Abstract
Hydrogen fuel is an interesting fossil fuel substitute due to its rich energy content, sustainable nature, and fuel efficiency. However, it is difficult to obtain. As a result, the demand for hydrogen has gone up significantly. Water electrolysis, hydrocarbon steam reforming, coal gasification, and partial oxidation techniques are all familiar approaches for producing hydrogen; however, they are not profitable because of high energy necessities. Compared to chemical approaches, biohydrogen gas processing has various advantages. Biological photolysis of water by algae and photo- and dark fermentation of organic resources, normally starch and sugars, by microbes, are the main biological processes used to produce hydrogen gas. The consecutive photo- and dark fermentation procedures are a relatively novel method to produce biohydrogen. In the manufacture of photo- and dark fermentative hydrogen, the prices of raw materials are a crucial concern. Carbohydrate-enriched substances like starch and cellulose-containing food and agricultural industry wastes, effluents from the olive mill, cheese whey, and baker’s yeast industry can be used for hydrogen making use of appropriate bioprocess techniques. Biological decomposable substances for hydrogen generation, as previously stated, provide low-cost energy generation including wastewater treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hydrogen, the lightest gas, is viewed as a potentially significant energy source since it is renewable, does not emit the “greenhouse gas” CO2 during ignition, liberates significant amounts of energy per gram weight, and is effectively transformed to electric power using fuel cells. The expanding energy demand and required energy supply as a result of the growing human population have inspired a lot of interest in new biofuel research and production in recent years (Ueno et al. 1995). Hydrogen fuel has a power density of 122 kJ/g. It is approximately 2.75 times that of conventional hydrocarbon fuels (Argun and Kargi 2010). Bio-H2 is H2 that is created from biological sources. As a result, it is regarded as one of the energy sources with the potential to partially replace conventional fossil-based fuels. Steam reforming, partial oxidation of oil, and coal gasification are all methods for producing hydrogen from fossil fuels and biomass. Nonbiological ways for producing hydrogen from H2O include thermal and thermochemical reactions, electrolysis, and photolysis. H2 is a gas that can be created physiologically. Many microbes create hydrogen as a result of reactions related to their energy metabolism. Dark fermentation (anaerobe microorganisms), photofermentation (with photoheterotrophic bacteria), fusion system of both, biological photolysis of water employing cyanobacteria and green algae, and water–gas shift reaction are all key biological hydrogen production mechanisms. Enzymes that produce hydrogen such as hydrogenase and nitrogenase regulate all processes (Azwar et al. 2014). Figure 1 depicts various materials and common hydrogen generation techniques used.
2 Industrial Waste Materials for Hydrogen Gas Synthesis
Coal, oil, natural gas, kerosene, propane, etc. are the sources of fossil fuels for hydrogen production. Steam reforming, partial oxidation, and coal gasification techniques are chemical methods used for hydrogen production (Higman and Tam 2014). All these chemical processes are described in Sect. 3.1. The obtainability, rate, carbohydrate content, and biodegradability of waste constituents utilized in biohydrogen generation are the most important factors to consider. Simple sugars like glucose, sucrose, and lactose are easily decomposable and are excellent hydrogen generation substrates. Clean carbohydrate sources, on the other hand, are costly raw materials for hydrogen synthesis. Agricultural and food processing trash comprising starch and cellulose, industrial wastewaters that are high in carbohydrates, and wastewater treatment plant sludge are the major raw materials utilized to produce hydrogen gas.
2.1 Agricultural and Food Industry Residues Comprising Starch and Cellulose
Agricultural and food leftovers are low-cost, carbohydrate-rich resources for the generation of biohydrogen. Biomass containing starch or cellulose can be subjected to enzymatic or acid hydrolysis to produce a highly concentrated sugar solution. It is then dark fermented by anaerobic acetogenic microbes for making H2, CO2, and volatile fatty acids. Volatile fatty acids formed by dark fermentation can be converted to H2 and CO2 by Rhodobacter sp. (photoheterotrophic bacteria). Starch and cellulose-containing substances are cheap raw materials found in agricultural wastes that seem to be the concentrated sources of carbohydrates (Yokoi et al. 2001; Liu and Shen 2004). For anaerobic fermentation of carbohydrates, pure cultures of bacteria such as Enterobacter sp., Clostridia sp., and a mixed culture of thermally treated anaerobic sludge can be adopted. Acid or enzymatic hydrolysis can convert starch to glucose and maltose, which can be transformed into hydrogen gas and organic acids (Han et al. 2016; Rangel et al. 2020).
Agricultural wastes containing cellulose require additional pretreatment. Before fermentation, cultivated wastes must be crushed and delignified using mechanical or chemical methods. The hemicellulose and cellulose components can be hydrolyzed into carbohydrates after they can subsequently be treated into hydrogen gas and organic acids.
2.2 Carbohydrate-Enriched Industrial Effluents
Dairy manufacturing, baker’s yeast, brewery, and olive mill effluents are examples of decomposable carbohydrate-comprising and nontoxic industrial wastes used for biohydrogen generation. Pretreatment of these sewer water may be necessary to eliminate unwanted constituents and restore nutritive balance. Using precise bio-processing methods, carbohydrate-enriched food sector sewages can be additionally treated to change the carbohydrate constituents to biological acids, and subsequently to hydrogen (Hao et al. 2015; Konstandopoulos et al. 2015).
2.3 Wastewater Treatment Plant Sewage Sludge
Large amounts of carbohydrates and proteins are present in wastewater treatment sludge. These wastes can then be used to yield methane or hydrogen gas. Additional wastes can be digested anaerobically in two phases. The primary phase (acidogenic phase) includes biological materials that will be transformed into organic acids, which can then be used to produce hydrogen gas in the next phase utilizing photoheterotrophic bacteria (Preethi et al. 2019).
3 Hydrogen Gas Production Techniques
Chemical and biological processes are used for hydrogen production. Heat and chemical reactions are used in thermochemical processes to release hydrogen from organic sources like fossil fuels and biomass, as well as from inorganic materials like water. Electrolysis or solar energy can also be used to divide water (H2O) into hydrogen (H2) and oxygen (O2). Biological activities can produce hydrogen in microorganisms like bacteria and algae.
3.1 Manufacture of Hydrogen Gas by Chemical Procedures
The majority of hydrogen (95%) is produced from fossil fuels through partial oxidation of methane, coal gasification, and natural gas steam reforming. Biomass gasification and water electrolysis are two other approaches to hydrogen making.
3.1.1 Natural Gas Reforming with Steam
Steam reforming is an ordinary gas-based hydrogen generation technique. This process is currently the most cost-effective way to obtain industrial hydrogen. The gas is heated to 700–1100 °C using nickel catalyst and steam. The heat-absorbing process breaks down methane gas into hydrogen (H2) and carbon monoxide (CO). The CO gas formed is then passed over iron oxide with steam to produce more H2 through a water–gas shift reaction. The disadvantage of this process is that it produces significant amounts of greenhouse gases like CO and CO2 in the atmosphere. Depending on the feedstock quality, 1 tonne of hydrogen creates CO2 of 9–12 tonnes (Collodi 2010).
The steam reforming plant is divided into four components, namely removal of sulfur and other impurities from the feedstock; it then transforms the feedstock components and vapor into syngas (mostly carbon monoxide and hydrogen) at elevated temperatures and reasonable pressures and performs syngas heat recovery using CO shift reactors to boost hydrogen generation and raw hydrogen purification using a pressure compression swing adsorption (PSA) unit for obtaining the desired quality.
The simplified chemical reactions are
In the adiabatic CO shift reactor vessel, the relatively exothermic water–gas shift reaction transforms carbon monoxide and steam to hydrogen and carbon dioxide:
3.1.2 Partial Oxidation Techniques
Partial oxidation is an exothermic reaction in which a small amount of oxygen is combined with natural gas or a heavy hydrocarbon fuel (heating oil). It is used to produce hydrogen from natural gas or other hydrocarbons. When a fuel-oxygen combination is partly ignited, a hydrogen-enriched syngas is formed. The water–gas shift process produces hydrogen and carbon monoxide (Mangold 2009):
3.1.3 Coal Gasification
The conversion of any carbonaceous fuel into a gaseous product with a desirable chemical heating value is known as gasification. The method of producing hydrogen from coal begins with partial oxidation, which involves adding some air to the coal, which produces carbon dioxide gas through typical burning. However, not enough heat is given to burn the coal—simply enough to heat the gasification reaction. Carbon dioxide can act as a gasifier. It becomes carbon monoxide when it reacts with the rest of the carbon in coal. The carbon monoxide in the gas stream now reacts with steam, resulting in hydrogen and carbon dioxide. Figure 2 displays the flowchart of the conversion of coal to hydrogen gas (Mangold 2009). Coal gasification can also be done on existing layers, with the coal being transformed to gaseous fuels without the need for intermediary mining activity. Underground gasification entails drilling boreholes that intersect the layers in at least two places and allowing gas to flow through the seam from one intersection to the next. A gasification agent, such as steam or compressed air, is introduced into the seam after the coal is fired at the bottom of one hole. Coal is converted to methane (synthetic natural gas) or other natural gases, and then gasified with carbon dioxide to generate liquid hydrocarbon fuels and carbon monoxide gas (Yu et al. 2015).
3.1.4 Electrolysis of Water
Electrolysis is a viable process for creating carbon-free fuel hydrogen using nuclear and renewable energy resources. It is a technique of dissociating water into oxygen and hydrogen using electricity. This reaction is carried out in electrolyzers. Electrolyzers have a cathode and an anode separated by an electrolytic solution, similar to fuel cells. Because of the various types of electrolyte materials and ionic species it conducts, different electrolyzers work in different ways:
Different electrolytic cells/electrolyzers used for hydrogen generation are membrane electrolyzers using polymer electrolytes, electrolyzers for the alkaline solution, and electrolyzers with solid oxide.
In the electrolyzer containing polymeric membrane, the electrolytic medium is a solid-specific soft substance. At the anodic compartment, water reacts to produce hydrogen ions (protons) and oxygen. Hydrogen ions selectively move through the electrolyzer to the cathode as electrons flow through an external connection. Hydrogen ions mix with electrons from the external circuit at the cathode to form hydrogen gas. Hydroxide ions (OH−) are transmitted from the cathode compartment to the anode compartment via the alkaline electrolyte, with hydrogen created at the cathode. The electrolyte used in electrolyzers is liquid alkaline sodium or potassium hydroxide solution. Solid oxide electrolyzers contain ceramic as the electrolyte produces hydrogen by transferring oxygen ions (O2−) at raised temperatures. Steam forms hydrogen gas and oxygen ions when it combines with electrons from the external circuit. Through the solid ceramic membrane, oxygen ions flow to the anode, where they react to form oxygen gas and produce electrons. The temperatures of solid oxide electrolyzers are high enough for solid oxide membranes to function properly (approximately 700–800 °C, related to 70–90 °C for PEM electrolyzers and less than 100 °C for commercial alkaline electrolyzers) (Sengodan et al. 2018).
However, these approaches are not sustainable because they rely on nonrenewable energy sources to manufacture hydrogen. As a result, it is critical to investigate hydrogen synthesis from renewable energy sources. Biological hydrogen generation processes are projected to consume a lesser amount of energy than thermochemical techniques of hydrogen manufacture since they work at ambient temperatures and pressures. Waste products can also be used on a carbon basis, making unused reutilizing more efficient. However, the proportion of hydrogen manufacture is modest, and this process’ technology has to be improved. Biological hydrogen generation is an innovative and promising technique to fulfill growing energy requirements as an alternative for fossil fuels because it produces a clean energy source and uses waste materials.
4 Biological Techniques for the Synthesis of Hydrogen Gas
Hydrogen formed from biological sources is called biohydrogen. Biohydrogen production methods are mainly classified as (1) photobiological methods, (2) fermentative methods, and (3) biocatalyzed electrolysis. Figure 2 shows a chart outline for producing biohydrogen from decomposable wastes and food sector effluents that contain cellulose/starch. It depicts biohydrogen production using an anaerobic photo- and dark fermentation (Kapdan and Kargi 2006).
4.1 Photobiological Methods
Photobiological methods include direct and indirect biophotolysis. The process that occurs in the presence of light is called direct biophotolysis and in the absence of light it is called indirect biophotolysis.
4.1.1 Direct Biophotolysis
In biological organisms, direct biophotolysis refers to the formation of hydrogen below the influence of sunlight. This mechanism is analogous to plant and algae photosynthesis. One of the most famous hydrogen-generating algae is Chlamydomonas reinhardtii. Marine green algae Chlorococcum littorale, Scenedesmus obliquus, and Chlorella fusca and green algae Platymonas subcordiformis all have the enzyme hydrogenase. Dunaliella salina and C. vulgaris showed no hydrogenase activity (Florin et al. 2001; Winkler et al. 2002; Guan et al. 2004).
The thylakoid membranes in algae and cyanobacteria’s chloroplasts are made up of chlorophyll pigments from both photosystems (PSI and PSII). Light energy is absorbed by the pigments, which raises electron energy levels from water oxidation through PSII to PSI to ferredoxin, where a portion of the light energy is promptly stored as hydrogen gas. The photosynthetic hydrogen generation method in direct biophotolysis involves H2O oxidation and transference of electrons to the hydrogenase enzyme, resulting in the manufacture of hydrogen. Although the method produces a 14:1 ratio of O2 and H2, it does not necessitate the fixation of CO2 or the storing of energy in metabolites produced by cells:
The above mechanism, however, can only work for a short period without the elimination of oxygen since the enzymatic reaction is strongly inhibited by oxygen, and hydrogenase gene expression is suppressed by it (Nguyen et al. 2008; Ghiasian 2019). In most hydrogenases, oxygen functions as a transcriptional repressor, a hydrogenase growth inhibitor, and an unalterable inhibitor of hydrogenase enzyme activity. The overall reactions of aerobic and anaerobic phases of photobiological processes involve
The H2O is divided into oxygen, electrons (e−), and protons (H+) in the aerobic phase (O2). As a result, the e− and H+ made by water in PSII are deposited as a range of metabolic products in the form of carbohydrates and proteins, and the e− and H+ are required for H2 manufacture. Hydrogenase produces H2 during the anaerobic phase. Under anaerobic conditions, [FeFe] hydrogenase is rapidly activated and catalyzes H+ to H2 reduction by using the e− donor ferredoxin. PSII donates to both indirect and direct e-source channels. Electrons generated by PSII’s water-splitting activity are carried into the electron transport chain (ETC) during the phase transition and eventually reach [FeFe] hydrogenase, which converts H to H2 (Najafpour et al. 2016).
Hydrogenase enzyme is found in green algae for hydrogen production, whereas enzymes (nitrogenase and hydrogenase) promote H2 synthesis in cyanobacteria. Both heterocystous nitrogen-fixing cyanobacteria and unicellular include the nitrogenase enzyme, which can be utilized to create hydrogen (Ghysels and Franck 2010).
4.1.2 Indirect Biophotolysis
In microalgae and cyanobacteria, indirect biophotolysis refers to the production of H2 from intracellular energy reserves such as starch and glycogen. As a result, there are two stages to this process: carbohydrate production in the light and carbohydrate fermentation in the dark for H2 generation (Huesemann et al. 2010; Ghiasian 2019).
In direct and indirect biophotolysis, the influence of an indirect and direct electron transference path is used to produce hydrogen.
Reduced substrates (starch in microalgae and glycogen in cyanobacteria) are collected during the photosynthetic O2 production and carbon dioxide fixation stages of the indirect biophotolysis process, and these are then used in a second stage for H2 creation under anaerobic conditions with carbon dioxide elimination. A diagram showing indirect electron transfer of biohydrogen production is shown in Fig. 3.
The overall hydrogen production can be shown as
4.2 Fermentative Methods
Fermentative methods include dark fermentation, photofermentation, and a combination of dark and photofermentation processes.
4.2.1 Photofermentation
Photofermentation can also yield hydrogen gas. In daylight, photosynthetic bacteria transform organic molecules such as lactic acids, butyric acids, and acetic acids into CO2 and H2. This procedure, however, needs anaerobic conditions. Depending on the physiological parameters of the microorganisms, both nitrogenase and hydrogenase enzymes are included in hydrogen generation during photofermentation. Four factors limit nitrogenase-facilitated photofermentation in purple non-sulfur bacteria:
-
The occurrence of an H2 absorption enzyme
-
Small photofermentation productivity for H2 making
-
The little turnover quantity of nitrogenase
-
The obtainability of organic acids
Nitrogenase and hydrogenase enzymes are active in purple non-sulfur bacteria. PNS bacteria generate hydrogenase in nitrogen-deficient environments. In an anaerobic situation, hydrogenase converts H2 to H+, electrons, and ATP for energy absorption. For photosynthetic bacteria to produce photofermentative H2, several parameters such as the amount of light, duration of light intensity, temperature, and inoculum age are critical (Ghysels and Franck 2010; Assawamongkholsiri and Reungsang 2015; Anwar et al. 2019).
Rhodobacter sp., for example, prefers temperatures between 31 and 36 °C. Using nitrogenase enzymes, these bacteria can change biological acids into CO2 and H2 under anaerobic circumstances. Supplementing micronutrients including molybdenum (Mo) and iron (Fe) in a Rhodobacter growing medium boosted photofermentative H2 generation. A chart for photofermentation of biohydrogen manufacture is shown in Fig. 4.
The reaction can be shown as
4.2.2 Dark Fermentation
Anaerobic bacteria thrive in the dark on carbohydrate-rich, hydrogen-producing substrates. Enterobacter, Bacillus, and Clostridium species have all been found to create hydrogen. Carbohydrates, particularly glucose, are the favored carbon bases for fermentation procedures that produce butyric and acetic acids as well as hydrogen. The chemical reactions involved can be shown as
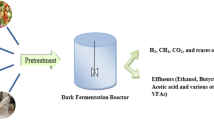
Dark fermentation procedures create mixed biogas that contains mostly carbon dioxide (CO2) and hydrogen, which also comprise smaller quantities of hydrogen sulfide (H2S), methane (CH4), and carbon monoxide (CO). Dark fermentation outperforms photofermentation since it does not need light and produces more energy due to the fermentation of sugar and carbs. Organic polymers can be hydrolyzed to monomers, which is followed by acetogenic transformation of monomers to alcohols, hydrogen, and organic acids. Even though dark fermentation produces more biohydrogen than photofermentation, it is more promising and advantageous. However, because organic biomass is required as a fuel, this method is highly expensive (Rafa et al. 2018; Sarangi and Nanda 2020). A schematic diagram for dark fermentation of biohydrogen production is displayed in Fig. 5.
Temperature, pH, hydraulic retention period, partial pressure of H2/CO2, volatile fatty acids, and inorganic content are all factors that influence hydrogen manufacture in dark fermentation. The temperature of the process has a direct impact on microbial growth, metabolic activity, and amount of hydrogen generation. The mesophilic region (25–40 °C), thermophilic region (40–65 °C), severe thermophilic region (65–80 °C), and hyperthermophilic region (>80 °C) are among the bacteria that produce hydrogen through dark fermentation. Dark fermentation techniques work best at temperatures between 35 and 55 °C. When compared to mesophilic circumstances, bacterial species produce more biohydrogen under high thermophilic conditions. The limited pressure of hydrogen in the bioreactor is another factor that affects hydrogen generation. The transference of hydrogen from the fluid to the gas phase increases as the limited pressure in the bioreactor falls. The alternative significant limit for biohydrogen manufacture via the dark fermentation procedure is the hydraulic retention period. By selecting acid-producing bacteria, short HRTs are utilized to clean up methanogens in a characteristic continuous stirred-tank reactor (CSTR) (Rangel et al. 2020).
4.3 Integration of Photo- and Dark Fermentation Processes
Many of the secondary products formed by dark fermentation could be employed as probable substrates for future transformation to hydrogen using other procedures, resulting in total biomass conversion and increased hydrogen production. As a result, implementing combined procedures for using the secondary products, particularly organic biological acids, is a viable strategy for achieving near-complete transformation of organic biomass while lowering surplus formation. Dark fermentation changes carbohydrates to organic biological acids in the first step of such combined bioconversion procedures for hydrogen production, and the second step employs these organic biological acids to produce methane and hydrogen as final products using a variety of methods. Purple non-sulfur bacteria are thought to have potential because of their capacity to transform dark fermentation by-products such as organic acids into hydrogen. Many researchers have explored the products containing organic acids after dark fermentation utilizing an integrated method such as dark fermentation and photofermentation (DF-PF) (Toledo-Alarcón et al. 2018; Rangel et al. 2020). The overall integrated dark and photofermentation are depicted in Fig. 6. Throughout the combination of DF-PF routes, special attention is paid to the following factors:
-
Convenient acceptance of photofermentation over dark fermentation
-
Farming of various microbes for photo- and dark fermentation in a common reactor
-
Membrane segregation of mutual fermentation procedures
Various products generated from the integrated pathway can be expressed as chemical equations given below:
Dark fermentation:
Photofermentation:
Lactic acid:
Propionic acid:
Butyric acid:
4.4 Biocatalyzed Electrolysis
Other energy forms such as electrical energy can be used to oxidize the effluent of the dark fermentation process to make hydrogen instead of solar energy. The anodic compartment of an electrolyzer cell is formed by a bioreactor containing acetate, while bacteria in the cathodic compartment create protons and electrons. The platinum electrode acts as the cathode and liberates hydrogen.
The reactions at anode and cathode can be summarized as
Chemical and biological methods used for hydrogen generation have their advantages and disadvantages. Table 1 describes various processes involved, organisms used, and advantages and disadvantages of methods used for hydrogen generation.
5 Conclusion
Hydrogen fuel is recognized as the “energy source of the future” when compared to fossil fuels. It is a low-carbon, high-energy resource. Chemical processes using high temperatures, such as partial oxidation of fossil fuels and steam reforming of hydrocarbons, are energy consuming and costly. Biological approaches for hydrogen production have different advantages, such as functioning under moderate circumstances and precise conversions. However, the most significant restrictions of biohydrogen generation are the expense of raw materials. The use of starch or cellulose-containing solid wastes and carbohydrate-rich and food-industry effluents for biohydrogen production is an intriguing option. To improve the “recent advances” in biohydrogen production, much research and development are required.
References
Anwar M et al (2019) Recent advancement and strategy on bio-hydrogen production from photosynthetic microalgae. Bioresour Technol 292(June):121972. https://doi.org/10.1016/j.biortech.2019.121972
Argun H, Kargi F (2010) Bio-hydrogen production from ground wheat starch by continuous combined fermentation using annular-hybrid bioreactor. Renew Energy 35(12):6170–6178. https://doi.org/10.1016/j.ijhydene.2010.03.132
Assawamongkholsiri T, Reungsang A (2015) Photo-fermentational hydrogen production of Rhodobacter sp. KKU-PS1 isolated from an UASB reactor. Electron J Biotechnol 18(3):221–230. https://doi.org/10.1016/j.ejbt.2015.03.011
Azwar MY, Hussain MA, Abdul-wahab AK (2014) Development of biohydrogen production by photobiological, fermentation and electrochemical processes: a review. Renew Sust Energ Rev 31(November):158–173. https://doi.org/10.1016/j.rser.2013.11.022
Collodi G (2010) Hydrogen production via steam reforming with CO2 capture. Chem Eng Trans 19(August):37–42. https://doi.org/10.3303/CET1019007
Florin L, Tsokoglou A, Happe T (2001) A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J Biol Chem 276(9):6125–6132. https://doi.org/10.1074/jbc.M008470200
Ghiasian M (2019) Biophotolysis-based hydrogen production by cyanobacteria. Springer International Publishing, Heidelberg. https://doi.org/10.1007/978-3-030-14463-0_5
Ghysels B, Franck F (2010) Hydrogen photo-evolution upon S deprivation stepwise: an illustration of microalgal photosynthetic and metabolic flexibility and a step stone for future biotechnological methods of renewable H2 production. Photosynth Res 106(1–2):145–154. https://doi.org/10.1007/s11120-010-9582-4
Guan Y et al (2004) Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem Eng J 19(1):69–73. https://doi.org/10.1016/j.bej.2003.10.006
Han W et al (2016) Biohydrogen production from enzymatic hydrolysis of food waste in batch and continuous systems. Sci Rep 6(July):1–9. https://doi.org/10.1038/srep38395
Hao H-TN, Karthikeyan OP, Heimann K (2015) Bio-refining of carbohydrate-rich food waste for biofuels. Energies 8:6350–6364. https://doi.org/10.3390/en8076350
Higman C, Tam S (2014) Advances in coal gasification, hydrogenation, and gas treating for the production of chemicals and fuels. Chem Rev 5(April):1–36
Huesemann MH et al (2010) Hydrogen generation through indirect biophotolysis in batch cultures of the nonheterocystous nitrogen-fixing cyanobacterium Plectonema boryanum. Appl Biochem Biotechnol 162(1):208–220. https://doi.org/10.1007/s12010-009-8741-6
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microb Technol 38(5):569–582. https://doi.org/10.1016/j.enzmictec.2005.09.015
Konstandopoulos AG et al (2015) Production of hydrogen from renewable resources. SciVerse ScienceDirect 5:97–117. https://doi.org/10.1007/978-94-017-7330-0
Liu G, Shen J (2004) Effects of culture and medium conditions on hydrogen production from starch using anaerobic bacteria. J Biosci Bioeng 98(4):251–256. https://doi.org/10.1016/s1389-1723(04)00277-4
Mangold K-M (2009) Introduction to hydrogen technology (Roman J. Press, K. S. V. Santhanam, Massoud J. Miri, Alla V. Bailey, and Gerald A. Takacs). ChemSusChem 2(8):781–781. https://doi.org/10.1002/cssc.200900109
Najafpour MM et al (2016) Manganese compounds as water-oxidizing catalysts: From the natural water-oxidizing complex to nanosized manganese oxide structures. Chem Rev 116(5):2886–2936. https://doi.org/10.1021/acs.chemrev.5b00340
Nguyen AV et al (2008) Transcriptome for photobiological hydrogen production induced by sulfur deprivation in the green alga Chlamydomonas reinhardtii. Eukaryot Cell 7(11):1965–1979. https://doi.org/10.1128/EC.00418-07
Preethi et al (2019) Biohydrogen production from industrial wastewater: An overview. Bioresour Technol Rep 7(April):100287. https://doi.org/10.1016/j.biteb.2019.100287
Rafa Ł et al (2018) Hydrogen production from biomass using dark fermentation. Renew Sustain Energy Rev 91(April):665–694. https://doi.org/10.1016/j.rser.2018.04.043
Rangel C et al (2020) Hydrogen production by dark fermentation process: effect of initial organic load. Chem Eng Trans 79(February):133–138. https://doi.org/10.3303/CET2079023
Sarangi PK, Nanda S (2020) Biohydrogen production through dark fermentation. Chem Eng Technol 4:601–612. https://doi.org/10.1002/ceat.201900452
Sengodan S et al (2018) Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew Sust Energ Rev 82(July 2016):761–780. https://doi.org/10.1016/j.rser.2017.09.071
Toledo-Alarcón J, Capson-Tojo G, Marone A (2018) Basics of bio-hydrogen production by dark fermentation. In: Bioreactors for microbial biomass and energy conversion 5(10), pp 199–220. doi:https://doi.org/10.1007/978-981-10-7677-0
Ueno Y et al (1995) Biological production of hydrogen from cellulose by natural anaerobic microflora. J Ferment Bioeng 79(4):395–397. https://doi.org/10.1016/0922-338X(95)94005-C
Winkler M et al (2002) Isolation and molecular characterization of the [Fe]-hydrogenase from the unicellular green alga Chlorella fusca. Biochim Biophys Acta 1576(3):330–334. https://doi.org/10.1016/S0167-4781(02)00239-7
Yokoi H et al (2001) Microbial hydrogen production from sweet potato starch residue. J Biosci Bioeng 91(1):58–63. https://doi.org/10.1016/S1389-1723(01)80112-2
Yu HY et al (2015) A facile one-pot route for preparing cellulose nanocrystal/zinc oxide nanohybrids with high antibacterial and photocatalytic activity. Cellulose 22(1):261–273. https://doi.org/10.1007/s10570-014-0491-0
Acknowledgments
Reshmy R and Raveendran Sindhu acknowledge the Department of Science and Technology for sanctioning projects under DST WOS-B scheme.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Unni, R., Reshmy, R., Madhavan, A., Binod, P., Pandey, A., Sindhu, R. (2022). Methods of Biological Hydrogen Production from Industrial Waste. In: Kuddus, M., Yunus, G., Ramteke, P.W., Molina, G. (eds) Organic Waste to Biohydrogen. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-19-1995-4_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-1995-4_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1994-7
Online ISBN: 978-981-19-1995-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)