Abstract
Biochar is a prominent adsorbent for environmental remediation. The physicochemical properties responsible for adsorption can be significantly enhanced by using physical, chemical, and biological treatments of biochar. The biological treatment methods are advantageous in terms of cost-effectiveness and reduced secondary pollutants. The present chapter summarizes the need, methodology, modification mechanism of biological modification of biochar, and its implementation for environmental remediation. The biologically modified biochar can be obtained by either ex situ (pyrolysis of anaerobically digested residue) or in situ (using extracellular enzymes) technologies. The process includes colonization and biofilm formation by microorganisms on biochar surface and attachment of microbes. Biologically modified biochar metabolizes organic/inorganic contaminants and helps in adsorption, biodegradation, and bio-adsorption simultaneously.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
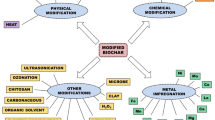
Generally, the pristine biochar has less adsorption capacity for the removal of contaminants as compared to modified biochar. Different modification methods are developed to increase the adsorption capacity of biochar for its utilization in soil remediation, energy storage, and wastewater remediation. These practices for the production of engineered or modified biochar are termed as biochar engineering (Ok et al. 2015). So, engineered/modified biochar can also be defined as a derivative of biochar with improved specific surface area, porosity, cation exchange capacity, and surface functional groups through biological, physical, chemical, or combination of these methods. The improved physicochemical properties provide significantly better adsorption capacity of modified biochar as compared to pristine biochar (Rajapaksha et al. 2016; Yao et al. 2013a, b, c).
Biological modification using earthworms is emerging as a potential method of biochar modification for increment in surface area, better pore size distribution, surface functional groups, and adsorption capacity for heavy metal contamination in soil. The enzymes generated by gut epithelium of earthworm and other symbionts are catalase, b-Deglucuronidase, alkaline phosphatase, d-aminolevulinate dehydratase, and superoxide dismutase. The biochar particles bind with these enzymes through mucus. So, this process can be employed where earthworms can ingest the substrate and discharge the enzyme coated modified biochar. This biologically modified biochar consists of enormous exo-enzymes (molecular ligands). The ex situ modification can be performed by activation of waste/sludge obtained as cow dung, leaf litter, anaerobically digested biomass, etc. This chapter explains in detail the methodology, mechanism, applications, and need for biological modification of biochar through metabolic enzymes.
2 Biological Modification Techniques
The metabolic pathways possessed by microorganisms, viz. Aeromonas, Cellulosimicrobium, Chloroflexi, Shewanella, Streptomyces, etc. (Mohammadipanah and Dehhaghi 2017; Wink et al. 2017), enable the biochar to integrate with various organic compounds and result in usable metabolites (Dehhaghi et al. 2019a, b) and value-added products (Dehhaghi et al. 2018, 2020; Sajedi et al. 2018). Due to their microscopic size, they can penetrate into pores of biochar and develop a non-washable rigid structure of biofilm. The biological modification process includes colonization and biofilm formation by microorganisms on biochar surface. The general mechanism of pollutant removal by biologically modified biochar is illustrated in Fig. 1. Initially, microbes get attached to biochar surface through sticky extracellular polymers and the contaminants get attached to it through molecular diffusion. These organic and inorganic pollutants get metabolized by microbes through various bio-electrochemical and biochemical reactions (Sharma et al. 2020). The biofilms perform degradation and removal of various inorganic, organic, and biological pollutant (Simpsosn 2008; Bouabidi et al. 2019; Sharma et al. 2020). A biologically modified biochar performs pollutant remediation in several steps, viz biofilm formation followed by biodegradation, desorption, and diffusion of contaminants at biochar–biofilm and air/soil/water interface (Wurzer et al. 2019).
Biological treatment enhances the desired physicochemical and functional properties of biochar. It enables the simultaneous adsorption of contaminants on biochar scaffold and their degradation by inoculated microorganisms. For example, several microorganisms (viz. Clostridium, Paenibacillus, Aeromonas, Cellulosimicrobium, Chloroflexi, Shewanella, etc.) possess bio-adsorbent characteristics for heavy metals (Hamedi et al. 2015; Mohammadipanah et al. 2015). The microbial colonization on biochar facilitates the adsorption of inorganic contaminants (heavy metals) with removal and degradation of organic contaminants (naphthenic acid) simultaneously (Frankel et al. 2016). It was observed that the biochar-active biofilm can efficiently perform adsorption and degradation (about 98% of carbamazepine) as compared to conventional sand-active biofilm (about 7% of carbamazepine) in a sewage treatment plant. The caffeine, ranitidine, and metoprolol adsorption characteristics were found similar for both conventional and biochar-based scaffolds (Dalahmeh et al. 2018).
2.1 Interaction of Biochar with Intestinal Enzymes of Earthworms
Earthworms can significantly change the physicochemical and biological properties of consumed substrates (Jones et al. 1994; Yuvaraj et al. 2019). The gut of earthworm/manure worm has various anaerobic (Clostridium, Paenibacillus, Aeromonas, Cellulosimicrobium, Chloroflexi, Shewanella, and Streptomyces) and aerobic (Photobacterium, Pseudomonas, and Bacillus) bacteria that releases many enzymes (Hong et al. 2011). The gut of Perionyx millardi, Drawida willsi, Drawida calebi, Dichogaster bolaui, and Pontoscolex corethrurus type of earthworms have sufficient quantity of phosphatase, lipase, urease, amylase, chitinase, protease, and cellulose enzymes (Parle 1963; Mishra and Dash 1980; Baskaran et al. 1986; Mishra 1993). The anterior portion of earthworms have higher enzyme secretion as compared to the posterior portion. It is due to that fact that fore-gut and stomach of earthworms have the enzyme secreting parts (Tillinghast and MacDonnell 1973). Mishra and Dash (1980) and Nakajima et al. (2003) have asserted that the cellulase, amylase, protease, and lipase are the most secreted enzymes from intestinal cells of earthworms. Urbasek and Pizl (1991) have stated that the mid-gut of Lumbricus terrestris earthworm releases 20 enzymes from three different sections, namely typhlosole, peripheral intestinal epithelium, and peripheral chloragocytes. More than 44% of enzymes (such as b-Deglucuronidase, superoxide dismutase, catalase, d-aminolevulinate dehydratase, and alkaline phosphatase) are produced in peripheral chloragogen. Also, the maximum proportion (about 70%) of glutamate dehydrogenase, isocitrate dehydrogenase, NADH, and NADPH diaphorase was also found in the mid-gut of earthworm. Similar to a tubular reactor, the gut also maintains the optimum temperature to avoid enzyme inactivation. During vermicomposting, the urease, dehydrogenase, acid, and alkali phosphatases catalytic activity increases initially and thereafter decreases as optimum concentration is reached. This phenomenon specifies earthworms as bioreactors for organic waste with microorganisms (Balachandar et al. 2020; Karmegam et al. 2019).
The interaction with earthworm enzymes can be a potential mode for biochar surface modification. An enormous quantity of organic waste can be ingested by earthworms and also an abundant amount of metal ions can get accumulated in chloragogen tissues of earthworms (Yuvaraj et al. 2020). The implementation of biochar with earthworms can significantly minimize the heavy metal concentration in soils (Shaaban et al. 2018; Sun et al. 2016). The abiotic components in biochar can enrich the soil with minerals and earthworms can induce the degradation process. The biochar particles bind with gut enzymes through mucus of earthworms (Urbasek and Pizl 1991). The mucus ejected from gastrointestinal epithelial cells consists of amino acids, mucopolysaccharides, and glycoproteins (Zhang et al. 2016a, b). The other enzyme originated from earthworm gut such as alkaline phosphatase, amylase, nitrate reductase, cellulose, and acid phosphatase can induce microbial growth. Therefore, the intestines of earthworms and symbionts can be seen as potential sources of extracellular enzymes for biochar activation.
The co-application of biochar with earthworms was found to contribute significantly to soil nutrient enrichment (Ameloot et al. 2013; Puga et al. 2015). During vermicomposting, the ingested biochar by earthworms interacts with intestinal enzymes and is discharged with humus-like substances (Domene 2016). Sanchez-Hernandez (2018) have experimented with Aporrectodea caliginosa and Lumbricus terrestris earthworms in biochar mixed soil and harvested b-glucosidase, alkaline phosphatase, and carboxylesterase enzyme coated biochar released by earthworms. In another experiment, Sanchez-Hernandez et al. (2019) mixed 2.5–5% (w/w) biochar with soil and interacted with Lumbricus terrestris earthworms, and obtained enzyme coated biochar on the top of soil surface. The carboxylesterases induce biological modification/activation of biochar and can effectively remediate organophosphorus-contaminated soils. It can be explained by binding of carboxylesterases with oxygen analogs of organophosphorus (Wheelock et al. 2008).
2.2 Pyrolysis of Anaerobically Digested (AD) Waste
Apart from microorganism incubation, the biologically modified biochar can also be produced from the residues obtained after anaerobic digestion (AD) of biomass. The biochar produced from AD residue possesses a higher specific surface area, anion exchange capacity (AEC), cation exchange capacity (CEC), hydrophobicity, alkaline pH, and more negative surface charge as compared to conventional biochar (Yao et al. 2018). These variations in properties might be attributed to the alteration of redox potential and pH values of biomass during anaerobic digestion (Inyang et al. 2010). The enhanced AEC and CEC facilitate the utilization of biologically modified biochar for sequestration of both positive and negative ions from water. The higher cation adsorption capacity of AD biochar is due to the strong negative surface functional groups and negative zeta potential. The presence of strongly negative surface functional groups in modified biochar (due to negative zeta potential of AD waste) increases the cation adsorption capacity. The emerging industrial applications of modified biochar enhance the economic and environmental feasibility of biochar production from AD residue (Dehhaghi et al. 2019b; Tabatabaei et al. 2019). Another biological approach for biochar modification includes the utilization of mineral enriched biomass through bioaccumulation for the production of modified biochar (Yao et al. 2013b; Wang et al. 2017). This process results in value-added biochar nanocomposites and provides a safe disposal method for hyper-accumulating plants. Several studies on biochar production from bagasse stillage waste sugar beet residue, dairy waste, animal waste, and sewage sludge digested slurry were performed at different pyrolysis temperatures (300–1000 °C) under an inert atmosphere (Ma et al. 2018; Inyang et al. 2012; Yao et al. 2011, 2015, 2017a, b). Another study by Inyang et al. (2010) reported the comparative analysis of biochar produced from sugarcane bagasse and AD bagasse. These studies imply that the BET surface area of biochar produced from digested biomass was slightly higher than that produced from biomass pyrolysis. It facilitates the efficient utilization of modified biochar as a low-cost adsorbent for soil amendment, water holding capacity, and soil quality improvement that leads to sequestration of atmospheric carbon. It was concluded that the organic functional groups present in biochar and AD biochar were mainly hydroxyl, alkene, and aromatic groups. The major difference in organic groups was evident only as the presence of carbonyl groups in AD biochar (Inyang et al. 2010). Based on the physicochemical characterization, it is evident that AD biochar has higher adsorption and ion-exchange capacity relative to undigested biomass residues. Scanning electron microscopy (SEM) of AD biochar indicated the presence of several prismatic, hexagonal crystalline structures, and the pore diameter was found similar to the wood-based activated carbon (Ma et al. 2018; Gundogdu et al. 2013; Inyang et al. 2012). Studies indicate the significant effect of different AD biomass on the physicochemical properties of AD biochar. The AD biochar also possesses good heavy metal adsorption capacity from aqueous solutions. The results concluded that the animal waste AD biochar acquire a stronger affinity to Pb2+ (99%), Cu2+ (98%), and weaker affinity to Cd2+ (57%), Ni2+ (26%) as compared to sugar beet residue AD biochar (Inyang et al. 2012). Batch experiments of soil remediation indicated that the pH and coexisting anions in initial solution can significantly affect the phosphate adsorption capacity of AD biochar (Yao et al. 2011). The comparative analysis of biochar, anaerobically digested biochar (DBC), and commercially activated carbon (AC) asserted that DBC is the most efficient lead adsorbent in aqueous solutions. The lead adsorption capacity of DBC (653.9 mmol/kg) was twice of AC (395.3 mmol/kg) and several times greater than that of BC (31.3 mmol/kg). Despite lower surface area of DBC, the lead adsorption capacity of DBC was observed higher than AC and BC. This phenomenon suggests the involvement of other mechanisms along with surface adsorption. Post-adsorption analysis using X-ray diffraction (XRD) and SEM identified lead minerals on the DBC surface as cerrussite—[PbCO3] and hydrocerrussite—[Pb3(CO3)2(OH)2]. These mineral crystals were not observed on the BC or AC after Pb adsorption. It concluded that the lead adsorption capacity of DBC also depends partly on the precipitation mechanism. The precipitation of cerrussite and hydrocerrussite on DBC surface is due to the presence of specific organic functional groups (O=C=O) and high pH. Another study by Yao et al. (2011) proposed the predominance of adsorption over precipitation mechanism during the phosphate removal from aqueous solutions. High metal removal efficiency of biochar made from digested biomass suggests that it could be considered an efficient method of “biological activation” to produce biochar-based adsorbents.
3 Effect of Biological Modification
3.1 On Microbial Properties
Biochar application in soil induces the stabilization of organic matter and the exchange of electrons between microbial cells and organic matter (Fang et al. 2014). It can significantly affect the enzyme activities and community structure of microbes. These parameters can be examined using quantitative real-time polymerase chain reaction (q-PCR), ergosterol extraction, next-generation sequencing, phospholipid fatty acid quantitation (PLFA), gradient gel electrophoresis (DGGE), and fluorescence in situ hybridization (FISH) (Chen et al. 2013; Hale et al. 2014; Mackie et al. 2015; Rousk et al. 2009). Actinobacteria, Acidobacteria, Verrucomicrobia, and Gemmatimonadetes were observed to adopt high-throughput sequencing techniques in biochar-treated soils (Mackie et al. 2015; Nielsen et al. 2014). The different theories for the effect of biochar on microbial activity are explained by several researches. The first concept is that the high specific surface area with well-developed pore structure avails vacant space for microorganisms (Quilliam et al. 2013). Another research by Joseph et al. (2013) stated that the microorganisms extract the essential nutrients for their development from biochar. The biochar enhances the properties of substrate (such as pH, moisture, and aeration conditions) to alter its habitation (Quilliam et al. 2013). Another theory identified that biochar minimizes the toxicity to microorganisms by adsorbing the soil pollutants (Stefaniuk and Oleszczuk 2016).
3.2 On Biochar Properties
Biochar consists of various essential nutrients such as sodium, potassium, nitrogen, magnesium, and phosphorus for the enrichment of soil nutrients (Chathurika et al. 2016). With enriched soil nutrients, the rhizobacterial population increases which further leads to higher enzyme availability in soil. The enzyme adsorption depends on surface functional groups of biochar. The force (other than Coloumb force) between neutral protein molecules and polar disaccharides is linked to the neutral region of biochar surface. It leads to the biological activation of biochar through enzymes (Lammirato et al. 2011). Also, the biochar surface contains a significant amount of microalgae variants (Klebsormidium flaccidum and filamentous Cyanobacteria) that increase the activation process. Some extracellular enzymes (oxidoreductase enzyme) bind covalently with biochar surface and this biologically modified biochar can be efficiently implemented for heavy metal adsorption (Naghdi et al. 2018). The microbial activation is limited to bench-scale studies and biological activation of biochar through earthworms is been considered as a cost-effective method.
4 Mechanisms Involved in Biological Modification of Biochar
4.1 Biological Modification Through Intestinal Enzymes of Earthworms
The posterior part of earthworms has several enzyme-secreting glands and discharged digestive enzymes break the fed organic matter (Kaushik and Garg 2004). The earthworms can consume a diverse variety of substrates that can be divided into three classes (anecic, epigeic, and endogeic) according to their feeding habits (Domínguez and Edwards 1997; Huang et al. 2014). Epigeic earthworms (such as Eisenia fetida, Eudrilus eugeniae, and Perionyx excavatus) are most efficient in the degradation of complex organic substances and are recommended for biological modification of biochar (Khatua et al. 2018; Karmegam et al. 2021; Ananthavalli et al. 2019). Figure 2 depicts the process of biological modification of biochar by earthworms. The biological modification takes place in two stages. In the first stage (active stage), earthworms grind the consumed material in gut section, the gut-secreted enzymes crack the complex substances, and in the second stage (maturation stage), the earthworm releases biologically modified biochar with humus like substance (Gomez-Brandon et al. 2011; Gomez-Brandon and Domínguez 2014; Lores et al. 2006). During the active stage, the consumed organic material gets ground in gizzard, gut epithelium releases multiple enzymes, and induces biochemical reactions (Nozaki et al. 2009) for different enzymes, microbes, beneficial nutrients, and biologically modified biochar (Balachandar et al. 2021; Domínguez et al. 2019). Therefore, the efficiency of vermicomposting increases by mixing organic waste with biochar (El-Naggar et al. 2019; Malinska et al. 2017). Along with the biological modification of biochar, the process also alters microbial properties by improving moisture availability, aeration level, toxicity adsorption, nutrient establishment, and pH neutralization (Ge et al. 2019; Zhu et al. 2017a, b; Quilliam et al. 2013; Ennis et al. 2012; Jeffery et al. 2011). The gut enzymes have various biomolecules which can be used as catalyst and affect soil pollutants (Burns et al. 2013; Gianfreda et al. 2016). The implementation of biochar increases the stability of enzymes and the biochar particles bind with extracellular enzymes due to highly affinitive surface functional groups. The ionic interactions and van der Waals forces are major contributors to binding (De Oliveira et al. 2000). It can be concluded from the above-mentioned studies that the co-implementation of biochar and earthworms is a feasible method for remediation of metal contamination. Various environmental researchers stated that the soil invertebrates (such as earthworms) efficiently produce biologically activated biochar with the use of gut enzymes. The experimental study of Zhu et al. (2017a, b) on cow manure-based vermi-modified biochar evidenced effective adsorption of Pb2+ and Cd2+ ions.
4.2 Biological Modification by Pyrolysis of Anaerobically Digested (AD) Biomass
The anaerobically digested waste can be efficiently converted to biochar by drying and pyrolysis. The studies concluded that the wood biomass components hemicellulose, cellulose, and lignin decompose at temperature ranges 200–325 °C, 240–375 °C, and 280–500 °C, respectively (Prins et al. 2006; Downie et al. 2009; Wani et al. 2021). The mechanism of biological modification by pyrolysis of AD biomass includes the degradation of different components (hemicellulose, cellulose, and lignin) present in the feedstock. The characteristics of resultant biologically modified biochar depend greatly on pyrolysis temperature, heating rate, heating time, raw material characteristics, inert gas flow rate, etc.
5 Applications of Biologically Modified Biochar
Biochar-based nanocomposites have been extensively utilized in heavy metal adsorption (for example, As(III), As(V), Pb(II), Cr (VI), Cd(II), Cu(II), and Hg(II)) from wastewater. The adsorption capacity of various inorganic contaminants vary with different nanomaterials, contaminant concentration, and biochar substrate (Li et al. 2016; Wang et al. 2017; Yao et al. 2013a, b; Zhang and Gao 2013; Zhang et al. 2013). Biologically modified biochar is generally used in advanced water remediation for biodegradation and adsorption of organic, inorganic, and biological contaminants which cannot be separated in primary and secondary water remediation (Çeçen and Aktas 2011) (Table 1).
With increased population and food demand, the use of chemicals in agricultural sector has been significantly increased in past decades. Therefore, there is a need to develop a safe and efficient soil remediation technique for contaminated soil. It is been evident from several studies that biochar with high specific surface area, oxygen containing surface functional groups, cation exchange capacity can be efficient inactivating, stabilizing, and adsorbing agent for even highly heavy metals concentrated soils (Beesley et al. 2011; Park et al. 2011; Uchimiya et al. 2010a, b, 2011a). The heavy metal stabilization capacity is higher for alkaline soil pH and higher intraparticle diffusion (Rees et al. 2014). The pristine biochar constitutes good adsorption capacity for heavy metals which significantly increases upon biochar modification. There are some lab scale observations on heavy metal adsorption using modified biochar but large-scale experimentation is still rare. Traditional technologies (precipitation, ion exchange, packed-bed filtration, electro-coagulation, membrane filtration) for heavy metal removal from wastewater were found to be effective in reducing pollutant concentrations. Though, these technologies involve high-cost and disposal problems. The bio-adsorbents are suggested as low cost alternative for wastewater treatment (Demirbas 2008; Sud et al. 2008).
Heavy metal contamination in soil has been a serious environmental problem in recent time (Alloway 2013). Biochar is generally an alkaline substance which can increase soil pH and promote stabilization of heavy metal contamination. Biologically treated biochar have significantly higher specific surface area and microbial biofilm which provide high pollutant adsorption capacity (Ahmad et al. 2014). Apart from adsorption, application of biologically treated biochar is also beneficial for agricultural soil due to increased microbial growth, bio-adsorption, and degradation of heavy metal and organic pollutants. The biologically modified biochar helps in retaining the soil nutrients (Yao et al. 2011).
6 Advantages and Limitations of Biological Modification of Biochar
The biologically modified biochar is not only a potential substitute for activated carbon in environmental remediation but also avails an additional advantage of sustainable carbon sink (Yoder et al. 2011; Laer et al. 2015). The contaminant removal efficiency of activated carbon, biochar and biologically modified biochar differ significantly due to dependence on adsorption capacity, bio-adsorption capacity, specific surface area, and pore size distribution. These parameters assert the suitability of biologically modified biochar due to high bio-adsorption, biodegradation, and adsorption along with positive environmental impact. The biological treatment of biochar is cost-effective and eco-friendly process as compared to physical and chemical activation. The other activation methods require high initial investment and produce secondary pollutants (emissions, chemical wastes, etc.) during activation (Sanchez-Hernandez et al. 2019). Also, there is no need for biochar regeneration after phosphate removal from soil because the phosphate-laden biochar consists of valuable nutrients and can be utilized as a slow-release fertilizer and for carbon sequestration (Yao et al. 2011). Thus, the implementation of biologically modified biochar eliminates the drudgery and cost associated with regeneration process.
7 Conclusions
This chapter summaries the feasibility, efficiency, and cost-effectiveness of biologically modified biochar. Raw materials and production process significantly alter the physicochemical and functional properties of biochar. Biologically modified biochar facilitates the effective biodegradation and biosorption through various complex mechanisms. The safe, cost-effective production process of biological modification avails agricultural, environmental, and economic sustainability. The co-implementation of earthworms and biochar is a feasible method for microbial growth, biochar modification, and soil nutrient enrichment. The biological modification of biochar through extracellular enzymes paved the path for efficient environmental remediation. There is a need for detailed study using statistical tools and mathematical modeling to accurately correlate the control parameters and properties of biologically modified biochar.
References
Ahmad M, Rajapaksha A.U, Lim J.E, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Alloway BJ (2013) Heavy metals in soils, heavy metals in soils. https://doi.org/10.1007/978-94-007-4470-7_10
Ameloot N, Graber ER, Verheijen FGA, De Neve S (2013) Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci 64:379–390. https://doi.org/10.1111/ejss.12064
Ananthavalli R, Ramadas V, John Paul J.A, Karunai Selvi B, Karmegam N, (2019) Seaweeds as bioresources for vermicompost production using the earthworm, Perionyx excavatus (Perrier). Bioresour Technol 275:394–401. https://doi.org/10.1016/j.biortech.2018.12.091
Balachandar R, Baskaran L, Yuvaraj A, Thangaraj R, Subbaiya R, Ravindran B, Chang SW, Karmegam N (2020) Enriched pressmud vermicompost production with green manure plants using Eudrilus eugeniae. Bioresour Technol 299:122578. https://doi.org/10.1016/j.biortech.2019.122578
Balachandar R, Biruntha M, Yuvaraj A, Thangaraj R, Subbaiya R, Govarthanan M, Kumar P, Karmegam N (2021) Earthworm intervened nutrient recovery and greener production of vermicompost from Ipomoea staphylina e an invasive weed with emerging environmental challenges. Chemosphere 263:128080
Baskaran S, Palanichamy S, Arunachalam S, Balasubramanian MP (1986) Changes of acid and alkaline phosphatases during the development of the tropical earthworm Pontoscolex corethrurus. Natl Sci Acad Lett 9:159–162
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environmental pollution 1; 159(12):3269–3282
Bouabidi ZB, El-Naas MH, Zhang Z (2019) Immobilization of microbial cells for the biotreatment of wastewater: a review. Environ Chem Lett 17:241–257
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234. https://doi.org/10.1016/j.soilbio.2012.11.009
Çeçen F, Aktas Ö (2011) Activated carbon for water and wastewater treatment: integration of adsorption and biological treatment. John Wiley & Sons
Chathurika JAS, Kumaragamage D, Zvomuya F, Akinremi OO, Flaten DN, Indraratne SP, Dandeniya WS (2016) Woodchip biochar with or without synthetic fertilizers affects soil properties and available phosphorus in two alkaline, chernozemic soils. Can J Soil Sci 96:472–484. https://doi.org/10.1139/cjss-2015-0094
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44. https://doi.org/10.1016/j.apsoil.2013.05.003
Chen S, Rotaru A, Shrestha PM, Malvankar NS, Liu F, Fan W, Nevin KP, Lovley DR (2014) Promoting interspecies electron transfer with biochar. Sci Rep 4:5019
Dalahmeh S, Ahrens L, Gros M, Wiberg K, Pell M (2018) Potential of biochar filters for onsite sewage treatment: adsorption and biological degradation of pharmaceuticals in laboratory filters with active, inactive and no biofilm. Sci Total Environ 612:192–201
De Oliveira PC, Alves GM, De Castro HF (2000) Immobilisation studies and catalytic properties of microbial lipase onto styrene-divinylbenzene copolymer. Biochem Eng J 5:63–71. https://doi.org/10.1016/S1369-703X(99)00061-3
Dehhaghi M, Kazemi Shariat Panahi H, Guillemin GJ (2019a) Microorganisms, tryptophan metabolism, and kynurenine pathway: a complex interconnected loop influencing human health status. Int J Tryptophan Res 12:1178646919852996
Dehhaghi M, Tabatabaei M, Aghbashlo M, Kazemi Shariat Panahi H, Nizami A-S (2019b) A state-of-the-art review on the application of nanomaterials for enhancing biogas production. J Environ Manage 251:109597. https://doi.org/10.1016/j.jenvman.2019.109597
Dehhaghi M, Mohammadipanah F, Guillemin GJ (2018) Myxobacterial natural products: an under-valued source of products for drug discovery for neurological disorders. Neurotoxicology 1(66):195–203
Dehhaghi M, Panahi HK, Jouzani GS, Nallusamy S, Gupta VK, Aghbashlo M, Tabatabaei M (2020) Anaerobic Rumen fungi for biofuel production. In: Fungi in fuel biotechnology, pp. 149–175. Springer, Cham
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157(2–3):220–229
Domene X (2016) A critical analysis of meso- and macrofauna effects following biochar supplementation. In: Biochar application: essential soil microbial ecology. Elsevier Inc., pp. 268–292. https://doi.org/10.1016/B978-0-12-803433-0.00011-4
Domínguez J, Aira M, Kolbe AR, Gomez-Brandon M, Perez-Losada M (2019) Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-46018-w
Domínguez J, Edwards CA (1997) Effects of stocking rate and moisture content on the growth and maturation of Eisenia andrei (Oligochaeta) in pig manure. Soil Biol Biochem 29:743–746. https://doi.org/10.1016/S0038-0717(96)00276-3
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar, biochar for environmental management—science and technology. In: Lehmann J, Joseph S (eds) London and Washington DC, Earthscan, pp 13–29
El-Naggar A, Lee SS, Rinklebe J, Farooq M, Song H, Sarmah AK, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS (2019) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337:536–554. https://doi.org/10.1016/j.geoderma.2018.09.034
Ennis CJ, Evans AG, Islam M, Ralebitso-Senior TK, Senior E (2012) Biochar: carbon sequestration, land remediation, and impacts on soil microbiology. Crit Rev Environ Sci Technol 42:2311–2364. https://doi.org/10.1080/10643389.2011.574115
Fang G, Gao J, Liu C, Dionysiou DD, Wang Y, Zhou D (2014) Key role of persistent free radicals in hydrogen peroxide activation by biochar: implications to organic contaminant degradation. Environ Sci Technol 48:1902–1910
Frankel ML, Bhuiyan TI, Veksha A, Demeter MA, Layzell DB, Helleur RJ, Hill JM, Turner RJ (2016) Removal and biodegradation of naphthenic acids by biochar and attached environmental biofilms in the presence of co-contaminating metals. Biores Technol 1(216):352–361
Ge X, Cao Y, Zhou B, Wang X, Yang Z, Li MH (2019) Biochar addition increases subsurface soil microbial biomass but has limited effects on soil CO2 emissions in subtropical moso bamboo plantations. Appl Soil Ecol 142:155–165
Gianfreda L, Rao MA, Scelza R, de la Luz Mora M (2016) Role of enzymes in environment cleanup/remediation. In: Dhillon G, Kaur S (eds) Agro-industrial wastes as feedstock for enzyme production: apply and exploit the emerging and valuable use options of waste biomass. Academic Press, London, UK, pp 133–155
Gomez-Brandon M, Domínguez J (2014) Recycling of solid organic wastes through vermicomposting: microbial community changes throughout the process and use of vermicompost as a soil amendment. Crit Rev Environ Sci Technol 44:1289–1312. https://doi.org/10.1080/10643389.2013.763588
Gomez-Brandon M, Domínguez J, Lores M, G omez-Brandon M, Perez-Díaz D, Domínguez J (2011) The microbiology of vermicomposting. In: Edwards CA, Arancon NQ, Sherman R (eds) Vermiculture technology: earthworms, organic wastes, and environmental management. CRC Press, Taylor & Francis Group, LLC, Boca Raton, Fl, USA, pp 53–66. https://doi.org/10.1080/10643389.2013.763588
Gundogdu A, Duran C, Senturk HB, Sovlak M (2013) Physicochemical characteristics of a novel ctivated carbon production from tea industry waste. J Anal Appl Pyr 104:249–259
Hale L, Luth M, Kenney R, Crowley D (2014) Evaluation of pinewood biochar as a carrier of bacterial strain Enterobacter cloacae UW5 for soil inoculation. Appl Soil Ecol 84:192–199. https://doi.org/10.1016/j.apsoil.2014.08.001
Hamedi J, Dehhaghi M, Mohammdipanah F (2015) Isolation of extremely heavy metal resistant strains of rare actinomycetes from high metal content soils in Iran. Int J Environ Res 1:9(2)
Hong SW, Kim IS, Lee JS, Chung KS (2011) Culture-based and denaturing gradient gel electrophoresis analysis of the bacterial community structure from the intestinal tracts of earthworms (Eisenia fetida). J Microbiol Biotechnol 21:885–892
Huang K, Li F, Wei Y, Fu X, Chen X (2014) Effects of earthworms on physicochemical properties and microbial profiles during vermicomposting of fresh fruit and vegetable wastes. Bioresour Technol 170:45–52. https://doi.org/10.1016/j.biortech.2014.07.058
Inyang M, Gao B, Ding W, Pullammanappallil P, Zimmerman AR, Cao X (2011) Enhanced lead sorption by biochar derived from anaerobically digested sugarcane bagasse. Sep Sci Technol 46(12):1950–1956
Inyang M, Gao B, Pullammanappallil P, Ding W, Zimmerman AR (2010) Biochar from anaerobically digested sugarcane bagasse. Bioresource Technol 1;101(22):8868–8872
Inyang M, Gao B, Yao Y, Xue YW, Zimmerman AR, Pullammanappallil P, Cao XD (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour Technol 110:50–56
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187. https://doi.org/10.1016/j.agee.2011.08.015
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Joseph S, Graber ER, Chia C, Munroe P, Donne S, Thomas T, Nielsen S, Marjo C, Rutlidge H, Pan GX, Li L, Taylor P, Rawal A, Hook J (2013) Shifting paradigms: development of high-efficiency biochar fertilizers based on nanostructures and soluble components. Carbon Manag 4:323–343. https://doi.org/10.4155/cmt.13.23
Karmegam N, Vijayan P, Prakash M, John Paul JA (2019) Vermicomposting of paper industry sludge with cowdung and green manure plants using Eisenia fetida: a viable option for cleaner and enriched vermicompost production. J Clean Prod 228:718–728. https://doi.org/10.1016/j.jclepro.2019.04.313
Karmegam N, Jayakumar M, Govarthanan M, Kumar P, Ravindran B, Biruntha M (2021) Precomposting and green manure amendment for effective vermitransformation of hazardous coir industrial waste into enriched vermicompost. Bioresour Technol 319:124136. https://doi.org/10.1016/j.biortech.2020.124136
Kaushik P, Garg VKK (2004) Dynamics of biological and chemical parameters during vermicomposting of solid textile mill sludge mixed with cow dung and agricultural residues. Bioresour Technol 94:203–209
Khatua C, Sengupta S, Krishna Balla V, Kundu B, Chakraborti A, Tripathi S (2018) Dynamics of organic matter decomposition during vermicomposting of banana stem waste using Eisenia fetida. Waste Manag 79:287–295. https://doi.org/10.1016/j.wasman.2018.07.043
Laer VT, Smedt PD, Ronsse F, Ruysschaert G, Boeckx P, Verstraete W, Buysse J (2015) Legal constraints and opportunities for biochar: a case analysis of EU law. GCB Bioenergy 7(1):14–24
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by b-glucosidase from Aspergillus niger. Soil Biol Biochem 43:1936–1942. https://doi.org/10.1016/j.soilbio.2011.05.021
Li J, Zhao P, Li Y, Tian Y, Wang Y (2016) Systems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci Rep 15;5(1):1–5
Lores M, Gomez-Brandon M, Perez-Díaz D, Domínguez J (2006) Using FAME profiles for the characterization of animal wastes and vermicomposts. Soil Biol Biochem 38:2993–2996. https://doi.org/10.1016/j.soilbio.2006.05.001
Ma Q, Song W, Wang R, Zou J, Yang R, Zhang S (2018) Physicochemical properties of biochar derived from anaerobically digested dairy manure. Waste Manage 79(2018):729–734
Mackie KA, Marhan S, Ditterich F, Schmidt HP, Kandeler E (2015) The effects of biochar and compost amendments on copper immobilization and soil microorganisms in a temperate vineyard. Agric Ecosyst Environ 201:58–69. https://doi.org/10.1016/j.agee.2014.12.001
Malinska K, Golanska M, Caceres R, Rorat A, Weisser P, Slezak E (2017) Biochar amendment for integrated composting and vermicomposting of sewage sludge e the effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour Technol 225:206–214. https://doi.org/10.1016/j.biortech.2016.11.049
Mishra PC, Dash MC (1980) Digestive enzymes of some earthworms. Experientia 36:1156–1157. https://doi.org/10.1007/BF01976096
Mishra SL (1993) Digestive enzymes of the tropical earthworm Perionyx millardi. J Ecobiol 5:77–79
Mohammadipanah F, Dehhaghi M (2017) Classification and taxonomy of actinobacteria. In: Biology and biotechnology of actinobacteria. Springer, Cham, pp 51–77
Mohammadipanah F, Hamedi J, Schumann P, Spröer C, del Carmen Montero-Calasanz M, Klenk HP,(2015) Saccharothrix ecbatanensis sp. nov., an actinobacterium isolated from soil. Int J Syst Evol Microbiol 65(Pt_12):4544–4549
Naghdi M, Taheran M, Brar SK, Kermanshahi-pour A, Verma M, Surampalli RY (2018) Pinewood nanobiochar: a unique carrier for the immobilization of crude laccase by covalent bonding. Int J Biol Macromol 115:563571. https://doi.org/10.1016/j.ijbiomac.2018.04.105
Nakajima N, Sugimoto M, Ishihara K (2003) Earthworm-serine protease: characterization, molecular cloning, and application of the catalytic functions. J Mol Catal B Enzym 23:191–212. https://doi.org/10.1016/S1381-1177(03)00082-1
Nielsen S, Minchin T, Kimber S, van Zwieten L, Gilbert J, Munroe P, Joseph S, Thomas T (2014) Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agric Ecosyst Environ 191:73–82. https://doi.org/10.1016/j.agee.2014.04.006
Nozaki M, Miura C, Tozawa Y, Miura T (2009) The contribution of endogenous cellulase to the cellulose digestion in the gut of earthworm (Pheretima hilgendorfi: Megascolecidae). Soil Biol Biochem 41:762–769. https://doi.org/10.1016/j.soilbio.2009.01.016
Ok YS, Uchimiya SM, Chang SX, Bolan N (2015) Biochar: Production, characterization, and applications. CRC Press
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451. https://doi.org/10.1007/s11104-011-0948-y
Parle JN (1963) Micro-organisms in the intestines of earthworms. J Gen Microbiol 31:1–11
Perez-Mercado LF, Lalander C, Berger C, Dalahmeh SS (2018) Potential of biochar filters for onsite wastewater treatment: effects of biochar type, physical properties and operating conditions. Water 10:1835–1843
Prado A, Berenguer R, Esteve-Núñez A (2019) Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer. Carbon N. Y. 146:597–609
Prins MJ, Ptasinski KJ, Janssen FJJG (2006) Torrefaction of wood: Part 1. weight loss kinetics. J Anal Appl Pyrol 77(1):28–34
Puga AP, Abreu CA, Melo LCA, Beesley L (2015) Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J Environ Manag 159:86–93. https://doi.org/10.1016/j.jenvman.2015.05.036
Quilliam RS, Glanville HC, Wade SC, Jones DL (2013) Life in the “charosphere”—does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol Biochem 65:287–293. https://doi.org/10.1016/j.soilbio.2013.06.004
Rajapaksha AU, Chen SS, Tsang DCW, Zhang M, Vithanage M, Mandal S, Gao B, Bolan NS, Ok YS (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291. https://doi.org/10.1016/j.chemosphere.2016.01.043
Rees F, Simonnot MO, Morel JL (2014) Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur J Soil Sci 65(1):149–161
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596. https://doi.org/10.1128/AEM.02775-08
Sajedi H, Mohammadipanah F, Shariat PH, K, (2018) An image analysis-aided method for redundancy reduction in differentiation of identical actinobacterial strains. Future Microbiol 13:313–329. https://doi.org/10.2217/fmb-2016-0096
Sanchez-Hernandez JC (2018) Biochar activation with exoenzymes induced by earthworms: a novel functional strategy for soil quality promotion. J Hazard Mater 350:136–143. https://doi.org/10.1016/j.jhazmat.2018.02.019
Sanchez-Hernandez JC, Cares XA, Perez MA, del Pino JN (2019) Biochar increases pesticide-detoxifying carboxylesterases along earthworm burrows. Sci Total Environ 667:761–768. https://doi.org/10.1016/j.scitotenv.2019.02.402
Shaaban M, Van Zwieten L, Bashir S, Younas A, Núnez-Delgado A, Chhajro MA, Kubar KA, Ali U, Rana MS, Mehmood MA, Hu R (2018) A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J Environ Manag 228, 429–440. https://doi.org/10.1016/j.jenvman.2018.09.006
Sharma A, Jamali H, Vaishnav A, Giri BS, Srivastava AK (2020) Chapter 15—microbial biofilm: an advanced eco-friendly approach for bioremediation. In: Yadav MK, Singh BP (eds) New and future developments in microbial biotechnology and bioengineering: microbial biofilms. Elsevier, pp 205–219
Simpson DR (2008) Biofilm processes in biologically active carbon water purification. Water Res 42:2839–2848
Stefaniuk M, Oleszczuk P (2016) Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge-amended soil. Environ Pollut 218:242–251. https://doi.org/10.1016/j.envpol.2016.06.063
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—a review. Bioresour Technol 99(14):6017–6027
Sun K, Kang M, Ro K.S, Libra J.A, Zhao Y, Xing B, (2016) Variation in sorption of propiconazole with biochars: the effect of temperature, mineral, molecular structure, and nano-porosity. Chemosphere 142:56–63. https://doi.org/10.1016/j.chemosphere.2015.07.018
Tabatabaei M, Aghbashlo M, Dehhaghi M, Panahi HK, Mollahosseini A, Hosseini M, Soufiyan MM (2019) Reactor technologies for biodiesel production and processing: a review. Prog Energy Combust Sci 74:239–303
Tan Z, Lin CS, Ji X, Rainey TJ (2017a) Returning biochar to fields: a review. Appl Soil Ecol 116:1–1
Tan Z, Wang Y, Zhang L, Huang Q (2017b) Study of the mechanism of remediation of Cd-contaminated soil by novel biochars. Environ Sci Pollut Res 24:24844–24855. https://doi.org/10.1007/s11356-017-0109-9
Tillinghast EK, MacDonnell PC (1973) The distribution of ammonia-generating enzymes along the intestine of the earthworm, Lumbricus terrestris L. J Exp Zool 185:153–158. https://doi.org/10.1002/jez.1401850203
Uchimiya M, Chang S, Klasson KT (2011a) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190(1–3):432–441
Uchimiya M, Lima IM, Klasson KT, Wartelle LH (2010a) Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere 80(8):935–940. https://doi.org/10.1016/j.chemosphere.2010.05.020 Epub 2010 Jun 12 PMID: 20542314
Uchimiya M, Lima IM, Thomas Klasson K, Chang S, Wartelle LH, Rodgers JE (2010b) Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J Agric Food Chem 58(9):5538–5544. https://doi.org/10.1021/jf9044217 PMID: 20402510
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011b) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59(6):2501–2510
Urbasek F, Pizl V (1991) Activity of digestive enzymes in the gut of five earthworm species (Oligochaeta Lumbricidae). Rev Ecol Biol Sol 28:461–468
Wang G, Wang S, Sun W, Sun Z, Zheng S (2017) Synthesis of a novel illite@ carbon nanocomposite adsorbent for removal of Cr(VI) from wastewater. J Environ Sci (china) 57:62–71. https://doi.org/10.1016/j.jes.2016.10.017
Wani I, Ramola S, Garg A, Kushvaha V (2021) Critical review of biochar applications in geo-engineering infrastructure: moving beyond agricultural and environmental perspectives. Biomass Convers. Biorefin. https://doi.org/10.1007/s13399-021-01346-8
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178
Wink J, Mohammadipanah F, Panahi HK, (2017) Practical aspects of working with actinobacteria. In: Biology and biotechnology of actinobacteria. Springer, Cham, pp 329–376.
Wurzer C, Masek O, Sohi S (2019) Synergies in sequential biochar systems. In: Advanced carbon materials from biomass: an overview. Green carbon ENT book, pp 147–159
Yao Y, Gao B, Chen J, Yang L (2013a) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47(15):8700–8708. https://doi.org/10.1021/es4012977 Epub 2013 Jul 25 PMID: 23848524
Yao Y, Gao B, Chen J, Zhang M, Inyang M, Li Y, Alva A, Yang L (2013b) Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: characterization and phosphate removal potential. Bioresour Technol 138:8–13. https://doi.org/10.1016/j.biortech.2013.03.057 Epub 2013 Mar 19 PMID: 23612156
Yao H, Lu J, Wu J, Lu Z, Wilson PC, Shen Y (2013c) Adsorption of Fluoroquinolone Antibiotics by Wastewater Sludge Biochar: Role of the Sludge Source. Water Air Soil Pollut 224:1370
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao X, Pullammanappallil P, Yang L (2011a) Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresour Technol 102:6273–6278
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao X, Pullammanappallil P, Yang L (2011b) Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J Hazard Mater 190(2011):501–507
Yao Y, Gao B, Wu F, Zhang C, Yang L (2015) Engineered biochar from biofuel residue: characterization and its silver removal potential. ACS Appl Mater Interfaces 7(19):10634–10640. https://doi.org/10.1021/acsami.5b03131 Epub 2015 May 8 PMID: 25923987
Yao Y, Zhang Y, Gao B, Chen RJ, Wu F (2017a) Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse. Environ Sci Pollut Res 25(26):25659–32566
Yao Y, Zhang Y, Gao B, Chen R, Wu F (2017b) Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-8849-0
Yao Y, Zhang Y, Gao B, Chen RJ, Wu F (2018) Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse. Environ Sci Pollut Control Ser 25:25659–25667
Yoder J, Galinato S, Granatstein D, Garcia-Pérez M (2011) Economic tradeoff between biochar and bio-oil production via pyrolysis. Biomass Bioenergy 35:1851–1862
Yuvaraj A, Karmegam N, Tripathi S, Kannan S, Thangaraj R (2020) Environmentfriendly management of textile mill wastewater sludge using epigeic earthworms: bioaccumulation of heavy metals and metallothionein production. J Environ Manag 254:109813. https://doi.org/10.1016/j.jenvman.2019.109813
Yuvaraj A, Thangaraj R, Maheswaran R (2019) Decomposition of poultry litter through vermicomposting using earthworm Drawida sulcata and its effect on plant growth. Int J Environ Sci Technol 16:7241–7254. https://doi.org/10.1007/s13762-018-2083-2
Zhang D, Chen Y, Ma Y, Guo L, Sun J, Tong J (2016a) Earthworm epidermal mucus: rheological behavior reveals drag-reducing characteristics in soil. Soil Tillage Res. 158:57–66. https://doi.org/10.1016/j.still.2015.12.001
Zhang F, Wang X, Xionghui J, Ma L (2016b) Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ Pollut 216:575–583. https://doi.org/10.1016/j.envpol.2016.06.013
Zhang M, Gao B (2013) Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem Eng J 226:286–292
Zhang P, Sun H, Yu L, Sun T (2013a) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J Hazard Mater 244:217–224
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013b) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20(12):8472–8483
Zhu N, Zhang J, Tang J, Zhu Y, Wu Y (2018) Arsenic removal by periphytic biofilm and its application combined with biochar. Bioresour Technol 248:49–55
Zhu W, Du W, Shen X, Zhang H, Ding Y (2017a) Comparative adsorption of Pb2þ and Cd2þ by cow manure and its vermicompost. Environ Pollut 227:89–97
Zhu X, Chen B, Zhu L, Xing B (2017b) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115. https://doi.org/10.1016/j.envpol.2017.04.032
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Azad, D., Pateriya, R.N., Arya, R., Sharma, R.K. (2022). Biological Treatment for Biochar Modification: Opportunities, Limitations, and Advantages. In: Ramola, S., Mohan, D., Masek, O., Méndez, A., Tsubota, T. (eds) Engineered Biochar. Springer, Singapore. https://doi.org/10.1007/978-981-19-2488-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-2488-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2487-3
Online ISBN: 978-981-19-2488-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)