Abstract
Thermal barrier coatings (TBCs) are particularly designed to detain the degradation of material as a result of high heat flux. Thermal barrier coating is a process in which fine particles of a material in non-metal or metal form are deposited on the surface of a substrate in semi-molten or molten state. During service, degradation in the form of thermal or mechanical performance of a component can happen. Therefore, it is very important to understand the material requirements and failures of TBCs. In this paper, the properties of material to be used in TBC and the failure mechanisms caused by hot corrosion, residual stress, sintering reaction, calcium-magnesium-aluminosilicate attack and interdiffusion and oxidation are discussed. This review correlates the properties and structure of TBCs and reasons of failures which would be beneficial for designing TBC for future applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Thermal barrier coatings
- Thermal and mechanical properties of thermal barrier coatings
- Hot corrosion
- Oxidation
- Sintering
- Residual stress
1 Introduction
By decelerating the extent and rate of deprivation experienced by the component, operating efficiency of a power producing unit or gas turbine may be improved. Failure of a component can be avoided by the use of claddings or protective coatings that are resistant to the high-temperature deprivation [1]. Nevertheless, it is not recommended to use claddings because it can change the microstructure of the basic substrate that can expose it to the other means of failure [2]. For this reason, thermal spray coatings are broadly used, and thermal barrier coatings (TBCs) are particularly designed to detain the degradation of material as a result of high heat flux [3]. Many researchers have suggested to use TBCs for protection of gas turbines from various failures [4,5,6]. Thermal spray coating is the most adaptable process that can be useful for apparatuses of any dimension. In contrast to the laser cladding process and welding, this coating technique does not change the microstructure of substrate.
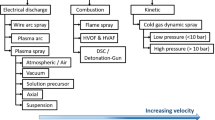
Thermal spray is the general name for a coating process, in which a material is melted by using hot gaseous medium and at the same time deposited onto a substrate with high velocity where it deposits to form required coating. The material to be coated can be deposited in various states including wire, powders, rods, suspension or molten form. Torch is used to convert particles depending on the energy supplied into a hot gas stream. Fuel and oxygen recommended by manufacturer of spray gun are mixed in the mixing zone of gun and then directed on the way by carrier gas to combustion chamber. Chemical reaction occurs during ignition in combustion chamber due to which heat energy is released. Pressure inside combustion chamber increases as the combustion continues and hot gases start to flow with the high velocity. The fuel used is acetylene, propylene, oxygen or propane, and as carrier gas, nitrogen or other inert gas is used. A chemical reaction between acetylene and oxygen gases and nitrogen as carrier gas is presented in Eq. 1.
Thermal spray coatings were first commercialized by Schoop who devised devices to melt zinc or tin and to project metal in molten form by using compressed air in 1910. After that in the mid of 1920s, thermal spraying started to be used in fifteen countries [7]. In 1947, the first paper on ceramic coatings for turbine blades was published by NACA. In 1948, frit coating was tested on turbine blades of an engine. Later on, in 50s and 60s, this coating was tested by air force. In 1960, thermal barrier coatings made from zirconia were used in nozzles of rocket engine. Sal Grisaffe conducted research on thermal spray and founded the first group of coatings in 1960. In the mid of 1970, modern thermal spray coating was developed which consisted of 12 YSZ top-coat and NiCrAlY bond coat in a research-based gas turbine engine [8, 9]. Until now, much of the work has been done on thermal barrier coatings but there is very less literature available, in which properties to be considered while selecting the materials, failures of coatings and methods to avoid these failures are discussed [10, 11].
In this review article, first section contains details about the history and working of thermal spray coatings. Second section deals with the requirements of a material to be used in thermal barrier coatings. In the third section, properties of top-coat, bond coat, substrate and thermally grown oxide layer are described that must be kept in mind while selecting the materials. In the last section, failures of TBC coatings and methods to measure and avoid these failures are discussed.
2 Material Requirements for TBCs
The factors for efficiency improvement of mechanical systems especially gas turbines are high operating efficiency, reduced emissions and extended operating life. The efficiency of a gas turbine depends on the temperature difference between inlet and outlet. For every increase of 56 °C in the turbine inlet temperature, there will be an increase of 1.5% efficiency and 10% work output [12]. In order to meet these situations, by increasing the entry temperature of turbine to enhance the thermodynamic efficiency which is very effective, there is a chance of extreme operating environment as a result of increase in operating temperature, that can damage the core parts [13]. In a modern gas turbine, the temperature of inlet may reach 1600 °C [14]. The temperature of components can be reduced to nearly 100–300 °C, by using the coatings which have low thermal conductivity on parts like blades. Many features like no phase alteration between working temperature and room temperature, excessive melting point, chemical inertness, low thermal conductivity, better adherence to substrate, thermal expansion coefficient match with substrate, lower sintering rate [15], capital, thickness, surface roughness requirement, performance and process cost per part must be kept in mind while selecting a TBC process [16].
3 Material Properties
3.1 Top-Coat
The main purpose of top-coat is to offer thermal insulation between the hot gases and the substrate. This can be achieved by using low thermal conductivity material [17]. Low thermal conductivity is achieved by pores inside the top coat material, but porosity must not be greater than 8% [13]. Its thickness ranges from 100 to 400 µm. Also, topcoat should have following properties:
-
It should be phase stable when exposed to high temperature, because phase transformation and volume change can occur which can damage the material [18].
-
It must be resistant to erosion attack and sintering when subjected to high temperature in the presence of oxidized environment [19].
-
It must have resistance to fracture and tolerance of strain throughout the process of thermal cycling.
-
It should be thermodynamically compatible with the material of TGO for better adhesion.
-
There should be match of coefficient of thermal expansion between top-coat and substrate in order to avoid strain during thermal cycling.
-
Its stiffness and elastic modulus should not increase when subjected to high temperature (greater than 1200 °C).
Figure 1 shows different alloys of substrate and coating materials. It can be seen that for Ni-based alloys, zirconia is the best material for top-coat because it has low thermal conductivity and its coefficient of thermal expansion is matching with the Ni alloys.
Thermal conductivity and coefficient of thermal expansion of materials used for top coat [17]
3.2 Bond Coat
Bond coats are applied directly to the substrate before top-coat to avoid oxidation at high temperature. This coat provides resistance for substrate against oxidation by means of a protective TGO layer which is developed on the bond coat and grows slowly when it gets oxidized at high temperature. Generally, bond coat is made of two basic types of alloys. The first one is platinum-modified Ni aluminide, and second one is MCrAlY. Yield strength of MCrAlY bond coat is higher than platinum aluminide bond. Generally, its thickness varies from 75 to 200 µm. A bond coat should have following properties:
-
It should have high creep strength to avoid failure due to compressive stress originated in the TGO. These stresses are developed in the TGO if the cooling rate is extremely high, and due to compressive stress, tensile stresses are produced which can cause spallation of top coat [20].
-
It should have moderate strength and ductility at both low and high temperatures. If the bond coat is weak and not much ductile, then it can be unstable and TGO buckling can be the cause of crack in the layer [21].
-
The oxidation property of bond coat should be such that it can produce and maintain the TGO layer normally (alpha-alumina).
-
Its chemical composition should be homogeneous [22].
A bond coat is also anticipated to lessen the interdiffusion with the substrate based on Ni superalloy [23].
3.3 Thermally Grown Oxide
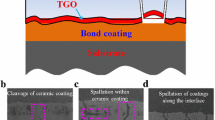
At temperature above 700 °C, the bond coat starts to get oxidized and a third layer called thermally grown oxide starts to develop between the topcoat and bond coat. The thickness of TGO is between 1 and 10 µm, and the maximum value can be 20 µm [24]. If the thickness is more than 5 µm, then it can introduce high magnitude stresses and can cause detachment of coating which is the reason of premature failure of coating. An ideal bond coat should have a TGO layer of α-Al2O3 because its oxygen ion diffusivity is very low, and it is an excellent barrier against diffusion which stops further oxidation of bond coat. It should have following properties:
-
For the top coat to be thermodynamic stable at high temperature for long time, the phase of TGO material must be compatible with it [17].
-
The rate of its growing should be slow and stable at high temperature. If the growing rate is fast, then thick layer will be formed which can cause spallation [17].
-
It should not allow oxygen to diffuse through it. Otherwise, oxygen will diffuse through this layer to the substrate material and oxidize it [22].
-
It must be dense and mechanically strong to have resistance against fracture at high cycle loads.
3.4 Substrate
It is the actual material of blade in gas turbine on which coating is deposited. As it is exposed to high temperature and mechanical load for long period, it should have high fatigue and creep strength, hot corrosion and oxidation resistance and ductility. On the other hand, its thermal conductivity should be high because higher thermal conductivity will help to dissipate heat at higher rate that is necessary to cool the blade [13]. Ni-based superalloys are currently being used along with 11 or 10 alloying elements as a substrate for applications where temperature is more than 800 °C [25]. Figure 2 is the schematic diagram of a TBC system which shows the structure and composition.
4 Types of Failures in TBCs
In case of gas turbines, the temperature can reach to 1500 °C. Typically, the material used for coating is made of YSZ which is stable up to 1200 °C. Above this temperature, coating starts to damage. Due to which, lifetime of a turbine is decreased at high temperature. There are many types of damages which can occur during service life of a turbine. These failures are listed below.
4.1 Hot Corrosion
Corrosion of superalloys or materials in the presence of oxidizing gas due to molten salt at high temperature of range 700–925 °C is called hot corrosion. The rate of oxidation is fast at this temperature when alloys and metals are contaminated with salts. As a result of which, protective oxide layer breaks down and salts reach the metal surface causing degradation [26]. Due to this degradation, bond coat and topcoat are both affected due to the porosity of APS method in YSZ coating. Through the pores and cracks, molten salt infiltrates the YSZ and reacts with bond coat [27]. It is different from corrosion at low temperature. If the hot corrosion occurs at temperature equal to melting point of salt layers, then it is hot corrosion of type-1 and the corrosion occurring at the temperature less than the melting point of salt layers is called type-2 hot corrosion. Corrosion in topcoat is because of the occurrence of lead (Pb) and vanadium in the fuel. Despite from the fuel or air, corrosion can also be produced after combustion due to the contaminants in the fuel that deposit on the surface.
4.2 Residuary Stress
These are produced during coating deposition process. There are two main reasons of residual stresses in thermal coating. First one is rapid cooling, in which the temperature of particles is reduced from higher to the temperature of substrate during deposition. The stress induced in this type is tensile in nature. The second reason for stress is the mismatch between coefficient of thermal expansion of substrate and coating. When the temperature of coating reaches from average temperature after deposition to the room temperature, the residual stresses are produced due to the difference in thermal shrinkage of coating and substrate. If coefficient of thermal expansion of substrate is greater than coating, then stresses produced are compressive and tensile stresses are produced for opposite case [28]. Compressive stresses are favorable to a certain limit than tensile stresses because tensile stress is more dangerous and can cause initiation of crack and adhesion loss. Besides this, there are other reasons for residual stresses like coating thickness and temperature, speed of deposition, phase transformation, thermally grown oxide and grain morphology. The various methods to examine these stresses include modeling, diffraction technique, spectroscopy technique, high energy X-ray, curvature measurement and finite element modeling [29].
4.3 Sintering Reaction
Sintering can be the cause of failure in case of thermal barrier coatings if the temperature of application is greater than 1000 °C. Microstructure of coating is altered due to sintering which includes change of properties such as thermal conductivity, hardness and elastic modulus. Sintering occurs at high temperature during service that results an increase in hardness and elastic modulus, and this is dangerous for coating system because it fills the pores of coating due to which thermal conductivity of top coat increases. Stiffness also increases due to sintering which increases thermal stress that leads to spallation of coating. This issue is common for YSZ coatings at temperature greater than 1000 °C. The problem of sintering can be controlled by using appropriate powder and time in a deposition technique as well as coating material and stabilizer [29]. Nanostructured coating is found to be suitable for this problem.
4.4 Calcium-Magnesium-Aluminosilicate Attack
In a gas turbine, when temperature reaches to higher value, coating on blades starts to be corroded because of silicates in molten form which is comprised of calcium-magnesium-aluminosilicate abbreviated as CMAS. During the service of a turbine, CMAS sticks on the topcoat of blade in solid form, and when the temperature reaches above the melting point (1200 °C) of CMAS, it starts to penetrate through the microstructure of topcoat in molten form. As a result, elastic modulus of ceramic top coat increases, which causes high stress. Crack is initiated and propagated in top coat due to mismatch in the thermal expansion coefficient between CMAS and ceramic top coat [29]. Many authors have investigated the relationship between stress and CMAS penetration depth. They found that the regions which are away from CMAS have low stress than the regions which are close to it. Also, as the depth of penetration increases, strain energy of the layer decreases and this results in degradation of the coating layer during thermal cycling [30]. The failure due to CMAS can be avoided by following ways:
-
(a)
to increase the surface area of top-coat by modifying pore geometry
-
(b)
to enhance the level of porosity
-
(c)
to select new and appropriate materials for the top-coat
-
(d)
to block the CMAS penetration by using dense layer upon the top-coat surface.
4.5 Interdiffusion and Oxidation
During the thermal cycle fatigue period at high temperature, the bond coat starts to get oxidized and thermally grown oxide layer begins to develop at the interface of bond coat and topcoat. If this situation continues for long time, then oxide layer thickness will increase due to which stresses will develop. There are two reasons for these stresses; first one is mismatch of the coefficient of thermal expansion between TGO and the coating layer, and second reason is the conversion of metal from high density to oxide of low density that generates growth stress. Residual stresses in TGO are the sum of stress produced due to mismatch of CTE and stress due to TGO growth. There is a limit of thickness for oxide layer growth which is 5 µm. When the value of this thickness is greater than this value, stresses of high magnitude will be introduced which will detach the top coat along with oxide layer from bond coat and life of coating will end [31]. There is also a chance of chemical failure due to low thickness of TGO. This failure is due to the low aluminum amount in bond coat. When the amount of aluminum is low, the protective oxide layer which is made of α-alumina develops no longer and other alloying elements will start to be oxidized. In the absence of α-alumina oxide layer, oxygen may diffuse into the bond coat which is known as internal oxidation. Due to this diffusion, the mismatch of coefficient of thermal expansion between bond coat and top coat made of YSZ is reduced, and hence, thermal stresses are also reduced [32].
5 Conclusion
In this review, a brief history of thermal barrier coatings and working of thermal spray process is discussed. The main points are as follows:
-
While selecting a TBC process, many features like no phase alteration between working temperature and room temperature, excessive melting point, chemical inertness, low thermal conductivity of top-coat, better adherence to substrate, thermal expansion coefficient match with substrate, lower sintering rate, thickness, surface roughness requirement, performance and process cost per part must be kept in mind.
-
The failures associated with TBC are hot corrosion, sintering reaction, oxidation and interdiffusion, residual stress and CMAS attack.
-
The problem of sintering can be controlled by using appropriate powder and time in a deposition technique as well as coating material and stabilizer.
-
By selecting new and appropriate materials for the topcoat and using dense layer upon the topcoat surface, CMAS attack can be controlled.
-
There should be match between coefficient of thermal expansion of TGO and the coating layer to avoid interdiffusion and oxidation.
References
Wang L, Zhao Y, Zhong X et al (2014) Influence of “island-like” oxides in the bond-coat on the stress and failure patterns of the thermal-barrier coatings fabricated by atmospheric plasma spraying during long-term high temperature oxidation. J Therm Spray Technol 23:431–446
Yakout M, Elbestawi MA, Veldhuis SC et al (2019) Density and mechanical properties in selective laser melting of Invar 36 and stainless steel 316L. J Mater Process Technol 266:397–420
Ziaei-Asl A, Ramezanlou MT et al (2019) Thermo-mechanical behavior of gas turbine blade equipped with cooling ducts and protective coating with different thicknesses. Int J Mech Sci 150:656–664
Wee S, Do J, Kim K et al (2020) Review on mechanical thermal properties of superalloys and thermal barrier coating used in gas turbines. Appl Sci 10:5476
Miller RA et al (2009) History of thermal barrier coatings for gas turbine engines. National Aeronautics and Space Administration
Reed RC et al (2008) The superalloys: fundamentals and applications. Cambridge University Press
Zhang G, Fan, Xu R et al (2018)Transient thermal stress due to the penetration of calcium-magnesium-alumino-silicate in EB-PVD thermal barrier coating system.Ceram Int44:12655–12663
Rani S, Agrawal AK, Rastogi V et al (2017) Failure analysis of a first stage IN738 gas turbine blade tip cracking in a thermal power plant. Case Stud Eng Fail Anal 8:1–10
Sun J, Fu Q-G, Yuan R-M et al (2017) Corrosion and thermal cycling behavior of plasma sprayed thermal barrier coatings on die steel. Mater Des 114:537–545
Leyens C, Wright IG, Pint BA et al (2000) Hot corrosion of an EB-PVD thermal-barrier coating system at 950°C. Oxid Met 54:401–424
Breeze P et al (2016) Chapter 4—Gas turbines. In: Gas turbine power generation, pp 31–42
Yakout M, Elbestawi MA, Veldhuis SC et al (2018) On the characterization of stainless steel 316L parts produced by selective laser melting. Int J Adv Manuf Technol 95:1953–1974
Bose S et al (2007) Chapter 5—High-temperature corrosion. In: High temperature coatings, pp 53–70
Cook L, Wolfenden A, Brindley W et al (1994) Temperature dependence of dynamic Young’s modulus and internal friction in LPPS NiCrAlY. J Mater Sci 29:5104–5108
Liu Q, Huang S, He A et al (2019) Composite ceramics thermal barrier coatings of yttria stabilized zirconia for aero-engines. J Mater Sci Technol 35:2814–2823
Pollock T, Lipkin D, Hemker K et al (2012) Multifunctional coating interlayers for thermal-barrier systems. MRS Bull 37:923–931
Feuerstein A, Knapp J, Taylor T et al (2008)Technical and economical aspects of current thermal barrier coating systems for gas turbine engines by thermal spray and EBPVD: a review.J Therm Spray Technol 17:199–213
Evans AG, Mumm D, Hutchinson J et al (2001) Mechanisms controlling the durability of thermal barrier coatings. Prog Mater Sci 46:505–553
Karlsson AM, Xu T, Evans A et al (2002) The effect of the thermal barrier coating on the displacement instability in thermal barrier systems. Acta Mater 50:1211–1218
Ghadami F, Aghdam ASR, Ghadami SJV et al (2020) Microstructural characteristics and oxidation behavior of the modified MCrAlX coatings: a critical review. Vacuum 109980
Mehboob G, Liu M-J, Xu T et al2020)A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime.Ceram Int 46:8497–8521
Habibi MH, Wang L, Liang J et al (2013) An investigation on hot corrosion behavior of YSZ-Ta2O5 in Na2SO4+V2O5 salt at 1100°C. Corros Sci 75:409–414
Thakare JG, Pandey C, Mahapatra M et al (2020) Thermal barrier coatings—a state of the art review. Met Mater Int
Tan JC et al (1997) Optimisation of the HVOF thermal spray process for coating, forming and repair of components. Dublin City University
Wu S, Zhao Y, Li W et al (2021) Research progresses on ceramic materials of thermal barrier coatings on gas turbine. Coatings 11:79
Clarke D, Levi C et al (2003) Materials design for the next generation thermal barrier coatings. Annu Rev Mater Res 33:383–417
Miller RA et al (1997) Thermal barrier coatings for aircraft engines: history and directions. J Therm Spray Technol 6:35–42
Chen Y et al (2015) Study of bond coats for thermal barrier coating applications
Rana N, Mahapatra MM, Jayaganthan R et al (2015) High-temperature oxidation and hot corrosion studies on NiCrAlY coatings deposited by flame-spray technique. J Therm Spray Technol 24:769–777
Soares C et al (2015) Chapter 3—Gas turbine configurations and heat cycles. In: Gas turbines, 2nd edn, pp 93–171
Ali MY, Nusier SQ, Newaz GM et al (2001) Mechanics of damage initiation and growth in a TBC/superalloy system. Int J Solids Struct 38:3329–3340
Arif AFM, Al-Athel KS, Mostaghimi J et al (2017) 3.4 Residual stresses in thermal spray coating, pp 56–70
Acknowledgements
The authors are highly obliged to Center of Corrosion Research (CCR), Advanced and Functional Materials (AFM) and Universiti Teknologi PETRONAS (Research Graduate Assistantship Scheme).
Funding
This research funding was supported by research fund: YUTP (015LC0-195) by Universiti Teknologi PETRONAS (UTP), 32610 Seri Iskandar, Perak Darul Ridzuan, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Raza, A., Ahmad, F., Badri, T.M., Raza, M.R., Malik, K., Ali, S. (2023). Selection of Materials Based on Thermo-Mechanical Properties of Thermal Barrier Coatings and Their Failures—A Review. In: Emamian, S.S., Awang, M., Razak, J.A., Masset, P.J. (eds) Advances in Material Science and Engineering. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-19-3307-3_22
Download citation
DOI: https://doi.org/10.1007/978-981-19-3307-3_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3306-6
Online ISBN: 978-981-19-3307-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)