Abstract
Non-degradable pollutants have emerged as a consequence of industrialization, population increment, and changing lifestyles, endangering human well-being and the environment. Biological techniques based on microorganisms are gaining popularity as an environmentally beneficial and cost-effective way to reduce pollution. Microorganisms may thrive in a variety of environments and create metabolites that degrade and change contaminants, allowing contaminated places to be organically revived. For a greater knowledge of biological and life sciences, multiple technologies have begun to be integrated into metagenomics. Technology such as metagenomics is now being used to develop strategies for studying the ecology and variety of microbes, as well as its application in the environment. Metagenomics is a novel and rapidly expanding discipline of environmental biology that provides a strong tool for accessing information on the genomes of environmental microorganisms and entire microbial communities. The application of metagenomics in environmental surveying and bioremediation is becoming more usual. In recent years, a number of functional metagenomics techniques have been used to investigate a wide range of resistant microbial degradation mechanisms. In a metagenomic investigation, it is critical to identify and screen metagenomes from the polluted location. These procedures are well-known for their effectiveness in eliminating many types of contaminants. These strategies may change rapidly as technology develops, but the once that focus on the best ways to improve bioremediation of the contaminated places will be the most successful. Culture-independent molecular approaches, on the other hand, can disclose very relevant information on the metagenome of environmental microorganisms, which play a key role in biogeochemical cycles and the breakdown and detoxification of environmental pollutants. These high-throughput studies would assist in the discovery of novel species for bioremediation, as well as providing new and interesting insights into their primary biodegradative processes at the molecular level. In this chapter, we are attempting to convey an overview that how functional one of the finest bioremediation adaptations that leads to the development of a clean non-toxic environment is metagenomics. We also went through the metagenomics analysis methods with respect to bioremediation. In addition to this, we provide an overview of examples of metagenomics in bioremediation which have recently been reported. Furthermore, our study clarifies the widespread use of metagenomes formed from metagenomics communities, which are capable of comprehending environmental pollutants and poisons.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Pollution is a worldwide issue, since numerous natural and manmade substances have been introduced into places where they are endangering human and ecosystem health (Fig. 1.1). The destruction, conversion, or stabilization of these substances by organisms, usually bacteria and plants, is known as bioremediation. When natural organisms at a contaminated site remove toxins on their own, the site’s toxicity can simply be surveilled as decrement in pollutant or changed into a less hazardous form (Verma and Jaiswal 2016). In many circumstances, though, intervention can speed up the bioremediation process. The most typical methods for aiding clean-up are the insertion of stimulating alterations on site (e.g. micronutrients, organic material) and the transport of contaminates to treatment centres that are not on-site.
The different places depicting soil and water contamination as a result of industries’ dumping of inadequately treated or untreated wastewater (Chandra et al. 2015)
Microorganisms are frequently the most important players in bioremediation (Baker and Herson 1994). High-resolution genomic data is required to figure out how toxins and medicines alter the diverse microbial communities that exist in natural environments (Metzker 2009).
Many of the taxa and enzymes involved in bioremediation are unknown, despite the fact that some taxonomic groupings have been related to the presence of specific contaminants. Since there are many different microbial species that can coexist in single gramme of soil, pollution that is chemically similar to that of natural components in soil (where many species compete for carbon, nutrients and energy) consume as a source for survive. If the contaminant is complicated or fabricated in origin, there may be none indigenous strains effective of metabolizing it or decreasing its toxicity straight away (Boopathy 2000).
Although many bio remediating microorganisms have been isolated from many sources, including contaminated areas, it is now widely recognised that the knowledge gained from these isolates is insufficient to completely understand the functioning of complex microbial communities. More comprehensive genetic data from natural habitats is needed to acknowledge how pollution impacts microbial communities as a whole, and whether bioremediation may be improved further (Lee 2013). Large-scale, culture-independent research, which is essential to attain this aim, is now achievable because of the advent of new high-throughput sequencing methods.
Bioremediation techniques can be divided into three types based on the degree of intervention. Natural attenuation is the least invasive method, in which pollutants are detoxified through natural processes by native species. This method is appealing because it does not require any expensive or perhaps harmful additions. However, natural attenuation rates in many systems may be excessively sluggish and unresponsive to dangers to the environment and health (Kumar et al. 2018). Biostimulation makes use of natural organisms, but it aims to speed up biodegradation by removing some environmental constraints. This is frequently accomplished by supplementing with restricted nutrients. Biostimulation can nevertheless cause sluggish biodegradation in some situations (Jain and Bajpai 2012).
The native microbial community’s incapacity to digest the pollutant of concern could be the cause of the poor biodegradation rates. To address this problem, during bioaugmentation, a foreign organism or enzyme may be introduced to a system to increase biodegradation rates. Because a foreign organism is introduced into an ecosystem, this method is the most invasive. In some cases, however, bioaugmentation has proven to be the most effective method of clean-up. One prevalent worry with bioaugmentation is that foreign species may not be able to thrive in the contaminated environment.
To most effectively drive bioremediation processes, it is necessary to acknowledge the microbial populations entangled in bioremediation, not just the end results and rates of pollutant breakdown. Changes in the characteristics and actions of a microbial community may have an impact on the fate of a pollutant in the environment since microorganisms are the drivers of bioremediation. Recent research has used next-generation sequencing techniques to learn more about the microbial communities participating in various bioremediation strategies (Jilani and Altaf Khan 2004). These methods have substantially increased our knowledge of the bioremediation techniques using microorganisms, as well as the influence of various contaminated clean-up response options. Molecular biology and metagenomics have dramatically advanced our knowledge of the biological systems present in these polluted settings, as well as our understanding of the microbial world in many situations. We intend to give a brief overview of metagenomic approaches and discuss how they have been utilized to better understand damaged ecosystems and guide environmental rehabilitation best practises.
2 Bioremediation
Bioremediation is a waste management technology in which biological organisms are used to remove or neutralize pollutants in the environment. The “biological” species include microscopic organisms like algae, fungi, and bacteria, as well as the “remediation” of the issue’s treatment. Microorganisms thrive in a diverse range of environments across the biosphere. They thrive in a variety of environments, including plants, animals, soil, water, the deep sea, and the frozen ice. Microorganisms are the ideal candidates to function as our environmental stewards because of their sheer numbers and desire for a wide spectrum of pollutants (Kumar et al. 2022).
Bioremediation technologies became widely used and are still increasing at an exponential rate today. Because of its environmentally benign characteristics, bioremediation of polluted places has proven to be effective and trustworthy. Recent advancements in bioremediation techniques have occurred in the last two decades, with the ultimate goal of successfully restoring damaged areas in an economical and environmentally beneficial manner. Different bioremediation approaches have been developed by researchers to recover polluted ecosystems (Jan et al. 2003). Most of the issues related with pollution biodegradation and bioremediation can be solved by indigenous microorganisms found in disturbed areas.
Bioremediation has a number of advantages over chemical and physical remediation approaches, including being environmentally benign and cost-effective.
Bioremediation works by reducing, mineralizing, degrading, detoxifying, or transforming more hazardous contaminants into less toxic ones. Agrochemicals, chlorinated compounds, dyes, heavy metals, pesticides, nuclear waste, organic halogens, plastics, greenhouse gases, xenobiotic compounds, hydrocarbons, and sludge are among the pollutants that can be removed. Toxic waste is removed from a polluted environment using cleaning techniques. Through the all-inclusive and action of microorganisms, bioremediation is heavily involved in the eradication, degradations, detoxification, or immobilization of various physical dangerous chemicals and chemical waste from the surrounding (Chauhan and Singh 2015).
3 History of Bioremediation
Bioremediation technique is known since the 1940s. Scientists already knew that certain bacteria have potential to degrade petroleum hydrocarbons.
George M. Robinson pioneered bioremediation in the 1960s. In 1968, while working as an engineer in California, Robinson arranged the clean-up of the first large-scale microbiological oil spill. He employed bioremediation to clean-up sewage, spills, and leach fields, as well as control odours and pests. Microbes are being employed to remediate oil spills, sewage, contaminated soil, and boost crop production. Bug cultures developed by George Robinson or one of his co-workers are used by almost every firm in this market (Taylor and Reimer 2008).
By the 1970s, tremendous progress had been made in this field of study. Nature, microbiologists understood, had an answer. Scientists knew that if the right amount of nutrients, like nitrogen, oxygen, and phosphate, could be added in the contaminated wells, the bacteria would multiply and remove the toxic gasoline faster and more efficiently than physical methods ever could.
Bioremediation has been utilized in a number of well-known clean-ups, including the 1989 Exxon Valdez oil spill in Alaska. Microbes assisted the many volunteers who worked to clean up the 11 million gallons of spilt oil by breaking down the oil as a food source. Engineers can help speed up the process of a pollutant spill by using particular bacteria, reducing environmental damage.
4 Bioremediation Successes
Bioremediation has been a huge success for the US Geological Survey. Their use of bioremediation has aided in the safe and effective clean-up of several spills, some of which were highly toxic, as well as the improvement of bioremediation knowledge and expertise. The following are a few of their accomplishments.
-
1.
Chlorinated Solvents, New Jersey:
In the heavily industrialized Northeast, chlorinated solvents are a particularly prevalent pollutant. Microorganisms can employ chlorinated chemicals as oxidants when other oxidants are unavailable because their metabolic processes are so flexible. United States Geological Survey (USGS) scientists at Picatinny Arsenal, New Jersey, have extensively recorded such changes, which can naturally cure solvent contamination of ground water.
-
2.
Pesticides, San Francisco Bay Estuary:
The poisoning of water bodies by pesticides is a major concern across the US. In field and laboratory study in the Sacramento River and San Francisco Bay, the impacts of biological and non-biological processes in degrading commonly used pesticides such as thiobencarb, carbofuran, melinite, and methyl parathion have been proven.
-
3.
Gasoline Contamination, Galloway, New Jersey:
Contamination by gasoline in the US gasoline is arguably the most prevalent contamination of ground water. Rapid microbial degradation of gasoline pollutants has been documented at this location, demonstrating the importance of activities in the unsaturated zone in contaminant degradation.
-
4.
Sewage Effluent, Cape Cod, Massachusetts:
In the United States, sewage effluent is commonly disposed of in septic drain fields. Systematic observations of a sewage effluent plume at Massachusetts Military Reservation provided the first reliable field and laboratory data of how rapidly natural microbial communities reduce nitrate pollution in a shallow aquifer.
-
5.
Crude Oil Spill Bemidji, Minnesota:
A pipeline which carries the crude oil exploded near Bemidji, Minnesota, in 1979, contaminating the underlying aquifer. The harmful compounds seeping from the crude oil were rapidly destroyed by natural microbial communities, according to USGS scientists who studied the site. Significantly, the plume of contaminated ground water stopped increasing after a few years as rates of microbial degradation and contaminant leaching were found to be in balance. This was the earliest and best case of intrinsic bioremediation, in which contaminated ground water is remedied by naturally existing microbial processes without the need for human intervention (Atlas and Bragg 2009).
-
6.
Agricultural Chemicals in the Midwest:
In several Midwestern states, agricultural chemicals have an impact on the chemical quality of ground water. The fate of nitrogen fertilizers and pesticides in ground and surface waterways has been studied in the Midwest. Many common pollutants, such as the herbicide atrazine, are destroyed through microbial degradation and non-biological mechanisms.
5 Mechanism of Bioremediation
Microbes have great potential to interact with chemicals both chemically and physically, causing structural alterations or total mineralization of the contaminants. The ability to digest and detoxify inorganic and organic contaminants in the environment is possessed by a large variety of bacteria, fungus, and actinomycetes genera (Thapa et al. 2012). Organic contaminants are biodegraded by microorganisms through two processes: (1) primary metabolism and (2) co-metabolism. The usage of the substrate as a source of carbon and energy has been termed as the primary metabolism of an organic substance. This substrate acts as an electron donor, allowing bacteria to proliferate. Organic contaminants are completely degraded as a result of this procedure (Fig. 1.2).
Mechanism of bioremediation (Boll et al. 2014)
Major classes of factors influencing the bioremediation (Kumar et al. 2018)
Co-metabolism, also known as gratuitous metabolism, co-oxidation, or accidental or free metabolism, occurs frequently in nature, where degradation activities are accompanied by the transformation of additional molecules, such as xenobiotics. The metabolism of an organic material that isn’t a source of carbon and energy or a necessary nutrient and can only occur in the presence of a main substrate is referred to as “co-metabolism.” Co-metabolism must be employed to remediate a xenobiotic-contaminated region when a molecule cannot serve as a source of carbon and energy owing to its chemical structure, which does not activate the needed catabolic enzymes. When microbial activity develops at a contaminated environment, it is a common occurrence. The enzymes of developing group of tissues and the manufacture of cofactors required for enzymatic reactions, such as hydrogen donors for oxygenase, are requirements for metabolic transformation (Leitão 2009).
The most typically reported pollutants to co-metabolize are PAHs with more than five aromatic rings, chlorinated biphenyls, chlorine mono-aromatics, and chlorinated aliphatic hydrocarbons. A technique may leverage aerobic or anaerobic metabolism of heterotrophic bacteria, depending on the pollutant of interest and the media. Aerobic metabolism, commonly known as aerobic respiration, is the utilization of oxygen (O2) as a reactant to convert a portion of the carbon in a pollutant to carbon dioxide (CO2), with the remaining carbon being utilized to regenerate new cell mass. Aerobic metabolism is more extensively employed and can be beneficial for hydrocarbons and other organic compounds.
Indigenous microorganisms in contaminated areas possess great tolerance for harmful contaminants and use them as a nitrogen, carbon, or energy source, degrading them into simple products up to total mineralization (Varma et al. 2011). Contaminated habitats, on the other hand, frequently contain a combination of organic and inorganic substances. If the concentrations of readily biodegradable pollutants in the matrices are too low, they may go undetected or disintegrate very slowly. The octanol water partition coefficient (log Kow), acidity constant (pKa), aqueous solubility (Sw), octanol solubility, and pollutant concentration are all physicochemical characteristics that influence the absorption of organic pollutants by plants and microorganisms. The arrival and transfer of organic contaminants inside the cellular state of microorganisms is determined by the octanol water partition coefficient.
Some bacteria are unable to mineralize these contaminants because the enzymes utilized for degradation do not recognize them as a substrate. They may contain substitution groups such as amino, carbamoyl, halogens, methoxy, nitro, and sulfonate, and may be chemically and physiologically highly stable. Furthermore, the chemicals may be water-insoluble in some situations and remain adsorbed to the soil’s exterior matrix. Furthermore, the large molecular size of persistent organic pollutants (POPs) and the absence of permease in cells of microbes may hinder their uptake by cells. As a result, there could be a variety of reasons for ineffective biodegradation in contaminated areas. Microbial bioavailability is a fundamental concept in evaluating all bioremediation strategies. Bioavailability refers to the amount of a pollutant in soil that may be taken up or changed by living organisms. Simply put, a contamination cannot be rectified if it is so tightly bound up in the solid matrix that microorganisms seem unable to access it. The amount of bioavailable pollutant is determined by two main factors: mass transfer and cell intrinsic activity (Sharma 2012). Bioavailability varies by species and organism, and in situ microbial breakdown of organic pollutants is influenced by contaminant bioavailability and microbe catabolic activity. The rate and extent of contamination removal vary depending on the pollutant of concern and site-specific variables. Several factors influence clearance rates, including pollutant dispersion and concentration, contaminant concentrations, indigenous microbial populations and response kinetics, pH, temperature, nutrition availability, and moisture content. Many of these variables are dependent on the site and the local microbial community, making them difficult to control.
6 Microorganisms Used in Bioremediation
Due to their exceptional metabolic capacities and ability to grow under a wide range of environmental conditions, microorganisms are extensively spread throughout the biosphere. Microorganisms’ dietary adaptations might be used to aid pollution biodegradation (Paul et al. 2005). Bioremediators are biological agents that are utilized in contaminated site bioremediation. Bacteria, archaea, and fungus are among the most effective bioremediators. Because of their benefits over traditional remediation processes, microorganisms play an essential role in pollutant removal in soil, water, and sediments (Singh et al. 2014). Microorganisms are helping to restore the natural environment and prevent pollution (Agrawal et al. 2021). Microbes have two characteristics that make them suited for the remediation process: flexibility and biological system. Microbial activity is primarily reliant on carbon. The bioremediation process was carried out in several conditions by a microbial consortia. Nitrosomonas, Achromobacter, Arthrobacter, Pseudomonas, Flavobacterium, Mycobacterium, Alcaligenes, Bacillus, Corynebacterium, Xanthobacter, and other microbes are among them. Bacteria are varied creatures that make great biodegradation and bioremediation players. Because bacteria have few universal toxins, any given substrate is likely to be broken down by an organism if given the correct conditions (anaerobic versus aerobic environment, sufficient electron acceptors or donor, etc.).
6.1 Fungi
Currently, bacteria are used in most bioremediation applications, with fungi being used in only a few cases. It can be transferred into bioremediation applications that can break down organic molecules and lower metal hazards. Fungi have an edge over bacteria in some circumstances, not only in terms of metabolic variety, but also in terms of environmental robustness. They can oxidize a wide range of compounds and withstand tough environmental conditions such as low moisture and high pollutant concentrations. As a result, fungi have the potential to be a very strong agent in soil bioremediation, and particularly diverse species, such as White Rot Fungi, have been a hot research area. The discovery of the white rot species Phanerochaete chrysosporium’s ability to digest many important environmental toxins in 1985 sparked interest in using fungi as a potential therapy for contaminants. The ability of these fungi to digest complicated compounds like lignin is their greatest distinguishing attribute. Other white rot fungus species were later identified to have similar properties. Furthermore, white rot fungi are useful because their hyphal extension degrades lignin extracellularly. This enables them to access soil contaminants that other species are unable to, as well as increase the surface area available for enzymatic interaction. These low-cost fungi can withstand a wide range of environmental conditions, including pH, temperature, and moisture content (Watanabe 2001). While many microbial organisms employed in bioremediation require the environment to be preconditioned before they can thrive, white rot fungi can be used directly in most systems since they disintegrate due to nutrient restriction.
6.1.1 Phanerochaete Chrysosporium
The first fungus P. chrysosporium was to be linked to the breakdown of organic pollutants. Pesticides, polycyclic aromatic hydrocarbons (PAHs), dioxins, carbon tetrachloride, and a wide range of other pollutants have all been demonstrated to have significant bioremediation potential. Among fungal systems, P. chrysosporium has become a model for bioremediation. Other well-known white rot fungus include Pleurotus ostreatus and Trametes versicolor.
7 Factors Affecting Bioremediation
Controlling bioremediation process is a complicated system involving numerous variables. These characteristics include the presence of a microbial population capable of degrading pollutants, the availability of toxicants to the microbial population, and environmental parameters (temperature, soil type, oxygen, pH, and nutrient availability). Microbial activity, degradative enzyme activity, and hydrocarbon breakdown in general are all affected by these factors. This suggests that bacterial bioremediation could be more successful and efficient if these variables are tweaked, controlled, and managed (Lee 2013).
7.1 Biotic Factors
7.1.1 The Availability of Bacteria That Degrade Hydrocarbons
In hydrocarbon-contaminated soils, microorganisms that break down these hydrocarbons are abundant and also widespread. The bacteria’s capacity to quickly adapt to a hydrocarbon-polluted environment and utilize the pollutant as a sole energy and carbon source for metabolism and development is the reason for this. In a polluted environment, the presence of naturally occurring probable hydrocarbon-degrading organisms has a direct relationship with the efficiency of hydrocarbon biodegradation. These bacteria have metabolic activity and may break down hydrocarbon pollutants in either an aerobic or anaerobic environment. However, the types and nature of hydrocarbon pollutants, as well as the surrounding environmental circumstances, have an impact on their abundance and diversity in terms of species. (Fomina and Gadd 2014). As a result, the presence of naturally occurring appropriate bacteria is critical for microbial therapeutic activities to be implemented.
7.1.2 Competition and Cooperation Among Bacteria
Bacterial cooperation and competition could be a key motivator for survival and stability among microbic communities on a specific home turf. Microorganisms that degrade hydrocarbons compete for survival in hydrocarbon-contaminated environments. Microorganism competition, whether interspecies or intraspecies, may, nonetheless, be a limiting factor in biodegradation efficacy. For example, hydrocarbon-degrading fungi and bacteria may compete for the activity of organic compound pollution as carbon sources as well as various limited resources for metabolism and growth. Furthermore, some hydrocarbon-degrading microbic species create compounds that prevent other hydrocarbon-degrading bacteria from growing and expanding. Exogenous bacteria are commonly used in analysis to break down organic chemical pollutants; however, they do not appear to be always efficient. As a result, the interdependency of microbic population is crucial for bioremediation to achieve success. Some hydrocarbon-degrading microbic consortia are incontestible to make synergistic connections for full degradation. Thus, microorganism consortia will give vital info for in place bioremediation of organic compound-related pollutions by providing comprehensive views regarding modification of their hydrocarbon biodegradation capabilities.
7.1.3 Exogenous and Indigenous Hydrocarbon-Degrading Bacteria
Bacteria, either naturally existing or introduced, can eliminate hydrocarbon contaminants. Native bacterial populations, on the other hand, are better at mineralizing hydrocarbon pollutants than imported inocula for long-term bioremediation effectiveness. Degrading a wide range of hydrocarbon components from contaminated sites necessitates a microorganism with high stability and physiological adaptations to local nutrition and environmental conditions. When compared to foreign microbial consortia, indigenous bacteria shows higher rate of degradation. This is due to the fact that foreign bacteria cannot withstand abiotic stress and must rely on the soil environment to survive and multiply. As a result, for reliable and low-cost bacterial bioremediation, native bacterial isolates of certain strains or consortia derived from hydrocarbon-contaminated areas are recommended.
7.1.4 Number of Hydrocarbon-Degrading Bacteria
The quantity of bacteria in the soil that digest hydrocarbon pollutants is a significant factor in their decomposition. If the region has not previously been polluted by hydrocarbon pollutants, the quantity and variety of microorganisms that can degrade hydrocarbons are substantially smaller than the total number of naturally accessible microorganisms. Bacteria that degrade hydrocarbons, on the other hand, have a better chance of living in hydrocarbon-polluted environments than bacteria that do not. (Fantroussi and Agathos 2005). This difference is significant since microorganisms that degrade hydrocarbons are known to have efficient hydrocarbon degradative enzymes as well as a variety of metabolic pathways. The higher the population of bacteria that degrade hydrocarbons, the faster they degrade. As a result, determining the quantity of possible microorganisms in contaminated areas is critical to achieving effective bacterial remediation.
7.1.5 Redox Potential of the Bacteria
To perform biological functions, maintain cell structure, and reproduce, bacteria require energy. A redox mechanism within bacterial cells produces this energy physiologically. By increasing the electron transfer from electron donors to electron acceptors, bacteria catabolize hydrocarbon contaminants as a source of energy in aerobic or anaerobic bioremediation. Redox potentials are critical for enhancing bacterial growth and facilitating the bacterial energy production (respiration) by oxidation of hydrocarbon-derived contaminants. Many organic contaminants, such as hydrocarbons, have a sluggish rate of degradation due to their high redox potential, according to studies. In aerobic environments, oxygen is required for the enzymatic activation of aromatic hydrocarbons as monooxygenases or dioxygenases, as well as serving as a final electron acceptor. Aromatic compounds are best example for giving electron as it is known to have large Gibbs free energy change of oxidation of aromatic compounds with various electron acceptors required for bacterial growth. Furthermore, chemicals having a high reduction potential (such as perchlorate and chlorate) are good electron acceptors for microbial metabolism in anaerobic bioremediation processes. At extensively polluted areas, electron donors (hydrocarbons) outnumber electron acceptors’ oxidation potential. As a result, the availability of certain electron acceptors can affect the respiration and biodegradation processes. As a result of the low redox potential and depletion of electron acceptors, bacterial breakdown may be inhibited. This is due to the reduced redox potential, which causes anoxic conditions and slows biodegradation.
7.1.6 Effect of Biosurfactants
Biodegradation becomes a concern when pollution caused by hydrocarbons is dumped into the soil. Because of their high soil adsorption and lack of bioavailability, in that case it shows limited microbial degradation. This problem has been addressed with the use of inorganic and organic surface-active compounds. Chemically generated surfactants have been worked to increase the solubility of hydrocarbons through a process known as emulsification. Ionic, non-ionic, biological, and combination surfactants were used to remediate hydrocarbon contaminants. Chemically synthesized (inorganic) surfactants, on the other hand, are not suggested for future as they should not be used since they are resistant to secondary contamination, are primarily harmful to the environment, and have little to no influence on the efficiency of hydrocarbon biodegradation. This suggests that biosurfactants derived from natural sources (plants or microorganisms) are appropriate since they are biodegradable and have beneficial characteristics, nontoxic, have a high specificity under difficult conditions, and are better adapted to breakdown. To emulsify and readily absorb the hydrocarbon pollutants, hydrocarbon-degrading bacteria produce such surface-active biological chemicals extracellularly. Biosurfactants include glycolipids, lichenysin, and surfactin, as well as lipopeptides, fatty acids, phospholipids, polymeric biosurfactants, lipoproteins, and neutral lipids. Enterobacter sp., Burkholderia sp., Bacillus sp., Pseudomonas sp., Aeromonas sp., Acinetobacter sp., Micrococcus sp., Rhodococcus sp., and several halophile species are among the microorganisms that create them. Biosurfactants help hydrocarbon breakdown by assisting in the solubilization and desorption of contaminants, as well as altering the properties of bacteria cell surfaces (Meysami and Baheri 2003). The surface tension and interfacial tension of the water/oil or water/air interaction can be reduced by biosurfactants. As a result, the hydrocarbon substrate’s surface area increases, making emulsification simpler, and the entire process increases the substrate’s availability for absorption and metabolism.
7.2 Abiotic Factors
7.2.1 Contaminant Physical and Chemical Properties
A microbial bioremediation approach is usually necessary to figure out how possible microorganisms interact with hydrocarbon pollutants. The chemical and physical characteristics of hydrocarbon pollutants can impact the biodegradation, transportation, and metabolism of a single strain or consortia of bacteria. This is owing to the contaminated environment’s composition, concentration, molecular size, structure, toxicity, and unpredicted hydrocarbon molecules. Hydrocarbon-degrading bacteria’s occurrence, stability, and biological activity are all affected by these factors. Polyaromatic hydrocarbons and highly condensed cycloalkane compounds are also more resistant to microbial degradation than unbranched alkanes and lighter PAHs. The contaminant’s non-degradation potential is due to their nature, which accounts for their solubility, bioavailability, toxicity to bacteria due to lipid membrane disruption, and inability to split the ring for degradation. To assess the rate of hydrocarbon biodegradation, it is necessary to understand the chemical properties, physical condition, and toxicity of hydrocarbon pollutants, as well as their fate.
7.2.2 Hydrocarbon Concentration
The rates of transformation, absorption, and mineralization of bacterial biodegradation are affected by the quantities of hydrocarbon pollutants. Because of the heavy and undispersed nature of high concentration contaminants, they are poisonous and have a severe impact on the growth and production of biomass of degraders, necessitating a protracted treatment period. Furthermore, it was discovered that hydrocarbon concentrations greater than 5% inhibit microbial decomposition and may disrupt the C:N:P ratio and oxygen availability. Similarly, extremely low hydrocarbon concentrations prevent biodegradation by repressing bacterial metabolic genes that produce degradation enzymes, resulting in a lack of carbon supply or availability to support microbial growth. As a result, the presence of an ideal concentration for complete mineralization from contaminated settings is required for bacterial biodegradation of hydrocarbons.
7.2.3 Nutrient Availability
Although microorganisms can be found in contaminated soil, they are unlikely to be in sufficient numbers to allow for clean up of the site. To aid indigenous microorganisms, biostimulation generally entails the provision of oxygen and nutrients. These nutrients are the fundamental components of life, allowing bacteria to produce the enzymes required to break down pollutants. Nitrogen, phosphorus, and carbon will be required by all of them.
Furthermore, excessive concentrations of hydrocarbon pollutants may affect the NPK ratio, resulting in oxygen deficiency. In general, the growth of hydrocarbon-using bacteria in the soil is hampered by an insufficient supply (either too much or too little) and/or the absence of mineral nutrients. As a result, ensuring that contaminated soils receive the proper quantity of nutrients (P and N) is crucial for efficient biodegradation of hydrocarbon. As a result, biodegradation of hydrocarbon depends on soil environment modifications, and the maximum rate of breakdown can be accelerated by providing the appropriate nutrients. Carbon is the most basic element in living creatures, and it is needed in higher quantities than other elements. pH, temperature, and moisture all have an impact on microbial growth and activity. Temperature influences the rate of numerous biological reactions, and for every 10 degrees Celsius rise in temperature, the rate of many of them doubles. The cells, on the other hand, perish at a specific temperature. A plastic covering can be used to boost solar heating in late spring, summer, and autumn. To promote microbial activity for hydrocarbon breakdown, sufficient moisture is required in hydrocarbon-contaminated areas (Grath et al. 1994).
7.2.4 Oxygen Availability
Bacteria that break down hydrocarbons can breathe both in the availability and non-availability of oxygen. At the terminal, sub-terminal, and bi-terminal levels of aromatic hydrocarbon pollutants, the presence of oxygen in the soil acts as the last chemical reactant and electron acceptor for oxidation and ring breakage. Therefore, it is a limiting factor for aerobic bioremediation. Studies show that destroying 1 mg/mL hydrocarbon pollutants requires 3.2 mg/mL oxygen, even if the total biomass of potential hydrocarbon-degrading bacteria is not taken into account. Successful biodegradation requires 10–40% oxygen. As a result, aerobic catabolism produces a faster rate of biodegradation than anaerobic metabolism.
7.2.5 Moisture Availability
The transportation medium for the nutrients of soil and elimination of bacterial waste products into soil particles is soil moisture and has an impact on hydrocarbon bioavailability, aeration, the character and number of soluble materials, diffusion processes, osmotic pressure, pH, gas transfer, and soil toxicity. The porosity and water holding capacity of soil are diminished when it contains hydrocarbon pollutants. In the end, this environment reduces microbial activity because bacterial and soil water activities are directly related, meaning that as moisture content falls, so do bacterial activities, and conversely, when soil moisture levels are high, oxygen transport is limited. As a result, the availability of water for microbial development and metabolism limits hydrocarbon breakdown in terrestrial environments. As a result, appropriate moisture availability in the ranges of 50–75%, 30–90%, and 50–80% is required for hydrocarbon biodegradation.
Extreme moisture levels, on the other hand, are detrimental to microbial development and metabolism. This is because, rather than creating anaerobic soil conditions, oxygen diffusion in the soil is reduced and aerobic hydrocarbon breakdown is hindered. To promote microbial activity for hydrocarbon breakdown, sufficient moisture in hydrocarbon-contaminated locations is required.
7.2.6 Bioavailability
The rate of contaminant absorption and metabolism, as well as the rate of contamination transport to the cell, determines how quickly microbial cells can convert toxins during bioremediation. In most polluted sediments, this appears to be the case. After 50 years, polluting explosives in soil have not degraded. A range of physio-chemical processes, including absorption and desorption, diffusion, and dissolution, influence a contaminant’s bioavailability. The sluggish mass transfer to the degrading bacteria reduces the bioavailability of pollutants in soil. When the rate of mass transfer is zero, contaminants become unavailable. Aging or weathering is the term used to describe the decline in bioavailability with time. It could be caused by:
-
1.
Contaminants are incorporated into natural organic matter through chemical oxidation reactions.
-
2.
Slow diffusion through very small pores and absorption into organic substances.
-
3.
The formation of semi-rigid films with a high resistance to NAPL-water mass transfer around non-aqueous-phase liquids (NAPL).
The introduction of food-grade surfactants, which increase the availability of pollutants for microbial breakdown, can help solve these bioavailability issues. (Chakraborty et al. 2012).
8 Bioremediation Types
Bioremediation procedures can be used both ex situ and in situ at the application site. The type of contaminant, the volume and depth of contamination, the ecosystem type, the cost, and environmental policies are all factors to consider when choosing a bioremediation technique. Abiotic factors such as nutrient concentrations and oxygen, pH, temperature, and other abiotic variables impact the effectiveness of bioremediation procedures.
8.1 Ex Situ Bioremediation
Ex situ approaches entail excavating contaminants from polluted places and bringing them to a treatment facility. Ex situ bioremediation procedures are frequently chosen depending on the depth of contamination, the type of pollutant, the degree of pollution, the cost of treatment, and the location of the contaminated site. Ex situ bioremediation procedures are likewise governed by performance requirements.
8.1.1 Treatment in the Solid Phase
Solid-phase bioremediation is a form of an ex situ method that requires digging and stacking contaminated soil. Organic wastes, such as animal manures, leaves, and farm wastes, are included, as well as industrial, household, and municipal wastes. Pipelines positioned throughout the heaps encourage bacterial growth. For ventilation and microbial respiration, air must flow through the pipes. Solid-phase systems require a lot of space and take a long time to clean up compared to slurry-phase techniques. Land farming, biopile, composting, and other solid-phase treatment methods are examples.
8.1.2 Slurry-Phase Bioremediation
When compared to alternative treatment methods, slurry-phase bioremediation is a faster technique. In the bioreactor, polluted soil is mixed with nutrients, water, and oxygen to produce the suitable environment for microbes to break down the contaminants in the soil. Separation of rubbles and stones from polluted sediment is part of this process. The amount of water added is determined by the amount of pollutants present, the rate of biodegradation, and the soil’s physicochemical parameters. The soil is removed and dried when this process is completed using centrifuges, vacuum filters, and pressure filters. The next step is to dispose of the soil and treat the resulting fluids in advance.
8.2 In Situ Bioremediation
These methods entail treating contaminated matter at the source of the contamination. It doesn’t require any excavation and creates little to no soil disturbance. In comparison to ex situ bioremediation approaches, these procedures should be quite cost-effective. Bioventing, biopharming, and phytoremediation are examples of in situ bioremediation processes that might be enhanced, but intrinsic bioremediation and natural attenuation may not. Chlorinated solvents, heavy metals, dyes, and hydrocarbons have successfully treated using in situ bioremediation approaches.
9 Bioremediation Approaches for Environmental Clean-Up
9.1 Ex Situ Bioremediation Approaches
9.1.1 Biopile
Above-ground layering of recovered hazardous soil is followed by oxygenation and nutrient replenishing to improve bioremediation by microbial metabolic activities. Aeration, irrigation, fertilizers, leachate collection, and treatment bed systems are all part of this technology. The cost-effectiveness of this one-of-a-kind ex situ technique, which allows for precise control of operative biodegradation factors including pH, nutrition, temperature, and aeration, is rapidly being examined. The biopile is used to treat low-molecular-weight contaminants that are volatile, and it may also be utilized to clean up contaminated very cold harsh situations which makes it versatile. Additionally, warm air can be fed into the biopile design to give both air and heat, allowing for improved bioremediation. To speed up the restoration process, bulking agents such as straw sawdust, bark or wood chips, and other organic elements have been added to a biopile build. Despite the fact that biopile systems are linked to other methods for ex situ bioremediation in the field, such as land farming, bioventing, and biopharming, engineering, maintenance, and operation that is dependable costs, and a lack of electricity at remote locations, preventing continuous air circulation in polluted heaped soil via an air pump, are all obstacles. Additionally, during bioremediation, excessive air heating can cause soil dryness, which limits microbial activity and favours volatilization rather than biodegradation. On-halogenated VOCs, fuel hydrocarbons, SVOCs, and pesticides are all treated with this system. The efficiency of the method will vary, and it may only be applicable to certain substances within specific contamination classes.
9.1.2 Biofilter
A biofilter consists of a huge media bed through which contaminants travel and are digested by microbes. They are one of the oldest methods of environmental clean-up. They are utilized in the waste water treatment and air pollution management. Peat, bark, gritty dirt, or plastic shapes are some of the materials utilized as bed medium. The trickling filter is a common type of biofilter that is used to treat a variety of waste waters, and waste that has been converted into liquid. A trickling filter consists of a vertical tank with a support rack filled with aggregate, ceramic, or plastic media and a vertical pipe in the middle with a rotating connection and spray nozzles on the top end. A spray arm is connected to the rotary connection and has spray nozzles fitted along its length for waste water distribution. On the surface, microorganisms forms biofilm that serve as the packaging for the breakdown of pollutants in the effluent. Contaminants bind to the surface of the media, where they are destroyed by microorganisms. Specific bacteria strains can be added into the filter, and optimal conditions can be created to break down specific substances more efficiently.
9.1.3 Land Farming
Land farming is one of the most successful bioremediation technologies due to its low equipment needs. It is most frequent in ex situ bioremediation, although it may also occur in some in situ bioremediation environments. Because of the treatment site, this element is considered. Contamination depth is crucial factor in land farming, which can be done by ex situ or in situ. Contaminated soils are excavated and tilled on a regular basis in land farming, and the form of bioremediation depends on the treatment site. Because it has more in common with other ex situ bioremediation techniques, ex situ bioremediation happens when hazardous soil is removed and treated on-site.
Excavated contaminated soils are often carefully placed above the ground surface on a fixed layer support to facilitate aerobic biodegradation of the contaminant by autochthonous microorganisms. Overall, land farming bioremediation is a straightforward design and implementation technology that requires little financial investment and may be utilized to repair huge amounts of polluted soil with minimal environmental impact and energy consumption.
9.1.3.1 Composting
Composting is a controlled biological process in which organic pollutants are transformed to harmless, stable metabolites by microbes. To adequately compost soil polluted with harmful organic pollutants, thermophilic temperatures must typically be maintained. Temperatures rise due to heat produced by microbes during the breakdown of organic material in garbage. In most situations, this is accomplished by the use of naturally occurring microbes. To enhance the porosity of the mixture to be decomposed, soils are dug and blended with bulking agents and organic additions such as wood chips, animal, and vegetal wastes. Maintaining oxygenation, watering as needed, and regularly monitoring moisture content and temperature all contribute to maximum degrading efficiency. Composting in windrows is generally thought to be the most cost-effective method. Meanwhile, it may be the source of the most fugitive emissions. Off-gas control may be required if volatile organic compound (VOC) or semivolatile organic compound (SVOC) pollutants are present in soils (Perpetuo et al. 2011).
The composting technique can be used on biodegradable organic compound contaminated soils and lagoon sediments. Aerobic, thermophilic composting has been shown in full-scale programmes to reduce explosives, ammonium picrate, and related toxicity to acceptable levels. PAH-contaminated soil can also benefit from aerobic, thermophilic composting. Composting materials and equipment are all commercially available.
9.1.4 Bioreactor
A bioreactor is a vessel that uses a series of biological reactions to turn basic materials into specific products. Different operational modes exist in batch, fed-batch, sequencing batch, continuous, and multistage bioreactors. Bioreactors are good for the growth of bioremediation. A bioreactor is loaded with dirty samples for the clean-up procedure. Ex situ bioremediation technologies provide a number of benefits over bioreactor-based treatment of contaminated soil. The use of a bioreactor-based bioremediation technique with greater control of aeration, agitation, pH, substrate, and temperature, and bioremediation time is sped up by increasing inoculum concentrations. The capacity to manage and modify biological reactions in a bioreactor is shown by the ability to regulate and adjust process parameters. Bioreactor designs are versatile enough to allow for optimal biological degradation while minimizing abiotic losses. In soil and groundwater, bioreactors are typically used to treat VOCs and fuel hydrocarbons. Pesticides are less effective in this procedure. The technique was utilized to treat soil that included trinitrotoluene (TNT) and Research Department eXplosive (RDX) in one application. It operated in both aerobic and anaerobic settings in the lab, with a significant reduction in pollutant concentration. In addition, intermediate by-products were degraded.
Hazardous by-products formed during the decomposition of some chlorinated solvents can also be degraded using in situ bioreactors. Adapted bacteria in this sort of bioreactor mineralize the organic molecules of interest. A biological support medium is used to capture the bacteria. A vapour extraction system can be utilized in conjunction with an in situ immobilized bioreactor system. Basic bioreactors are a well-established technique for the treatment of municipal and industrial wastewater. Fuels can be treated in bioreactors, which are commercially accessible. Explosive chemicals have been tested in the laboratory. A novel method for treating halogenated VOCs, SVOCs, pesticides, polychlorinated biphenyls (PCBs), and other chemicals is to sequence anaerobic/aerobic bioreactors. As contaminated groundwater travels through the reactor, these strategies accelerate degradation. This method has been used to remediate organic compounds in various leaking underground storage tanks and industrial locations with great success.
9.2 In Situ Bioremediation Approaches
9.2.1 Bioventing
Bioventing is a potentially innovative way for promoting spontaneous in situ biodegradation of any aerially degradable chemical in soil by providing oxygen to the soil microorganisms already there. Bioventing, in contrast to soil vapour vacuum extraction, employs low air flow rates to give just enough oxygen to keep microbial activity going. The most common way of delivering oxygen is direct air injection into residual pollution in soil. In addition to adsorbed fuel residues, volatile compounds are biodegraded when vapours travel slowly through biologically active soil.
Bioventing techniques have effectively treated petroleum hydrocarbons, non-chlorinated solvents, some insecticides, wood preservatives, and other organic contaminants.
Inorganic pollutants cannot be destroyed by bioremediation, but it may be used to modify their valence state and causing inorganic adsorption, absorption, accumulation, and concentration in micro- and macro-organisms. While still in their infancy, these solutions hold a lot of potential for stabilizing or removing inorganics from soil. Two critical requirements must be satisfied for bioventing to be successful. To maintain aerobic conditions, sufficient volumes of air must circulate through the soil; second, bacteria that degrade hydrocarbons naturally must be available in sufficient densities to accomplish satisfactory biodegradation rates. The initial testing will determine the air permeability of the soil as well as in situ respiration rates. Microbial activity in soil is known to be influenced by basic nutrients, temperature, moisture (e.g. nitrogen and phosphorus), and pH. Despite the fact that soil pH studies show that the optimal pH range for microbial activity is 6 to 8, microbial respiration has been seen at all locations, even in soils outside of this range. The ideal moisture level in the soil is quite soil-specific. Too much moisture in the soil might reduce air permeability and oxygen delivery. A lack of moisture inhibits microbial activity. However, in extremely dry conditions, irrigation or humidification of the injected air may be able to accelerate biodegradation. Bioventing breaks down pollutants more quickly in the summer, although some remediation can occur in soil temperatures as low as 0 °C (Prasad 2014).
The bulk of the necessary gear is easily available, and bioventing is becoming increasingly common. Bioventing is becoming increasingly popular among remediation experts, particularly when combined with soil vapour extraction (SVE). Bioventing, like other biological treatments, takes a long time to remediate a site because of the particular soil and chemical characteristics of the contaminated medium.
9.2.2 Biosparging
Biosparging is an in situ remediation approach that involves supplying oxygen and nutrients to polluted soils in order to encourage aerobic biodegradation of contaminants by indigenous microorganisms. In order to induce in situ aerobic biological activity, biosparging involves pumping pressurized air or gas into a polluted zone. This technology targets chemical substances that can be biodegraded under aerobic conditions and is used to treat soluble and residual contaminants in the saturated zone. By giving oxygen to the microorganisms and increasing the interactions between air, water, and the aquifer, the injection of air promotes the development of the aerobic microbial population and thereby enhances the bioavailability of pollutants. The goal of a biosparging system is to increase pollutant biodegradation while minimizing volatile and semi-volatile organic compound volatilization. The air injection flow rate is designed to give the amount of oxygen needed to improve bacterial contamination degradation. However, some volatilization may occur, necessitating air capture and treatment, depending on the operation mode and design chosen. The injection method and gas composition are two of the most important variables to consider when designing a biosparging system. Vertical or horizontal wells, as well as trenches or reactive barriers, can be used to inject gas. Nutrients are also injected below the water table to boost microbial degradation activity, causing pollutants to be destroyed or transformed. The microbial community adjusts to the changing chemical and geochemical circumstances. When pollutant concentrations meet treatment targets, the treatment is terminated. In situ biosparging has the potential to be used in isolated northern locations where material delivery and injection equipment mobilization are a problem. Biodegradation can be hampered by cold temperatures, and microbial activity may only occur during the summer months, thus treatment could take years. Because temperatures are essentially consistent throughout the year, microbial activity may be possible in deep soil. Treatment time for biosparging is very varied, depending on the qualities of the contamination, the natural bacterial population, and the physical and chemical characteristics of the contaminated site. Under ideal circumstances, treatment times of 6 months to 2 years are common.
9.2.3 Bioslurping
Bioslurping combines bioventing with vacuum-assisted free-product pumping to extract free-product from groundwater and soil, as well as to bioremediate soils.
Bio-slurping comprises employing vacuum-enhanced extraction/recovery, vapour extraction, and bioventing all at the same time to cope with light non-aqueous phase liquid (LNAPL) contamination. Vacuum extraction/recovery removes free product and some groundwater from the vadose zone, while vapour extraction removes high volatility vapours from the vadose zone, and bioventing improves aerobic biodegradation in the vadose zone and capillary fringe.
The bioslurping system consists of a well into which an adjustable-length “slurp tube” is attached. The slurp tube is lowered into the LNAPL layer and connected to a vacuum pump, which starts pumping to remove free product as well as some groundwater (vacuum-enhanced extraction/recovery). The vacuum-induced negative pressure zone in the well aids LNAPL flow toward the well, dragging LNAPL trapped in microscopic pore gaps above the water table. When the LNAPL level lowers somewhat in response to pumping, the slurp tube begins to suck in and remove vapours (vapours extraction). The removal of vapour enhances air movement across the unsaturated zone, increasing oxygen concentration and improving aerobic bioremediation (bioventing). When mounding occurs, the slurp returns to sucking LNAPL and groundwater since the introduced vacuum raises the water table a little. The liquid from the slurp tube (both product and groundwater) is sent to an oil/water separator, while the vapours are sent to a liquid vapour separator. Aboveground water and vapour treatment systems may be incorporated if necessary. In other cases, however, system design adjustments have made it possible to release groundwater and vapour collected by bioslurping without treatment. LNAPL and vapour recovery are directly connected with the degree of vacuum, according to field tests of bioslurping systems. When bioslurping was compared to traditional LNAPL recovery methods, it was shown that bioslurping outperformed both skimming and dual-pump methods. Bioslurping has been said to have cheaper project costs (because of less groundwater extraction and the fact that vapour and groundwater may not require treatment) and less aquifer “smearing” than other LNAPL recovery/treatment processes. Potential “biofouling” of well screens owing to active aeration is highlighted as a disadvantage of bioslurping, as is the lack of treatment of residual LNAPL contamination in saturated soils.
10 Metagenomics
As the name indicates, metagenomics is concerned with the metadata of many genomes in order to offer rapid and exact information on the composition and dispersion of an interacting microbial community in an environment, as well as their evolutionary history. Metagenomics has been referred to as environmental DNA library (eDNA library), community genomics, environmental genomics, soil microbial DNA library, community genome analysis, whole-genome shotgun sequencing, and a variety of other terms.
Metagenomics adds to the toolkit for studying non-cultured species (Tringe and Rubin 2005). This new field proposes a method for examining microbial communities as a whole rather than individual members. Metagenomics is the process of extracting DNA from a community in order to pool all of the organisms’ genomes. These genomes are typically divided and cloned into a culturable organism to produce metagenomics libraries, which are then analysed based on DNA sequence or functionalities given on the surrogate host by metagenomics DNA (Garfield and Merton 1979).
Targeted metagenomics and shotgun metagenomics are two common types of metagenomic methods. The diversity of a single gene is investigated in targeted metagenomics or microbiomics to determine the entire sequences of a specific gene in a given environment. The most typical use of targeted metagenomics is to look at the phylogenetic diversity and relative frequency of a single gene in a sample. To understand the taxonomic richness of an environment, microbial ecologists frequently use small subunit rRNA sequencing. It can also be used to explore how environmental toxins affect the structure of microbial communities. Environmental DNA is isolated for targeted metagenomics, and the gene of interest is PCR (polymerase chain reaction) amplified with primers designed to amplify the largest diversity of sequences for that gene.
Next-generation sequencing is used to sequence the amplified genes. Next-generation sequencing, which can explore hundreds of samples at once, generates thousands of small subunit rRNA reads per sample. The universality of the PCR primers employed for the investigation limits targeted metagenomics, which captures the diversity of a specific gene of interest. In addition, different bioinformatics analyses have the ability to distort total diversity estimations. Targeted metagenomics provides the benefit of giving a comprehensive inventory of the microbial species present in a collection of samples, as well as in-depth analyses of microbial diversity changes before and after a disturbance. Shotgun metagenomics uses genomic sequencing to analyse an environmental community’s whole genetic complement. In this procedure, environmental DNA is extracted and subsequently fragmented to form sequencing libraries. After that, the libraries are sequenced to ascertain the sample’s entire genetic content. Shotgun metagenomics is a strong tool for determining a microbial community’s functional potential. The depth of sequencing is generally the most limiting factor in shotgun metagenomics. To get a comprehensive inventory of the genes in an environmental sample, deep sequencing is typically necessary. A thorough study of a community’s functional potential necessitates a thorough examination of every creature’s genetic material. Shotgun metagenomics frequently over-samples the dominant bacteria in a community while sparingly sampling the genetic content of the community’s low-abundance members.
A phylogenetic anchor is used in several studies to relate a functional gene to a taxonomic categorization. With metagenomics sequencing, this can be challenging until enough sequencing depth is reached and the reads can be effectively assembled into suitably lengthy contigs. Several recent reviews have attempted to highlight the major phases in metagenomics as well as the numerous potential hazards (Kumavath and Deverapalli 2013).
11 Metagenomics in Bioremediation Process
Metagenomics is an approach for analysing directly extracted genetic material in environmental samples. Metagenomics study gives information on non-cultivable species’ microbial populations in a niche habitat using sequence and function-based research approaches (Kumar et al. 2020, 2021). Gene clusters for pollutant-degrading enzymes may be encoded, and novel bacteria can be identified (Table 1.1). Metagenomics techniques can also be used to detect and monitor microbes. Metagenomics is a technique for studying DNA taken from ambient sources without isolating and cultivating microbes. This method was first used to find new microbial products and to sample microbial diversity from various habitats in the environment. To remove pollution from the environment, microbes are used in bioremediation. This method, which uses microorganisms to clear hazardous pollutants, is both ecologically friendly and practical (Shah et al. 2011).
Bioremediation approaches for environmental clean-up (Harekrushna and Kumar 2012)
Microorganisms not only help to regulate biogeochemical processes in the environment to keep humans healthy and to alleviate and stimulate plant diseases, but they also assist in the removal of poisons from the environment. The goal is to study uncultivated microorganisms in order to get a better understanding of the genuine microbial community, including their functions, collaboration, interactions, and adaption to new environments. Metagenomics is a topic of study that is continually expanding and evolving. Sequence-based and feature-based metagenomic techniques are the two most common types. Currently, these tactics have increased our understanding of the environment of non-culturable bacteria, giving us a better understanding of the microbial world. The function-focused metagenomics method encourages the identification of novel genes and enables for genetic analysis, both of which are intriguing prospects for uncovering new ecosystems. Metagenomics research includes gene recognition, explanation of entire metabolic processes, genome assembly, and sequence-based identification of species from diverse populations (Chen and Murrell 2010). Microbial diversity and particular genes found via metagenomics research that have the potential to act as pollution indicators are also included in the bioremediation process. Several microbes play a crucial role in pollution clean-up thanks to bioactive compounds and enzymes discovered utilizing metagenomics methods. Bacteroidetes, Firmicutes, Actinobacteria, Spirochetes, Chloroflexi, Proteobacteria, Acidobacteria, and Patescibacteria are some of the most common microbial groups that can withstand high metal concentrations (Watanabe 2001). Microbial enzymes, metabolites, and bioactive chemicals, all of which have a role in water treatment, can also be discovered using metagenomics approaches. Using metagenomics approaches, many enzymes were discovered in chlorinated biphenyl-polluted soils, activated sludge, oil-contaminated water, wastewater, cow rumen, chemically contaminated soils, and compost wastewater. Alkane enzymes, carboxylesterases, dioxygenases, esterases, laccases, monooxygenases, phenol-degrading enzymes, polyaromatic and hydrocarbon-degrading enzymes, trichlorophenol hexadecane hydrolyzing enzymes, and trichlorophenol hexadecane hydrolyzing enzymes are some of the enzymes that remove toxins. The metagenomic analysis looks for novel bacteria-producing genes and assists in the identification of new processes and approaches to improve clean-up strategies (Wood 2008).
12 Metagenomics Research in a Contaminated Environment
12.1 Sampling from Contaminated Site
Biological replicates are necessary for statistical data analysis in order to examine the microbial communities’ geographical and temporal variability in diverse settings like soils and sediments. Indeed, physical features might alter locally in the latter, affecting the structure of the microbes. Microbial population is usually substantially less in polluted habitats than in pristine environments, and it is heavily influenced by the type of contamination present and the history of contamination. The complex interaction between nutrients, organic contamination, and hydrological processes generates geographic heterogeneity of resident bacteria in groundwater habitats. The study of fine scale heterogeneity offers a lot of potential for accurately assessing biodegradation rates and designing pollutant fate models (Lovey 2003).
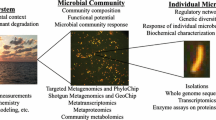
Environmental metagenomics will provide information on community composition and potential activity on the basis of samples obtained at certain date and place, but not on resilience or change resistance. In certain circumstances, a large sequencing depth can compensate for a small number of sequenced samples, resulting in an obvious microbial configuration at the studied site (Röling 2015). A common metagenomic approach is shown as flow chart in Fig. 1.6.
A common metagenomic approach is depicted as flowchart (Franzosa et al. 2015)
12.2 Extracting the DNA from Contaminated Samples
In all metagenomics research, DNA derivation is a critical step. Impurities in recovered DNA, natural components of soil/sediment, and soil contaminants should be eliminated to guarantee that it is representative of microbes found in the investigated environment. Stable Isotope Probing (SIP) has been integrated with metagenomics in pollution biodegradation research for bioremediation applications. The most essential considerations in deciding which process to utilize for DNA extraction are the number of microorganisms in the sample and the level of contamination. Many approaches for recovering high-quality, high-quantity, and high-molecular-weight DNA from the environment have been developed. Either the cells are lysed in the sample or they are eliminated before lysis. The process is known as direct extraction because DNA is extracted from inside the sample matrix in the first scenario. When used on samples with a low cell density and a low level of contamination, this method provides more DNA than the second. Because DNA is extracted from pristine cells that are not exposed to the sample matrix, the second method is known as indirect extraction. When possible, the latter extraction is favoured since it lowers the simultaneously extraction of inhibitory compounds in the sample, which might interfere with subsequent steps (e.g. cloning, polymerase chain reaction, and sequencing). Due to cell mortality during matrix purification, DNA yield is lower than in direct extraction. Extracting DNA from polluted settings remains difficult, despite the different technologies available. Metals and aromatic hydrocarbons, for example, have a negative impact on bacterial activity, resulting in lower bacterial biomass and, as a result, a lower yield of recoverable DNA. In this procedure, the phage phi29 polymerase is employed to amplify DNA using random hexamers at a constant temperature.
12.3 Metagenome Analysis
12.3.1 Targeted Metagenomics Using a Library
Cloning of ambient metagenomics DNA and screening of the clones for a desired function are the steps in library-based targeted metagenomics. It has been frequently used to pollute settings in recent years in order to identify the gene pool implicated in microbial degradation processes. Library-based targeted metagenomics restricts metagenome analysis to certain tasks, resulting in the context of a larger range of genes associated to the researched ecological role, even if there aren’t many of them, when as compared to complete DNA sequencing.
12.3.1.1 Creating a Metagenomics Library
The availability of commercial kits that include host cells and vectors with optimized methods for the many types of vectors that could be used in metagenomics has made the construction of metagenomic libraries easier. Multiple copy number vectors enable “massive” rDNA creation for later study. Overexpression of some genes, on the other hand, could be fatal to the host. Vectors with a single copy (e.g. BAC) ensure consistent preservation of a vast number of DNA segments and lower DNA output. Single-copy to high-copy-number vectors that are inducible were developed as a consequence, combining the aid of both types of vectors while also overcoming their limits in a single system.
12.3.1.2 Screening of Metagenomic Clones
There are two types of metagenomics library screening approaches: techniques based on sequences and methods based on functions.
12.3.1.2.1 Screening Based on a Sequence
This method is based on comparing recovered ambient DNA to sequences currently stored in databases. It is heavily influenced based on what we know about previously found genes. It usually entails utilizing PCR or hybridization-based methods to find clones with a conserved section within the targeted gene family or functional class of proteins. The large number of pollutant degradation gene sequences obtained from in situ has contributed in the development of degenerate probes and primers to help us better understand pollutant degradation processes. The search for sequences encoding a degradative gene of interest in gene databases is the first step in developing such primers. The alignment of the recovered sequences indicates areas that are both conserved and varied (Dettmer et al. 2007). PCR amplicons that have been tagged with radioactive fluorescent (e.g. digoxigenin) or antigenic (e.g. digoxigenin) molecules are known as hybridization probes. Hybridization conditions are empirically tweaked to uncover alternative sequences deviating from the original probe. Furthermore, in metagenomics libraries, the employment of a group of probes that are all aimed at the same thing, different GOI may have the advantages of
-
1.
increasing the likelihood of finding a single gene,
-
2.
in a single experiment, the identical hybridization conditions are applied to a large number of clones, and,
-
3.
increasing the likelihood of catching interesting target gene sequences.
12.3.1.2.2 Function-Driven Sequence
The function-driven screening is not reliant on previously identified sequences. It is solely dependent on the manifestation of a biological characteristic in the host cell. It has a better chance of finding novel and orthologous genes. Screening is typically done in polluted locations by selecting growing clones in minimal media augmented with the pollutant as the carbon or energy source. In this case, screening is necessary based on previous information of the target degradation process, as it is essential to construct a viable genetically modified host (Valiente and Pesole 2012).
To recognize the development of metagenomics clones more quickly, a chromogenic substrate such as MTT (3- (4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) can be used in conjunction with the selective medium. Bacterial respiration converts it to the purple chemical formazan. When the target enzyme creates a colourless output, MTT comes in handy. Other substrates, such as indole and catechol, generate a coloured response, making it easy to identify clones that contain enzymes involved in aromatic compound oxidation. Apart from those substrates, scientists have only made intermittent attempts to thrive selective media for recognizing clones that produce degradative enzymes. This is due to a present dearth of knowledge about the substrates (and products) that many of these enzymes can metabolize.
12.4 Direct Sequencing of Metagenomics
The genetic material found in a sample collected from environmental is sequenced without first constructing a metagenomic clone library. To acquire a better understanding of the ecosystem of microbes with less laboratory work, bioinformatics capabilities and extensive DNA sequence databases are necessary.
The high-throughput sequencing of an individual gene is another technique to examine environmental metagenomes without pre-cloning. This method doesn’t allow the retrieval of entire information contained in a metagenome, but it allows for better sequencing depth for the targeted functions. For example, targeted sequencing of 16S rRNA gene amplicons is routinely used in (preliminary) investigations of contaminated environments. 16S rRNA gene surveys can reveal a lot about microbial dynamics in these kinds of places. In comparison to library-based metagenomics, direct sequencing is becoming more popular in environmental investigations. This is due to the fact that the former has profited from an increased speed and throughput sequencing of DNA by orders of magnitude, while the latter has suffered from relatively slow developments in screening methods (Handelsman 2004).
12.5 Next-Generation Sequencing
A technological aspect common to all NGS technologies is massive parallel sequencing of clonally amplified or single DNA molecules spatially separated in a flow cell. There are numerous great comparison studies of NGS platforms that provide important information in terms of features of analysis and applications. Illumina just released the MiniSeq which is proved to be cost-effective and user-friendly sequencer. The Illumina and other NGS technologies’ short read lengths, on the other hand, are a limiting factor in genome completeness, variant calling, genome de novo assembly, and read mapping to reference genomes (Handelsman 2005). MiniSeq’s cheaper cost and speed come at the expense of a little for samples; there is an increase in inaccuracy and underperformance with less diversity and questionable base discrimination. It is due to the fact that the number of scanning channels has been lowered to reduce fluorophore consumption (Liu et al. 2012).
12.6 Bioinformatics Analysis
NGS sequencing of environmental DNA is becoming faster while also becoming less expensive, and new, superior sequencing equipment is launched almost every other year. NGS technologies generate massive volumes of data, but developments in analysis are lacking. Only dominant strains or tiny genomes could previously be reconstructed from complex metagenomes, but that is set to change according to computational advances that will allow individual genomes of rare species to be reconstructed (Kumar and Khanna 2010). Using metagenomics to answer ecological problems necessitates a thorough examination of the sequences retrieved.
12.7 Assembly
Assembly is the process of reassembling a genome using sequenced reads that are first turned into contigs and then scaffolds. The two techniques to genome assembly are de novo genome assembly, which reconstructs the genome from read data, and reference-guided assembly /comparative assembly, which assembles sequencing, reads using reference sequences (Tijssen 2002).
12.8 Binning
Binning is the process of grouping genomic reads/fragments that are presumably from the same organism based on sequence-related signals acquired over time, such as phylogenetic signals. Binning’s purpose is to assign sequences to “bins” that match to their taxonomic rank. Binding can also help with the assembly of previously difficult-to-cultivate genomes. To do so, a variety of bioinformatics tools are already accessible. There are two ways to do the binning procedure: supervised and unsupervised. Statistical classification methods for read categorization, such as similarity/distance matrix models or hidden Markov models, are used in supervised approaches for training. Unsupervised approaches, on the other hand, sort items into bins based on their composition, eliminating the necessity for a reference database. These techniques are typically futile since the vast majority of microorganisms discovered in environmental samples are usually unknown or can’t be grown.
12.9 Annotation
The foundation for microbiological sequence functional annotations is BioCyc, COG, KEGG, NCBI genomes, SEED, Pfam, and other databases. Because functional annotations employ homology-based techniques similar to those used for taxonomy assignment, the availability of previously recognized sequences acts as a limitless. Only 20–50% of a metagenomics sequence can be annotated in its entirety, according to current estimates. Smaller datasets, however, can benefit from manual curation to enhance annotation accuracy. The genomic segments are annotated in two steps. Following the identification of genes of interest, taxonomic neighbours and probable gene functions are assigned. In a nutshell, there are two major paths to choose.
13 Metagenomics in Bioremediation: Current Challenges and Future
NGS appears to be difficult due to a lack of appropriate sequence descriptions from a higher number of microorganisms present in various habitats. Due to varying relative numbers of various community members within a population, certain genomes may be covered by thousands of sequences while others are only covered by a few of sequencing reads or none at all. These considerations, together with the project’s budget, help to decide how much sequencing work is done on a project. Millions to billions of short reads are generated by the most prevalent metagenome sequencing technologies now in use (Kumar et al. 2020).
Estimates of community diversity are routinely conducted before metagenomics sequencing experiments. While these efforts (which usually involve rRNA gene amplicon analysis) might be valuable for community study, when it comes to strain level diversification or population heterogeneity, they might be deceiving. Another challenge with metagenomics assembly is that, despite advances in assembly algorithms and computer hardware technology, the assembly of such enormous volumes of complex data can soon surpass the memory constraints of any machine. The community’s natural diversity and variations identified within the population contribute to this problem, which is amplified as there may be inconsistencies in the sequencing (even at very low levels) in the sequencing data. Metagenomics is a systematic approach to genetic analysis of microbial populations. This gives a glance into the “Uncultured Microbiota’s” microbial community. Bioremediation has always adapted new scientific and technological breakthroughs to create healthier ecosystems, and metagenomics is one of the best adaptations ever. In a metagenomic investigation, identifying and screening metagenomes from contaminated environments is critical. The second section focuses on recent multiple case studies that illustrate how metagenomics can be used in bioremediation. The third section, as a result, discusses metagenomic bioremediation in various polluted habitats, such as soil and water. Starting with a full understanding of metagenomic screening, FACS, and several advanced metagenomic sequencing methodologies, diverse sequences and function-based metagenomic strategies and tools are available (Joshi et al. 2014). Many experiments that were previously unthinkable are now possible because of advances in technology that promote metagenomic study. Companies like PacBio and Nanopore are working on sequencers that can read Kbs of DNA, enabling for continuous genome construction in mixed populations.
The convergence of a number of high-throughput approaches will allow researchers to look at the relationships between species composition, gene density, gene expression, chemical reactions, and protein production in polluted surroundings. SIP is exposing a substrate to heavy isotope-labeled compounds and allowing microbes to digest it and integrate the labelled atoms into biological components such as DNA, RNA, and phospholipids. To separate the “heavy” (labelled) DNA from the “light” (unlabeled) DNA in DNA-SIP, all DNA from a treated sample is collected and centrifuged in CsCl gradients. This approach has a lot of potential for finding functionally active microorganisms, especially those involved in pollutant degradation, according to a recent assessment. Using SIP-metagenomics analyses of polluted substrates, the active response genes and species may be retrieved from the massive quantity of background genetic information from the original, uncontaminated soil. One of the following big metagenomics projects is expected to be the discovery of a core microbiome. To put it another way, what genes and species can be found in a certain habitat as well as different settings. It will be crucial to establish if there are critical genes and organisms that do indeed respond favourably to the addition of a contaminant in order to accomplish successful clean-up in the setting of bioremediation. Outside of this common core, genes that are favoured must be the result of extra environmental or stochastic factors (Simon and Daniel 2009). Many contemporary genomic investigations depend on snapshots of genetic information in environmental samples, despite the fact that many microbial communities are constantly changing due to microorganisms’ rapid pace of development. A metagenomics analysis of metal-contaminated groundwater revealed that pollution has decreased biological diversity and metabolic complexity to near-zero levels after 50 years. Despite the finding of all necessary metabolic pathways, there were 10 times fewer OTUs and a commensurate loss in metabolic complexity compared to a neighboring background site. Long-term monitoring of how evolution selects genes in polluted settings will certainly benefit the research and treatment of chronically contaminated areas, albeit massive volumes of data would demand a solution to the human-processing bottleneck first.
14 Conclusion
As sequencing costs fall, the value of high-throughput 16S rRNA sequencing and metagenomics grows. These methods allow researchers to investigate the impact of various bioremediation interventions on the native microbial ecosystem in greater detail. This allows these techniques to be fine-tuned for certain microorganism groups that are crucial to the bioremediation process. Metagenomics has the ability to guide the adoption of remediation technologies in order to achieve rapid and minimum invasive pollutant removal. Moving forward, a thorough understanding of the main taxa and routes indulge in many of these processes is critical. The ease with which data may be sequenced has resulted in the identification of several uncultured phyla and gene families with unknown functions. This is also true in contaminated situations. To further understand the bacteria engaged in these processes, metagenomic methods must be combined with traditional culture-based methodologies. Changes in microbial or gene diversity as a result of the reaction to a disturbance are frequently investigated using current techniques. Genetic and biochemical studies on model organisms are required to gain a deterministic view of the community’s response or the underlying metabolic mechanisms involved in reacting to these perturbations. The dominating species in these environments are typically distantly related to these model organisms. We will have a better comprehension of the metagenomic data sets obtained from these areas if we can identify ecologically relevant organisms from the ecosystems of interest. The application of 16S rRNA sequencing and metagenomics to guide bioremediation tactics and acquire deep insights into microbial responses to pollution or remediation procedures has a lot of potential. A more comprehensive picture of the bioremediation basis emerges when these approaches are paired with pure-culture analyses of environmental microorganisms.
References
Agrawal N, Kumar V, Shahi SK (2021) Biodegradation and detoxification of phenanthrene in in-vitro and in-vivo conditions by a newly isolated ligninolytic fungus Coriolopsis byrsina strain APC5 and characterization of their metabolites for environmental safety. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15271-w
Atlas R, Bragg JR (2009) Bioremediation of marine oil spills: when and when not - the Exxon Valdez experience. Microb Biotechnol 2:213–221
Baker KH, Herson DS (1994) Bioremediation. McGraw-Hill, Inc., New York
Boll M, Löffler C, Morris BEL, Kung JW (2014) Anaerobic degradation of homocyclic aromatic compounds via arylcarboxyl-coenzyme A esters: organisms, strategies and key enzymes. Environ Microbiol 16:612–627
Boopathy R (2000) Factors limiting bioremediation technologies. Bioresour Technol 74:63–67
Chakraborty R, Wu CH, Hazen TC (2012) Systems biology approach to bioremediation. Curr Opin Biotechnol 23:483–490
Chandra R, Kumar V, Yadav S (2015) Microbial degradation of lignocellulosic waste and its metabolic products. In: Chandra R (ed) Environmental waste management. CRC Press, Boca Raton
Chauhan A, Singh J (2015) Biodegradation of DDT. J Textile Sci Eng 5:1–8
Chen Y, Murrell JC (2010) When metagenomics meets stable-isotope probing: progress and perspectives. Trends Microbiol 18:157–163
Dettmer K, Aronov PA, Hammock BD (2007) Mass spectrometry-based metabolomics. Mass Spectrom Rev 26:51–58
Fantroussi S, Agathos SN (2005) Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol 8:268–275
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Franzosa EA, Hsu T, Sirota-Madi A, Shafquat A, Abu-Ali G, Morgan XC et al (2015) Sequencing and beyond: integrating molecular ‘omics’ for microbial community profiling. Nat Rev Microbiol 13:360–372
Garfield E, Merton RK (1979) Citation indexing: its theory and application in science, technology, and humanities, vol 8. Wiley, New York
Grath SP, Chaudri AM, Giller KE (1994) Summary 15th world congress of soil science. Acapulco, México
Handelsman J (2004) Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 68(4):669–685
Handelsman J (2005) Sorting out metagenomes. Nat Biotechnol 23(1):38–39
Harekrushna S, Kumar DC (2012) A review on: bioremediation. Int J Res Chem Environ 2(1):13–21
Jain PK, Bajpai V (2012) Biotechnology of bioremediation- a review. Int J Environ Sci 3:535–549
Jan B, Beilen V, Neuenschwunder M (2003) Rubredoxins involved in alkane degradation. J Bacteriol 184(1722–1732):97
Jilani S, Altaf Khan M (2004) Isolation, characterization and growth response of pesticides degrading bacteria. J Biol Sci 4(15–20):96
Joshi MN et al (2014) Metagenomics of petroleum muck: revealing microbial diversity and depicting microbial syntrophy. Arch Microbiol 196(8):531–544
Kumar M, Khanna S (2010) Diversity of 16S rRNA and dioxygenase genes detected in coal-tar-contaminated site undergoing active bioremediation. J Appl Microbiol 108:1252–1262
Kumar V, Shahi SK, Singh S (2018) Bioremediation: an eco-sustainable approach for restoration of contaminated sites. In: Microbial bioprospecting for sustainable development. Springer, Singapore, pp 115–136
Kumar V, Thakur IS, Singh AK, Shah MP (2020) Application of metagenomics in remediation of contaminated sites and environmental restoration. In: Shah M, Rodriguez-Couto S, Sengor SS (eds) Emerging technologies in environmental bioremediation. Elsevier. https://doi.org/10.1016/B978-0-12-819860-5.00008-0
Kumar V, Singh K, Shah MP, Singh AK, Kumar A, Kumar Y (2021) Application of omics technologies for microbial community structure and function analysis in contaminated environment. In: Shah MP, Sarkar A, Mandal S (eds) Wastewater treatment: cutting edge molecular tools, techniques & applied aspects in waste water treatment. Elsevier, Cambridge, MA. https://doi.org/10.1016/B978-0-12-821925-6.00013-7
Kumar V, Agrawal S, Shahi SK, Motghare A, Singh S, Ramamurthy PC (2022) Bioremediation potential of newly isolated Bacillus albus strain VKDS9 for decolourization and detoxification of biomethanated distillery effluent and its metabolites characterization for environmental sustainability. Environ Technol Innov 26:102260. https://doi.org/10.1016/j.eti.2021.102260
Kumavath RN, Deverapalli P (2013) Scientific swift in bioremediation: an overview. Intech Publishers
Lee JH (2013) An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol Bioprocess Eng 18(431):439
Leitão AL (2009) Potential of Penicillium species in the bioremediation field. Int J Environ Res Public Health 6(4):1393–1417
Liu L, Li YH, Li S, Hu N, He Y, Pong R et al (2012) Comparison of next-generation sequencing systems. J Biomed Biotechnol 2012:251364. https://doi.org/10.1155/2012/251364
Lovey DR (2003) Cleaning up with genomics: applying molecular biology to bioremediation. Nat Rev Microbiol 1:35–44
Metzker ML (2009) Sequencing technologies-the next generation. Nat Rev Genet 11(1):31–46
Meysami P, Baheri H (2003) Prescreening of fungi and bulking agents for contaminated soil bioremediation. Adv Environ Res 7(881–887):149
Paul D, Pandey G, Pandey J, Jain RK (2005) Accessing microbial diversity for bioremediation and environmental restoration. Trends Biotechnol 23:135–142
Perpetuo EA, Souza CB, Nascimento CAO (2011) Engineering bacteria for bioremediation. In: Carpi A (ed) Progress in molecular and environmental bioengineering—from analysis and modeling to technology applications. InTech, Rijeka, pp 605–632
Prasad R (2014) New approaches and insights into bioremediation of hazardous waste. Rev Environ Health 29(1–2):33–35
Röling WF (2015) Maths on microbes: adding microbial ecophysiology to metagenomics. Microb Biotechnol 8(1):21–22. https://doi.org/10.1111/1751-7915.12233
Shah V, Jain K, Desai C, Madamwar D (2011) Metagenomics and integrative – omics’ technologies in microbial bioremediation: current trends and potential applications. In: Metagenomics: current innovations and future trends. Caister Academic Press, Norfolk, pp 211–240
Sharma S (2012) Bioremediation: features, strategies and applications. Asian J Pharm Life Sci 2(2):202–213
Simon C, Daniel R (2009) Achievements and new knowledge unraveled by metagenomic approaches. Appl Microbiol Biotechnol 85(2):265–276
Singh R, Singh P, Sharma R (2014) Microorganism as a tool of bioremediation technology for cleaning environment: a review. Proc Int Acad Ecol Environ Sci 4(1):1–6
Taylor E, Reimer D (2008) Oil persistence on beaches in Prince William sound—a review of SCAT surveys conducted from 1989 to 2002. Mar Pollut Bull 43:458–474
Thapa B, Kumar A, Ghimire A (2012) A review on bioremediation of petroleum hydrocarbon contaminants in soil. Kathmandu Univ J Sci Eng Technol 8(1):164–170
Tijssen RJ (2002) Science dependence of technologies: evidence from inventions and their inventors. Res Policy 31(4):509–526
Tringe SG, Rubin EM (2005) Metagenomics: DNA sequencing of environmental samples. Nat Rev Genet 6(11):805–814
Valiente G, Pesole G (2012) Bioinformatics approaches and tools for metage nomic analysis. Editorial. Brief Bioinform 13(6):645
Varma R, Turner A, Brown MT (2011) Bioaccumulation of metals by Fucus ceranoides in estuaries of South West England. Mar Pollut Bull 62(11):2557–2562
Verma JP, Jaiswal DK (2016) Book review: advances in biodegradation and bioremediation of indust yerial waste. Front Microbiol 6:1–2
Watanabe K (2001) Microorganisms relevant to bioremediation. Curr Opin Biotechnol 12(3):237–241
Wood TK (2008) Molecular approaches in bioremediation. Curr Opin Biotechnol 19:572–578
Zhang C, Bennett GN (2005) Biodegradation of xenobiotics by anaerobic bacteria. Appl Microbiol Biotechnol 67(5):600–618
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sokal, S., Palsania, P., Kaushik, G. (2022). Bioremediation and Functional Metagenomics: Advances, Challenges, and Opportunities. In: Kumar, V., Thakur, I.S. (eds) Omics Insights in Environmental Bioremediation. Springer, Singapore. https://doi.org/10.1007/978-981-19-4320-1_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-4320-1_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4319-5
Online ISBN: 978-981-19-4320-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)