Abstract
The electrolytes in batteries are one of the vital components that can decide the overall performance of any secondary storage device. The conventionally used liquid electrolytes possess many safety hazards while operating at extreme conditions of temperature and climate. Polymer based solid electrolytes are identified as one of the key candidates that can offer safety, good battery performance, flexible and compact battery design. This chapter gives an overview of the various polymer based electrolytes that are currently under research and development for use in LIBs. Prior to explaining the polymer based electrolytes, the general properties of electrolytes are outlined with some of the conventionally used liquid electrolyte system and their characteristics. Special emphasis is given to solid polymer electrolytes, Gel polymer electrolytes, the ion transport mechanism in these systems and their characteristics are highlighted with examples from literature.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Li-Ion batteries (LIBs) have become an indispensable part of our daily power requirements enabling the use of wide range of mobile electronic devices and tools. Electrolytes serve as key components in Li-Ion battery technology as they are vital in making the electronic devices conductive by permitting the transport of ions from cathode to anode upon charging and reverse process during discharge. In LIBs, electrolytes undertakes the role of a medium that enables hassle free movement of Li-Ions back and forth between the cathode and anode and hence should possess high ionic conductivity. The basic components of an electrolyte in a battery include soluble salts, acids or other bases in liquid, gelled and dry forms.
The electrolytes used currently in batteries are the outcome of many years of research and development and play a significant role in providing good performance for various applications. Significant innovations in battery chemistry and technology is the need of the hour to cater to the novel challenges in energy storage requirements for hybrid electric vehicles, power tools and stand-by power sources for communications and modern aircrafts. The batteries looked-for require higher voltages and higher energy content. Moreover, they are prone to exposure to extremes of temperature with the requisite of providing long cycle, storage life and assured user safety. A new class of electrolytes is essential to cater to these requirements. The emerging electrolytes are not only expected to impart good ionic conduction over a wide range of ambient temperatures but also cater to the need of superior chemical stability and compatibility with the more reactive electrode materials in order to realize higher battery-specific energy and power. Considering these factors, research and technology is favoring the use of high specific energy electrode materials, such as layer–layer composite, layer–spinel composites made of Li[Co, Ni, Mn]O2 (Mizushima et al. 1980; Rougier et al. 1996; Hy et al. 2014), layer-olivine phosphates like LiCoPO4 (Lloris et al. 2002) having specific capacity in the range of 250–300 mAh/g in comparison to 140 mAh/g for LiCoO2 cathodes. There is also demand for higher capacity Li alloy-based anodes such as Li–Sn (Xu et al. 2013) and Li–Si (Ryou et al. 2013) alloys. The present state-of-art electrolytes based on lithium hexafluorophosphate (LiPF6) dissolved in linear and cyclic carbonate solvent mixtures with functional additives have turned out to be insufficient in these new higher energy density electrochemical systems and no compromise over capacity or power could be thought about (Xu 2004). A step further ahead, researchers began utilizing sulfur or air as an even higher theoretical capacity cathode pairing with pure Li as an anode to achieve even higher energy density. The need of compatible electrolytes is inevitable for developing such higher energy density systems. Hence the development and usage of new electrolytes will depend on their compatibility with the as mentioned electrode materials which in effect count on a proper understanding of electrolytes and their interaction with the electrodes.
Most of the electrolytes in Li based batteries employ aprotic or non-aqueous organic carbonate solvents like Ethylene Carbonate (EC) in which Li salts are dissolved. Other electrolyte systems make use of lithium salts dissolved in ionic liquids (IL), solids/ceramics, polymers, and aqueous based systems which have their own merits but what limits their utilization to an extent are their lower ionic conductivities, higher production costs, safety and toxicity concerns (Aurbach 1999; Henderson 2014; Gores et al. 2011).
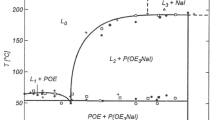
2 Electrolyte Properties
Early research on electrolytes used in Li-Ion batteries (LIBs) relied mainly on Li salts with few anions such as hexafluoroarsenate (AsF6−), perchlorate (ClO4−), hexafluorophosphate (PF6−), tetrafluoroborate (BF4−) and trifluoromethanesulfonate or triflate (Tf) (CF3SO3−) (Fig. 1) (Pistoia 1971; Desjardins et al. 1985; Besenhard and Eichinger 1976; Koch 1981). Most of these anions were initially used based on the understanding of their ability to generate strong superacids which are considered to be stronger than sulfuric acid (Olah et al. 2009). The stronger the acidity, the weaker the coordination of the anions with the associated protons (H+ cations). Therefore the ionic association tendency of anions to coordinate Li+ cations will be moreresulting in the replacement of the acid protons with Li+ cations giving rise to the corresponding lithium salts (Koppel et al. 1994; Barthel et al. 1996). With more advancement in LIB technology, lithium salts like LiAsF6 and LiClO4 became less popular due to safety ad toxicity issues. It was also found that the electrolytes made from LiTf suffered from low conductivity issues. Gradually with the advent of graphite anodes and commercialization of LIBs, LiPF6 and LiBF4 dissolved in aprotic solvents like EC or dimethylene carbonate (DMC), became more popular with ultimately LiPF6 gaining prominence as the most widely used Li salt in electrolytes (Huang et al. 1994; Johansson 2007; Dudley et al. 1991). In fact it is not the best candidate in any of its properties of paramount importance to batteries like: ionic conductivity-relatively good, hygroscopic, thermal stability-somewhat poor but its success as the most dominant salt arises from the fact that it strikes the best balance of these properties and enables the formation of an appropriate Solid Electrolyte Interface (SEI) along with solvents such as EC or DMC developed on the graphite anode in addition to a suitable protective layer formed on the aluminum cathode current collector. The use of LiBF4 is also common since it operates well in LIBs at extreme temperatures (−20–80 °C) but exhibits only moderate conductivity (Takami et al. 2002; Zhang et al. 2003). All this clearly indicates the intricacies of the criteria to be fulfilled by the Li salt with regard to the several components of the cell (Andersson et al. 2002).
Electrolyte in LIBS must fulfill an extensive and challenging range of properties some of these as listed below.
I. Solubility. A reasonably high lithium salt solubility in the electrolyte solvents is a prerequisite to provide adequate charge carriers for rapid ionic conduction, as well as to prevent precipitation. It is vital to differentiate the solubility of salt from crystalline solvate formation. A salt may be readily soluble, but also leads to a crystalline solvate phase with a high melting point (Tm) giving rise to the solvates formed in the electrolyte which gradually extracts the salt from the electrolyte solution affecting the conductivity to drop. This becomes more evident in the case of cell operation at lower temperatures (Younesi et al. 2015). The Li dissolution in non-aqueous solvents, occurs through solvent-Li+ interfaces (Scheers et al. 2010). The interaction of solvent and Li+ involves two processes; (i) Li+ gets dissociated from the anion, overcoming the lattice energy of the salt and (ii) s coordination bonds between Li+ ions and the electron-lone pairs of the solvent molecules are subsequently formed. The extent of Li+–anion interaction is extremely crucial besides solvent properties like viscosity and polarity. A strong cation–anion interactions lead to high lattice energies resulting in poor solubility of the salts in relevant aprotic solvents which is why many simple salts like Li2O, LiF, LiCl etc. are restricted from electrolyte usage. Li salts like triflates made of weakly coordinating anions (WCA) to reduce Li+–anion interaction are also preferred. They are developed through delocalization of negative charge over the whole anion via electron-drawing substituents like –F or –CF3 (Johansson et al. 2004).
II. Ionic Conductivity. A high Li+ cation transport rate is crucial for high power output as the Li+ cation mobility within the bulk electrolyte often serves as one of the main sources of impedance for the battery. The selection of a lithium salt’s anion is an influencing factor that determines the electrolyte’s conductivity. This is due to the variations in the Li+ cation solvation and ionic association interactions due to the differences in anion structure and coordination strength. Although electrolyte conductivity is the paramount parameter to be considered, it is actually the Li+ cation mobility that is calculated from the product of the total ionic conductivity and Li+ cation transference or transport number (i.e., TLi+, tLi+). This value tells about the measure of the fraction of the current contributed by the Li+. It is estimated to be less than 0.5 in non-aqueous electrolytes from impedance measurements. The electrolyte conductivity increases as a function of salt concentration with increase in solvation of Li salt in solvent as the number of charge carriers increases. Based on Stokes–Einstein equation, the ionic conductivity of liquid electrolytes depends on the dissociation of ions in the solvents and could be improved by enhancing the ion-dissociation in solvents with high dielectric constant and low viscosity.
where D = Diffusion coefficient, \(\eta\) = solvent viscosity, k = Boltzman Coefficient, T = Temperature, R = solute radius. In totality, the conductivity of any aprotic/nonaquoeus Li-salt based electrolyte depends on the salt concentration, anion, solvent composition and the temperature. For instance, the conductivity of LiClO4 in propylene carbonate (PC) attains a maximum value of 1.5 mS cm−1 at a low salt concentration of 0.08 M, with further increase in the concentration results in a drop in the conductivity. The same conductivity of 15 mS cm−1 is achieved in a solvent combination of EC/PC at a salt concentration of 1 M (Chen et al. 2000).
III. SEI Formation. The SEI refers to a passivation layer formed at the interface between the electrode surface and the electrolyte when the both electrolyte components and electrode material(s) degrade or react (Zaghib et al. 1995). Only a limited amount of materials is expected to react, with the as formed SEI layer restricting further electrode–electrolyte reactions and aiding the smooth movement of Li+ cations within the electrolytes and electrodes offering low impedance. The lithium salt(s), whether as a bulk salt or an additive, can considerably influence the SEI’s components properties, and stability (Ryou et al. 2012). The popularity of graphite anode relies on the formation of this SEI which acts as a barrier for electron transfer between anode and the electrolyte resulting in kinetic stability of the cell and prevents exfoliation that occurs due to solvent intercalation during cycling.
IV. Stability. Electrolytes must, in general, be stable in the sense that it has to be non-reactive with all other cell components within the electrochemical potential window for the battery charge/discharge reactions (Lee et al. 2005). Electrolytes also need to be stable at extremes of temperatures, to deliver thousands of charge/discharge cycles with low capacity fading. From a thermodynamic stability point of view, battery should contain an electrochemical stability window (ESW), located beyond the oxidation and reduction potential window of the cathode and anode respectively. The difference between the LUMO and HOMO of the electrolyte defines the theoretical ESW which is determined by the coordination between Li-salt and solvent. ESW is strongly temperature dependent with even a slight increase in temperature, in some cases, resulting in a significant decrease in stability (Zheng et al. 2005). The electrolyte need to be stable in contact with the reaction products developed during cycling. This is mostly relevant in the case of Li–S, Li–O2 in which new reaction species or intermediate products are formed in each discharge cycle which can degrade the electrolyte and cause battery failure unlike in conventional LIBs based on intercalation electrodes. Many F-containing anions hydrolyze upon exposure to water, specifically at elevated temperatures giving rise to the formation of HF which can degrade the electrolyte and also cause serious safety concerns. This leads to additional costs associated with the salt’s synthesis, storage, and handling. The formation of HF could strongly impact the cycling behavior and lifetime of batteries when performed at high temperature and/or at high potential windows (>4.8 V versus Li/Li+) (Lux et al. 2012).
V. Donor Number. For any battery electrolyte, Guttmann Donor number (DN). (Gutmann 1976), is an often used quantitative measure for solvents showing their electron donating properties and hence ability to interact with acceptors such as protons, Li+ and other cations thereby solvating them. The higher DN, the higher basicity of the solvent resulting in stronger interaction with Li+ and hence most anions have relatively small DN. For instance, the DN for the PF6− and AsF6− is 2.5 with tetrabutyl ammonium (TBA+) as the counter cation in PC at 25 °C measured using a solvatochromic method while that of ClO4− is 8.4 under the same conditions. This is one of the reasons for lesser solubility of ClO4− in PC compared to PF6−. In recent studies, DN is found to be significant in the case of Li–O2 batteries where it is found to influence the, discharge reaction pathways and the capacity of the cells (Jeong et al. 2008). In the case of Li–S cells, it influences the solubility of the intermediate polysulphide species formed in every discharge cycle (Suo et al. 2013).
2.1 Conventional Non-Aqueous Liquid Electrolytes
The electrolyte system in any battery system assumes the role of a medium for the transport of ions between the pair of electrodes. As discussed in the previous section, vast majority of the electrolytes used in secondary batteries is made up of electrolytic solutes (salts) dissolved in organic liquids operating in the service temperature range. Being sandwiched in between the electrodes and in close interaction with them, this interface between electrolyte and electrodes direct the performance of a battery system. In batteries, the chemical behavior of the cathode and anode determines the energy output whereas the electrolyte controls the rate of mass flow within the battery and tells us how fast the energy could be released. All the Faradaic processes happen at the electrodes and no net chemical change should occur to the electrolyte during the operation of the battery. A typical non-aqueous liquid electrolyte used in rechargeable lithium make use of LiPF6 dissolved in a mixture of ethylene carbonate (EC)/propylene carbonate and linear carbonates like dimethyl carbonate (DMC), diethyl carbonate (DEC) (Tarascon and Guyomard 1994). A mixture of two or more solvents is preferred over single solvent formulation primarily because individual formulation often does not alone meet the diverse and contradictory requirement of battery operation. Hence solvents with varied chemical and physical properties are used together to perform various functions simultaneously. A mixture of salts is however not used since anion choice is limited and performance improvement is not readily demonstrated. An ideal electrolyte solvent must adhere to the following criteria:
-
It should have a high dielectric constant and dissolve sufficient concentration of salts.
-
Possess low viscosity (ŋ) to facilitate smooth ion transport.
-
Remain inert to all cell components especially the charged surface of cathode and anode during cell operation.
-
Should have a low melting point (Tm) and high boiling point (Tb) and remain liquid over a wide range of temperature. The flash point also needs to be high (Tf) so that it remains safe.
The use of a non-aqueous solvent that has active protons and solvate lithium salts quite efficiently is often ruled out because the strongly reducing anode and strongly oxidizing cathode which initiates the reduction of proton and the oxidation of the corresponding anions fall within 2.0–4.0 V versus Li whereas the charged potential of the cathode and anode in rechargeable lithium devices ranges between 3.0–4.5 V and 0.0–0.2 V respectively. Hence solvents with polar groups which have sufficiently good solvation like carbonyl (C=O), nitrile (C=N), sulfonyl (S=O) and ether linkage (–O–) are conventionally considered (Fry 1989).
The interest in alkyl carbonates as solvents gained prominence with development in Li-ion batteries. Propylene carbonate (PC) with its high anodic stability made it a promising solvent in first generation of commercial Li-ion cells with LiCoxO2 as cathode and petroleum coke as anode (Harris 1958). However, EC with its high dielectric constant and low viscosity brought the real renaissance in using alkyl carbonates as lithium electrolyte solvents since it was understood that it could not only improve the bulk ionic conductivity but also interfacial properties like less polarization on various cathode surfaces (Surampudi et al. 1993). EC assumed a unique place in electrolyte system when Dahn et al. in 1990 studied the fundamental difference between the two on how they affected the reversibility of Li-Ion intercalation/deintercalation on graphite anodes. EC was found to be more effective in the formation of an effective SEI on graphite anode that prevented further electrolyte decomposition on the electrode. On the other hand, with PC co-intercalation, the graphite anode eventually exfoliated and disintegrated. PC was also found to favor the formation of Li dendrite like structures via its reaction with Li and caused non uniformity on Li surface. This gradually resulted in the formation of Li crystals which are isolated from the lithium anode during the discharge processes. These dendrites as well as isolated lithium crystals react with the solvent and cause serious safety hazards and internal shorts (Dahn et al. 1991). The first commercialized rechargeable lithium battery used a combination of EC/PC mixture as the solvent in which LiPF6 was dissolved. Attempts to use ethers as solvents did not gain much momentum as they were unstable against oxidation caused on the surface of the charged cathode. It was realized that the electrolyte must have a oxidative stability up to ~5 V vs Li for a Li-Ion cell that uses graphite as anode and LiMO2 metal oxides as cathode (4 V), the. In order to achieve this, the use of a linear carbonate, dimethyl carbonate (DMC) as cosolvent with EC was formulated (Guyomard and Tarascon 1993). Linear carbonates can form homogenous mixture with EC at any ratio and the resultant mixed electrolytes take the synergistic advantages of both solvents like high anodic stability of EC on cathode surfaces, high lithium solvation of EC, melting temperature suppression of EC, low viscosity, hence better ion transport and high ionic conductivity of DMC are imparted to the resultant electrolyte. Such a mixture of solvents also benefit from high electrochemical stability window: it remains stable up to 5 V on a spinel cathode. Other linear carbonates like diethyl carbonate (DEC), ethylmethyl carbonate (EMC), propylmethyl carbonate (PMC) etc. were also used. These mixture of solvents made of EC and linear carbonates with LiPF6 emerged as state of art electrolyte in Li-Ion cells (Ein-Eli et al. 1996; Chu et al. 1997; Koetschau et al. 1995). Another interesting way of improving the electrolyte performance was by the introduction of fluorine atoms into the solvent molecules. Fluorinated organic solvents exhibit different physical properties compared to commonly used organic solvents due to the high electronegativity, superior ionic potential and poor polarizability of the fluorine atom. In truth, partially a fluorinated organic solvent demonstrates high polarity compared to perfluoro-organic solvents. Direct fluorination of EC and PC were carried out and improvement in the cycling efficiency was obtained (McMillan et al. 1999). Since fluorine was involved in the process, there were concerns regarding the formation of HF and subsequent safety hazards which limited their usage in practical application. Table 1 mentions the various Li salts and their conductivities in commonly employed solvents.
2.2 Characteristics of High Voltage Electrode–Electrolyte Interface
LIBs always seek improvement in the energy and power density, cycle life and safety to range their applications from small scale like in electronic de vices to large scale such as in pure-electric vehicles. Increasing the specific capacity and operating potential of the cathode materials is one of the key approaches to promote the energy and power density of LIBs Therefore many novel cathode materials have been successfully proposed such as nickel-rich layered oxides (LiNi1−xMxO2 M = Co, Mn and Al), lithium-rich layered oxides (Li1+xM1−xO2 M = Mn, Ni, Co, etc.) and high-voltage spinel oxides (LiNi0.5Mn1.5O4) (Rozier and Tarascon 2015; Song et al. 2017; Kim et al. 2016) However, these cathode materials suffer from poor cycling stability at higher potentials which practically hinders its application in LIBs. The continuous electrolyte oxidation is mainly responsible for the capacity fading of high voltage LIBs. The commercialized carbonate-based electrolyte oxidizes when the cell operating voltage goes beyond 4.5 V, resulting in continuous increase in the interfacial reaction resistance and generation of harmful by-products, like HF, which hastens the dissolution of transition metal ions from cathode materials. Improving the interfacial stability of high voltage cathode/electrolyte is crucial for the practical application of high voltage LIBs (Kim et al. 2016; Park et al. 2014). The redox stability of electrolytes (containing lithium salt and mix-solvents) depends not only on the lowest oxidation/reduction stability solvent, but also on the stability of the component that lies next to the electrode surface. Moreover, the composition of the interfacial electrolyte near the electrode depends strongly on the electrode material. The electrolyte component of the most widely used electrolyte (1 M LiPF6/EC:DMC = 3:7) near a graphite electrode was extensively studied by Vatamanu et al. (2012) using molecular dynamic (MD) simulation and observed that upon increasing the electrode potential i.e., charging process, the less polar DMC solvent is partially replaced in the interfacial electrolyte layer by the more polar ethylene carbonate (EC) solvent. At higher potential values, the surface becomes affluent in the minority EC than in DMC. This result elucidates that the detected electrolyte oxidation decomposition products arises mainly from EC than DMC, though the former shows higher oxidation stability than the latter. Sulfone-based electrolytes have high oxidation stability compared to conventional carbonates. The interfacial electrolyte component of trimethyl sulfonate (TMS)/DMC mixed-solvent doped with LiPF6 salt was also studied consequently. TMS was shown to have higher polarity and stronger binding energy with Li+ than that of DMC. As a result, when the negative electrode potential increases, the electrode surface gets loaded with higher proportions of TMS molecules. On the other hand at positive electrode/cathode surface, the ratio of TMS/DMC was found to be similar to the bulk components (Xing et al. 2012). The change in the interfacial electrolyte component as a function of electrode potential obtained from the MD simulation helps to predict the electrochemical stability of the electrolyte, and more importantly, to design an ideal interfacial electrolyte layer with high reaction inertness, or to form a high stability solid electrolyte interphase (SEI) film and cathode electrolyte interphase (CEI) film on the anode and cathode surfaces, respectively (Watanabe et al. 2008).
The critical role of anions in affecting the electrolyte oxidation stability has also received considerable attention in recent years. The oxidation decomposition rate of propylene carbonate (PC)-based electrolyte greatly depends on the lithium salts being used, indicating that salt anions are in fact involved in electrolyte oxidation. Moreover, studies also reveal that apart from more polar solvent, a higher accumulation of PF6–anions on the positively charged surfaces can also be observed, signifying the possibility of anions participating in the oxidation reaction with the neighboring polar solvent (Arakawa and Yamaki 1995). The idea to replace carbonate-based electrolytes with high anodic stability salt and a solvent could drastically solve the instability of the high voltage cathode/electrolyte interphase. However, the practical application of these novel electrolytes in LIBs takes longer since they must consider compatibility with the graphite anode and the Al current collector, influence on interphasal reaction kinetics, safety, cost etc. Majority of the novel non-carbonate/LiPF6 electrolytes appear in a highly concentrated state, which shows improved anodic stability than in a dilute state. Dahn et al. (Xia et al. 2016) showed that LiPF6/EC-free-linear alkyl carbonate-based electrolytes with a small amount of film-forming additive, such as vinylene carbonate (VC), achieved higher capacity retention of high voltage LIBs. They also suggest that further optimizing the linear alkyl carbonate electrolytes with appropriate co-additives may open a plausible path to the successful commercial utilization of high voltage LIBs. Without using EC as co-solvent and replacing LiPF6 with a high stable salt lithium bis(fluorosulfonyl)-amide (LiFSA), Wang et al. (2016) reported stable and fast charge/discharge cycling of 5 V LiNi0.5Mn1.5O4/graphite cells. The high concentration LiFSA/DMC electrolyte with salt-to-solvent molar ratio of 1:1.1 shows great stability, with high voltage cathode, graphite anode and an Al current collector, in effect giving a significantly improved cyclic performance of high voltage LIBs as shown in Fig. 2.
Performance of a high-voltage LiNi0.5Mn1.5O4|natural graphite battery. Charge–discharge voltage curves of LiNi0.5Mn1.5O4|graphite full cell using a a commercial 1.0 mol dm−3 LiPF6/EC:DMC (1:1 by vol) electrolyte and b a lab-made superconcentrated 1:1.1 LiFSA/DMC electrolyte at a C/5 rate and 40 °C. c Discharge capacity retention of the full cells at a C/5 rate. d Discharge capacity of the full cell at various C-rates and 25 °C. All charge–discharge cycling tests were conducted with a cut-off voltage of 3.5–4.8 V. The 1C-rate corresponds to 147 mA g−1 on the weight basis of the LiNi0.5Mn1.5O4 electrode (Wang et al. 2016)
Sun et al. (2017) proposed the use of three novel half borate lithium salts, such as lithium difluoro-2-methyl-2-fluoromalonaoborate (LiDFMFMB), lithium difluoro-2-ethyl-2-fluoromalonaoborate (LiDFEFMB), and lithium difluoro-2-propyl-2-fluoromalonaoborate (LiDFPFMB) (Fig. 3) in high voltage LIBs. The ionic conductivity of the half borate lithium salts occur in the following order of LiDFMFMB > LiDFEFMB > LiDFPFMB. As the alkyl chain length of the anions increase, the mobility of the anions is found to increase.
Cyclic voltammetry was performed to investigate the redox stability of these new salts on a natural graphite anode and a high voltage spinel LiNi0.5Mn1.5O4 (LNMO) cathode, which showed that three new salts reduced/oxidized on the graphite/LNMO surface when the electrode potential initially decreased/increased, and significantly, created SEI and CEI film on the graphite and LNMO surfaces, respectively. Therefore, the new salt-based electrolytes presented good cycling stability with high columbic efficiencies in both graphite and LNMO based half-cells and full cells. Another successful way of improving the characteristics of interfacial stability of high voltage cathode is the use of CEI film-forming additives. Such electrolyte additive does not bring any change in the physicochemical properties of the electrolyte like its ionic conductivity or viscosity. Some of the additives, in addition to improving the interfacial stability of graphite/electrolyte also enhance the thermal stability of LiPF6, taking up HF and reducing the risk of flammability of electrolytes. For instance, an electrolyte additive based on a highly fluorinated phosphate ester structure tris(hexafluoro-iso-propyl) phosphate (HFiP) was found to stabilize carbonate-based electrolytes on 5 V LiNi0.5Mn1.5O4 cathode surfaces (Cresce and Xu 2011). The sacrificial oxidation of HFiP occurs before oxidation of the bulk electrolyte components, passivizing the reactive sites on the cathode surface, resulting in improved cyclic stability of the LiNi0.5Mn1.5O4/Li half-cell. In addition, HFiP also provided excellent protective SEI on the graphitic anode. Such protective CEI layer prevents electrolyte decomposition and cathode degradation.
3 Solid Polymer Electrolytes (SPEs): Components and Ion Transport Studies
The need for electric vehicles (EVs) has become indispensable in a view to reduce greenhouse emissions and the use of fossil fuels. LIBs have been identified as one of the best options to power such large scale applications with essential requirements like high energy density, exceptional safety, long lifecycle, and extensive operating temperature range (Goodenough and Kim 2010). It is well understood that the electrolyte sandwiched between the anode and the cathode has a key role to play towards the success of LIBs used for powering EVs. In extreme thermal, mechanical and electrical conditions, the use of flammable/combustible organic electrolytes pose a threat to the safety features of LIBs (Tarascon and Armand 2001). A sustainable strategy to evade this limitation of existing LIBs is to replace the state-of-the-art, alkyl carbonate-based liquid electrolytes, with solid electrolytes (SEs), which paves a way towards building intrinsically safer, and possibly eco-friendly, lithium battery systems. SE materials mainly include solid polymer electrolytes (SPEs), inorganic solid electrolytes (ISEs), and their composite/hybrid versions (Kalhoff et al. 2015). In this section we mostly stick on to solid polymer electrolytes (SPEs) and their possibilities as electrolyte membranes.
3.1 Components of SPEs
SPEs offer advantages of cost effectiveness, ease of processing to fabricate ultra-thin films, facile patterning and integration to give flexible battery design. SPEs also rule out the possibility of the use of separators which act as membrane separating cathode and anode to avoid shorting. Solid polymer electrolytes (SPEs) comprises of lithium salts (Li+, X−, X− = monovalent anion) with low dissociation energy, polymer matrices with high donor number (DN), solid additives like Al2O3 as fillers, low-molecular-weight plasticizers like, PEGDME (Arya and Sharma 2017; Fan et al. 2018). The polymer electrolytes in which plasticizers are added to enhance ionic transport to impart more ionic conductivity falls under the category of Gel polymer electrolytes (GPEs) though they can be broadly treated as a sub category of SPEs (Song et al. 1999). Lithium salts embedded on polymer matrices, and/or other solid additives, have improved mechanical properties and lesser fire-induced hazards compared to those added with plasticizers (GPEs), though the former tends to be less ionic conductive. SPEs possess many merits over conventional liquid electrolytes such as low reactivity (kinetically) with polarized electrodes, no risk of electrolyte leakage and release of harmful/toxic gases, slower Li dendrite formation. In contrast to ISEs which have better ionic conductivity than SPEs, the SPEs can attenuate the interfacial resistance and improve the electrode–electrolyte compatibility (Bachman et al. 2016) (Fig. 4).
3.2 Ionic Transport Mechanism in SPE
The chemical properties of SPEs are intrinsically different from that of liquid organic electrolytes. In conventional liquid electrolytes, both cations and anions are mobile and contribute to the ionic conductivity. The mobility of these ions also varies in solvents and hence individual contribution to conductivity varies over a wide range. The ions solvate and move in the solvent, hence by improving the ionic dissociation in the solvents one can improve the ionic conductivity. On the other hand, only one type of ion is mobile in SPEs and in order for ion transport it requires to overcome the electrostatic forces and other interaction with the lattice or polymer structure in general. Ionic mobility and diffusion coefficient vary with compositional variation in the polymer, in other words ionic conductivity is highly dependent on the amorphous/crystalline structural frame of the host polymer matrix. The space provided by the free volume of the polymer allows the ions to move within the matrix and conductivity above the glass transition temperature could be achieved where the ions are free to move. Polymers thus act as host for ionic transport. Ionic transport could be viewed to have diffusive liquid-like behavior in SPE (Druger et al. 1983; Harris et al. 1986; Bunde et al. 2005). Ionic conductivity of the SPE is measured by electrochemical impedance spectroscopy (EIS) in a certain frequency range using an AC impedance analyzer. It is understood that the ionic conduction mechanism in polyether based media occur via amorphous phases. The local structural relaxations associated to the glass transition occurring in these amorphous domains contribute to the ionic motion. It is understood that the lithium ions are coordinated on the segments of a polymeric chain by ether oxygen atoms in a similar manner in which they complex with crown ethers or other oligo-ether based solvents (Druger et al. 1983). A continuous segmental rearrangement occur with the gradual replacement of ligands in the solvation medium of Li-Ions giving rise to the long range net displacement of Li-Ions.
We need to understand the ionic transport mechanism at macroscopic level, i.e., ionic conductivity-temperature dependence and at microscopic level i.e. Li+ transport mechanism. In 1889, Arrhenius proposed an empirical Eq. (2) for the description of temperature (T, K) dependence of reaction rate (k, rate constant) (Stoeva et al. 2003). This equation has been extended to express the relationship between ionic conductivity (σ, S cm−1) and temperature in Eq. (3). In both equations, Ea, and R represent, activation energy (J mol−1), and ideal gas constant (J mol−1 K−1), respectively. In 2009, Frech et al. (Petrowsky and Frech 2009) gave a modified Arrhenius equation that introduces the temperature dependence of the dielectric constant (ε(T)) into the pre-exponential factor in order to terminate the non-Arrhenius factors as in Eq. (4).
These are mostly applied to inorganic electrolytes with ordered structures (such as Li3N, and β-Al2O3), as well as in crystalline polymer electrolytes. However, for amorphous materials such as silicates, phosphates and chalcogenide glasses and, polymer electrolytes in particular, the σ–T dependences deviate from the typical Arrhenius behavior (i.e. a linear correlation between the logarithm of conductivity and the inverse of temperature). In these cases, the Vogel–Tamman–Fulcher (VTF) and Williams–Landel–Ferry (WLF) equations are adopted as given in Eqs. (5) and (6) respectively (Ikeda and Aniya 2010; Williams et al. 1955). In both expressions, A, B, C1, and C2 are constants, T0 is the ideal glass transition temperature, and Ts is a reference temperature. Effectively, the WLF equation can be converted into the VTF equation when C1 = B/(T − Ts) and C2 = Ts − T0.
It was Armand (1986) who suggested the use of VTF equation to correlate the σ–T dependence of Li-ion conducting polymer electrolytes. VTF equation is extensively used for studying the ionic transport phenomena in SEs for rechargeable LIBs.
In general, the Arrhenius equation gives a microscopic illustration of ionic transport whereby it is achieved by the hopping of ionic species from existing occupied sites to vacant sites. For instance, in the crystalline LiAsF6/poly(ethylene oxide) (PEO) electrolyte, the ion motion is mainly contributed by the hopping of Li+ cations in the PEO semi-helical skeleton. That is, ion transport is facilitated by intra- or interchain hopping via the Li–O bonds breaking/forming (Armand 1986). By contrast, the VTF equation depicts the segmental motion of a polymer chain, instead of the diffusivity or mobility of the ion species. According to the free-volume theory developed by Cohen and Turnbull (1959), molecular transport occurs only at the moment when voids have a volume larger than a certain critical volume. The expansivity of the materials with the increase of temperature yields free volume, which allows the ionic carriers, solvated molecules, or polymer chains to move. Ratner and Shriver (1988) developed a dynamic percolation model for explaining ion transport in polymer electrolytes (PEs). This microscopic model features the ionic motion via hopping between neighboring positions, where the Li+ cations are transferred by a complete exchange of ligand surrounded in the local coordination environment as in Fig. 5.
Diffusion pathway of Li+ in PEO according to dynamic percolation model by Ratner et al. Reprinted with permission from M. A. Ratner and D. Shriver: Ion Transport in Solvent-Free Polymers. Chem. Rev. 88(1). (Reprinted with permission from (Ratner and Shriver 1988), copyright (1988)
The mechanism of ion transport in these electrolytes is rather complicated, due to the co-existence of multiple phases and structures, thus different models are required for simulating the diffusion of ionic species and accompanying mechanism. However, for PEs, there is a general agreement that lowering the glass transition temperature Tg crucial for increasing the mobility of polymer segments, thus leading to an improved ionic conductivity.
3.3 Polyethylene Oxide (PEO) Based and Non PEO Based Electrolytes
Polyethylene oxide (PEO) is a poly ether compound with chemical structure H–(O–CH2–CH2)n–OH. It is also known as Polyethylene glycol (PEG) based on the molecular weight of PEO. PEO has a molecular weight of 20,000 g mol−1 whereas PEG is an oligomer of ethylene oxide with MW < 20,000 g mol−1. PEO based materials are considered potential candidates of polymer hosts in SPEs for realizing high energy density secondary Li-Ion batteries. They have several advantages like ease of processability high safety, good electrochemical stability, cost effectiveness and exceptional compatibility with Li salts (Jung et al. 2017). The repeating units of PEO, ethylene oxide (–CH2CH2O–), are appropriately spaced ether solvating units (low entropy change) that have excellent solvation abilities (DN = 22), thus permitting the formation of the favored lithium salt/PEO complex and providing a sufficient concentration of charge carriers. Moreover, it presents optimal conditions for Li+ dissociation, and high chain flexibility However, they suffer from insufficient ionic conductivity due to the semi-crystalline nature of the ethylene Oxide (EO) chains. The stiff structure restrains the ionic transition especially at low temperature. Nevertheless they are still one of the best choices of polymer host material and research is always on to develop approaches to reduce the crystallinity of the structure and hence improve the ionic conductivity. This includes polymer blending, modifying and making PEO derivatives etc. (Judez et al. 2017; Devaux et al. 2015; Khurana et al. 2014).
It was the study by Wright (1975) regarding the ionic conductivity of PEO complexes with alkali metal salts and later Armand (1986) suggestion that PEO could be used in electrochemical devices that paved the way for the development of SPE to be used in Li batteries.
The ionic conductivity of the PEO is contributed by the ionic transport happening in the amorphous region of PEO, thereby the ionic motion ability in the PEO segment need to be increased (Fig. 6a). The Li-Ions are understood to move in the PEO chain via both interchain and intrachain hopping as illustrated in Fig. 6b. The ionic conductivity reached practically useful values (~10−4 S/cm) at temperatures of 60–80 °C (Armand et al. 1979). Most commonly used and traditional approach adopted to improve the problem of ionic conductivity is the use of plasticizer to reduce the crystallinity and increase the amorphous phase content of Li salt in polymer electrolytes. Such a use of plasticizer, most commonly organic aprotic solvents deviate them from SPEs and make them GPEs which are not regarded as pristine SPEs.
Adopting a chemical modification of the polymer matrix as is one of the feasible approach. Poly(styrene-b-ethylene oxide) block copolymers have been widely studied and comprising PS segment confers mechanical stiffness and a PEO block provides solvation and transport properties. As the PS block content increases, both Tm and crystallinity (χc) decrease regardless of the length of the PEO chain, while the Young’s modulus and tensile strength increase. The decreased melting transition compared to PEO could allow the operating of polymer cells at relatively low temperatures (25 °C) (Ping et al. 2017). Chintapalli et al. (2016) studied the conductivity of a PS-b-PEO/LiTFSI system with high salt content and found the maximum ionic conductivity of the copolymer to be about twice that of the corresponding PEO based one. Another strategy for overcoming the above-mentioned challenges involves cross-linking of PEO with other polymers. For example, cross-linked PE and PEO enhance the ionic conductivity of SPEs to as high as 2.3 × 10−4 S cm−1 at 25 °C, a value that was proportionally increased with the addition of low molecular weight PEGDME as plasticizer (Khurana et al. 2014).
Addition of inorganic fillers to reduce the crystallinity is a possible strategy to improve the conductivity of PEO based electrolytes. It is remarkable that the overall performance of an SPE does not rely only on the chosen polymer host, but also on the selection of Li salt. Generally, anions with highly delocalized negative charge are selected as counterparts of lithium salts, which could improve the amorphicity and ionic conductivity of SPEs. Among all the investigated candidates, LiN(CF3SO2)2 known as lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) has been employed as a dominative salt in SPEs, due to its low lattice energy (Tm = 233 °C) and highly delocalized negative charge of the anion, which promotes the dissociation and dissolution of lithium salt in the polymer host, thus enhancing the ionic conductivity (Fig. 7) (Judez et al. 2018; Judez et al. 2017; Zhang et al. 2014).
Chemical structure of a PE-PEO cross-linked SPE with LiTFSI as salt. https://pubs.acs.org/doi/10.1021/ja502133j. (Reprinted with permission from (Judez et al. 2018), copyright (2014)
In spite of the great advances in the performance of PEO-based batteries, the poor compatibility between PEO-based electrolytes and high-voltage electrodes (>4 V versus Li/Li+) due to the substandard anodic stability of PEO paves way for alternative polymer matrices. Among the variety of candidates to replace PEO, polycarbonates (PCs), are identified as feasible materials (Mindemark et al. 2018). Generally, polycarbonate-based polymer electrolytes show higher transference number TLi+ due to the weaker carbonyl group oxygen–Li+ interaction and/or the absence of chelating effects. Unlike EO-based PEs, PC-based PEs show poor conductivities at a low-salt concentration, although a high salt concentration results in a strong plasticizing effect and therefore fast Li-ion transport and hence improved conductivity. Tominaga and Yamazaki (2014) proved that high conductivity and lithium transference number TLi+ (0.54) values were obtained for poly(ethylene carbonate) (PEC)/LiFSI electrolytes, which were further improved upon the addition of 1 wt% TiO2 nanoparticles. Within this family of polymers, acrylate derivatives with the ester moiety have been widely studied. Poly(methyl methacrylate) (PMMA)-based SPEs, showing enhanced interface stability, is restricted by their poor mechanical flexibility (Tg ≈ 100 °C). The use of a more flexible ether-modified polysiloxane partner in a blend with LiTFSI could render a more conductive (3.1 × 10−6 at 20 °C) and flexible (Tg of PMMA, 62 °C) polymer electrolytes (Cznotka et al. 2015).
Poly(acrylonitrile) (PAN) and poly(vinylidene fluoride) (PVdF) based host matrices, guarantee good mechanical properties and in general lead to higher stabilities towards electrochemical oxidation (Sharma and Thakur 2013; Tamilselvi and Hema 2016). However, conductivity problems may arise from their semi-crystalline structure. Thus, they are mainly applied to enhance the mechanical properties of other materials as well as in combination with fillers. Research on composite/hybrid electrolyte which consists of inert (inactive) or Li-conducting inorganic ceramic filler (active), embedded in an organic polymer matrix is steadily gaining popularity in SPEs. Such systems have advantage of either providing high ionic conductivity at low temperatures from ceramic phase when active, or of interfacial interactions between the nano-filler and the polymer, giving rise to lower crystallinity fraction, lower Tg and higher TLi+ even when inactive. They have synergistic interfacial properties and improved mechanical stability derived from the polymeric structure (Sharma and Thakur 2010).
In conclusion, while the primary importance of the polymer host is indisputable, it has been observed that those can be tuned by physical and chemical processes leading to better properties of the SPEs (ionic conductivity, thermal properties and stability towards electrochemical oxidation). Furthermore, the structural nature of the lithium salt assumes a pivotal role in elevating the electrochemical performance of cells.
4 Gel Polymer Electrolytes (GPEs)
It is well realized from previous sections that the highly flammable nature of liquid electrolytes result in fires or explosions in cases of unusual heat generation such as short circuits or local overheating. Polymer electrolytes are understood to be safe and more reliable electrolyte systems. However, poor ionic conductivity at room temperature is one of the limiting factors in most of the SPEs which regulates their practical application in LIBs at ambient temperature. For this reason, most of the recent research has been engrossed on gel polymer electrolytes (GPEs) that exhibit higher ionic conductivities than SPEs. GPE is defined as an electrolyte that consists of a lithium salt dissolved in polymer along with suitable amount of organic solvent in it. The ionic conductivities of GPEs can be improved to almost the same level of liquid electrolyte by incorporating a lithium salt and a large amount of organic solvent into a matrix polymer that can form a stable and free-standing film. Inorganic filler is often used as the plasticizer to enhance the amorphous phase in the polymer. The electrochemical and mechanical properties of GPEs are dependent on the type and composition of the constituents (i.e. lithium salt, organic solvent, polymer and inorganic filler).
4.1 Constituents of GPEs
Any GPE consist of the Lithium salt dissolved in the organic solvent that is incorporated into the polymer matrix by various techniques like solution casting, hot melting, and immersion of porous membrane of the polymer matrix into the liquid electrolyte etc. (Wang et al. 2014; Kong et al. 2007; Kim et al. 2008). Most commonly used Li salts in liquid electrolytes such as LiPF6, LiClO4 and LiBF4 are employed out of which LiPF6 offers overall good physicochemical properties in terms of good Li dissociation and ionic conductivity though superficial thermal stability. A major shortcoming of LiPF6 is low thermal stability and high moisture sensitivity as compared with other lithium salts. It decomposes at high temperature and produces highly acidic PF5 gas that reacts with water to generate HF. Therefore, the GPEs prepared with LiPF6 exhibit high ionic conductivity but low thermal stability. LiClO4 has safety concerns due to the fact that it is highly reactive with most organic species at high temperature and high charging current (Hossain 1995). Nonetheless, it is still being used in preparing GPEs. On the other hand, LiCF3SO3 is thermally stable, non-toxic and insensitive to moisture as compared with LiPF6. Its critical limiting factor is low ion conductivity as compared with other lithium salts, which is due to its low dissociation in organic solvents. Li(CF3SO2)2N has high ionic conductivity, thermal stability and is a safe salt. The high ion conductivity can be credited to its high degree of dissociation. The high dispersion of anionic charge marks it more easily dissociated into cations and anions. It melts at around 236 °C without thermal decomposition. However this salt is known to corrode Al current collector which restricts its application (Schmidt et al. 2001).
The organic solvents must meet the usual criteria as in the case of liquid electrolyte like high dielectric constant, low viscosity, electrochemically stability in the potential range of 0–4.5 V versus Li/Li+, low melting temperature, high boiling point, low volatility to remain safe. A synergistic effect in ionic conductivity is attained when cyclic carbonates and linear carbonates are mixed, as the advantages of each individual solvent are imparted to the resultant mixture. Among various cyclic carbonate-based solvents for preparing the GPEs, EC is the basic and indispensable component, as it can form an effective protective film on a graphitic anode which inhibits any further electrolyte decomposition on the surface of the anode. Such protective film formation cannot be realized with PC as a result of which exfoliation of graphite occurs due to the co-intercalation of PC into the graphite. Organic solvents usually undergo decomposition due to their tendency to reduce at low potential. This can be overcome by adding a small amount of additives such as vinylene carbonate (VC) and fluoroethylene carbonate (FEC). They also help to form an even and ionic-conductive solid electrolyte interphase (SEI) layer at the surface of a carbon anode.
The role of the polymer is to sustain a solid-state film that supports ion migration in organic solvents. Numerous types of polymers, such as PEO, polyacrylonitrile (PAN), poly(vinylidene fluoride) (PVdF), poly(vinylidene fluoride-co-hexafluoropropylene) (PVdF–HFP), poly(methyl methacrylate) (PMMA) and poly(vinyl chloride) (PVC), are mainly used in developing GPES (Fig. 8) (Weston and Steele 1982; Watanabe et al. 1982; Capiglia et al. 2001; Sukeshini et al. 1996).
The ionic conductivities of the most widely used PEO-based polymer electrolytes is found to improve upon addition of plasticizers. In addition to reducing the crystallinity and increasing the segmental motion of the polymer chain, the organic plasticizers can also enhance ion dissociation. Low-molecular-weight polyethers, organic solvents and ionic liquids are generally used as plasticizers for the purposes. Adding poly(ethylene glycol) (PEG) as a low-molecular-weight polyether to PEO–LiCF3SO3 complexes increased the ionic conductivity, however, the existence of hydroxyl end groups negatively affected the interfacial properties at the electrode and electrolyte interface. An increase of ionic conductivity with the addition of PEG can be attributed to the decrease in the crystallinity and the increase of free volume in PEO (Ito et al. 1987). A GPE containing PEO and a conventional LiPF6-based liquid electrolyte was prepared by in situ polymerization using UV irradiation. The gel polymer electrolyte revealed a high ionic conductivity value of 3.3 × 10−3 S cm−1 and lithium transference number of 0.76 at room temperature. A Li/LiFePO4 cell using this GPE exhibited good cycling stability with excellent capacity retention of 81% after 500 cycles.
The presence of polar side chain in PAN attracts lithium ions and organic solvents, making it suitable as a matrix polymer for preparing GPEs. Abraham et al. prepared a GPE comprising of EC, PC, LiClO4 immobilized in PAN which exhibited a high ionic conductivities of 1.1 × 10−3 and 1.7 × 10−3 S cm−1 at −10 °C and 20 °C, respectively (Li et al. 2017). As the PAN matrix is devoid of oxygen atoms, a lithium transference number higher than 0.5 was also reported GPEs based on PAN are prepared by soaking the porous PAN membrane in organic electrolytes such as LiPF6–EC/DMC, LiBF4–EC/DMC and LiClO4–EC/DMC. High ionic conductivities of the order of 2.0 × 10−3 S cm−1 and sufficient electrochemical stability over 5.0 V could be achieved which paves its application in high-voltage lithium-ion polymer batteries. The preparation of PAN-based GPEs usually involves the heating of PAN dissolved in EC or PC at high temperatures. When PAN and lithium salt are fully dissolved in organic solvents, the solution is cast and then subjected cooling at room temperature. Regardless of several advantages of PAN-based GPEs, comprising high ionic conductivity, electrochemical stability high lithium transference number, the poor compatibility with lithium metal has inhibited their practical applications (Abraham and Alamgir 1990).
PVdF and its copolymer are potential host polymers for preparing GPEs due to their unique properties like high dielectric constant which allows a high degree of salt dissociation and thus a high concentration of charge carriers, high oxidative stability due to the strong electron-withdrawing –CF2– group. It was understood from a study by Tsuchida et al. that the ionic conductivities of the GPEs consisting of PVdF, LiClO4 and plasticizers like EC, PC, DMF, γ-BL, PEG, PPG, was strongly influenced by the ionic mobility within the material. Hence, the viscosity of the plasticizer was the governing parameter rather than its dielectric constant for achieving high ionic conductivity (Min et al. 2003). PVdF–HFP copolymer is rich in amorphous domains which could entrap large amounts of liquid electrolyte, and crystalline regions capable of offering enough mechanical support for the processing of free-standing films, eliminating the need for cross-linking. The porous PVdF–HFP membrane is initially prepared under atmospheric conditions and GPE is obtained by injecting liquid electrolyte into the porous membrane. While PVdF homopolymer does not swell well, the presence of HFP quite increases the electrolyte uptake. A solution casting of PVdF–HFP containing 12% of HFP in carbonate solutions resulted in films capable of swelling up to 60% by volume, which maintained good mechanical properties and high ionic conductivity (1 × 10−3 S cm−1) (Capiglia et al. 2001). A porous and chemically cross-linked GPE based on PVdF–HFP copolymer as a matrix was reported by Cheng et al. (2004). In this study, PEG was used as a plasticizer and polyethylene glycol methacrylate (PEGDMA) as a chemical cross-linking oligomer. GPEs based on PVdF–HFP/PEG/PEGDMA with a composition in the ratio 5:3:2 demonstrated a high ambient ionic conductivity of 1 × 10−3 S cm−1 and a high tensile modulus value of 52 MPa due to the presence of porous network structures. They are found to be electrochemically stable up to 5.0 V versus Li/Li+ in the presence of 1 M LiPF6–EC/DEC (Cheng et al. 2004). PMMA based GPE was first reported by Iijima et al. who found that PMMA could be used as a gelation agent and an ionic conductivity of the order of 10−3 S cm−1 could be achieved at 15 wt% PMMA. A main disadvantage of PMMA-based GPEs is their poor mechanical strength when plasticized with large amounts of organic solvents, which limits their application as electrolytes in rechargeable lithium batteries (Rajendran and Uma 2000). Some studies proved that copolymerization of PMMA with other polymers gave rise to GPEs with better mechanical and electrical properties. GPEs based on PMMA copolymerized with polyacrylonitrile, polystyrene and polyethylene to form a terpolymer showed enhanced mechanical strength to develop free-standing films. The ionic conductivity reached 1.4 × 10−3 S cm−1 in the GPE containing 27 wt% terpolymer, 64 wt% LiClO4–EC/PC and 9 wt% fumed silica at room temperature to give homogeneous films that exhibited good mechanical properties (Kim and Sun 1998). Since an amorphous polymer structure is always desirable for achieving high ionic conductivity, amorphous PVC finds use as a host polymer for GPEs. PVC complexed with LiTFSI and plasticized using organic solvents such as dibutyl phthalate (DBP) and dioctyl adipate (DOA) is used as GPE. The plasticizing effect was understood in terms of reduction in the mechanical modulus by about one to two orders of magnitude compared to pure PVC. Ionic conductivities varied from 10−7 to 10−4 S cm−1 at 25 °C when the weight ratio of PVC was changed from 0.67 to 0.17. The lithium transference number varied from 0.54 to 0.98 depending on the composition and temperature (Sukeshini et al. 1996). The GPE developed with 15 wt% PVC, 40 wt% EC, 40 wt% PC and 5 wt% LiClO4 exhibited an ionic conductivity of 1.2 × 10−3 S cm−1 at 20 °C. PVC-based GPEs is known to display high mechanical strength even if the content of plasticizing solvents is very high (Alamgir and Abraham 1993). PVC could also be used to enhance the mechanical strength of PMMA-based GPEs. The GPEs based on PVC/PMMA blend offered a phase-separated structure consisting of a PVC-rich phase and an electrolyte-rich phase. At the blend ratio of PVC/PMMA = 3/2, it was observed that the mechanical properties and ionic conductivity increased. At this ratio, the polarity of the liquid electrolyte was elevated and the domain size of the electrolyte-rich phase reduced. The lithium-ion cell assembled with carbon anode, blended GPE and LiCoO2 cathode gave a discharge capacity of 111 mAh g−1 at 2C-rate with good capacity retention (Kim et al. 2000).
Another major component of interest in GPEs is the inorganic fillers that are integrated into the polymer matrix to improve the electrical and mechanical properties. GPEs usually exhibit high ionic conductivities exceeding 10−3 S cm−1 at room temperature upon the addition of large amount of liquid electrolyte into a matrix polymer. However, incorporating larger amounts of liquid electrolyte can be detrimental to mechanical properties of GPEs and cause poor compatibility with the lithium electrode. This can be overcome using inorganic fillers such as SiO2, Al2O3, γ-LiAlO2, TiO2, ZrO2, CeO2 and BaTiO3 (Borghini et al. 1995; Liu et al. 2003; Woo et al. 2018). The particle size of the inorganic filler is an important parameter that affects the ion transport and ionic conductivity. Park et al. demonstrated that incorporating Al2O3 as a filler augmented the electrochemical stability of the PEO-based polymer electrolyte. The hydrogen bonding between surface group of Al2O3 and the perchlorate anion in the Li salt resulted in the stability enhancement. A composite polymer electrolyte made with two fillers Al2O3 and BaTiO3 showed better ionic conductivity which can be attributed to both the reduction of crystallinity and the increase in charge carrier concentration. Ferroelectric inorganic fillers such as BaTiO3 possess permanent dipole and they are capable of reducing the interfacial resistance between the electrode and electrolyte. A composite GPE was prepared by an in situ cross-linking reaction between reactive SiO2 particles dispersed in the PAN membrane and electrolyte precursor containing tri(ethylene glycol)diacrylate. The cross-linked composite polymer electrolyte encapsulated electrolyte solution efficiently without leakage and offered good thermal stability in addition to favorable interfacial characteristics toward electrodes. Inorganic clay with high strength and stiffness also finds application as inert filler for reinforcing the mechanical strength of GPEs (Shin et al. 2015).
4.2 Ionic Conduction in GPES
GPEs inherited majority of their properties and merits like ionic conduction and electrochemical stability on carbonaceous anode and metal oxide cathode from bulk liquid electrolytes; nonetheless features like safety, tolerance against electrical and mechanical instabilities and dimensional stability are dealt by the polymer matrix encapsulating them. The ionic mobility and hence the ion conduction is determined mainly upon the motion of ions in the liquid electrolyte which is embedded in the solid polymer matrix. Most of the GPEs are understood to consist of a two-phase separated gel consisting of swollen polymer chains and the Li salt solution in organic solvents retained in the cavities of porous polymer. Both these phases contribute to carrier migration. Gelation happens in a stepwise manner in which the pores gets filled with electrolyte solution initially followed by their penetration into polymer to swell the polymer chains. The porosity and pore size of the polymer thus determine the swelling condition of the gel. The interaction between the carriers and polymer chains in the swollen polymer affects the carrier concentration and mobility and hence the ionic conductivity. As far as the polymer substrate is concerned, its porosity, pore size, amorphousness, chain structure and degree of polymerization matters while from the aspect of electrolyte solution, the degree of dissociation of Li salt, viscosity, concentration and dielectric constant would attribute to the carrier migration characteristics. A porous polymer membrane allows smooth movement of electrolyte solution trapped in the cavities into the polymer from resulting in homogenous network chains appropriate for ion transport (Figs. 9 and 10) (Saito et al. 2003).
An illustration of composite GPE based on PMMA/polystyrene with SiO2 as inorganic fillers. Liquid electrolyte is 1 M LiTFSI in TEGDME. (Reprinted with permission from (Woo et al. 2018), copyright (2017)
SEM photograph of GPE composed of PVdF–HFP polymer. The porous network enables smooth movement of electrolytes trapped in the cavities into the polymer and hence improves ionic conductivity. (Reprinted with permission from (Saito et al. 2003), copyright (2003)
4.3 Characteristics of GPEs
The essential parameters that determine the efficacy of GPEs include their ionic conductivity, electrochemical stability, Lithium transference number and the mechanical strength to find practical large scale application in Li batteries. Ionic conductivity of GPEs is the most important characteristic that affects battery performance. The ionic conductivity of a GPE for lithium-ion battery application should be higher than 10−3 S cm−1 at room temperature. The migration of lithium ions between two electrodes and diffusion of lithium ions in the electrodes are crucial for the fast charge–discharge of LIBs at high current rates. Unless a rapid and facile transport of Li-Ions occur between the electrodes, a high capacity cannot be achieved. Ionic conductivity of the GPE can be measured by electrochemical impedance spectroscopy (EIS) in a certain frequency range using an AC impedance analyser. For conductivity measurement, GPE film is sandwiched between two blocking electrodes such as stainless-steel electrodes and the bulk resistance (Rb) is obtained by the intercept on the real axis of AC impedance spectrum (Nyquist plot) of the cell (Oh et al. 2006). The ionic conductivity can be calculated by equation:
where \(\sigma\) is the ionic conductivity, \(t\) is the thickness of the gel polymer electrolyte, film; \(A\) is the area of gel polymer electrolyte in contact with blocking electrodes.
The operating voltage of the LIB is decided by the electrochemical stability window of the electrolyte. Linear sweep voltammetry (LSV) is employed to evaluate the electrochemical stability window of the GPE, which is the range of potential that does not involve in oxidative and reductive decomposition reactions. A potentiostat scans the potential of a working electrode at a constant rate with respect to the reference electrode. When the current increases rapidly, it gives a decomposition voltage of the electrolyte. A platinum or stainless-steel electrode is often used as a working electrode, and lithium metal is used as reference and counter electrodes. GPEs should be electrochemically stable up to at least 4.5 V versus Li/Li+, because the lithium-ion batteries with lithium metal oxide cathodes such as LiCoO2, LiNiO2 and LiNixCoyMn1−x−yO2 reach a voltage of 4.3 V when fully charged. Since the GPE is sandwiched between anode and cathode in the cell, no chemical reactions should occur when the GPE come into direct contact with electrodes. So like the conventional liquid electrolytes and SPEs, the GPEs should also continue to be inert to the charged surfaces of the electrodes during cell operation.
Lithium ions are the charge carriers in LIBS and henceforth they should have a higher mobility than the anions. The fraction of the current carried by Li+ ions can be determined from the lithium transference number (tLi+) given by the equation.
The conductivity ratio is also expressed in terms of ionic mobility since lithium salt dissociates to form the same number of cations and anions. When the lithium transference number is low, the overall resistance of the cell increases due to the concentration polarization of the anions in the electrolyte, resulting in reduced capacity and poor power density. The lithium transference number in the GPE usually ranges between 0.2 and 0.5. Various methods are employed to measure transference number such as AC impedance, DC polarization measurement, Tubandt’s method, Hittorf’s method and the pulsed field gradient NMR (PFG NMR) technique. The most commonly used method for determining the lithium transference number is a combination of AC impedance and DC polarization measurements of the symmetric Li/gel polymer electrolyte/Li cell using the following equation:
where \(V\) is the applied voltage to the cell, \(I_0\) and \(I_s\) are the initial and steady state current, respectively, and \(R_0\) and \(R_s\) are the interfacial resistances before and after the polarization, respectively. The cell is polarized by applying a DC voltage and the time evolution of the current flow is monitored. The initial and steady-state currents flowing in the cell are monitored. AC impedance measurements of the cell are done before and after DC polarization and the initial and steady-state interfacial resistances are measured. The lithium transference number is dependent on various factors including temperature, salt type and concentration, types of polymer, organic solvent and inorganic filler (Jung et al. 2017). The anions can be effectively covalent bonded to polymer backbone making it immobile and thereby improving the Li transference number. GPE not only function as the electrolyte but also as a separator between two electrodes in the cell. The mechanical strength of GPE plays a vital role in the manufacture of batteries. To facilitate easy fabrication of battery, with reproducibility along with mechanical reliability during assembling, storage and usage, the mechanical stability of GPE is the most important factor to be considered when battery technology scales from laboratory to mass production. Most of the GPEs reported so far have exhibited poor mechanical properties, which has restricted their application without a supportive membrane in LIBs. Increasing the amount of organic solvent improves ionic conductivity but leads to a decrease in mechanical strength. While considering this connection, ionic conductivity and mechanical properties should be optimized within a suitable range. The mechanical strength of the GPEs can be augmented by addition of inorganic fillers and in situ chemical cross-linking (Evans et al. 1987). The thickness of GPE is also an important factor that affects battery performance such as rate capability and energy density. By enhancing the mechanical strength of GPEs, the thickness can be reduced and battery could be made more compact. In general, the film thickness should be less than 30 μm taking it into account, the thickness of conventional polyolefin separators used in LIBs. The mechanical strength of a GPE can be evaluated from the strain–stress curve by tensile test. For maintaining good interfacial characteristics and better contacts with electrodes in the cell during charge and discharge cycles, GPE should also retain adequate adhesive properties and flexibility. Thermally stability of GPEs also serves as a major requirement to have a suitable temperature range of operation in Li batteries (Zhang et al. 2011; Park et al. 2017). A comparison of conventional liquid electrolytes, SPEs and GPEs is illustrated in Table 2.
5 Summary
The importance and role of electrolytes used in LIBs and general properties required for any electrolyte in LIBs are explained. The various electrolyte salts and solvents that are conventionally used to offer the demand of high voltage electrode–electrolyte interface for practical and large scale application is outlined. The ionic transport mechanism in SPEs is rather complicated and follows a different route as compared with conventional liquid electrolytes which restricts its ionic conductivity. But taking in to account the merits of safety, ability to develop compact and flexible battery designs, relevance of SPEs to meet the future application in electric vehicles is realized with focus on PEO based and non-PEO based electrolytes. GPEs on the other hand could overcome many of the demerits of SPEs mainly the ionic conductivity since they have organic liquid electrolytes incorporated into the polymer skeleton encapsulating them. The use of inorganic fillers also helped to improve the amorphousness of the polymer to facilitate proper ion migration and transport. Ionic conductivity comparable to liquid electrolytes of the order of 10−3 S cm−1 could be achieved in GPEs which pave way for its usage in large scale applications. The mechanical and thermal stability of these systems offer possibilities for flexible compact battery design. With advantages of safety, cost-effectiveness and flexible battery design, SPEs is expected to dominate the electrolyte technology and there by its usage in secondary energy storage devices sometime in the near future.
References
Abraham KM, Alamgir M (1990) Li+-conductive solid polymer electrolytes with liquid-like conductivity. J Electrochem Soc 137(5):657
Alamgir M, Abraham KM (1993) Li ion conductive electrolytes based on poly(vinyl chloride). J Electrochem Soc 140(6):L96–L97
Andersson AM, Herstedt M, Bishop AG, Edstrom K (2002) The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes. Electrochim Acta 47(12):1885–1898
Arakawa M, Yamaki J (1995) Anodic oxidation of propylene carbonate and ethylene carbonate on graphite electrodes. J Power Sources 54(2):250–254
Armand M (1986) Polymer electrolytes. Annu Rev Mater Res 16:245–261
Armand MB, Chabagno JM, Duclot MJ (1979) Polyethers as solid electrolytes, in fast ion transport in solids: electrodes and electrolytes. Proc Int Conf 131–136
Arya A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23(3):497–540
Aurbach D (1999) Non aqueous Electrochemistry, 1st edn. Marcel Dek, New York
Bachman JC, Muy S, Grimaud A, Chang H-H, Pour N, Lux SF, Paschos O, Maglia F, Lupart S, Lamp P (2016) Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev 116(1):140–162
Barthel J, Gores HJ, Kraml L (1996) Effects of electronegative substituents of anions on ion-pair formation. Temperature dependence of the conductivity of lithium fluoroacetate and alkali-metal acetate solutions in dimethyl sulfoxide. J Phys Chem 100(4):1283–1287
Besenhard JO, Eichinger G (1976) High energy density lithium cells: part I. Electrolytes and anodes. J Electroanal Chem Interfacial Electrochem 68(1):1–18
Borghini MC, Mastragostino M, Passerini S, Scrosati B (1995) Electrochemical and structural studies of petroleum coke in carbonate‐based electrolytes. J Electrochem Soc 142(7):2118–2122
Bunde A, Dieterich W, Maass P, Meyer M (2005) Ionic transport in disordered materials. In: Heitjans P, Kärger J (eds) Diffusion in condensed matter. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-30970-5_20
Capiglia C, Saito Y, Kataoka H, Kodama T, Quartarone E, Mustarelli P (2001) Structure and transport properties of polymer gel electrolytes based on PVdF–HFP and LiN(C2F5SO2)2. Solid State Ion 131(3):291–299
Chen HP, Fergus JW, Jang BZ (2000) The effect of ethylene carbonate and salt concentration on the conductivity of propylene carbonate|lithium perchlorate electrolytes. J Electrochem Soc 147(2):399–406
Cheng CL, Wan CC, Wang YY (2004) Preparation of porous, chemically cross-linked, PVdF-based gel polymer electrolytes for rechargeable lithium batteries. J Power Sources 134(2):202–210
Chintapalli M, Le TNP, Venkatesan NR, Mackay NG, Rojas AA, Thelen JL, Chen XC, Devaux D, Balsara NP (2016) Structure and ionic conductivity of polystyrene-block-poly(ethylene oxide) electrolytes in the high salt concentration limit. Macromolecules 49(5):1770–1780
Chu AC, Josefowicz JY, Farrington GC (1997) Electrochemistry of highly ordered pyrolytic graphite surface film formation observed by atomic force microscopy. J Electrochem Soc 144(12):4161–4169
Cohen MH, Turnbull D (1959) Molecular transport in liquids and glasses. J Chem Phys 31(5):1164–1169
Cresce A, Xu K (2011) Electrolyte additive in support of 5 V Li ion chemistry. J Electrochem Soc 158(3):A337–A342
Cznotka E, Jeschke S, Vettikuzha P, Wiemhöfer HD (2015) Semi-interpenetrating polymer network of poly(methyl methacrylate) and ether-modified polysiloxane. Solid State Ion 274:55–63
Dahn JR, von Sacken U, Juzkow MW, Al‐Janaby H (1991) Rechargeable LiO2/carbon cells. J Electrochem Soc 138(8):2207–2211
Desjardins CD, Cadger TG, Salter RS, Donaldson G, Caser EJ (1985) Lithium cycling performance in improved lithium hexafluoroarsenate/2‐methyl tetrahydrofuran electrolytes. J Electrochem Soc 132(3):529–533
Devaux D, Glé D, Phan TNT, Gigmes D, Giroud E, Deschamps M, Denoyel R, Bouchet R (2015) Optimization of block copolymer electrolytes for lithium metal batteries. Chem Mater 27(13):4682–4692
Druger SD, Nitzan A, Ratner MA (1983) Dynamic bond percolation theory: a microscopic model for diffusion in dynamically disordered systems. I. Definition and one-dimensional case. J Chem Phys 79(6):3133–3142
Druger SD, Ratner MA, Nitzan A (1983) Polymeric solid electrolytes: dynamic bond percolation and free volume models for diffusion. Solid State Ion 9–10:1115–1120
Dudley JT, Wilkinson DP, Thomas G, LeVae R, Woo S, Blom H, Horvath C, Juzkow MW, Denis B, Juric P, Aghakian P, Dahn JR (1991) Conductivity of electrolytes for rechargeable lithium batteries. J Power Sources 35(1):59–82
Ein‐Eli Y, Thomas SR, Koch VR (1996) New electrolyte system for Li‐ion battery. J Electrochem Soc 143(9):L195–L198
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13):2324–2328
Fan L, Wei S, Li S, Li Q, Lu Y (2018) Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv Energy Mater 8(11):1702657, 1–31
Fry AJ (1989) Synthetic organic electrochemistry, 2nd edn. Wiley, London
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603
Gores HJ, Barthel J, Zugmann S, Moosbauer D (2011) Handbook of battery materials. Wiley-VCH Verlag GmbH &Co., KGaA, Weinheim, Germany
Gutmann V (1976) Solvent effects on the reactivities of organometallic compounds. Coord Chem Rev 18(2):225–255
Guyomard D, Tarascon JM (1993) Rechargeable Li1+xMn2O 4/carbon cells with a new electrolyte composition: potentiostatic studies and application to practical cells. J Electrochem Soc 140(11):3071–3081
Harris WS (1958) Electrochemical studies in cyclic esters. PhD thesis, University of California, Berkeley, CA
Harris CS, Nitzan A, Ratner MA, Shriver DF (1986) Particle motion through a dynamically disordered medium: the effects of bond correlation and application to polymer solid electrolytes. Solid State Ion 18–19:151–155
Henderson WA (2014) Electrolytes for lithium and lithium-ion batteries, 1st edn. Springer, New York
Hossain S (1995) Linden D (ed) Handbook of batteries, 2nd edn, ch 36. McGraw-Hill, New York
Huang CK, Surampudi S, Shen DH, Halpert G (1994) Effect of electrolyte composition on carbon electrode performance. Proc Electrochem Soc 94(4):105–110
Hy S, Felix F, Rick J, Su W-N, Hwang BJ (2014) Direct in situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li[NixLi(1−2x)/3Mn(2−x)/3]O2 (0 ≤ x ≤0.5). J Am Chem Soc 136(3):999−1007
Ikeda M, Aniya M (2010) Bond strength—coordination number fluctuation model of viscosity: an alternative model for the Vogel–Fulcher–Tammann equation and an application to bulk metallic glass forming liquids. Materials 3:5246–5262
Ito Y, Kanehori K, Miyauchi K, Kodu T (1987) Ionic conductivity of electrolytes formed from PEO–LiCF3SO3 complex low molecular weight poly(ethylene glycol). J Mater Sci 22:1845–1849
Jeong SK, Seo HY, Kim DH, Han HK, Kim JG, Lee YB, Iriyama Y, Abe T, Ogumi Z (2008) Suppression of dendritic lithium formation by using concentrated electrolyte solutions. Electrochem Commun 10(4):635–638
Johansson P (2007) Electronic structure calculations on lithium battery electrolyte salts. Phys Chem Chem Phys 9(12):1493–1498
Johansson P, Nilsson H, Jacobsson P, Armand M (2004) Novel Hückel stabilised azole ring-based lithium salts studied by ab initio Gaussian-3 theory. Phys Chem Chem Phys 6:895–899
Judez X, Zhang H, Li C, González-Marcos JA, Zhou Z, Armand M, Rodriguez-Martinez LM (2017) Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte for all solid-state Li–S cell. J Phys Chem Lett 8(9):1956–1960
Judez X, Zhang H, Li C, González-Marcos JA, Zhou Z, Armand M, Rodriguez-Martinez LM (2017) Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte for all solid-state Li–S cell. J Phys Chem Lett 8:1956–1960
Judez X, Piszcz M, Coya E, Li C, Aldalur I, Oteo U, Zhang Y, Zhang W, Rodriguez-Martinez LM, Zhang H (2018) Stable cycling of lithium metal electrode in nanocomposite solid polymer electrolytes with lithium bis(fluorosulfonyl)imide. Solid State Ion 318:95–101
Jung YC, Park MS, Kim DH, Ue M, Eftekhari A, Kim DW (2017) Sci Rep 7:17482
Jung YC, Lee SM, Choi JH, Jang SS, Kim DW (2017) Room-temperature performance of poly(ethylene ether carbonate)-based solid polymer electrolytes for all-solid-state lithium batteries. Sci Rep 7:17482
Kalhoff J, Eshetu GG, Bresser D, Passerini S (2015) Safer electrolytes for lithium-ion batteries: state of the art and perspectives. ChemSusChem 8(13):2154–2175
Khurana R, Schaefer J, Archer LA, Coates GW, Schaefer JL, Coates GW (2014) Suppression of lithium dendrite growth using cross-linked polyethylene/polyethylene oxide electrolytes: a new approach for practical lithium–metal polymer batteries. J Am Chem Soc 136(20):7395–7402
Kim DW, Sun YK (1998) Polymer electrolytes based on acrylonitrile‐methyl methacrylate‐styrene terpolymers for rechargeable lithium‐polymer batteries. J Electrochem Soc 145(6):1958–1964
Kim HT, Kim KB, Kim SW, Park JK (2000) Li-ion polymer battery based on phase-separated gel polymer electrolyte. Electrochim Acta 45(24):4001–4007
Kim W, Cho JJ, Kang Y, Kim DW (2008) Study on cycling performances of lithium-ion polymer cells assembled by in situ chemical cross-linking with star-shaped siloxane acrylate. J Power Sources 178(2):837–841
Kim S, Cho W, Zhang XB, Oshima Y, Choi JW (2016) A stable lithium-rich surface structure for lithium-rich layered cathode materials. Nat Commun 7(13598):1–8
Kim U-H, Lee EJ, Yoon CS, Myung ST, Sun YK (2016) Compositionally graded cathode material with long-term cycling stability for electric vehicles application. Adv Energy Mater 6(22):1601417
Koch VR (1981) Status of the secondary lithium electrode. J Power Sources 6(4):357–370
Koetschau I, Richard MN, Dahn JR, Soupart JB, Rousche JC (1995) The electrochemical society, orthorhombic LiMnO2 as a high capacity cathode for Li‐ion cells. J Electrochem Soc 142(9):2906–2910
Kong L, Zhan H, Li Y, Zhou Y (2007) In situ fabrication of lithium polymer battery basing on a novel electro-polymerization technique. Electrochem Commun 9(10):2557–2563
Koppel IA, Taft RW, Anvia F, Zhu S-Z, Hu LQ, Sung K-S, Desmarteau DD, Yagupolskii LM, Yagupolskii YL, Igat’ev NV, Kondratenko NV, Volkonskii AY, Vlasor VM, Notario R, Maria PC (1994) The gas-phase acidities of very strong neutral Brønsted acids. J Am Chem Soc 116(7):3047–3057
Lee H, Cho JJ, Kim J, Kim HJ (2005) Comparison of voltammetric responses over the cathodic region in LiPF6 and LiBETI with and without HF. J Electrochem Soc 152(6):A1193–A1198
Li W, Pang Y, Liu J, Liu G, Wang Y, Xia Y (2017) A PEO-based gel polymer electrolyte for lithium ion batteries. RSC Adv 7:23494–23501
Liu Y, Lee JY, Hong L (2003) Morphology, crystallinity, and electrochemical properties of in situ formed poly(ethylene oxide)/TiO2 nanocomposite polymer electrolytes. J Appl Polym Sci 89(10):2815–2822
Lloris JM, Pérez Vicente C, Tirado JL (2002) Improvement of the electrochemical performance of LiCoPO4 5 V material using a novel synthesis procedure. Electrochem Solid-State Lett 5(10):A234–A237
Lux SF, Lucas IT, Pollak E, Passerini S, Winter M, Kostecki R (2012) The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem Commun 14(1):47–50
McMillan R, Slegr H, Shu ZX, Wang W (1999) Fluoroethylene carbonate electrolyte and its use in lithium ion batteries with graphite anode. J Power Sources 81–82:20–26
Min HS, Ko JM, Kim DW (2003) Preparation and characterization of porous polyacrylonitrile membranes for lithium-ion polymer batteries. J Power Sources 119–121:469–472
Mindemark J, Lacey MJ, Bowden T, Brandell D (2018) Beyond PEO-alternative host materials for Li+-conducting solid polymer electrolytes. Prog Polym Sci 81:114–143
Mizushima K, Jones PC, Wiseman PJ, Goodenough JB (1980) LixCoO2(0 < x < −1): a new cathode material for batteries of high energy density. Mater Res Bull 15(6):783–789
Oh JS, Kang Y, Kim DW (2006) Lithium polymer batteries using the highly porous membrane filled with solvent-free polymer electrolyte. Electrochim Acta 52(4):1567–1570
Olah GA, Prakash GKS, Molnár Á, Sommer J (2009) Superacid chemistry, 1st edn. Wiley, Hoboken, NJ
Park JS, Meng X, Elam JW, Hao S, Wolverton C, Kim C, Cabana J (2014) Ultrathin lithium-ion conducting coatings for increased interfacial stability in high voltage lithium-ion batteries. Chem Mater 26(10):3128–3134
Park SR, Jung YC, Shin WK, Ahn KH, Lee CH, Kim DW (2017) Cross-linked fibrous composite separator for high performance lithium-ion batteries with enhanced safety. J Membr Sci 527:129–136
Petrowsky M, Frech R (2009) Temperature dependence of ion transport: the compensated Arrhenius equation. J Phys Chem B 113(17):5996–6000
Ping J, Pan H, Hou PP, Zhang MY, Wang X, Wang C, Chen J, Wu D, Shen Z, Fan XH (2017) Solid polymer electrolytes with excellent high-temperature properties based on brush block copolymers having rigid side chains. ACS Appl Mater Interfaces 9(7):6130–6137
Pistoia G (1971) Nonaqueous batteries with LiClO4–ethylene carbonate as electrolyte. J Electrochem Soc 118(1):153–158
Rajendran S, Uma T (2000) Characterization of plasticized PMMA–LiBF4 based solid polymer electrolytes. Bull Mater Sci 23:27–29
Ratner MA, Shriver DF (1988) Ion transport in solvent-free polymers. Chem Rev 88(1):109–124
Rougier A, Gravereau P, Delmas C (1996) Optimization of the composition of the Li1−zNi1+zO2 electrode materials: structural, magnetic, and electrochemical studies. J Electrochem Soc 143(4):1168–1175
Rozier P, Tarascon JM (2015) Review-Li-rich layered oxide cathodes for next-generation Li-ion batteries: chances and challenges. J Electrochem Soc 162(14):A2490–A2499
Ryou MH, Kim J, Lee I, Kim S, Jeong YK, Hong S, Ryu JH, Kim TS, Park JK, Lee H, Choi JW (2013) Mussel-inspired adhesive binders for high-performance silicon nanoparticle anodes in lithium-ion batteries. Adv Mater 25(11):1571–1576
Ryou MH, Lee JN, Lee DJ, Kim WK, Jeong YK, Choi JW, Park JK, Lee YM (2012) Effects of lithium salts on thermal stabilities of lithium alkyl carbonates in SEI layer. Electrochim Acta 83:259–263
Saito Y, Stephan AM, Kataoaka H (2003) Ionic conduction mechanism of lithium gel polymer electrolytes investigated by conductivity and diffusion coefficient. Solid State Ion 160:149–153
Scheers J, Johanssona P, Szczecinski P, Wieczorek W, Armand M, Jacobsson P (2010) Benzimidazole and imidazole lithium salts for battery electrolytes. J Power Sources 195(18):6081–6087
Schmidt M, Heider U, Kuehner A, Oesten R, Jungnitz M, Ignatev N, Sartori P (2001) Lithium fluoroalkylphosphates: a new class of conducting salts for electrolytes for high energy lithium-ion batteries. J Power Sources 97/98:557–560
Sharma AL, Thakur AK (2010) Improvement in voltage, thermal, mechanical stability and ion transport properties in polymer–clay nanocomposites. J Appl Polym Sci 118(5):2743–2753
Sharma AL, Thakur AK (2013) Plastic separators with improved properties for portable power device applications. Ionics 19(5):795–809
Shin WK, Yoo JH, Choi W, Chung KY, Jang SS, Kim DW (2015) Cycling performance of lithium-ion polymer cells assembled with a cross-linked composite polymer electrolyte using a fibrous polyacrylonitrile membrane and vinyl-functionalized SiO2 nanoparticles. J Mater Chem A 3:12163–12170
Song JY, Wang YY, Wan CC (1999) Review of gel-type polymer electrolytes for lithium-ion batteries. J Power Sources 77(2):183–197
Song BH, Li WD, Oh SM, Manthiram A (2017) Long-life nickel-rich layered oxide cathodes with a uniform Li2ZrO3 surface coating for lithium-ion batteries. ACS Appl Mater Interfaces 9(11):9718–9725
Stoeva Z, Martin-Litas I, Staunton E, Andreev YG, Bruce PG (2003) Ionic conductivity in the crystalline polymer electrolytes PEO6:LiXF6, X = P, As, Sb. J Am Chem Soc 125(15):4619–4626
Sukeshini AM, Nishimoto A, Watanabe M (1996) Transport and electrochemical characterization of plasticized poly(vinyl chloride) solid electrolytes. Solid State Ion 86–88:385–393
Sun XG, Wan S, Guang HY, Fang YX, Reeves KS, Chi M, Dai S (2017) New promising lithium malonatoborate salts for high voltage lithium ion batteries. J Mater Chem A 5:1233–1241
Suo L, Hu YS, Li H, Armand M, Chen L (2013) A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat Commun 4(1481):1–9
Surampudi S, Shen DH, Huang CK, Narayanan SR, Attia A, Halpert G, Peled E (1993) Effect of cycling on the lithium/electrolyte interface in organic electrolytes. J Power Sources 43(1–3):21–26
Takami N, Ohsaki T, Hasebe H, Yamamoto M (2002) Laminated thin Li-ion batteries using a liquid electrolyte. J Electrochem Soc 149(1):A9–A12
Tamilselvi P, Hema M (2016) Structural, thermal, vibrational, and electrochemical behavior of lithium ion conducting solid polymer electrolyte based on poly(vinyl alcohol)/poly(vinylidene fluoride) blend. Polym Sci Ser A 58:776–784
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Tarascon JM, Guyomard D (1994) New electrolyte compositions stable over the 0 to 5 V voltage range and compatible with the Li1+xMn2O4/carbon Li-ion cells. Solid State Ion 69(3):293–305
Tominaga Y, Yamazaki K (2014) Fast Li-ion conduction in poly(ethylenecarbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem Commun 50(34):4448–4450
Vatamanu J, Borodin O, Smith GD (2012) Molecular dynamics simulation studies of the structure of a mixed carbonate/LiPF6 electrolyte near graphite surface as a function of electrode potential. J Phys Chem C 116(1):1114–1121
Wang X, Gong C, He D, Xue Z, Chen C, Liao Y, Xie X (2014) Gelled microporous polymer electrolyte with low liquid leakage for lithium-ion batteries. J Membr Sci 454:298–304
Wang JH, Yamada Y, Sodeyama K, Chiang CH, Tateyama Y, Yamada A (2016) Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat Commun 7:12032
Watanabe M, Kanba M, Nagaoka K, Shinohara I (1982) Ionic conductivity of hybrid films based on polyacrylonitrile and their battery application. J Appl Electrochem 27(11):4191–4198
Watanabe Y, Kinoshita SI, Wada S, Hoshino K, Morimoto H, Tobishima SI (2008) Electrochemical properties and lithium ion solvation behavior of sulfone–ester mixed electrolytes for high-voltage rechargeable lithium cells. J Power Sources 179(2):770–779
Weston JE, Steele BCH (1982) Effects of preparation method on properties of lithium salt–poly(ethylene oxide) polymer electrolytes. Solid State Ion 7(1):81–88
Williams ML, Landel RF, Ferry JD (1955) The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J Am Chem Soc 77(14):3701–3707
Woo HS, Moon YB, Seo S, Lee HT, Kim DW (2018) Semi-interpenetrating polymer network composite gel electrolytes employing vinyl-functionalized silica for lithium–oxygen batteries with enhanced cycling stability. ACS Appl Mater Interfaces 10(1):687–695
Wright PV (1975) Electrical conductivity in ionic complexes of poly(ethylene oxide). Br Polym J 7(5):319–327
Xia J, Petibon R, Xiong D, Ma L, Dahn JR (2016) Enabling linear alkyl carbonate electrolytes for high voltage Li-ion cells. J Power Sources 328:124–135
Xing LD, Vatamanu J, Borodin O, Smith GD, Bedrov D (2012) Electrode/electrolyte interface in sulfolane-based electrolytes for Li ion batteries: a molecular dynamics simulation study. J Phys Chem C 116:23871–23881
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4417
Xu L, Kim C, Shukla AK, Dong A, Mattox TM, Milliron DJ, Cabana J (2013) Monodisperse Sn nanocrystals as a platform for the study of mechanical damage during electrochemical reactions with Li. Nano Lett 13(4):1800−1805
Younesi R, Veith GM, Johansson P, Edstrom K, Veggea T (2015) Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S. Energy Environ Sci 8(7):1905–1922
Zaghib K, Tatsumi K, Abe H, Ohsaki T, Sawada Y, Higuchi S (1995) Electrochemical behavior of an advanced graphite whisker anodic electrode for lithium-ion rechargeable batteries. J Power Sources 54(2):435–439
S.S. Zhang., K. Xu., and T.R. Jow.: Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte. J Solid State Electrochem. 7, 147–151(2003).
Zhang P, Yang LC, Li LL, Ding ML, Wu YP, Holze R (2011) Enhanced electrochemical and mechanical properties of P(VDF–HFP)-based composite polymer electrolytes with SiO2 nanowires. J Membr Sci 379(1–2):80–85
Zhang H, Li L, Feng W, Zhou Z, Nie J (2014) Polymeric ionic liquids based on ether functionalized ammoniums and perfluorinated sulfonimides. Polymer 55(16):3339–3348
Zheng H, Qin J, Zhao Y, Abe T, Ogumi Z (2005) Temperature dependence of the electrochemical behavior of LiCoO2 in quaternary ammonium-based ionic liquid electrolyte. Solid State Ion 176(29):2219–2226
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ravi, S., Jayaraj, M.K. (2022). An Overview of Polymer Based Electrolytes for Li-Ion Battery Applications. In: Jayaraj, M.K., Antony, A., Subha, P.P. (eds) Energy Harvesting and Storage. Energy Systems in Electrical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-19-4526-7_8
Download citation
DOI: https://doi.org/10.1007/978-981-19-4526-7_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4525-0
Online ISBN: 978-981-19-4526-7
eBook Packages: EnergyEnergy (R0)