Abstract
Animal research provides a major contribution to the discovery of new compounds and its mechanism of action. It also deals with the pharmacokinetics profile and determination of safe dose of a compound which is to be tested in humans. There is a necessity to choose an appropriate animal model for preclinical research in order to carry out a clinical trial. Research can be performed on already existing validated animal model or by validating a newer model. Validation criteria of an animal model changes from one to another based on the purpose of the model (fit-for-purpose). Face validity, predictive validity and construct validity ensures the closeness of the animal model to humans. In addition to these validity, few more criteria have been added to assess and optimise the animal model, i.e. epidemiology, symptomatology, natural history, end points, genetics, and biochemical parameters, pharmacological and histological features. There is no single animal model which can satisfy all types of validity for any disease. Even though shortcomings are inevitable, these models pave way for the safer research study in humans. One can choose an animal model closer to an ideal one. Thus validation plays a crucial role in translation of animal research to humans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Animal research provides a major contribution to the discovery of new compounds and its mechanism of action. It also deals with the pharmacokinetics profile and determination of safe dose of a compound which is to be tested in humans. Various animal species have also been used to study the disease pathogenesis. Animal welfare organisations have made the regulatory bodies in the field of animal research to follow stringent ethical guidelines while handling them. 3R has been followed as a guideline (Reduce, Replace and Refine).
2 Animal Model
One of the definition for animal model has been given by Held based on Wessler’s original definition: “a living organism in which normative biology or behaviour can be studied, or in which a spontaneous or induced pathological process can be investigated, and in which the phenomenon in one or more respects resembles the same phenomenon in humans or other species of animal.”
2.1 History –Animals in Research
The use of animals for purposes like understanding the human physiology date backs to ancient period. Till date it is being used judiciously for the betterment of human life (Fig. 12.1).
2.2 Classification of Animal Model
Animal models are classified based on various factors (disease -course, symptoms) (Tables 12.1, 12.2, and 12.3).
2.3 Utilization of Animal Models in Scientific Field
The ultimate use of animal models is to deal with the translation of the data obtained from the same to humans for the better health care. Extrapolation of the results from one species to the other is based on the evolution and the morphological and physiological similarity. The toxicity data obtained through animal models are used to determine the safer dose in humans. Certain information from these models do not get translated because of lack of relevance in humans. Significant contribution have been achieved by animal models to research and development:
-
Type I diabetes treated with insulin - first demonstrated in the dog (Banting and McLeod)
-
Evaluation of ex vivo, in vitro, and in silico models to attain better validation of extrapolated data from animal models
-
Experimental design and methodology—crucial role to get valid extrapolation
Preclinical research is a preliminary level where the drug is studied before entering the clinical phase.
2.4 Ideal Animal Model
Any animal model should have these following characteristic features:
-
1.
Appropriateness to human disease
-
2.
Translation to humans
-
3.
Genetic uniformity or closer to humans
-
4.
Cost and availability- cheap & easily available
-
5.
Generalizability of the results
-
6.
Ethical consideration should be addressed
3 Validation of Animal Model
Validation means the relation between test score and its quality to measure. It should cover internal validity (replicability), generalizability, predictive and construct validity. There is a necessity to choose a proper and appropriate animal model for preclinical research in order to carry out a clinical trial which will eventually yield results through which humankind can be benefitted. Hence selection of the same requires validation. Research can be performed on already existing validated animal model or by validating a newer animal model.
Animal model is being called so only when it has been validated whereas it is called as putative animal model if it is yet to undergo validation. Validation criteria of an animal model changes from one to another based on the purpose of the model (fit-for-purpose). It is impossible for a single animal model to achieve validity with all criteria. In order to obtain the maximum number of validity criteria, optimal combination of models should be considered.
Criteria for valid animal model includes:
-
1.
Face validity
-
2.
Construct validity
-
3.
Predictive validity
The above mentioned validity criteria comes under the broader component called external validity.
3.1 Face Validity
The symptoms induced in animal model should match the symptoms of humans, i.e. phenotypic similarity. Example Type I Diabetes mellitus in humans and insulin requiring animal (BB rats). These two conditions respond well to the insulin therapy. Most of the spontaneous animal models exhibit face validity.
3.2 Construct Validity
Presence of homology between the human and animal model on genomic level is called construct validity. Transgenic disease models comes under this category. Animal model with this validity will give inputs about the occurrence of a disease condition with the effects of genetic change and interaction of gene with the environment.
3.3 Predictive Validity
It shows how much an animal model can predict an unknown aspect of the human disease or its therapy. It major contribution is by assessing the cure of a disease. It do not go in for specific cause of a disease and its treatment.
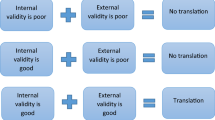
All these three criteria were proposed by Wilner. These three criteria falls under external validity and provides basis for generalisation of the results obtained from animal studies to humans. Belzung and Lemoine proposed a nine validity criteria in case of models related to psychiatric disorders. Tricklebank and Garner suggested other criterias in addition to Wilner’s—internal validity (third variable influencers), external validity (results can be generalized), convergent validity, discriminant validity. It is mostly not possible to have an animal model with all these above criteria/validity, but researchers insist the presence of predictive validity which is the most important one.
Any animal model is said to be valid if it resembles humans with respect to the above said aspects as depicted in the figure (Fig. 12.2).
3.4 Internal Validity
Internal validity means the results or outcomes among animals (different treatment groups) varies only with respect to the intervention. If so those animal models are considered to be with adequate internal validity. Internal validity may be reduced if there is any flaw in the design of animal model and conduct of the study. Certain bias (selection bias, performance bias, attrition bias and detection bias) will affect the internal validity.
-
Reliability—consistent results (similar experimental condition).
-
Replicability—reproducibility.
-
High replicability & reliability—good internal validity.
3.5 Factors Affecting Internal Validity
-
1.
Rearing conditions
-
2.
Housing conditions
-
3.
Social hierarchy
-
4.
Gender
-
5.
Age
-
6.
Time of testing
-
7.
Day-night cycle
-
8.
Health condition of research animal
-
9.
Calibration of research equipment
These factors need to be addressed to increase the internal validity (Table 12.4).
Animal models can either be holistic or reductionist. Holistic model- the model as a whole (symptoms, behaviour, underlying mechanism, etc.) is comparable to human targets. Reductionist model- any specific feature of the animal model is alone comparable to the human targets.
5 Validation Process for Predictive Validity
Among face validity, predictive validity and construct validity, main focus of validation process is on predictive validity. The following steps are involved in the validation process:
-
1.
Test development: Development of diagnostic/therapeutic intervention to predict the outcome of the same in a specific disease
-
2.
Pre-validation: Test is performed in two or more laboratory (relevance & reliability)
-
3.
Validation: Test must be relevant and also reliable for more than one purpose. Data analysis should be done followed by further evaluation.
-
4.
Independent assessment: The study data and conclusion must be published after peer reviewing the same and should be done by the independent panel.
-
5.
Regulatory body acceptance
This validation process can also be performed in a retrospective manner, not necessary to be unidirectional all the time. But retrospective method found to show less reliability.
6 Framework to Identify Models of Disease (FIMD)
-
This framework was done by Ferreira GS et al.
-
Initial step for validation of animal models
-
Identify the aspect in humans which need to be simulated in animals
-
Selection of appropriate disease model is necessary which predicts (outcome in humans) accurately
-
Parameters significant for validation of animal model should be identified
-
Eight domains were included to select an optimal animal model (Fig. 12.6)
Level of Validation:
-
1.
Insufficient validation (0–40% of definitive answer)
-
2.
Slightly validated (41–60% of definitive answer)
-
3.
Moderately validated (61–80% of definitive answer)
-
4.
Highly validated (81–100% of definitive answer)
Definitive answer—All answer except unclear for the above questions in each domain (Table 12.5).
7 Evaluation of Valid Animal Model
Animal model can be evaluated by comparing it with the data acquired from humans. This happens only if the research has progressed from preclinical to clinical trials. Repeatability and reproducibility can be analysed in this evaluation (Tables 12.6 and 12.7).
Discrepancy to clinical state is 3 if all three components (organism, complexity & disease simulation) shows discrepancy when compared with human disease condition, similarly discrepancy to clinical state is two if any of the two components did not match the human disease.
8 Limitations of Animal Model
-
Certain clinical endpoints (biomarkers) are not reproduced with animal models hence following research process (preclinical study) is not achieved in few disease conditions.
-
Quality of life cannot be assessed with the help of animal model.
-
Ideal animal model do not exist.
-
Experimental group with less number of samples exhibit insufficient power.
-
Duration of follow up in animal models varies with the human.
-
Disparity between animal and humans in the drug metabolic pathways.
9 Conclusion
Animal model need to be selected wisely based on the human disease condition and the predictive outcome. There are many animal models available for a single disease condition, appropriate model (proper validation) is selected based on the criteria of concern. Even though shortcomings are inevitable, these models pave way for the safer research study in humans. One can choose an animal model closer to an ideal one. Thus validation plays a crucial role in translation of animal research to humans.
Bibliography
Andersen ML, Winter LMF. Animal models in biological and biomedical research – experimental and ethical concerns. An Acad Bras Cienc. 2017;91(Suppl 1):1–13.
Bailey J, Balls M. Recent efforts to elucidate the scientific validity of animal-based drug tests by the pharmaceutical industry, pro-testing lobby groups, and animal welfare organisations. BMC Med Ethics. 2019;20(16):1–7.
Denayer T, Stohr T, Van Roy M. Animal models in translational medicine: validation and prediction. New Horiz Transl Med. 2014;2:5–11.
Ericsson AC, Crim MJ, Franklin CL. A brief history of animal modeling. Mo Med. 2013;110(3):201–5.
Ferreira GS, Veening-Griffioen DH, Boon WPC, Moors EHM, Gispen-de Wied CC, Schellekens H, et al. A standardised framework to identify optimal animal models for efficacy assessment in drug development. PLoS One. 2019;14(6):1–17.
Ferreira GS, Veening-Griffioen DH, Boon WPC, Moors EHM, Meer PJK. Levelling the translational gap for animal to human efficacy data. Animals. 2020;10(1199):1–13.
Hau J. Animal models. In: Hau J, Hoosier GLV, editors. Handbook of laboratory animal science. 2nd ed. Boca Raton: CRC Press; 2003. p. 1–11.
Hau J. Animal models for human diseases. In: Conn PM, editor. Sourcebook of models for biomedical research. Totowa, NJ: Humana Press Inc; 2008. p. 3–8.
McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2013;87(1):1–39.
Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I. Where is the evidence that animal research benefits humans? BMJ. 2014;328:514–7.
Robinson NB, Krieger K, Khan F, Huffman W, Chang M, Naik A, Yongle R, Hameed I, Krieger K, Girardi LN, Gaudino M. The current state of animal models in Research: a review. Int J Surg. 2019;10:1–21.
Sam-Dodd F. Strategies to optimize the validity of disease models in the drug discovery process. Drug Discov Today. 2006;11(7):355–63.
Sjoberg EA. Logical fallacies in animal model research. Sjoberg Behav Brain Funct. 2017;13(3):1–13.
Varga OE, Hansen AK, Sandoe P, Olsson IAS. Validating animal models for preclinical research: a scientific and ethical discussion. ATLA. 2010;38:245–8.
Worp HB, Howells DW, Emily Sena ES, Porritt MJ, Rewell S, Collins VO, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Subramanian, U. (2022). Validation of Animal Models. In: Lakshmanan, M., Shewade, D.G., Raj, G.M. (eds) Introduction to Basics of Pharmacology and Toxicology. Springer, Singapore. https://doi.org/10.1007/978-981-19-5343-9_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-5343-9_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5342-2
Online ISBN: 978-981-19-5343-9
eBook Packages: MedicineMedicine (R0)