Abstract
With the global rise in incidences of ulcerative colitis and various dysbiosis in the gut microbiota, there has been a growing demand to understand the development and cause behind this disease. Numerous findings have been predicted that gut microbiome is involved in the development of the ulcerative colitis and could also delay the healing process. Studies suggest that the penetration of gut bacteria inside the intestinal wall cause the release of interferon gamma, tumor necrotic factors, interleukins-1 and 6 (IFN-γ, TNF-α, IL-1, and IL-6) that produce reactive oxygen species which further damages the intestinal lining and at the top delays the healing process. The major role is played by the regulatory T cells (Tregs) and the formation of interleukin-10 (IL-10) via GPR43. The Treg cells are stimulated by IL-10 that causes activation of macrophages. Many pro-inflammatory mediators such as Th-1 and Th-17 are produced in response to invasive gut microorganisms (e.g., TNF-α, IFN-γ) by enhancing the transcription of significant genes. This chapter focuses on all the alternative pathophysiology and pathogenesis and management related to ulcerative colitis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ulcerative colitis
- Epithelial restitution
- Intestinal wound healing
- Pathophysiology
- H. pylori
- Mycobacterium avium complex
- TNF-α
- Treg cells
1 Introduction
Ulcerative colitis is a very common inflammatory disease (long lasting); it is characterized by non-infectious inflammation that affects the colon and rectum. The condition is characterized by continuous inflammation that causes deep ulcerations on the rectum. Several factors can elicit ulcerative colitis—gut bacteria, immune system, genetic factors, and eating habits. The pathogenesis of ulcerative colitis is not very clearly understood, but new techniques and research over the intestinal microbiota have revealed a lot of information (Shen et al. 2018; Ohkusa and Koido 2015; Thoreson and Cullen 2007). To take a deep dive into this topic, we tried to get all the recent data and clinical trials to come up with a solid conclusion. Ulcerative colitis comes under the umbrella of inflammatory disease—chronic condition, idiopathy inflammatory disease. With all the latest research and data, it is quite clear that thickness of mucus and any alteration in its composition cause stress and can misfold the mucus associated with the protein. Any irregularities in the immune response involving the innate as well as the adaptive immunity can cause macroscopic lesions (Ordás et al. 2012; Keshteli et al. 2019). Ulcerative colitis can be characterized by the presence of pANCA (primary sclerosing cholangitis) antibodies and the isoform of 1 and 5 human tropomyosin or a bloody diarrhea. That include mycobacterium avium complex or its subspecies paratuberculosis aka MAP (Fries and Comunale 2011).
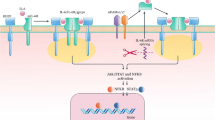
Other factors that play the major roles are diet and sucrose intake which maximize the risk of ulcerative colitis (UC). However, a diet rich in PUFA (polyunsaturated fatty acids) and vitamins/minerals has shown to surely reduce the chance of ulcerative colitis. Various reasonable theories for the etiology of gastrointestinal microbiota, microbiota metabolites formation, immune system changes, and gastric mucosal integrity have also been postulated (Kapel 1950; Gitter et al. 2001). The local complication of ulcerative colitis is colon cancer, rupture of the bowel, and massive hemorrhage. Many studies have confirmed that any inflammation can happen due to misfunctioning of M-cellsM-cells can causes antigen sampling that further lead to translocation of microbial peptide. Microbial peptide then further stimulate the immune cells to release interferon gamma, tumor necrotic factors, interleukins-1 and 6 (IFN-γ, TNF-α, IL-1 & IL-6), which produces reactive oxygen species, that involved in the ill effect of ulcerative colitisUlcerative colitis (UC) (Fig. 12.1) (Kevans et al. 2016; Martinez et al. 2008). The products of anaerobic respiration are thought to be produced by reactive oxygen species. In the course of interaction between the colon/rectum and the pathogen, the inflammation is limited to the mucosa and submucosa levels only. Neutrophils accumulated can be found infiltrating the crypts, forming crypt abscesses, and also the accumulation of phagocytes in the lamina propria, most notably neutrophilic granulocytes, which upon activation release large quantities of reactive oxygen species (ROS) that are cytotoxic to the mucosal cells as well as epithelial cells (Zhang et al. 2017; Nemoto et al. 2012; Angelberger et al. 2013). These compounds could be utilized by facultative anaerobes to overrun, resulting in microbial populations being reduced. This dysbiosis microbiome could promote the formation of fungus which aggravate inflammatory response by activating the type-I T helper (TH1) pathway through chitin and glucan antigen-presenting cells (APCs). In addition, microorganism dysbiosis has been linked to an increase in bacteriophage species and activity, which can affect the overall microbiome through elevated levels of dimethyl sulfoxide, tetramethyl ammonium oxalate, and trimethylamine N-oxide (Sun et al. 2016; Paramsothy et al. 2019).
Potential significance of short-chain fatty acids (SCFAs) produced from fiber in maintaining gut microflora has been established, and SCFA acts as an energy source for intestinal mucosa. In addition, SCFAs also control the functioning of the gut wall as well as the immune response by signaling through G-protein-coupled receptors (GPCRs). Through the GPR43 receptor, SCFAs induce the development of regulatory T cells (Treg), stimulating the formation of cytokines (IL)-10. In addition, SCFA also stimulate IL-18 synthesis, which is important for anti-inflammation as well as epithelium healing, by facilitating upregulation in intestinal cells via GPR43. SCFAs potentially influence the functioning of the gut wall by inducing the expression of adhesion molecules as well as promoting the mucin production (MUC2) (Byndloss et al. 2019; De Leon et al. 2013; Bjerrum et al. 2010). The systemic complications that are caused by the ulcerative colitis are conjunctivitis, mouth ulcer, fatty liver, large joint arthritis, venous thrombosis, etc.
2 Role of Microorganism in the Pathophysiology of Ulcerative Colitis
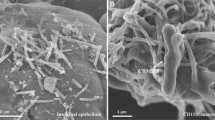
Bacteria in the gut are more than important as ever, based on our limited diet we get. The intestinal lining especially of colon and rectum need to regenerate more quickly than any other cells or tissues in our body; the gut bacteria will produce different amino acid vitamins and proteins that will help repair these intestinal linings. The gastrointestinal microbiome plays an important mechanism in strengthening the gut immunity as along the way it also stimulates the lymphocytes for the expansion of colon, and it also prevents lymphocyte apoptosis. Studies show that with the lack of these bacteria in the intestine, the healing process is strongly hampered, which will further cause ulcer or inflammation to enhance (Fig. 12.2).
Some bacteria will selectively stimulate interleukin-12 productions like the Gram-positive bacteria, whereas some will induce interleukin-4 production like the Gram-negative bacteria (Marteau et al. 2004; Hendrickson et al. 2002). Mostly in small intestinal samples from patient populations with ulcerative colitis, Gram-negative anaerobic bacteria, particularly Escherichia coli and Fusobacterium varium, also with the existence of Peptostreptococcus encroachment, the possibilities of inflammatory response would also significantly raise, and so will drastic microorganism invasions of the mucous membranes, in contrast to healthy individuals (Macfarlane et al. 2004; Tamboli et al. 2004; Ohkusa et al. 2002). The relationships between respiratory epithelium as well as native microbial species (flora) are the focus of the emerging techniques. A few of them can now be used to identify ulcerative colitis from other conditions (e.g., anti-Peptostreptococcus anaerobius Ab-antibody) as well as colitis (e.g., anti I2-from Pseudomonas fluorescens antibody or antibody to an outer membrane porin of E. coli—anti-ompC) (Martinez et al. 2008). The bacterium composition of a gut activates epithelium and lymphatic tissues inside the gastrointestinal among both systemic and local immune function. MAP is eliminated from the feces of sick animals and released in their milk. Thus, there are two ways for MAP to cause infection: fecal contamination transfer via contaminated water and intake of infected breastmilk or items manufactured from contaminated milk (Head and Jurenka 2003; Hindryckx et al. 2016; Bjerrum et al. 2010).
One other possible way of inflammation and an early onset of ulcerative colitis is through the impairment of metabolism of epithelial cells (Borruel et al. 2002). The way it happens is that the anaerobic bacteria by the fermentation process ingest the carbohydrates and proteins to a SCFA which acts as a main source of energy for them. This fatty acid also works as an energy source for intestinal cells, and any variation in this energy can cause ulcerative colitis. The bacteria Desulfovibrio desulfuricans can cause an excess of hydrogen sulfide that acts as a toxin and leads to ulcerative colitis (Roediger 1980; Guo et al. 2020). Many RT-PCR assessments employing 16S rRNA-based species PCR primers revealed that Rhodococcus erythropolis, Clostridium, Methanobrevibacter smithii, Desulfovibrio (sulfate-reducing bacteria, SRB), Enterobacteria, type E Clostridium perfringens, Enterococcus faecalis, and enterohepatic Helicobacter species were substantially enhanced throughout UC (Frank et al. 2007) (Table 12.1).
-
1.
Helicobacter pylori: It is a common bacterium that can elicit ulcerative colitis. It is a Gram-negative microaerophilic bacteria with curved or spiral flagellated flagella. Although the mechanism is not clear, some consider it to be due to the immune regulation caused by H. pylori. Patients infected with H. pylori have higher levels of Foxp3, a T-cell regulatory marker that may help to prevent the progression of inflammatory bowel disease. A long-term infection of colon via the H. pylori causes partial or complete loss of parietal cells, and H. pylori has been detected in colon mucosa and the colonic tissues (Jin et al. 2013; Thomson et al. 2011).
-
2.
Mycobacterium avium Complex: MAP is indeed a pathogen discovered in the feces of animals. It infects and induces systemic infection in a broad range of animals, known as Johne’s (“Yo-knees”) disorder. Numerous systematic review and publication reviews had already indicated that the MAP species is consistently linked to Crohn’s disease. A modest dosage of the MAP microorganism, or even many microorganisms infected a person at every given age, causes Crohn’s disease, whereas a large dose of MAP causes ulcerative colitis (Pierce 2010; Bibiloni et al. 2006; Ohkusa and Koido 2015).
-
3.
Fusobacterium varium: One of the findings has suggested that diagnostic accuracy and ELISA titer of antibody against F. varium was dramatically higher in individuals having active UC than other individuals or control in a cohort study which comprised patients had active UC, CD, ischemic colitis, colonic adenocarcinomas, and normal individuals. Furthermore, individuals with UC had considerably greater immunohistochemistry identification for F. varium throughout the intestinal mucosa than some other participants. Furthermore, in vivo investigations revealed that F. varium is responsible for generating extremely high levels of butyric acid that induces gastrointestinal ulcers in animals that are comparable to those seen in UC patients (Ohkusa et al. 2003).
3 Role of Microorganisms in the Management of Ulcerative Colitis
Human gut is the home for millions of bacteria. Some of these bacteria that live in the colon and rectum in the pH from 5 to 7 are Bacteroides, Clostridium, Streptococcus, Enterococcus, γ-Proteobacteria, Lactobacillus, Fusobacteria, Eubacterium, and Peptostreptococcus. The microbiota in the gut helps with the immuno-modulation of both innate and adaptive immunity. The cell types like effectors Treg cells (T regulatory), IgA-forming B cells, group 3 innate lymphoid cells, dendritic cells, and the lamina propria all help in the immune modulatory function. Pathogenic microbiome drives the development of pro-inflammatory mediators (e.g., TNF-α, IFN-γ) via upregulation of key genetic sequences; when nonpathogenic bacteria invade intestinal mucosal wall of normal individuals, cells of the immune system generate regulating mediators (e.g., transforming growth factor as well as IL-10). It should really be noted how certain bacterial species inhibit the generation of proinflammatory mediators as well as cause stimulated macrophages eventually die via apoptotic mechanism (Borruel et al. 2002).
Their important function is to help with the synthesis of Vitamin- K as well as numerous vitamin-B substances that have key metabolic roles inside the intestinal flora, also they help to produce acetate, propionate, and butyrate. Butyrate emerges as a crucial power source for mammalian enterocytes, may trigger death in carcinoma cells, and can stimulate colonic glucose production, all of which shows the benefit on glucose and caloric expenditure. Because butyrate is needed by epithelium to utilize substantial O2 via oxidative mechanism, ischemia occurs, which promotes oxygenation homeostasis inside the intestines and prevents gut microbial dysbiosis. It has been demonstrated that some microbial members of Bacteroides species conjugated linoleic acid (CLA) may be synthesized that helps with antidiabetic, antiatherogenic, antiobesogenic, hypocholesterolemic, and immune-modulating characteristics (Devillard et al. 2009; Sepehri et al. 2007).
Around 99% of intestinal microbiome comprises of four phyla—Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (Li et al. 2015). The anatomy of mucus layer has Muc2 as a polymeric sheet along with goblet cells that secretes bioactive molecules. The mucus layer of inner colonic is different from intestinal epithelial cells, and the bacteria in this reason do not trigger immune response. Although the inner mucus layer is not sterile and can come in contact of few bacteria, prolonged bacterial exposure has been associated with harmful immunological response, which could also misbalance MUC2 production as well as the features and function of the innermost mucous membrane (Bhinder et al. 2014). It has been seen that in case of ulcerative colitis, the thickness of mucus layer often decreases that makes it easy for the bacteria to reach epithelium and create an immune response.
An outermost layer is substantially highly elastic and has a greater amount than the internal lining due to proteolytic processing of Muc2 mucin. It produces large amount of mucin and mostly bacteria colonize around them. Thus, trails and studies have proven that Muc2 mucin helps build a mucous barrier/layer that prevent the entry of bacteria into the colon epithelium, and any abnormalities in mucus wall can lead to the ulcerative colitis. Furthermore, numerous research studies focused on mouse ulcerative animals, including animals lacking Muc2 mucin as well as IL-10, which were immunostained to evaluate the Muc2 production throughout dextran sodium sulfate-treated animals. Microbial location in such mammal mucous was investigated, both bacterium and beads seemed to permeate the innermost mucous membrane. Any imbalance in the dysbiosis of gut and the bacterial clearance cause inflammatory response and can lead to ulcerative colitis (Swidsinski et al. 2005; Mayer 2000; Guarner and Malagelada 2003). Studies have shown that there is a decrease in Bacteroidetes and Firmicutes, while Proteobacteria and Actinobacteria increases in the mucosal inflammation (Li et al. 2015). Patients with ulcerative colitis have also shown reduction in Butyricicoccus bacteria in the gut, and the oral administration of B. pullicaecorum improved the morphological as well as histopathological measures. The study also reported health benefits as well as lower rates of intestine myeloperoxidase (MPO) and inflammatory cytokines (IL-12, TNF-α) levels. Moreover, in a Caco-2 cell line, supernatant generated from Butyricicoccus pullicaecorum cultures reduced the reduction of intestinal epithelial resistivity (TER) and enhanced IL-8 production generated with TNF-α as well as IFN γ (Eeckhaut et al. 2013) (Table 12.2).
-
1.
Bifidobacterial species: Among 30 species, Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium lactis, Bifidobacterium breve, as well as Bifidobacterium adolescents act as a good source of energy in the gut. These bacteria synthesize the short-chain fatty acid, e.g., propionate and acetate which is further utilized by the intestinal cells to produce energy. Also, the Bifidobacterium species helps with the metabolism of oxalate that prevents the formation of kidney stones (Macfarlane and Macfarlane 2003; Sidhu et al. 1998).
-
2.
Escherichia coli: Escherichia coli is frequently located in the lower human as well as mammalian gut. Whenever E. coli colonizes at the larger intestinal of humans, this could improve digestion, nutritional processing, and uptake, as well as vitamin K synthesis. The mode of action and the nonpathogenic E. coli strain hypothesized throughout this research have been trying to block receptor sites to stop the establishment of adhesive microbes, antagonistic activity against pathogenic and nonpathogenic enterobacteria, most likely through the development of therapeutic drugs, and changes inside the pH as well as variable components of both the small intestinal lumen (Kotlowski et al. 2007; Sha et al. 2013). Studies also show that a random increase in the E. coli population might result in greater adherence scores than harmful E. coli colonies from normal subjects (Verma et al. 2010).
-
3.
Lactobacillus species: Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus paracasei, Lactobacillus gasseri, Lactobacillus reuteri, Lactobacillus salivarius, and Lactobacillus helveticus are the advantageous Lactobacillus genera. Lactobacillus is a Gram-positive, facultative anaerobic, rod-shaped bacterium. These lactic acid bacteria are involved in lactase synthesis, e.g., Lactobacillus plantarum (Mann and Li 2014). Bifidobacterium and Lactobacillus strains interact with TLR2 and/or TLR9 to both improve the functioning of the gastrointestinal epithelial layer and enable Treg cell conversions through CD103+ DCs (Mann and Li 2014).
-
4.
Faecalibacterium prausnitzii: These bacteria are found to have the anti-inflammatory properties. These bacteria produce butyrate which is indeed a main energy source for them and can activate intestinal gluconeogenesis. In fecal microbiota of the ulcerative colitis patients, there is a reduced number of F. prausnitzii. It is essential to maintain the population of F. prausnitzii for a healthy gut (Machiels et al. 2014).
4 Conclusion
With all the above study and data, we can easily predict that the microbiota plays a very crucial role when it comes to the development and repair of the gut and the health of our immunity. Human immunity also has a significant impact on the activity of the intestinal wall. A wide range of bacteria in gut and their population are responsible for the normal functioning. We have studied different mechanisms by which gut microbiota and its population difference can induce or heal the ulcerative colitis. Further research should focus to figure out the exact population difference or alteration of gut microbiota that can induce ulcerative colitis. Bacteria such as Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, Streptococcus salivarius subsp. thermophiles, and probiotic Escherichia coli have been demonstrated to lower inflammatory response as well as maintain recovery in ulcerative colitis patients. The microbiota in the gut helps with the immuno-modulation of both innate and adaptive immunity. Several cell types such as effector T regulatory cells, IgA forming B cells, group 3 innate lymphoid cells, dendritic cells, and the lamina propria help in the immuno-modulatory functions. Pro-inflammatory mediators are produced by invasive intestinal bacteria. Several clinical trials have been conducted and have revealed a positive effect on healing and treatment of ulcerative colitis. As our knowledge and understanding about different gut microbiota grow, it will help us better analyze our current medicine prescribed and how and what can be done to help keep the balance between the gut microbiota. These advancements will lead to an improved probiotic therapy that will restore the condition at the earliest.
References
Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Berry D (2013) Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 108:1620–1630
Barroso FAL, De Jesus LCL, De Castro CP, Batista VL, Ferreira Ê, Fernandes RS, De Barros ALB, Leclerq SY, Azevedo V, Mancha-Agresti P, Drumond MM (2021) Intake of Lactobacillus delbrueckii (pExu:hsp65) prevents the inflammation and the disorganization of the intestinal mucosa in a mouse model of mucositis. Microorganisms 9:107
Bhinder G, Stahl M, Sham HP, Crowley SM, Morampudi V, Dalwadi U, Ma C, Jacobson K, Vallance BA (2014) Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses. Infect Immun 82:3753–3763
Bibiloni R, Mangold M, Madsen K, Fedorak R, Tannock G (2006) The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn’s disease and ulcerative colitis patients. J Med Microbiol 55(Pt 8):1141–1149
Bjerrum JT, Nielsen OH, Hao F, Tang H, Nicholson JK, Wang Y, Olsen J (2010) Metabonomics in ulcerative colitis: diagnostics, biomarker identification, and insight into the pathophysiology. J Proteome Res 9:954–962
Bohr UR, Glasbrenner B, Primus A, Zagoura A, Wex T, Malfertheiner P (2004) Identification of enterohepatic Helicobacter species in patients suffering from inflammatory bowel disease. J Clin Microbiol 42:2766–2768
Borruel N, Carol M, Casellas F, Antolín M, De Lara F, Espín E, Naval J, Guarner F, Malagelada JR (2002) Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut 51:659–664
Bullock NR, Booth JC, Gibson GR (2004) Comparative composition of bacteria in the human intestinal microflora during remission and active ulcerative colitis. Curr Issues Intest Microbiol 5:59–64
Byndloss MX, Litvak Y, Bäumler AJ (2019) Microbiota-nourishing immunity and its relevance for ulcerative colitis. Inflamm Bowel Dis 25:811–815
De Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL (2014) Regulation of intestinal immune responses through TLR activation: implications for pro- and prebiotics. Front Immunol 5:60–60
De Leon LM, Watson JB, Kelly CR (2013) Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 11:1036–1038
De Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS (2017) Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 152:1–12
Devillard E, McIntosh FM, Paillard D, Thomas NA, Shingfield KJ, Wallace RJ (2009) Differences between human subjects in the composition of the faecal bacterial community and faecal metabolism of linoleic acid. Microbiology (Reading) 155:513–520
Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R, Vermeire S, Van Immerseel F (2013) Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62:1745–1752
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104:13780–13785
Fries W, Comunale S (2011) Ulcerative colitis: pathogenesis. Curr Drug Targets 12:1373–1382
Fyderek K, Strus M, Kowalska-Duplaga K, Gosiewski T, Wedrychowicz A, Jedynak-Wasowicz U, Sładek M, Pieczarkowski S, Adamski P, Kochan P, Heczko PB (2009) Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J Gastroenterol 15:5287–5294
Gitter AH, Wullstein F, Fromm M, Schulzke JD (2001) Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 121:1320–1328
Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H (2009) Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 137:495–501
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet 361:512–519
Guo XY, Liu XJ, Hao JY (2020) Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis 21:147–159
Head KA, Jurenka JS (2003) Inflammatory bowel disease Part 1: ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern Med Rev 8:247–283
Hendrickson BA, Gokhale R, Cho JH (2002) Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 15:79–94
Hindryckx P, Jairath V, D'haens, G. (2016) Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol 13:654–664
Jess T, Simonsen J, Nielsen NM, Jørgensen KT, Bager P, Ethelberg S, Frisch M (2011) Enteric salmonella or campylobacter infections and the risk of inflammatory bowel disease. Gut 60:318–324
Jin X, Chen Y-P, Chen S-H, Xiang Z (2013) Association between Helicobacter pylori infection and ulcerative colitis--a case control study from China. Int J Med Sci 10:1479–1484
Kalischuk LD, Inglis GD, Buret AG (2009) Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog 1:2
Kapel O (1950) Ulcerative colitis in pregnancy and labor. Ugeskr Laeger 112:721–723
Keshteli AH, Madsen KL, Dieleman LA (2019) Diet in the pathogenesis and management of ulcerative colitis; a review of randomized controlled dietary interventions. Nutrients 11:1498
Kevans D, Tyler AD, Holm K, Jørgensen KK, Vatn MH, Karlsen TH, Kaplan GG, Eksteen B, Gevers D, Hov JR, Silverberg MS (2016) Characterization of intestinal microbiota in ulcerative colitis patients with and without primary sclerosing cholangitis. J Crohns Colitis 10:330–337
Kotlowski R, Bernstein CN, Sepehri S, Krause DO (2007) High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 56:669–675
Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A, Schreiber S (2011) Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 141:227–236
Li G, Yang M, Zhou K, Zhang L, Tian L, Lv S, Jin Y, Qian W, Xiong H, Lin R, Fu Y, Hou X (2015) Diversity of duodenal and rectal microbiota in biopsy tissues and luminal contents in healthy volunteers. J Microbiol Biotechnol 25:1136–1145
Macfarlane S, Macfarlane GT (2003) Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72
Macfarlane S, Furrie E, Cummings JH, Macfarlane GT (2004) Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clin Infect Dis 38:1690–1699
Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S (2014) A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63:1275–1283
Mann ER, Li X (2014) Intestinal antigen-presenting cells in mucosal immune homeostasis: crosstalk between dendritic cells, macrophages and B-cells. World J Gastroenterol 20:9653–9664
Marteau P, Lepage P, Mangin I, Suau A, Doré J, Pochart P, Seksik P (2004) Review article: gut flora and inflammatory bowel disease. Aliment Pharmacol Ther 20(Suppl 4):18–23
Martinez C, Antolin M, Santos J, Torrejon A, Casellas F, Borruel N, Guarner F, Malagelada JR (2008) Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am J Gastroenterol 103:643–648
Mayer L (2000) Mucosal immunity and gastrointestinal antigen processing. J Pediatr Gastroenterol Nutr 30(Suppl):S4–S12
Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri M, Moreno LA, Martin-Matillas M, Campoy C, Martí A, Moleres A, Delgado M, Veiga OL, García-Fuentes M, Redondo CG, Sanz Y (2009) Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes 33:758–767
Nemoto H, Kataoka K, Ishikawa H, Ikata K, Arimochi H, Iwasaki T, Ohnishi Y, Kuwahara T, Yasutomo K (2012) Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci 57:2955–2964
Ohkusa T, Koido S (2015) Intestinal microbiota and ulcerative colitis. J Infect Chemother 21:761–768
Ohkusa T, Sato N, Ogihara T, Morita K, Ogawa M, Okayasu I (2002) Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J Gastroenterol Hepatol 17:849–853
Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N (2003) Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 52:79–83
Ohkusa T, Nomura T, Terai T, Miwa H, Kobayashi O, Hojo M, Takei Y, Ogihara T, Hirai S, Okayasu I, Sato N (2005) Effectiveness of antibiotic combination therapy in patients with active ulcerative colitis: a randomized, controlled pilot trial with long-term follow-up. Scand J Gastroenterol 40:1334–1342
Ohkusa T, Kato K, Terao S, Chiba T, Mabe K, Murakami K, Mizokami Y, Sugiyama T, Yanaka A, Takeuchi Y, Yamato S, Yokoyama T, Okayasu I, Watanabe S, Tajiri H, Sato N (2010) Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol 105:1820–1829
Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ (2012) Ulcerative colitis. Lancet 380:1606–1619
Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, Paramsothy R, Walsh AJ, Van Den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Lin E, Borody TJ, Wilkins MR, Colombel JF, Mitchell HM, Kaakoush NO (2019) Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 156:1440–1454.e2
Pierce ES (2010) Ulcerative colitis and Crohn’s disease: is Mycobacterium avium subspecies paratuberculosis the common villain? Gut Pathog 2:21
Rhodes JM (1996) Unifying hypothesis for inflammatory bowel disease and associated colon cancer: sticking the pieces together with sugar. Lancet 347:40–44
Roediger WE (1980) The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet 2:712–715
Sepehri S, Khafipour E, Bernstein CN, Coombes BK, Pilar AV, Karmali M, Ziebell K, Krause DO (2011) Characterization of Escherichia coli isolated from gut biopsies of newly diagnosed patients with inflammatory bowel disease. Inflamm Bowel Dis 17:1451–1463
Sepehri S, Kotlowski R, Bernstein CN, Krause DO (2007) Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis 13:675–683
Sha S, Xu B, Wang X, Zhang Y, Wang H, Kong X, Zhu H, Wu K (2013) The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn Microbiol Infect Dis 75:245–251
Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY (2018) Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol 24:5–14
Sidhu H, Hoppe B, Hesse A, Tenbrock K, Brömme S, Rietschel E, Peck AB (1998) Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet 352:1026–1029
Sun D, Li W, Li S, Cen Y, Xu Q, Li Y, Sun Y, Qi Y, Lin Y, Yang T, Xu P, Lu Q (2016) Fecal microbiota transplantation as a novel therapy for ulcerative colitis: a systematic review and meta-analysis. Medicine (Baltimore) 95:e3765
Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H (2005) Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43:3380–3389
Tamboli CP, Neut C, Desreumaux P, Colombel JF (2004) Dysbiosis in inflammatory bowel disease. Gut 53:1–4
Ternhag A, Törner A, Svensson A, Ekdahl K, Giesecke J (2008) Short- and long-term effects of bacterial gastrointestinal infections. Emerg Infect Dis 14:143–148
Thomson JM, Hansen R, Berry SH, Hope ME, Murray GI, Mukhopadhya I, Mclean MH, Shen Z, Fox JG, El-Omar E, Hold GL (2011) Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities? PLoS One 6:e17184
Thoreson R, Cullen JJ (2007) Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am 87:575–585
Verma R, Verma AK, Ahuja V, Paul J (2010) Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J Clin Microbiol 48:4279–4282
Zhang S-L, Wang S-N, Miao C-Y (2017) Influence of microbiota on intestinal immune system in ulcerative colitis and its intervention. Front Immunol 8:1674–1674
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, S., Aggarwal, P., Sharma, S., Ravichandiran, V. (2022). Microorganisms in Pathogenesis and Management of Ulcerative Colitis (UC). In: Dwivedi, M.K., Sankaranarayanan, A., Kemp, E.H., Shoenfeld, Y. (eds) Role of Microorganisms in Pathogenesis and Management of Autoimmune Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-19-4800-8_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-4800-8_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4799-5
Online ISBN: 978-981-19-4800-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)