Abstract
Steel structures used in oil and gas industries suffer many catastrophic failures due to hydrogen evolution, which originates from corrosion processes and cathodic protection in their service settings. The use of ferrous steel to construct these structures diminishes their structural stability. Offshore pipeline systems include moisture and molecular water reduction caused by cathodic protection. Synergistic hydrogen concentration and stress create a situation of hydrogen embrittlement in susceptible steels, leading to very brittle circumstances. The present paper extensively discusses the different sources of hydrogen attack and the associated failure mechanisms. The most recent work done by researchers has been reviewed; widely accepted techniques to assess the impact of hydrogen on steel pipelines are also presented.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The build-up of carbon in the environment, which contributes to global warming, is causing increasing concern. Technologically sophisticated nations, China and India, are heavy oil consumers. Due to their high living standards, these major consumer nations utilize fossil fuels and derivatives to sustain their economies. Significant indications of human-caused climate change have emerged because of the high exploitation of the earth’s crust’s carbon. In the present era, hydrogen has served as an excellent fuel and energy storage since it is the most prevalent element in the universe. However, the effects of hydrogen on material properties, including hydrogen embrittlement (HE), offer technical difficulties in terms of storage and transportation. Materials suitable for hydrogen production, distribution, storage, and application are required to establish a clean and sustainable hydrogen economy. First, the article sheds light on the process that causes HE. The current study approaches and findings have also been examined [1].
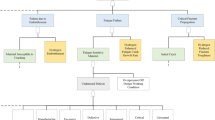
2 Mechanism of HE
HE of a material may be attributed to several different mechanisms (Fig. 1). Some of the most important mechanisms will be explored at length in the present section [2].
2.1 Hydrogen-Enhanced Decohesion Mechanism (HEDE)
Troiano initially proposed this technique in 1959, and it is the simplest of all [3]. It relies on introducing a hydrogen atom into a fracture to reduce the material’s cohesive strength near the crack’s tip. Iron’s 3rd cell has a weaker 3d interatomic connection because of a hydrogen 1s electron. There will be decohesion when the critical crack tip opening displacement (CTOP) is attained. Hydrogen atoms diffuse throughout the material at the crack tip, diminishing interatomic or cohesive strength. This causes cleavage-like fractures because hydrogen atoms are readily accessible throughout the material when applied forces. A material’s surface energy is lowered when its cohesive strength diminishes, resulting in reduced fracture stress and a lower-than-acceptable fracture rate.
2.2 Hydrogen-Enhanced Local Plasticity Model (HELP)
This technique was initially introduced in 1972 and has since gained widespread acceptance. Dislocation mobility rises due to hydrogen accumulating at fracture tips in this model, which reduces dislocation motion resistance. As a result, dislocations function as transporters of deformations in a metal lattice. Since hydrogen reduces the yield stress locally, dislocation movement is feasible even at low-stress levels.
2.3 Adsorption-Induced Dislocation Emission (AIDE)
In principle, the approach is a mix of HEDE and HELP. In this process, hydrogen atoms from the solution adsorb on the surfaces of fracture points and other high-stress areas. Hydrogen adsorption at a fracture tip occurs in the HEDE mechanism, promoting dislocation injection from a crack tip, culminating in crack initiation by slip and micro-void generation by the HELP mechanism.
2.4 Hydrogen-Enhanced Macroscopic Ductility (HEMP)
When hydrogen is accessible in significant volumes (concentrations) and interacts with the whole specimen volume, it reduces steel’s yield strength owing to hydrogen diffusion and solid solution weakening by hydrogen atoms. As a result, a macroscopic enrichment in plasticity had developed throughout the whole specimen length (volume).
2.5 Hydrogen-Changed Microfracture Mode (HAM)
Previously, ductile materials became brittle when hydrogen altered their microfracture modes. Materials lost some of their ductility due to hydrogen charge, and their fracture modes have shifted from cup-and-cone to a more common brittle shear type. Another reason is that a high hydrogen cluster at the dislocation site aids the shear fracture mechanism. Hydrogen-assisted microfracture is the name of this shift in microfracture mode caused by hydrogen (HAM).
2.6 De-cohesive Hydrogen Fracture (DHF)
It is simply a brittle fracture that is aided by hydrogen or decohesion and ends with the material or specimen breaking apart. The ductile fracture triggered this fracture. The final fractography was found to include both brittle (fisheye) and flexible (MVC) characteristics during the final fracture surface inspection (by SEM or TEM). As a result, the combined action of these two microfracture processes results in the ultimate material fracture. The middle portion, often at inclusion, is born fish eyes. Fish eyeballs expand and fracture radially until they come into touch (get near) with a ductile zone or coalescence of micro-voids. The MVC fracture encloses these fish eyes. Hydrogen microfracture mechanisms for fisheyes and MVC compete in this sort of fracture, known as a mixed fracture mechanism.
2.7 Hydrogen-Assisted Micro-Void Coalescence (HAMVC)
As the name implies, micro-void coalescence (MVC) is a method of fracture that uses ductility. As far as the progression of a multi-vessel crack (MVC), several distinct stages are involved, from initial nucleation to ultimate coalescence and additional crack extension to the breakage of any residual ligament by shear. As a result of the hydrogen’s impact, materials suffer dislocation motion and localized plastic deformities. The fracture spread in a zigzag pattern as voids joined in the crack propagation direction. In the presence of hydrogen, an MVC dimple will form, but it will be brittle and difficult to process. Shallow shear dimples are caused by shear stress, which leads to final fracture. The presence of hydrogen causes some brittle intergranular fracture on the specimen’s edges. Micro-void coalescence aided by hydrogen is the term for this procedure (HDMC). Hydrogen, it turns out, affects the ductile MVC process. MVC shear dimples surround fisheyes seen in fracture propagation regions on rare occasions.
3 Literature Review
With the Devanathan—Stachurski technique, Xing et al. investigated the influence of temperature on bulk hydrogen concentration and diffusion. Experiments validated the theoretical models and suggested a temperature threshold of 320 K as an optimal temperature for fracture initiation and propagation. This research may be used to improve pipeline maintenance operations and extend their useful lives [4].
Tian et al. [5] investigated calcareous deposition, hydrogen penetration, and hydrogen embrittlement in artificial seawater with cathodic polarization. When hydrogen concentration is low, the preferred cracking path is lath boundary separation; when hydrogen concentration is high, the preferred cracking method is preceding austenite grain failure.
Cerniauskas et al. studied mixing hydrogen with natural gas (H2/NG) for transportation. This research develops cost functions for several pipeline reassignment techniques. 80% of the typical German pipeline network studied showed a technically feasible potential. The study of pipeline availability limitations in 2030 indicates a 30% decrease in transmission system’s cost compared to a newly constructed hydrogen pipeline system [6].
The study done by Zhou et al. investigates the deterioration process of X80 steel, a material often used in natural gas pipelines. The findings indicate that the tensile and yield strengths are not significantly affected by using hydrogen-mixed natural gas. This research lays the groundwork for a safe hydrogen percentage in natural gas for X80 pipeline steel [7].
Trautmann and colleagues studied hydrogen embrittlement due to the environment in hydrogen-natural gas transportation. This research lays the groundwork for a safe hydrogen percentage for X80 pipeline steel while carrying hydrogen mixed natural gas combined with other gases. Slow strain tensile tests are employed in this study to qualitatively investigate the degradation process of X80 steel, which is often used in natural gas pipelines, according to the results. After stretching, mechanical characteristics and fracture morphologies are examined in more detail [8].
Researchers Villalobos and coworkers studied the impact of hydrogen embrittlement (HE) on the mechanical characteristics of X-120 micro-alloyed steel. The decohesion and dislocation emission process of HE discovered via fractography study leads to HE nucleation and crack development. Findings revealed a martensite-bainite, acicular-ferrite, and maintained austenite microstructure with no discernible change in morphology following tempering treatment. However, when the tempering temperature rose, the mechanical qualities of the steel decreased [9].
The study by Shin et al. examined the impact of hydrogen embrittlement (HE) on the mechanical characteristics of X-120 micro-alloyed steel after a 10-min tempering treatment at 200, 400, or 600 ℃. Decohesion and dislocation emission of HE was discovered via fractography study, and these processes lead to HE nucleation and crack development. As the number of irreversible traps increased throughout the tempering process, there was a link between index brittleness, effective diffusion reduction, and apparent concentration rise [10].
In situations where CO2 coexists with other gases, cracking failure processes need more knowledge than in a pure CO2 environment. Carbon dioxide with H2S traces may cause hydrogen embrittlement and pipeline steel failure. The study by Silva et al. aims to have a closer look at an API 5L X65 steel’s laboratory performance in a CO2–H2S environment (a little sour) compared to that of the same material in a purely CO2 atmosphere [11].
According to Jiang et al., PH stainless steel (SS), 17–4, is often utilized in high-pressure corrosive environments. Some petrochemical pipeline valve stems are made from 17 to 4 PH SS to assure strength, impact toughness, and corrosion resistance. HE was shown to be a factor in the valve stem failing due to stress fracture. The tensile and fatigue properties of X65 pipeline steel have deteriorated because of hydrogen-driven blister cracking [12].
Singh et al. reported that tensile and fatigue specimens were electrochemically charged with hydrogen at 20 mA/cm2. Using the hydrogen generator caused HIC and blisters on the model. BWC and HIC have been found in Al–Si–O inclusions together. Hydrogen embrittlement is shown via an SSRT test and a fractographic analysis of X65 steel [13].
In alpha iron at ambient temperature, a phase transition from body-centered cubic (bcc) to face-centered cubic (fcc) phase was investigated by Xing et al. The propagation of the fracture was studied using molecular dynamics simulations. According to the findings, the phase shift may release strain energy and delay fracture development without hydrogen atoms. Before the crack’s tip, hydrogen atoms would form a nanosize hydrogen-rich region, blocking the phase change. Brittle fractures might develop as a result of this. A general formula may determine the hydrogen concentration at which no phase transition occurs. The risk level during the procedure may also be predicted using a simple crack development model [4].
When the deforming temperature and deforming strain in a hydrogen atmosphere were varied in different ways, Chen et al. studied the resistance of austenitic stainless steel to hydrogen environment embrittlement (HEE). Resistance to HEE may be improved by deforming at higher temperatures (100–300 ℃) and reduced by using more significant deforming stresses. Tremendous strain led to primary deformation twins, which helped cause strain-induced martensite to form and accelerate the growth of hydrogen-induced fractures [14].
In a hydrogen environment with structure–property-performance linkages, ferritic steels are subjected to mechanical stress (Martin et al.). When hydrogen is present in a ferritic steel, the structure is explained by sorption and dissociation processes, diffusion across lattice and grain boundaries, and hydrogen–steel interactions. Once numerous embrittlement processes have been thoroughly examined, steels’ performance in hydrogen environments may be assessed by looking at how well they function [15].
Using a mass diffusion model fueled by mechanical fields, Gobbi et al. simulated hydrogen embrittlement in plastics. Because the automatic reaction is dependent on the hydrogen concentration, continuum mechanics and mass transfer calculations are tightly connected. All of the code generated for this work is freely available to anybody who wants it [16].
Wang et al. investigated the impact of a quenching–tempering (QT) treatment on a reactor pressure vessel’s hydrogen embrittlement (HE) resistance. Studies using transmission electron microscopy and atom probe tomography confirmed the breakdown of M3C/VC carbides and the precipitation of M7C3 carbides. After QT treatment, HE sensitivity reduced to almost nothing. Lower M3C carbides, which serve as hydrogen trapping sites, were the primary cause of increased HE resistance. Using thermal desorption spectroscopy, it was discovered that the concentration of reversible hydrogen had dropped. The quantity of irreversible hydrogen (likely trapped in VC carbides) was also reduced, although this is not the cause of the improved HE quality [17].
Titov et al. studied hydrogen build-up and dispersion in high-corrosion pipeline steel. Axial stress tests and XRD measurements show corrosion on steel pipeline inner and outer surfaces. Hydrogen is produced when methane dissociates insignificantly. Measurements were made to determine the concentration of adsorbed hydrogen in the steel pipe bulk. Charpy specimens were subjected to national standards-required pendulum impact testing at room temperature. Materials testing revealed that hydrogen embrittlement significantly reduced the mechanical characteristics of steel specimens [18].
The influence of hydrogen on candidate fracture toughness (KQ) was studied by Wasim and Djukic using low carbon steel soaked in acidic hydrogen conditions for a year. The study is significant because of its practical use in evaluating steel durability against hydrogen environmentally aided cracking (HEAC) [19].
Three structural steel sheets were tested to see how hydrogen affected their mechanical properties (Alvarez et al.). It was found that hydrogen embrittlement could be accurately evaluated by performing a Small Punch Test on notched specimens. This study’s results were compared to standard fracture testing to discover which was superior. It took 21 h for gaseous hydrogen at 19.5 MPa and 450 C to burn in the first technique [20].
The possibility of transferring hydrogen via a natural gas pipeline was examined by Boukortt et al. To determine whether hydrogen embrittlement affects the mechanical properties of pipes, researchers conducted this investigation. The yield stress and ultimate strength proved to be less responsive to HE than previously thought. Fracture toughness and failure elongation, on the other hand, both decreased significantly [21].
Researchers (Alvarez et al.) investigated how the displacement rate affected fracture toughness of two structural steel grades. When the displacement rate is low, and steel yield strength is high, the fracture toughness is reduced because of internal hydrogen. To evaluate the fracture toughness of steel, hydrogen gas was used to pre-charge the specimens for 21 h at 19.5 MPa, and 450 ℃ and then typical air temperature tests were done [22].
Wasim and Djukic studied hydrogen-induced mechanical deterioration of low carbon steel on the macro, micro, and nanoscales. A link was discovered between hydrogen concentration and corrosion growth after 28 days after exposure to hydrogen-rich acidic environments. Microstructural analysis revealed that hydrogen penetration had deformed bigger grains, cracked surfaces, and formed blisters [23].
Researchers (Bouledroua et al.) found that blending gaseous hydrogen into a natural gas flow significantly impacted hydrogen permeation in a steel pipeline. Fractures may trigger pipeline collapse and hydrogen leaking. Explosions are much more probable due to the latter, making this phenomenon highly hazardous. A reliable technique is given to minimize the hazards associated with hydrogen embrittlement-induced pipe failure [24].
Nguyen et al. investigated the effects of low hydrogen partial pressure and methane gas on welded API X70 pipeline steel. The weld metal was more susceptible to hydrogen-induced fracture than the base metal. The smooth tensile specimen was used since the weld metal lost ductility under 10 MPa of a gas combination with 1% hydrogen [25].
4 Literature Summary
See Table 1
5 HE Assessment Methods and Instruments
Researchers often use fracture tests to characterize and evaluate the HE susceptibility of various pipe steels. In the process of evaluating the effect of hydrogen on the pipe material, the pipeline specimen is exposed to two different environments. In the first environment, the pipe specimens are tested in a hydrogen-free environment, and in the second, the pipe specimen is exposed to hydrogen. Afterward, the pipe specimen is tested for material tensile strength, fracture, and yield strength. The results are shown in Fig. 2. The figure depicts the probable outcomes of the test by showing the variation in stress versus strain profiles of un-convicted and hydrogen-charged pipe specimens [28]. It can be seen in the figure that the hydrogen-charged specimens result in a decline in pipe material strength tensile at failure and extension at the fracture point [29].
5.1 Constant Load Test
The test is carried out by applying stress to the hydrogen exposed pipe specimen. The test evaluates the maximum hydrogen that can diffuse into steel pipe without material failure [30]. Researchers like Hagihara [31]; Takagi et al. [32]; Scharf et al. [33] utilized the CLT tests to evaluate the susceptibility of HE to steel specimens. In 2005, Takagi et al. [32] exploited the test to assess the HE susceptibility of AHSS that was first quenched and then tempered. In the process conducted, the specimen was first cathodic charged in a water solution that contained NaCl and NH4SCN. The specimen was further coated by using Cadmium coating to minimize HE. After the fracture of the specimen caused due to CLT, the hydrogen content in the specimen was estimated using thermal desorption analysis. In the study of Chida et al. [34], Zinc coating was used instead of cadmium to test the HE sensitivity of low-alloy high-strength steels.
With an increase in hydrogen concentration, fracture stress was reduced. To determine HE resistance, the exposed zinc electro-galvanized DP1000 specimens were subjected to in situ hydrogen charging by Scharf et al. [33]. A “corrosive cup” filled with NaCl solution was used to charge the hydrogen, and a proof ring was used to provide a constant load. To determine the effect on HE of material pre-damage, hammering, cutting tools, and milling were used. Microcracks appeared only on specimens with shear-cut edges; milled edges were unaffected.
5.2 Slow Strain Rate Test (SSRT)
The strain rate in SSRT is typically 10–6 per second, which is comparatively much lower in magnitude than the one used in regular tensile testing. In the SSRT testing process, the applied force is steadily raised until the pipe specimen fractures. The process enhances stress-induced hydrogen diffusion, resulting in hydrogen build-up in a specimen based on the stress concentration.
There is evidence from Rehrl et al. [35] that strain rate significantly affects AHSS mechanical properties when hydrogen is present. Hydrogen did not affect mechanical properties at a strain rate of 20 per second. It was shown that at a slow strain rate of 10–5 per second, hydrogen-charged and hydrogen-free specimens both had a 25 and 90% decrease in their strength and elongation at fracture. When hydrogen is present, AHSS exhibits strain rate sensitivity. It is expected that SSRT results will be consistent with those of CLT in terms of hydrogen concentration and fracture stress because of the possibility for hydrogen diffusion (Chida et al.) [34]. Compared to CLT, the test time and variability of results are reduced significantly using SSRT. Because it always culminates in a material failure, the SSRT technique eliminates the problem of specifying a testing time.
Smooth or perforated specimens might be used for this test. Stress–strain curves are employed when evaluating cracking susceptibility, followed by scanning electron microscopy (SEM) and thermal desorption to determine hydrogen concentration.
As part of their investigation into whether or not the AHSS was susceptible to HE, Wang et al. [36,37,38] Cathodic hydrogen charging, Cd plating, and homogenization of hydrogen content were performed on the specimens, resulting in smooth and notched surfaces. The fracture stress decreased as the hydrogen diffusible quantity increased. An in situ hydrogen-charging SSRT method was used to determine a Cr–Mo steel’s vulnerability to high-energy hydrogen embrittlement (HEHE). Temper embrittled steel was shown to be safe at a hydrogen concentration of 0.064 parts per million (ppm).
5.3 Conventional Strain Rate Test
Conventional strain rate testing (CSRT) results in a specimen's hydrogen content corresponding to the hydrogen concentration acquired throughout the CLT and SSRT tests. A very high strain rate is used in the test, resulting in little hydrogen diffusion. Therefore, a fracture in a specimen’s hydrogen content corresponds to the average hydrogen content overall. The main benefit of this method is that it takes just a few minutes to test. On the other hand, hydrogen charge before testing must be very consistent. Chida et al. [34] used SSRT, CLT, and CSRT to investigate low-alloy steels’ HE susceptibility. Nominal fracture stress was shown to correlate with diffusible hydrogen concentration using SSRT and CLT, which allow for hydrogen diffusion throughout the experiments. Dislocations may have inhibited hydrogen’s ability to interact with dislocations, resulting in a lower HE susceptibility.
5.4 Stepwise Load Test
Another approach of evaluating HE susceptibility was presented by Takagi et al. [32]. When performing a stepwise load test, the specimen is subjected to a series of rising stress and stress holding phases. During the holding period, the hydrogen distribution almost achieves equilibrium. Researchers first applied 702 MPa of stress for 12 h, then 14 MPa for 2 h, with a holding duration of two and a half hours. Independency from specimen size and stress conditions are two advantages of this approach. Therefore, applying the test findings to components of varying dimensions and stress concentration factors makes it possible.
6 Material Selection to Prevent HE in Pipeline Structures
Based on the above discussion, it is clear that HE causes huge damage to pipelines. Hence, controlling the HE is of foremost importance. Understanding the correct source of hydrogen formation and the mechanism leading to HE can help find suitable coating materials that can prevent hydrogen embrittlement. Selecting the most appropriate coating material to prevent HE is therefore critical.
High-strength materials must be protected against hydrogen embrittlement (HE) by knowing the true source of hydrogen and the mechanism responsible for this phenomenon. Selecting the most appropriate material is critical to success when working in a hydrogen environment. The material design must be suited for the situation. Before processing, it is necessary to prevent notch formation, acute and regular variation, and residual stresses must be eliminated. The baking process was adopted to remove hydrogen absorbed and potentially cause damage or failure. There are several different ways to bake, and the temperature at which they are accomplished varies depending on the kind of material being baked. Pickling is a process that uses an acidic solution to remove scale and oxide compounds from a material. Various mechanical methods, including sanding, gritty blasting, and vapor blasting, will limit the amount of hydrogen released into the environment. Metal alloys and a protective film on the surface of the supporting structure may also be used to reduce the risk of exposure to harmful electromagnetic radiation (HE). A few coating processes include vacuum deposition, organic deposition, and mechanical plating (among others). The use of efficient inhibitors is also vital in this process. If titanium is easily available in considerable numbers, increasing the titanium percentage of hot-stamped boron steel may reduce the material’s high-temperature susceptibility to hydrogen embrittlement (HE) by creating titanium carbide in the material [21]. Some experts feel that aluminum alloying also has a positive effect on the HE impacts. Niobium and graphene coatings have also been shown to protect materials against high-energy physics.
In their studies, Kim and Kim [21] determined that the graphene layer serves as a robust hydrogen barrier and therefore is resilient even after being saturated with hydrogen. It was accomplished due to the increased diffusion length and the formation of the C–h bond during the hydrogen charging the atmosphere. Cadmium is a metal used in steel to prevent hydrogen diffusion during the production process. The hydrogen diffusion coefficient in cadmium and tin is lesser than the hydrogen diffusion coefficient in ferrite.
Steel is coated with a nickel layer to prevent hydrogen from leaking into the atmosphere. Different coatings, such as titanium dioxide and titanium carbide (TiC), have been shielded against hydrogen transfer. According to Cwiek, coatings comprised platinum, copper, lead, silver, aluminum, and gold may reduce hydrogen diffusion inside the steel structure itself. The outer skin of oxides and nitrides, according to him, may also serve as a deterrent to hydrogen diffusion, decreasing the quantity of hydrogen absorbed [39]. The practical operation of the coating relies on the sturdiness of the coating and the existence of coating faults. Furthermore, the service’s condition is critical [40].
There is localized tension in the coating, they could probably wear off, and hydrogen will seep onto the base metal. Although the diffusing layer of nitrogen and carbon is very effective in lowering the rate of fracture spread, it is not effective at eliminating hydrogen-induced environmental embrittlement in the material under study. The possibility of HE is reduced as long as excellent adhesion, an adequate coating level, and a coating free of flaws are provided [29].
Some elements that produce hydrogen may induce hydrogen to infiltrate the structure of a substance if they are exposed to a hydrogen environment. The elements in question are Te, Sn, S, Hg, Se, Pb, As, and Bi. They aid in the admission of hydrogen into the material, and if they are already present in the material, they divide the grain boundary, making them severely prohibited [39].
6.1 Electroplating Using Different Metals to Prevent HE
Researchers have used different materials to avoid HE. Electroplating with zinc and cadmium provides corrosion resistance for structural steel with high tensile strength. However, this plating process is also responsible for entering hydrogen into steel and its genesis. This has been accomplished by heating the plated steel to between 190 and 230 ℃ over 10–24 h to decrease the corrosion. As a result of this, diffusible hydrogen is released from the substance.
When building the model, heat treatment was believed not to affect the mechanical properties of steel and alloys. According to Hillier’s research on alloying zinc with nickel, this method reduced hydrogen penetration through steel. As a result of the initial nickel deposit, a nickel coating is produced over the material, and this layer prevents hydrogen from getting into the substance. Hydrogen’s diffusivity coefficient in nickel is 5 * 10–11 m2/s, a very low diffusion rate. A lot of the time, hydrogen can get through the coating. This depends on how quickly hydrogen moves through the coating and how thick it is. In certain cases, hydrogen adsorbed on the surface interacts with other hydrogen molecules to form a new molecule, which escapes before penetrating the coating by forming bubbles. Cadmium is required for the recombination of hydrogen in coatings and reduces hydrogen entry.
6.2 Using Cadmium and Titanium as Electroplate Metals
This plating method has been around for a long time, developed in 1960 [41]. In the beginning, the required results were achieved using a cadmium cyanide solution in combination with a titanium compound solvable in cyanide. The titanium concentration of the deposit varied from 0.1 to 0.5 percent if the treatment was performed appropriately. When it comes to high-stress settings, it has long been used to coat threaded rods and gear actuation cylinders and associated shafts. Non-cyanide solution with titanium concentrations ranging from 0.1e0.7% was created. Non-cyanide solutions do not change the composition of titanium compounds. Protective coatings on high-quality instrument steel, high-strength structural steel, and spring wire have been successfully applied using the non-cyanide solution rather than cyanide solution [42].
6.3 Other Methods to Prevent HE
Ion plating, also known as physical vapor deposition, eliminates hydrogen embrittlement. The risk of embrittlement is reduced since this technique was performed in a vacuum. It has been used in aviation for more than two decades. Unlike prior electroplating procedures, this ion aluminum coating approach has been shown to preserve the surface better [43]. A thin coating of Pt, Cu, or electroless nickel is injected as a protective layer [43]. Preventing hydrogen penetration may be accomplished by coatings made of Au, Sn, and certain Sn and Pb alloys, for example [44,45,46,47].
Utilizing a protective film thickness of 0.0015 mm, hydrogen penetration in iron may be prevented when using Pt coating. Cu was likewise more efficacious than Ni at decreasing hydrogen migration in iron [43]. A further study reveals hydrogen cracking may be prevented by applying lead coatings to specified steels in a specific environment [43,44,45,46,47,48].
The electrodeposited nickel was protected against hydrogen ambient embrittlement by coating it with copper or gold, as seen in the illustration. It has been discovered that the ductility of electrodeposited nickel is not affected by the application of either copper or gold coatings [45]. The presence of alumina creates a barrier to hydrogen diffusion. This approach deposits alumina on the steel and deposits a one-millimeter-thick layer of crystallized aluminum oxide [49]. At 800 ℃, it has been found that temperature does not affect this coating and that it continues to work. This process creates a protective layer of enriched alumina on the steel’s surface. This alumina can reduce steel's hydrogen permeability and rate of hydrogen permeation by order of magnitude when contrasted to bare steel.
To diffuse ions into an environment, the surface layer of an amorphous material is unique in that it provides an adequate barrier. This is the novel phosphorus ion implantation technology [50]. The passage of hydrogen through the disordered structure and the penetration of hydrogen from it to the base material are very challenging for hydrogen. Santos observed that the amorphous iron base alloy’s hydrogen diffusion rate is slower and lower than ferrite steel’s [51].
6.4 Corrosion Inhibitors for Preventing Hydrogen Embrittlement
Several compounds and mixes may inhibit, prevent, or decrease corrosion when applied at low concentrations and in a harsh environment to a protective system. Following processes are often used to explain the working of ow an inhibitor works [52].
-
Adhesion of the inhibitor to the metal's surface results in a protective coating that acts as an inhibitor or combines the effects of both the metal’s surface and its own.
-
The inhibitor leads to a formation of a film by oxide protection of the base metal.
-
A complex product is formed when the inhibitor combines with a corrosive component present in the aqueous medium.
To minimize the apparent corrosion rate, inhibitors build up as a coating on the metal surface. Inhibitory chemicals, of which they are the most prevalent, are classified as a subclass. The most prevalent types of inhibitors of the barrier layer are adsorption-type inhibitors. Adsorbed onto the metal surface, these organic molecules often establish a strong connection. As the adsorption process is finished, the apparent corrosion rate reduces.
7 Conclusion
HE is a critical issue in the oil and gas business. Pipelines and related facilities suffer significant losses while transporting hydrogen. The main function of HE is to diminish material ductility and make it brittle. The deterioration of mechanical characteristics is caused by hydrogen embrittlement. We discussed the causes and mechanisms of hydrogen embrittlement in our review research. The diffusion of hydrogen causes fracture growth in materials. The crack growth rate is primarily determined by the stress concentration factor and the loading frequency; when the loading frequency decreases, the possibilities of fatigue crack growth of sample rise. This research also explored how the microstructure and loading condition significantly impacts high-strength steel's HE vulnerability. Material selection is critical for lowering the HE. The use of alloys such as Al, titanium, and Mn may help to avoid hydrogen embrittlement.
References
Hardie D, Charles EA, Lopez AH (2006) Hydrogen embrittlement of high-strength pipeline steels. Corros Sci 48(12):4378–4385
Lynch S (2012) Hydrogen embrittlement phenomena and mechanisms. Corros Rev 30(3–4):105–123
Troiano AR (1959) Delayed failure of high strength steels. Corrosion 15(4):57–62
Xing X, Zhang H, Cui G, Liu J, Li Z (2019) Hydrogen inhibited phase transition near crack tip–An atomistic mechanism of hydrogen embrittlement. Int J Hydrogen Energy 44(31):17146–17153
Tian H, Xin J, Li Y, Wang X, Cui Z (2019) Combined effect of cathodic potential and sulfur species on calcareous deposition, hydrogen permeation, and hydrogen embrittlement of a low carbon bainite steel in artificial seawater. Corros Sci 158:108089
Cerniauskas S, Junco AJC, Grube T, Robinius M, Stolten D (2020) Options of natural gas pipeline reassignment for hydrogen: cost assessment for a Germany case study. Int J Hydrogen Energy 45(21):12095–12107
Zhou D, Li T, Huang D, Wu Y, Huang Z, Xiao W, Wang Q, Wang X (2021) The experiment study to assess the impact of hydrogen blended natural gas on the tensile properties and damage mechanism of X80 pipeline steel. Int J Hydrogen Energy 46(10):7402–7414
Trautmann A, Mori G, Oberndorfer M, Bauer S, Holzer C, Dittmann C (2020) Hydrogen uptake and embrittlement of carbon steels in various environments. Materials 13(16):3604
Villalobos JC, Del-Pozo A, Mayen J, Serna S, Campillo B (2020) Hydrogen embrittlement suscetibility on X-120 microalloyed steel as function of tempering temperature. Int J Hydrogen Energy 45(15):9137–9148
Shin HS, Bae KO, Baek UB, Nahm SH (2019) Establishment of an in-situ small punch test method for characterizing hydrogen embrittlement behaviors under hydrogen gas environments and new influencing factor. Int J Hydrogen Energy 44(41):23472–23483
Silva SC, Silva AB, Gomes JP (2021) Hydrogen embrittlement of API 5L X65 pipeline steel in CO2 containing low H2S concentration environment. Eng Fail Anal 120:105081
Jiang T, Zhong J, Zhang X, Wang W, Guan K (2020) Hydrogen embrittlement induced fracture of 17–4 PH stainless steel valve stem. Eng Fail Anal 113:104576
Singh V, Singh R, Arora KS, Mahajan DK (2019) Hydrogen induced blister cracking and mechanical failure in X65 pipeline steels. Int J Hydrogen Energy 44(39):22039–22049
Chen X, Ma L, Zhou C, Hong Y, Tao H, Zheng J, Zhang L (2019) Improved resistance to hydrogen environment embrittlement of warm-deformed 304 austenitic stainless steel in high-pressure hydrogen atmosphere. Corros Sci 148:159–170
Martin ML, Connolly MJ, DelRio FW, Slifka AJ (2020) Hydrogen embrittlement in ferritic steels. Appl Phys Rev 7(4):041301
Gobbi G, Colombo C, Miccoli S, Vergani L (2018) A weakly coupled implementation of hydrogen embrittlement in FE analysis. Finite Elem Anal Des 141:17–25
Wang Y, Hu S, Li Y, Cheng G (2019) Improved hydrogen embrittlement resistance after quenching–tempering treatment for a Cr–Mo-V high strength steel. Int J Hydrogen Energy 44(54):29017–29026
Titov AI, Lun-Fu AV, Gayvaronskiy AV, Bubenchikov MA, Bubenchikov AM, Lider AM, Syrtanov MS, Kudiiarov VN (2019) Hydrogen accumulation and distribution in pipeline steel in intensified corrosion conditions. Materials 12(9):1409
Wasim M, Djukic MB (2020) Hydrogen embrittlement of low carbon structural steel at macro-, micro-and nano-levels. Int J Hydrogen Energy 45(3):2145–2156
Álvarez G, Zafra A, Belzunce FJ, Rodríguez C (2021) Hydrogen embrittlement testing procedure for the analysis of structural steels with small punch tests using notched specimens. Eng Fract Mech 253:107906
Boukortt H, Amara M, Meliani MH, Bouledroua O, Muthanna BGN, Suleiman RK, Sorour AA, Pluvinage G (2018) Hydrogen embrittlement effect on the structural integrity of API 5L X52 steel pipeline. Int J Hydrogen Energy 43(42):19615–19624
Álvarez G, Peral LB, Rodríguez C, García TE, Belzunce FJ (2019) Hydrogen embrittlement of structural steels: effect of the displacement rate on the fracture toughness of high-pressure hydrogen pre-charged samples. Int J Hydrogen Energy 44(29):15634–15643
Wasim M, Djukic MB, Ngo TD (2021) Influence of hydrogen-enhanced plasticity and decohesion mechanisms of hydrogen embrittlement on the fracture resistance of steel. Eng Fail Anal 123:105312
Bouledroua O, Hafsi Z, Djukic MB, Elaoud S (2020) The synergistic effects of hydrogen embrittlement and transient gas flow conditions on integrity assessment of a precracked steel pipeline. Int J Hydrogen Energy 45(35):18010–18020
Nguyen TT, Park JS, Kim WS, Nahm SH, Beak UB (2020) Environment hydrogen embrittlement of pipeline steel X70 under various gas mixture conditions with in situ small punch tests. Mater Sci Eng, A 781:139114
Ohaeri E, Eduok U, Szpunar J (2019) Relationship between microstructural features in pipeline steel and hydrogen assisted degradation. Eng Fail Anal 96:496–507
Xing X, Zhou J, Zhang S, Zhang H, Li Z, Li Z (2019) Quantification of temperature dependence of hydrogen embrittlement in pipeline steel. Materials 12(4):585
Rudomilova D, Prošek T, Luckeneder G (2018) Techniques for investigation of hydrogen embrittlement of advanced high strength steels. Corros Rev 36(5):413–434
Dwivedi SK, Vishwakarma M (2018) Hydrogen embrittlement in different materials: a review. Int J Hydrogen Energy 43(46):21603–21616
Chida T, Hagihara Y, Akiyama E, Iwanaga K, Takagi S, Ohishi H et al (2014) Comparison of constant load, SSRT, and CSRT methods for hydrogen embrittlement evaluation using round bar specimens of high strength steels. Tetsu-to-Hagane 100(10):1298–1305
Hagihara Y (2012) Evaluation of delayed fracture characteristics of high-strength bolt steels by CSRT. ISIJ Int 52(2):292–297
Takagi S, Toji Y, Yoshino M, Hasegawa K (2012) Hydrogen embrittlement resistance evaluation of ultra high strength steel sheets for automobiles. ISIJ Int 52(2):316–322
Scharf R, Muhr A, Luckeneder G, Larour P, Mraczek K, Rehrl J et al (2016) Hydrogen embrittlement of DP‐1000 flat steel sheet: influence of mechanical properties, specimen geometry, pre‐damaging and electrolytically zinc galvanizing. Mater Corros 67(3):239–250
Chida T, Hagihara Y, Akiyama E, Iwanaga K, Takagi S, Hayakawa M et al (2016) Comparison of constant load, SSRT and CSRT methods for hydrogen embrittlement evaluation using round bar specimens of high strength steels. Isij Int 56(7):1268–1275
Rehrl J, Mraczek K, Pichler A, Werner E (2014) The impact of Nb, Ti, Zr, B, V, and Mo on the hydrogen diffusion in four different AHSS/UHSS microstructures. Steel Res Int 85(3):336–346
Wang M, Akiyama E, Tsuzaki K (2005) Crosshead speed dependence of the notch tensile strength of a high strength steel in the presence of hydrogen. Scripta Mater 53(6):713–718
Wang MQ, Akiyama E, Tsuzaki K (2006) Fracture criterion for hydrogen embrittlement of high strength steel. Mater Sci Technol 22(2):167–172
Wang M, Akiyama E, Tsuzaki K (2007) Effect of hydrogen on the fracture behavior of high strength steel during slow strain rate test. Corros Sci 49(11):4081–4097
Ćwiek J (2010) Prevention methods against hydrogen degradation of steel. J Achievements Mater Manuf Eng 43(1):214–221
Hollenberg GW, Simonen EP, Kalinin G, Terlain A (1995) Tritium/hydrogen barrier development. Fusion Eng Des 28:190–208
Takata K (1963) Japanese patents SHO-35 18260 (1960) and SHO-38 20703
Fannin ER, Muehlberger DE (1978) Ivadizer applied aluminum coating improves corrosion protection of aircraft. McDonnell Aircraft Company, p 26
Chatterjee SS, Ateya BG, Pickering HW (1978) Effect of electrodeposited metals on the permeation of hydrogen through iron membranes. Metall Trans A 9(3):389–395
Perng TP, Johnson MJ, Altstetter CJ (1988) Hydrogen permeation through coated and uncoated WASPALOY. Metall Trans A 19(5):1187–1192
Chandler WT, Walter RJ, Moeller CE, Carpenter HW (1978) Effect of high-pressure H on electrodeposited Ni. Plat Surf Finish 65(5):63e70
Robinson SL, SL R, WA S, AD A (1979) The role of brush plating in future hydrogen and transmission systems
Begeal DR (1975) The permeation and diffusion of hydrogen and deuterium through Rodar, tin—coated Rodar, and solder—coated Rodar. J Vac Sci Technol 12(1):405–409
Freiman L, Titov V (1956) The inhibition of diffusion of hydrogen through iron and steel by surface films of some metals. Zh Fiz Khim 30:882
Levchuk D, Koch F, Maier H, Bolt H (2004) Deuterium permeation through Eurofer and α-alumina coated Eurofer. J Nucl Mater 328(2–3):103–106
Ensinger W, Wolf GK (1989) Protection against hydrogen embrittlement by ion beam mixing. Nucl Instrum Methods Phys Res, Sect B 39(1–4):552–555
Dos Santos DS, De Miranda PV (1997) J Mater Sci 32:6311e5
Soudani M, Hadj Meliani M, El-Miloudi K, Bouledroua O, Fares C, Benghalia MA et al (2018) Efficiency of green inhibitors against hydrogen embrittlement on mechanical properties of pipe steel API 5L X52 in hydrochloric acid medium. J Bio- Tribo-Corros 4(3):1–11
Nam TH, Lee JH, Choi SR, Yoo JB, Kim JG (2014) Graphene coating as a protective barrier against hydrogen embrittlement. Int J Hydrogen Energy 39(22):11810–11817
Wang SS, Chai JK, Shui YM, Liang JK (1981) Cd–Ti electrodeposits from a noncyanide bath. Plat Surf Finish 68(12):62–64
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Arya, A.K., Gautam, S., Yadav, S. (2022). Impact of Hydrogen Embrittlement in Pipeline Structures—A Critical Review. In: Mukherjee, K., Layek, R.K., De, D. (eds) Tailored Functional Materials. Springer Proceedings in Materials, vol 15. Springer, Singapore. https://doi.org/10.1007/978-981-19-2572-6_31
Download citation
DOI: https://doi.org/10.1007/978-981-19-2572-6_31
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2571-9
Online ISBN: 978-981-19-2572-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)