Abstract
Plants respond and adapt to various environmental conditions through morphological, anatomical, and physiological adaptations at the cellular and plant level. These morphological, anatomical, and physiological adaptations help the plant to cope up with the environmental variations and the stress created by those variations. Among these adaptations, morphological and physiological adaptive traits are the most well-studied traits in most crops, including model crops. Drought and salinity stresses are the major abiotic stress factors affecting yield loss worldwide. Sugarcane with 12–18 months of crop cycle is not flexible enough to avoid unfavorable environmental conditions and faces all climatic variability throughout the year. In sugarcane development, about 80% of the sugar accumulates during tillering and grand growth period. Abiotic stresses during these growth stages critically affect sugarcane yield. Both leaf and root anatomical plasticity in crops play an important role in imparting tolerance to various abiotic stresses such as drought, salinity, oxidative stress, high and low temperature. An increase in the leaf cuticle thickness and increase in leaf epidermal thickness are reported to be the anatomical traits in drought-tolerant sugarcane varieties. Intact bulliform cells, bulliform cell area, chloroplast content, and chloroplast ultrastructure, especially the length, width, and width/length of chloroplasts, are reported to be effective indexes for drought-resistant sugarcane variety. Roots are the actual site that requires the highest plasticity during drought combined with high temperature to ensure continuous water movement through the soil-plant-atmosphere continuum. The efficiency of soil water uptake by the root system determines the rate of transpiration and above-ground performance. Increased root length, reduced cortical layer, increased protoxylem poles, increased metaxylem vessels, and reduced metaxylem diameter, which provides better hydraulic resistance, are some of the adaptive root traits reported in sugarcane under drought conditions. This chapter provides an overview of these leaf and root anatomical traits conferring tolerance to various abiotic stresses in sugarcane.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
6.1 Introduction
Sugarcane (Saccharum officinarum L.) (Poaceae) is an economically important crop used for approximately 80% of sugar production globally. Due to the high biomass production, sugarcane is also increasingly used as a source of bioenergy crop. However, a lack of water often limits sugarcane production, specifically at the critical growth stages such as formative and grand growth stages (Naik 2001; Silva et al. 2008; Tammisola 2010; Verma et al. 2019a, b, 2021a, b, c). In India, sugarcane cultivation is experiencing drought in tropical and subtropical regions and depends on supplementary irrigation for growth.
Abiotic stress is a recurrent problem in sugarcane that affects the quantity and quality of its yield. It is estimated that about 2.94 lakh ha is affected by drought in India, and about 2.5 lakh ha is affected by waterlogging (Misra et al. 2020), while nearly 9 mha sugarcane area is reported to be affected by salinity (Brindha et al. 2019). These abiotic stresses disturb the metabolism, growth, and development of sugarcane crop and finally leads to yield loss (Shrivastava and Srivastava 2016; Verma et al. 2020a, b, 2021d). Drought stress is one of the most destructive among all abiotic stresses since sugarcane is known to be a water-loving crop (Zingaretti et al. 2012; Lakshmanan and Robinson 2013; Verma et al. 2021c). Drought stress simultaneously affects several morphological and physiological traits in sugarcane, thereby causing the reduction in overall growth and crop productivity (Yardanov et al. 2003). Sugarcane needs a lot of water during the tillering and grand growth phase (Ramesh 2000). Plants have evolved to adapt to any stress conditions through various morphological, anatomical, and physiological mechanisms. Understanding these mechanisms will not only provide clues towards the crop adaptation to various stress conditions, but it will also help us develop improved tolerant genotypes (Chandler and Bartels 2008; Verma et al. 2019a).
The structural adaptations through leaf and root anatomical features help the plant respond and adapt to limited resources (Matsuda and Rayan 1990). The structural transformations in the leaf are more crucial for plants to survive under drought conditions, which help the plant to protect the photosynthetic machinery and minimize water loss under drought. Adaptive anatomical features of leaves are directly linked to CO2 assimilation rates and photosynthetic efficiency (Terashima et al. 2001). Some leaf traits such as leaf area are reported to contribute to yields in sugarcane directly. Leaf area is another essential characteristic to maximize solar radiation interception and is directly associated with carbon fixation (Sinclair et al. 2004). However, the root is the first organ to sense and respond to water dehydration in soil (Ferreira et al. 2017). Due to their functions in nutrient and water uptake, the anatomical adaptations among root traits also play an important role in determining sustainable yield under stress.
6.2 Leaf Anatomy and Drought Tolerance
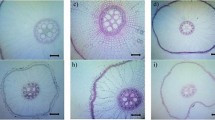
Any stress condition impacts the internal structure, reflecting the poor physiological performance of a crop (Pan et al. 2011). The leaf is the first organ to reflect physiological performance, and leaf anatomy and physiology directly correlate with plant drought resistance (Wang et al. 2006). Table 6.1 summarizes the leaf anatomical features studied so far and reported to be important markers for drought resistance in sugarcane.
6.3 Stomatal Density and Size
Stomata with a pair of specialized guard cells surrounding a central pore provide access to the mesophyll cells (Grantz et al. 1987). Stomata play a crucial role in regulating water use and carbon uptake; hence stomatal structures are most extensively studied for plant water use efficiency and drought tolerance (Grantz et al. 1987; Bertolino et al. 2019; Hetherington and Woodward 2003).
Stomatal conductance is regulated in plants through substantial crosstalk between guard cell turgor pressure and stomatal pore aperture movement (Grantz et al. 1987; Kollist et al. 2014). Under reduced soil moisture, high temperature, or light intensity, the guard cell turgor pressure decreases, which results in reduced stomatal aperture and conductance (Schroeder et al. 2001; Mustilli et al. 2002; Tombesi et al. 2015; Bartlett et al. 2016; McAdam and Brodribb 2016). By reducing the stomatal aperture and conductance, plants improve water conservation, but often at the expense of reduced photosynthesis (Flexas and Medrano 2002).
Although the stomatal aperture is significant for stomatal conductance and photosynthesis under water-limiting conditions, stomatal density and stomatal size play an important role (Bertolino et al. 2019; Verma et al. 2020). Stomatal density and size have shown correlation to drought resistance in sugarcane (Zhang et al. 2015). Due to reduced stomatal size, loss in stomatal conductance has been linked to higher water conservation under water deficit conditions (Zhang et al. 2003). Verma et al. (2020) have reported reductions in the stomatal density and stomatal aperture size in sugarcane plant leaves under drought to reduce water loss. Further Si application has enhanced stomatal density and aperture size under drought stress.
Smaller stomata can reduce the total leaf pore area, and smaller cells permit faster aperture response (Franks and Beerling 2009; Drake et al. 2013; Lawson and Blatt 2014). The more rapid stomatal response has shown maximum Water Use Efficiency (WUE) under fluctuating light conditions than prolonged water stress (Drake et al. 2013; McAusland et al. 2016; Kardiman and Ræbild 2018). Along with the stomatal size, the shape of guard cells and subsidiary cells are also proposed to affect stomatal functioning for water use efficiency and drought tolerance (Lawson and Vialet-Chabrand 2019).
Any stomatal damage affects carbon uptake, leading to the loss of photosynthetic machinery and reduced crop yield. Several authors have reported an increase in stomatal density and a decrease in size as an adaptive character during drought stress (Nawazish et al. 2006; Taratima et al. 2019). Few authors have also reported anatomical features such as more veins and lesser stomata per unit area in leaf to be closely related with sugarcane drought resistance (Mo and Zhou 1984; Meneses Rodriguez 1985; Malik 1986; Xu 1986).
6.4 Enlargement of Bulliform and Epidermal Cells
Bulliform cells are the water-storing epidermal cells present in the upper surface of leaves and play an essential role in regulating the rate of transpiration. Under moisture stress, bulliform cells assist in leaf rolling to avoid water loss through transpiration. Leaf rolling and reduced transpiration are related to plants’ drought resistance (Baranova 1987). The inefficiency of bulliform cells in leaf rolling and reduction in bulliform cell area under drought is considered as a susceptible character in sugarcane (Zhang et al. 2015; Taratima et al. 2019). With the water loss from the leaf, the perimeter/area ratio in bulliform cells is reported to reduce under drought. It is also noticed that the smaller ratio of perimeter and area is better for material and energy conversion (Wang et al. 2009; Zhang et al. 2015; Taratima et al. 2019).
Other important anatomical modifications reported in sugarcane under drought stress are enlargement of bulliform cells and epidermal cells, widened vesicles in bulliform cells, and bulliform cells with thin cell walls (Nawazish et al. 2006; Taratima et al. 2019). Under drought stress conditions, the pit of sclerenchyma cell walls is also reported to increase (Bosabalidis and Kofidis 2002).
6.5 Thickening of Leaf Lamina and Cuticle Layer
In sugarcane, the thickening of adaxial and abaxial cuticles covering the epidermis happens under both drought and salinity (Mo and Zhou 1984; Taratima et al. 2019). Along with the cuticle layer, increased lignification of cells around the vascular bundle is found in drought-resistant sugarcane varieties (Zhu et al. 2010). Strong lignification around the vascular bundle protects the conducting tissues under drought stress.
6.6 Other Anatomical Features
The size of bundle-sheath cells and vascular bundles gets modified under drought stress in sugarcane (Wu et al. 2011). Under moisture, increase in the vascular bundle size improves water and food transportation efficiency (Bosabalidis and Kofidis 2002). The number of vessels per unit area in sugarcane roots and stems is positively correlated with drought resistance (Tan 1988). Under severe drought stress, plasmolysis of chloroplasts is reported in sugarcane (Zhang et al. 2015). Movement of chloroplasts towards the center of the cell, change in shape, and increase in starch content are shown in susceptible sugarcane genotypes (Zhang et al. 2015). Reduced length, width, and width/length of chloroplasts are effective indexes for drought and salinity (Wu et al. 2011).
6.7 Root Anatomical Traits
Roots are the organs to detect moisture stress, and the physiological and molecular signals to induce resistance are sent by the roots (Atkinson and Urwin 2012). These root system signals help the plant adapt through various biological mechanisms to maintain optimal growth and yield under stress conditions (Sieburth and Lee 2010). Roots not only initiate the molecular signaling, but also modify the root architecture and anatomical traits, which contributes to enhance above-ground performance. Root System Architecture (RSA) plays a vital role in the agronomic performance of a crop. The adaptive plasticity in root anatomical helps to maintain photosynthesis and stomatal regulation, resulting in better yield under stress conditions (Chimungu et al. 2014a, b, 2015; Kadam et al. 2015).
In sugarcane, the relationship between root and shoot growth under diverse conditions has shown a positive correlation, and the efficient root traits also determine to stalk dry weight (Glover 1967; Smith et al. 1999; Ferreira et al. 2017). Sugarcane root system is highly divergent, comprising of highly branched sett roots (roots originating from the sett), shoot roots (main roots originating directly from the shoot), and deep rope roots formed by the agglomeration of shoot roots (Lynch 2013; Valarmathi et al. 2020). Sett roots arise from root eyes of setts within 24 h after planting that are required essentially for settling development and eventually degrades after 30–40 days. Shoot roots are stable, thicker, and fleshier permanent roots that provide strong anchorage developed from shoot bases 5–7 days after planting. These roots penetrate deeper soil beyond 1.5 m providing access to deep soil water reserves. The development of these root types strongly contributes to the performance of the above-ground parts (Gregory 2006). In sugarcane, extensive root systems support physiological and morphological traits of the above-ground parts under early drought stress (Khonghintaisong et al. 2018; Smith et al. 2005). Among the Root System Architecture (RSA), deep rooting is an extensively studied and reported root trait under stress conditions. Tolerant sugarcane genotypes have a long root system compared to susceptible genotypes under both drought and salinity stress conditions (Kumar et al. 2017; Khonghintaisong et al. 2018; Ogbaga et al. 2020). The genotypes with deep and extensive root systems are selected as water stress-tolerant genotypes (Smith et al. 2005). Long roots result in better water uptake, a desirable trait to extract deep soil moisture when water is limiting (Tardieu et al. 1992; Blum 2005; Tardieu 2012). At the cellular level, increased biosynthesis of lignin has one of the most crucial reactions under water-limiting conditions. The increased biosynthesis of lignin leads to cell-wall thickening of the vascular tissues, endodermis, and exodermis (Enstone et al. 2002; Naseer et al. 2012).
Anatomically monocot roots are characterized by the presence of two highly suberized layers called endodermis and pericycle. These two cell layers play a significant role in selective absorption as well as mineral and water uptake (Vásquez 2003). The pericycle is the meristematic layer, the source of lateral roots and surrounds the vascular bundle or stele (Richards and Passioura 1981). The major challenge for the plant under moisture stress is to protect the root water-conducting tissues from hydraulic pressure. Another challenge is to protect the meristematic layer pericycle for the growth of lateral roots. These two modifications are achieved either by lignifying the cells surrounding the vascular cylinder or by reducing the diameter of the xylem vessels. Only three authors have so far worked on the anatomical structures of sugarcane roots under drought conditions (Queiroz-Voltan et al. 1998; Chaves et al. 2009; da Cruz Maciel et al. 2015). The anatomical features studied in sugarcane are described in detail in separate sections.
6.8 Reduced Xylem Diameter
It is reported that continuous drought intensifies the imbalance between water transport and transpiration (through stomata and cuticles). This imbalance develops highly negative water potential and increases xylem tension, leading to bubble formation or cavitation of the vessel elements. Cavitation interrupts the flow through the xylem elements and may reduce the stomatal conductance, rate of photosynthesis, and, consequently, growth (Tyree and Sperry 1989). Under moisture stress conditions, this is the first symptom that directly affects the hydraulic system. To avoid this problem, the major adaptive root plasticity in the root system is making the hydraulic system more resistant and preventing cavitation (Kadam et al. 2015). Studies have demonstrated that the adaptive plasticity of xylem elements is the key to improve water use efficiency. The efficiency of the xylem hydraulic conductance shows direct relation to drought resistance and sustained yield. Reduced metaxylem diameter is very common in plants under water stress, and reductions in diameter of the metaxylem elements result in greater resistance to water flow (Passioura 1982; Melo et al. 2007). The tolerant sugarcane genotype RB867515 showed reduced vessel diameter under drought conditions (da Cruz Maciel et al. 2015). Several studies have also reported early stomatal closure as an adaptive mechanism that prevents xylem cavitation (Tardieu and Davies 1993; Plaut et al. 2012). Two major anatomical root traits have been reported to increase hydraulic root resistance: reduced xylem diameter and increased xylem number (Richards and Passioura 1981; Plaut et al. 2012).
6.9 Increased Exodermal Layer
Exodermis is the unicellular cell layer below the outermost epidermal layer in roots. Both epidermis and exodermis serve as apoplasmic barriers to transport water and ions to the inner vascular cylinder (Enstone et al. 2002; Enstone and Peterson 2005). The increased exodermal layer acts as a barrier for oxygen and water movement (Colmer 2003). On the other hand, a thin exodermis allows free radical oxygen and water movement. The rhizosphere, with better-aerated conditions, protects the roots against phytotoxins (Armstrong et al. 2000; Soukup et al. 2002). The low oxygen levels also stimulate ethylene synthesis, which inhibits root elongation. da Cruz Maciel et al. (2015) showed that roots of susceptible sugarcane genotypes had the highest number of exodermis.
6.10 Thin-Walled Exodermis
The deposition of suberin in the cell wall of the exodermis makes the layer thicker. The suberin layer acts as a barrier and prevents the radial loss of oxygen to the rhizosphere. In contrast, the barrier increases the longitudinal diffusion of oxygen in the aerenchyma (Soukup et al. 2002). Similar to the condition in increased exodermal layer, a thick-walled exodermis reduces the aeration in roots. It is also shown that the suberized exodermis reduces the flow of water and minerals from epidermis to cortex and the vascular cylinder (Prado 2005). A drought-tolerant sugarcane genotype RB867515 with thin-walled exodermis has been shown to facilitate water movement and maintain productivity under reduced moisture (Prado 2005; Ferreira et al. 2007; da Cruz Maciel et al. 2015).
6.11 Reduced Cortical Layer
The cell layer forms the cortex in between the exodermis and the stele. The reduced cortical layer is an adaptive trait in roots under drought conditions. It has been shown in several crops that reduced cortical cell layer reduces the metabolic costs of root growth and maintenance. Reduction in the cortical layer reduces the root volume, which has more metabolic demand than the stele region (Lynch 2013; Chimungu et al. 2014a, b). Reduced root volume decreases the metabolic demand under resource-limiting conditions. The drought tolerance is improved by reducing the metabolism cost, enabling continuous root growth and deeper soil exploration. Deeper soil exploration gives better water acquisition from the deeper soil reserves for better yield under water stress (Chimungu et al. 2014a, b; da Cruz Maciel et al. 2015).
6.12 Cortical Lysigenous Aerenchyma
The phytohormone ethylene triggers the formation of lysigenous aerenchyma in plants subjected to abiotic stress conditions (Bouranis et al. 2007). Aerenchyma develops intercellular spaces in the cortical layer. The reduced cortical layer filled with aerenchyma is found to be an adaptive character under drought as well as waterlogging conditions. The presence of aerenchyma is reported to have two important roles under drought conditions such as (1) it prevents the sudden shrinking of cortical cells due to the change in hydric potential and (2) the air spaces in the aerenchyma layer help in avoiding excess loss of water from the compact cortical layer (Melo et al. 2007). Aerenchyma cells facilitate better O2 diffusion, which helps to maintain aerobic respiration and cellular metabolism in roots (Vasellati et al. 2001; Bouranis et al. 2007; Melo et al. 2007). The presence of aerenchyma in the roots of sugarcane genotypes tolerant to drought has been reported (da Cruz Maciel et al. 2015).
6.13 Endodermis with U-Thickening
The endodermis is the outermost safety layer surrounding the stele and functions as an apoplasmic layer in regulating the movement of water, ions, and hormones into and out of the vascular system. In sugarcane under drought conditions, the anticlinal and inner periclinal walls of endodermal layers were found to be thickened (da Cruz Maciel et al. 2015). This is called U-thickening, which is more in the tolerant genotype than the susceptible sugarcane genotypes (da Cruz Maciel et al. 2015; Valarmathi unpublished data). Endodermal thickening is reported to play an important role in the conduction of water and photosynthates under both salinity and drought stress conditions. The thickening of endodermal cells helps to protect the vascular cylinder from damage due to hydraulic resistance and also prevents excess water loss from the stele region. Increased lignification of root endodermal cell wall is found to be one of the major salinity tolerance strategies in the roots of halophytes (Barzegargolchini et al. 2017).
6.14 Sclerification of Pericycle
As already mentioned, the pericycle is the meristematic layer, which is the source of lateral roots and surrounds the vascular bundle or stele (Richards and Passioura 1981). A common feature of the roots of monocots is the sclerification of the pericycle under drought and salinity stress (da Cruz Maciel et al. 2015). The sclerification of the pericycle helps to protect the vascular cylinder and increases the hydraulic resistance, while it reduces the morphogenic ability of this layer to form lateral roots (Ferri et al. 2000; Raven et al. 2008). In sugarcane, sclerified pericycle is reported intolerant genotypes under drought conditions (da Cruz Maciel et al. 2015). Sclerification of pericycle may prevent the cellular damage during stress, once the cessation of stress if the pericycle is intact, new roots will arise.
6.15 Conclusion
Sugarcane is an economically important crop for sugar and bioenergy production. Abiotic stress factors such as drought, salinity, high temperature, and waterlogging impact sugarcane productivity. Drought and salinity stresses are considered as one of the most deleterious stresses affecting sugarcane yield losses. Developing a tolerant genotype is essential to sustain sugarcane production under extreme environmental conditions. Studying the physiological, anatomical, and molecular changes during stress is essential to develop a tolerant genotype with a holistic approach. Very limited studies have been carried out to understand the anatomical tolerance mechanisms in sugarcane. However, the details given in this chapter show that anatomical feature of sugarcane leaf and root responds to stress conditions, and they also help in imparting tolerance to sugarcane crops. These traits can be used as a marker trait to identify the most stress-resistant genotype.
References
Armstrong W, Cousins D, Armstrong J, Turner D, Beckett P (2000) Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot 86(3):687–703
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63(10):3523–3543
Baranova MA (1987) Historical development of the present classification of morphological types of stomates. The Botn Rev 53(1):53–79
Bartlett MK, Klein T, Jansen S, Choat B, Sack L (2016) The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc Nat Acad Sci 113(46):13098–13103
Barzegargolchini B, Movafeghi A, Dehestani A, Mehrabanjoubani P (2017) Increased cell wall thickness of endodermis and protoxylem in Aeluropus littoralis roots under salinity: the role of LAC4 and PER64 genes. J Plant Physiol 218:127–134
Bertolino LT, Caine RS, Gray JE (2019) Impact of stomatal density and morphology on water-use efficiency in a changing world. Front Plant Sci 10:225
Blum A (2005) Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56(11):1159–1168
Bosabalidis AM, Kofidis G (2002) Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci 163(2):375–379
Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ (2007) Lysigenous aerenchyma development in roots–triggers and cross-talks for a cell elimination program. Int J Plant Developl Biol 1(1):127–140
Brindha C, Vasantha S, Arunkumar R (2019) The response of sugarcane genotypes subjected to salinity stress at different growth phases. J Plant Stress Physiol 5:28–33
Chandler JW, Bartels D (2008) Drought: avoidance and adaptation. In: Encyclopedia of water science, 2nd edn. CRC Press, Boca Raton, pp 222–224. ISBN 978-0-8493-9627-4
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560
Chimungu JG, Brown KM, Lynch JP (2014a) Large root cortical cell size improves drought tolerance in maize. Plant Physiol 166(4):2166–2178
Chimungu JG, Brown KM, Lynch JP (2014b) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol 166(4):1943–1955
Chimungu JG, Loades KW, Lynch JP (2015) Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea mays). J Exp Bot 66(11):3151–3162
Colmer T (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26(1):17–36
da Cruz Maciel JR, de Oliveira D, Fadin DA, das Graças Sajo M, Pedroso-de-Moraes C (2015) Morpho-anatomical characteristics conferring drought tolerance in roots of sugar cane genotypes (Saccharum L., Poaceae). Braz J Bot 38(4):951–960
Drake PL, Froend RH, Franks PJ (2013) Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 64(2):495–505
Enstone DE, Peterson CA (2005) Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant Cell Environ 28(4):444–455
Enstone DE, Peterson CA, Ma F (2002) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21(4):335–351
Ferreira E, Ventrella M, Santos J, Barbosa M, Silva A, Procópio S et al (2007) Leaf blade quantitative anatomy of sugarcane cultivars and clones. Planta Daninha 25:25–34
Ferreira TH, Tsunada MS, Bassi D, Araújo P, Mattiello L, Guidelli GV et al (2017) Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front Plant Sci 8:1077
Ferri A, Lluch C, Ocana A (2000) Effect of salt stress on carbon metabolism and bacteroid respiration in root nodules of common bean (Phaseolus vulgaris L.). Plant Biol 2(04):396–402
Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann Bot 89(2):183–189
Franks PJ, Beerling DJ (2009) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proce Nat Acad Sci 106(25):10343–10347
Glover J (1967) The simultaneous growth of sugarcane roots and tops in relation to soil and climate. In: Proc 41st Annu Congr S Afr Sugar Technol Ass, p 143–158
Grantz D, Moore P, Zeiger E (1987) Stomatal responses to light and humidity in sugarcane: prediction of daily time courses and identification of potential selection criteria. Plant Cell Environ 10(3):197–204
Gregory PJ (2006) Roots, rhizosphere and soil: the route to a better understanding of soil science? Eur J Soil Sci 57(1):2–12
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424(6951):901–908
Hölttä T, Mencuccini M, Nikinmaa E (2009) Linking phloem function to structure: analysis with a coupled xylem–phloem transport model. J Theor Biol 259(2):325–337
Kadam NN, Yin X, Bindraban PS, Struik PC, Jagadish KS (2015) Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water deficit stress than rice? Plant Physiol 167(4):1389–1401
Kardiman R, Ræbild A (2018) Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol 38(5):696–705
Khonghintaisong J, Songsri P, Toomsan B, Jongrungklang N (2018) Rooting and physiological trait responses to early drought stress of sugarcane cultivars. Sugar Tech 20(4):396–406
Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203(1):44–62
Kumar GH, Babuvishwanath H, Purohit R, Sahu P, Rana R (2017) Investigations on mechanical properties of glass and sugarcane fiber polymer matrix composites. Mater Today Proc 4(4):5408–5420
Lakshmanan P, Robinson N (2013) Stress physiology: abiotic stresses. Physiology, biochemistry, and functional biology, Sugarcane, pp 411–434
Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164(4):1556–1570
Lawson T, Vialet-Chabrand S (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytol 221(1):93–98
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112(2):347–357
Malik K (1986) Some anatomical characteristics of sugarcane varieties in relation to drought resistance. Agric Res 11(1):43–49
Matsuda K, Rayan A (1990) In: Katterman F (ed) Anatomy: a key factor regulating plant tissue response to water stress. Environmental injury to plants. Academic Press, San Diego, pp 63–88
McAdam SA, Brodribb TJ (2016) Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiol 171(3):2008–2016
McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol 211(4):1209–1220
McDowell NG, Sevanto S (2010) The mechanisms of carbon starvation: how, when, or does it even occur at all? New Phytol 186(2):264–266
Melo HC, Castro EM, Soares ÂM, Melo LA, Alves JD (2007) Alterações anatômicas e fisiológicas em Setaria anceps Stapf ex Massey e Paspalum paniculatum L. sob condições de déficit hídrico. Hoehnea 34:145–153
Meneses Rodriguez S (1985) Diagnosis of drought tolerance in sugarcane cultivars by the determination of electrolyte leakout [Cuba]. Revista ATAC (Cuba)
Misra V, Solomon S, Mall A, Prajapati C, Hashem A et al (2020) Morphological assessment of water stressed sugarcane: a comparison of waterlogged and drought affected crop. Saudi J Biol Sci 27(5):1228–1236
Mo JR, Zhou CS (1984) Physiological bases for sugarcane cultivation and breeding. Fujian, Fuzhou, China, pp 329–334
Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14(12):3089–3099
Naik GR (2001) Sugarcane biotechnology. Science Publishers
Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012) Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Nat Acad Sci 109(25):10101–10106
Nawazish S, Hameed M, Naurin S (2006) Leaf anatomical adaptations of Cenchrus ciliaris L. from the Salt Range, Pakistan against drought stress. Pak J Bot 38(5):1723–1730
Ogbaga CC, Amir M, Bano H, Chater CC, Jellason NP (2020) Clarity on frequently asked questions about drought measurements in plant physiology. Sci African 8:e00405
Pan G, Gao M, Hu G, Wei Q, Yang X, Zhang W et al (2011) Impacts of climate change on agricultural production of China. J Agro-Environ Sci 30(9):1698–1706
Passioura J (1982) Water in the soil-plant-atmosphere continuum. In: Physiological plant ecology II. Springer, pp 5–33
Plaut JA, Yepez EA, Hill J, Pangle R, Sperry JS, Pockman WT et al (2012) Hydraulic limits preceding mortality in a piñon–juniper woodland under experimental drought. Plant Cell Environ 35(9):1601–1617
Prado HD (2005) Ambientes de produção de cana-de-açúcar na região Centro-Sul do Brasil. Informações Agronômicas 110:12–17
Queiroz-Voltan RB, Paradela FO, Carelli MLC, Fahl JI (1998) Aspectos estruturais de cafeeiro infectado com Xylella fastidiosa. Bragantia 57:23–33
Ramesh P (2000) Effect of different levels of drought during the formative phase on growth parameters and its relationship with dry matter accumulation in sugarcane. J Agron Crop Sci 185(2):83–89
Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH et al (2008) Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol 506(5):745–758
Richards RA, Passioura JB (1981) Seminal root morphology and water use of wheat I. Environmental effects. Crop Sci 21(2):249–252
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Ann Rev Plant Biol 52(1):627–658
Shrivastava A, Srivastava S (2016) Diversity of the germplasm of Saccharum species and related genera available for use in directed breeding programmes for sugarcane improvement. Curr Sci:475–482
Sieburth LE, Lee DK (2010) BYPASS1: how a tiny mutant tells a big story about root-to-shoot signaling. J Integr Plant Biol 52(1):77–85
Silva MA, Soares RAB, Landell MGA, Campana MP (2008) Agronomic performance of sugarcane families in response to water stress. Bragantia 67(3):655–661
Sinclair T, Gilbert R, Perdomo R, Shine J Jr, Powell G, Montes G (2004) Sugarcane leaf area development under field conditions in Florida, USA. Field Crops Res 88(2–3):171–178
Smith JP, Lawn RJ, Nable RO (1999) Investigations into the root:shoot relationship of sugarcane, and some implications for crop productivity in the presence of sub-optimal soil conditions. Proc Aust Soc Sugar Cane Technol 21:108–113
Smith D, Inman-Bamber N, Thorburn P (2005) Growth and function of the sugarcane root system. Field Crops Res 92(2–3):169–183
Soukup A, Votrubová O, Čížková H (2002) Development of anatomical structure of roots of Phragmites australis. New Phytol 153(2):277–287
Tammisola J (2010) Towards much more efficient biofuel crops-can sugarcane pave the way? GM Crops 1(4):181–198
Tan Y (1988) Study of membrane fatty acids and permeability in sugarcane leaves in relation to drought resistance. J Fujian Agril College China 17(3):211–215
Taratima W, Ritmaha T, Jongrungklang N, Raso S, Maneerattanarungroj P (2019) Leaf anatomical responses to drought stress condition in hybrid sugarcane leaf (Saccharum Officinarum ‘KK3’). Malaysian App Biol 48(3):181–188
Taratima W, Ritmaha T, Jongrungklang N, Maneerattanarungroj P, Kunpratum N (2020) Effect of stress on the leaf anatomy of sugarcane cultivars with different drought tolerance (Saccharum officinarum, Poaceae). Rev Biol Trop 68(4):1159–1170
Tardieu F (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63(1):25–31
Tardieu F, Davies WJ (1993) Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ 16(4):341–349
Tardieu F, Bruckler L, Lafolie F (1992) Root clumping may affect the root water potential and the resistance to soil-root water transport. Plant Soil 140:291–301
Terashima I, Miyazawa S, Hanba YT (2001) Why are sun leaves thicker than shade leaves? – consideration based on analyses of CO, diffusion in the leaf. J Plant Res 114:93–105
Tombesi S, Nardini A, Frioni T, Soccolini M, Zadra C, Farinelli D et al (2015) Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci Rep 5(1):1–12
Tyree MT, Sperry JS (1989) Vulnerability of xylem to cavitation and embolism. Ann Rev Plant Biol 40(1):19–36
Valarmathi R, Mahadevaswamy H, Preethi K, Narayan JA, Appunu C, Rahman H (2020) Characterization and in silico analysis of RTCS gene from sugarcane encoding LOB Protein family of transcription factors: a key regulator of shoot borne root initiation. J Sugarcane Res 10(1):12–23
Vasellati V, Oesterheld M, Medan D, Loreti J (2001) Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann Bot 88(3):355–360
Vásquez M (2003) Anatomy and morphology of monocotyledonous and dicotyledonous roots. In: Banana root system: towards a better understanding for its productive management. Proceedings of an International Symposium held in San José, Costa Rica on 3–5 November 2003. International Network for the Improvement of Banana and Plantain (INIBAP). p 37–42
Verma KK, Singh RK, Song QQ, Singh P, Zhang B-Q, Song X-P, Chen G-L, Li YR (2019a) Silicon alleviates drought stress of sugarcane plants by improving antioxidant responses. Biomed J Sci Tech Res 17:002957. https://doi.org/10.26717/BJSTR.2019.17.002957
Verma KK, Wu K-C, Singh P, Malviya MK, Singh RK, Song X-P, Li YR (2019b) The protective role of silicon in sugarcane under water stress: photosynthesis and antioxidant enzymes. Biomed J Sci Tech Res 15:002685. https://doi.org/10.26717/BJSTR.2019.15.002685
Verma KK, Song X-P, Zeng Y, Li D-M, Guo D-J, Rajput VD et al (2020) Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 5(37):24145–24153
Verma KK, Anas M, Chen Z, Rajput VD, Malviya MK, Verma CL, Singh RK, Singh P, Song XP, Li YR (2020a) Silicon supply improves leaf gas exchange, antioxidant defense system and growth in sugarcane responsive to water limitation. Plan Theory 9:1032. https://doi.org/10.3390/plants9081032
Verma KK, Singh P, Song X-P, Malviya MK, Singh RK, Chen G-L, Solomon S, Li YR (2020b) Mitigating climate change for sugarcane improvement: role of silicon in alleviating abiotic stresses. Sugar Tech 22(5):741–749
Verma KK, Song XP, Tian DD, Guo DJ, Chen ZL, Zhong CS, Nikpay A, Singh M, Rajput VD, Singh RK, Minkina T, Li YR (2021a) Influence of silicon on biocontrol strategies to manage biotic stress for crop protection, performance and improvement. Plan Theory 10:2163. https://doi.org/10.3390/plants10102163
Verma KK, Song XP, Tian DD, Singh M, Verma CL, Rajput VD, Singh RK, Sharma A, Singh P, Malviya MK, Li YR (2021b) Investigation of defensive role of silicon during drought stress induced by irrigation capacity in sugarcane: physiological and biochemical characteristics. ACS Omega 6:19811–19821
Verma KK, Song XP, Verma CL, Chen ZL, Rajput VD, Wu KC, Liao F, Chen GL, Li YR (2021c) Functional relationship between photosynthetic leaf gas exchange in response to silicon application and water stress mitigation in sugarcane. Biol Res 54:15. https://doi.org/10.1186/s40659-021-00338-2
Verma KK, Song XP, Zeng Y, Guo DJ, Sing M, Rajput VD, Malviya MK, Wei KJ, Sharma A, Li DP, Chen GL, Li YR (2021d) Foliar application of silicon boosts growth, photosynthetic leaf gas exchange, antioxidative response and resistance to limited water irrigation in sugarcane (Saccharum officinarum L.). Plant Physiol Biochem 166:582–592
Wang L, Zhang T, Ding S (2006) Effect of drought and rewatering on photosynthetic physioecological characteristics of soybean. Acta Ecol Sinica 26(7):2073–2078
Wang W-B, Kim Y-H, Lee H-S, Kim K-Y, Deng X-P, Kwak S-S (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47(7):570–577
Wu L-L, Liu Z-L, Wang J-M, Zhou C-Y, Chen K-M (2011) Morphological, anatomical, and physiological characteristics involved in development of the large culm trait in rice. Aust J Crop Sci 5(11):1356–1363
Xu Y (1986) Sugarcane callus physiological responses to water stress and drought resistance. Thesis, Guangxi Agricultural College, Guangxi University, Nanning, Guangxi, China
Yardanov I, Velikova V, Tsonev T (2003) Plant responses to drought and stress tolerance. Bulg J Plant Physiol:187–206
Zhang L, Brook JR, Vet R (2003) A revised parameterization for gaseous dry deposition in air-quality models. Atmos Chem Phys 3(6):2067–2082
Zhang Q, Wang M, Hu J, Wang W, Fu X, Liu J-H (2015) PtrABF of Poncirus trifoliata functions in dehydration tolerance by reducing stomatal density and maintaining reactive oxygen species homeostasis. J Exp Bot 66(19):5911–5927
Zhu L, Xing Y, Yang L, Li Y, Yang R, Mo L (2010) Effects of water stress on leaf water and chlorophyll fluorescence parameters of sugarcane seedling. Agric Sci Technol-Hunan 11(5):17–21
Zingaretti SM, Rodrigues FA, Graça J, Pereira L, Lourenço MV (2012) Sugarcane responses at water deficit conditions. In: Mofizur IM, Hasegawa RH (eds) Water stress. IntechOpen, pp 255–276. https://doi.org/10.5772/30986
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Valarmathi, R., Mahadeva Swamy, H.K., Appunu, C., Kambale, R., Sudhagar, R. (2022). Anatomy of Tolerance Mechanisms in Sugarcane Crop to Abiotic Stresses. In: Verma, K.K., Song, XP., Rajput, V.D., Solomon, S., Li, YR., Rao, G.P. (eds) Agro-industrial Perspectives on Sugarcane Production under Environmental Stress. Springer, Singapore. https://doi.org/10.1007/978-981-19-3955-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-3955-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3954-9
Online ISBN: 978-981-19-3955-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)