Abstract
Microgreens are 7–21-day-old seedlings of certain crop species which are harvested at first true leave stage manually or mechanically cutting the seedlings 5–10 mm above the growing media surface. Microgreens are considered as high-value functional foods as these are the storehouse of various antioxidants and certain minerals like K, Ca, Fe, and Zn. Microgreens have gained a lot of attention and popularity over last few years as a novel food, mainly due to their unique flavor, color, texture, and nutritional profiles. Recent studies have revealed that microgreens are richer than mature greens in some vitamins, sugars, and antioxidants, including carotenoids. The consumption of microgreens also appears to be associated with multiple health benefits like reduced risk of cardiovascular disease, possibly due to prevention of hypercholesterolemia, and also provides protection against inflammatory processes, oxidative stress, and chronic diseases. Until now, microgreens have gained market mostly in the western countries; however, in other parts of the world, this is gaining foothold, especially in the urban and peri-urban settings. Rapid growth cycle, limited space requirement, rich flavor, diverse color, and highly economic produce make microgreens a dietary alternative that may contribute to the nutritional security of a large population. Success of microgreens technology will largely depend on the collective and collaborative efforts from the industry and researchers in the food chemistry, biochemistry, genetics, and human nutrition working to enhance the production of secondary metabolites. In this chapter, we have comprehensively covered various functional and nutritional aspects of a number of microgreens which are popularly being grown and consumed across the globe.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
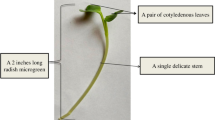
In an era of fast-paced lifestyle, humans require easily available, and healthy food options at affordable prices (Cohen and Garrett 2010). In this context, microgreens are nutrient-rich food crops which can be produced from a number of crops like vegetables, herbs, grains, or even some wild species. The term “microgreens” signify the very small (“micro”) and delicate seedlings (mostly of “green” color) of certain crop species which are consumed either raw or partially cooked. Microgreens are also called “vegetable confetti” which are generally 7–21-day-old tender immature greens of 5–10 cm height (Fig. 7.1) having three major parts, viz. stem, cotyledonary leaf, and a pair of true leaves (Xiao et al. 2012; Sun et al. 2013). The global market of microgreens can be segmented mainly into four broad categories, viz. (1) green types like Brassicaceae (cabbage, broccoli, etc.), Asteraceae (lettuce, chicory, etc.), Amaranthaceae (amaranth, spinach, etc.), Cucurbitaceae (cucumber, melons, etc.), Lamiaceae (basil, mint, etc.), and others (lentils, mung bean, leeks, etc.); (2) farm types (outdoor farming, greenhouse farming, vertical farming); (3) end uses (food and beverages, cosmetics, etc.); and (4) geographical region-based (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) (Globe Newswire Europe 2018; Samuolienė et al. 2013a).
The United States of America (U.S.A.) is a major producer of microgreens in the global market which is followed by Canada and Mexico. In terms of geography, North America is leading the microgreens market with a share of nearly 50% in terms of dollar sales in 2019. The large-scale microgreens farming and consumption (mostly in the restaurants) in the U.S.A. are supporting the microgreens market in this region (https://www.datamintelligence.com). During the 5-year period of 2020–2025, the global microgreens market is anticipated to grow at a CAGR (compound annual growth rate) of 7.5–8.0% (www.researchandmarkets.com), while in U.S.A. it is projected to register a CAGR of 10.1% (www.reportlinker.com). Overall, the market of indoor farming, including the hydroponic system kits for microgreens, was assessed to have a worth of nearly 25.40 billion US dollars in the year 2017 and is expected to reach the value of 40.25 billion US dollars (Globe Newswire Europe 2018).
Microgreens, as a culinary delight, were first reported in late 1980s by the chefs of some restaurants in San Francisco, California (USA), especially in imparting color and flavor to the cuisines. It has then gained attention during last decade as an innovative cooking constituent (Treadwell et al. 2010). The popularity of microgreens is also due to their uniquely varied colors, delicate structures, textures, and flavors, which are required for the garnishing of salads, sandwiches, soups, etc. (www.agresearchmag.ars.usda.gov). In addition, microgreens are loaded with an array of phytonutrient constituents possessing potential bioactive functions (Sun et al. 2013; Mishra et al. 2022; Xiao et al. 2012).
Microgreens are sometimes misconstrued as sprouts and baby greens or baby leaf. However, sprouts, microgreens, and baby leaf are of different food categories which are of special interest due to their unique sensorial and nutritional properties. According to definition of European Union (2013), “sprouts” are the “product obtained from the germination of seeds and their development in water or another medium, harvested before the development of true leaves and which is intended to be eaten whole, including the seed” (EU/208/2013), while “baby leaf” is the “young leaves and petioles of any crops (including Brassica) harvested up to 8 true leaf stage” (EU 752/2014). However, till now there is no “legal definition” for “microgreens,” and it is still a marketing or a commercial term (Treadwell et al. 2010). The fine differences between these terminologies are presented in the Table 7.1.
Microgreens differ from sprouts as the former requires light, a growing medium (both soil or soilless), more growth period, and shoots are the edible portion. Nevertheless, compared to baby greens, microgreens need less growth period, do not necessitate any agrochemicals, and may be marketed without cutting of the seedlings (with growing media), which extends their shelf life window (www.botanicalinterests.com). However, all the three are preferably consumed as raw (Di Gioia et al. 2017a). When microgreens are grown in some solid medium (soil, peat, vermiculite, etc.), they pose very little risk of microbial contamination compared to that of sprouts (Di Gioia et al. 2017a).
Microgreens can be comfortably grown either at home or at commercial scale under controlled environmental conditions (greenhouses) or even under open conditions, irrespective of the season (Ebert et al. 2015, 2017) in a variety of growing medium (soil, soilless), depending on the scale of production. The growing media is very crucial for the proper germination and growth of microgreens. The desired physical properties of the solid growing media include nearly 85% porosity, water holding capacity between 55 and 70% of the total volume, and aeration to the extent of 20–30% of total volume for the roots (Abad et al. 2001). The growing media can be organic (peat, coir, etc.) or inorganic (like perlite and vermiculite). The most commonly used growing substrates for the microgreens production are vermiculite, peat, sand, and perlite either individually or in combination depending on the species grown (Di Gioia et al. 2015). Desired chemical properties of the media include a pH range of 5.5–6.5 and electrical conductivity below 500 μS/cm and should be free of any heavy metal and microbial contamination such as Salmonella and E. coli (Di Gioia et al. 2015, 2017a). Microgreens are generally grown in the plastic trays, of various sizes having depth of 3–5 cm, which is required for placing the ample growing medium to support the microgreens till it reaches the harvesting stage. Based on the type of microgreens grown, the base of the tray can be with or without holes, more often with holes for facilitation of the drainage (Di Gioia et al. 2015). The trays should be placed on a leveled surface on the benches (movable or static) (Di Gioia et al. 2017a).
Special attention is required while harvesting the microgreens to avoid the sticking of any growing media particles and seed integuments which in certain species tend to remain attached (Di Gioia et al. 2015). Immediately after the harvest, microgreens are washed and cooled (1–5 °C) (Kyriacou et al. 2016) or alternatively marketed in trays or growing plants are packed with the growing media (Di Gioia et al. 2017a). Thus, postharvest handling is very crucial for extending the shelf life of the microgreens which otherwise are highly perishable. A method of shelf life extension of microgreens, to at least 10 days, has been patented by Sasuga (2014). The most important parameters for the storage of microgreens are temperature (18–24 °C) and relative humidity (40–60%) (Hodges and Toivonen 2008) as these factors significantly affect the tissue electrolytic leakage and influence microbial contamination (Kou et al. 2013).
Phytonutrient content is reportedly varying with the changing growth stage of the plants, and a decrease has been observed from the seedling (sprout/microgreen) to the fully grown stage. Seed germination enhances the nutritive value of the plants by activation of the enzymes which reduces or even eliminates the antinutritional factors, especially in the legumes (Bau et al. 1997; Mubarak 2005). During germination, there is breakdown of fibrous components which are bound to vitamins, minerals, and amino acids, and thus, the availability of these desired phytochemicals including micronutrients like Fe and Zn increases. In addition, germination also eliminates the flatulence-causing agents (Bird 2014).
Considering a few days of photosynthesis, microgreens are reported to contain much higher contents of various antioxidants, vitamins, and minerals than the sprouts. In general, microgreens contain nearly 4–6 times more nutrients than that of their mature leaves. Microgreens contain relatively high phytonutrients (ascorbic acid, β-carotene, α-tocopherol, and phylloquinone) and minerals (Ca, Mg, Fe, Mn, Zn, Se, and Mo) and less nitrate contents than their mature counterparts (Pinto et al. 2015; Xiao et al. 2012). In this context, microgreens are one of the novel food products which could also be considered as functional and nutraceutical foods for the health-conscious consumers (Kyriacou et al. 2016). Microgreens offer a great potential to become a food of choice to achieve nutritional security of a broad range of settlements due to their ease in the cultivation under varied environmental conditions.
7.2 Crop Species Suitable for Microgreens Cultivation
The crop species used for the production of microgreens are generally of intense color and flavor and rich in various phytochemicals such as antioxidants and vitamins. In addition, the selected crop species are of such type which can be consumed raw as seedlings. A range of crops can be used for the production of microgreens which include beet, broccoli, flax, kale, peas, and radish. The most commonly used species are from the crop families like Amaranthaceae, Amaryllidaceae, Apiaceae, Asteraceae, Brassicaceae, Chenopodiaceae, Cucurbitaceae, Fabaceae, and Lamiaceae.
Some members of the solanaceous family (tomato, brinjal, and pepper) are not considered edible as they contain various antinutrients (Di Gioia et al. 2015) and thus cannot be grown as the microgreens. Even among the edible species, there are various other factors like palatability, flavor, smell, texture, and color that are key traits for the consumer acceptability, while companies/producers of the microgreens look for attractive colors, shapes, flavor, and shelf life (Di Gioia et al. 2015). From the commercial perspective, the selection of microgreens species should be based on the availability of good quality untreated seeds with high and homogeneous germination and have least unit cost of the seeds (Di Gioia et al. 2017a). A comprehensive list of microgreens under cultivation across different countries is presented in Tables 7.2 and 7.3.
7.3 Growth Conditions and Quality of Microgreens
A number of factors that regulate the overall quality of microgreens and phytochemical contents are (1) genetic variability (between and within the taxa), (2) environmental impact (temperature, light quality, and quantity, photoperiod, etc.), and (3) genotype and environmental interaction. However, these factors have not yet been investigated very critically for a large number of species used in the production of microgreens. Very high variations in the bioactive contents largely depend upon both the genetics of the trait and also on the prevailing environmental conditions (Kader 2008). Hitherto, the effect of light quality and quantity is one of the most investigated parameters which is known to regulate the overall biochemical composition of the microgreens.
7.3.1 Light Spectra and the Biochemical Composition of Microgreens
The morphophysiology of microgreens is highly influenced by the light conditions as it significantly regulates the biosynthesis and accumulation of various phytochemicals (Delian et al. 2015). Light quality has more definite effects over light intensity or photoperiod in the regulation of growth and physiology of the microgreens (Bian et al. 2015). Instead of natural lighting, growers prefer to use high-pressure sodium (HPS) lamps (~150 μmol/m2/s) (Vaštakaitė and Viršilė 2015). However, research is exploring the utility of light-emitting diode (LED)-based illumination for the optimal microgreens cultivation (Agarwal and Gupta 2016), as these can be matched with the plant photoreceptors to optimize the production of various phytochemicals (Morrow 2008). LED-based systems are environment friendly over HPS, and they allow even light dispersal over any conventional lighting systems (Agarwal and Gupta 2016; Morrow 2008).
Moreover, the light quality in terms of photon flux and photoperiod (wavelength) can help in the improvement of some biochemicals, thereby regulating the functional quality of microgreens (Kyriacou et al. 2016). Supplementation with green light and standard LED illumination (blue/red/far-red) has shown the improvement in the carotenoid content of the mustard microgreens and red pak choi/tatsoi microgreens, respectively (Brazaitytė et al. 2015a). Three-day application of supplementary red light (pre-harvest) has recorded better antioxidant levels in some microgreens (Samuolienė et al. 2012). Red, blue, or mix of theses lights were found more effective over white or yellow light alone in reducing the undesirable nitrates contents in the microgreens (Ohashi-Kaneko et al. 2007; Qi et al. 2007). Nevertheless, the precise molecular or biochemical mechanisms regulating the spectral quality-induced variations in the bioactive compounds of the microgreens have not been uncovered yet (Kyriacou et al. 2017).
Light quality is known to affect various plant growth, color, flavor, and nutrition-related parameters (Kyriacou et al. 2016). Alrifai et al. (2019) found red, blue, and combined lights are more effective than the white light for better photosynthesis and metabolism. Better antioxidant profile of lentil and wheat was recorded when supplemented with green light (510 nm) (Samuolienė et al. 2011), while better mineral profile was observed in beet microgreens (Brazaitytė et al. 2018). Green LED lighting produced different anthocyanin levels in the microgreens produced from the green and red genotypes of the same species (Carvalho and Folta 2016). However, red and blue lights showed improved phenolic content and free radical scavenging activity in the green and red ocimum cultivars, respectively (Lobiuc et al. 2017). Amber light (595 nm) supplementation enhanced antioxidants in radish sprouts (Samuolienė et al. 2011), while supplementation of short-term red LED lighting could alter the antioxidant composition in some of the microgreens including ocimum, amaranth, brassica, spinach, broccoli, beetroot, and green peas (Samuolienė et al. 2012). In general, supplemental light wavelengths are reported to cause enhanced production of various bioactive compounds, which could play protective role against mild photooxidative stress, in a number of microgreens species.
Vaštakaitė et al. (2015b) imposed photostress by both insufficient and an excess of blue light, which has resulted in the biosynthesis of various protective antioxidants. Differential synthesis of various phytochemicals and antioxidant activity under similar supplemental lighting is dependent on genotype and the season of cultivation (Turner et al. 2020). The presence of relative cloud cover, day length of the microgreen growing period, and incident light angle are some of the key factors affecting both light quality and quantity which suggests the importance of the supplemental light while growing microgreens (Turner et al. 2020).
Ultraviolet (UV) spectra, which fall beyond the visible spectra, are also reported to influence the physiological responses of the plants, and UV-A (320–400 nm) spectrum is considered least harmful (Brazaitytė et al. 2015b). A number of studies have shown that by changing the spectral composition, certain targeted phytochemicals content of the microgreens can be modified (Table 7.4).
Besides the wavelength, even changes in the light pulse frequency can also influence the overall plant developmental and photosynthetic activities (Ani et al. 2014; Vaštakaitė et al. 2017, 2018). Same light quality with varying irradiance levels may cause differential effects on overall nutritional quality of microgreens. High-light conditions cause increased photosynthesis in the growing microgreens, which in turn reduces the susceptibility to photodamage. However, low-light conditions increase the number of light-harvesting complexes for the optimized light utilization (Walters 2005).
Photoperiod also affects the accumulation of various phytochemicals in the microgreens, and it interacts with both quality and intensity of the light used for growth and development of microgreens. Effect of light intensity on the growth and nutrition of microgreens is well known, but the effect of photoperiod has not been thoroughly investigated. However, the effect of photoperiod on the nutrient composition of baby spinach was reported by Lester et al. (2010, 2013). In case of pea-derived microgreens, Wu et al. (2007) studied the effects of 96-h continuous illumination based on blue, red, and white LEDs on biosynthesis and accumulation of various phytochemicals. In-depth research is required to unravel the mechanism regulating the induction of secondary metabolites synthesis and light-associated signal transduction pathways in different microgreens species (Kyriacou et al. 2016).
Environmental conditions of high-altitude regions (Leh, India), especially regions having wide temperature amplitude, PAR, and UV-B content, cause differential nutrient profile of the lentil and mung bean microgreens when compared to that of microgreens grown in plains (Delhi, India) (Priti et al. 2021). In general, better antioxidant profiles were recorded from the samples grown in Leh. Various enterprises have entered into the venture of growing the microgreens under indoor conditions, in a multilayer system, under artificial lighting having desired level of radiation (nearly 100 μmol/m2/s of photosynthetically active radiation) for photosynthesis. At times, natural sunlight is integrated with supplemental lighting, with control on light intensity and quality, for the production of microgreens having enhanced nutritional composition (Kopsell and Sams 2013; Samuolienė et al. 2013b).
7.4 Biochemical Composition of Microgreens
Different microgreens species have varied flavor and are also quite rich in different bioactive contents, and hence, there is a need to identify the genotypes which can fulfill both the taste and nutritional priorities (Xiao et al. 2015a). Sprouts and microgreens are also used as dietary supplements (Kovacs 1996) and functional foods which can minimize the risk of various diet-related diseases (Tang et al. 2014). In this section, the details of biochemical composition of microgreens including vitamins, carotenoids, total sugars, minerals, and antioxidants are presented (Table 7.5).
7.4.1 Antioxidants and Vitamins
A study of 25 diverse microgreens species at USDA and University of Maryland revealed nearly 10 times more antioxidant contents over their mature counterparts (Xiao et al. 2012). The total antioxidant capacity (TAC) in radish microgreens increased nearly 1.7 times under high light (HL) (4.6 ± 0.6 mg/g DW) over normal light (NL) (2.6 ± 0.7 mg/g DW), while in kale the increase was nearly 2.5 times under HL (9.2 ± 1.8 mg/g DW) over NL (3.6 ± 0.5 mg/g DW) (Goble 2018). A set of 20 mung bean and lentil genotypes each, when grown as microgreens under plain-altitude (Delhi) and high-altitude (Leh) conditions, showed significant genotypic variations for ascorbic acid, tocopherol, carotenoids, flavonoid, total phenolics, antioxidant activities (DPPH, FRAP), peroxide activity, proteins, enzymes (peroxidase and catalase), micronutrients, and macronutrients contents (Priti et al. 2021).
The dark-green microgreens such as those derived from spinach, kale, and broccoli are known to possess relatively high phylloquinone (or vitamin K1), which is an essential component required for the coagulation of blood (Olson 1984). Xiao et al. (2012) have reported the phylloquinone content in the range of 0.6 (spinach) to 4.1 (amaranth) μg/g fresh weight (FW) among 25 microgreens species. The phylloquinone content in the mature amaranth, basil, and red cabbage was reported as 1.14, 0.41, and 0.04 μg/g FW, respectively (Haytowitz et al. 2002). Vitamin C (ascorbic acid) is considered as an essential nutrient (Machlin and Bendich 1987), and the range of total ascorbic acid (TAA) was recorded from 20.4 (sorrel) to 147.0 (red cabbage) mg/100 g FW (Xiao et al. 2012). In mung bean microgreens, the mean vitamin C content was recorded 2.7-fold higher (Ebert et al. 2017) over their mature counterparts.
The vitamin E family includes various isomers of tocopherols (Brigelius-Flohé and Traber 1999) which are present in microgreens. Green daikon radish when grown as microgreen showed maximum tocopherol (α:87.4 and γ:39.4 mg/100 g FW), while golden pea tendrils exhibited minimum (α:4.9; γ:3.9 mg/100 g FW), which are quite higher than those of fully grown spinach (α:2.0; γ:0.2 mg/100 g FW) (USDA-ARS 2018b). β-Carotene acts as a precursor of vitamin A, having key role in the vision (Mayne 1996), and is also having antioxidant function (Sies and Stahl 1995). The β-carotene content varied from 0.6 mg/100 g FW (golden pea tendrils and popcorn shoots) to 12.1 mg/100 g FW (red sorrel), and hence, most of the microgreens are considered as richer source of β-carotene (Choe et al. 2018). Other carotenoids like lutein and zeaxanthin (Bone et al. 1997) act as antioxidants (Sujak et al. 1999). Very high lutein/zeaxanthin content was recorded in cilantro (10.1 mg/100 g FW), while lower values were observed for popcorn-derived microgreens (1.3 mg/100 g FW). Similarly, cilantro microgreens showed higher violaxanthin content (7.7 mg/100 g FW), while popcorn microgreens showed the lower values (0.9 mg/100 g FW) (Xiao et al. 2012).
In red cabbage microgreens, the average vitamin C content was found six folds more (147 and 23.5 mg/100 g FW), a 400-fold more vitamin E (24.1 and 0.06 mg/100 g FW), and nearly 60-fold more vitamin K (2.4 vs 0.04 μg/g FW) over their mature counterpart (Xiao et al. 2012). Thus, the recommended daily intake (European Food Safety Authority) of vitamin C (60 mg), E (13 mg), and vitamin K (70 μg) for a medium weight adult can be met from nearly 41 g red cabbage microgreens, 15 g of green radish microgreens, and 17 g of garnet amaranth, respectively (Di Gioia and Santamaria 2015).
7.4.2 Sugars
Relatively high sugar content (10.3 g/kg) was recorded for the microgreens of China rose radish, while red amaranth recorded 1.7 g/kg of fresh microgreens (Xiao et al. 2012). However, mature vegetables recorded higher sugar content (red amaranth recorded 17 g/kg (USDA-ARS 2018a). High light (HL)-induced biofortification strategy was used for the kale and radish microgreens (Xonti et al. 2020). Radish microgreens accumulated nearly 9 times more total starch under HL (191.9 ± 30.1 mg/g DW) over normal light (NL) conditions (20.9 ± 5.2 mg/g DW), whereas in kale the increase was nearly threefold under HL (106.2 ± 18.2 mg/g DW) over NL (35.7 ± 15.4 mg/g DW) (Goble 2018).
7.4.3 Mineral Content
Microgreens are considered as an excellent source of minerals (Weber 2017; Waterland et al. 2017). Broccoli microgreens have 1–2 times more minerals such as P, K, Mg, Mn, Zn, Fe, Ca, Na, and Cu over their mature counterparts (Weber 2017). Similarly, Waterland et al. (2017) also found more dietary mineral content in kale microgreens on a dry weight basis. Among various minerals, microgreens are considered as a good source of K and Ca (Di Gioia et al. 2017a). Especially, the brassica and basil microgreens contain very high nitrates (over 4000 mg/kg FW) under low sunlight, while Na content is generally very low, which makes microgreens as low-Na food (Di Gioia and Santamaria 2015).
Minerals in the microgreens are also directly related to their abundance in the growth medium or the nutrient solution. Thus, the overall nutritional composition of the microgreens can be enhanced through fortification of growing media with certain micronutrients, while some undesirable elements such as Na and nitrates can be reduced (Di Gioia and Santamaria 2015). The most abundant elements recorded were in the order of K, P, and Ca in mung bean microgreens and K, Ca, and P in the lentil microgreens (Priti et al. 2021).
Agronomic biofortification was attempted for Fe and Zn through enrichment of nutrient media by iron sulfate (0, 10, 20, 40 mg/L) and zinc sulfate (0, 5, 10, 20 mg/L) for Brassicaceae (arugula, red cabbage, and red mustard) microgreens. Application of Zn (10 mg/L) through media had resulted in 281% increase in Zn content over control, while Fe enrichment (20 mg/L) increased its content 278% over control. Thus, for biofortification of microgreens, soilless system or hydroponics is considered the most suitable and handy (Di Gioia et al. 2019). Application of more than 20 mM calcium (as calcium chloride) was found toxic for the cultivation of radish microgreens under hydroponic system. However, application of 5.0 and 10.0 mM Ca gave maximum shoots (%), hypocotyl length and also an increase is observed for the average fresh weight per plant and total Ca accumulation in radish microgreens (Goble 2018). Przybysz et al. (2016) demonstrated that microgreens may be enriched with Mg and Fe.
7.4.4 Others
Microgreens undergo the process of germination and hence are characterized with low phytate levels and more mineral bioavailability (Liang et al. 2009). The bioactive compounds such as polyphenols and glucosinolates which are known to have a role in the prevention of various chronic diseases (Del Rio et al. 2013; Dinkova-Kostova and Kostov 2012) are found more in red cabbage microgreens (71.01 and 17.15 μmol/g, respectively) than the mature ones (50.58 and 8.30 μmol/g, respectively) (Huang et al. 2016a). Despite several studies confirming the superior nutritional content of the microgreens over their mature counterparts, detailed investigations are required to analyze the genotypic and environmental factors regulating their nutritional composition (Choe et al. 2018).
7.5 Diverse Scope of Microgreens
7.5.1 Microgreens as Functional Foods
Microgreens are being used as a functional food in the prevention of diseases like obesity, cancer, cardiovascular diseases (CVD), and type 2 diabetes mellitus (Choe et al. 2018). Health-promoting effect of red cabbage microgreens was reported by Huang et al. (2016a). For the patients ailing with impaired kidney function, the hydroponic nutrient solution (in which microgreens are grown) can be tailored to have low or no potassium so that the resultant microgreens from such a system are low in potassium (Renna et al. 2018). Similarly, Se-supplemented hydroponic solution resulted in the Se fortified basil microgreens which are also having increased antioxidant capacity (Puccinelli et al. 2019). Rocket microgreens are known to be the excessive N accumulator; thus, they can be grown under hydroponic system having limited N content so that their content can be regulated in their produce (Bulgari et al. 2017).
The desulfoglucosinolate content in the red cabbage microgreens (17.15 μmol/g DW) was much higher over their mature counterparts (8.30 μmol/g DW) (Huang et al. 2016a), which can mediate NF-κB signaling pathway. The red cabbage microgreens showed the ability to lower the liver lipids by attenuating the C-reactive protein (CRP) and tumor necrosis factor (TNF-α) (Huang et al. 2016a). NF-κB can induce the pro-inflammatory genes like TNF-α, IL-1β, IL-6, and IL-8 (Tak and Firestein 2001). Polyphenols can interfere with the NF-κB signaling pathways by inhibiting phosphorylation or ubiquitination of kinases (Gupta et al. 2010), and they can also inhibit the interaction of NF-κB subunits with target DNA (Ruiz and Haller 2006). Microgreens contain flavonoids such as kaempferol and quercetin which can suppress the COX-2 activity (Mittal et al. 2014). Thus, microgreens can regulate the process of ROS generation and scavenging, thereby influencing NF-κB and other signaling pathways (Choe et al. 2018). Considerable flavonoids contents are reported in the brassica-based microgreens which may influence Nrf2 pathway and inflammation (Busbee et al. 2013). Microgreens analyses confirmed them as the rich sources of natural AhR ligands like quercetin and I3C (indole-3-carbinol) which can regulate the AhR-mediated immune pathways. Thus, microgreens do have a significant role in the regulation of inflammation-associated pathways (Choe et al. 2018). Thus, the consumption of microgreens is supposedly having beneficial effect in the prevention of diseases like obesity, CVD, and diabetes via regulation of inflammation. The effects of microgreens could be due to the presence of compounds like I3C and metabolites like β-carotene and retinoic acid which can suppress the adipogenesis and lipid metabolism (Choi et al. 2013; Berry et al. 2012). Microgreens also have the potential to modulate cancer progression via regulation/inhibition of various pathways including modulation of xenobiotic metabolisms (Choe et al. 2018).
The gut microbiome is considered as a key component for the regulation of human health. Flavonoids (kaempferol, quercetin, apigenin, quercetin, catechin, puerarin, etc.) are known to regulate the gut microbiota composition and thus have role in the prevention of disease development (Clemente et al. 2012; Huang et al. 2016b). Microgreens being rich in flavonoids are likely to regulate the gut microbiome, which needs further in-depth studies. Microgreens rich in various bioactive compounds like flavonoids, indoles, and isothiocyanates are known to provide protection against inflammation and oxidative stress and thus prevent the various chronic diseases including cancers through miRNA and/or DNA methylation and histone modification pathways (Choe et al. 2018). Brassica microgreens are rich in compounds like sulforaphane, phenethyl isothiocyanate, and I3C which may regulate promoter and histone methylation and also the activities of different miRNAs (Wagner et al. 2013). Food-derived bioactive compounds like tocopherols, quercetin, curcumin, resveratrol, and lycopene are also known to affect DNA methylation and histone modification (Shankar et al. 2013; Simpkins et al. 1999; Huang et al. 2012) and thereby restore Nrf2 expression, which impart the protection against prostate cancer (Yu et al. 2010).
7.5.2 Microgreens as Space Food
The extended stay of humans in space requires proper diet to the space travelers with least supply from the earth (Perchonok et al. 2012). A range of stress effects like weight loss, change in the blood composition, and radiation-induced stress are commonly encountered by the space travelers (Vergari et al. 2010; Cohu et al. 2014; Kyriacou et al. 2017). Prevention of such stress calls for the food-based antioxidant supply (Wan et al. 2006), for which microgreens seems an excellent option. Since, microgreens can be grown on board during the mission; therefore, future space missions aim to produce carotenoid-rich food as a part of space life support systems (SLSS) (Perchonok et al. 2012).
A major challenge for adapting agricultural practices in the space is reduced gravity (or microgravity) that impacts fluid and gas distribution around the plants (Kuang et al. 2000); transpiration rates tend to increase (under hypobaric conditions); irradiance levels are low (≤300 μmol/m2/s) requiring supplemental lighting which is energy demanding for the space farm (Salisbury and Bugbee 1988). Fresh microgreens can be directly harvested by crew members, and their production can be done on any synthetic media with little or no nutrient supplementation (Perchonok et al. 2012; Nyenhuis and Drelich 2015; Kyriacou et al. 2017). Microgreens in general have a low photon flux requirement compared to long-cycle crops. Further, the use of LED lights can reduce the overall power demand per unit of crop area (Poulet et al. 2014, 2016).
7.5.3 Microgreens for the Skin Care Formulation
Microgreens are also used in various skin care formulations due to the abundance of antioxidants and vitamins. The microgreen-enriched formulations provide cleansing, exfoliating and detoxification properties which help in the nourishment, repair, and protection of the skin (https://magazine.lneonline.com/breaking-news-microgreens/).
7.5.4 Microgreens for Nutritional Security
The trans-Himalayan part of cold-arid region covers nearly 80,000 km2 land area. The Ladakh region of trans-Himalayas harbors more than 90% of the cold desert of India. In such remote areas, due to extreme long winter, the agricultural season is very short for a period from May to September months (Bhoyar et al. 2011a, 2012). In addition, altitudinal variations, seasonal climate, and weather make it very difficult to standardize the package of practices for the year-round cultivation of different crops in such harsh conditions (Bhoyar et al. 2011b; Singh et al. 2009). Among various climatic factors, temperature imposes serious restrictions on the cropping pattern and production techniques of this region (Bhoyar et al. 2010; Mishra et al. 2009). Human settlement in these far-flung areas is even up to 4200 m altitudes, which requires year-round healthy diets at affordable prices (Cohen and Garrett 2010). For enhanced crop production and to ensure the nutritional security in these harsh areas, various comprehensive research programs are required (Bhoyar et al. 2018; Mishra and Singh 2010). One novel but potential strategy is the optimization of nutrient-rich microgreen technology for such conditions (Singh et al. 2020). Although a range of crops such as beet, broccoli, flax, kale, peas, and radish can be used for microgreen lentil, brassica and mung bean may be the cheapest and quickest among all. In addition, growing these crop species is relatively easy for the microgreens purpose over many other crop species.
7.6 Food Safety of Microgreens
During germination, the seeds release a mix of carbohydrates and peptides which attract a number of microbes present in the rhizosphere, thus making microgreens more prone to the microbial contamination than their mature counterparts (Warriner et al. 2003). Microbial load was generally found more for the sprouts over microgreens (Xiao et al. 2014). However, more microbial contamination has been recorded for the hydroponically grown microgreens over soil or media-grown ones (Riggio et al. 2019), which could be due to the constant warm temperature and humid conditions maintained for the hydroponic system. Although studies on the survival and growth of pathogens on microgreens are limited (Table 7.6), such studies are abundant for sprouts (Turner et al. 2020).
Microbial contamination can be easily overcome by the use of good agricultural practices like use of uninfected seeds, seed treatment, use of clean utensils, and use of UV for the disinfection of hydroponic system (Riggio et al. 2019). Application of Trichoderma harzianum Rifai (strain KRL-AG2 G41) and T. virens (strain G-41) (ThTv) to either seed ball or to the growth media was found effective in reducing the damping-off (Pythium aphanidermatum (Edson) Fitzp.) in the beet microgreens at 14 days after planting (Pill et al. 2011). Safer microgreens can also be produced deploying blue and UV wavelength lights as these have the antimicrobial properties (Kim et al. 2016; Maclean et al. 2009; McKenzie et al. 2014; Turner et al. 2020). As microgreens are very delicate in nature, it is almost impossible to eliminate the microbial contamination using any sanitization treatment. A recent study revealed that the pathogenic bacteria like Salmonella spp. and Listeria spp. were not detected in the mungbean, lentil and Indian mustard microgreens when stored for certain duration under 4 °C conditions in the refrigerator. Similarly, total aerobic bacteria (TAB), yeast and mould (Y&M), Shigella spp., and E. coli were recorded well within the limit to cause any human illness in these microgreens. Washing of the microgreens for 2 min with double distilled water showed some reduction microbial load of these microgreens (Priti et al. 2022).
Although no food-borne outbreak associated with the microgreen consumption is reported, still they are considered as the vehicle of bacterial pathogens (Xiao et al. 2015b). It also warrants more attention to study the survival and proliferation of food-borne pathogens on the microgreens and stored under different conditions for different periods (Di Gioia et al. 2017a).
7.7 Consumption of Microgreens
Fresh microgreens form an extraordinary ingredient of taste and aesthetics for all kinds of energy drinks. Microgreens can invigorate any liquid creation, raising the bar for freshness, flavor, and overall nutritional composition, especially the antioxidant level. Mung bean and lentil-based microgreens are assumed to be the viable and cheapest option for the novel microgreens-based products. There are a number of ways in which the microgreens can be consumed either raw or after cooking or stir-frying (Fig. 7.2). Some of the most common recipes in which microgreens are used are as follows:
-
Green salads: Microgreens are best when consumed raw as salads, since these are loaded with antioxidants, minerals, and delicate flavor.
-
Juices/smoothies: Any microgreen is considered good for juicing or smoothies, but wheatgrass is considered the most used one in 1:3 ratio of microgreens:juice.
-
Sandwiches and wraps: These are another raw option for consumption (radish, arugula microgreens).
-
Burgers: Spring onion, radish, lentil, and mung bean microgreens.
-
Pizza: Sprinkle of peppery microgreens.
-
Pasta with raw or stir-fried microgreens.
-
Omelette with microgreens: Any fresh microgreens will work.
-
Dhokla with microgreens: Any fresh microgreens (preferably brassica) will work.
-
Noodles: Flavored with microgreens.
-
Cooking with microgreens: Some microgreens are good for cooking; while some need to be tossed in at the very last second (radish microgreens), others can stand up to a little heat.
The consumption of microgreens is not limited to any specific recipe, but can be consumed in a number of ways as per the regional food preparations (Fig. 7.2).
Microgreens as dry formulation have the ability to contribute to the nutritional security as they can be very easily made available at any part of the world (Ebert et al. 2015, 2017). The dehydration may be performed following cold raw dehydration process to retain full nutritional content of microgreens, and thus, these can be considered a raw food product. The dehydrated microgreens are unique because of their nutritional qualities. It can be used in smoothies, soups, salads, salad dressings, eggs, baked goods, etc. A number of products are now available in the market as dried formulations like tea (broccoli microgreen-based) or dressing. Microgreen-based energy drinks appear an easy way to add more healthy foods into our diet. These dried microgreen-based products are claimed to be packed with nutrients with added benefits of intense taste (https://drinkmicrotea.com).
7.8 Conclusions and Prospects
Balanced nutrition depends on the availability, accessibility, and utilization of quality foods, including microgreens. In recent decades, the consumption of calorie-rich diets which are high in fat and carbohydrates and low in protein has led to increased rates of diabetes, hypertension, and obesity in developing countries, prompting a call for serious changes in dietary patterns. Considering the burgeoning nutritional needs of our population, novel approaches like microgreen-based formulations seem a viable option for nutritional security. It has immense potential to be used as energy drinks and food additives at commercial scale. Thus, enhancing the quality of food using various microgreen-based formulations appears an option to tackle the nutritional security of our population.
The microgreens cultivation is now attracting the greenhouse growers so that the consumer need can be fulfilled especially in the urban settlements (Chandra et al. 2012). As a novel food crop, microgreens cultivation is still in infancy, especially in the developing and underdeveloped countries. However, constantly expanding research data for a number of microgreens species is unfolding their immense potential as superfood (Kyriacou et al. 2016; Xiao et al. 2012).
Currently, microgreens are mainly being used as a fresh flavor ingredient in the cuisines of upscale restaurants. As per the National Restaurant Association, microgreens are going to be considered as a culinary trend across the world. Nearly 51% of the chefs have predicted microgreens as a hot trend in the US eateries (Globe Newswire Report 2020). A few species of microgreens have been explored, and its cost of cultivation or the availability of untreated seeds has not yet been seriously considered. In addition, studies on the varietal or genotypic differences for the nutritional composition or for the shelf life studies have not been focused properly.
Research on postharvest storage should be intensified so as to have crop species-specific storage strategies. Augmentation of phytonutrient content through fortification of growth media should be explored as an alternative strategy of reaping more from same microgreens. Optimization of sanitization and drying techniques should be intensively studied which will help in the formulation of various novel storable food products. In-depth fundamental research for ensuring the food safety of microgreens and microgreens-based products should be done for their quick acceptance by the food industry.
Some of the key researchable areas include the following:
-
1.
Variations in the nutritional composition of microgreens under different altitudinal and environmental conditions and under different drying conditions.
-
2.
Identification of superior genotype(s) of microgreen when grown under different growth conditions (e.g., light, altitude, temperature, photoperiod).
-
3.
Identification of factors responsible for the variations in the nutritional profile of microgreens when grown under different growth conditions using multiple OMICs approaches.
-
4.
Optimization of cold dehydration and raw dehydration process of various microgreens for maximum nutrition retention.
-
5.
Development of storable microgreen-based products such as microgreens powder and microgreen-based energy drink and its nutritional and medicinal characterization.
-
6.
Region or country-specific selection and optimization of microgreens species with added cost economics for their large-scale commercialization.
-
7.
Studies on the bioactive compounds of microgreens for their health-promoting effects in humans and estimation of bioavailability of microgreens bioactive components.
References
Abad M, Noguera P, Bures S (2001) National inventory of organic wastes for use as growing media for ornamental potted plant production: case study in Spain. Bioresour Technol 77:197–200
Agarwal A, Gupta SD (2016) Impact of light-emitting diodes (LEDs) and their potential effects on plant growth and development in controlled-environment plant production systems. Curr Biotechnol 5:28–43. https://doi.org/10.2174/2211550104666151006001126
Alrifai O, Hao X, Marcone MF et al (2019) Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J Agric Food Chem 67:6075–6090. https://doi.org/10.1021/acs.jafc.9b00819
Ani NN, Ahmad R, Zain CM (2014) Effect of RGB LED pulse lights in photomorphogenesis of Brassica chinensis. In: Paper presented at 2nd international conference on agriculture and biotechnology, Phuket, Thailand
Bau H, Villaume C, Nicolas JP et al (1997) Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soya bean (Glycine max) seeds. J Sci Food Agric 73:1–9
Berry DC, Desantis D, Soltanian H et al (2012) Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 61(5):1112–1121
Bhoyar M, Mishra GP, Singh R et al (2010) Effects of various dormancy breaking treatments on the germination of wild caper (Capparis spinosa L.) seeds from the cold arid desert of trans-Himalayas. Indian J Agric Sci 80(7):620–624
Bhoyar M, Mishra GP, Singh R et al (2011a) Ethno-botany of traditional wild edible plants from cold arid desert of Ladakh-potential source of winter vegetables. Indian Forester 137(8):1029–1033
Bhoyar MS, Mishra GP, Naik PK et al (2011b) Estimation of antioxidant activity and total phenolics among natural populations of Capparis spinosa leaves collected from cold arid desert of trans-Himalayas. Aust J Crop Sci 5(7):912–919
Bhoyar MS, Mishra GP, Naik PK et al (2012) Genetic variability studies among natural populations of Capparis spinosa from cold arid desert of trans-Himalayas using DNA markers. Nat Acad Sci Lett 35(6):505–515
Bhoyar MS, Mishra GP, Naik PK et al (2018) Evaluation of antioxidant capacities and total polyphenols in various edible parts of Capparis spinosa L. collected from trans-Himalayas. Def Life Sci J 3(3):30–36
Bian ZH, Yang QC, Liu WK (2015) Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: a review. J Sci Food Agric 95:869–877
Bird E (2014) Lentils: gems in the treasure state. Montana State University, Bozeman
Bone RA, Landrum JT, Friedes LM et al (1997) Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res 64(2):211–218
Brazaitytė A, Jankauskienė J, Novičkovas A (2013) The effects of supplementary short-term red LEDs lighting on nutritional quality of Perilla frutescens L. microgreens. Rural Dev 6:54–58
Brazaitytė A, Sakalauskienė S, Samuolienė G et al (2015a) The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem 173:600–606. https://doi.org/10.1016/j.foodchem.2014.10.077
Brazaitytė A, Viršilė A, Jankauskienė J et al (2015b) Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int Agrophys 29:13–22. https://doi.org/10.1515/intag-2015-0004
Brazaitytė A, Vaštakaitė V, Viršilė A et al (2018) Changes in mineral element content of microgreens cultivated under different lighting conditions in a greenhouse. Acta Hortic 1227:507–516
Brigelius-Flohé R, Traber MG (1999) Vitamin E: function and metabolism. FASEB J 13(10):1145–1155
Bulgari R, Baldi A, Ferrante A et al (2017) Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N Z J Crop Hortic Sci 45:119–129. https://doi.org/10.1080/01140671.2016.1259642
Busbee PB, Rouse M, Nagarkatti M et al (2013) Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev 71(6):353–369
Carvalho SD, Folta KM (2016) Green light control of anthocyanin production in microgreens. In: Currey CJ, Lopez RG, Runkle ES (eds) Proceedings of the VIII international symposium on light in horticulture, vol 1134. ISHS, Leuven, pp 13–18. https://doi.org/10.17660/ActaHortic.2016.1134.2
Chandra D, Kim JG, Kim YP (2012) Changes in microbial population and quality of microgreens treated with different sanitizers and packaging films. Hortic Environ Biotechnol 53(1):32–40
Choe U, Yu LL, Wang TTY (2018) The science behind microgreens as an exciting new food for the 21st century. J Agric Food Chem 66:11519–11530. https://doi.org/10.1021/acs.jafc.8b03096
Choi Y, Um SJ, Park T (2013) Indole-3-carbinol directly targets SIRT1 to inhibit adipocyte differentiation. Int J Obes 37(6):881–884
Clemente JCAC, Ursell LK, Parfrey LW et al (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270
Cohen MJ, Garrett JL (2010) The food price crisis and urban food (in) security. Environ Urban 22:467–482
Cohu CM, Lombardi E, Adams WW III et al (2014) Increased nutritional quality of plants for long-duration spaceflight missions through choice of plant variety and manipulation of growth conditions. Acta Astonaut 94:799–806. https://doi.org/10.1016/j.actaastro.2013.10.009
Craver JK, Gerovac JR, Lopez RG (2017) Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica microgreens. J Am Soc Hortic Sci 142(1):3–12. https://doi.org/10.21273/JASHS03830-16
Del Rio D, Rodriguez-Mateos A, Spencer JP et al (2013) Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18(14):1818–1892
Delian E, Chira A, Badulescu L et al (2015) Insight into microgreens physiology. Sci Pap Ser B Hortic 59:447–454
Di Gioia F, Santamaria P (2015) The nutritional properties of microgreens. In: Di Gioia F, Santamaria P (eds) Microgreens. Eco-logica editore, Bari, pp 41–47
Di Gioia F, Mininni C, Santamaria P (2015) How to grow microgreens. In: Di Gioia F, Santamaria P (eds) Microgreens: microgreens: novel fresh and functional food to explore all the value of biodiversity. ECO-Logica srl, Bari, pp 51–79
Di Gioia F, Renna M, Santamaria P (2017a) Sprouts, microgreens and “baby leaf” vegetables. In: Yildiz F, Wiley R (eds) Minimally processed refrigerated fruits and vegetables. Springer, Boston
Di Gioia F, De Bellis P, Mininni C et al (2017b) Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. J Sci Food Agric 97:1212–1219. https://doi.org/10.1002/jsfa.7852
Di Gioia F, Petropoulos SA, Ozores-Hampton M et al (2019) Zinc and iron agronomic biofortification of Brassicaceae microgreens. Agronomy 9:677. https://doi.org/10.3390/agronomy9110677
Dinkova-Kostova AT, Kostov RV (2012) Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 18(6):337–347
Ebert AW, Wu TH, Yang RY (2015) Amaranth sprouts and microgreens—a homestead vegetable production option to enhance food and nutrition security in the rural–urban continuum. In: Proceedings of the regional symposium on sustaining small-scale vegetable production and marketing systems for food and nutrition security (SEAVEG 2014), 25–27 February 2014, Bangkok, Thailand, pp 233–244
Ebert AW, Chang CH, Yan MR et al (2017) Nutritional composition of mungbean and soybean sprouts compared to their adult growth stage. Food Chem 237:15–22
Ghoora MD, Haldipur AC, Srividya N (2020) Comparative evaluation of phytochemical content, antioxidant capacities and overall antioxidant potential of select culinary microgreens. J Agric Food Res 2:100046. https://doi.org/10.1016/j.jafr.2020.100046
Globe Newswire Report (2020) United States microgreens market-growth, trends and forecast (2020–2025). https://www.reportlinker.com/p05916379/?utm_source=GNW. https://www.globenewswire.com/news-release/2020/07/02/2057048/0/en/United-States-Microgreens-Market-Growth-Trends-and-Forecast-2020-2025.html. Accessed 31 Dec 2020
Goble CC (2018) Effects of calcium fertilization on growth, yield, and nutrient content of hydroponically grown radish microgreens. MSU graduate theses, vol 3328. https://bearworks.missouristate.edu/theses/3328
Gupta SC, Sundaram C, Reuter S et al (2010) Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta Gene Regul Mech 1799(10–12):775–787
Haytowitz DB, Peterson J, Booth S, USDA-ARS (2002) Phylloquinone (vitamin K) content of vegetables and vegetable products. https://www.ars.usda.gov/ARSUserFiles/80400525/Articles/ift2002_vitk.pdf
Hodges DM, Toivonen PMA (2008) Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol Technol 48:155–162
Huang Y, Khor TO, Shu L et al (2012) A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J Nutr 142(5):818–823
Huang H, Jiang X, Xiao Z et al (2016a) Red cabbage microgreen lower circulating LDL, liver cholesterol and inflammatory cytokines in mice fed a high fat diet. J Agric Food Chem 64:9161–9171. https://doi.org/10.1021/acs.jafc.6b03805
Huang J, Chen L, Xue B et al (2016b) Different flavonoids can shape unique gut microbiota profile in vitro. J Food Sci 81(9):H2273
Jones-Baumgardt C, Llewellyn D, Ying Q et al (2019) Intensity of sole-source light-emitting diodes affects growth, yield, and quality of Brassicaceae microgreens. HortScience 54:1168–1174
Kader AA (2008) Perspective flavor quality of fruits and vegetables. J Sci Food Agric 88:1863–1868
Kim MJ, Mikš-Krajnik M, Kumar A et al (2016) Inactivation by 405 ± 5 nm light emitting diode on Escherichia coli O157:H7, Salmonella typhimurium, and Shigella sonnei under refrigerated condition might be due to the loss of membrane integrity. Food Control 59:99–107
Kopsell DA, Sams CE (2013) Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J Am Soc Hortic Sci 138:31–37
Kopsell DA, Pantanizopoulos NI, Sams CE et al (2012) Shoot tissue pigment levels increase in “Florida broadleaf” mustard (Brassica juncea L.) microgreens following high light treatment. Sci Hortic 140:96–99. https://doi.org/10.1016/j.scienta.2012.04.004
Kou L, Luo Y, Yang T et al (2013) Postharvest biology, quality and shelf life of buckwheat microgreens. Food Sci Technol 51:73–78
Kovacs J (1996) Composition of dehydrated powdered mung bean sprout and plant fiber for use as dietary supplement in healthcare. U.S. Patent no. 5,487,894. U.S. Patent and Trademark Office, Washington, DC
Kuang A, Xiao Y, McClure G et al (2000) Influence of microgravity on ultrastructure and storage reserves in seeds of Brassica rapa L. Ann Bot 85:851–859. https://doi.org/10.1006/anbo.2000.1153
Kyriacou MC, Rouphael Y, Di Gioia F et al (2016) Micro-scale vegetable production and the rise of microgreens. Trends Food Sci Technol 57:103–115. https://doi.org/10.1016/j.tifs.2016.09.005
Kyriacou MC, De Pascale S, Kyratzis A et al (2017) Microgreens as a component of space life support systems: a cornucopia of functional food. Front Plant Sci 8:1587. https://doi.org/10.3389/fpls.2017.01587
Kyriacou MC, El-Nakhel C, Pannico A et al (2019) Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front Plant Sci 10:1501. https://doi.org/10.3389/fpls.2019.01501
Lester GE, Makus DJ, Hodges DM (2010) Relationship between fresh-packaged spinach leaves exposed to continuous light or dark and bioactive contents: effects of cultivar, leaf size, and storage duration. J Agric Food Chem 58:2980–2987. https://doi.org/10.1021/jf401461z
Lester GE, Makus DJ, Hodges DM et al (2013) Summer (subarctic) versus winter (subtropic) production affects spinach (Spinacia oleracea L.) leaf bionutrients: vitamins (C, E, folate, K1, provitamin A), lutein, phenolics, and antioxidants. J Agric Food Chem 61:7019–7027. https://doi.org/10.1021/jf401461z
Liang J, Han BZ, Nout MR et al (2009) Effect of soaking and phytase treatment on phytic acid, calcium, iron and zinc in rice fractions. Food Chem 115(3):789–794
Lin KH, Huang MY, Huang WD et al (2013) The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. Var. Capitata). Sci Hortic 150:86–91. https://doi.org/10.1016/j.scienta.2012.10.002
Lobiuc A, Vasilache V, Oroian M et al (2017) Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 22:2111. https://doi.org/10.3390/molecules22122111
Loedolff B, Brooks J, Stander M et al (2017) High light bio-fortification stimulates de novo synthesis of resveratrol in Diplotaxis tenuifolia (wild rocket) microgreens. Funct Foods Health Dis 7:859–872. https://doi.org/10.31989/ffhd.v7i11.380
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1(6):441–445
Maclean M, MacGregor SJ, Anderson JG et al (2009) Inactivation of bacterial pathogens following exposure to light from a 405-nm light-emitting diode array. Appl Environ Microbiol 75:1932–1937
Mayne ST (1996) Beta-carotene, carotenoids, and disease prevention in humans. FASEB J 10(7):690–701
McKenzie K, Maclean M, Timoshkin IV et al (2014) Enhanced inactivation of Escherichia coli and Listeria monocytogenes by exposure to 405 nm light under sub-lethal temperature, salt and acid stress conditions. Int J Food Microbiol 170:91–98
Mishra GP, Singh SB (2010) Frozen gene bank at frozen mountain. Curr Sci 98(4):466
Mishra GP, Singh R, Bhoyar M et al (2009) Capparis spinosa: unconventional potential food source in cold arid deserts of Ladakh. Curr Sci 96(12):1563–1564
Mishra GP, Ankita, Aski MS et al (2022) Morphological, molecular, and biochemical characterization of a unique lentil (Lens culinaris Medik.) genotype showing seed-coat color anomalies due to altered anthocyanin pathway. Plants 11(14):1815. https://doi.org/10.3390/plants11141815
Mittal M, Siddiqui MR, Tran K et al (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20(7):1126–1167
Morrow RC (2008) LED lighting in horticulture. HortScience 43:1947–1950
Mubarak AE (2005) Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem 89(4):489–495
Nyenhuis J, Drelich JW (2015) Essential micronutrient biofortification of sprouts grown on mineral fortified fiber mats. Int Sch Sci Res Innov 9:981–984
Ohashi-Kaneko K, Takase M, Kon N et al (2007) Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ Cont Biol 45:189–198. https://doi.org/10.2525/ecb.45.189
Olson R (1984) The function and metabolism of vitamin K. Annu Rev Nutr 4(1):281–337
Park HK, Kushad MM, Feng H (2013) Survival of Escherichia coli O157:H7 strain 87–23 on arugula, kale, lettuce and mizuna microgreens, and comparison of leaf surface morphology for mature greens and microgreens. In: Presented at poster session, IAFP annual meeting, Charlotte, NC, USA
Perchonok MH, Cooper MR, Catauro PM (2012) Mission to mars: food production and processing for the final frontier. Ann Rev Food Sci Technol 3:311–330. https://doi.org/10.1146/annurev-food-022811-101222
Pill WG, Collins CM, Gregory N et al (2011) Application method and rate of Trichoderma species as a biological control against Pythium aphanidermatum (Edson) Fitzp. in the production of microgreen table beets (Beta vulgaris L.). Sci Hortic 129:914–918
Pinto E, Almeida AA, Aguiar AA et al (2015) Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J Food Compos Anal 37:38–43
Poulet L, Massa GD, Morrow RC et al (2014) Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sci Space Res 2:43–53. https://doi.org/10.1016/j.lssr.2014.06.002
Poulet L, Fontaine JP, Dussap CG (2016) Plant’s response to space environment: a comprehensive review including mechanistic modelling for future space gardener. Bot Lett 163:337–347. https://doi.org/10.1080/23818107.2016.1194228
Priti, Mishra GP, Dikshit HK et al (2021) Diversity in phytochemical composition, antioxidant capacities, and nutrient contents among mungbean and lentil microgreens when grown at plain-altitude region (Delhi) and high-altitude region (Leh–Ladakh), India. Front Plant Sci 12:812. https://doi.org/10.3389/fpls.2021.710812
Priti, Sangwan S, Kukreja B et al (2022) Yields optimization, microbial load analysis, and sensory evaluation of mungbean (Vigna radiata L.), lentil (Lens culinaris subsp. culinaris), and Indian mustard (Brassica juncea L.) microgreens grown under greenhouse conditions. PLoS ONE 17(5):e0268085. https://doi.org/10.1371/journal.pone.0268085
Przybysz A, Wrochna M, Małecka-Przybysz M et al (2016) Vegetable sprouts enriched with iron: effects on yield, ROS generation and antioxidative system. Sci Hortic 203:110–117
Puccinelli M, Malorgio F, Rosellini I et al (2019) Production of selenium biofortified microgreens from selenium-enriched seeds of basil. J Sci Food Agric 99:5601–5605. https://doi.org/10.1002/jsfa.9826
Qi LD, Liu SHQ, Xu L et al (2007) Effects of light qualities on accumulation of oxalate, tannin and nitrate in spinach. Trans Chin Soc Agric Eng 4:201–205. https://doi.org/10.3969/j.issn.1002-6819.2007.4.040
Reed E, Ferreira CM, Bell R et al (2018) Plant-microbe and abiotic factors influencing Salmonella survival and growth on alfalfa sprouts and Swiss chard microgreens. Appl Environ Microbiol 84:1–11. https://doi.org/10.1128/aem.02814-17
Renna M, Castellino M, Leoni B et al (2018) Microgreens production with low potassium content for patients with impaired kidney function. Nutrients 10:675–688. https://doi.org/10.3390/nu10060675
Riggio GM, Wang Q, Kniel KE et al (2019) Microgreens—a review of food safety considerations along the farm to fork continuum. Int J Food Microbiol 290:76–85. https://doi.org/10.1016/j.ijfoodmicro.2018.09.027
Ruiz PA, Haller D (2006) Functional diversity of flavonoids in the inhibition of the proinflammatory NF-kappaB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J Nutr 136(3):664–771
Salisbury FB, Bugbee B (1988) Plant productivity in controlled environments. HortScience 23:293–299
Samuolienė G, Urbonavičiūtė A, Brazaitytė A et al (2011) The impact of LED illumination on antioxidant properties of sprouted seeds. Cent Eur J Biol 6:68–74. https://doi.org/10.2478/s11535-010-0094-1
Samuolienė G, Brazaitytė A, Sirtautas R et al (2012) The impact of supplementary short-term red LED lighting on the antioxidant properties of microgreens. Acta Hortic 956:649–655. https://doi.org/10.17660/ActaHortic.2012.956.78
Samuolienė G, Brazaitytė A, Sirtautas R et al (2013a) LED illumination affects bioactive compounds in Romaine baby leaf lettuce. J Sci Food Agric 93:3286–3291
Samuolienė G, Brazaitytė A, Jankauskienė J et al (2013b) LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent Eur J Biol 8:1241–1249. https://doi.org/10.2478/s11535-013-0246-1
Samuolienė G, Brazaitytė A, Viršilė A et al (2016) Red light-dose or wavelength dependent photoresponse of antioxidants in herb microgreens. PLoS One 11(9):e0163405. https://doi.org/10.1371/journal.pone.0163405
Samuolienė G, Viršilė A, Brazaitytė A et al (2017) Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem 228:50–56. https://doi.org/10.1016/j.foodchem.2017.01.144
Sasuga DG (2014) Providing microgreens e.g. celery product with significantly longer shelf life than most other microgreens products. Patent WO2014117034-A2; US2014212549-A1
Shankar S, Kumar D, Srivastava RK (2013) Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol Ther 138(1):1–17
Sies H, Stahl W (1995) Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr 62(6):1315S–1321S
Simanavičius L, Viršilė A (2018) The effects of led lighting on nitrates, nitrites and organic acids in tatsoi. Res Rural Dev 2:95–99. https://doi.org/10.22616/rrd.24.2018.057
Simpkins SB, Bocker T, Swisher EM et al (1999) MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet 8(4):661–666
Singh R, Mishra GP, Ahmed Z et al (2009) Effect of various greenhouse structures on yield and quality of different table tomato varieties under cold arid desert of trans-Himalayas. Indian J Agric Sci 79(4):243–247
Singh N, Aditika RS et al (2020) Vegetable microgreens farming in high-altitude region of trans-Himalayas to maintain nutritional diet of Indian troops. Proc Natl Acad Sci 90:743–752
Sujak A, Gabrielska J, Grudziński W et al (1999) Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys 371(2):301–307
Sun J, Xiao Z, Lin LZ et al (2013) Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMSn. J Agric Food Chem 61:10960–10970
Tak PP, Firestein GS (2001) NK-kappaB: a key role in inflammatory diseases. J Clin Investig 107(1):7–11
Tang D, Dong Y, Ren H et al (2014) A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem Cent J 8:4
Treadwell D, Hochmuth R, Landrum L et al (2010) Microgreens: a new specialty crop (p. HS1164). University of Florida, Gainesville
Turner ER, Luo Y, Buchanan RL (2020) Microgreen nutrition, food safety, and shelf life: a review. J Food Sci 85(4):870–882. https://doi.org/10.1111/1750-3841.15049
USDA-ARS (2018a) Basic report: 11203, cress, garden, raw, National Nutrient Database for standard reference legacy release. https://ndb.nal.usda.gov/ndb/search/list?qlookup=11203
USDA-ARS (2018b) Full report (all nutrients): 11457, spinach, raw, National Nutrient Database for standard reference legacy release. https://ndb.nal.usda.gov/ndb/foods/show/3167?fgcd=&manu=&lfacet=&format=Full&count=&max=35&offset=&sort=&qlookup=11457. Accessed Apr 2018
Vaštakaitė V, Viršilė A (2015) Light emitting diodes (LEDs) for higher nutritional quality of Brassicaceae microgreens. In: Conference on ‘research for rural development’. Held at Jelgava, Latvia, 11, pp 111–117
Vaštakaitė V, Virsile A, Brazaityte A et al (2015a) The effect of UV-A supplemental lighting on antioxidant properties of Ocimum basilicum L. microgreens in greenhouse. In: Proceedings of the 7th international scientific conference rural development, pp 1–7
Vaštakaitė V, Viršilė A, Brazaitytė A et al (2015b) The effect of blue light dosage on growth and antioxidant properties of microgreens. Sodininkystė ir Daržininkystė 34:25–35
Vaštakaitė V, Viršilė A, Brazaitytė A et al (2017) Pulsed light-emitting diodes for a higher phytochemical level in microgreens. J Agric Food Chem 65:6529–6534
Vaštakaitė V, Viršilė A, Brazaitytė A et al (2018) Pulsed light increases the phytochemical level of basil microgreens. Acta Hortic 1227:579–584
Vergari F, Tibuzzi A, Basile G (2010) An overview of the functional food market: from marketing issues and commercial players to future demand from life in space. In: Giardi MT, Rea G, Berra B (eds) Bio-farms for nutraceuticals. Springer, Boston, pp 308–321
Wagner AE, Terschluesen AM, Rimbach G (2013) Health promoting effects of Brassica-derived phytochemicals: from chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxidative Med Cell Longev 2013:1–12
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447. https://doi.org/10.1093/jxb/eri060
Wan XS, Ware JH, Zhou Z et al (2006) Protection against radiation induced oxidative stress in cultured human epithelial cells by treatment with antioxidant agents. Int J Rad Oncol Biol Phys 64:1475–1481. https://doi.org/10.1016/j.ijrobp.2005.11.024
Wang Q, Kniel KE (2016) Survival and transfer of murine norovirus within a hydroponic system during kale and mustard microgreen harvesting. Appl Environ Microbiol 82:705–713. https://doi.org/10.1128/aem.02990-15
Wang L, Luo Y, Nou X (2015) Proliferation of Listeria monocytogenes during microgreen production. In: Presented at poster session, international association for food protection annual meeting, Portland, OR, USA. https://iafp.confex.com/iafp/2015/webprogram/Paper9430.html
Warriner K, Ibrahim F, Dickinson M et al (2003) Internalization of human pathogens within growing salad vegetables. Biotechnol Genet Eng Rev 20:117–136. https://doi.org/10.1080/02648725.2003.10648040
Waterland NL, Moon Y, Tou JC et al (2017) Mineral content differs among microgreen, baby leaf, and adult stages in three cultivars of kale. HortScience 52(4):566–571
Weber CF (2017) Broccoli microgreens: a mineral-rich crop that can diversify food systems. Front Nutr 4:7. https://doi.org/10.3389/fnut.2017.00007
Wright KM, Holden NJ (2018) Quantification and colonisation dynamics of Escherichia coli O157:H7 inoculation of microgreen species and plant growth substrates. Int J Food Microbiol 273:1–10. https://doi.org/10.1016/j.ijfoodmicro.2018.02.025
Wu M, Hou C, Jiang C et al (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101:1753–1758
Xiao Z, Lester GE, Luo Y et al (2012) Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J Agric Food Chem 60:7644–7651. https://doi.org/10.1021/jf300459b
Xiao Z, Nou X, Luo Y et al (2014) Comparison of the growth of Escherichia coli O157:H7 and O104:H4 during sprouting and microgreen production from contaminated radish seeds. Food Microbiol 44:60–63. https://doi.org/10.1016/j.fm.2014.05.015
Xiao Z, Lester GE, Park E et al (2015a) Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: microgreens. Postharvest Biol Technol 110:140–148
Xiao Z, Bauchan G, Nichols-Russell L et al (2015b) Proliferation of Escherichia coli O157:H7 in soil-substitute and hydroponic microgreen production systems. J Food Prot 78:1785–1790. https://doi.org/10.4315/0362-028x.jfp-15-063
Xonti A, Hunter E, Kulu N et al (2020) Diversification of health-promoting phytochemicals in radish (Raphanus raphanistrum) and kale (Brassica oleracea) micro-greens using high light bio-fortification. Funct Foods Health Dis 10(2):65–81
Ying Q, Kong Y, Zheng Y (2020) Applying blue light alone, or in combination with far-red light, during night time increases elongation without compromising yield and quality of indoor-grown microgreens. HortScience 55(6):876–881. https://doi.org/10.21273/HORTSCI14899-20
Yu S, Khor TO, Cheung KL et al (2010) Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One 5(1):e8579
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mishra, G.P. et al. (2022). Microgreens: A Novel Food for Nutritional Security. In: S. V., R., Praveen, S. (eds) Conceptualizing Plant-Based Nutrition. Springer, Singapore. https://doi.org/10.1007/978-981-19-4590-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-4590-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4589-2
Online ISBN: 978-981-19-4590-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)