Abstract
Despite many advancements that have been made in the field of cardiovascular research, heart failure is still associated with a poor prognosis and high mortality worldwide. The discovery that adult cardiomyocytes have an extremely low turnover rate has prompted researchers to investigate stem cells as a therapeutic option for cardiac regeneration. With stem cell therapy now approaching its third decade, numerous clinical trials have been completed and questions surrounding stem cell therapy are starting to be answered. It is now fairly well established that stem cell therapy has a positive safety profile, but only has produced neutral to moderately positive clinical outcomes. It is also clear that current stem cell therapies lack a significant ability to engraft and remuscularize the myocardium, suggesting cardiac repair occurs primarily through paracrine signaling mechanisms. Innovative strategies involving cardiac bioengineering, cell-free biomolecules, and combination therapies likely hold the key to advancing the field to the next stage. Looking ahead, we remain cautiously optimistic that stem cell therapeutics will have a significant place in the future of cardiovascular treatments, but there are still many questions that need to be answered before routine clinical application is possible.
MRH and IW collected the data and wrote this chapter. RA devised the project, developed the main conceptual ideas and edited the manuscript. All authors revised and approved the final version
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Cardiovascular disease is a significant worldwide health issue that consistently ranks among the leading causes of death and disability-adjusted life years lost (Abbafati et al. 2020). Cardiovascular disease is an umbrella term used for numerous cardiac and peripheral vascular pathologies including heart failure. Heart failure (HF) is a global epidemic that impacts the quality of life, life expectancy, and the economics of the health care system. In the USA and Europe, HF is the leading cause of hospitalization with over one million admissions as a primary diagnosis (Ambrosy et al. 2014). Moreover, it represents up to 2% of total hospitalizations in these regions, with minor improvements in post-discharge mortality rates. It is estimated that there are over six million people in the USA living with HF, and this number is expected to increase to over eight million by 2030 (Mozaffarian et al. 2016; Virani et al. 2021). Globally, HF affects 26 million people, imposing an immense burden on international health care systems and society as a whole (Cheung and Jahan 2020).
Heart Failure
Heart failure is a complex clinical syndrome characterized by structural and functional abnormalities which impair ventricular filling or ejection of blood (Gaglianello et al. 2016). These changes in cardiac function are highly problematic as they result in inadequate perfusion of vital organs and peripheral tissues. This mechanical pump failure consequently results in an inability of the heart to meet the body’s metabolic demands. Heart failure is commonly classified as HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF). HFrEF is defined as a left ventricular ejection fraction (LVEF) of less than 40%. Conversely, HFpEF is defined as an LVEF greater than 50% (Gaglianello et al. 2016). These classifications are essential, as they provide the information necessary to determine the appropriate management strategy and prognosis of the disease. Another important point to consider is the clinical severity of HF, which is graded based on the New York Heart Association (NYHA) functional classification. It defines four functional classes of heart failure – from class 1 being the least severe to class IV being the most severe (Inamdar and Inamdar 2016). Patients can move between classes relatively quickly as this classification system focuses on symptoms only. Moreover, the American College of Cardiology and the American Heart Association (ACC/AHA) HF staging system was created to complement the NYHA functional classification. With the ACC/AHA classification, there is no moving backward to prior stages. Once symptoms have developed, the patient is in stage C HF and will never again be classified in stage B. Together, the NYHA functional classification and the ACC/AHA clinical tools are useful in estimating the progression and future outcome of the disease.
The causes of heart failure are numerous – coronary artery disease, diabetes, hypertension, idiopathic cardiomyopathies, pressure overload, volume overload, cardiotoxic drugs, metabolic conditions, and inflammatory and hereditary conditions are common culprits. Nonetheless, the most common etiology of HF remains myocardial infarction (MI) (Tanai and Frantz 2015). Myocardial infarction results in ischemia, necrosis of cardiomyocytes (CMs), and fibrotic scar tissue formation (Mouton et al. 2018). The extent of CM damage depends on the duration of ischemia and the size of the zone of infarction. If the MI is not treated on time, severe structural and functional impairments ensue. Following an MI, compensatory mechanisms such as activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system work together to maintain adequate cardiac output and blood flow to vital organs (Gaglianello et al. 2016). Over time, these responses are often insufficient as they result in fluid retention and adverse remodeling of the chambers of the heart. Cardiac remodeling typically results in CM hypertrophy, resulting in decreased cardiac contractility. Following these changes, a progressive decline in cardiac function is observed until the heart ultimately fails to pump completely (Tables 1 and 2).
Due to the high quality of medical care and revascularization techniques such as coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI), survival rates following MI have increased (Skinner and Cooper 2011; Ambrosy et al. 2014). However, as patient survival increases, cases of HF are rapidly growing. Symptoms of HF are frequently homogenous, yet the progression of the disease and the pathological processes are diverse, warranting a holistic approach to treatment. It is recommended that patients incorporate both pharmacological and nonpharmacologic therapies to improve the functional capacity of the heart – reducing its workload (Gaglianello et al. 2016). Triple therapy, consisting of diuretics, angiotensin-converting enzyme inhibitors (ACE inhibitors), and beta-blockers, are some of the most common symptom management strategies for HF patients and have been particularly beneficial in HFpEF. Moreover, implantable cardiac defibrillators and ventricular assist devices are options for more severe cases. Heart transplantation is considered the gold standard for treatment, yet it yields a high cost, immune reactions are common, and there remains a major discrepancy between the availability of donors and recipients (Rojas and Haverich 2019; Mohite et al. 2015; Liu et al. 2020). Despite many therapeutic options available, the prognosis of HF remains poor, with a 5-year mortality rate of around 50% and a 10-year survival rate of only 20% (Gaglianello et al. 2016). These statistics warrant a treatment that can stop the progressive nature of the disease while restoring cardiac function. Due to the limited endogenous regeneration potential of the heart, stem cells as a therapeutic option to treat HF have gained interest globally. In the last few decades, stem cells have been extensively studied to treat patients living with HF.
Regenerative Potential of Stem Cells
The use of stem cells to generate healthy cells or replace diseased cells is not a novel idea. Historically, doctors have performed stem cell transplants – also known as bone marrow transplants. In these transplants, stem cells replace cells that have been damaged by disease or chemotherapy and serve as a vehicle for the recipient of these cells to fight off various forms of hematological cancers – leukemia, lymphoma, and multiple myeloma. In regenerative medicine, the unique potential of stem cells is based on two important qualities: the ability to self-replicate and the potential to differentiate into mature, functional cell phenotypes (Zakrzewski et al. 2019).
Stem cells can be divided into two broad categories – pluripotent stem cells (PSCs) and adult stem cells. Adult stem cells are more differentiated and are limited to give rise to only certain sets of cells or tissues of the body (Singh et al. 2016). These can be broken down into derivatives from the bone marrow, cardiac tissue, skeletal muscle, umbilical cord, and adipose tissue. Human ESCs are termed pluripotent, meaning that they can divide into any cell type found in the body. These cells are incredibly versatile and can be used to repair or regenerate damaged or diseased tissues and organs. However, they are limited by important ethical implications as they arise from the blastocyst of an embryo. Novel protocols have been developed to reprogram adult stem cells to have properties of embryonic stem cells (Takahashi and Yamanaka 2006). The cells termed induced pluripotent stem cells (iPSCs) were developed by genetic reprogramming techniques to alter the potency level of mature somatic cells.
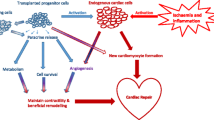
When considering stem cells as an option for cardiac regeneration, some cells are more specialized than others. Also, they can secrete different levels or combinations of various bioactive messengers, and some have different functions altogether through the expression of various cell markers (Sobhani et al. 2017). Stem cells can be transplanted directly into the heart, allowing engraftment, direct differentiation, and replacement of diseased cells. Conversely, some stem cells trigger a paracrine effect, involving the secretion of various chemical messengers that stimulate the patient’s nearby cells to repair damaged tissue. To recognize and compare the therapeutic potential of the various stem cell types, it is important to understand their therapeutic potential along with the mechanisms in which they work. To date, ESCs, iPSCs, and various adult stem cell lineages such as bone-marrow-derived stem cells (BMDCs), cardiac stem cells (CSCs), and skeletal myoblasts (SMs) have either been tested or are currently undergoing clinical trials in the treatment of cardiac disease (Fig. 1).
Endogenous Cardiac Regeneration
It is well known that the human heart lacks any significant regenerative capacity in response to myocardial injury or chronic disease. This explains why myocardial injury is so problematic. An acute left ventricular MI that kills over 25% of the CMs in the LV can ultimately lead to chronic heart failure (Murry et al. 2006). Although the heart may not adequately repair itself after injury, it is becoming increasingly clear that the heart is not a postmitotic organ. Research in the last couple of decades has revealed that CM renewal does occur, albeit at a very slow rate. The current consensus is that in a healthy, adult human heart, the CM turnover rate is approximately 0.5–1% per year, and this rate decreases over the life span (Eschenhagen et al. 2017). A study using carbon 14 dating techniques in human cardiomyocyte DNA demonstrated that adult CM turnover takes place at a rate of 1% annually at age 20 and 0.3% at age 75. This equates to approximately 50% of the entire cell population being turned over in one’s lifespan (Bergmann et al. 2009). This evidence was corroborated in a separate study that again demonstrated that CM renewal is greatest during the neonatal period and decreases in adulthood to less than 1% annually. Of note, turnover rates of cardiac endothelial cells and mesenchymal cells (MSCs) were slightly higher, at >15% and <4%, respectively (Bergmann et al. 2015).
Interestingly, anecdotal evidence supports the claim that cardiac regeneration in neonatal populations may be higher than initially thought. In 2015, a case report was published on a newborn child with thrombotic occlusion of the left anterior descending artery for >20 h, causing severe acute myocardial infarction. The myocardial damage was marked by elevated troponin levels, electrocardiogram, echocardiography, and cardiac angiography abnormalities. Miraculously, within weeks the child’s heart function began to recover, and at 1-year post-MI, there was no distinguishable difference in heart function or morphology compared to a healthy 1-year-old infant. A similar phenomenon of functional cardiac repair has been demonstrated in newborn murine models of severe MI and cardiac apex resection (Haubner et al. 2012; Porrello et al. 2011). This raises a pertinent question regarding the mechanism of new cardiomyocytes regeneration. Unfortunately, the answer remains elusive. Researchers hypothesize that new cardiomyocytes possibly originate from three potential sources: resident cardiac progenitor cells, proliferation of preexisting cardiomyocytes, or migration of extra-cardiac stem cells to the myocardium (Eschenhagen et al. 2017). We also do know that during embryonic development the heart enlarges primarily through CM proliferation. One month after birth, CM numbers are already at their highest and will remain constant across one’s lifetime. In the second decade of life, cardiac growth occurs via cellular hypertrophy, and an increase of cardiomyocyte DNA content via polyploidization (Bergmann et al. 2015).
Determining the source and the extent of normal physiologic cardiac regeneration and renewal of CMs post-injury is a delicate but critical task. Identifying pathways that could be manipulated and amplified for therapeutic purposes remains a key goal for future research. Given that the endogenous turnover of CMs is relatively low, prevention of proapoptotic pathways immediately after cardiac injury and the stimulation of endogenous proliferative pathways may be the key to the cardiac regeneration puzzle. For example, researchers have recently begun investigating the Hippo-YAP pathway, with the hope of manipulating this intrinsic regenerative mechanism to influence cardiac regeneration.
Outcomes of Clinical Studies
Successful stem cell therapy depends on a multitude of outcomes that are observed in patients post-administration. Improvements in mortality, morbidity, and quality of life indexes such as the NYHA functional class, Minnesota Living with HF Questionnaire (MLHFQ), and distance on the six-min walk test with exercise capacity are commonly taken into consideration. In terms of heart function, stem cells should be able to improve left ventricular ejection fraction (LVEF), decrease end-systolic volume (ESV), end-diastolic volume (EDV), atrial natriuretic peptide (ANP), and brain natriuretic peptide (BNP). Lastly, the successful application of stem cells in HF relies heavily on the capacity for engraftment and survival of such cells into the host myocardium, the potential for revascularization and angiogenesis, and the capacity to electromechanically couple with resident CMs, allowing them to beat as a functional syncytium (Gerbin and Murry 2015). Most importantly, successful therapy needs to demonstrate a favorable safety profile, with minimal to no adverse events. Common concerns would be any cardiovascular events, cardiac arrhythmias, immune rejection, and teratoma formation. Past, present, and future clinical trials using various types of stem cells as a treatment for cardiac disease, and specifically HF, will be discussed in the following section in detail.
Pluripotent Stem Cells
In an attempt to regenerate the myocardium, some researchers have adopted an upstream approach to cellular-based therapies, using pluripotent stem cells (PSCs) and differentiating them into cardiac progenitors or functional CMs. The two basic approaches to creating PSC-derived CMs begin with either the extraction of human ESCs or the creation of iPSCs from mature somatic cell types. PSCs possess the unique ability of unlimited self-renewal and the ability to differentiate into functional progenitor cell types (Kadota and Shiba 2019). These two unique qualities allow researchers to differentiate a significant number of PSCs into functional CMs (Kadota and Shiba 2019). The newly generated CMs can then be implanted into the damaged tissue, with the ultimate goal of integration into the host myocardium. Recently, there has been increasing preclinical evidence accumulating on the efficacy of both iPSCs and human ESCs in the treatment of HF. Researchers have demonstrated that human PSCs can be successfully differentiated into functional CMs that display necessary electrophysiologic properties, calcium handling abilities, and contractile proteins (Kadota et al. 2013). Various animal models including rat, pig, and nonhuman primates have been used to show that human-ESC-derived-CMs can functionally engraft into the host myocardium and improve contractile function (Kadota et al. 2020).
To date, the phase I ESCORT trial (NCT02057900) conducted out of France is the only published clinical trial investigating human PSC-CMs in humans with HF (Menasché et al. 2018). This trial investigated the safety and feasibility of implanting human-ESC-derived CD15+ Isl-1+ cardiac progenitor cells (hESC-CP) in patients with severe ischemic left ventricular dysfunction. Six patients had a fibrin patch embedded with hESC-CPs transplanted epicardially as an adjunct to CABG. Safety was measured at 1-year follow-up for three primary endpoints: cardiac or off-target tumors assessed by CT and PET scan imaging, arrhythmias detected by serial interrogations of cardioverter-defibrillators, and alloimmunization assessed by detection of donor-specific antibodies. During 1-year follow-up, none of the patients exhibited any arrhythmias, and no tumors were detected in any of the patients included in the follow-up. Three patients developed low-level donor-specific antibodies; however, none of the patients had any clinically relevant complications due to the alloimmunization. Although efficacy was not a primary outcome in the study, all patients at the 1-year endpoint demonstrated symptomatic improvement from the baseline determined by a decrease in the NYHA functional class score and improvements in distance during the 6-min walk test. Patients also showed a modest increase in LVEF and a significantly improved left ventricular wall motion score measured via transthoracic echocardiogram. Although there is only one completed clinical trial using PSC-CMs in humans, there are currently two ongoing clinical trials utilizing iPSCs-CMs and likely several others in the near future. The first trial is an open-label clinical trial in China (HEAL-CHF, NCT03763136) assessing the safety and feasibility of direct epicardial injection of allogeneic human iPSCs-CMs in five patients with HF. The second clinical trial was launched in Japan in 2020 to implant cellular sheets of iPSCs-CMs into patients with ischemic cardiomyopathy. There are relatively few known details about the trial, but it is enrolling ten patients over 3 years and aims to assess the efficacy and safety of iPSC-CMs in humans at the 1-year postoperative stage (Cyranoski 2018).
There remain some real concerns regarding the use of iPSC-CMs in clinical settings. Preclinical trials in porcine and nonhuman primate models demonstrated nonlethal transient ventricular arrhythmias posttransplantation assessed by electro-anatomical mapping (Shiba et al. 2016; Romagnuolo et al. 2019). Incidentally, the arrhythmias were shown to be of graft-site origin. Two proposed mechanisms are believed to be contributing to the ectopic pacing: immaturity of the neomyocytes and automaticity (Kadota et al. 2020). No arrhythmias were observed in the human clinical trial. Besides arrhythmias, poor long-term engraftment of PSC-CMs remains a primary concern and this issue has been discussed in depth in a later section of the chapter. Lastly, immune rejection of the allogeneic donor cells is a potential problem that necessitates immunosuppression. However, Menasché et al. (2018) have demonstrated that three patients developed alloimmunization, but it was clinically silent.
Skeletal Myoblasts
Skeletal myoblasts (SMs) are harvested under the basal lamina of muscle fibers. This cell lineage is an attractive option for regenerative therapy as it provides an abundant source of readily available cells with myogenic differentiation potential (Haider et al. 2004a). Moreover, they can be easily expanded undifferentiated in culture to achieve the required number for cell-based therapy (Sim et al. 2003). Interestingly, SMs are immuno-privileged and need only transient immunosuppression for successful cross-species transplantation (Haider et al. 2004b). These data have led to first-in-man allogenic SMs transplantation in patients (Law et al. 2003). Preclinical trials found that SMs could improve cardiac function by differentiating into functional CMs in animal models (Taylor et al. 1998; Chiu et al. 1995; Ye et al. 2007). SMs are excellent carriers of therapeutic genes due to their robust nature. Hence, they have been genetically modulated for the angiomyogenic repair of the heart in experimental settings (Lei et al. 2007, 2011). They have also been preconditioned to enhance their post-engraftment survival and improve their reparability (Niagara et al. 2007; Elmadbouh et al. 2011). SMs have also been reprogrammed to develop induced pluripotent stem cells for myocardial repair, however, not without the possibility of myocardial tumorigenesis (Ahmed et al. 2011a, b).
Researchers quickly pushed SMs into human clinical trials with hopes to achieve similar results (Menasché et al. 2001; Sim et al. 2003; Siminiak et al. 2004). Unfortunately, researchers could not reproduce the successful findings in human clinical trials. The MARVEL study tested the intramyocardial (IM) injection of SMs in a sample of 14 patients, but ultimately resulted in an increased risk of developing ventricular tachycardia (Povsic et al. 2011). In addition, there were no significant improvements in LVEF or Minnesota Living with HF scores at the 6-month follow-up. The SEISMIC trial investigated the IM transplantation of autologous SMs in a cohort of 40 patients. It also found that LVEF and global heart function improvements were not statistically significant at 6 months following administration (Duckers et al. 2011). Serious adverse events were common, yet there were no differences in incidence between the cell therapy group and the control group.
Similar to the MARVEL and SEISMIC studies, the MAGIC trial also administered SMs via IM route. In a sample of 120 patients, it was found that SMs did not improve LVEF or global cardiac function (Menasché et al. 2008). This was confirmed on a 5-year follow-up (Brickwedel et al. 2014). More significant risks of arrhythmias were noted upon administration of SMs and this was determined to be the primary concern regarding this cell lineage. Skeletal myoblasts are deprived of gap junctions and therefore lack electromechanical coupling potential, forming the basis of arrhythmia formation (Reinecke et al. 2002; Abraham et al. 2005). Though more studies with larger sample sizes could indicate whether or not SMs could be incorporated in HF treatment in the future, researchers have transitioned away from this phenotype due to their apparent lack of efficacy and safety concerns.
Bone Marrow-Derived Stem Cells
Bone marrow-derived stem cells are one of the most heavily tested cells in the treatment of cardiac disease to date. Researchers believe that autologous bone marrow mononuclear cells (BMMNCs) can improve heart function through angiogenesis and direct cardiomyocyte regeneration within the myocardium (Hu et al. 2011). The first ever published clinical trial using autologous (BMMNCs) included 21 patients with chronic HF who received the cells via transendocardial route. There were no safety concerns, and after 4 months, significant increases in LVEF, reductions in ESV, and improvements in perfusion and myocardial contractility were observed (Perin et al. 2003). Other clinical trials, such as the TOPCARE-CHD, showed significant improvements in global cardiac function, regional contractility, and mortality besides a decrease in ANP and BNP in response to transcoronary BMMNCs injection (Assmus et al. 2007). Similarly, improvements in LVEF, infarct zone, exercise capacity, NYHA functional class, and long-term mortality were observed up to 5 years after intracoronary (IC) administration of autologous bone marrow cells in the STAR-heart study (Strauer et al. 2010). The initial success of bone marrow-derived stem cells (BMDSCs) prompted the creation of more extensive phase II trials such as the FOCUS-CCTRN and the CELLWAVE, where autologous BMMNCs were administered via transendocardial and IC route, respectively. The initial excitement created by the smaller clinical trials data was short lived, as no significant improvements in LVEF, maximal oxygen consumption, infarct size, and reversibility of ischemia were observed in the larger phase II trials (Perin et al. 2012; Assmus et al. 2013). In the TAC-HFT trial, patients received either transendocardial injections of autologous BMMNCs, autologous MSCs, or placebo. Results showed that only MSC-based therapy decreased infarct size and improved the 6-min walk test distance and regional function of the heart (Heldman et al. 2014). No improvement was noted in LVEF. The Cardio133 clinical trial reported significant adverse events in patients receiving CD133(+) bone marrow cells delivered via CABG. Although some improvements in scar size and perfusion were observed, it was found that the injection of CD133(+) cells had neither effect on clinical symptoms of HF nor global LV function (Nasseri et al. 2014).
A systematic review and meta-analysis including 1907 participants and a total of 38 randomized controlled trials found low-quality evidence that BMDSCs improved LVEF and mortality during short-term and long-term follow-up (Fisher et al. 2016a). Moreover, there was little evidence that BMDSCs improved NYHA functional class in HF patients. Though periprocedural adverse events were uncommon and serious adverse events were rare, there is no current consensus on whether or not this cell type is truly efficacious in improving outcomes. Fisher et al. applied the trial sequential analysis of two Cochrane reviews to overcome the limitations of meta-analyses (Fisher et al. 2016b). Randomized controlled trials using autologous BMDSCs to 2739 patients with acute MI and 1094 patients with HF were included in the analysis. It was found that although there is insufficient evidence to determine the treatment effect in acute MI, there is solid evidence that the administration of autologous BMDSCs reduced mortality and rehospitalization for those with HF. It was also found that BMDSCs did not improve LVEF by more than a mean difference of 4% in patients with acute MI or HF (Fisher et al. 2016b). Results of this analysis must be confirmed by an adequately powered double-blind phase III trial. Given these marginally positive findings, there is currently no consensus on whether or not BMDSCs will have a future role in treating HF patients. However, BMDSCs seem to have a favorable safety profile as there are generally few safety concerns.
Mesenchymal Stem Cells
Mesenchymal stem cells are a subtype of BMDSCs. Though found primarily in the bone marrow, they are also located in other areas of the body, including adipose tissue, blood, and the umbilical cord, commonly referred to as Wharton’s jelly (Menasché 2018; Mathiasen et al. 2020; Zhao et al. 2015). Among the different BMDSCs types, MSCs primarily act via paracrine signaling mechanisms and, for this reason, seem to show great promise for regeneration of the myocardium (Menasché 2018). Paracrine signaling allows for pro-angiogenic effects by releasing vascular endothelial growth factor (VEGF), insulin growth factor-1 (IGF-1), and stem cell growth factor (Natsumeda et al. 2017; Liu et al. 2020). Moreover, the paracrine effect stimulates the proliferation and differentiation of endogenous cardiac progenitor cells (CPCs) (Natsumeda et al. 2017). These CPCs have pro-angiogenic capabilities and the potential to promote the differentiation of existing cardiac cells, though evidence of their long-term engraftment is lacking (Williams et al. 2013). In addition to paracrine signaling, MSCs can directly contribute to vascular proliferation and direct myocardial regeneration on a greater scale (Tehzeeb et al. 2019; Lalu et al. 2018). This cell lineage also has important repair properties through immunomodulation, anti-fibrotic, pro-angiogenic, and anti-oxidative effects (Turner et al. 2020). One unique characteristic of MSCs is that they do not express major histocompatibility complex class II (MHC II) antigens. Hence, MSCs are good candidates for allogeneic sourcing, as they are nonimmunogenic (Nair and Gongora 2020). Together, these qualities make MSCs great contenders for treating chronic HF. A meta-analysis investigating 52 preclinical studies showed that MSCs were moderately associated with significant improvements in LVEF and showed that cardiac cell therapy is safe and not associated with increased mortality (van der Spoel et al. 2011). A recent systematic review investigated the impact of MSCs as a treatment for nonischemic dilated cardiomyopathy (Hoeeg et al. 2020). In total, 27 studies were included; however, most studies involved preclinical animal models (3 clinical and 24 preclinical trials). Of these, 21 of the included trials tested bone marrow-derived MSCs, 4 tested human umbilical cord MSCs, and 3 tested adipose tissue-derived MSCs. It was found that bone marrow, umbilical cord, and adipose tissue-derived MSC treatment can improve cardiac function in nonischemic dilated cardiomyopathy through main mechanisms of anti-fibrotic and anti-apoptotic effects, angiogenesis, and immunomodulation. This suggests that independent of the cell origin, all MSC types share synonymous mechanisms of action. All three clinical trials and 22 of the 24 preclinical trials reported improvements in cardiac function following administration of MSCs.
Thus far, human clinical trials utilizing MSCs in HF have yielded exciting results. The results of the first placebo-controlled trial, MSC-HF, indicated that the IM injection of autologous MSCs is safe, reduces hospital admissions, and improves myocardial function (Nigro et al. 2018). In addition, the TAC-HFT trial suggested that MSCs were superior to BMMNCs in reducing infarct size and improving regional heart function in patients with chronic HF (Heldman et al. 2014). Other studies like the POSEIDON trial compared the transendocardial delivery of autologous versus allogeneic MSCs in patients with HF. Interestingly, both autologous and allogeneic MSCs improved LV function while reducing infarct size and adverse cardiac remodeling without any safety concerns highlighted (Hare et al. 2012). Five years later, results of the POSEIDON-DCM trial were released. Overall, it was demonstrated that more significant improvements were observed in allogeneic MSCs versus autologous MSCs in patients with nonischemic dilated cardiomyopathy. There were more significant improvements in quality of life, functional capacity scores such as the NYHA functional class and MLHFQ, the 6-min walk test, and improvements in ejection fraction (Hare et al. 2017). Just like the POSEIDON trial, the transendocardial delivery of both autologous and allogeneic MSCs in the POSEIDON-DCM trial had no notable safety concerns. Though more extensive trials are required to determine which cell source is more favorable, evidence supports the superiority of allogeneic MSCs regarding the efficacy and endothelial function. Another trial led by Mathiasen et al. (2020) administering MSCs via IM injection resulted in improvements in LVEF, stroke volume, and myocardial mass in patients with HF. A larger trial, the DREAM-HF trial with 566 patients enrolled, was recently completed. It evaluated the efficacy of allogeneic mesenchymal precursor cells (MPCs) in patients with advanced chronic HF. Results are pending.
A systematic review and meta-analysis investigating the efficacy and safety of MSCs in ischemic and nonischemic cardiomyopathies determined that of the 29 clinical trials, the vast majority demonstrated improvements in LVEF, LVESV, NYHA functional class, and exercise capacity without significant safety concerns (Poulin et al. 2016). Patients who received stem cells as an adjunct to CABG had the most remarkable improvements in LVEF, which justifies the role of catheter-based revascularization. Based on this data, it seems that MSC therapy may be a feasible option in improving cardiac function while decreasing adverse cardiac remodeling in patients with HF. Another systematic review and meta-analysis reviewed the safety and efficacy of MSCs in 12 trials focused on ischemic HF. There were significant improvements in LVEF; however, there were no significant improvements in mortality. Additionally, several studies within the meta-analysis showed an increase in quality of life and physical performance. However, quality of life and performance status were inconsistently reported in studies, which limited the ability to provide conclusions (Lalu et al. 2018). Most importantly, there seems to be no association between treatment with MSCs and adverse events, suggesting a favorable safety profile.
Other descendants of MSCs such as cardiopoietic and umbilical cord MSCs have been studied in clinical trials. These are more specialized cells derived from a pure MSC lineage. Cardiopoietic stem cells are derived from MSCs in the bone marrow and are of particular interest in regenerative medicine. One of the first using cardiopoietic cells, the C-CURE trial, demonstrated significant improvements in LVEF, quality of life, and lower LVESV 2 years after administration into the heart with no safety concerns noted (Bartunek et al. 2013). This small-scale study catalyzed the formation of the CHART-1 trial, which had a greater sample size of 351 individuals. Interestingly, this study shared similar results to the C-CURE trial, indicating that cardiopoietic cells have the potential to provide long-lasting benefits toward cardiac function in those with HF (Teerlink et al. 2017; Bartunek et al. 2017). Wharton’s jelly, a gelatinous substance present within the umbilical cord, is rich in MSCs, which have been tested in various clinical trials. Of these, a study led by Zhao et al. (2015) studied the delivery of umbilical cord MSCs via IC route in combination with various medications, such as ACE inhibitors or ARBs, beta-blockers, diuretics, and digoxin. Twenty-four hours after transplantation, symptoms of HF such as cough, dyspnea, and chest tightness were alleviated, though no improvements were noted in LVEF. There were some improvements in the 6-min walk test, NT-pro BNP levels were significantly lower than the control group, and improvements in mortality rates were observed. Given these positive results, it is crucial to link these improvements with the medications given in combination with the MSCs. In the RIMECARD trial, the intravenous (IV) infusion of umbilical cord MSCs were found to improve LVEF but did not reduce LVESV or LVEDV (Bartolucci et al. 2017). No significant safety concerns were noted.
Though MSCs have massive potential in regenerative medicine, there is a need for larger, international clinical trials to fully elucidate the field of MSC therapy in humans living with HF. The surge of incoming clinical trials, including the first phase III clinical trial, should help clarify the true therapeutic potential of MSCs in HF. Put together, the meta-analysis by Lalu et al. demonstrated that MSCs could improve LVEF and enhance the quality of life and performance, that too without any major safety concerns (Lalu et al. 2018).
Cardiac Stem Cells
Cardiac stem cells (CSCs) were among the most heavily researched cells in cardiac regenerative medicine. Clinical research led by Dr. Piero Anversa showed great promise for the application of CSCs to treat heart failure. He claimed that CSCs produced functional myocardial changes and they were a viable option to treat heart failure. These claims sparked a great interest in the medical community and the public (Chien et al. 2019). Many researchers attempted to replicate Anversa’s studies albeit without success. It was soon discovered that the field of CSCs was heavily compromised and Anversa and his group were accused of scientific misconduct. As a result, the Brigham and Women’s Hospital along with the Harvard Medical School took action by launching an investigation on Anversa’s work. In 2014, the SCIPIO clinical trial using c kit+ CSCs was retracted, and by 2018, the investigations revealed that 31 publications contained falsified or fabricated data (Ozkan 2019). The National Institute of Health also stepped in by suspending the CONCERT-HF trial due to its lack of scientific foundations (Bolli et al. 2018). This clinical trial was the first to evaluate the combination of CSCs and MSCs as a treatment of HF.
To date, c-kit+ CSCs and cardiosphere-derived cell (CDC) phenotypes have been studied in clinical trials. The CADUCEUS trial demonstrated that the IC injection of CDCs reduced scar tissue size and improve regional contractility and heart mass on MRI (Makkar et al. 2012). There were no differences in ESV, EDV, and LVEF. There were no significant adverse events, alluding to a favorable safety profile for CDCs. The TAC-HFT-II trial will soon compare therapy with autologous MSCs alone vs MSCs combined with c-kit+ CSCs (Bolli et al. 2018). Without question, the implications of Piero Anversa’s 31 retracted studies will have a long-lasting effect on the field of CSCs. The findings of these investigations have created a significant distrust of the scientific community and discredited the current advancements made in this field. Although other clinical trials are currently investigating the feasibility and efficacy of CSCs and cardiac-derived stem cells, clinical benefit has yet to be demonstrated for patients. Moreover, CSC isolation is invasive as it requires a heart biopsy, and culture requires many days before injecting adequate numbers (Nigro et al. 2018). In the future, it is of utmost importance that rigorous scientific standards are followed when conducting clinical trials to protect the integrity of research and protect patients (Tables 3 and 4).
Understanding the Factors Affecting Cell-Based Therapy
Though the safety profile of stem cells appears to be satisfactory, their overall efficacy is, at best, modest. For successful cell therapy to treat HF, a greater understanding of the factors surrounding the application of cellular treatment is warranted.
Engraftment, Survival, and Rejection
One of the most critical impediments in stem cell therapy is their ability to engraft and survive in the heart post-administration, while avoiding immune rejection from the recipient. Both preclinical and clinical trials show that cell retention in the heart 24 h post-administration does not exceed 10% (Hou et al. 2005; Aicher et al. 2003; Blocklet et al. 2006). This is likely due to a poor engraftment potential along with the rapid washout of cells once they are injected into the heart (Terrovitis et al. 2009). These shortcomings have prompted the development of improved cell retention approaches, such as plugging the injection site with a fibrin compound to prevent backflow of cells, and transplantation of constructed cell sheets besides the use of bioengineered natural or synthetic polymers (Chiu et al. 2012; Terrovitis et al. 2009). Autoimmune rejection of transplanted cells is another crucial risk to mitigate, mainly when the source is allogeneic. This has resulted in a push for cells that require minimal to no immunosuppression, such as ESC-derived cells, MSCs, and MPCs. Future research focusing on cell retention while decreasing the risk of immune rejection will continue to improve the efficacy and feasibility of stem cell therapy for HF.
Dosage
The optimal dosage of stem cells to reach therapeutic effect is still unknown, and the evidence is conflicting. Studies show large variations in dosing from 1 × 106 to 2 × 108 cells per dose administered per patient (Madonna et al. 2014). Though these include a large number of cells administered to a patient, it is not enough, as on average, one billion CMs are lost following an MI (Robey et al. 2008). There is a need for larger doses of stem cells to be administered if the goal is to replace lost CMs following MI. However, this may not be the case, as several clinical trials have demonstrated that smaller doses of stem cells are more effective than higher doses, possibly due to increased paracrine and cytoprotective factors release, activation of angiogenesis, and induced cardiomyocyte hypertrophy (Nigro et al. 2018). Whereas large doses of stem cells may increase the potential for remuscularization, cell engraftment and survival are pretty low, and large doses may aggregate in areas of the heart, thus increasing the risk of arrhythmias (Prockop and Olson 2007). Also, the exact timing of cell administration has not been confirmed – though it is hypothesized that the longer the time interval between MI and administration of stem cells, the less the patient is likely to benefit. Current and future clinical trials will hopefully address these controversies and challenges.
Cell Type
Many different cell types have been tested in clinical trials, yet there is no current consensus on the type that is best suited for cardiac regeneration. In an ideal world, the perfect stem cell would be able to proliferate, engraft, and survive in ischemic areas, along with the ability to induce paracrine effects to stimulate endogenous cardiac regeneration. Moreover, it would have the potential to contract and electromechanically couple with the host CMs. At the moment, no cell has met all of these expectations in clinical trials (Gerbin and Murry 2015). The quality of the cell in question is also important to consider, as older age and preexisting comorbidities limit cell potency and regenerative potential (Nigro et al. 2018). Based on these findings, allogeneic sources of cells may present greater advantages over autologous sources. It is noteworthy that allogeneic cells can be cultivated and isolated from younger, healthy donors devoid of comorbidities and genetic defects. Allogeneic cells can be screened for quality and stored as an “off the shelf” product, making them readily available for acute applications. Lastly, allogeneic stem cells are more appealing in the eyes of the pharmaceutical industry as they provide superior profit margins versus autologous stem cells (Nigro et al. 2018). However, one must be wary of this conflict of interest, as we cannot sacrifice the quality of the product over the financial interests of external parties.
Currently, MSCs have been of particular interest in trials due to their ease of isolation and extraction, their multipotent differentiation potential, low immunogenicity, and their potential to trigger paracrine effects (Menasché et al. 2018; Nigro et al. 2018). Recent research has focused on more pure forms of stem cells, such as CSCs to increase the regenerative potential. Though CSCs have been shown to improve some aspects of cardiac function in patients with HF, the isolation of autologous CSCs is an invasive procedure, culture takes time, and the field of CSCs is heavily compromised (Nigro et al. 2018; Ozkan 2019). Some derivatives of iPSCs, such as cardiomyocyte-derived from human iPSCs, pose some risk of tumorigenicity (Nigro et al. 2018). For the moment, the most promising types of stem cells for cardiac regeneration appear to be iPSCs and ESCs. Alternatively, different approaches to cellular therapy may involve combining different cell types to increase efficacy. The rationale behind combining cells revolves around the activation of various regenerative pathways due to the underlying physiologic role of each cell type. Preclinical trials have shown promising results. It was found that the combination of MSCs and CPCs in the treatment of chronic HF improved cardiac outcomes versus MSCs alone or placebo (Williams et al. 2013; Karantalis et al. 2015; Natsumeda et al. 2017). Though there were some differences between the three trials, there were improvements in EF, contractility, cell retention, and decreases in infarct size versus MSCs administered alone. Since MSCs alone act primarily via paracrine signaling, they stimulate angiogenesis and the proliferation, differentiation, and migration of endogenous CPCs to the heart (Natsumeda et al. 2017). However, the evidence on long-term engraftment is lacking (Williams et al. 2013). Other preclinical studies investigated the utility of epicardial patches containing human iPSC-derived CMs combined with human MSCs versus administering either of the cell type alone. It was found that the combination therapy group showed more significant improvements in EF, cardiac fibrosis, and capillary density (Park et al. 2019). Larger randomized, double-blind trials with more extended follow-up periods are warranted to determine which combination of cell types will yield the remarkable improvements and reduce safety concerns in HF patients. Alternatively, cardiac regeneration may not rely on stem cells after all. Current studies are now investigating the use of cell-free strategies due to concerns that the administration of stem cells results in poor cellular retention rate. This approach is discussed in greater detail in section “Cell-Free Strategies.”
Route of Administration
An effective route of delivery is as important as the type of cell in question. It is one of the essential factors in successful stem cell treatment, as it can affect the potency of the cells, their retention, engraftment, and survival in the recipient’s heart (Turner et al. 2020; Nigro et al. 2018). Successful delivery of cells will depend on the ability of the cells to migrate to the target area of the heart, their engraftment potential, and the ability to function in synchrony with the heart’s natural rhythm without interference. Overall, an optimal delivery method should ensure the survival of the cells. It must be well tolerated by the patient, and the procedure should be relatively easy to perform by a clinician.
Preclinical studies have utilized various methods of delivery such as transendocardial, retrograde intracoronary sinus, open surgical epicardial injection, IM, IC, IV, and, more recently, 3D scaffolding with the help of cardiac patches (Bruyneel et al. 2016; Nakamura and Murry 2019; Mazzola and Di Pasquale 2020). The most common routes of administration include, IM, IC, IV, transendocardial, and 3D scaffolding.
Intramyocardial injection entails a direct injection of stem cells in a targeted area of the heart. These cells are usually injected along the borders of infarcted heart tissue as this provides better blood and oxygen supply for the cells to survive. This method ensures adequate blood supply to the cells, an essential component for their survival (Campbell and Suzuki 2012). This method of delivery provides the highest rate of retention, greatest engraftment, and remuscularization potential but fails to produce a significant paracrine effect (Nakamura and Murry 2019; Campbell and Suzuki 2012). This technique is more invasive and risks myocardial perforation, vascular injury, arrhythmia, and embolism. Moreover, it can be challenging to distinguish between infarcted tissue and normal myocardium. For this reason, a skilled clinician is required.
The intracoronary infusion of stem cells is the most common and safer delivery method in clinical trials. This is primarily due to the central role of catheter-based revascularization of the heart in MI (Nakamura and Murry 2019; Tehzeeb et al. 2019). Clinical trials have deemed this method superior to the IM route as it promotes the paracrine effect, is less invasive, and ensures high cell survival rates due to the rich oxygen and nutrient content in the coronary circulation. The IC injection of cells is considered to decrease the risk of inflammation and damage to the myocardium post-transplantation, while allowing for a uniform distribution of cells in the target area (Campbell and Suzuki 2012). Given these slight advantages, IC injection is associated with long-term minimal cell retention due to rapid washout in humans, resulting in inefficient remuscularization of the heart (Nakamura and Murry 2019). Moreover, large doses of cells cannot be delivered via the IC route due to risk of obstruction of the coronary arteries, ultimately resulting in ischemia and myocardial cell death (Freyman et al. 2006; Goussetis et al. 2006; Nakamura and Murry 2019).
Although the IV approach has demonstrated positive safety parameters and is one of the least invasive methods, it is less efficacious than the IM and IC route (Freyman et al. 2006; Menasché 2018). This lack of efficacy is primarily due to a lack of cell retention, and engraftment and the majority of the cells remaining trapped in the lungs. Most of the cells are eliminated by phagocytic cells in the reticuloendothelial system (Freyman et al. 2006; Bruyneel et al. 2016; Turner et al. 2020). Moreover, there are concerns about vascular occlusion that can quickly occur with systemic delivery. To improve the effectiveness of the IV method, the approaches that will enhance the cellular homing mechanisms to the heart are essential.
The transendocardial route is the most challenging technique to execute, yet it can avoid the need for open-heart surgery – reducing periprocedural risks. It has shown tremendous potential for cell retention as it deposits stem cells directly into the myocardium (Tehzeeb et al. 2019; Turner et al. 2020). Like the IM route, there is a risk for ventricular rupture and arrhythmia formation. Though some small preclinical and clinical trials have shown the safety and efficacy of the transendocardial route, larger, more robust clinical trials are necessary to evaluate the long-term success of this method.
Bioengineering of cellular materials has recently entered the field of regenerative medicine. It involves culturing and implanting stem cells in a three-dimensional vehicle to improve the rate of cell differentiation and survival at the site of the cell graft. One of the current goals is to create a scaffold that mimics the microenvironment of the heart. This scaffold can be grafted with the cell type of choice and administered to the heart (Mazzola and Di Pasquale 2020). One of the first applications of fibrin patches was in the ESCORT trial, where patches embedded with human ESC-derived CPCs were implanted epicardially during CABG (Menasché et al. 2018). This novel method of stem cell delivery has been validated in preclinical studies and yields high cell retention rates (Park et al. 2019). It was also reported that sheets containing MSCs could increase cell retention while amplifying paracrine effects to regenerate damaged heart tissue (Narita et al. 2013). Biomaterials such as collagen, hyaluronic acid, alginate, and a large variety of synthetic polymers have shown variable advantages and disadvantages (Mazzola and Di Pasquale 2020). Though the optimal combination of scaffolding materials and stem cells has yet to be confirmed in clinical trials, PSCs are better candidates in creating functional heart tissue since they have a more significant potential to integrate into the myocardium (Liu et al. 2018; Oikonomopoulos et al. 2018). MSCs combined with bioengineered materials will also hold a promising approach for heart repair. Currently, hydrogel-based scaffolds and cell sheet engineering are being studied (Oikonomopoulos et al. 2018). Cell sheets and scaffolds also avoid the risk of myocardial injury caused by direct injection.
Though many methods of delivery exist, the degree of cell retention within the myocardium remains very low. Though some preclinical studies have compared various routes of delivery, the results in human studies are pending. It is believed that the most effective method will likely be dependent on the type of cell in question, as all have their benefits and limitations (Turner et al. 2020). Intravenous delivery of stem cells is minimally invasive but has negligible cell retention potential. Intracoronary infusion of cells is the most commonly used method but presents a risk of coronary artery occlusion. The intramyocardial route has the greatest potential for cell retention, lacks paracrine effects, and requires skilled clinicians. The transendocardial route poses great potential for cell retention, yet is challenging to approach and requires skilled and experienced clinicians (Fig. 2). The type of stem cell, patient characteristics, and the degree of cardiac disease may all play a role in determining which route of administration is optimal for the patient in question (Nigro et al. 2018) (Table 5).
Hippo-YAP Pathway
It is well established that the adult heart lacks significant endogenous regenerative potential. For this reason, researchers have focused on manipulating pathways that regulate CMs proliferation to amplify endogenous cardiac regeneration. Particular attention has been paid to the Hippo signaling pathway – an evolutionary homeostatic mechanism that controls organ size (Wang et al. 2018). The primary mechanism of this pathway is to restrict cardiomyocyte proliferation once the heart has fully developed to maintain its optimal size. This pathway also inhibits cardiac regeneration, which is problematic when tissue damage occurs. Research has focused on the Hippo-YAP (Yes-associated protein) pathway to reactivate cardiac regeneration by regulating cardiomyocyte proliferation and differentiation. YAP is a downstream effector of the Hippo pathway and is responsible for triggering transcription of cell-proliferating genes while suppressing apoptotic genes. These two characteristics are instrumental in determining regenerative potential (Xin et al. 2011). Normally, YAP is inhibited by the Hippo signaling pathway, restricting cellular growth and organ size (Wang et al. 2018). It is clear that manipulating the Hippo-YAP pathway effectively enhances fetal and neonatal cardiac proliferation and regeneration; however, the question remains if YAP activation can stimulate adult CMs’ proliferation and mitigate cardiac cell death (Lin et al. 2014).
Researchers have shown that 28-day-old YAP transgenic mice post-MI had a 2.5-fold increase in cardiomyocyte proliferation, a decrease in scar size and an improvement in cardiac function compared to control mice (Xin et al. 2013). Similar results were found with the inactivation of the Hippo pathway, which led to increased DNA synthesis and cytokinesis (Heallen et al. 2013). However, the 2.5-fold increase in CM proliferation is still 20-fold lower than the observed rate in wild-type mice. This suggests that alternative mechanisms, such as mitigation of fibrosis, reduction in apoptosis, and decreasing inflammation, may contribute a large portion of the therapeutic benefit (Xin et al. 2013). Notably, the Hippo-YAP pathway stands out from other paracrine growth factors as it promotes cardiomyocyte proliferation without the possibly deleterious effects of cardiac hypertrophy (Lin et al. 2014). More detailed studies are required to confirm the manipulation of the Hippo-YAP pathway. Nevertheless, it may be a feasible tool for treating cardiac injury and triggering endogenous cardiac regeneration in humans.
Ethical Issues in Regenerative Medicine
In an attempt to lower the global burden of cardiovascular diseases, stem cells have quickly gained momentum in research and are being explored at an unprecedented rate. However, their use generates various ethical and political issues not commonly seen in other treatment modalities. The most prominent ethical issue arises from the morality of using human embryonic stem cells in research. The creation of a human ESC line involves the extraction of the inner cell mass from the blastocyst during 5–7 days of fetal development, which results in the destruction of the human embryo and the potential for human life. This ethical dilemma begs the question of when does human life truly begin. Some believe that human life begins at conception, whereas others believe that it begins further into development or even at birth (Lo and Parham 2009). A current strategy to circumvent this issue involves only using embryos that have initially been produced for reproductive purposes. Although this may ease the ethical burden for some, it is a near-impossible task appeasing all the parties involved in the matter. Alternatively, the use of other stem cell types eliminates the ethical concerns regarding the destruction of potential human life. Currently, iPSCs are seen as an attractive alternative to ESCs. They are derived from adult stem cells and genetically programmed back to a higher state of potency, making them a strong contender to the ESC. Adult stem cells can also be viable options, but their use is limited, and they lack the level of potency seen in ESCs and iPSCs (Barile et al. 2007).
Another concern regarding the use of stem cells is the growing trend of “stem cell tourism,” which describes the practice of patients seeking expensive, unproven stem cell treatments at private clinics around the world. These treatments are frequently promoted as a definite cure; however, they often lack sufficient data on clinical efficacy or safety (Ryan et al. 2010). This dangerous trend has resulted in the opening of unregulated clinics that exploit their patients for their profits, sometimes charging fees of $30,000 for unproven treatments (Regenberg et al. 2009). Unfortunately, the public is often ill-equipped to gauge whether or not treatments offered in clinics are safe and credible, and only 29% of clinics that offer stem cell treatments are accredited to do so (Connolly et al. 2014). Although the applications of stem cells are pretty exciting, cardiac regeneration is still a relatively novel concept. Particularly for HF treatment, many variables must be accounted for before stem cells can make their way into medical practices. It is imperative that scientific standards and methodologies remain at the highest degree to avoid breaches of scientific integrity, as previously seen in the study of CSCs.
What Does the Future Hold for Cardiac Regenerative Medicine?
Cell-Free Strategies
Data from preclinical and clinical trials seems to come to a reasonably consistent conclusion that low cellular retention rates and the inability to generate new CMs adequately remain a concern with current cellular strategies (Maghin et al. 2020). In addition, it postulated that a large portion of the therapeutic benefit of stem cells is derived from the biological factors that they secrete rather than direct cellular differentiation (Park et al. 2018). Recently, much attention has been paid to utilizing a cell-free approach to cardiac regeneration via administering a “cocktail” of cardio-protective paracrine signaling molecules. This combination of factors secreted by progenitor or stem cell populations includes cytokines, growth factors, and miRNAs and has been termed the “secretome.” Several strategies to deliver these paracrine factors include direct administration of individual growth factors, use of endogenous extracellular vesicles secreted by various cell types, delivery of cultured medium of stem cells, and the overexpression of proteins via modified mRNA (Liew et al. 2020).
One of the first studies that truly corroborated the validity of the paracrine hypothesis was the use of a conditioned medium of cultured human MSCs into a porcine model of acute MI (Timmers et al. 2007). It was demonstrated that a single IC injection of human MSC cultured medium into an ischemic porcine model was associated with a 60% reduction in infarct size and improvement in both systolic and diastolic cardiac function (Timmers et al. 2007). Precisely, it is thought that small EVs or exosomes may contribute a significant portion of the stem cell secretome’s regenerative effects (Kishore and Khan 2016). EVs is a collective term that describes small phospholipid MVs secreted by cells and contain biologically active compounds such as proteins, growth factors, RNA, and biolipids (Simons and Raposo 2009). Although they may be used interchangeably in the literature, exosomes refer to a subclass of extracellular vesicles that range from 40 to 100 nm and originate as endosomes (Simons and Raposo 2009). However, the source of extracellular vesicles is not limited to MSCs. A study was published delivering exosomes derived from CDCs into a porcine model of acute MI (Gallet et al. 2017). The pigs received either IC or open-chest IM injection of either placebo vehicle or exosomes 4 weeks post-MI. They demonstrated that pigs receiving CDC-derived exosomes had a preserved LV volume and LVEF, as well as histologic improvements in vessel density and cardiomyocyte hypertrophy.
Interestingly, a separate study investigated the efficacy of a mixture of human iPSC-derived CMs, endothelial cells, and smooth muscle cells versus only exosomes extracted from these PSC-derived cell types (Gao et al. 2018). It was found that cardiac outcomes such as LV function, angiogenesis, infarct size, and wall stress were similar in both the exosome group and cell group, and both were significantly improved compared to MI without treatment group. Moreover, exosome therapy did not increase the frequency of arrhythmia, a primary concern from studies investigating human iPSC-derived CMs in a primate model (Shiba et al. 2016; Romagnuolo et al. 2019). These findings suggest that exosomes alone could potentially be as effective as stem cells in the treatment of cardiac pathologies. However, human clinical trials will need to be completed on exosome use before these conclusions can be definitively made.
The direct administration of cellular growth factors appears to be the most simplistic strategy for delivering paracrine factors to the heart. Two standard strategies to induce growth factor gene expression include the direct injection of recombinant proteins or the administration of viral vectors or plasmids containing the growth factor encoded gene (Spannbauer et al. 2020). Most preclinical studies have focused on several key growth factors: vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), although many others have also been investigated (Liew et al. 2020). A similar strategy revolves around using RNA, including coding messenger (mRNA) and noncoding RNA (ncRNA). One of the first studies that demonstrated this concept involved the epicardial or IC administration of VEGF and effectively improved myocardial blood flow and enhanced regional ventricular function in a porcine model of chronic ischemia (Lopez et al. 1998). In another study, porcine models of chronic ischemic heart disease were co-transfected with VEGF-A and platelet-derived growth factor B (PDGF-B) plasmids (Kupatt et al. 2010). The pigs co-transfected with VEGF-A and PDGF-B showed significant neovascularization and improved regional and global myocardial function. In gene therapy, there have been over 150 human clinical trials investigating the effects of various therapeutic molecules on cardiac angiogenesis (Cannatà et al. 2020).
However, there remains no gene therapy that has been proven to be successful in achieving clinical benefit. There have also been several landmark clinical trials that specifically investigated the use of gene therapy in the treatment of HF. The main targets in these trials were various components of the calcium handling system within CMs (Cannatà et al. 2020). The first key clinical trial was the phase 1/2 CUPID trial, which aimed to restore the sarcoplasmic Ca2+ ATPase (SERCA2a) in 39 patients with HF (Jaski et al. 2009; Jessup et al. 2011). The SERCA2a gene was delivered via cDNA in an adenovirus vector (AAV-1) during a single IC infusion. Patients were stratified into a low dose, medium dose, high dose, or placebo group. Initial results were promising, as a positive safety profile was confirmed, and there was a trend toward symptomatic improvement and a significant reduction in cardiovascular events at 12 months (Jessup et al. 2011). This prompted a follow-up with the much larger randomized, placebo-controlled phase II CUPID2 clinical trial, which enrolled 250 patients with HF (Greenberg et al. 2016). Unfortunately, results were disappointing as patients given AAV-1/SERCA2a did not have an improvement in cardiovascular events. A similar story can be told with a different target, the stromal cell-derived factor-1 (SDF-1). The SDF-1 growth factor promotes cell survival and recruitment, triggers angiogenesis, and increases tissue repair (Chung et al. 2015).
Initial phase I clinical trials tested the safety and efficacy of an endomyocardial injection of a DNA plasmid encoding SDF-1 into 19 patients with ischemic cardiomyopathy (Penn et al. 2013). The trial demonstrated a positive safety profile and qualitative symptomatic improvement. However, the larger, randomized phase II STOP-HF trial fell short and could not reproduce the positive results seen in the earlier trials (Chung et al. 2015). However, this does not mean that all hope is lost as several other targets have either shown initial promise or are currently in clinical trials (Hulot et al. 2016). Interestingly, an ongoing phase I clinical trial investigates the combination of VEGF-A, SDF-1 alpha, and S100 calcium-binding protein A1 as triple gene therapy in patients with end-stage HF and an implantable left ventricular assist device (NCT03409627). The ongoing EPICCURE study will be the first clinical trial to administer mRNA into the human heart (NCT03370887). It aims to investigate the safety and efficacy of AZD8601, a VEGF-A mRNA formulated in an injectable saline solution. Twenty-four patients with moderately decreased EF will be given a round of 30 epicardial injections of either 30 mg, 3 mg AZD8061, or placebo during a CABG procedure and followed for 6 months.
Cell-free strategies appear to overcome potential concerns surrounding conventional stem cell therapies, particularly from a translational perspective. These include safety concerns such as immune compatibility and rejection, as well as practical concerns such as accessibility, cost-effectiveness, and time-consuming procedures (Maghin et al. 2020). The necessary next steps include determining the optimal route of administration of these factors. In summary, noncellular regenerative therapies such as single growth factors, RNAs, or combination therapies such as exosomes appear to have significant myocardial reparative capacity. Exosomes circumvent many practical issues such as cell retention and immunogenicity and could potentially serve as an alternative to the cell-based approach in the future.
Bioengineering
The lack of understanding of the optimal cell type, dosage, route of administration compounded by low cell retention and survival, poor engraftment, and ineffective differentiation of progenitor cells post-transplantation are some of the most significant barriers encountered in clinical trials. Tissue engineering may hold the key to improving cell delivery and retention. The extracellular matrix (ECM) is now understood to be a dynamic microenvironment that contains complex networks of various proteins and growth factors and provides physical and mechanical signals to modulate cell behavior and differentiation (Maghin et al. 2020). Two strategies to bioengineer a matrix include creating a synthetic microenvironment or extracting ECM from tissues and decellularizing the matrix to create a cell-free product (Domenech et al. 2016). Since the cell microenvironment plays such an essential role in cell behavior, researchers have aimed at creating constructs that mimic the environment to improve cell culture systems and improve effectiveness of stem cell transplantation. In the last several years, 3D culture systems have improved differentiation techniques to create cell phenotypes similar to those in native tissues (Mazzola and Di Pasquale 2020). Several studies have demonstrated that differentiation of human PSCs or iPSCs-CMs in 3D culture systems improved the level of cell maturation (Ronaldson-Bouchard et al. 2018; Correia et al. 2018). Ronaldson-Bouchard et al. (2018) showed that early iPSCs-derived CMs cultured on a 3D hydrogel subjected to electrical and mechanical stimulation showed a similar gene expression profile, sarcomere length, density of mitochondria, and functional calcium handling similar to mature adult cells.
A separate strategy in tissue engineering revolves around implanting the bioengineered tissue construct with stem cells or biologically active molecules, which is believed to increase cellular retention rates and lengthen the duration of regenerative paracrine signaling (Micheu 2019). Tissue constructs known as cardiac patches were created by adding stem cells to natural or synthetic biomaterials such as fibrin, collagen, alginate, or even a natural decellularized ECM and mimicking the biomechanical or electrical signaling they would receive in vivo (Mazzola and Di Pasquale 2020). These cardiac patches can be directly transplanted onto the epicardial surface of the heart and serve as a temporary scaffold to enable cell engraftment into the host heart. Thus, the choice of biomaterial for the scaffold is important in determining the functional survival, engraftment, and proliferation of implanted cells (Mazzola and Di Pasquale 2020). Preclinical trials have shown that administration of cells embedded in patch construct improved cellular engraftment and decreased the number of cells needed for the same graft size and functional benefit by tenfold compared to cell injection studies (Weinberger and Eschenhagen 2021). Efficacy and safety have also been demonstrated in a porcine model of MI. Researchers showed that a cardiac muscle patch produced from human iPSC-derived CMs, smooth muscle cells, and endothelial cells loaded onto a fibrin scaffold induced significant improvements in LV function, infarct size, and protective effects on endogenous CMs (Gao et al. 2018). Since tissue engineering is a relatively novel concept in cardiac regeneration, there have been relatively few clinical trials investigating the use of cardiac scaffolds. The groundbreaking ESCORT trial discussed earlier demonstrated the effective and safe use of a fibrin patch embedded with human ESC-derived CPCs (Menasché et al. 2018).
Likewise, a recent clinical trial out of Japan compared the use of human umbilical cord mesenchymal stromal cells embedded in a collagen hydrogel versus cell treatment only in patients with chronic ischemic heart disease (NCT02635464). Fifty patients were randomized into either cell/hydrogel group, cell only, or placebo, and were given a single IM injection during a CABG procedure. Results showed that the collagen hydrogel treatment was a safe and feasible delivery option, and the collagen/cell combination decreased mean scar size at 12 months. However, results were not statistically significant (He et al. 2020). However, improvements in LVEF, NYHA functional class, viable heart mass, and quality of life measured by the MLHFQ were noted. Moreover, LVESV and LVEDV decreased. This study, to our knowledge, is the first ever to establish that the use of collagen hydrogel in humans is safe and feasible for cell delivery. These findings will provide a basis for future clinical trials in the future.
Recently, researchers have created scaffold-free cell sheets which can be directly implanted onto the myocardium without transplantation of a biomaterial in addition to the cells. This technology involves culturing a monolayer or multilayer of cells on a thermoresponsive polymer surface, and then removing the individual sheets of cells to combine them and create 3D cardiac grafts (Zhang 2015). However, this recent phenomenon has been well proven in various preclinical animal models but has yet to be tested in the clinical trial setting. Because of the low rates of cellular retention and cardiac remuscularization involved with direct cell injection, alternative methods needed to improve cardiac regeneration. Many believe that combination therapy holds the key to improving the efficacy of cardiac regeneration through enhanced cell engraftment and also improved paracrine factor signaling. This likely involves not only the combination of cells embedded in scaffolds, but also the involvement of biologically active molecules such as growth factors, RNAs, and exosomes. It seems inevitable that future research will investigate the implantation of a cardiac construct containing several different cell types and signaling molecules as a cell sheet or embedded in a biologically active scaffold (Fig. 3).
Conclusion and Future Perspectives
As the field of cardiac cell therapy approaches its third decade, we have yet to have a single stem cell type that the FDA approves for the treatment of heart disease (Cingolani 2019). However, it appears that we are finally gaining a greater understanding of the issues that have plagued the discipline for so long. Through a plethora of clinical trials, we have witnessed that it is incredibly difficult to remuscularize the failing heart. Cellular retention and survival rates have been incredibly low in clinical trials. In response, the field has shifted its mechanistic hypothesis of how cardiac cell therapy provides the observed therapeutic benefits. Most researchers now believe that stem cells provide the vast majority of their cardiac benefit via paracrine signaling rather than directly producing new CMs to remuscularize the heart. However, there appears to be some variation between specific cell types and method of delivery.
Thus far, numerous clinical trials have shown that cardiac cell therapy generally has a favorable safety profile. The SafeCell Heart meta-analysis demonstrated no adverse events associated with the use of MSCs in heart disease (Lalu et al. 2018). There are concerns surrounding the arrhythmogenicity of stem cells primarily within the skeletal myoblast lineage. Nonetheless, all cell types should be thoroughly investigated for arrhythmogenic risk before applied in large-scale clinical settings (Chen et al. 2020).
Most clinical trials to date have demonstrated neutral to marginally positive results in terms of clinical efficacy. Interestingly, a trial sequential analysis in 2016 revealed that there is firm evidence supporting that bone marrow-derived stem cell therapies do reduce the risk of rehospitalization and mortality in patients with HF (Fisher et al. 2016b). The SafeCell Heart meta-analysis also demonstrated that patients who receive MSCs therapy have a significantly improved LVEF (Lalu et al. 2018). Nonetheless, there has only ever been one phase III clinical trial investigating cellular therapy in heart disease and the results are still pending. This does not imply that stem cells have no clinical value in heart disease. Rather, we have not found the optimal cell type or delivery system for these stem cells. Large comparative clinical trials will need to be performed before reaching any conclusions about the effectiveness of regenerative therapies compared to our conventional pharmacologic treatments currently used for HF.
Clinical research to date has set the foundations for a technological breakthrough in cardiac cell therapy. In particular, we believe that future research in three specific areas will produce a generational breakthrough in cardiac regeneration. First, cell-free sources of cardiac regeneration such as exosomes, growth factors, and RNAs may provide a potential therapeutic approach without some of the safety concerns associated with cellular therapies. A cell-free strategy may also have practical advantages in terms of scalability, availability, and reduced cost. Second, combining cell therapy with bioengineering scaffolds or tissue constructs may provide a way to increase cell engraftment rates and prolong the survival of cells so that their beneficial paracrine effects can be sustained for longer. Lastly, the use of pluripotent cells to derive cell phenotypes of choice could be an excellent strategy for creating functional CMs or endothelial cells that can directly engraft into the myocardium and synchronize with the host cells. In addition, combination therapy of multiple PSC-derived cells types has already been proven in clinical trials to provide successful cardiac outcomes.
In summary, future research must establish the optimal cell type, route of delivery, and dosage to improve the efficacy to levels needed for implementation at the clinical level. In addition, studies should have long-term follow-up periods to truly identify the therapeutic risks and potential of cardiac cell therapy. Tailoring the cell therapy to the patient in question would be likely the most productive approach, as many differences exist between patients such as age, the severity of HF, immune status, and comorbidities. In addition, combination approaches consisting of pharmacological, cell-free paracrine strategies, and stem cell therapy will likely provide superior, sustainable results for patients. Regenerative therapies are still far from being implemented as a mainstay in the clinic; however, incredible progress has been in the field in the last several years. We remain cautiously optimistic that innovative techniques such as bioengineering, exosome therapy, and combination therapies will propel the field into unprecedented territory in the years to come.
Abbreviations
- AAV:
-
Adeno-associated virus
- ACC/AHA:
-
American College of Cardiology and the American Heart Association
- ANP:
-
Atrial natriuretic peptide
- BMDSC:
-
Bone marrow-derived stem cell
- BMMNC:
-
Bone marrow mononuclear cell
- BNP:
-
Brain natriuretic peptide
- CABG:
-
Coronary artery bypass grafting
- CDC:
-
Cardiosphere-derived cell
- CDCP:
-
Center for Disease Control and Prevention
- CM:
-
Cardiomyocyte
- CPC:
-
Cardiac progenitor cell
- CSC:
-
Cardiac stem cell
- ECM:
-
Extracellular matrix
- EDV:
-
End diastolic volume
- EF:
-
Ejection fraction
- ESC:
-
Embryonic stem cell
- ESV:
-
End systolic volume
- FGF:
-
Fibroblast growth factor
- hESC-CP:
-
Human embryonic stem cell cardiac progenitor
- HF:
-
Heart failure
- HFpEF:
-
HF with preserved ejection fraction
- HFrEF:
-
HF with reduced ejection fraction
- IC:
-
Intracoronary
- IGF-1:
-
Insulin-like growth factor-1
- IM:
-
Intramuscular
- iPSC:
-
Induced-pluripotent stem cell
- IV:
-
Intravenous
- LV:
-
Left ventricular
- LVEDV:
-
Left ventricular end diastolic volume
- LVEF:
-
Left ventricular ejection volume
- LVESV:
-
Left ventricular end systolic volume
- MI:
-
Myocardial infarction
- MLHFQ:
-
Minnesota Living with HF Questionnaire
- MPC:
-
Mesenchymal precursor cell
- MSC:
-
Mesenchymal stem cell
- NYHA:
-
New York Heart Association
- PCI:
-
Percutaneous coronary intervention
- PDGF-B:
-
Platelet-derived growth factor B
- PSC:
-
Pluripotent stem cell
- SDF-1:
-
Stromal cell-derived factor-1
- SM:
-
Skeletal myoblast
- VEGF:
-
Vascular endothelial growth factor
References
Abbafati C, Machado DB, Cislaghi B, Salman OM, Karanikolos M, McKee M, Abbas KM et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, Li RA et al (2005) Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res 97:159–167. https://doi.org/10.1161/01.RES.0000174794.22491.a0
Ahmed RPH, Haider KH, Buccini S, Shujia J, Ashraf M (2011a) Reprogramming of skeletal myoblasts for induction of pluripotency for tumor free cardiomyogenesis in the infarcted hear. Circ Res 109:60–70
Ahmed RPH, Ashraf M, Buccini S, Shujia J, Haider KH (2011b) Cardiac tumorigenic potential of induced pluripotent stem cells in immunocompetent host: a note of caution. Regen Med 6:171–178
Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T et al (2003) Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 107:2134–2139. https://doi.org/10.1161/01.CIR.0000062649.63838.C9
Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S et al (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63:1123–1133. https://doi.org/10.1016/j.jacc.2013.11.053
Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E et al (2007) Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res 100:1234–1241. https://doi.org/10.1161/01.RES.0000264508.47717.6b
Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A et al (2013) Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA 309:1622–1631. https://doi.org/10.1001/jama.2013.3527
Barile L, Chimenti I, Gaetani R, Forte E, Miraldi F, Frati G, Messina E et al (2007) Cardiac stem cells: isolation, expansion and experimental use for myocardial regeneration. Nat Clin Pract Cardiovasc Med 1:S9–S14. https://doi.org/10.1038/ncpcardio0738
Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P et al (2017) Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ Res 121:1192–1204. https://doi.org/10.1161/CIRCRESAHA.117.310712
Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B et al (2013) Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 61:2329–2338. https://doi.org/10.1016/j.jacc.2013.02.071
Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, … CHART Program (2017) Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 38:648–660. https://doi.org/10.1093/eurheartj/ehw543
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102. https://doi.org/10.1126/science.1164680
Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL et al (2015) Dynamics of cell generation and turnover in the human heart. Cell 161:1566–1575. https://doi.org/10.1016/j.cell.2015.05.026
Blocklet D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E et al (2006) Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells 24:333–336. https://doi.org/10.1634/stemcells.2005-0201
Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, Yang PC, … Cardiovascular Cell Therapy Research Network (CCTRN) (2018) Rationale and design of the CONCERT-HF trial (combination of mesenchymal and c-kit+ cardiac stem cells as regenerative therapy for heart failure). Circ Res 122:1703–1715. https://doi.org/10.1161/CIRCRESAHA.118.312978
Borow KM, Yaroshinsky A, Greenberg B, Perin EC (2019) Phase 3 DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ Res 125:265–281. https://doi.org/10.1161/CIRCRESAHA.119.314951
Brickwedel J, Gulbins H, Reichenspurner H (2014) Long-term follow-up after autologous skeletal myoblast transplantation in ischaemic heart disease. Interact Cardiovasc Thorac Surg 18:61–66. https://doi.org/10.1093/icvts/ivt434
Bruyneel AA, Sehgal A, Malandraki-Miller S, Carr C (2016) Stem cell therapy for the heart: blind alley or magic bullet? J Cardiovasc Transl Res 9:405–418. https://doi.org/10.1007/s12265-016-9708-y
Campbell NG, Suzuki K (2012) Cell delivery routes for stem cell therapy to the heart: current and future approaches. J Cardiovasc Transl Res 5:713–726. https://doi.org/10.1007/s12265-012-9378-3
Cannatà A, Ali H, Sinagra G, Giacca M (2020) Gene therapy for the heart lessons learned and future perspectives. Circ Res 126:1394–1414. https://doi.org/10.1161/CIRCRESAHA.120.315855
Chen K, Huang Y, Singh R, Wang ZZ (2020) Arrhythmogenic risks of stem cell replacement therapy for cardiovascular diseases. J Cell Physiol 235:6257–6267. https://doi.org/10.1002/jcp.29554
Cheung MM, Jahan N (2020) Can stem cells improve left ventricular ejection fraction in heart failure? A literature review of skeletal myoblasts and bone marrow-derived cells. Cureus 12:e11598. https://doi.org/10.7759/cureus.11598
Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL (2019) Regenerating the field of cardiovascular cell therapy. Nat Biotechnol 37:232–237. https://doi.org/10.1038/s41587-019-0042-1
Chiu RC, Zibaitis A, Kao RL (1995) Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg 60:12–18
Chiu LL, Iyer RK, Reis LA, Nunes SS, Radisic M (2012) Cardiac tissue engineering: current state and perspectives. Front Biosci (Landmark Ed) 17:1533–1550. https://doi.org/10.2741/4002
Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, Silver KH et al (2015) Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized phase II trial. Eur Heart J 36:2228–2238. https://doi.org/10.1093/eurheartj/ehv254
Cingolani E (2019) MY APPROACH to stem cell therapy for heart failure patients: not all cells are created equally. Trends Cardiovasc Med 29:374. https://doi.org/10.1016/j.tcm.2019.02.005
Connolly R, O’Brien T, Flaherty G (2014) Stem cell tourism – a web-based analysis of clinical services available to international travellers. Travel Med Infect Dis 12:695–701. https://doi.org/10.1016/j.tmaid.2014.09.008
Correia C, Koshkin A, Duarte P, Hu D, Carido M, Sebastião MJ, Gomes-Alves P et al (2018) 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol Bioeng 115:630–644. https://doi.org/10.1002/bit.26504
Cyranoski D (2018) ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 557:619–620. https://doi.org/10.1038/d41586-018-05278-8
Domenech M, Polo-Corrales L, Ramirez-Vick JE, Freytes DO (2016) Tissue engineering strategies for myocardial regeneration: acellular versus cellular scaffolds? Tissue Eng Part B Rev 22:438–458. https://doi.org/10.1089/ten.TEB.2015.0523
Duckers HJ, Houtgraaf J, Hehrlein C, Schofer J, Waltenberger J, Gershlick A, Bartunek J et al (2011) Final results of a phase IIa, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: the SEISMIC trial. EuroIntervention 6:805–812. https://doi.org/10.4244/EIJV6I7A139
Elmadbouh I, Haider KH, Ashraf M, Chachques J-C (2011) Preconditioning of human skeletal myoblast with stromal cell-derived factor-1α promotes cytoprotective effects against oxidative and anoxic stress. Int J Stem Cells 4:50–60
Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M et al (2017) Cardiomyocyte regeneration: a consensus statement. Circulation 136:680–686. https://doi.org/10.1161/CIRCULATIONAHA.117.029343
Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2016a) Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev 12. https://doi.org/10.1002/14651858.CD007888.pub3
Fisher SA, Doree C, Taggart DP, Mathur A, Martin-Rendon E (2016b) Cell therapy for heart disease: trial sequential analyses of two Cochrane reviews. Clin Pharmacol Ther 100:88–101. https://doi.org/10.1002/cpt.344
Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M et al (2006) A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27:1114–1122. https://doi.org/10.1093/eurheartj/ehi818
Gaglianello NA, Mahr C, Benjamin I (2016) Heart failure and cardiomyopathy. In: Benjamin IJ, Griggs RC, Wing EJ, Fitz JG (eds) Andreoli and Carpenter’s Cecil essentials of medicine, 9th edn. Elsevier/Saunders, Philadelphia, pp 55–66
Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E et al (2017) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38:201–211. https://doi.org/10.1093/eurheartj/ehw240
Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R et al (2018) Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 137:1712–1730. https://doi.org/10.1161/CIRCULATIONAHA.117.030785
Gerbin KA, Murry CE (2015) The winding road to regenerating the human heart. Cardiovasc Pathol 24:133–140. https://doi.org/10.1016/j.carpath.2015.02.004
Goussetis E, Manginas A, Koutelou M, Peristeri I, Theodosaki M, Kollaros N, Leontiadis E et al (2006) Intracoronary infusion of CD133+ and CD133−CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells 24:2279–2283. https://doi.org/10.1634/stemcells.2005-0589
Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D et al (2016) Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387:1178–1186. https://doi.org/10.1016/S0140-6736(16)00082-9
Haider KH, Tan T, Aziz S, Chachques JC, Sim EKW (2004a) Myoblast transplantation for cardiac repair: a clinical perspective. Mol Ther 9:14–23
Haider KH, Jiang SJ, Aziz S, Lei Y, Law PK, Sim EKW (2004b) Effectiveness of transient immunosupression using cyclosporine for xenomyoblast transplantation for cardiac repair. Transplant Proc 36:232–235
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M et al (2012) Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 308:2369–2379. https://doi.org/10.1001/jama.2012.25321
Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A et al (2017) Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol 69:526–537. https://doi.org/10.1016/j.jacc.2016.11.009
Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM (2012) Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 4:966–977. https://doi.org/10.18632/aging.100526
He X, Wang Q, Zhao Y, Zhang H, Wang B, Pan J, Li J et al (2020) Effect of intramyocardial grafting collagen scaffold with mesenchymal stromal cells in patients with chronic ischemic heart disease: a randomized clinical trial. JAMA Netw Open 3:e2016236. https://doi.org/10.1001/jamanetworkopen.2020.16236
Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF (2013) Hippo signaling impedes adult heart regeneration. Development 140:4683–4690. https://doi.org/10.1242/dev.102798
Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M et al (2014) Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 311:62–73. https://doi.org/10.1001/jama.2013.282909
Hoeeg C, Frljak S, Qayyum AA, Vrtovec B, Kastrup J, Ekblond A, Follin B (2020) Efficacy and mode of action of mesenchymal stem cells in non-ischemic dilated cardiomyopathy: a systematic review. Biomedicines 8:570. https://doi.org/10.3390/biomedicines8120570
Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC et al (2005) Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112:I150–I156. https://doi.org/10.1161/CIRCULATIONAHA.104.526749
Hu S, Liu S, Zheng Z, Yuan X, Li L, Lu M, Shen R et al (2011) Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: a single-center, randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 57:2409–2415. https://doi.org/10.1016/j.jacc.2011.01.037
Hulot JS, Ishikawa K, Hajjar RJ (2016) Gene therapy for the treatment of heart failure: promise postponed. Eur Heart J 37:1651–1658. https://doi.org/10.1093/eurheartj/ehw019
Inamdar AA, Inamdar AC (2016) Heart failure: diagnosis, management and utilization. J Clin Med 5:62. https://doi.org/10.3390/jcm5070062
Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, … Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators (2009) Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J Card Fail 15:171–181. https://doi.org/10.1016/j.cardfail.2009.01.013
Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, … Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators (2011) Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124:304–313. https://doi.org/10.1161/CIRCULATIONAHA.111.022889
Kadota S, Shiba Y (2019) Pluripotent stem cell-derived cardiomyocyte transplantation for heart disease treatment. Curr Cardiol Rep 21:73. https://doi.org/10.1007/s11886-019-1171-3
Kadota S, Minami I, Morone N, Heuser JE, Agladze K, Nakatsuji N (2013) Development of a reentrant arrhythmia model in human pluripotent stem cell-derived cardiac cell sheets. Eur Heart J 34:1147–1156. https://doi.org/10.1093/eurheartj/ehs418
Kadota S, Tanaka Y, Shiba Y (2020) Heart regeneration using pluripotent stem cells. J Cardiol 76:459–463. https://doi.org/10.1016/j.jjcc.2020.03.013
Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C et al (2015) Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J Am Coll Cardiol 66:1990–1999. https://doi.org/10.1016/j.jacc.2015.08.879
Kishore R, Khan M (2016) More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res 118:330–343. https://doi.org/10.1161/CIRCRESAHA.115.307654
Kupatt C, Hinkel R, Pfosser A, El-Aouni C, Wuchrer A, Fritz A, Globisch F et al (2010) Cotransfection of vascular endothelial growth factor-A and platelet-derived growth factor-B via recombinant adeno-associated virus resolves chronic ischemic malperfusion role of vessel maturation. J Am Coll Cardiol 56:414–422. https://doi.org/10.1016/j.jacc.2010.03.050
Lalu MM, Mazzarello S, Zlepnig J, Dong YYR, Montroy J, McIntyre L, Devereaux PJ et al (2018) Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell heart): a systematic review and meta-analysis. Stem Cells Transl Med 7:857–866. https://doi.org/10.1002/sctm.18-0120
Law PK, Sim EKW, Haider KH, Fang G, Chua FK, Kakuchaya T, Repin VS, Bockeria LA (2003) Chapter 17. Myoblast genome therapy and the regenerative heart. In: Kipshidze N, Serruys P (eds) Handbook of cardiovascular cell transplantation. Martin Dunitz, London, pp 241–257
Lei Y, Haider KH, Toh WC, Beng W, Tan R, Law PK, Su L et al (2007) Transplantation of nanoparticle based skeletal myoblasts over-expression vascular endothelial growth factor-165 for cardiac repair. Circulation 116:I-113–I-120
Lei Y, Zhang W, Su L-P, Haider KH, Poh K-K, Galupo MJ, Songco G et al (2011) Nanoparticle based delivery of hypoxia-regulated VEGF transgene system combined with myoblast engraftment for myocardial repair. Biomaterials 32:2424–2431
Liew LC, Ho BX, Soh BS (2020) Mending a broken heart: current strategies and limitations of cell-based therapy. Stem Cell Res Ther 11:138. https://doi.org/10.1186/s13287-020-01648-0
Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL et al (2014) Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res 115:354–363. https://doi.org/10.1161/CIRCRESAHA.115.303632
Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L et al (2018) Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 36:597–605. https://doi.org/10.1038/nbt.4162
Liu Z, Mikrani R, Zubair HM, Taleb A, Naveed M, Baig MMFA, Zhang Q et al (2020) Systemic and local delivery of mesenchymal stem cells for heart renovation: challenges and innovations. Eur J Pharmacol 876:173049. https://doi.org/10.1016/j.ejphar.2020.173049
Lo B, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30:204–213. https://doi.org/10.1210/er.2008-0031
Lopez JJ, Laham RJ, Stamler A, Pearlman JD, Bunting S, Kaplan A, Carrozza JP et al (1998) VEGF administration in chronic myocardial ischemia in pigs. Cardiovasc Res 40:272–281. https://doi.org/10.1016/s0008-6363(98)00136-9
Madonna R, Ferdinandy P, De Caterina R, Willerson JT, Marian AJ (2014) Recent developments in cardiovascular stem cells. Circ Res 115:e71–e78. https://doi.org/10.1161/CIRCRESAHA.114.305567
Maghin E, Garbati P, Quarto R, Piccoli M, Bollini S (2020) Young at heart: combining strategies to rejuvenate endogenous mechanisms of cardiac repair. Front Bioeng Biotechnol 8:447. https://doi.org/10.3389/fbioe.2020.00447
Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS et al (2012) Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379:895–904. https://doi.org/10.1016/S0140-6736(12)60195-0
Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, Ekblond A et al (2020) Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail 22:884–892. https://doi.org/10.1002/ejhf.1700
Mazzola M, Di Pasquale E (2020) Toward cardiac regeneration: combination of pluripotent stem cell-based therapies and bioengineering strategies. Front Bioeng Biotechnol 8:455. https://doi.org/10.3389/fbioe.2020.00455
Menasché P (2018) Cell therapy trials for heart regeneration – lessons learned and future directions. Nat Rev Cardiol 15:659–671. https://doi.org/10.1038/s41569-018-0013-0
Menasché P, Hagège AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K et al (2001) Myoblast transplantation for heart failure. Lancet 357(9252):279–280. https://doi.org/10.1016/S0140-6736(00)03617-5
Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT et al (2008) The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117:1189–1200. https://doi.org/10.1161/CIRCULATIONAHA.107.734103
Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, Cacciapuoti I et al (2018) Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 71:429–438. https://doi.org/10.1016/j.jacc.2017.11.047
Micheu MM (2019) Moving forward on the pathway of cell-based therapies in ischemic heart disease and heart failure – time for new recommendations? World J Stem Cells 11:445–451. https://doi.org/10.4252/wjsc.v11.i8.445
Mohite PN, Sabashnikov A, Simon AR, Weymann A, Patil NP, Unsoeld B, Bireta C et al (2015) Does CircuLite Synergy assist device as partial ventricular support have a place in modern management of advanced heart failure? Expert Rev Med Devices 12:49–60. https://doi.org/10.1586/17434440.2015.985208
Mouton AJ, Rivera OJ, Lindsey ML (2018) Myocardial infarction remodeling that progresses to heart failure: a signaling misunderstanding. Am J Physiol Heart Circ Physiol 315:H71–H79. https://doi.org/10.1152/ajpheart.00131.2018
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB (2016) American Heart Association Statistics Committee; Stroke Statistics Subcommittee Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 26:e38–360. https://doi.org/10.1161/CIR.0000000000000350
Murry CE, Reinecke H, Pabon LM (2006) Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol 47:1777–1785. https://doi.org/10.1016/j.jacc.2006.02.002
Nair N, Gongora E (2020) Stem cell therapy in heart failure: where do we stand today? Biochim Biophys Acta Mol Basis Dis 1866:165489. https://doi.org/10.1016/j.bbadis.2019.06.003
Nakamura K, Murry CE (2019) Function follows form – a review of cardiac cell therapy. Circ J 83:2399–2412. https://doi.org/10.1253/circj.CJ-19-0567
Narita T, Shintani Y, Ikebe C, Kaneko M, Campbell NG, Coppen SR, Uppal R et al (2013) The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol Ther 21:860–867
Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C et al (2014) Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J 35:1263–1274. https://doi.org/10.1093/eurheartj/ehu007
Natsumeda M, Florea V, Rieger AC, Tompkins BA, Banerjee MN, Golpanian S, Fritsch J et al (2017) A combination of allogeneic stem cells promotes cardiac regeneration. J Am Coll Cardiol 70:2504–2515. https://doi.org/10.1016/j.jacc.2017.09.036
Niagara MI, Haider KH, Jiang S, Ashraf M (2007) Pharmacological preconditioning renders skeletal myoblasts resistant to oxidative stress and enhances their cardiac repair ability via expression of paracrine factors after transplantation. Circ Res 100:545–555
Nigro P, Bassetti B, Cavallotti L, Catto V, Carbucicchio C, Pompilio G (2018) Cell therapy for heart disease after 15 years: unmet expectations. Pharmacol Res 127:77–91. https://doi.org/10.1016/j.phrs.2017.02.015
Oikonomopoulos A, Kitani T, Wu JC (2018) Pluripotent stem cell-derived cardiomyocytes as a platform for cell therapy applications: progress and hurdles for clinical translation. Mol Ther 26:1624–1634. https://doi.org/10.1016/j.ymthe.2018.02.026
Ozkan J (2019) Piero Anversa and cardiomyocyte regeneration. Eur Heart J 40:1036–1037. https://doi.org/10.1093/eurheartj/ehz096
Park SR, Kim JW, Jun HS, Roh JY, Lee HY, Hong IS (2018) Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther 26:606–617. https://doi.org/10.1016/j.ymthe.2017.09.023
Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ et al (2019) Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun 10:3123. https://doi.org/10.1038/s41467-019-11091-2
Penn MS, Mendelsohn FO, Schaer GL, Sherman W, Farr M, Pastore J, Rouy D et al (2013) An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res 112:816–825. https://doi.org/10.1161/CIRCRESAHA.111.300440
Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI et al (2003) Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 107:2294–2302. https://doi.org/10.1161/01.CIR.0000070596.30552.8B
Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV et al (2012) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 307:1717–1726. https://doi.org/10.1001/jama.2012.418
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080. https://doi.org/10.1126/science.1200708
Poulin MF, Deka A, Mohamedali B, Schaer GL (2016) Clinical benefits of stem cells for chronic symptomatic systolic heart failure: a systematic review of the existing data and ongoing trials. Cell Transplant 25:1911–1923. https://doi.org/10.3727/096368916X692087
Povsic TJ, O’Connor CM, Henry T, Taussig A, Kereiakes DJ, Fortuin FD, Niederman A et al (2011) A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J 162:654–662. https://doi.org/10.1016/j.ahj.2011.07.020
Prockop DJ, Olson SD (2007) Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood 109:3147–3151. https://doi.org/10.1182/blood-2006-03-013433
Regenberg AC, Hutchinson LA, Schanker B, Mathews DJ (2009) Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells 27:2312–2319. https://doi.org/10.1002/stem.132
Reinecke H, Poppa V, Murry CE (2002) Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol 34:241–924. https://doi.org/10.1006/jmcc.2001.1507
Robey TE, Saiget MK, Reinecke H, Murry CE (2008) Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol 45:567–581. https://doi.org/10.1016/j.yjmcc.2008.03.009
Rojas SV, Haverich A (2019) Heart failure: ventricular assist devices and cardiac transplantation: a review of current surgical innovations. Der Chirurg; Zeitschrift fur alle Gebiete der Operativen Medizen 90:110–116. https://doi.org/10.1007/s00104-018-0774-3
Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A, Qi X et al (2019) Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep 12:967–981. https://doi.org/10.1016/j.stemcr.2019.04.005
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K et al (2018) Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556:239–243. https://doi.org/10.1038/s41586-018-0016-3
Ryan KA, Sanders AN, Wang DD, Levine AD (2010) Tracking the rise of stem cell tourism. Regen Med 5:27–33. https://doi.org/10.2217/rme.09.70
Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T et al (2016) Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538:388–391. https://doi.org/10.1038/nature19815
Sim EKW, Lei Y, Jiang SJ, Lim YL, Ooi OC, Haider KH (2003) Skeletal myoblast transplant in heart failure. J Card Surg 18:319–327
Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzeźniczak J, Rozwadowska N, Kurpisz M (2004) Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J 148(3):531–537. https://doi.org/10.1016/j.ahj.2004.03.043
Simons M, Raposo G (2009) Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581. https://doi.org/10.1016/j.ceb.2009.03.007
Singh VK, Saini A, Kalsan M, Kumar N, Chandra R (2016) Describing the stem cell potency: the various methods of functional assessment and in silico diagnostics. Front Cell Dev Biol 4:134. https://doi.org/10.3389/fcell.2016.00134
Skinner JS, Cooper A (2011) Secondary prevention of ischaemic cardiac events. BMJ Clin Evid 2011:0206
Sobhani A, Khanlarkhani N, Baazm M, Mohammadzadeh F, Najafi A, Mehdinejadiani S, Sargolzaei AF (2017) Multipotent stem cell and current application. Acta Med Iran 55:6–23
Spannbauer A, Mester-Tonczar J, Traxler D, Kastner N, Zlabinger K, Hašimbegović E, Riesenhuber M et al (2020) Large animal models of cell-free cardiac regeneration. Biomolecules 10:1392. https://doi.org/10.3390/biom10101392
Strauer BE, Yousef M, Schannwell CM (2010) The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail 12:721–729. https://doi.org/10.1093/eurjhf/hfq095
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Tanai E, Frantz S (2015) Pathophysiology of heart failure. Compr Physiol 6:187–214. https://doi.org/10.1002/cphy.c140055
Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD et al (1998) Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 4:929–933. https://doi.org/10.1038/nm0898-929
Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, … CHART Investigators (2017) Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail 19:1520–1529. https://doi.org/10.1002/ejhf.898
Tehzeeb J, Manzoor A, Ahmed MM (2019) Is stem cell therapy an answer to heart failure: a literature search. Cureus 11:e5959. https://doi.org/10.7759/cureus.5959
Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK et al (2009) Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 54:1619–1626. https://doi.org/10.1016/j.jacc.2009.04.097
Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ et al (2007) Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1:129–137. https://doi.org/10.1016/j.scr.2008.02.002
Turner D, Rieger AC, Balkan W, Hare JM (2020) Clinical-based cell therapies for heart disease – current and future state. Rambam Maimonides Med J 11. https://doi.org/10.5041/RMMJ.10401
van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyöngyösi M, Sluijter JP, Cramer MJ et al (2011) Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res 91:649–658. https://doi.org/10.1093/cvr/cvr113
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2021) Heart disease and stroke Statistics – 2021 update: a report from the American Heart Association. Circulation 143:e254–e743. https://doi.org/10.1161/CIR.0000000000000950
Wang J, Liu S, Heallen T, Martin JF (2018) The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol 15:672–684. https://doi.org/10.1038/s41569-018-0063-3
Weinberger F, Eschenhagen T (2021) Cardiac regeneration: new hope for an old dream. Annu Rev Physiol 83:59–81. https://doi.org/10.1146/annurev-physiol-031120-103629
Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR et al (2013) Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127:213–223. https://doi.org/10.1161/CIRCULATIONAHA.112.131110
Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA et al (2011) Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal 4:ra70. https://doi.org/10.1126/scisignal.2002278
Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER et al (2013) Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A 110:13839–13844. https://doi.org/10.1073/pnas.1313192110
Ye L, Haider KH, Jiang S, Ling LH, Ge R, Law PK, Sim EKW (2007) Reversal of myocardial injury using genetically modulated human skeletal myoblasts in a rodent cryoinjured heart model. Eur J Heart Fail 7(6):945–952
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10:68. https://doi.org/10.1186/s13287-019-1165-5
Zhang J (2015) Engineered tissue patch for cardiac cell therapy. Curr Treat Options Cardiovasc Med 17:399. https://doi.org/10.1007/s11936-015-0399-5
Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN (2015) Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res 14:3010–3017. https://doi.org/10.4238/2015.April.10.11
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Rheault-Henry, M., White, I., Atoui, R. (2022). Unraveling the Mystery of Regenerative Medicine in the Treatment of Heart Failure. In: Haider, K.H. (eds) Handbook of Stem Cell Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-19-2655-6_16
Download citation
DOI: https://doi.org/10.1007/978-981-19-2655-6_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2654-9
Online ISBN: 978-981-19-2655-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences