. Virus Res 118:23–30
Yoshioka H, Shiraishi T, Yamada K et al (1990) Suppression of pisatin production and ATPase activity in pea plasma membranes by orthovanadate, verapamil and a suppressor from Mycosphaerella pinodes. Plant Cell Physiol 31:1139–1146
You T, Liu D, Chen J et al (2018) Effects of metal oxide nanoparticles on soil enzyme activities and bacterial communities in two different soil types. J Soils Sediments 18:211–221
Yu J, Penaloza-Vazquez A, Chakrabarty AM et al (1999) Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol Microbiol 33:712–720
Yu X, Lund SP, Scott RA et al (2013) Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc Natl Acad Sci U S A 110:E425–E434
Zeng W, He SY (2010) A prominent role of the flagellin receptor FLAGELLINSENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153:1188–1198
Zhang X, Yuan Y-R, Pei Y et al (2006) Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20:3255–3268
Zhang CZ, Liu YY, Sun XC et al (2008) Characterization of a specific interaction between IP-L, a tobacco protein localized in the thylakoid membranes, and tomato mosaic virus coat protein. Biochem Biophys Res Commun 374:253–257
Zhu SF, Gao F, Cao XS et al (2005) The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms. Plant Physiol 139:1935–1945
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pattanayak, S.P., Bose, P., Sunita, P. (2023). Interaction Between Nanoparticles and Phytopathogens. In: Fernandez-Luqueno, F., Patra, J.K. (eds) Agricultural and Environmental Nanotechnology. Interdisciplinary Biotechnological Advances. Springer, Singapore. https://doi.org/10.1007/978-981-19-5454-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-5454-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5453-5
Online ISBN: 978-981-19-5454-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)