Abstract
Dye-sensitized solar cells (DSSCs) based on redox electrolyte solution set the limitation and restriction on its fabrication. Moreover, when this redox electrolyte comes in contact with photoanode causes its corrosion. The finding of ionic conductivity in polymer material complex with salt has given a breakthrough in the formation of DSSC devices. Polymer electrolytes, especially Polyvinylidene fluoride (PVDF), have attained considerable interest due to its some exceptional properties like thermal stability, chemical resistance, and excellent mechanical strength. In the present work, dye-sensitized solar cell has been assembled using electrolyte system composed of PVDF as host polymer, Ethylene Carbonate as plasticizer, LiI: I2 as redox, and couple and graphite as filler; TiO2 modified with CuO photoanode in order to provide inherent energy barrier and natural cocktail dye as sensitizer. The obtained solar cell conversion efficiency was about 2.27% with using an irradiation of 100 mW/cm2 at 25 °C.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Dye-sensitized solar cells (DSSCs) are third generation solar cells based on dye molecules which absorb light energy and convert into electrical energy. These cells are gaining lot of attention due to their low cost and easy fabrication. The main components of DSSCs are semiconductor electrode (TiO2, ZnO, or SnO2), counter electrode (Pt or C), dye as sensitizers, and electrolyte as redox mediator [1, 2]. Scientists are doing lot of research on improving its components and optimization so that efficiency comparable to silicon-based solar cell can be achieved.
The commonly used redox electrolyte used for DSSCs comprises iodide/triiodide (I−/I3−) redox couple in an organic solvent. But it suffers from major drawback as the organic solvent used leaks and vaporizes with time. Therefore, scientists are focusing their attention in the solidification of electrolyte such as inorganic or organic hole conductors, ionic liquids, and polymer electrolyte. Various polymers such as polyethylene oxide (PEO) and polyvinyl alcohol (PVA). are being employed as electrolyte in DSSCs [3, 4]. In the present study, we have prepared polymer electrolyte based on Polyvinylidene fluoride (PVDF) with graphite as filler to improve the conductivity of the electrolyte. Polyvinylidene fluoride (PVDF) can be considered as a suitable for forming polymer electrolyte as it has some exceptional properties like thermal stability, chemical resistance, excellent mechanical strength, and photo-electrochemical stability under potential application [5, 6].
Generally synthetic dyes based on heavy metals have been employed as sensitizers to achieve high efficiency. However, these dyes have expensive production and complicated synthesis. Moreover, heavy metals used in their production causes environmental pollution. Therefore use of environmental friendly dyes such as natural dyes as photosensitizers in DSSC is gaining lot of attention. Natural pigments containing carotenoids, chlorophyll, betalains, and flavonoids which are found in various parts of plants such as vegetable, fruits, flowers, and leaves have been successfully used as photosensitizer in DSSCs [7, 8].
The photoanode is another very important component of DSSCs and is generally made up of nanocrystalline semiconductor. Generally TiO2 is widely used to make photoanode as it is nontoxic, low cost, and widely available. But TiO2 suffers from major drawback as it large band gap of 3.2 eV, so it can utilize only 6% of the total solar radiation. Studies have shown that the addition of various metal oxides like SnO2, ZnO, WO3, CeO2, etc., can improve the photocatalytic or photoelectrolytic activities of TiO2 [9, 10]. Due to the interesting physical properties of CuO, it has received considerable interest. The wide application of CuO as a gas sensor, and photochromic and electrochromic material makes it an interesting candidate for the modification of the TiO2 photoelectrode [11].
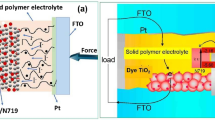
In the present work, we fabricated DSSC with polymer electrolyte system composed of PVDF as a host polymer, LiI: I2 as a redox couple, EC as plasticizer, graphite as filler with cocktail dye, and CuO modified TiO2 photoanode. The properties like short-circuit photocurrent density (JSC), open circuit voltage (Voc), fill factor (ff) conversion efficiency, and stability of the fabricated DSSC have been studied.
15.2 Experimental
15.2.1 Materials
For the preparation of natural dye, fresh black grapes and spinach leaves were purchased from the local market. Titanium Isopropoxide (Ti [OCH (CH3)2]4), Copper (II) Chloride (CuCl2·6H2O), Ethylene Carbonate (EC), Propylene Carbonate (PC), Lithium iodide, and Iodine were purchased from Sigma Aldrich. Co. Ltd. Analytical grade solvents like Ethanol, Propanol were purchased from Merck Ltd.
15.2.2 Preparation of Polymer Electrolyte
For the preparation of polymer electrolyte film, we have employed well-known solution cast technique. To prepare thin films, appropriate amount of PVDF was dissolved in the solvent. LiI: I2 was also dissolved in the same polymeric solution. Tetrahydrofuran (THF) and dimethylsulphoxide (DMSO) in a suitable ratio was used as common solvent and stirring medium. The polymeric solution with salt and graphite filler along with Ethylene Carbonate was casted in the glass petridish. The graphite powder was synthesized by ball milling machine for 48 h at rotation speed of 300 rpm. For the evaporation of solvent, petridish was closely packed for 40 h at room temperature then a thick viscous solution was obtained, which was further dried at room temperature for 8 h. This film was further dried in incubator for controlled evaporation followed by vacuum drying to obtain the solvent free standing film.
15.2.3 Synthesis of TiO2–CuO Photoelectrode
The TiO2 and CuO nanopowders were prepared by sol–gel process. First, TiO2 colloidal solution was prepared by adding Ti [OCH(CH3)2]4 to propanol drop by drop, and then, deionized water was added for the duration of 10 min. A white precipitate was formed during addition, and then, 1 ml of 70% HNO3 was added to the mixture. The mixture was then stirred for 15 min at 80 ºC, and at the same time, propanol together with some water was allowed to evaporate resulting in TiO2 colloidal solution. For the preparation of CuO, colloidal solution CuCl2·6H2O (0.2 M) and 1 ml of glacial acetic acid are added to the aqueous solution and heated to 100 ºC with constant stirring. Then in this heated solution, 8 M NaOH is added till pH value reaches 7. The large amount of precipitate is formed quickly, resulting in a CuO colloidal solution. For the preparation of TiO2–CuO admixed nanopowder, CuO solution was slowly added to the TiO2 colloidal solution under vigorous stirring condition for 6 h. The resulting gel was dried and calcinied at 450 ºC to get TiO2–CuO nanopowder.
ITO conductive glass plates with a sheet resistance of 15–20 Ω/cm2 were first cleaned in a detergent solution using an ultrasonic bath for 15 min, rinsed with water and ethanol, and then dried. In order to obtain TiO2–CuO film, TiO2–CuO nanopowder pastes were deposited on the ITO conductive glass by doctor-blade technique. The film on the substrate was annealed in oven at 150 °C for 5–10 min.
15.2.4 Extraction and Purification of Dye
The cocktail dye was extracted from black grapes, and the chlorophyll dye was extracted from spinach leaves. The black grapes and spinach leaves were cut into pieces washed and soaked separately in ethanol for one week, and then, it was crushed. Solutions were filtered out, and filtrates were concentrated in rotavapor at 40 °C. After that prepared extracts were refined by chromatogram method, and the extracts were blended at volume ratio of 1:1 to serve as natural cocktail dye sensitizer for DSSC. Chromatogram technique was used for the purification of dye.
15.2.5 Structural Characterization
The structural and morphological characterization of TiO2–CuO nanomaterial was carried out through XRD by employing a Philips PW 1710 diffractometer (CuKα radiation) equipped with a graphite monochromater and scanning electron microscope (JEOL-JXA-8100 EPMA).
In order to explore the spectral response, the absorption spectra of cocktail dye-coated TiO2–CuO on quartz glass substrates was carried out through double-beam spectrophotometer (Systronics 2201).
The XRD pattern of the prepared polymer electrolyte film was recorded between 2θ values 20–80° at room temperature using Phillips PW 1710 diffractometer. The optical micrograph of the film was recorded using computer-controlled polarizing microscope (LEICA DMLP).
Impedance measurements were carried out using Biologic SP-150. During all the measurements, humidity level was maintained at ~55% (constant).
Fabrication and cell performance measurement of DSSC—TiO2–CuO-based electrode—was immersed in an ethanol solution containing a natural cocktail dye for 10–12 h. Dye-sensitized working electrode and counter electrode were assembled to form a solar cell by sandwiching a chitosan-based polymer electrolyte containing redox couple. All electrochemical measurements were carried out by a Biologic SP-150. A Xenon-Mercury lamp (Oriel Corporation, USA) was used as illumination source the intensity of which was adjusted and fixed at 100 mW/cm2.
15.3 Results and Discussion
The XRD pattern of 99 {80 PVDF- 20 LiI: I2}: 1 graphite film prepared by solution cast technique is shown in Fig. 15.1 (normalized). The XRD pattern of pure PVDF film shows sharp and intense peaks at 18.3º, 19.9º, and 26.6º, respectively. However, for pure graphite film, it is at 25º. From the comparative study of the XRD patterns, it is clear that the addition of graphite in the polymer matrix reduces the intensity of the main peak followed by broadening of the peak area, which is an indication of increase in the degree of amorphousity. This provides a better medium for fast protonic conduction of ions resulting in better conductivity of the film. The intercalation of the polymer chain with filler increases the interlayer spacing which results in increase in the amorphous nature of the film.
The optical micrograph of pure PVDF and 99 {80 PVDF - 20 LiI: I2}: 1 graphite film prepared by solution cast technique at 80X magnification are shown in Fig. 15.2a, b. In pure PVDF film, several pores with a lamellar distribution can be seen. The addition of salt and filler drastically changed the microstructure of PVDF. The addition of filler connects these pores to each other due to lower surface energy resulting in smooth surface of the film. The disappearance of these pores is advantageous for interfacial contact between the polymers and salt. The connectivity of the pores is favorable for the transportation of proton and thus resulting in improved ionic conductivity.
Figure 15.3 shows that the ambient ac conductivity of the PVDF-based polymer electrolyte reaches 10–3 S/cm. The conductivity increases linearly up to 5 kHz. According to well-known power law the ac conductivity of the sample depends on the frequency
where A is constant and ρ is the frequency exponent (ρ < 1). The ωρ power law is very frequently observed in a wide range of materials among which is polymer.
The crystal structure and surface morphology of the prepared TiO2–CuO nanoparticles were investigated by XRD and SEM analyses. X-ray diffraction pattern of the prepared TiO2–CuO nanoparticles is shown in Fig. 15.4. The particles are found to be nanocrystalline in nature, and the crystallite size is obtained by the well-known Debye–Scherrer’s equation. The crystallite size obtained using this formula is 20–35 nm.
The SEM image of the fabricated TiO2–CuO nanoparticles is shown in Fig. 15.5, the SEM image reveals the highly porous nature of the resulting nanoparticles having grain size 20–35 nm. The small grain size offers large surface area, which offers many docking sites for the dye molecules and these molecules can anchor numerously and harvest immense amount of light.
The absorption spectra of the cocktail dye-coated TiO2–CuO photoelectrode is shown in Fig. 15.6. The absorption peak is shifted toward the longer wavelength region which helps in improving the efficiency of the cell.
Figure 15.7 shows the cell performance of the PVDF-based DSSC with TiO2–CuO photoelectrode sensitized with cocktail dye. The photocurrent (Isc) was found to be 9.86 mA/cm2, and photovoltage (Voc) is 0.435 V. The conversion efficiency and fill factor of cocktail dye-coated TiO2–CuO photoanode were found to be 52% and 2.27%, respectively.
15.4 Conclusion
In the present work, we employed PVDF-based thin and mechanically stable polymer electrolyte for natural dye-sensitized-based DSSC. Ionic conductivity of the prepared electrolyte was found in the range of 10−5 S/Cm. The addition of graphite filler helped in improving the conductivity of the electrolyte. We have used TiO2–CuO photoelectrode for DSSC. TiO2 with CuO helps in improving the cell performance due to the reduced recombination rate of photoinjected electrons. In the present work, we have also explored the use of cocktail dye in DSSC. The cocktail dye prepared by 1:1 volume mixture of anthocyanin and chlorophyll dye obtained from black grapes and spinach leaves showed high absorption in the visible part of the solar spectrum. The dye showed better complexation with the photoelectrode, which aided in better charge transfer between the dye and the photoelectrode. Then, the cell performance of the TiO2–CuO coated DSSC is investigated. The photocurrent (Isc) was found to be 9.86 mA/cm2, and photovoltage (Voc) is 0.435 V. The conversion efficiency and fill factor of cocktail dye-coated TiO2–CuO photoanode were found to be 52% and 2.27%, respectively.
References
R. Sastrawan, J. Beier, U. Belledin, S. Hemming, A. Hinsch, R. Kern, C. Vetter, F.M. Petrat, A. Prodi-Schwab, P. Lechner, W. Hoffmann, A glass frit sealed dye sensitized solar cell module with integrated series connections. Solar Energy Mater. Solar Cells 90, 1680–1691 (2006)
I. Dincer, C. Acar, A review on clean energy solutions for better sustainability. Int. J. Energy Res. 39(5), 585–606 (2015)
P. Chawla, M. Tripathi, Nanocomposite polymer electrolyte for enhancement in stability of Betacyanin dye sensitized solar cell. ECS Solid State Lett. 4(1), 21–23 (2015)
Z. Seidalilir, R. Malekfar, H.P. Wu, J.W. Shiu, E.W.G. Diau, High performance and stable gel state dye sensitized solar cells using anodic TiO2 nanotube arrays and polymer based gel electroly. ACS Appl. Mater. 23, 12731 (2015)
S. Denizalti, A.K. Ali, C. Ela, M. Ekmekci, S.E. Ela, Dye sensitized solar cells using ionic liquids as redox mediators. Chem. Phys. Lett. 691, 373–378 (2018)
R. Wang, B. Ahmed, S.K. Raghuvanshi, Siddhartha, N.P. Sharma, J.B.M. Krishna, M.A. Wahab, Prog. Nanotechnol. Nanomater. 2, 42 (2013)
M. Kokkonen, P. Talebi, J. Zhou, S. Asgari, S.A. Soomro, F. Elsehrawy, J. Halme, S. Ahmad, A. Hagfelt, S.G. Hashmi, Advanced research trends in dye sensitized solar cells. J. Mater. Chem. A 9, 10527–10545 (2021)
F. Kabir, M.M.H. Bhuiyan, M.S. Rahaman, M.A. Khan, T. Ikegami, Development of dye sensitized solar cell based on combination of natural dyes extracted from Malabar spinach and red spinach. Results Phys. 102474 (2019)
R. Vittal, K.C. Hou, Zinc oxide based dye sensitized based solar cells: a review. Renew. Sustain0 Energy Rev. 920–935
P. Chawla, M. Tripathi, CeO2–TiO2 photoanode for solid state dye sensitized solar cell. Ionics 21(2), 541–546 (2015)
M. Tripathi, K. Pandey, S.D. Kumar, Surface modification of semiconductor photoelectrode for improved solar cell performance. Solar Energy Mater. Solar Cells 91, 1663–1668 (2007)
Acknowledgements
One of the authors is thankful to CSIR-India for the financial support in the form of Research Associate (RA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Chawla, P., Pooja, K., Tripathi, M. (2022). PVDF-Based Nanocomposite Polymer Electrolyte for Enhancement in Stability of Dye-Sensitized Solar Cells. In: Pandey, K.L., Priya, P.K., Yadav, U.K., Khandai, P.K. (eds) Proceedings of the National Workshop on Recent Advances in Condensed Matter and High Energy Physics. Springer Proceedings in Physics, vol 278. Springer, Singapore. https://doi.org/10.1007/978-981-19-2592-4_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-2592-4_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2591-7
Online ISBN: 978-981-19-2592-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)