Abstract
Sugar beet genome was first published in 2014. Before this, there were GMO cultivars of sugar beet commercially available. The possibility of genetic transformation, together with the complete characterization of the genome facilitates the use of molecular techniques as well as new breeding techniques for sugar beet improvement. Another important aspect is the fact that sugar beet is a recently domesticated crop, and there is a huge wild genetic diversity, which constitute a great genetic pool which can be used as a source of useful genes for introgressions or for designing new GMO crops. In this chapter, we will review all the knowledge available in GMO sugar beet, the recent advances and applications of biotechnology and novel breeding techniques in sugar beet improvement, and the use of sugar beet genes in other crops as well as the future prospects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
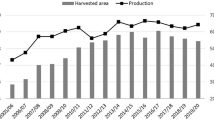
The world population is nowadays about 7.5 billion and it is increasing. Some models expect numbers of about nine billion in 2050. To feed this growing population, the agricultural yield must increase concomitantly. Sugar beet (Beta vulgaris L.) is one of the world’s main suppliers of calories in the diet, together with sugarcane. Worldwide 80% of sugar comes from sugarcane and 20% comes from sugar beet. In the 2019/20 period, the production was about 166.18 million tons and is expected to be about 182 million tons in 2020/21, and this produces about 35 million tons of sugar per year. Over 120 countries produce sugar. While sugarcane cultivation is concentrated in the tropics, sugar beet is cultivated in temperate climates mainly in the northern hemisphere in regions of Europe, North America, and some countries in Asia. The Russian Federation, France, the United States, Germany, and Turkey represent the main producers in 2018 (FAOSTAT 2018). Besides its pivotal role as a source of sugar, sugar beet cultivation has other important outcomes. Beta vulgaris is used as an energetic crop (Zabed et al. 2014), for animal feed (Evans and Messerschmidt 2017) and some varieties (i.e., Cicla) are cultivated as green-leaf vegetables, commonly known as Swiss chard.
Environmental stress and several pests greatly threat affect yield. Sugar beet is grown in temperate areas of the northern hemisphere where the crop is usually sown in early spring. This allows improving the root production and avoids summer drought and excessive heat at the plant maturity. In warmer climates or subtropical climates like southeast Asia, it is also possible to sow sugar beet in autumn (autumn sowing) or in other seasons, in order to anticipate harvest and escape drought and other environmental stresses. Sugar beet is also prone to pest of several kinds. Its cultivation is affected by weeds like the Redroot Pigweed (Amaranthus retroflexus), virus as beet necrotic yellow vein virus (BNYVV), bacteria such as Pseudomonas aptata, fungal disease as Cercospora, nematodes as Ditylenchus dipsaci, or insects like Aphis fabae; therefore, there is a great need to breed for more resistant Beta vulgaris cultivars.
There are pivotal achievements that have been obtained by classical breeding. Sugar beet is a recently domesticated plant created for the production of sugar (sucrose) from the beet used for animal feed in the 1800s. Classical breeding raised its sugar content from about 4-6% to the current 18% in 200 years (Mcgrath et al. 2018). Classical breeding has also made great achievements in traits of agronomical interest like the discovery and fixation of the monogerm gene-seed character (Savitsky 1950) which avoids multiple seeds germinating from a single fruit (utricle). Another major breeding achievement was the cytoplasmic male sterility for commercial varieties, which enabled the hybrid seed production, a trait that depends on a mitochondrial locus (Kitazaki et al. 2015). Hybrids present heterosis in root yield, among other traits (Ćurčić et al. 2017; Schwegler et al. 2014). Another achievement was the semitropical beet germplasm by increasing heat and bolting resistance which enables the cultivation in huge areas of India and southeast Asia (Srivastava 1995).
Abiotic stress is also a main limiting factor for yield. Soil salinity is a great constraint for productivity in many areas of cultivation (i.e., the Mediterranean basin, Iran, India, Southeast Asia, or the western United States). On the other hand, the advantage is that sugar beet is phylogenetically related to halophytic wood plants, being the most important the sea beet (Beta vulgaris ssp. Maritima) (Munns and Tester 2008). Sugar beet can stand concentrations up to 250 mM of sodium chloride in soil, which induces a 50% reduction in yield. In other major crops like beans, a 60 mM concentration of NaCl produces a similar reduction (Taïbi et al. 2021). Also, the defense system against salt stress is quite effective, for instance, in response to 300 mM sodium chloride solutions, beets are reported to suppress the generation of reactive oxygen species by transcriptional regulation (Hossain et al. 2017). The problem faced by farmers is that sugar beet is sensitive to salt stress at the seedling stage, and therefore, getting early vigor for salt stress tolerance is a great objective (Khayamim et al. 2014). The genotype “EL56” (PI 663211) is able to germinate in soils in up to 150 mM sodium chloride (McGrath 2011). Drought is also a problem faced in many cultivation areas. There are at least three loci described by quantitative trait locus (QTL) analysis suggested to be important for drought tolerance, but so far, the advances in sugar beet tolerance to drought stress are very limited (Rajabi and Borchardt 2015).
Also, classical breeding has focused on introgression disease and stress tolerance into novel germplasm and genotypes to offer solutions to farmers (Doney 1995; Panella et al. 2015). Thanks to that there are cultivars available with resistance or tolerance to predominant seedling diseases such as Pythium, Aphanomyces, and Rhizoctonia. There are also some cultivars resistant to the main root disease Rhizomania “crazy root,” due to the viral pathogen BNYVV (beet necrotic yellow vein virus). The main source of disease resistance genes for classical breeding, again, is the sea beet although some resistance has been found in wild beets, in fact, four different resistant genes (Rz1-5) isolated from different sources have been introgressed into commercial cultivars (Pavli et al. 2011b) although there are some strains that have broken the resistance (Biancardi and Tamada 2016). There have been some advances in resistance to nematodes and insects by classical genetic means (Zhang et al. 2008b). Therefore yield, pest resistance, and abiotic stress tolerance are the main objectives for the biotechnological improvement of sugar beet.
2 Sugar Beet Transformation
As we have seen classical plant breeding has improved the effectiveness of sugar beet for yield, pest, and to a very minor extent abiotic stress tolerance. It becomes clear that there is a need for biotechnological improvement. The first genetically engineered plant is considered to be tobacco and was reported in 1983 (Bevan et al. 1983). Attempts to transform sugar beet started almost immediately. The first was not successful as plants prove to be difficult to regenerate from transformed calluses (Harpster et al. 1988; Krens et al. 1988; Jacq et al. 1993) or cells (Wozniak and Owens 1989; Kallerhoff et al. 1990). Although transgene was incorporated in the plant genome, in most of these early reports stable transformation and reproducibility were not confirmed (Gurel et al. 2008).
Lindsey and Gallois (1990) described the first successful regeneration of a transformed sugar beet. Authors co-cultivated shoot-base tissues with Rhizobium radiobacter (formerly Agrobacterium tumefaciens) strain LBA4404 transformed with the gene nptII which confers resistance to the antibiotic kanamycin and either the chloramphenicol acetyltransferase (cat) gene (antibiotic resistance) or the β-glucuronidase (gusA) gene (enables staining). Shortly after the first genetically modified organism (GMO) sugar beet expressing an agronomically important trait (Herbicide tolerance) was described by D’Halluin (D’Halluin et al. 1992). In this case, the authors used callus derived from several organs transformed with R. radiobacter. As a selectable marker, they used (bar) (herbicide and bialaphos resistance) or a mutated acetolactate synthase (ALS) gene (herbicide resistance). These protocols were lengthy (2 years to obtain shoots) and showed a great genotype dependency. In fact, several years later it was described that easiness to transformation is a heritable trait (Kagami et al. 2016). The first genotype-independent protocol was described by (Fry et al. 1991) using cotyledonary explants inoculated with R. radiobacter containing different genes for herbicide or antibiotic tolerance. In this report, they confirmed the presence of transgenes, their mendelian segregation, and their functionality and the phenotype conferred (tolerance to herbicide). But the specific protocol was not published. Since then, other protocols based on callus transformation have been described (Kagami et al. 2015). Transformation with other techniques has been described, specifically, particle bombardment (Saunders et al. 1992; Hashimoto and Shimamoto 1999); polyethylene glycol (PEG)-mediated transformation of protoplasts (Hall et al. 1996), petiole explants of haploid and diploid sugar beet genotypes using Rhizobium rhizogenes and sonication (Klimek-Chodacka and Baranski 2014). Most of these techniques took advantage of the advances in somatic embryogenesis (Zhang et al. 2008a) and regeneration (Ninković et al. 2010). Transformation techniques are so advanced that there are also several molecular tools available to the community, like root-specific promoters (Padmanaban et al. 2016), a promoter from Swiss chard that directs petioles and roots preferential expression (Yu et al. 2015) and a promoter that drives expression specific to nematode-feeding sites (Thurau et al. 2003). There are other tools to modulate gene expression such as the lox gene (Wang et al. 2003), and there is also a description of a set of transgenic plants and cell lines of sugar beet transformed with the maize transposable elements Spm/dSpm which allows gene-tagging (Kishchenko et al. 2010). Moreover, heterologous expression of the Arabidopsis thaliana GRF5 (AtGRF5) in sugar beet callus cells greatly increases transformation efficiency (Kong et al. 2020).
3 Plastid Transformation
Another point of interest has been transforming plastids into sugar beet. This technique has many advantages, among them, the fact that pollen does not have chloroplast, and therefore, gene flow is prevented. The first description dates back to 2009 although a previous communication can be found in the literature (De Marchis et al. 2007) and was achieved from petioles of the line Z025 by biolistic bombardment. Chloroplast was transformed with a vector that directs genes to the rrn16/rps12 intergenic region, employing the aadA and the GFP genes as markers (De Marchis et al. 2009). The technique has been improved but is not a routine technique yet (De Marchis and Bellucci 2021), and all the available literature comes from the same laboratory.
4 Transient Expression
Although the transient expression is not a routine technique in agronomy, it is very useful for investigation purposes, mostly related to basic science. There is a recent description of a transient expression in sugar beet using Rhizobium radiobacter (Moazami et al. 2018). One of the most common strategies for transient expression is agroinfiltration in leaves with bacterial cultures of Rhizobium transformed with the transgene. This is the election technique, for instance, for subcellular localization of a given protein using confocal microscopy (Locascio et al. 2019b). In our laboratory, we have transiently transformed sugar beet leaves using the published protocol for lettuce and tomato (Wroblewski et al. 2005) with positive results (our unpublished observations) (Fig. 4.1).
5 Heterologous Genes Transformed in Sugar Beet
We have just reviewed the different methods for sugar beet transformation. It becomes clear that inserting exogenous genes in the sugar beet genome poses no technical problem. Transgenic sugar beets have existed for almost three decades with a wide variety of traits with different objectives.
5.1 GMO Sugar Beet for Improvement of Biotic Stress
5.1.1 Herbicide Tolerance
Beta vulgaris culture is strongly affected by weeds (May and Wilson 2006). Standard control techniques require herbicide spraying at different periods, so controlling weeds in sugar beet cultivation is difficult, expensive and increases the carbon and the water footprint of the cultivation, this reducing sustainability (Klenk et al. 2012; Gerbens-Leenes and Hoekstra 2012).
As mentioned, the first stable transformed GMO sugar beet was resistant to herbicide (D’Halluin et al. 1992). There is only three commercial sugar beet approved and all contain genes conferring tolerance to herbicides. Liberty LinkTM sugar beet (name: T120-7; code: ACS-BVØØ1-3) by Bayer crop science contains the gene pat encoding the phosphinothricin N-acetyltransferase (PAT) enzyme from Streptomyces viridochromogenes (resistance to glufosinate/phosphinothricin) and the nptII from Escherichia coli Tn5 transposon which confers tolerance to neomycin and kanamycin antibiotics. This variety was authorized in the United States in 1998, in Canada in 2000, and in Japan in 2001 (James 2011). The Roundup Ready™ sugar beet by Monsanto (name: H7-1; code: KM-ØØØH71-4) contains a single transgene, the cp4 epsps (aroA:CP4) from Rhizobium radiobacter strain CP4 conferring tolerance to glyphosate. This variety was approved in many countries worldwide for food, specifically Australia, Canada, China, Colombia, European Union, Japan, Mexico, New Zealand, Philippines, Russia, Singapore, South Korea, Taiwan, and the United States, but for cultivation only Canada and the United States (since 2005) and Japan (since 2007). Finally, the InVigor™ sugar beet (name: GTSB77 (T9100152); code: SY-GTSB77-8), by Novartis and Monsanto which contains three different genes, the mentioned cp4 epsps (aroA:CP4), the glyphosate oxidase goxv247 from Ochrobactrum anthropi strain LBAA that confers tolerance to glyphosate and the marker gene uidA (GUS) gene from Escherichia coli. This was authorized in the United States in 1998, New Zealand, and Australia in 2002 and Japan in 2003. From an economic and environmental perspective, the use of these varieties (mostly the Roundup Ready) has saved millions of tons of herbicide. Usually, a glyphosate-resistant GMO sugar beet requires about 2 kg ha−1, while non-GMO cultivars require about 6 kg ha−1 or higher, depending on the amount of weeds (Märländer 2005).
In 2012, there was a partial ban on the use of GMO sugar beet in the United States motivated by the case of Center for Food Safety v. Vilsack (CFS v. Vilsack), set in the U.S. District Court for the Northern District of California (CA District Court). The point was the presence of species like Beta macrocarpa and Beta vulgaris ssp. maritima in areas of California where RR sugar beet was planted, raising concerns on the possibility of transfer of the genes conferring tolerance to herbicides (McGinnis et al. 2010). Finally, following the different scientific reports, the USDA deregulated RR Sugar beet (Khan 2014). GMO sugar beets account for about 95% of the sugar beet cultivated in the United States (United States Department of Agriculture 2019). Given that the US imports 30% of sugar and produces 70%, and that sugar beet production is mainly for the sugar industry, which means that 66.5% of the sugar consumed in the United States comes from glyphosate-resistant GMO sugar beet. Similar numbers apply in Canada (Dillen et al. 2013). There are no reports of any adverse effects on human or animal health related to the use of GMO sugar beet. Studies show that after 2 weeks of the herbicide application only trace amounts of glyphosate are detected in roots or shoots, and in the crystalline sugar glyphosate is below the levels of detection (Barker and Dayan 2019).
Although not commercially available, there are also reports of transgenic sugar beet resistance to phosphinothricin with the bar gene (D’Halluin et al. 1992; Mishutkina et al. 2010), to imidazolinone by transformation with the als gene from Arabidopsis thaliana (a mutated acetolactate synthase). This mutation confers tolerance to some commercial herbicides such as imazethapyr (Pursuit®, BASF) (Kishchenko et al. 2011). There are also reports of crop trials of novel sugar beet varieties with tolerance to a specific acetolactate synthase (ALS) inhibitor herbicide bred by KWS using standard breeding techniques and branded Conviso Smart, but these varieties have not been marketed yet, and there are no published reports of the results of the assays (https://www.fwi.co.uk/arable/herbicide-tolerant-sugar-beet-trialled-uk). The herbicide-resistant sugar beet has been a great advance for sugar beet farmers but is the only one available in the market. Even though, there is a lot of investigation being performed in other traits.
5.1.2 Insect Resistance
Transgenic insect-resistant plants are in the market for a long time with great success in crops such as maize, cotton, soy (Koch et al. 2015), or brassicas (Poveda et al. 2020). These varieties have been developed mainly by transformation with the Bacillus thuringiensis insecticidal crystal proteins (ICPs) encoded by the cry gene family. Sugar beet plants transformed with cry1Ab (Shimamoto and Domae 2000), proved to be very effective protection against lepidopterans (Jafari et al. 2008) and the Egyptian leafworm (Spodoptera littoralis) (Sedighi et al. 2011). Sugar beet has also been transformed with cry1C (Kimoto and Shimamoto 2001), or with cry1C and cry2A (Lytvyn et al. 2014). A joint transformation with Cry1Ab and Cry1C protected against cabbage armyworm (Mamestra brassicae) (Kimoto and Shimamoto 2001).
5.1.3 Nematode Resistance
Sugar beet is very sensitive to nematode attack, among them Heterodera schachtii, the sugar beet cyst nematode (SBCN) is the one causing more damage to farmers (Villarías Moradillo 1999). The standard treatment depends on the use of chemicals with nematicide activity, but most of them are being banned or have undergone a strict regulation in many countries due to their environmental toxicity. Other strategies rely on resistant cultivars obtain by breeding, crop rotations (Joersbo 2007), or trap crops (Lathouwers et al. 2005). Another problem is that nematode pests are recalcitrant, as eggs can stay latent for years, even in adverse environments or in the absence of the host crop.
There was some success using classical breed to introduce the Hs1pro−1 gene from Beta procumbens in commercial sugar beet cultivars (Jung 1998), but this causes a severe yield penalty, perhaps due to linked genes that correlated with decreased yield (Heller et al. 1996). Using genetic engineering is a way to override the problem with the linked gene (Ali et al. 2017). The Hs1pro−1 gene from Beta procumbens was transformed in sugar beet, and the observed resistance was higher than in resistant cultivars developed by conventional breeding. GMO sugar beet expressing the SpTI-1 gene (a Kunitz-type trypsin inhibitor) exhibited similar phenotypes (Cai et al. 2003).
5.1.4 Fungal Resistance
The most important fungal diseases in sugar beet are Cercospora leaf spot (Cercospora beticola), root and crown rot (Rhizoctonia solani) and powdery mildew (Erysiphe betae, syn. E. polygoni) (Villarías Moradillo 1999). So far traditional control is based on integrated approaches, using both culture techniques, chemicals, or resistant varieties. There is not any GMO variety in the market with resistance to any fungal disease, indicating that from the biotechnological point of view there is still a need for improvement. There are several strategies which target the increase of the systemic acquired resistance by increasing the salicylic acid production or the hypersensitive response (with elicitors like the ones encodes by the avr genes). There are also attempts of transforming sugar beet with different defense proteins. The expression of a chitinase gene from pumpkin was able to increase chitinase activity and in some cases even suppressed some symptoms of fungal infection, as this protein is able to degrade the fungus cell wall (Hashimoto and Shimamoto 2001). Following a similar strategy, the polygalacturonase-inhibiting protein 2 (PvPGIP2) of common bean (Phaseolus vulgaris) protects GMO sugar beet against Rhizoctonia solani and Alternaria alternata (Goudarzi et al. 2015). This protein inhibits fungal polygalacturonase and therefore slows downs the ability of the fungus to degrade the plant cell wall (Mohammadzadeh et al. 2012). A recent report describes that overexpressing the native enzyme of Beta vulgaris (BvPGIP2) in hairy roots confers resistance to Fusarium oxysporum (Li and Smigocki 2019). The transformation with the chloroplastic and the cytosolic superoxide dismutase from tomato also increased resistance to C. beticola (Tertivanidis et al. 2004). Even a gene from a different species of this fungus, the cercosporin toxin export (CFP) gene from Cercospora kikuchii, has been transformed into sugar beet, but to date, there are no reports of whether transformed plants exhibited any phenotype (Kuykendall and Upchurch 2004). Another strategy consisted of the transformation of sugar beet with the Mannitol-1-Phosphate Dehydrogenase (mtlD) from E. coli. This increased the resistance to Alternaria alternata, Botrytis cinerea and Cercospora beticola although it depended on the transgenic line and the conditions of the assay (Goudarzi et al. 2016).
5.1.5 Viral Resistance
There are GMO commercial crops in the market whose trait is viral resistance (Fuchs and Gonsalves 2007), but this is not the case for sugar beet yet. Sugar beet is prone to viral diseases that cause great yield losses like rhizomania, caused by beet necrotic yellow vein virus (BNYVV), or can be infected by viruses such as beet western yellows virus (BWYV), beet yellows virus (BYV), or beet curly top virus (BCTV). There are descriptions of viral-resistant varieties using classical means (Scholten and Lange 2000). Genetic engineering has been used either expressing transgenes or using double-stranded DNA (ds-DNA) or interference RNA (RNAi) to interfere with the viral infection and dissemination, and therefore gain resistance (Lennefors et al. 2006). The expression of viral coat protein proved to be effective against BNYVV as early as in 1990 (Kallerhoff et al. 1990) and in hairy roots cultures (Ehlers et al. 1991), in protoplasts from transformed suspension cells (Mannerlöf et al. 1996) or in protoplast derived from guard cells (Lathouwers et al. 1997). Expression of the hrpZ gene of Pseudomonas syringae pv. Phaseolicola also conferred rhizomania resistance (Pavli et al. 2011a). In some cases, the virus resistance does not correlate with the level of expression of the transgene (Kerr 2005).
Another method to induce resistance is to disrupt the viral cell-to-cell movement. The usual strategy is to disrupt the P42, P13, and P15, triple gene cluster from BNYVV (Lauber et al. 1998; Erhardt et al. 2000). This has been done by designing an RNAi with a three-intron hairpin construct carrying parts of the BNYVV replicase gene (Pavli 2010) or a dsRNA derived from the replicase gene. This construct was able to confer protection to the roots of transformed plants (Pavli et al. 2010). Constructions using a similar strategy, but against other sites of the virus induced stronger resistance (Zare et al. 2015). Gene silencing against rhizomania has proved to be effective even in field tests (Safar et al. 2021). There is also a report describing the use of a single-chain variable fragment (scFv) specific to a major coat protein of virus, p21, with a construct that targeted the antibody to different organelles. After mechanical infection with BNYVV, the cytoplasmic construct showed the greatest efficiency in preventing the infection (Jafarzade et al. 2019). A different strategy has been found against BCTV. This infection provokes the generation of defective DNAs (D-DNA) which is suspected to be a plant response to attenuate symptoms. Transgenic plants expressing synthetic D-DNA presented attenuated symptoms (Horn et al. 2011).
6 Abiotic Stress
6.1 Drought Tolerance
In the current context of anthropogenic global warming, and the concomitant change in the precipitation regime the global aridity is increasing and affecting many sugar beet producing areas. Sugar beet is more tolerant to abiotic stress than most major crops, but under suboptimal water concentrations yield decreases drastically (Rajabi et al. 2007). There is no drought-resistant phenotypes of sugar beet identified so the use of classical breeding is very limited (Ebmeyer et al. 2021). A strategy to generate drought tolerance in crops is the transformation with genes able to increase the cellular content of osmolytes or osmoprotectans (small hydrophilic molecules that plants and other organism accumulates under drought stress conditions in order to avoid turgor loss) although in some cases with a severe yield penalty that compromises its market value and the utility for farmers (Van Camp 2005). There are only two cultivars in the market whose trait conferred by the transgene is drought tolerance: the DroughtGard® maize (Wang et al. 2015) and very recently the soybean expressing the HB4 transcription factor from sunflower (Ribichich et al. 2020). GMO plants transformed with the Bacillus subtilis gene, SacB accumulated fructans to low levels (0.5% of dry weight) but plants performed better under drought stress (dry weight +25–35% than control plants) and with no yield penalty under control conditions (Pilon-Smits et al. 1999).
6.2 Salt Tolerance
There are very few reports on GMO sugar beet resistant to salt stress. The first was authored by Yang et al. (2005). They introduced they introduced the AtNHX1 gene (a sodium proton vacuolar exchanger from Arabidopsis thaliana) and reported salt tolerance. Sugar beet plants transformed with a paralogue of the same gene (AtNHX3) accumulate more soluble sugar and less salt in storage roots (Liu et al. 2008). There is also a report in which authors describe the transformation of sugar beet with AVP1, the vacuolar H+-pyrophosphatase of Arabidopsis thaliana (Wang et al. 2012). And also, the co-expression of the sodium proton vacuolar antiporter (ZxNHX) and the vacuolar proton pyrophosphatase (ZxVP1-1) from the xerophyte plant Zygophyllum xanthoxylum increased the osmoregulatory mechanism and diminished sodium toxicity, and also accumulated a higher content of sugars (Wu et al. 2015). And although not directly related to salt stress, overexpression of the γ-glutamylcysteine synthetase-glutathione synthetase (StGCS-GS) from Streptococcus thermophilus, and enzyme of the glutathione biosynthetic pathway increased the tolerance sugar beet to grow under high cadmium, copper, and zinc (alone or in combination) and also the ability to accumulate these heavy metals, providing a useful tool for bioremediation (Liu et al. 2015).
7 Development and Metabolism
7.1 Bolting Resistance
Bolting resistance is a prime objective for breeders as in some areas such as California and the Mediterranean sugar beet is a winter crop, but as they do not suffer freezing temperatures, bolting is a serious problem. This is also a problem in subtropical or warm areas of India and southeast Asia. Bolting depends on the expression of flowering genes, most of them regulated or part of the gibberellic acid biosynthetic (GA) pathway. The use of GA inhibitors like paclobutrazol is a standard strategy to avoid bolting (Sadeghi-Shoae et al. 2014). Therefore, there is a huge interest in modulating GA response and production in sugar beet to avoid bolting, as this will expand the growing season and permit plants to accumulate higher yields. There have been some studies coping with the modulation of bolting-related genes, and some important QTL have been identified (Pfeiffer et al. 2014). The first attempts to develop bolting resist sugar beet by biotechnology consisted of the transformation with the repressor of gibberellin biosynthesis from Arabidopsis thaliana gai or by or deactivation of gibberellic acid by heterologous expression of the scarlet runner bean (Phaseolus coccineus) gene GA2ox1 (Mutasa-Gottgens et al. 2009).
B. vulgaris genome holds a vernalization-responsive FLC homolog (BvFL1), but opposite to what happens in other plants, its role is not important in bolting regulation, as plants transformed with iRNA to knock down BvFL1 did not eliminate the requirement for vernalization of biennial beets and did not have a major effect on bolting time after vernalization. Overexpression of BvFL1 only had a minor effect delaying the bolting after vernalization for about 1 week. Genes BvFT1 and BvFT2 are targets of regulation of BvFL1, and their protein product forms a module of regulation. These genes have antagonistic roles in the control of flowering (Pin et al. 2010). When these modules are downregulated, the phenotype is a several weeks delay in bolting, indicating some kind of redundancy in the BvFL1 function (Vogt et al. 2014). But so far, genetic engineering has not succeeded in creating a bolting-resistant genotype.
7.2 High Sucrose Yield
As sugar is the main product from sugar beet, increasing the yield is an obvious objective. This has been sought for long. First attempts consisted of knocking down the homolog of the SNF1 gene, a negative regulator of sucrose biosynthesis (Monger et al. 1995) or a maize sucrose-phosphate synthase (SPS) gene (Hashimoto and Shimamoto 1999), but the desired sugar yield increase was not obtained. Elliott et al. (1996) hypothesized that increasing the levels of cytokinin, a hormone related to development and mass accumulation, may increase sugar yield. They expressed a bacterial cytokinin biosynthesis gene, the isopentenyl transferase (ipt) (Snyder et al. 1999; Ivic et al. 2001). There was an effective increase in cytokinin accumulation, but also an inhibition of the taproot development and a minor sucrose accumulation.
7.3 Fructan Production
Sugar beet can be a source of molecules of industrial interest. For instance, fructans a fructose polysaccharide with many industrial applications (Turk and Smeekens 1999; Sévenier et al. 2002). There is a great interest in finding a cheap source of fructans, which includes the possibility to produce them from GMO sugar beet. First attempts consisted of using a gene from the traditional source of fructans, the Jerusalem artichoke (Helianthus tuberosus). The 1-sst gene of this crop, encoding a l-sucrose:sucrose fructosyl transferase was able to convert native sugar beet fructose to low molecular weight fructans in tap root cells and to a minor extent in leaves (Sévenier et al. 1998). Similar results were obtained when sugar beet was transformed with the 1-sst orthologue of onion (Allium cepa) and an additional gene of the fructan biosynthetic pathway (6g-fft, a fructan:fructan 6G-fructosyl transferase) (Weyens et al. 2004). Pilon-Smits et al. (1999) also tried to produce fructans in sugar beet by expressing the sacB bacterial gene, but as mentioned in a previous section, with very low yield and the drought tolerance phenotype was more interesting. There is also a report from transgenic beet transformed with PpFT1 and PpFT2, (two homologous sucrose:fructan 6-fructosyltransferases from timothy (Phleum pratense)) to produce levan, a molecule of the fructan family. Levans were successfully produced in GMO sugar beet, but the polymerization was much shorter than the polymerization obtained when levan was obtained from microorganisms (Matsuhira et al. 2014).
Finally, red beet has been used as a biofactory to develop test vaccines against type-I diabetes (Santoni et al. 2019), and there are also some reports of GMO crops designed for basic science, like the gene silencing of CYP76AD1, that blocks the red pigmentation of beets and induces a yellow coloration due to the accumulation of a betaxanthin pigment (Hatlestad et al. 2012) also the maize Ac transposase was expressed in sugar beet to confirm its alternative splicing in an heterologous system (Lisson et al. 2010).
8 Sugar Beet as a Source of Genes for Biotechnological Applications
Sugar beet genome was completed in 2014 (Dohm et al. 2014). This facilitates the identification of sugar beet genes useful for biotechnological applications, which has been a field of research interest in the last decades with some remarkable results.
8.1 Biotic Stress
There are some sugar beet genotypes that are resistant to pests. It means that could be a source of genes to transfer the resistance to plants in which interbred is not possible. This strategy has been used with the genes for nematode resistance BvcZR3 and BvHs1pro-1 that have been transformed into canola (Brassica napus) (Zhong et al. 2019) and tobacco obtaining resistance to nematodes (Sönmez et al. 2014). There are some sugar beet genes that have been transferred to other plants in order to get insect resistance. The serine proteinase inhibitor gene (BvSTI) has been expressed in Nicotiana benthamiana (Smigocki et al. 2008). In another report, Nicotiana transgenic plants overexpressing this gene were bioassayed against five lepidopteran pests with disparate effectiveness (Smigocki et al. 2013). There are also woody plants transformed with sugar beet genes. The silver birch (Betula pendula) has been transformed with the chitinase IV gene. The observed phenotype has been an increase in the fungal (Pappinen et al. 2002; Pasonen et al. 2004; Vihervuori et al. 2013) although in field trials transgenic trees suffered higher attacks from aphids than control plants (Vihervuori et al. 2008).
The gene codifying a germin-like protein (BvGLP-1), involved in nematode resistance, was transformed in Arabidopsis thaliana and conferred resistance to several pathogenic fungi (Verticillium longisporum and Rhizoctonia solani), without affecting beneficial fungus such as Piriformospora indica (Knecht et al. 2010). Rhizoctonia is a major problem of fungal origin for sugar beet cultivation. The overexpression of BvMLP1 and 3 major latex proteins in Arabidopsis thaliana resulted in less infectivity (Holmquist et al. 2021). Another strategy has been the overexpression of BvPGIP1 and BvPGIP2 Polygalacturonase-inhibiting proteins (PGIPs) that contain 11 leucine-rich repeat domains, contrary to most of the plants that only have 10 leucine-rich repeats. This construction conferred resistance in Nicotiana benthamiana (Li and Smigocki 2019).
8.2 Abiotic Stress
Sugar beet is a semi-domesticated crop that belongs to the Amaranthaceae family. Its closest cultivated relatives are spinach (Spinacia oleracea) and quinoa (Chenopodium quinoa). All three crops are suitable for poor soils or are able to resist environmental stress. It means that may be a source of useful genes to develop novel crops resistant to abiotic stress. One classical strategy is to test genes that have demonstrated the ability to confer salt stress in other organisms or which have some previous evidence that relates this gene to abiotic stress. For instance, S-adenosylmethionine decarboxylase is an enzyme involved in the biosynthesis of polyamines, and its overexpression increases the amounts of spermine and spermidine. This enzyme was identified in a proteomic study for M14, a salt-tolerant monosomic addition line obtained from the intercross between Beta vulgaris L. and Beta corolliflora Zoss proteins with a differential expression upon a salt treatment (Yang et al. 2013). The overexpression of this enzyme from conferred salt stress tolerance to Arabidopsis thaliana (Ji et al. 2019). Similarly, the overexpression of another enzyme of the same pathway, S-Adenosyl-l-Methionine Synthetase 2 increased abiotic stress tolerance (salt and oxidation) (Ma et al. 2017). Some other genes cloned from this germplasm have been assayed for their biotechnological potential against abiotic stress. The cysteine protease inhibitor, cystatin, increased salt tolerance when overexpressed in Arabidopsis (Wang et al. 2012) and also a monodehydroascorbate reductase (MDHAR), an enzyme that reduces monodehydroascorbate (DHA) to ascorbic acid (AsA), when overexpressed in Arabidopsis thaliana confers salt stress, longer roots, higher chlorophyll content, and higher AsA/DHA ratios (Li et al. 2020).
The overexpression of the glyoxalase I gene from the same cultivar increased tolerance to pleiotropic abiotic stresses (salt, drought, oxidation) in E. coli and tobacco (Wu et al. 2013). A proteinase inhibitor BvSTI has been expressed in the forage legume Lotus corniculatus L. increasing the resistance to salt stress salt and altering plant architecture (Savić et al. 2019).
There is a strategy that has been very useful to identify sugar beet genes, the screening in a heterologous system such as yeast (Locascio et al. 2019a). There are several reports of such screenings which have been performed using Beta vulgaris cDNA libraries (Serrano et al. 2003). This is a fast and straightforward methodology that can unveil novel genes to identify limiting factors for abiotic stress tolerance. This strategy allowed the cloning of BvCK2, the catalytic subunit of the casein kinase which conferred salt tolerance upon overexpression in yeast (Kanhonou et al. 2001), the translation initiation factor BveIF1A which conferred salt tolerance by overexpression in yeast and Arabidopsis thaliana (Rausell et al. 2003) and BvSATO1 (RNA-binding protein with RGG and RE/D motifs), BvSATO2 (paralogous to BvSATO1), BvSATO4 (RNA-binding protein), BvSATO5 (RNA-binding protein), and BvU2AF (U2snRNP AF protein) (Téllez et al. 2020), all of them conferred tolerance to salt stress when overexpressed in yeast.
The same library was overexpressed in an osmosensitive yeast strain and screened for osmotic stress tolerance. The results were a serine acetyl-transferase, an enzyme involved in the biosynthesis of cysteine form serine (Mulet et al. 2004) and class 2 non-symbiotic plant hemoglobin (BvHb2) (Salort et al. 2010). BvHb2 conferred tolerance to drought stress in yeast and Arabidopsis thaliana. When transformed in a horticultural crop, tomato (Solanum lycopersicum L.) also conferred resistance to drought induced withering. Another interesting aspect is that it also altered iron content by overexpression, increasing it in leaves and decreasing in fruit (Gisbert et al. 2020). BvHb2 has also been expressed in wild field cress (Lepidium campestre) and increased the seed oil content without altering its composition (Ivarson et al. 2017).
A different screening of the mentioned Beta vulgaris cDNA library for genes able to improve growth under cold conditions (10 °C) identified (BvCOLD1), a novel aquaporin gene not conserved in Arabidopsis thaliana and other model plants, which could only be found in evolutionarily related crops such as spinach or chinoa. Overexpression of BvCOLD1 conferred pleiotropic abiotic stress tolerance and increased growth under poor boron medium (Porcel et al. 2018). In the same screening, several genes related to endosomal vesicle transport (Salort and Salom 2009), a ring finger protein (Sanz Molinero et al. 2009), a protein from the PATELLIN family (Molinero et al. 2014), and a growth-regulating factor (Reuzeau et al. 2017) were also identified.
In some cases, transformation has been performed to investigate the role of a given gene. This has allowed knowing that BvCMO the gene encoding a choline monooxygenase is required for salt stress tolerance, as the transformation of sugar beet with an antisense construction to block the expression of this gene conferred a phenotype of salt sensitivity (Yamada et al. 2015). Similarly, Arabidopsis transformed with the tonoplast glucose exporter BvIMP exhibit decreased freezing tolerance and germination (Klemens et al. 2014).
8.3 Development and Metabolism
Most of the studies found in the literature are not interested in the biotechnological improvement of sugar beet but in gaining knowledge on the molecular mechanisms in sugar beet. For instance, the role of BvCOL1 as a photoperiod regulator was determined by its ability to complement Arabidopsis AtCOL1 and AtCOL2 mutants (Chia et al. 2008). Genetic engineering has been used as a tool to investigate sugar accumulation in sugar beets. BvSUT1, the gene that encodes the protein responsible for sucrose loading to the phloem, was transformed into yeast and expressed in oocytes of Xenopus laevis to investigate its mechanism (Nieberl et al. 2017). To investigate the subcellular localization of the betalain biosynthetic enzymes, those were expressed in tobacco (Chen et al. 2017). The sugar beet enzyme CYP716A was expressed in yeast to evaluate its ability to oxidize triterpenoids, molecules with several pharmacological and industrial applications (Suzuki et al. 2018).
The line M14 has also been a source of genes for studying the molecular biology of Beta vulgaris. For instance, the BvM14-MADSBOX was overexpressed in tobacco and led to severe phenotypic changes (Ma et al. 2011). The male sterility of the phenotype of some Beta vulgaris cultivars was mimicked in tobacco by expressing the mitochondrial ORF129 in tobacco with a mitochondrial targeting pre-sequence and a promoter for expression in flowers (Yamamoto et al. 2008). Transgenic sugar beet overexpressing bvORF20, a nuclear factor known as a restorer of fertility, was able to partially restore pollen fertility when overexpressed in cytoplasmic male sterile plants (Matsuhira et al. 2012) and has a complex, post-translational regulation which includes the interaction with the preSATP6 protein (Kitazaki et al. 2015).
Another strategy is that during harvesting the plant suffers wounds and this induces the expression of invertase genes which degrades sucrose, and therefore reducing yield. Overexpression of sugar beet invertase inhibitor BvC/VIF to block this postharvest decrease proved to be ineffective (Jansen 2009).
9 New Breeding Techniques in Sugar Beet
New breeding techniques are the hyperonym for a set of techniques that go beyond classical breeding (hybridization, mutagenesis, grafting) but cannot be considered GMO as do not involve the transformation of a gene into another species. Among these techniques, the most popular is CRISPR/Cas9 that consists of using a bacterial defense system to edit the genome of the host species in a specific site of the genome, defined by the guide RNA. Although there should be intense research in this field, there are not many descriptions published yet the use of CRISPR in sugar beet, nor there is information on a field trial with CRISPR sugar beet. There are descriptions of the design of a CRISPR system based in the BNYVV that allows transient expression of four different proteins in different tissues of the plant. This system has been used to transform Nicotiana benthamiana, Beta macrocarpa, and Beta vulgaris (Jiang et al. 2019). Also, considered a new breeding technique the TILLING (Targeting Induced Local Lesions in Genomes), the platform is a reverse genetics technology that is being used in Beta vulgaris to increase its agro-diversity (Kornienko and Butorina 2013).
10 Future Prospects
We have summarized in this book chapter the main advances in genetic engineering in sugar beet. It is clear that new breeding techniques are still starting and there is not much development yet. CRISPR, TILLING, and other strategies may be implemented. In a similar manner, we have described a lot of advances on developing GMO sugar beet for avoiding bolting, sugar yield increase, abiotic stress tolerance, or pest management, but farmers currently only have access to herbicide-resistant cultivars. There is a long way ahead, not only for scientists, breeders, and agro-bio companies but also for politicians to enable the use of these varieties that are already in hand. Let’s hope that in the close future some of the advances described in this chapter or some unexpected could help produce more and better food.
Abbreviations
- QTL:
-
Quantitative Trait Locus
- ALS:
-
Acetolactate Synthase
- AsA:
-
Ascorbic Acid
- BCTV:
-
Beet Curly Top Virus
- BNYVV:
-
Beet Necrotic Yellow Vein Virus
- BWYV:
-
Beet Western Yellows Virus
- BYV:
-
Beet Yellows Virus
- D-DNA:
-
Defective DNAs
- DHA:
-
Monodehydroascorbate
- ds-DNA:
-
Double-Stranded DNA
- GA:
-
Gibberellic Acid Biosynthetic
- GMO:
-
Genetically Modified Organism
- ICPs:
-
Insecticidal Crystal Proteins
- MDHAR:
-
Monodehydroascorbate Reductase
- mtlD:
-
Mannitol-1-Phosphate Dehydrogenase
- PAT:
-
Phosphinothricin N-Acetyltransferase
- PEG:
-
Polyethylene Glycol
- PvPGIP2 :
-
Polygalacturonase-Inhibiting Protein 2
- RNAi:
-
Interference RNA
- scFv:
-
Single-Chain Variable Fragment
- SPS:
-
Sucrose-Phosphate Synthase
- StGCS-GS:
-
γ-Glutamylcysteine Synthetase-Glutathione Synthetase
- TILLING:
-
Targeting Induced Local Lesions In Genomes
References
Ali MA, Azeem F, Abbas A, Joyia FA, Li H, Dababat AA (2017) Transgenic strategies for enhancement of nematode resistance in plants. Front Plant Sci 8:750. https://doi.org/10.3389/FPLS.2017.00750
Barker AL, Dayan FE (2019) Fate of glyphosate during production and processing of glyphosate-resistant sugar beet (Beta vulgaris). J Agric Food Chem 67:2061–2065. https://doi.org/10.1021/ACS.JAFC.8B05672
Bevan MW, Flavell RB, Chilton MD (1983) A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304:184–187. https://doi.org/10.1038/304184A0
Biancardi E, Tamada T (2016) Rhizomania. Springer International Publishing Switzerland. https://doi.org/10.1007/978-3-319-30678-0. isbn:978-3-319-30676-6
Cai D, Thurau T, Tian Y, Lange T, Yeh KW, Jung C (2003) Sporamin-mediated resistance to beet cyst nematodes (Heterodera schachtii Schm.) is dependent on trypsin inhibitory activity in sugar beet (Beta vulgaris L.) hairy roots. Plant Mol Biol 516(51):839–849. https://doi.org/10.1023/A:1023089017906
Chen N, Yu ZH, Xiao XG (2017) Cytosolic and nuclear co-localization of betalain biosynthetic enzymes in tobacco suggests that betalains are synthesized in the cytoplasm and/or nucleus of betalainic plant cells. Front Plant Sci 8(831):1–10. https://doi.org/10.3389/fpls.2017.00831
Chia TYP, Müller A, Jung C, Mutasa-Göttgens ES (2008) Sugar beet contains a large CONSTANS-LIKE gene family including a CO homologue that is independent of the early-bolting (B) gene locus. J Exp Bot 59:2735–2748. https://doi.org/10.1093/JXB/ERN129
Ćurčić Ž, Taški-Ajduković K, Nagl N (2017) Relationship between hybrid performance and genetic variation in self-fertile and self-sterile sugar beet pollinators as estimated by SSR markers. Euphytica 213:108. https://doi.org/10.1007/s10681-017-1897-1
D’Halluin K, Bossut M, Bonne E, Mazur B, Leemans J, Botterman J (1992) Transformation of sugarbeet (Beta vulgaris l.) and evaluation of herbicide resistance in transgenic plants. Biotechnology 10:309–314. https://doi.org/10.1038/nbt0392-309
De Marchis F, Bellucci M (2021) Plastid transformation in sugar beet: an important industrial crop. In: Methods in Molecular Biology, pp 283–290
De Marchis F, Wang Y, Bellucci M and Arcioni S (2007) Developing a method for sugar beet chloroplast transformation. Proceedings of the 51st Italian Society of Agricultural Genetics Annual Congress. Riva del Garda, Italy – 23/26 September, 2007
De Marchis F, Wang Y, Stevanato P, Arcioni S, Bellucci M (2009) Genetic transformation of the sugar beet plastome. Transgenic Res 18:17–30. https://doi.org/10.1007/s11248-008-9193-4
Dillen K, Demont M, Tillie P, Rodriguez Cerezo E (2013) Bred for Europe but grown in America: the case of GM sugar beet. New Biotechnol 30:131–135. https://doi.org/10.1016/J.NBT.2012.11.004
Dohm JC, Minoche AE, Holtgräwe D, Capella-Gutierrez S, Zakrzewski F, Tafer H, Rupp O, Sorensen TR, Stracke R, Reinhardt R, Goesmann A, Kraft T, Schulz B, Stadler PF, Schmidt T, Gabaldon T, Lehrach H, Weisshaar B, Himmelbauer H (2014) The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 505:546–549. https://doi.org/10.1038/nature12817
Doney DL (1995) USDA-ARS sugarbeet releases. J Sugar Beet Res 32:229–257. https://bsdf-assbt.org/wp-content/uploads/2017/04/JSBRVol32No4P229to257USDAARSSugarbeetReleases.pdf
Ebmeyer H, Fiedler-Wiechers K, Hoffmann CM (2021) Drought tolerance of sugar beet – Evaluation of genotypic differences in yield potential and yield stability under varying environmental conditions. Eur J Agron 125:126262. https://doi.org/10.1016/J.EJA.2021.126262
Ehlers U, Commandeur U, Frank R, Landsmann J, Koenig R, Burgermeister W (1991) Cloning of the coat protein gene from beet necrotic yellow vein virus and its expression in sugar beet hairy roots. Theor Appl Genet 81:777–782. https://doi.org/10.1007/BF00224989
Elliott MC, Chen DF, Fowler MR, Kirby MJ, Kubalakova M, Scott NW, Slater A (1996) Transgenesis-a scheme for improving sugar beet productivity. Russ J Plant Physiol 43:544–551
Erhardt M, Morant M, Ritzenthaler C, Stussi-Garaud C, Guilley H, Richards K, Jonard G, Bouzoubaa S, Gilmer D (2000) P42 movement protein of beet necrotic yellow vein virus is targeted by the movement proteins P13 and P15 to punctate bodies associated with plasmodesmata. Mol Plant-Microbe Interact MPMI 13:520–528. https://doi.org/10.1094/MPMI.2000.13.5.520
Evans E, Messerschmidt U (2017) Review: Sugar beets as a substitute for grain for lactating dairy cattle. J Anim Sci Biotechnol 8:25. https://doi.org/10.1186/s40104-017-0154-8
FAOSTAT (2018) http://www.fao.org/faostat/en/?#data/QC/visualize. Accessed 22 May 2020
Fry JE, Barnason AR, Hinchee M (1991) Genotype-independent transformation of sugarbeet using Agrobacterium tumefaciens. In: Molecular biology of plant growth and development, Third International Congress of Plant Molecular Biology, p 384
Fuchs M, Gonsalves D (2007) Safety of virus-resistant transgenic plants two decades after their introduction: lessons from realistic field risk assessment studies. Annu Rev Phytopathol 45:173–202. https://doi.org/10.1146/ANNUREV.PHYTO.45.062806.094434
Gerbens-Leenes W, Hoekstra AY (2012) The water footprint of sweeteners and bio-ethanol. Environ Int 40:202–211. https://doi.org/10.1016/J.ENVINT.2011.06.006
Gisbert C, Timoneda A, Porcel R, Ros R, Mulet JM (2020) Overexpression of BvHb2, a Class 2 non-symbiotic hemoglobin from sugar beet, confers drought-induced withering resistance and alters iron content in tomato. Agronomy 10:1754. https://doi.org/10.3390/agronomy10111754
Goudarzi A, Safaei N, Jafari M, Mahmoudi SB, Far MT (2015) Transformation of sugar beet with a bean chitinase gene and enhanced resistance to Alternaria alternata. Agric Biotechnol J 7:175–200. https://doi.org/10.22103/JAB.2015.1358
Goudarzi A, Jafari M, Safaie N, Mohammad Jafari S (2016) Transgenic sugar beet expressing a bacterial mannitol-1-phosphate dehydrogenase (mtlD) gene shows enhanced resistance to fungal pathogens. Sugar Tech 18:192–203. https://doi.org/10.1007/s12355-015-0379-9
Gurel E, Gurel S, Lemaux PG (2008) Biotechnology applications for sugar beet. CRC Crit Rev Plant Sci 27:108–140. https://doi.org/10.1080/07352680802202000
Hall RD, Riksen-Bruinsma T, Weyens GJ et al (1996) A high efficiency technique for the generation of transgenic sugar beets from stomatal guard cells. Nat Biotechnol 14:1133–1138. https://doi.org/10.1038/nbt0996-1133
Harpster MH, Townsend JA, Jones JDG, Bedbrook J, Dunsmuir P (1988) Relative strengths of the 35S cauliflower mosaic virus, 1′, 2′, and nopaline synthase promoters in transformed tobacco sugarbeet and oilseed rape callus tissue. Mol Gen Genet 2121(212):182–190. https://doi.org/10.1007/BF00322463
Hashimoto R, Shimamoto Y (1999) Growth of transgenic sugar beet plants with sucrose-phosphate synthase (SPS). In: Proc. Jpn. Soc. Sugar Beet Technol, pp 85–89
Hashimoto R, Shimamoto Y (2001) Transgenic sugar beet plants harboring a pumpkin chitinase gene demonstrating improved resistance to Rhizoctonia solani. In: Proc Jpn Soc Sugar Beet Technol, pp 24–28
Hatlestad GJ, Sunnadeniya RM, Akhavan NA, Gonzalez A, Goldman IL, McGrath M, Lloyd AM (2012) The beet R locus encodes a new cytochrome P450 required for red betalain production. Nat Genet 44:816–820. https://doi.org/10.1038/ng.2297
Heller R, Schondelmaier J, Steinrücken G, Jung C (1996) Genetic localization of four genes for nematode (Heterodera schachtii Schm.) resistance in sugar beet (Beta vulgaris L.). Theor Appl Genet 92:991–997. https://doi.org/10.1007/BF00224039
Holmquist L, Dölfors F, Fogelqvist J, Cohn J, Kraft T, Dixelius C (2021) Major latex protein-like encoding genes contribute to Rhizoctonia solani defense responses in sugar beet. Mol Gen Genomics 296:155–164. https://doi.org/10.1007/s00438-020-01735-0
Horn J, Lauster S, Krenz B, Krausb J, Frischmutha T, Jeskea H (2011) Ambivalent effects of defective DNA in beet curly top virus-infected transgenic sugarbeet plants. Virus Res 158:169–178. https://doi.org/10.1016/j.virusres.2011.03.029
Hossain MS, Persicke M, ElSayed AI, Kalinowski J, Dietz KJ (2017) Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. J Exp Bot 68:5961–5976. https://doi.org/10.1093/JXB/ERX388
Ivarson E, Leiva-Eriksson N, Ahlman A, Kanagarajan S, Bülow L, Zhu LH (2017) Effects of overexpression of WRI1 and hemoglobin genes on the seed oil content of Lepidium campestre. Front Plant Sci 7:2032. https://doi.org/10.3389/fpls.2016.02032
Ivic S, Sicher R, Smigocki A (2001) Growth habit and sugar accumulation in sugarbeet (Beta vulgaris L.) transformed with a cytokinin biosynthesis gene. Plant Cell Rep 208(20):770–773. https://doi.org/10.1007/S002990100389
Jacq B, Lesobre O, Sangwan RS (1993) Sangwan-Norreel BS (1993) Factors influencing T-DNA transfer in Agrobacterium-mediated transformation of sugarbeet. Plant Cell Rep 1211(12):621–624. https://doi.org/10.1007/BF00232811
Jafari M, Norouzi P, Malboobi MA, Ghareyazie B, Valizadeh M, Mohammadi SA, Mousavi M (2008) Enhanced resistance to a lepidopteran pest in transgenic sugar beet plants expressing synthetic cry1Ab gene. Euphytica 165:333–344. https://doi.org/10.1007/S10681-008-9792-4
Jafarzade M, Ramezani M, Hedayati F, Mokhtarzade Z, Zare B, Sabet MS, Norouzi P, Malboobi MA (2019) Antibody-mediated resistance to rhizomania disease in sugar beet hairy roots. Plant Pathol J 35:692–697. https://doi.org/10.5423/PPJ.OA.04.2018.0073
James C (2011) Brief 42: global Status of Commercialized Biotech/GM Crops: 2010. ISAAA Br
Jansen A (2009) Modifying post-harvest sucrose loss in sugar beet: Assessment of transgenic approaches—heiDOK. Ph.D. Dissertation. https://doi.org/10.11588/heidok.00010077
Ji M, Wang K, Wang L, Chen S, Li H, Ma C, Wang Y (2019) Overexpression of a S-Adenosylmethionine decarboxylase from sugar beet M14 increased Arabidopsis salt tolerance. Int J Mol Sci 20:1990. https://doi.org/10.3390/ijms20081990
Jiang N, Zhang C, Liu JY, Guo ZH, Zhang ZY, Han CG, Wang Y (2019) Development of beet necrotic yellow vein virus-based vectors for multiple-gene expression and guide RNA delivery in plant genome editing. Plant Biotechnol J 17:1302–1315. https://doi.org/10.1111/pbi.13055
Joersbo M (2007) Sugar Beet. In: Pua E-C, Davey MR (eds) Transgenic crops IV. Springer, Berlin Heidelberg, pp 355–379
Jung C (1998) Cloning and breeding utility of the gene Hs1 for nematode resistance from Beta procumbens. In: Comptes-Rendus des Congres de l’Institut International de Recherches Betteravieres (Belgium)
Kagami H, Kurata M, Matsuhira H, Taguchi K, Mikami T, Tamagake H, Kubo T (2015) Sugar beet (Beta vulgaris L.). Methods Mol Biol 1223:335–347. https://doi.org/10.1007/978-1-4939-1695-5_27
Kagami H, Taguchi K, Arakawa T, Kuroda Y, Tamagake H, Kubo T (2016) Efficient callus formation and plant regeneration are heritable characters in sugar beet (Beta vulgaris L.). Hereditas 153:12. https://doi.org/10.1186/s41065-016-0015-z
Kallerhoff J, Perez P, Bouzoubaa S, Ben Tahar S, Perret J (1990) Beet necrotic yellow vein virus coat protein-mediated protection in sugarbeet (Beta vulgaris L.) protoplasts. Plant Cell Rep 94(9):224–228. https://doi.org/10.1007/BF00232185
Kanhonou R, Serrano R, Palau RR (2001) A catalytic subunit of the sugar beet protein kinase CK2 is induced by salt stress and increases NaCl tolerance in Saccharomyces cerevisiae. Plant Mol Biol 47:571–579
Kerr SP (2005) Performance of rhizomania resistant sugar beet varieties in UK trials. Asp Appl Biol 76:21
Khan MFR (2014) The beet goes on-roundup ready sugar beet deregulated again in USA. Int Sugar J 116:131–133. http://www.agra-net.com/portal2/isj/
Khayamim S, Tavkol Afshari R, Sadeghian SY, Poustini K, Roozbeh F, Abbasi Z (2014) Seed germination, plant establishment, and yield of sugar beet genotypes under salinity stress. J Agric Sci Technol 16:779–790
Kimoto Y, Shimamoto Y (2001) Difference in toxicity to larvae of cabbage armyworm between transgenic sugar beet lines with Cry1Ab and Cry1C. Proc Jan Soc Sugar Beet Technol 43:20–23
Kishchenko EM, Komarnitskii IK, Kuchuk NV (2010) Transposition of the maize transposable element dSpm in transgenic sugar beets. Cytol Genet 444(44):200–205. https://doi.org/10.3103/S009545271004002X
Kishchenko EM, Komarnitskii IK, Kuchuk NV (2011) Transgenic sugar beet tolerant to imidazolinone obtained by Agrobacterium-mediated transformation. Cytol Genet 453(45):148–152. https://doi.org/10.3103/S0095452711030030
Kitazaki K, Arakawa T, Matsunaga M, Yui-Kurino R, Matsuhira H, Mikami T, Kubo T (2015) Post-translational mechanisms are associated with fertility restoration of cytoplasmic male sterility in sugar beet (Beta vulgaris). Plant J 83:290–299. https://doi.org/10.1111/tpj.12888
Klemens PAW, Patzke K, Trentmann O, Poschet G, Büttner M, Schulz A, Marten I, Hedrich R, Ekkehard Neuhaus H (2014) Overexpression of a proton-coupled vacuolar glucose exporter impairs freezing tolerance and seed germination. New Phytol 202:188–197. https://doi.org/10.1111/nph.12642
Klenk I, Landquist B, De Imaña OR (2012) The product carbon footprint of EU beet sugar (part I). Zuckerindustrie 137:169–177. https://doi.org/10.36961/SI12784
Klimek-Chodacka M, Baranski R (2014) A protocol for sonication-assisted Agrobacterium rhizogenes-mediated transformation of haploid and diploid sugar beet (Beta vulgaris L.) explants. Acta Biochim Pol 61:13–17. https://doi.org/10.18388/ABP.2014_1916
Knecht K, Seyffarth M, Desel C, Thurau T, Sherameti I, Lou B, Oelmüller R, Cai D (2010) Expression of BvGLP-1 encoding a germin-like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi. Mol Plant-Microbe Interact 23:446–457. https://doi.org/10.1094/MPMI-23-4-0446
Koch MS, Ward JM, Levine SL, Baum JA, Vicini JL, Hammond BG (2015) The food and environmental safety of Bt crops. Front Plant Sci 6:283. https://doi.org/10.3389/FPLS.2015.00283
Kong J, Martin-Ortigosa S, Finer J, Orchard N, Gunadi A, Batts LA, Thakare D, Rush B, Schmitz O, Stuiver M, Olhoft P, Pacheco-Villalobos D (2020) Overexpression of the transcription factor growth-regulating factor5 improves transformation of dicot and monocot species. Front Plant Sci 11:1389. https://doi.org/10.3389/FPLS.2020.572319
Kornienko AV, Butorina AK (2013) Induced mutagenesis in sugar beet (Beta vulgaris L.): obtained results and prospects for use in development of TILLING project. Biol Bull Rev 32(3):152–160. https://doi.org/10.1134/S2079086413020059
Krens FA, Zijlstra C, Wvd M, Jamar D, Huizing HJ (1988) Transformation and regeneration in sugar beet (Beta vulgaris L.) induced by ‘shooter’ mutants of Agrobacterium tumefaciens. Euphytica 393(39):185–194. https://doi.org/10.1007/BF00043382
Kuykendall LD, Upchurch RG (2004) Expression in sugar beet of the introduced cercosporin toxin export (CFP) gene from Cercospora kikuchii, the causative organism of purple seed stain in soybean. Biotechnol Lett 269(26):723–727. https://doi.org/10.1023/B:BILE.0000024096.22105.C3
Lathouwers J, Vandercappellen L, Lommel M, Rosquin I, Denys P, Bruyne E, Lefebvre M, Weyens G, Bleykasten C, Bouzoubaa S, Guilley H, Richards K, Jonard G (1997) Sugar beet transformation for rhizomania resistance: introduction and expression of different viral sequences. In: Comptes-Rendus des Congres de l’Institut International de Recherches Betteravieres (Belgium), vol 60, pp 491–495
Lathouwers J, Weyens G, Lefebvre M (2005) Transgenic research in sugarbeet. Genet Modif Sugar Beet:5–24
Lauber E, Bleykasten-Grosshans C, Erhardt M, Bouzoubaa S, Jonard G, Richards KE, Guilley H (1998) Cell-to-cell movement of beet necrotic yellow vein virus: I. Heterologous complementation experiments provide evidence for specific interactions among the triple gene block proteins. Mol Plant-Microbe Interact 11:618–625. https://doi.org/10.1094/MPMI.1998.11.7.618
Lennefors BL, Savenkov EI, Bensefelt J, Wremerth-Weich E, van Roggen P, Tuvesson S, Valkonen JPT, Gielen J (2006) dsRNA-mediated resistance to beet necrotic yellow vein virus infections in sugar beet (Beta vulgaris L. ssp. vulgaris). Mol Breed 184(18):313–325. https://doi.org/10.1007/S11032-006-9030-5
Li H, Smigocki AC (2019) Suppression of Fusarium oxysporum with recombinant polygalacturonase inhibiting proteins (BvPGIPs) extracted from sugar beet roots. Plant Cell Tissue Organ Cult (PCTOC) 136:197–203. https://doi.org/10.1007/s11240-018-1496-4
Li J, Li H, Yang N, Ma C, Li H (2020) Overexpression of a monodehydroascorbate reductase gene from sugar beet M14 increased salt stress tolerance. Sugar Tech 23:45–56. https://doi.org/10.1007/S12355-020-00877-0
Lindsey K, Gallois P (1990) Transformation of Sugarbeet (Beta vulgaris) by Agrobacterium tumefaciens. J Exp Bot 41:529–536. https://doi.org/10.1093/JXB/41.5.529
Lisson R, Hellert J, Ringleb M, Machens F, Kraus J, Hehl R (2010) Alternative splicing of the maize Ac transposase transcript in transgenic sugar beet (Beta vulgaris L.). Plant Mol Biol 74:19–32. https://doi.org/10.1007/s11103-010-9651-2
Liu D, An Z, Mao Z, Ma L, Lu Z (2015) Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus StGCS-GS in the presence of Cd, Zn and Cu alone or in combination. PLoS One 10(6):e0128824. https://doi.org/10.1371/journal.pone.0128824
Liu H, Wang Q, Yu M, Zhang Y, Wu Y, Zhang H (2008) Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ 31:1325–1334. https://doi.org/10.1111/j.1365-3040.2008.01838.x
Locascio A, Andrés-Colás N, Mulet JMJM, Yenush L (2019a) Saccharomyces cerevisiae as a tool to investigate plant potassium and sodium transporters. Int J Mol Sci 20:2133. https://doi.org/10.3390/ijms20092133
Locascio A, Marqués MCMC, García-Martínez G, Corratgé-Faillie C, Andrés-Colás N, Rubio L, Fernández JA, Véry AA, Mulet JM, Yenush L (2019b) BCL2-ASSOCIATED ATHANOGENE4 Regulates the KAT1 potassium channel and controls stomatal movement. Plant Physiol 181:1277–1294. https://doi.org/10.1104/pp.19.00224
Lytvyn DI, Syvura VV, Kurylo VV, Olenieva VD, Yemets AI, Blume YB (2014) Creation of transgenic sugar beet lines expressing insect pest resistance genes cry1C and cry2A. Cytol Genet 48:69–75. https://doi.org/10.3103/S0095452714020078
Ma C, Wang Y, Gu D, Nan J, Chen S, Li H (2017) Overexpression of S-adenosyl-L-methionine synthetase 2 from sugar beet M14 increased Arabidopsis tolerance to salt and oxidative stress. Int J Mol Sci 18:847. https://doi.org/10.3390/ijms18040847
Ma C, Wang Y, Wang Y, Wang L, Chen S, Li H (2011) Identification of a sugar beet BvM14-MADS box gene through differential gene expression analysis of monosomic addition line M14. J Plant Physiol 168:1980–1986. https://doi.org/10.1016/j.jplph.2011.05.027
Mannerlöf M, Lennerfors B-L, Tenning P (1996) Reduced titer of BNYVV in transgenic sugar beets expressing the BNYVV coat protein. Euphytica 903(90):293–299. https://doi.org/10.1007/BF00027479
Märländer B (2005) Weed control in sugar beet using genetically modified herbicide-tolerant varieties – a review of the economics for cultivation in Europe. J Agron Crop Sci 191:64–74. https://doi.org/10.1111/J.1439-037X.2004.00135.X
Matsuhira H, Kagami H, Kurata M, Kitazaki K, Matsunaga M, Hamaguchi Y, Hagihara E, Ueda M, Harada M, Muratasu A, Yui-Kurino R, Taguchi K, Tamagake H, Mikami T, Kubo T (2012) Unusual and typical features of a novel Restorer-of-fertility gene of sugar beet (Beta vulgaris L.). Genetics 192:1347–1358. https://doi.org/10.1534/genetics.112.145409
Matsuhira H, Kichi T, Tamagake H, Sato Y, Anzai H, Yoshida M (2014) High production of plant type levan in sugar beet transformed with timothy (Phleum pratense) 6-SFT genes. J Biotechnol 192:215–222. https://doi.org/10.1016/j.jbiotec.2014.09.025
May MJ, Wilson RG (2006) Weeds and weed control. Sugar beet:359–386
McGinnis EE, Meyer MH, Smith AG (2010) Sweet and sour: a scientific and legal look at herbicide-tolerant sugar beet. Plant Cell 22:1653. https://doi.org/10.1105/TPC.110.077198
McGrath JM (2011) Notice of release of EL56 sugar beet germplasm with high levels of tolerance to salinity during germination. USDA-ARS Germplasm Release
Mcgrath JM, Panella L, Mitchell J (2018) Plant breeding reviews. Plant Breed Rev. https://doi.org/10.1002/9781119521358
Mishutkina YV, Kamionskaya AM, Skryabin KG (2010) The creation of sugar beet transgenic plants expressing bar gene. Appl Biochem Microbiol 46:80–86. https://doi.org/10.1134/S000368381001014X
Moazami K, Mortazavi SE, Heidari B, Nouroozi P (2018) Agrobacterium-mediated transient assay of the gus gene expression in sugar beet. Annu Res Rev Biol 30:1–7. https://doi.org/10.9734/ARRB/2018/46049
Mohammadzadeh R, Zamani M, Motallebi M, Norouzi P, Jourabchi E, Benedetti M, De Lorenzo G (2012) Agrobacterium tumefaciens-mediated introduction of polygalacturonase inhibiting protein 2 gene (PvPGIP2) from Phaseolus vulgaris into sugar beet (Beta vulgaris L.). Aust J Crop Sci 6:1290–1297
Molinero AIS, Reuzeau C, Frankard V, Salom RS and Salort JMM (2014, August 21) Plants having enhanced yield-related traits and a method for making the same US patent 14175402, 21 August 2014
Monger W, Barrington PJ, Acaster AM, Halford NG, Ainsworth C, Thomas H (1995) A transgenic approach towards investigating carbon metabolism in sugar beet. Proc IIRB Congress 58:193–196
Mulet JM, Alemany B, Ros R, Calvete JJ, Serrano R (2004) Expression of a plant serine O-acetyltransferase in Saccharomyces cerevisiae confers osmotic tolerance and creates an alternative pathway for cysteine biosynthesis. Yeast 21:303–312. https://doi.org/10.1002/yea.1076
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Mutasa-Gottgens E, Qi A, Mathews A, Thomas S, Phillips A, Hedden P (2009) Modification of gibberellin signalling (metabolism & signal transduction) in sugar beet: analysis of potential targets for crop improvement. Transgenic Res 18:301–308. https://doi.org/10.1007/s11248-008-9211-6
Nieberl P, Ehrl C, Pommerrenig B, Graus D, Marten I, Jung B, Ludewig F, Koch W, Harms K, Flügge UI, Neuhaus HE, Hedrich R, Sauer N (2017) Functional characterisation and cell specificity of BvSUT1, the transporter that loads sucrose into the phloem of sugar beet (Beta vulgaris L.) source leaves. Plant Biol 19:315–326. https://doi.org/10.1111/PLB.12546
Ninković S, Djordjević T, Vinterhalter B, Uzelac B, Cingel A, Savić J, Radović S (2010) Embryogenic responses of Beta vulgaris L. callus induced from transgenic hairy roots. Plant Cell Tissue Organ Cult 1031(103):81–91. https://doi.org/10.1007/S11240-010-9757-X
Padmanaban S, Li H, Puthoff D, Smigocki A (2016) Beta vulgaris promoters for directed tissue-specific root transcription. Am. Soc. Sugar Beet Tech. Proc. 36. http://assbtproceedings.org/ASSBT2011Proceedings/Posters/Physilogy%20and%20Biotechnology/Padmanaban%20_Smigocki_%20_2_.pdf. Accessed 3 Mar 2016
Panella L, Campbell LG, Eujayl IA, Lewellen RT, McGrath JM (2015) USDA-ARS Sugarbeet releases and breeding over the past 20 years. J Sugar Beet Res 52. https://doi.org/10.5274/jsbr.52.3.40
Pappinen A, Degefu Y, Syrjälä L, Keinonen K, von Weissenberg K (2002) Transgenic silver birch (Betula pendula) expressing sugarbeet chitinase 4 shows enhanced resistance to Pyrenopeziza betulicola. Plant Cell Rep 20:1046–1051. https://doi.org/10.1007/S00299-002-0449-9
Pasonen H-L, Seppänen S-K, Degefu Y, Rytkönen A, von Weissenberg K, Pappinen A (2004) Field performance of chitinase transgenic silver birches (Betula pendula): resistance to fungal diseases. Theor Appl Genet 1093(109):562–570. https://doi.org/10.1007/S00122-004-1650-8
Pavli OI (2010) Molecular characterization of beet necrotic yellow vein virus in Greece and transgenic approaches towards enhancing rhizomania disease resistance. Wageningen University and Research. https://library.wur.nl/WebQuery/wurpubs/386941
Pavli OI, Kelaidi GI, Tampakaki AP, Skaracis GN (2011a) The HrpZ gene of Pseudomonas syringae pv. phaseolicola enhances resistance to rhizomania disease in transgenic Nicotiana benthamiana and sugar beet. PLoS One:6. https://doi.org/10.1371/journal.pone.0017306
Pavli OI, Panopoulos NJ, Goldbach R, Skaracis GN (2010) BNYVV-derived dsRNA confers resistance to rhizomania disease of sugar beet as evidenced by a novel transgenic hairy root approach. Transgenic Res 19:915–922. https://doi.org/10.1007/s11248-010-9364-y
Pavli OI, Stevanato P, Biancardi E, Skaracis GN (2011b) Achievements and prospects in breeding for rhizomania resistance in sugar beet. F Crop Res 122:165–172
Pfeiffer N, Tränkner C, Lemnian I, Grosse I, Müller AE, Jung C, Kopisch-Obuch FJ (2014) Genetic analysis of bolting after winter in sugar beet (Beta vulgaris L.). Theor Appl Genet 127:2479–2489. https://doi.org/10.1007/s00122-014-2392-x
Pilon-Smits EAH, Terry N, Sears T, Van Dun K (1999) Enhanced drought resistance in fructan-producing sugar beet. Plant Physiol Biochem 37:313–317. https://doi.org/10.1016/S0981-9428(99)80030-8
Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJL, Nilsson O (2010) An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 80(330):1397–1400. https://doi.org/10.1126/science.1197004
Porcel R, Bustamante A, Ros R, Serrano R, Mulet-Salort JM (2018) BvCOLD1: a novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ 41:2844–2857. https://doi.org/10.1111/pce.13416
Poveda J, Francisco M, Cartea ME, Velasco P (2020) Development of transgenic brassica crops against biotic stresses caused by pathogens and arthropod pests. Plan Theory 9:1664. https://doi.org/10.3390/PLANTS9121664
Rajabi A, Borchardt D (2015) QTL mapping for root yield and leaf traits in sugar beet (Beta vulgaris L.) under drought stress condition. Iran J Crop Sci 17(1):46–62
Rajabi A, Griffiths H, Ober ES, Kromdijk W, Pidgeon JD (2007) Genetic characteristics of water-use related traits in sugar beet. Euphytica 1602(160):175–187. https://doi.org/10.1007/S10681-007-9520-5
Rausell A, Kanhonou R, Yenush L, Serrano R, Ros R (2003) The translation initiation factor eIF1A is an important determinant in the tolerance to NaCl stress in yeast and plants. Plant J 34:257–267. https://doi.org/10.1046/j.1365-313x.2003.01719.x
Reuzeau C, Molinero AIS, Frankard V, Salom RS, Salort JMM (2017) Plants having increased yield-related traits by expressing a growth-regulating factor (GRF) polypeptide and method for making the same US Patent 9617557, 11 April 2017
Ribichich KF, Chiozza M, Ávalos-Britez S, Cabello JV, Arce AL, Watson G, Arias C, Portapila M, Trucco F, Otegui ME, Chan RL (2020) Successful field performance in warm and dry environments of soybean expressing the sunflower transcription factor HB4. J Exp Bot 71:3142–3156. https://doi.org/10.1093/jxb/eraa064
Rosa Téllez S, Kanhonou R, Castellote Bellés C, Serrano R, Alepuz P, Ros R (2020) RNA-binding proteins as targets to improve salt stress tolerance in crops. Agronomy 10:250. https://doi.org/10.3390/agronomy10020250
Sadeghi-Shoae M, Habibi D, Taleghani DF, Paknejad F, Kashani A (2014) Evaluation the effect of paclobutrazol on bolting, qualitative and quantitative performance in autumn sown-sugar beet genotypes in Moghan region. Int J Biosci 5:345–354. https://doi.org/10.12692/ijb/5.1.345-354
Safar S, Bazrafshan M, Khoshnami M, Behrooz AA, Hedayati F, Maleki M, Mahmoudi SB, Malboobi MA (2021) Field evaluation for rhizomania resistance of transgenic sugar beet events based on gene silencing. Can J Plant Pathol 43:179–188. https://doi.org/10.1080/07060661.2020.1783575
Salort JMM, Molinero AIS, Salom RS (2010) Method for producing transgenic plants with increased yield, comprising expressing of haemoglobin from Arabidopsis. U.S. Patent 7674953B2, 9 March 2010
Salort JMM, Salom RS (2009.) Protein for use in modifying abiotic stress tolerance in yeast US Patent 7612177, 3 November 2009
Santoni M, Bertini E, Zampieri R, Cuccurullo A, Commisso M, Gecchele E, Avesani L (2019) Transient expression in red beet of a biopharmaceutical candidate vaccine for type-1 diabetes. J Vis Exp. https://doi.org/10.3791/59298
Sanz Molinero AI, Reuzeau C, Frankard V, Mulet Salort JM and Salom RS (2009) Plants Having Enhanced Yield-Related Traits and a Method for Making the Same WO Patent WO/2009/068564. 4 June 2009
Saunders JW, Acquaah G, Renner KA, Doley WP (1992) Monogenic dominant sulfonylurea resistance in sugarbeet from somatic cell selection. Crop Sci 32:1357–1360. https://doi.org/10.2135/CROPSCI1992.0011183X003200060010X
Savić J, Nikolić R, Banjac N, Zdravković-Korać S, Stupar S, Cingel A, Ćosić T, Raspor M, Smigocki A, Ninković S (2019) Beneficial implications of sugar beet proteinase inhibitor BvSTI on plant architecture and salt stress tolerance in Lotus corniculatus L. J Plant Physiol 243:153055. https://doi.org/10.1016/j.jplph.2019.153055
Savitsky VF (1950) Monogerm sugar beets in the United States. Proc Am Soc Sugar Beet Technol 6:156–159
Scholten OE, Lange W (2000) Breeding for resistance to rhizomania in sugar beet: a review. Euphytica 1123(112):219–231. https://doi.org/10.1023/A:1003988003165
Schwegler DD, Gowda M, Schulz B, Miedaner T, Liu W, Reif JC (2014) Genotypic correlations and QTL correspondence between line per se and testcross performance in sugar beet (Beta vulgaris L.) for the three agronomic traits beet yield, potassium content, and sodium content. Mol Breed 341(34):205–215. https://doi.org/10.1007/S11032-014-0030-6
Sedighi L, Rezapanah M, Aghdam HR (2011) Efficacy of Bt transgenic sugar beet lines expressing cry1Ab Gene Against Spodoptera littoralis Boisd. (Lepidoptera: Noctuidae). J Entomol Res Soc 13:61–61
Serrano R, Montesinos C, Gaxiola R, Ríos G, Forment J, Leube M, Mulet JM, Naranjo MA, Roldán M, Vicente O, Kanhonou RA, Rausell A, Ros R (2003) Functional genomics of salt tolerance: the yeast overexpression approach. In: Proceedings of the International Symposium on managing Greenhouse Crops in Saline Environement. International Society Horticultural Science, pp 31–38. https://doi.org/10.17660/ActaHortic.2003.609.2
Sévenier R, der Meer IM, Bino R, Koops AJ (2002) Increased production of nutriments by genetically engineered crops. J Am Coll Nutr 21:199S–204S. https://doi.org/10.1080/07315724.2002.10719266
Sévenier R, Hall RD, van der Meer IM, Hakkert HJC, van Tunen AJ, Koops AJ (1998) High level fructan accumulation in a transgenic sugar beet. Nat Biotechnol 169(16):843–846. https://doi.org/10.1038/nbt0998-843
Shimamoto Y, Domae T (2000) Resistance to larvae of cabbage armyworm (Mamestra brassicae L.) in ICP gene transductant of sugarbeet [Beta vulgaris]. Proc Sugar Beet Res Association (Japan) 41:90–98
Smigocki AC, Ivic-Haymes S, Li H, Savić J (2013) Pest protection conferred by a Beta vulgaris serine proteinase inhibitor gene. PLoS One:8. https://doi.org/10.1371/journal.pone.0057303
Smigocki AC, Ivic-Haymes SD, Puthoff DP, Zuzga S (2008) Recent advances in functional genomics for sugar beet (Beta vulgaris L.) improvement: progress in determining the role of BvSTI in pest resistance in roots. Sugar Tech 10:91–98. https://doi.org/10.1007/S12355-008-0016-Y
Snyder GW, Ingersoll JC, Smigocki AC, Owens LD (1999) Introduction of pathogen defense genes and a cytokinin biosynthesis gene into sugarbeet (Beta vulgaris L.) by Agrobacterium or particle bombardment. Plant Cell Rep 18:829–834. https://doi.org/10.1007/S002990050669
Sönmez Ç, Elekcig̀olu IH, Yücel AM, Öktem HA (2014) Transgenic Nicotiana tabacum cultivar Samsun plants carrying the wild sugar beet Hs1pro1 gene have resistance to root-knot nematodes. Turk J Biol 38:200–207. https://doi.org/10.3906/biy-1306-35
Srivastava HM (1995) Sugarbeet pre-breeding in India. J Sugarbeet Res 32:99–111. https://doi.org/10.5274/JSBR.32.2.99
Suzuki H, Fukushima EO, Umemoto N, Ohyama K, Seki H, Muranaka T (2018) Comparative analysis of CYP716A subfamily enzymes for the heterologous production of C-28 oxidized triterpenoids in transgenic yeast. Plant Biotechnol 35:131. https://doi.org/10.5511/PLANTBIOTECHNOLOGY.18.0416A
Taïbi K, Ait Abderrahim L, Boussaid M, Bissoli G, Taïbi F, Achir M, Souana K, Mulet JM (2021) Salt-tolerance of Phaseolus vulgaris L. is a function of the potentiation extent of antioxidant enzymes and the expression profiles of polyamine encoding genes. South Afr J Bot 140:114–122. https://doi.org/10.1016/J.SAJB.2021.03.045
Tertivanidis K, Goudoula C, Vasilikiotis C, Hassiotou E, Perl-Treves R, Tsaftaris A (2004) Superoxide dismutase transgenes in sugarbeets confer resistance to oxidative agents and the fungus C. beticola. Transgenic Res 13:225–233. https://doi.org/10.1023/B:TRAG.0000034610.35724.04
Thurau T, Kifle S, Jung C, Cai D (2003) The promoter of the nematode resistance gene Hs1 pro−1 activates a nematode-responsive and feeding site-specific gene expression in sugar beet (Beta vulgaris L.) and Arabidopsis thaliana. Plant Mol Biol 52:643–660. https://doi.org/10.1023/A:1024887516581
Turk S, Smeekens SCM (1999) Genetic modification of plant carbohydrate metabolism. Appl Plant Biotechnol 71:100
United States Department of Agriculture (2019) 2017 Census of Agriculture United States summary and state data volume 1 - geographic area series - Part 51
Van Camp W (2005) Yield enhancement genes: seeds for growth. Curr Opin Biotechnol 16:147–153
Vihervuori L, Lyytikäinen-Saarenmaa P, Lu J, Pasonen HL (2013) Effects on lepidopteran herbivores of feeding on leaves of transgenic birch (Betula pendula) expressing the sugar beet chitinase IV gene. Eur J Entomol 110:253
Vihervuori L, Pasonen HL, Lyytikäinen-Saarenmaa P (2008) Density and composition of an insect population in a field trial of chitinase transgenic and wild-type silver birch (Betula pendula) clones. Environ Entomol 37:1582–1591. https://doi.org/10.1603/0046-225X-37.6.1582
Villarías Moradillo JL (1999) Compendio práctico del cultivo de la remolacha azucarera. Ediciones Agrotécnicas, S.L., Madrid
Vogt SH, Weyens G, Lefèbvre M, Bork B, Schechert A, Müller AE (2014) The FLC-like gene BvFL1 is not a major regulator of vernalization response in biennial beets. Front Plant Sci 5:146. https://doi.org/10.3389/fpls.2014.00146
Wang C, Burzio LA, Koch MS, Silvanovich A, Bell E (2015) Purification, characterization and safety assessment of the introduced cold shock protein B in DroughtGardTM maize. Regul Toxicol Pharmacol 71:164–173. https://doi.org/10.1016/j.yrtph.2014.12.014
Wang H, Kraus J, Dettendorfer J, Chua NH, Nehls R (2003) Marker gene elimination from transgenic sugarbeet by a chemically regulated Cre-lox system. Plant Biotechnol 2002 Beyond:229–231. https://doi.org/10.1007/978-94-017-2679-5_44
Wang X, Sun Y, Li G (2012) Agrobacterium-mediated transformation of sugar beet with vacuolar H+-ppase avp1 gene. Mol Plant Breed 9:370–375. https://agris.fao.org/agris-search/search.do?recordID=CN2012002147
Weyens G, Ritsema T, Van Dun K, Meyer D, Lommel M, Lathouwers J, Rosquin I, Denys P, Tossens A, Nijs M, Turk S, Gerrits N, Bink S, Walraven B, Lefèbvre M, Smeekens S (2004) Production of tailor-made fructans in sugar beet by expression of onion fructosyltransferase genes. Plant Biotechnol J 2:321–327. https://doi.org/10.1111/J.1467-7652.2004.00074.X
Wozniak CA, Owens LD (1989) Transformation of sugarbeet cell suspension cultures mediated by Agrobacterium tumefaciens. J Cell Biochem Suppl D 13:272
Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3:259–273. https://doi.org/10.1111/J.1467-7652.2005.00123.X
Wu C, Ma C, Pan Y, Gong S, Zhao C, Chen S, Li H (2013) Sugar beet M14 glyoxalase I gene can enhance plant tolerance to abiotic stresses. J Plant Res 126:415–425. https://doi.org/10.1007/s10265-012-0532-4
Wu GQ, Feng RJ, Wang SM, Wang CM, Bao AK, Wei L, Yuan HJ (2015) Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 confers enhanced salinity tolerance in chimeric sugar beet (Beta vulgaris L.). Front Plant Sci 6:581. https://doi.org/10.3389/FPLS.2015.00581
Yamada N, Takahashi H, Kitou K, Sahashi K, Tamagake H, Tanaka Y, Takabe T (2015) Suppressed expression of choline monooxygenase in sugar beet on the accumulation of glycine betaine. Plant Physiol Biochem 96:217–221. https://doi.org/10.1016/J.PLAPHY.2015.06.014
Yamamoto MP, Shinada H, Onodera Y, Komaki C, Mikami T, Kubo T (2008) A male sterility-associated mitochondrial protein in wild beets causes pollen disruption in transgenic plants. Plant J 54:1027–1036. https://doi.org/10.1111/j.1365-313X.2008.03473.x
Yang AF, Duan XG, Gu XF, Gao F, Zhang JR (2005) Efficient transformation of beet (Beta vulgaris) and production of plants with improved salt-tolerance. Plant Cell Tissue Organ Cult 83:259–270. https://doi.org/10.1007/S11240-005-6670-9
Yang L, Zhang Y, Zhu N, Koh J, Ma C, Pan Y, Yu B, Chen S, Li H (2013) Proteomic analysis of salt tolerance in sugar beet monosomic addition line M14. J Proteome Res 12:4931–4950. https://doi.org/10.1021/PR400177M
Yu ZH, Han YN, Xiao XG (2015) A PPO promoter from betalain-producing red Swiss chard, directs petiole- and root-preferential expression of foreign gene in anthocyanins-producing plants. Int J Mol Sci 16:27032–27043. https://doi.org/10.3390/IJMS161126011
Zabed H, Faruq G, Sahu JN, Azirun MS, Hashim R,1 and Boyce AN (2014) Bioethanol production from fermentable sugar juice. Sci World J 2014:957102 https://doi.org/10.1155/2014/957102
Zare B, Niazi A, Sattari R, Aghelpasand H, Zamani K, Sabet MS, Moshiri F, Darabie S, Daneshvar MH, Norouzi P, Kazemi-Tabar SK, Khoshnami M, Malboobi MA (2015) Resistance against rhizomania disease via RNA silencing in sugar beet. Plant Pathol 64:35–42. https://doi.org/10.1111/ppa.12239
Zhang CL, Chen DF, Kubalakova M, Zhang J, Scott NW, Elliott MC, Slater A (2008a) Efficient somatic embryogenesis in sugar beet (Beta vulgaris L.) breeding lines. Plant Cell Tissue Organ Cult 93:209–221. https://doi.org/10.1007/S11240-008-9364-2
Zhang CL, Xu DC, Jiang XC, Zhou Y, Cui J, Zhang CX, Chen DF, Fowler MR, Elliott MC, Scott NW, Dewar AM, Slater A (2008b) Genetic approaches to sustainable pest management in sugar beet (Beta vulgaris). Ann Appl Biol 152:143–156. https://doi.org/10.1111/J.1744-7348.2008.00228.X
Zhong X, Zhou Q, Cui N, Cai D, Tang G (2019) BvcZR3 and BvHs1pro-1 genes pyramiding enhanced beet cyst nematode (Heterodera schachtii schm.) resistance in oilseed rape (Brassica napus L.). Int J Mol Sci 20:1740. https://doi.org/10.3390/ijms20071740
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mulet, J.M. (2022). Shaping the Sugar Beet of Tomorrow: Current Advances in Sugar Beet Biotechnology and New Breeding Techniques. In: Misra, V., Srivastava, S., Mall, A.K. (eds) Sugar Beet Cultivation, Management and Processing. Springer, Singapore. https://doi.org/10.1007/978-981-19-2730-0_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-2730-0_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2729-4
Online ISBN: 978-981-19-2730-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)