Abstract
Recently, we reported that administration of Bifidobacteria resulted in increased concentrations of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in murine adipose tissue [1]. The objective of this study was to assess the impact of co-administration of Bifidobacterium breve NCIMB 702258 and the substrate for EPA, α-linolenic acid, on host fatty acid composition. α-Linolenic acid-supplemented diets (1%, wt/wt) were fed to mice (n = 8), with or without B. breve NCIMB 702258 (daily dose of 109 microorganisms) for 8 weeks. Two further groups received either supplement of B. breve alone or unsupplemented diet. Tissue fatty acid composition was assessed by gas liquid chromatography. Dietary supplementation of α-linolenic acid resulted in higher (P < 0.05) α-linolenic acid and EPA concentrations in liver and adipose tissue and lower (P < 0.05) arachidonic acid in liver, adipose tissue and brain compared with mice that did not receive α-linolenic acid. Supplementation with B. breve NCIMB 702258 in combination with α-linolenic acid resulted in elevated (P < 0.05) liver EPA concentrations compared with α-linolenic acid supplementation alone. Furthermore, the former group had higher (P < 0.05) DHA in brain compared with the latter group. These results suggest a role for interactions between fatty acids and commensals in the gastrointestinal tract. This interaction between administered microbes and fatty acids could result in a highly effective nutritional approach to the therapy of a variety of inflammatory and neurodegenerative conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammals can produce all but two of the fatty acids they require; thus linoleic acid (C18:2n-6, precursor of n-6 series of fatty acids) and α-linolenic acid (C18:3n-3, precursor of n-3 series of fatty acids) are essential dietary fatty acids. Although mammalian cells cannot synthesize these fatty acids, they can metabolize them into more physiologically active compounds through a series of elongation and desaturation reactions, in which linoleic acid is converted to arachidonic acid (C20:4n-6) and α-linolenic acid is metabolized to eicosapentaenoic acid (EPA) (C20:5n-3) via the action of the enzymes Δ6 desaturase, Δ5 desaturase and elongase [2]. The resulting highly unsaturated fatty acid metabolites play essential roles in cell membrane function, brain and nervous system development and function, and through the production of eicosanoids (thromboxanes, leukotrienes and prostaglandins) in the inflammatory process [2]. Eicosanoids derived from arachidonic acid, such as the 2-series prostaglandins and the 4-series leukotrienes are in general, regarded as being proinflammatory in nature [3, 4], whereas the eicosanoids derived from EPA, such as the 3-series prostaglandins and the 5-series leukotrienes are considered less inflammatory or even anti-inflammatory in nature [3–5]. Thus, by increasing the ratio of n-3 to n-6 fatty acids in the diet, and consequently favouring the production of EPA, the balance of eicosanoids can be shifted in a less inflammatory direction. EPA can be further metabolized to docosahexaenoic acid (DHA, C22:6n-3), which is one of the major n-3 polyunsaturated fatty acids (PUFA) in the brain. DHA is required for fetal brain development and is held to be critical in the newborn for appropriate development and intelligence [6]. Studies have also shown that DHA provides support to learning and memory events in animal models of Alzheimer’s disease [7] and brain injury [8].
The human gut is a diverse microbial ecosystem containing about 100 trillion microorganisms, comprised of more than 1,000 different species, whose collective genome, the microbiome, contains ~100-fold more genes than the entire human genome [9]. It has been well documented that the enteric microbiota play an important role in the health and well-being of the host, exerting effects on host lipid metabolism and acting as an environmental factor that contributes to development of obesity [10, 11]. In this respect, recent studies suggest that symbiosis between the microbiome and the host influences energy extraction from the diet. In addition, the promotion of fat deposition and influence on systemic inflammation have been proposed as mechanisms by which the microbiome contribute to obesity [12].
Little is known regarding the interplay between members of the enteric microbiota and fatty acids. However, some interactions between PUFAs and components of the indigenous gut microbiota and some probiotics have been reported, which might affect the biological roles of both. Recent studies by our group and others have reported that intestinal bacteria of human origin can convert linoleic and linolenic acids to bioactive isomers of conjugated linoleic acid (CLA) and conjugated α-linolenic acid, respectively [13–15]. Some bacteria of marine origin are also known to synthesise EPA and DHA de novo through the actions of polyunsaturated fatty acid synthase genes, which results in EPA and DHA being abundantly present in fish and fish oil [16–19]. Furthermore, it has been shown that administration of probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis Bb12) to pregnant women had an affect on placental fatty acid composition [20]. It has also been demonstrated that administration of formula supplemented with different probiotics (B. animalis subsp. lactis Bb12 and L. rhamnosus GG) to infants resulted in changes in serum fatty acid composition [21].
We have recently shown that feeding different animal species a CLA-producing Bifidobacterium of human origin (B. breve NCIMB 702258), in combination with linoleic acid as substrate, resulted in modulation of the fatty acid composition of the host, including significantly elevated concentrations of c9, t11 CLA in the liver. This study also demonstrated that oral administration of B. breve NCIMB 702258 to mice resulted in significantly higher concentrations of EPA and DHA in adipose tissue, coupled with reductions in the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [1]. The objective of this study was, therefore, to investigate the effects of co-administration of B. breve NCIMB 702258 and the substrate for EPA, α-linolenic acid, on fatty acid composition of different host tissues in mice.
Experimental Procedure
Preparation and Administration of B. breve NCIMB 702258
Rifampicin resistant variants of B. breve NCIMB 702258 were isolated by spread-plating ~109 colony forming units (CFU) from an overnight culture onto MRS agar (de Man, Rogosa & Sharpe; Difco Laboratories, Detroit, MI, USA) supplemented with 0.05% (wt/v) l-cysteine hydrochloride (98% pure; Sigma Chemical Co., St. Louis, MO, USA) (mMRS) containing 500 μg/ml rifampicin (Sigma Chemical Co., Poole, Dorset, UK). Following anaerobic incubation at 37 °C for 3 days, colonies were stocked in mMRS broth containing 40% (v/v) glycerol and stored at −80 °C. To confirm that the rifampicin resistant variant was identical to the parent strain, molecular fingerprinting using pulse-field gel electrophoresis (PFGE) was employed.
Prior to freeze drying, B. breve NCIMB 702258 was grown in mMRS by incubating overnight at 37 °C under anaerobic conditions. The culture was washed twice in phosphate buffered saline (PBS) and then resuspended at a concentration of ~1 × 1010 cells/ml in 15% (wt/v) trehalose (Sigma) in dH2O. One millilitre aliquots were freeze-dried using a 24 h programme (freeze temp. −40 °C, condenser set point −60, vacuum set point 600 mTorr). Each mouse that received B. breve consumed approximately 1 × 109 live microorganisms per day. This was achieved by resuspending appropriate quantities of freeze-dried powder in water which mice consumed ad libitum. Mice that did not receive the bacterial strain received placebo freeze-dried powder (15% wt/v trehalose in dH2O).
Animals and Treatment
Female BALB/c mice were purchased from Harlan Ltd. (Briester, Oxon, UK) at 8 weeks of age and were fed ad libitum with standard non-purified CRM(P) diet (Special Diets Services (SDS), Witham, Essex, UK) with free access to water at all times. The diet contained the following nutrient composition (wt/wt): nitrogen free extract (57.39%), crude protein (18.35%), moisture (10%), ash (6.27%), crude fibre (4.23%) and crude oil (3.36%), which consisted of saturated fatty acids: lauric acid (C12:0, 0.03%), myristic acid (C14:0, 0.14%), palmitic acid (C16:0, 0.33%) and stearic acid (C18:0, 0.06%), monounsaturated fatty acids: myristoleic acid (C14:1, 0.02%), palmitoleic acid (C16:1, 10%) and oleic acid (C18:1, 0.87%), polyunsaturated fatty acids: linoleic acid (C18:2n-6, 0.96%), linolenic acid (C18:3n-3, 0.11%) and arachidonic acid (C20:4n-6, 0.11%). Mice were maintained at four per cage and kept in a controlled environment at 25 °C under a 12-h-light/12-h-dark cycle. All laboratory animal experiments were performed according to the guidelines for the care and use of laboratory animals approved by the Department of Health and Children of the Irish Government.
One week after arrival, the mice were divided into four groups (A–D, n = 8) and subjected to the following dietary treatments daily: Group A received standard nonpurified CRM(P) diet supplemented with 1% α-linolenic acid (C18:3n-3, wt/wt, triglyceride bound form, Larodan Fine Chemicals AB, Malmo, Sweden) in combination with approximately 1 × 109 live B. breve NCIMB 702258 per mouse, Group B received standard non-purified CRM(P) diet supplemented with 1% α-linolenic acid and placebo freeze-dried powder, Group C received standard nonpurified CRM(P) diet and ~1 × 109 live B. breve NCIMB 702258, Group D received standard nonpurified CRM(P) diet and placebo freeze-dried powder. For α-linolenic acid treatment, a powdered diet (milled standard non-purified CRM(P) pellets) was blended with the α-linolenic acid to yield a concentration of approximately 90 mg α-linolenic acid per mouse per day (based on studies by Bassaganya-Riera et al. [22] who reported an optimal intake of fatty acids of 1 g/100 g per day). All prepared diets were stored at −20 °C and fresh diets were provided twice weekly. Following 8 weeks on experimental diets, the animals were sacrificed by cervical dislocation. Liver, adipose tissue and brain were removed from the carcasses, blotted dry on filter paper, weighed and frozen in liquid nitrogen. All samples were stored at −80 °C until processed.
Microbial Analysis
Fresh faecal samples were taken directly from the anus of each mouse every second week for microbial analysis. Large intestinal contents were also sampled at sacrifice for enumeration of the administered B. breve strain. Microbial analysis of B. breve NCIMB 702258 was performed by pour plating onto mMRS agar supplemented with 100 μg of mupirocin (Oxoid)/ml and 100 μg rifampicin (Sigma)/ml. Agar plates were incubated anaerobically at 37 °C for 72 h. Anaerobic environments were created using CO2 generating kits (Anaerocult A; Merck, Darmstadt, Germany) in sealed gas jars.
Lipid Extraction and Fatty Acid Analysis
Lipids were extracted according to the method of O’Fallon et al. [23]. Briefly, tissue samples were cut into 1.5-mm rectangular strips and placed into a screw-cap Pyrex culture tube together with 0.7 ml of 10 mol/l KOH in dH2O and 5.3 ml of MeOH. The tubes were incubated in a water bath at 55 °C for 1.5 h with vigorous hand-shaking every 20 min. After cooling below room temperature, 0.58 ml of 12 mol/l of H2SO4 in dH2O was added. The tubes were mixed by inversion and with precipitated K2SO4 present incubated again at 55 °C for 1.5 h with hand-shaking every 20 min. Fatty acid methyl esters (FAME) were recovered by addition of 3 ml hexane and vortex mixed and separated by gas liquid chromatography (Varian 3400, Varian, Walnut Creek, CA, USA fitted with a flame ionisation detector) using a Chrompack CP Sil 88 column (Chrompack, Middleton, The Netherlands, 100 m × 0.25 mm i.d., 0.20 μm film thickness) and He as the carrier gas. The column oven was initially programmed at 80 °C for 8 min, and increased at 8.5 °C/min to a final column temperature of 200 °C. The injection volume was 0.6 μl, with automatic sample injection on a SPI 1093 splitless on-column temperature programmable injector. Data were recorded and analysed on a Minichrom PC system (VG Data System, Manchester, UK). Peaks were identified with reference to retention times of fatty acids in a standard mixture. All fatty acid results are shown as means ± standard error means (SEM) g/100 g FAME.
Statistical Analysis
Results in the text, tables and figures are presented as means per group ± SEM. Data were analysed using analysis of variance (ANOVA) followed by Tukey’s post hoc test using GraphPad InStat for Windows (GraphPad Software, La Jolla, CA, USA) in order to assess if differences between treatment groups (A–D) were significant. Probability values of P < 0.05 were set as a threshold for statistical significance.
Results
Microbial Analysis
The administered B. breve NCIMB 702258 was recovered in faeces from all mice that received the strain, within 2 weeks of feeding, confirming gastrointestinal transit and survival of the strain. Stool recovery of B. breve NCIMB 702258 was approximately 4 × 105 CFU/g faeces by week 8 of the trial in mice that received B. breve in combination with α-linolenic acid (group A) and approximately 2.2 × 106 CFU/g faeces in mice that received B. breve without α-linolenic acid (group C) (data not shown). The B. breve strain was detected in large intestinal contents at ~4.6 × 105 CFU/g in mice that received B. breve and α-linolenic acid (group A) and ~1.4 × 106 CFU/g in mice that received B. breve alone (group C). B. breve NCIMB 702258 was not isolated from any of the mice within group B (administered α-linolenic acid alone) or group D (unsupplemented).
Tissue Fatty Acid Composition
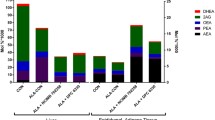
Oral administration of B. breve NCIMB 702258 and/or α-linolenic acid (C18:3n-3) did not significantly influence body weight throughout the trial period. Supplementation of α-linolenic acid, either in combination with B. breve or in the absence of the B. breve strain (group A and group B) resulted in tenfold higher concentrations of α-linolenic acid and EPA (C20:5n-3) in liver (P < 0.05; Table 1) and adipose tissue (P < 0.05; Table 2) compared with groups that did not receive the fatty acid supplement (group C and group D). In addition, the α-linolenic acid supplemented groups exhibited significantly higher concentrations of docosapentaenoic acid (DPA, C22:5n-3) in liver (P < 0.05; Table 1) and adipose tissue (P < 0.05; Table 2), significantly higher concentrations of DHA (C22:6n-3) in liver (P < 0.05; Fig. 2) and significantly lower concentrations of arachidonic acid (C20:4n-6) in liver (P < 0.05; Table 1), adipose tissue (P < 0.05, Table 2) and brain (P < 0.05, Table 3) compared with groups that did not receive fatty acid supplementation (group C and group D). The arachidonic acid/EPA ratios in liver and adipose tissue were approximately 30-fold and 20-fold lower, respectively, in the α-linolenic acid supplemented groups (group A and B) compared with unsupplemented controls (group D) (P < 0.05). In addition, the n-6/n-3 ratio was significantly lower in all tissues except the brain of animals supplemented with α-linolenic acid (group A and B) (P < 0.05; Table 1 and 2).
Administration of B. breve in combination with α-linolenic acid resulted in significant changes in the fatty acid composition of host liver and brain in comparison to animals that were administered α-linolenic acid alone. Mice that received B. breve in combination with α-linolenic acid (group A) exhibited on average, 23% more EPA (C20:5n-3) and 20% more dihomo-γ-linolenic acid (C20:3n-6) in the liver compared with the group that was administered α-linolenic acid alone (group B) (P < 0.05; Fig. 1; Table 1). Group A also exhibited a 12% higher concentration of DHA (C22:6n-3) in brain (P < 0.05; Fig. 2), as well as numerically, though not significantly, higher concentrations of DHA (C22:6n-3) in adipose tissue and liver (27 and 16%, respectively) compared with group B (Fig. 2). In addition, mice that received B. breve without α-linolenic acid (group C), exhibited numerically, though not significantly, higher concentrations of DHA (C22:6n-3) in brain tissue in comparison to unsupplemented controls (group D) (Fig. 2).
Oral administration of B. breve, both in combination with α-linolenic acid and without α-linolenic acid supplementation (group A and C), also resulted in significantly higher concentrations of arachidonic acid (C20:4n-6) and stearic acid (C18:0) incorporated in the liver compared to mice that did not receive B. breve (group B and group D) (P < 0.05; Table 1).
Discussion
The influence of dietary PUFA on phospholipids, their eicosanoid derivatives and the transmembrane-signalling lipid rafts into which they are arranged provide multiple targets for the dietary modulation of the balance of inflammatory mediators in the human gut. In addition, gut mucosal inflammation is now recognised as being heavily influenced by the gastrointestinal microbiota [24, 25]. The delicate balance of inflammatory mediators derived from PUFA may be readjusted by members of the indigenous gut microbiota. In this study, we investigated how co-administration of B. breve NCIMB 702258 and the substrate for EPA, α-linolenic acid affected the EPA and DHA concentrations of different host tissues. We found that dietary supplementation of B. breve NCIMB 702258 in combination with α-linolenic acid resulted in the modulation of host fatty acid composition, and, specifically, resulted in significantly higher EPA and dihomo-γ-linolenic acid concentrations in liver and higher DHA in brain compared to mice that received α-linolenic acid without microbial supplementation. Some recent studies have shown that the gut microbiota modifies a number of lipid species in serum, adipose tissue and liver [26] as well as the eye lipidome of mice [27] when compared to germ-free mice, suggesting that interactions between intestinal bacteria and fatty acids occur.
Supplementation of α-linolenic acid, both in combination with B. breve and in the absence of the B. breve strain, resulted in significant increases in EPA and DHA in the liver and adipose tissue, at the expense of arachidonic acid. Since EPA replaces arachidonic acid as an eicosanoid precursor in cell membranes of platelets, erythrocytes, neutrophils, monocytes and hepatocytes [28], this results in a reduced synthesis of inflammatory eicosanoids from arachidonic acid and, subsequently, elevated production of anti-inflammatory eicosanoids from EPA. This alteration towards a more anti-inflammatory profile could be of importance in a variety of chronic inflammatory settings that are of high prevalence in Western societies such as inflammatory bowel disease (IBD), rheumatoid arthritis, cardiovascular disease, obesity, Alzheimer’s disease and certain psychiatric diseases such as depression, which are characterised by an excessive production of arachidonic acid-derived eicosanoids [29–33]. Moreover, since excessive intake of n-6 PUFA, characteristic of modern Western diets, could potentiate inflammatory processes and so could predispose to, or exacerbate associated diseases, increasing the intake of α-linolenic acid and/or EPA may have a protective effect. A recent study using IL-10 knock-out mice (mice that spontaneously develop colitis) demonstrated significantly reduced colonic inflammation of mice that were fed fish oil (enriched in EPA and DHA) compared with mice that were fed n-6 PUFA-rich corn oil [34].
Oral administration of B. breve, both in combination with α-linolenic acid and without α-linolenic acid supplementation, resulted in significantly higher amounts of arachidonic acid incorporated in liver compared to mice that did not receive B. breve. Given that the administration of B. breve resulted in significantly higher concentrations of long-chain PUFA such as EPA, DHA and arachidonic acid, administration of this strain resulted in an increase in the levels of unsaturation within fatty acids. Interestingly, it was previously shown that a mixture of probiotics (Bacillus subtilis, B. natto, B. megaterium, B. thermophilus, Lactobacillus acidophilus, L. plantarum, L. brevis, L. casei, Streptococcus faecalis, S. lactis, S. thermophilus, Clostridium butyricum, Saccharomyces cerevisiae and Candida utilis) increased the activity of liver Δ6-desaturase in rats, which resulted in increased amounts of arachidonic acid derived from linoleic acid [35]. Consequently, in our study, the increased levels of long-chain PUFA in B. breve supplemented groups may have arisen from the reported properties of probiotics in regulating desaturase activity involved in the metabolism of fatty acids to their longer-chain unsaturated derivatives. B. breve NCIMB 702258 may also have influenced the mechanisms of PUFA uptake to the intestinal epithelium in the present study. It was recently demonstrated that exposure of L. plantarum WCFSI to human intestinal mucosa induced an upregulation of genes involved in fatty acid uptake, i.e. CD36 and microsomal triglyceride transfer protein (MTP) [36].
Quantification of the numbers of bacteria of the B. breve strain monitored in the feces of individual mice confirmed gastrointestinal transit and survival of B. breve NCIMB 702258. Faecal recovery was approximately 2 × 106 CFU/g feces in mice that received B. breve without α-linolenic acid and approximately 4 × 105 CFU/g feces in mice that received B. breve in combination with α-linolenic acid. The fecal recovery of B. breve NCIMB 702258 in mice that received B. breve without α-linolenic acid is consistent with our previous study [1], however the fecal recovery of B. breve was reduced in the presence of α-linolenic acid. Since free PUFA have been shown to be antibacterial and to inhibit the growth of bacteria [37–39] and adhesion of bacteria to intestinal surfaces [40], this might explain the lower numbers of B. breve obtained from mice that received supplementation of α-linolenic acid compared to mice that did not receive fatty acid supplementation.
Since the effect of the combined B. breve and α-linolenic acid intervention on EPA- and DHA concentrations was greater than that of α-linolenic acid intervention alone, this effect could be attributed to B. breve NCIMB 702258 and suggests that feeding a metabolically active strain can influence the fatty acid composition of host tissues. In conclusion, the present study shows that the administration of B. breve NCIMB 702258 is associated with alterations in the fatty acid composition of host liver and brain, including elevated concentrations of EPA and DHA. This interaction between administered microbes and n-3 fatty acids could result in more efficient probiotic preparations, which may be beneficial for a range of immunoinflammatory disorders as well as having significance for the promotion of neurological development in infants.
Abbreviations
- ANOVA:
-
Analysis of variance
- CFU:
-
Colony forming units
- CLA:
-
Conjugated linoleic acid
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FAME:
-
Fatty acid methyl esters
- IBD:
-
Inflammatory bowel disease
- IFN-γ:
-
Interferon-γ
- MTP:
-
Microsomal triglyceride transfer protein
- MRS:
-
de Man, Rogosa and Sharpe
- PBS:
-
Phosphate buffered saline
- PUFA:
-
Polyunsaturated fatty acids
- PFGE:
-
Pulse-field gel electrophoresis
- SDS:
-
Special diets services
- SEM:
-
Standard error mean
- TNF-α:
-
Tumor necrosis factor-α
References
Wall R, Ross RP, Shanahan F, O’Mahony L, O’Mahony C, Coakley M, Hart O, Lawlor P, Quigley EM, Kiely B, Fitzgerald GF, Stanton C (2009) The metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am J Clin Nutr 89:1393–1401
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 52:885–897
Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST (2003) Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA 100:1751–1756
Robinson JG, Stone NJ (2006) Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol 98:39–49
Salem N, Wegher B, Mena P, Uauy R (1996) Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA 93:49–54
Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka J, Shido O (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem 81:1084–1091
Wu A, Ying Z, Gomez-Pinilla F (2004) The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 19:1699–1707
O’Hara AM, Shanahan F (2006) The gut as a forgotten organ. EMBO Rep 7:688–693
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101:15718–15723
Marchesi J, Shanahan F (2007) The normal intestinal microbiota. Curr Opin Infect Dis 20:508–513
Tsai F, Coyle WJ (2009) The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep 11:307–313
Barrett E, Ross RP, Fitzgerald GF, Stanton C (2007) Rapid screening method for analyzing the conjugated linoleic acid production capabilities of bacterial cultures. Appl Environ Microbiol 73:2333–2337
Lee K, Paek K, Lee HY, Park JH, Lee Y (2007) Antiobestity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol 103:1140–1146
Coakley M, Ross RP, Nordgren M, Fitzgerald G, Devery R, Stanton C (2003) Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J Appl Microbiol 94:138–145
Orikasa Y, Yamada A, Yu R, Ito Y, Nishida T, Yumoto I (2004) Characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. strain SCRC-2738. Cell Mol Biol 50:625–630
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Russell NJ, Nichols DS (1999) Polyunsaturated fatty acids in marine bacteria—a dogma rewritten. Microbiology 145:767–779
Yano Y, Nakayama A, Saito H, Ishihara K (1994) Production of docosahexaenoic acid by marine bacteria isolated from deep sea fish. Lipids 29:527–528
Kaplas N, Isolauri E, Lampi AM, Ojala T, Laitinen K (2007) Dietary counselling and probiotic supplementation during pregnancy modify placental phospholipid fatty acids. Lipids 45:865–870
Kankaanpää PE, Yang B, Kallio HP, Isolauri E, Salminen SJ (2002) Influence of probiotic supplemented infant formula on composition of plasma lipids in atopic infants. J Nutr Biochem 13:364–369
Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff U, Hontecillas R (2004) Activation of PPARγ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 127:777–791
O’Fallon JV, Busboom JR, Nelson ML, Gaskins CT (2007) A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J Anim Sci 85:1511–1521
Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H (2002) Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54
Favier C, Neut C, Mizon C, Cortot A, Colombel JF, Mizon J (1997) Fecal beta-D-galactosidase production and Bifidobacteria are decreased in Crohn’s disease. Dig Dis Sci 42:817–822
Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu PV, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, Bäckhed F (2009) The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res doi:10.1194/jlr.M002774
Oresic M, Seppänen-Laakso T, Yetukuri L, Bäckhed F, Hänninen V (2009) Gut microbiota affects lens and retinal lipid composition. Exp Eye Res 89(5):604–607
Simopoulos AP (2003) Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet 92:1–22
Dinan T, Siggins L, Scully P, O’Brien S, Ross P, Stanton C (2008) Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J Psychiatr Res 43:471–476
Jupp J, Hillier K, Elliott DH, Fine DR, Bateman AC, Johnson PA, Cazaly AM, Penrose JF, Sampson AP (2007) Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis 13:537–546
Wallace JL (2001) Prostaglandin biology in inflammatory bowel disease. Gastroenterol Clin North Am 30:971–980
James MJ, Gibson RA, Cleland LG (2000) Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 71:343S–438S
Simopoulos AP, Leaf A, Salem N (2000) Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 63:119–121
Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN (2007) Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J Nutr 137:200–204
Fukushima M, Yamada A, Endo T, Nakano M (1999) Effects of a mixture of organisms, Lactobacillus acidophilus or Streptococcus faecalis on delta-6 desaturase activity in the livers of rats fed a fat and cholesterol-enriched diet. Nutrition 15:373–378
Troost FJ, van Baarlen P, Lindsey P, Kodde A, de Vos WM, Kleerebezem M, Brummer RJ (2008) Identification of the transcriptional response of human intestinal mucosa to Lactobacillus plantarum WCFSI in vivo. BMC Genomics 9:374–388
Nieman C (1954) Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev 18(2):147–163
Laser H (1951) Adaptation of Bacillus subtilis to fatty acids. Biochem J 49(5):lxvi–lxvii
Kelsey JA, Bayles KW, Shafii B, McGuire MA (2006) Fatty acids and monoacylglycerols inhibit growth of Staphylococcus aureus. Lipids 41(10):951–961
Kankanpää PE, Salminen SJ, Isolauri E, Lee YK (2001) The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol Lett 194:149–153
Acknowledgments
The authors acknowledge the technical assistance of Seamus Aherne for fatty acid analysis, Frances O’Brien and Grainne Hurley for assistance with the murine trial. The authors are supported, in part, by Science Foundation Ireland (SFI), the Irish Ministry for Food and Agriculture, the Higher Education Authority and the Health Research Board of Ireland and the Irish Government under the National Development Plan 2000–2006.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wall, R., Ross, R.P., Shanahan, F. et al. Impact of Administered Bifidobacterium on Murine Host Fatty Acid Composition. Lipids 45, 429–436 (2010). https://doi.org/10.1007/s11745-010-3410-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3410-7