Abstract
The metabolic flexibility of some photosynthetic microalgae enables them to survive periods of anaerobiosis in the light by developing a particular photofermentative metabolism. The latter entails compounds of the photosynthetic electron transfer chain and an oxygen-sensitive hydrogenase in order to reoxidize reducing equivalents and to generate ATP for maintaining basal metabolic function. This pathway results in the photo-evolution of hydrogen gas by the algae. A decade ago, Melis and coworkers managed to reproduce such a condition in a laboratory context by depletion of sulfur in the algal culture media, making the photo-evolution by the algae sustainable for several days (Melis et al. in Plant Physiol 122:127–136, 2000). This observation boosted research in algal H2 evolution. A feature, which due to its transient nature was long time considered as a curiosity of algal photosynthesis suddenly became a phenomenon with biotechnological potential. Although the Melis procedure has not been developed into a biotechnological process of renewable H2 generation so far, it has been a useful tool for studying microalgal metabolic and photosynthetic flexibility and a possible step stone for future H2 production procedures. Ten years later most of the critical steps and limitations of H2 production by this protocol have been studied from different angles particularly with the model organism Chlamydomonas reinhardtii, by introducing various changes in culture conditions and making use of mutants issued from different screens or by reverse genomic approaches. A synthesis of these observations with the most important conclusions driven from recent studies will be presented in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of some unicellular green algae to photo-evolve H2 gas was discovered in the early 1940s by Gaffron and Rubin (1942). Multiple screenings revealed the occurrence of a reversible hydrogenase enzyme in several algal lineages including some red algal species and diatoms (Frenkel and Rieger 1951; Meyer 2007), but light-induced H2 evolution was only detected in a selected group of green algae. The latter includes the chlorophycean genera Chlamydomonas, Chlorococcum, Desmodesmus, Lobochlamys, Pseudokirchneriella, Scenedesmus, the prasinophycea Platymonas subcordiformis and the trebouxiacea Chlorella pyrenoidosa and Oocystis sp. (Winkler et al. 2002; Guan et al. 2004; Skjånes et al. 2008; Timmins et al. 2009a; Meuser et al. 2009). The hydrogenase is linked to the photosynthetic electron transport chain at the level of ferredoxin (Florin et al. 2001; Winkler et al. 2009). The presence of a hydrogenase pathway enables some microalgae to dissipate excess electrons from the photosynthetic electron chain in conditions when common photosynthetic electron acceptors (CO2, O2) are lacking or sink processes (Calvin cycle, photorespiration, Mehler reaction) are suppressed.
The flow of electrons through this alternative valve results in the production of hydrogen gas, an attractive and environmentally friendly energy carrier, which currently makes the hydrogenase pathway the most studied auxiliary photosynthetic electron transfer pathway in green algae. A major obstruction toward biotechnological application of this feature is the extreme oxygen sensitivity of the hydrogenase (Abeles 1964; Erbes et al. 1979; Cohen et al. 2005). As a result of this sensitivity, H2 photo-evolution mostly appears as a transient phenomenon, incompatible with normal steady-state oxygenic photosynthesis.
H2 photo-evolution in green algae can rely on two electron transport pathways to the hydrogenase. One involves the photo-oxidation of water and the transport of electrons through both PSII and PSI. This pathway is characterized by its sensitivity to DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) and the simultaneous production of H2 and O2 (Spruit 1954; Bishop and Gaffron 1963). Therefore, H2 production by this mechanism can only be transient unless oxygen consumption capacity (through respiration) exceeds PSII-dependent O2 generation. The other pathway uses reducing equivalents from oxidative carbon metabolism, which serve to reduce intersystem electron carriers in such way that only PSI is involved in H2 photo-evolution (Frenkel 1952; Kaltwasser and Gaffron 1964).
The chlorophycea Chlamydomonas reinhardtii produces the highest levels of H2 from water reported so far and has been used extensively as a model organism for studying algal H2 metabolism. The isolation of the hydrogenase-encoding genes (Happe and Naber 1993; Happe and Kaminski 2002; Forestier et al. 2003) as well as the discovery of hydrogen evolution under sulfur (S) deprivation in C. reinhardtii gave new impulses for both basic and biotechnology-oriented research in this field (Melis et al. 2000).
A decade ago Melis and coworkers discovered that S deprivation induces anaerobiosis and sustained H2 production in light-exposed C. reinhardtii cultures (Melis et al. 2000). S-deficiency also imposes a major stress on the algae, thus limiting the potential H2 production yields. The application of this protocol, however, offered valuable information regarding the mechanisms underlying H2 production in green algae and the flexibility of the photosynthetic apparatus in response to nutritional and anaerobic stress conditions. The key events taking place during S deprivation and their known or putative importance for H2 production will be discussed in this review.
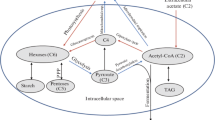
The hydrogen production process in S-deprived medium is characterized by a number of major metabolic transitions (Zhang et al. 2002; Timmins et al. 2009b). S is an essential macroelement incorporated into sulfolipids, polysaccharides, proteins, and electron transport carriers. Algal cells have only restricted storage capacity for S. This results in a rather fast response to the lack of S in the media. During the first 24 h of S depletion, cells stop dividing and start to accumulate large energy stores in the form of starch (Fig. 1) and lipids. This may be an adaptive response to the lack of S for synthesis of amino acids and other cell components required for growth. In such conditions, carbon is stored in a form that is readily available when S becomes available again. A typical part of mineral stress response (S, P, N) is the decline of photosynthetic capacity (Grossman 2000). For cultures maintained in closed vessels the decline of photosynthesis during S deprivation will eventually lead to the unusual state of anaerobiosis in the light (Fig. 1). S-deficiency also activates more specific processes allowing for better scavenging of S from internal and external stores.
Schematic representation of the evolution of some physiological parameters during a typical batch H2 experiment with S-deprived C. reinhardtii. The figure does not show a particular experiment but was assembled from data of several published studies on H2 evolution (Melis et al. 2000; Zhang et al. 2002; Timmins et al. 2009b). Y-axis units are not shown, and the evolution of the parameters is depicted on a relative time-scale that represents the different metabolic conditions experienced by the algae during a typical H2 experiment
Cells undergo a second metabolic switch at the onset of anaerobiosis. The algal cells start breaking down endogenous substrates (starch and some amino acids) and enter fermentative metabolism. The latter is characterized by a decrease in pH of the medium and increases in amounts of fermentation products like ethanol and formate (Timmins et al. 2009b) (Fig. 1). Concomitantly, a FeFe-hydrogenase is induced and the algae start to evolve hydrogen gas in the light.
The final stage is characterized by a slower rate of starch breakdown, stabilization of pH, and a reduction in rate of H2 production. The latter is often interpreted as a response to depleted energy supplies, but observations have shown that the medium still contains acetate and the cells still have starch and TAG reserves (Timmins et al. 2009b) (Fig. 1).
The establishment of anaerobiosis during S deprivation
Different mechanisms may contribute to the inhibition of photosynthetic O2 evolution in S deplete conditions (Fig. 2). Their relative importance is still matter of debate. It is known that S deprivation has a general effect on the transcription of chloroplast genes (Irihimovitch and Yehudai-Resheff 2008; Irihimovitch and Stern 2006), which can cause a decrease of photosynthetic rate. This occurs by means of the SNF1-related protein kinase 2.2, a serine/threonine kinase previously known as SAC3 (for Sulfur Acclimation 3) (Davies et al.1999; Irihimovitch and Stern 2006; Gonzalez-Ballester et al. 2008). In addition to this general effect, the PSII repair cycle or frequent replacement of the D1/32-kDa reaction-center protein in the PSII complex is most probably impaired by the inability of chloroplasts to carry out high rates of de novo protein biosynthesis due to scarcity of the S-containing amino acids methionine and cysteine (Wykoff et al. 1998; Zhang et al. 2002). However, the degradation of D1 protein is not the first effect of S-starvation but is preceded by a massive degradation of Rubisco (ribulose 1,5 bisphosphate carboxylase), which declines by about 80% in the first 24 h of S deprivation and becomes undetectable after 60 h of starvation (Zhang et al. 2002). Although this prompt Rubisco degradation makes good sense from a physiological point of view since it is the most abundant protein and a major reserve of reduced S in the algal cell, the underlying mechanism of its degradation remains unidentified. As it is the case for a number of abiotic stresses, oxidative modification of specific residues on Rubisco could mark the enzyme for degradation during S deprivation (Parry et al. 2007; Moreno et al. 2008). Loss of CO2 fixing ability by Rubisco, and the Calvin cycle was reported to slow down the repair cycle of photodamaged PSII complexes (Takahashi and Murata 2005, 2006) and may therefore stimulate the loss of PSII activity during S deprivation. From experiments on spinach chloroplasts, Takahashi and Murata concluded that in case of a dysfunctional Calvin cycle O2 replaces CO2 as final electron acceptor, leading to enhanced formation of reactive oxygen species that abolish chloroplast protein translation. For instance they observed that inhibition of the D1 repair cycle by Calvin cycle interruption was bypassed when its electron sink function was restored by addition of the Calvin cycle intermediate 1,3-bisphosphoglycerate, which accepts electrons from NADPH. In addition, they showed that impairment of chloroplast translation, and D1 turn-over depend on the presence of O2 and could be stimulated by H2O2.
Owing to a sudden drop of active PSII reaction centers, S-deprived C. reinhardtii cultures isolated from the atmosphere become anoxic in the light, a condition that enables sustained light-driven H2 evolution. The drop in PSII activity may be caused by three S deprivation triggered responses represented by the three branches in the above scheme
Better knowledge of the signals and mechanisms that cause the down-regulation of photosynthetic electron transport during S deprivation may allow us in the future to trigger them selectively without actual induction of a general nutrient stress that would lower metabolic rates and H2 yields. Another option for circumventing the harsh effect of a general S starved condition on the cells was proposed (Chen and Melis 2004; Chen et al. 2005). It was shown in these works that down-regulating the expression of the chloroplast sulfate transporter caused limiting sulfate nutrient uptake by the chloroplast and a localized S deprivation condition that initiates H2-photoproduction in sulfate-replete media.
Reaching the state of anaerobiosis in the culture not only depends on declining photosynthetic rates but also requires sufficient mitochondrial oxygen consumption. Most hydrogen production studies therefore make use of photoheterotrophic cultures with acetate. Acetate assimilation supports the transition of microalgae to anaerobic conditions by supplying the respiration with reducing equivalents (Fouchard et al. 2005; Kosourov et al. 2007). This is reflected in the fast acetate uptake during the first 40 h of S deprivation, while only little acetate is consumed during the hydrogen production phase (Timmins et al. 2009b). In recent surveys of green algal species, only those strains with both hydrogenase activity and the ability to utilize acetate achieved anaerobiosis rapidly (Skjånes et al. 2008; Meuser et al. 2009). However, for future economical and ecological purpose, H2 should be produced from light energy only. If no acetate is present in the medium, the anaerobic condition required for H2 evolution can eventually be reached and maintained by (enhanced) inhibition of PSII photochemistry. This can be achieved either by adding DCMU to the culture (after maximal accumulation of starch) (Fouchard et al. 2005) or by applying a light protocol (low light intensity before and high light after transfer to S-deprived condition) that increases PSII photoinhibition upon S deprivation (Tsygankov et al. 2006; Tolstygina et al. 2009).
Acetate is not required as substrate for starch accumulation if the media is supplemented with bicarbonate or bubbled with CO2 (2%) (Fouchard et al. 2005; Tsygankov et al. 2006) and thus the starch accumulation depends on Rubisco and an active Calvin cycle. However, the starch accumulated in high CO2 or the presence of bicarbonate is not able to supply sufficient reductant for supporting the high respiration rates necessary for the anaerobic switch (Fouchard et al. 2005; Tsygankov et al. 2006). The underlying mechanism that permits accumulation of substantial amounts of starch when amounts of Rubisco and photosynthetic activity are concomitantly declining rapidly is not yet understood.
Hydrogen production during S deprivation
Induction of the hydrogenase
The induction of the hydrogenase in vivo not only requires anaerobiosis, but is also dependent on a metabolic condition that requires either light or the presence of starch, the latter in case that the induction happens in the dark (Posewitz et al. 2004; Chochois et al. 2009). The necessity of one or the other of these factors can probably be explained by the significant amounts of energy needed for hydrogenase translocation to the chloroplast and its subsequent maturation (Happe et al. 1994; Happe and Kaminski 2002).
The dependence of dark anaerobic hydrogenase induction on starch can be ascribed to the fact that starch, or more precisely starch derived glucose as glycolytic substrate, is necessary for the generation of ATP in a dark anaerobic environment. Acetate present as organic carbon substrate in the media can only be metabolized following ATP-dependent entry into the Krebs cycle. ATP generation through acetate therefore mostly depends on oxidative phosphorylation, which is inactive during anaerobiosis in the dark. Alternatively, it was also suggested (Chochois et al. 2009) that dark anaerobic hydrogenase induction could depend on a signal that translates the cell metabolic condition to the chloroplast. The physiological state of the cell can be sensed by the chloroplast via the redox state of the PQ pool, which can be modified by non-photochemical PQ reduction (Jans et al. 2008; Desplats et al. 2009). In the light, the dependence of hydrogenase induction on starch can be circumvented, probably because ATP is generated by the chloroplast. It was indeed shown that in absence of starch, hydrogenase induction in S-deprived cultures is independent of PSII function but requires the formation of a trans-thylakoidal proton gradient (Chochois et al. 2009) generated by cyclic electron transport around PSI, a finding that supports the hypothesis of the ATP-dependence of hydrogenase induction.
H2 production through the direct (PSII-dependent) and indirect (PSII-independent) pathway
Photobiological H2 evolution in C. reinhardtii is driven by two different pathways that can supply reductants (i.e., reduced ferredoxin) for hydrogen production in the light (Fig. 3): a direct pathway involving PSII and an indirect, PSII-independent pathway, which relies on a non-photochemical reduction of plastoquinones (Fouchard et al. 2005; Melis 2007). Initially, Melis et al. (2000) assumed that hydrogen production in S-deprived cultures is driven by reductants supplied by photosynthesis in two phases. In the first phase, which corresponds to the initial aerobic stage of the S-deprived culture, photosynthesis would drive the storage of reduced carbon (starch) and ATP derived from water splitting. In a second anaerobic phase when PSII activity is down, starch consumption would provide reducing equivalents for the indirect pathway that operates with PSI but not with PSII.
The rate of light-sustained H2 evolution during S deprivation is determined by the available sources of reductant and the presence of electron sinks, which compete with the FeFe hydrogenase for photoreductant. During S deprivation, the two main chloroplast sinks for photoreductant are eliminated and the hydrogenase becomes an important electron sink for the thylakoid electron transfer chain in the light, accepting electrons from ferredoxin. In anoxic conditions, the photosynthetic apparatus also undergoes a state transition, which is characterized by a shift of mobile LHC antenna complexes from PSII to PSI reaction centers and a reorganization of the electron transporting complexes in the thylakoid membrane to favor cyclic electron transport around PSI. Starch forms an important source for the PSII-independent reductant supply for the hydrogenase. However, remaining mitochondrial activity can also be a sink for starch-derived reductants and becomes an important competitor for the PSII-independent pathway of H2 production. Electron fluxes are illustrated by red arrows in the scheme. Processes or fluxes that are stimulated in anoxic, S-deprived algal cells are marked with a +, the ones that are inhibited with a −
Subsequent observations showed that the supply of reducing equivalents for H2 evolution does not entirely occur according to this strict two-phase scheme. Some experimenters observed H2 evolution in S-deprived C. reinhardti cultures that is primarily driven by residual PSII activity (Kosourov et al. 2003; Antal et al. 2003) suggesting a rather limited dependence of H2 production on non-photochemical PQ reduction by starch derived reducing equivalents. It was indeed reported recently that starch deficient mutants are capable of producing H2 under S deprivation up to levels close to the ones reached with WT strains (Chochois et al. 2009). Only in presence of DCMU, a condition in which a residual H2 production is observed in the WT, do starch deficient mutants show impaired H2 capacity. On the one hand, this suggests that electron supply via non-photochemical PQ reduction is minor if not redundant for H2 evolution. At this point the work of Chochois et al. (2009) contrasts with observations by Jans et al. (2008) that H2 production ability is significantly reduced in cell lines deficient in chloroplast NAD(P)H-PQ oxidoreductase Nda-2 (Jans et al. 2008), the key enzyme of the PSII-independent pathway. However, it cannot be excluded that the role of Nda-2 in the H2 evolution process is related to its function in cyclic electron transport around PSI, which (see previous paragraph) may be required in order to obtain full induction (or activation) of the hydrogenase during anaerobiosis in the light. The ability of S-deprived Nda-2 RNAi lines to develop WT levels of in vitro hydrogenase activity should therefore be verified.
The work of Chochois et al. (2009) also confirms the importance of starch as the principal source of reducing equivalents for the PSII-independent H2 evolution pathway, thereby minimizing a role of other possible sources of reducing equivalents such as acetate or lipids (the latter being overproduced in starchless mutants like sta6 (Wang et al. 2009)). On the other hand, it was also shown that externally supplied glucose could be used to generate additional H2 in a mutant strain in which an hexose symporter from Chlorella kessleri was expressed (Doebbe et al. 2007).
It should be noted that the relative importance of the direct, PSII-dependent and of the indirect pathway may vary according to the experimental conditions. For instance in photoautotrophic H2 protocols, which require a more suppressed PSII activity in order to compensate for the limited oxygen consumption in absence of acetate, the PSII-independent pathway may become a more important source of reductant for the hydrogenase (Kosourov et al. 2007). We would also like to draw the attention to the fact that many of the conclusions concerning the relative importance of PSII-dependent and PSII-independent reductant supply for H2 evolution are driven from studies with the PSII inhibitor DCMU (Antal et al. 2003; Fouchard et al. 2005; Hemschemeier et al. 2008; Chochois et al. 2009). DCMU is used to suppress the direct, PSII-dependent pathway in order to determine the capacity of the indirect pathway. Results of these studies should be interpreted carefully since the presence of DCMU abolishes O2 evolution, and thereby triggers another metabolic switch of the cells in which they shift from a micro-aerobic state with a still active mitochondrial oxidative phosphorylation to a situation of complete anaerobiosis leading to more pronounced ATP depletion.
Factors that limit the rate of hydrogen production: competing electron sinks, dependence on mitochondrial respiration, and trans-thylakoid proton gradient formation
Elimination of electron sinks is a prerequisite for sustained H2 evolution
The hydrogenase competes with different electron sinks (Fig. 3), in particular ferredoxin-NADP-oxidoreductase as an interface with the Calvin cycle. Sufficient suppression of competing electron sink capacity during the production phase is a prerequisite for sustainable H2 evolution. The loss of CO2 fixation ability by Rubisco and the Calvin cycle may indeed be one of the critical effects of S deprivation for triggering H2 production (Zhang et al. 2002; Hemschemeier et al. 2008). The importance of a decreased sink capacity of the Calvin cycle was confirmed with mutants showing lowered rates of net photosynthesis issued from a screen based on the Winkler test (Rühle et al. 2008). One of the selected mutants becomes anaerobic in sulfur replete-sealed cultures at moderate light exposure around 60 μmol m−2 s−1. However, this anaerobic condition was not sufficient to trigger H2 evolution. Only when the Calvin cycle was additionally inhibited with glycolaldehyde did this mutant produce H2 for 6 h at a rate superior to the one observed in S-deprived WT. These authors also confirmed that Calvin cycle competition with H2 evolution seems largely suppressed during S deprivation given the fact that glycolaldehyde treatment did not further stimulate H2 evolution rates in a S-deprived WT strain.
Contrasting results were obtained with two mutants depleted of Rubisco complexes. The absence of significant hydrogen production in the Rubisco mutant strain CC-2653 reported by White and Melis (2006) under S deprivation contrasts with the observations of Hemschemeier et al. (2008) who reported H2 production with a Rubisco deficient mutant strain, CC-2803 under S-deprived but also in presence of S. From the effect of DCMU treatments, Hemschemeier et al. (2008) concluded that hydrogen was essentially produced by the PSII-dependent direct pathway in CC-2803. These contrasting results remain essentially unexplained, even though the two mutants are not equivalent (with CC-2683 being a partial and CC-2803 a complete RbcL deletion mutant) and display different rates of respiration (White and Melis 2006; Hemschemeier et al. 2008).
Besides CO2 reduction through the Calvin cycle, O2 reduction through the Mehler reaction may also compete with hydrogen photo-evolution. Lee and Greenbaum (2003) indeed indicated that very low amounts of O2 (<5,000 ppm) do lower hydrogen evolution rates by forming an additional electron acceptor and not by inhibiting hydrogenase activity.
The ambiguous role of mitochondria during the H2 evolution phase
H2 production in S-deprived C. reinhardtii cultures is limited by its dependence on mitochondrial respiration. In order to maintain the anaerobic conditions necessary for hydrogenase activity, residual PSII oxygenic activity should be kept below the O2 uptake capacity by mitochondria, a condition that is achieved during S deprivation. This implies that H2 evolution driven by the direct, PSII-dependent pathway could be improved with engineered strains that display higher mitochondrial respiration rates in an adapted protocol leading to less suppressed PSII activity. Kruse (2005) described a mutant (stm6) that shows improved H2 producing ability as a result of a knock-out of moc1. Moc1 is a nuclear encoded factor involved in the assembly of the mitochondrial respiratory chain in the light (Schönfeld et al. 2004). The pleiotropic phenotype of stm6 displays several characteristics that may result in higher H2 production, such as its tendency to store large starch amounts and its reduced capacity to perform state I–state II transitions. However, most likely of greater importance for the improved H2 ability of stm6 is its enhanced oxygen consumption capacity which probably enables it to reach higher PSII driven H2 evolution rates (Kruse 2005).
On the other hand, mitochondrial respiration and the indirect, PSII-independent H2 evolution pathway both depend on consumption of reducing equivalents derived from the Krebs cycle. Therefore, they may be in competition with each other. Such a competition was indeed revealed by the stimulatory effect of inhibitors of mitochondrial electron transport transport myxothiazol (complex III inhibitor) and SHAM (inhibitor for the mitochondrial alternative oxidase AOX) on H2 evolution. Addition of these inhibitors resulted in a doubling of the H2 evolution rate by the PSII-independent pathway (in presence of DCMU) (Antal et al. 2009). A study by Cardol et al. (2003) nicely illustrated the redox tuning between chloroplast and mitochondria with a battery of mutants affected in different complexes (I, III, IV, I + III, and I + IV) of the respiratory chain. These mutants would be useful for further exploring the importance of respiration and oxidative phosphorylation in the context of H2 photo-evolution.
The limits imposed by mitochondrial respiration seem to compromise further improvement of the H2 photo-evolution capacity of C. reinhardti. In order to significantly increase H2 production ability, strains should be engineered to have higher O2 uptake capacity as is the case in the mutant stm6. However, higher respiration rates would increase mitochondrial consumption of reducing equivalents derived from C-metabolism and would therefore limit the amount of reducing equivalents available for supporting H2 evolution through the indirect PSII-independent pathway. Wu et al. (2010) showed that O2 evolved from PSII activity can also be trapped without consumption of reducing equivalents. In this study, the dependence of the anoxic conditions on mitochondrial consumption could be partly circumvented by local buffering of the evolved O2 with O2 trapping proteins. Their findings were inspired on a natural situation in leguminous nodules, where the O2-sensitive nitrogenase is kept active under atmospheric conditions due to the abundance of leghemoglobins (lbs). These pigments accumulate in the soybean root nodules and buffer at a very low tension the free O2 around nitrogenase containing bacteroids (Ott et al. 2005). By transferring the coding region of both the ferrochelatase gene, hemH, from Bradyrhizobium japonicum, and the leghemoglobin gene, lba, from Glycine max, into the chloroplast of C. reinhardtii, transgenic strains that accumulate leghemoglobine in the chloroplast were created. These strains more rapidly consumed O2 and displayed increased H2 production in S-free medium (Wu et al. 2010). This result demonstrates the potential of local buffering of O2 in the chloroplast in order to help overcoming the incompatibility of oxygenic photosynthesis and H2 photoproduction and opens new possibilities for the development of more efficient photoautotrophic H2 production protocols in S-deplete or even S-replete conditions.
H2 production is limited by proton gradient formation
In S-replete, dark-adapted C. reinhardtii suspensions, PSII activity is kept at its full potential. In this condition, maximal H2 evolution rates are observed but only transiently because of the concomitant O2 evolution by PSII, which inhibits the hydrogenase. However, when in those conditions PSII activity is suppressed by DCMU, the residual H2 evolution supported by non-photochemical PQ reduction remains transient despite of the absence of O2 evolution. In the latter situation, H2 evolution becomes sustainable by addition of the ionophore FCCP that uncouples electron transport from proton gradient formation (Cournac et al. 2002). The proton gradient thus appears to restrict PSII-independent H2 evolution. Recently, Antal et al. (2009) found that the uncoupling agent FCCP also stimulated H2 evolution in S-deprived conditions, particularly when the latter depended on the indirect (PSII-independent) pathway. There is no clear explanation of this effect but several mechanisms may account for it:
-
Acidification of the lumen impairs cytb6f mediated oxidation of plastoquinol (Finazzi 2002).
-
Depletion of the stromal proton pool that can be reduced by the hydrogenase.
-
A decreased rate of NADPH oxidation (stroma side) at alkaline pH due to the electrostatic repulsion between the negative charges of the phosphate group of NADPH and the phospholipids of the membrane (Edman et al.1985). This would explain why the indirect pathway of H2 photo-evolution is more stimulated by uncoupling agents than the PSII-dependent pathway.
-
ATP depletion upon dissipation of the proton gradient. A likely function of the hydrogenase pathway in S-deprived anaerobic conditions is to generate ATP for the metabolic needs of the cell. The H2 production process may therefore be subject to regulation by ATP. In relation with this possibility, Zhang and Melis (2002) noticed that H2 evolution rates in S-deprived culture could be stimulated by mild salinity stress, a condition that leads to increased ATP demand.
Cyclic electron transport around PSI limits H2 evolution
The flexible photosynthetic apparatus of C. reinhardtii can quickly balance the relative light absorption fluxes through PSII and PSI by adjusting the antenna sizes of the respective photosystems. A majority of antennae associated with PSII in state I will be shuttled to PSI during a shift to state II. Chlamydomonas reinhardtii displays exceptionally large state transition ability when compared to higher plants. This enables this alga to adapt its chloroplast metabolism to diverse physiological conditions (Wollman 2001; Cardol et al. 2009).
Such a major metabolic shift is observed during anaerobiosis when the sink capacity of the mitochondria for cellular reductants is suppressed and cellular ATP pools are rapidly depleted. Reduction of PQ pool in anaerobiosis triggers the signal for a transition to state 2. State 2 transition in C. reinhardtii is also marked by a shift of thylakoid electron fluxes from a linear mode between PSII and ferredoxin to a cyclic mode where electrons circle around PSI and cytb6f for the exclusive generation of ATP without producing reducing equivalents (Finazzi et al. 2002). This way, the chloroplast is transformed into a major source of ATP in response to cellular ATP depletion (Finazzi et al. 2002; Cardol et al. 2009). During the process of anaerobic H2 evolution cyclic electron transport hence forms an additional electron transport pathway with which the hydrogenase must compete.
Two pathways for cyclic electron transport around PSI operate in C. reinhardtii thylakoids, distinguishable by their sensitivity to the inhibitor antimycin A. The antimycin sensitive pathway involves direct PQ reduction by ferredoxin mediated by a membrane bound ferredoxin quinone reductase (FQR) whereas the alternative pathway is driven by FNR-mediated NADP reduction followed PQ reduction catalyzed by a type 2 NDH (NDA2). If NDA2 expression is silenced by RNAi, H2 production ability in S-deprived conditions is reduced by 50%, which most likely highlights its role in the PSII-independent electron supply of the hydrogenase (Jans et al. 2008). Contrastingly, blocking FQR-dependent cyclic electron transport with antimycin A resulted in a major increase (more than 200%) of H2 evolution rate (Antal et al. 2009). This may point at some kind of competition between the hydrogenase and FQR for reduced ferredoxin. Part of this inhibition could also be caused by a limitation of electron transport due to the proton gradient generated by cyclic electron transport (see previous paragraph).
The high H2 producing phenotype of the mutant stm6 (Kruse 2005), cited earlier on, was ascribed to its increased O2 uptake capacity. However, the fact that this mutant displays a pleiotropic phenotype cannot be neglected. Some other aspects of this phenotype might to some extent account for the increased H2 productivity. For instance, this mutant is also locked in state 1 and therefore inhibited in cyclic electron transport. But since no improved H2 production ability was reported for other state transitions mutants, like Stt7, the stimulation of H2 evolution in the stm6 mutant is more likely due to other aspects of its complex phenotype, probably in relation to altered mitochondrial activities as earlier suggested.
The end of hydrogen production
Analysis of the fermentative metabolome of algal cells undergoing the H2 production process in S-deprived batch cultures (Hemschemeier and Happe 2005; Timmins et al. 2009b) shed some light on what could eventually determine the end of the process. These studies showed that neither the carbon reserves (starch, triacylglycerides), accumulated during the early stages of S deprivation, nor the acetate from the medium is consumed entirely during the anaerobic phase. Therefore, it seems unlikely that the end of H2 evolution can be ascribed to depletion of carbon or energy reserves. It might rather result from the continuous stress resulting from the sulfur depletion, or be initiated by the accumulation of fermentation products (such as ethanol and formate, produced by the pyruvate formate lyase pathway (Hemschemeier and Happe 2005)), which might either be toxic or limit the rate of fermentative pathways.
Laurinavichene et al. (2008) as well as Kosourov and Seibert (2009) reported that the duration of H2 evolution can be significantly extended by immobilisation of the algal cells in thin alginate films. This approach offers several advantages. Toxic products of fermentation can be washed away while low amounts of S can be supplied with fresh medium in order to sustain basic metabolism that supports the subsequent H2 evolution. With continuous supply of low sulfate amounts, the duration of continuous H2 production could be maintained for 90 days (Laurinavichene et al. 2008). Another, unexpected, side effect of this technique was observed. It appeared that H2 evolution ability of algal cells trapped in alginate films becomes less susceptible to inactivation by atmospheric O2 (Kosourov and Seibert 2009). Immobilized cells in vials containing 21% O2 in the headspace, evolved up to 67% of the H2 gas produced under anaerobic conditions. Crucial to this increased O2 oxygen tolerance appears to be the presence of acetate in the medium for supporting high respiration rates and the separation of the entrapped cells from O2 in the liquid and headspace by the alginate polymer (Kosourov and Seibert 2009).
Conclusions
The introduction of a protocol for sustained H2 photo-evolution (Melis et al. 2000) based on the metabolic adaptations of some green algae to S deprivation has inspired a multitude of studies, mostly with the model organism C. reinhardtii. These studies expanded our knowledge on the successive steps that determine rates and duration of the observed H2 photo-evolution and provided insight in the flexibility of the photosynthetic apparatus in stress conditions. They are now starting to form a basis for the development of alternative protocols with actual potential for renewable energy generation. The following elements can be pointed out as key factors for sustainable H2 photo-evolution:
-
Although incompatible with the susceptibility of the hydrogenase to O2 and despite the existence of pathways for non-photochemical PQ reduction, the oxygenic PSII activity directly supplies most of the reductants needed for H2 production in wild type cells and should therefore be compensated by sufficient O2 consumption or buffering.
-
Loss or suppression of sink capacity for photoreductant other than the hydrogenase appears as another prerequisite to insure increased rates of H2 photo-evolution.
Moreover, the knowledge and protocols accumulated in studies with C. reinhardtii serve as paradigm for the exploration of nature’s pool of algal species capable of light-sustained H2 evolution. Not surprisingly, when evaluated under conditions optimized for the model organism C. reinhardtii, other algal species usually show inferior H2 production ability to the latter model organism, which in some cases seems due to the absence of acetate uptake capacity and in others relate to differences in fermentative metabolism (Skjånes et al. 2008; Meuser et al. 2009). Further ecological screening for new H2 producing algal species will probably require that molecular approaches are combined with hydrogen production tests that fit a wide variety of anoxic metabolisms.
References
Abeles FB (1964) Cell-free hydrogenase from Chlamydomonas. Plant Physiol 39:169–176
Antal TK, Krendeleva TE, Laurinavichene TV, Makarova VV, Ghirardi ML, Rubin AB, Tsygankov AA, Seibert M (2003) The dependence of algal H2 production on photosystem II and O2 consumption activities in sulfur-deprived Chlamydomonas reinhardtii cells. Biochim Biophys Acta 1607:153–160
Antal TK, Volgusheva AA, Kukarskih GP, Krendeleva TE, Rubin AB (2009) Relationships between H2 photoproduction and different electron transport pathways in sulfur-deprived Chlamydomonas reinhardtii. Int J Hydrogen Energy 34:9087–9094
Bishop NI, Gaffron H (1963) On the interrelation of the mechanisms for oxygen and hydrogen evolution in adapted algae. In: Kok B, Jagendorf AT (eds) Photosynthetic mechanisms of green plants, Nat Acad Sci Publ 1145, pp 441–451
Cardol P, Gloire G, Havaux M, Remacle C, Matagne R, Franck F (2003) Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol 133:2010–2020
Cardol P, Alric J, Girard-Bascou J, Franck F, Wollman F, Finazzi G (2009) Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proc Natl Acad Sci USA 106:15979–15984
Chen H, Melis A (2004) Localization and function of SulP, a nuclear-encoded chloroplast sulfate permease in Chlamydomonas reinhardtii. Planta 220:198–210
Chen H, Newton AJ, Melis A (2005) Role of SulP, a nuclear-encoded chloroplast sulfate permease, in sulfate transport and H2 evolution in Chlamydomonas reinhardtii. Photosynth Res 84:289–296
Chochois V, Dauvillée D, Beyly A, Tolleter D, Cuiné S, Timpano H, Ball S, Cournac L, Peltier G (2009) Hydrogen production in Chlamydomonas: photosystem II-dependent and -independent pathways differ in their requirement for starch metabolism. Plant Physiol 151:631–640
Cohen J, Kim K, Posewitz M, Ghirardi ML, Schulten K, Seibert M, King P (2005) Molecular dynamics and experimental investigation of H2 and O2 diffusion in [Fe]-hydrogenase. Biochem Soc Trans 33:80–82
Cournac L, Mus F, Bernard L, Guedeney G, Vignais P, Peltier G (2002) Limiting steps of hydrogen production in Chlamydomonas reinhardtii and Synechocystis PCC 6803 as analysed by light-induced gas exchange transients. Int J Hydrogen Energy 27:1229–1237
Davies JP, Yildiz FH, Grossman AR (1999) Sac3, an Snf1-like serine/threonine kinase that positively and negatively regulates the responses of Chlamydomonas to sulfur limitation. Plant Cell 11:1179–1190
Desplats C, Mus F, Cuiné S, Billon E, Cournac L, Peltier G (2009) Characterization of Nda2, a plastoquinone-reducing type II NAD(P)H dehydrogenase in Chlamydomonas chloroplasts. J Biol Chem 284:4148–4157
Doebbe A, Rupprecht J, Beckmann J, Mussgnug JH, Hallmann A, Hankamer B, Kruse O (2007) Functional integration of the HUP1 hexose symporter gene into the genome of C. reinhardtii: impacts on biological H2 production. J Biotechnol 131:27–33
Edman K, Ericson I, Möller IM (1985) The regulation of exogenous NAD(P)H oxidation in spinach (Spinacia oleracea) leaf mitochondria by pH and cations. Biochem J 232:471–477
Erbes DL, King D, Gibbs M (1979) Inactivation of hydrogenase in cell-free extracts and whole cells of Chlamydomonas reinhardi by oxygen. Plant Physiol 63:1138–1142
Finazzi G (2002) Redox-coupled proton pumping activity in cytochrome b6f, as evidenced by the pH dependence of electron transfer in whole cells of Chlamydomonas reinhardtii. Biochemistry 41:7475–7482
Finazzi G, Rappaport F, Furia A, Fleischmann M, Rochaix J, Zito F, Forti G (2002) Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep 3:280–285
Florin L, Tsokoglou A, Happe T (2001) A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J Biol Chem 276:6125–6132
Forestier M, King P, Zhang L, Posewitz M, Schwarzer S, Happe T, Ghirardi ML, Seibert M (2003) Expression of two [Fe]-hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. Eur J Biochem 270:2750–2758
Fouchard S, Hemschemeier A, Caruana A, Pruvost J, Legrand J, Happe T, Peltier G, Cournac L (2005) Autotrophic and mixotrophic hydrogen photoproduction in sulfur-deprived Chlamydomonas cells. Appl Environ Microb 71:6199–6205
Frenkel AW (1952) Hydrogen evolution by the flagellate green alga Chlamydomonas moewusii. Arch Biochem 38:219–230
Frenkel AW, Rieger C (1951) Photoreduction in algae. Nature 167:1030
Gaffron H, Rubin J (1942) Fermentative and photchemical production of hydrogen in algae. J Gen Physiol 26:219–240
Gonzalez-Ballester D, Pollock SV, Pootakham W, Grossman AR (2008) The central role of a SNRK2 kinase in sulfur deprivation responses. Plant Physiol 147:216–227
Grossman A (2000) Acclimation of Chlamydomonas reinhardtii to its nutrient environment. Protist 151:201–224
Guan Y, Zhang W, Deng M, Jin M, Yu X (2004) Significant enhancement of photobiological H2 evolution by carbonylcyanide m-chlorophenylhydrazone in the marine green alga Platymonas subcordiformis. Biotechnol Lett 26:1031–1035
Happe T, Kaminski A (2002) Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur J Biochem 269:1022–1032
Happe T, Naber JD (1993) Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur J Biochem 214:475–481
Happe T, Mosler B, Naber JD (1994) Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. Eur J Biochem 222:769–774
Hemschemeier A, Happe T (2005) The exceptional photofermentative hydrogen metabolism of the green alga Chlamydomonas reinhardtii. Biochem Soc Trans 33:39–41
Hemschemeier A, Fouchard S, Cournac L, Peltier G, Happe T (2008) Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks. Planta 227:397–407
Irihimovitch V, Stern DB (2006) The sulfur acclimation SAC3 kinase is required for chloroplast transcriptional repression under sulfur limitation in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103:7911–7916
Irihimovitch V, Yehudai-Resheff S (2008) Phosphate and sulfur limitation responses in the chloroplast of Chlamydomonas reinhardtii. FEMS Microbiol Lett 283:1–8
Jans F, Mignolet E, Houyoux P, Cardol P, Ghysels B, Cuiné S, Cournac L, Peltier G, Remacle C, Franck F (2008) A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc Natl Acad Sci USA 105:20546–20551
Kaltwasser H, Gaffron H (1964) Effects of carbon dioxide and glucose on photohydrogen production in Scenedesmus. Plant Physiol 39(Suppl):xiii
Kosourov SN, Seibert M (2009) Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng 102:50–58
Kosourov S, Seibert M, Ghirardi ML (2003) Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol 44:146–155
Kosourov S, Patrusheva E, Ghirardi ML, Seibert M, Tsygankov A (2007) A comparison of hydrogen photoproduction by sulfur-deprived Chlamydomonas reinhardtii under different growth conditions. J Biotechnol 128:776–787
Kruse O (2005) Improved photobiological H2 production in engineered green algal cells. J Biol Chem 280:34170–34177
Laurinavichene TV, Kosourov SN, Ghirardi ML, Seibert M, Tsygankov AA (2008) Prolongation of H2 photoproduction by immobilized, sulfur-limited Chlamydomonas reinhardtii cultures. J Biotechnol 134:275–277
Lee J, Greenbaum E (2003) A new oxygen sensitivity and its potential application in photosynthetic H2 production. Appl Biochem Biotechnol 106:303–313
Melis A (2007) Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226:1075–1086
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122:127–136
Meuser JE, Ananyev G, Wittig LE, Kosourov S, Ghirardi ML, Seibert M, Dismukes GC, Posewitz MC (2009) Phenotypic diversity of hydrogen production in chlorophycean algae reflects distinct anaerobic metabolisms. J Biotechnol 142:21–30
Meyer J (2007) [FeFe] hydrogenases and their evolution: a genomic perspective. Cell Mol Life Sci 64:1063–1084
Moreno J, García-Murria MJ, Marín-Navarro J (2008) Redox modulation of Rubisco conformation and activity through its cysteine residues. J Exp Bot 59:1605–1614
Ott T, van Dongen JT, Günther C, Krusell L, Desbrosses G, Vigeolas H, Bock V, Czechowski T, Geigenberger P, Udvardi MK (2005) Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr Biol 15:531–535
Parry MAJ, Keys AJ, Madgwick PJ, Carmo-Silva AE, Andralojc PJ (2007) Rubisco regulation: a role for inhibitors. J Exp Bot 59:1569–1580
Posewitz MC, Smolinski SL, Kanakagiri S, Melis A, Seibert M, Ghirardi ML (2004) Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii. Plant Cell 16:2151–2163
Rühle T, Hemschemeier A, Melis A, Happe T (2008) A novel screening protocol for the isolation of hydrogen producing Chlamydomonas reinhardtii strains. BMC Plant Biol 8:107
Schönfeld C, Wobbe L, Borgstädt R, Kienast A, Nixon P, Kruse O (2004) The nucleus-encoded protein MOC1 is essential for mitochondrial light acclimation in Chlamydomonas reinhardtii. J Biol Chem 279:50366–50374
Skjånes K, Knutsen G, Källqvist T, Lindblad P (2008) H2 production from marine and freshwater species of green algae during sulfur deprivation and considerations for bioreactor design. Int J Hydrogen Energy 33:511–521
Spruit CJP (1954) Photoproduction of hydrogen and oxygen in Chlorella. In: Proceeding of the First International Photobiology Congress, Amsterdam, pp 323–327
Takahashi S, Murata N (2005) Interruption of the Calvin cycle inhibits the repair of Photosystem II from photodamage. Biochim Biophys Acta 1708:352–361
Takahashi S, Murata N (2006) Glycerate-3-phosphate, produced by CO2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta 1757:198–205
Timmins M, Thomas-Hall SR, Darling A, Zhang E, Hankamer B, Marx UC, Schenk PM (2009a) Phylogenetic and molecular analysis of hydrogen-producing green algae. J Exp Bot 60:1691–1702
Timmins M, Zhou W, Rupprecht J, Lim L, Thomas-Hall SR, Doebbe A, Kruse O, Hankamer B, Marx UC, Smith SM, Schenk PM (2009b) The metabolome of Chlamydomonas reinhardtii following induction of anaerobic H2 production by sulfur depletion. J Biol Chem 284:23415–23425
Tolstygina IV, Antal TK, Kosourov SN, Krendeleva TE, Rubin AB, Tsygankov AA (2009) Hydrogen production by photoautotrophic sulfur-deprived Chlamydomonas reinhardtii pre-grown and incubated under high light. Biotechnol Bioeng 102:1055–1061
Tsygankov AA, Kosourov SN, Tolstygina IV, Ghirardi ML, Seibert M (2006) Hydrogen production by sulfur-deprived Chlamydomonas reinhardtii under photoautotrophic conditions. Int J Hydrogen Energy 31:1574–1584
Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryotic Cell 8:1856–1868
White AL, Melis A (2006) Biochemistry of hydrogen metabolism in Chlamydomonas reinhardtii wild type and a Rubisco-less mutant. Int J Hydrogen Energy 31:455–464
Winkler M, Heil B, Heil B, Happe T (2002) Isolation and molecular characterization of the [Fe]-hydrogenase from the unicellular green alga Chlorella fusca. Biochim Biophys Acta 1576:330–334
Winkler M, Kuhlgert S, Hippler M, Happe T (2009) Characterization of the key steps of light driven hydrogen production in Chlamydomonas reinhardtii. J Biol Chem 284:36620–36627
Wollman F-A (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20:3623–3630
Wu S, Huang R, Xu L, Yan G, Wang Q (2010) Improved hydrogen production with expression of hemH and lba genes in chloroplast of Chlamydomonas reinhardtii. J Biotechnol 146:120–125
Wykoff DD, Davies JP, Melis A, Grossman AR (1998) The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117:129–139
Zhang L, Melis A (2002) Probing green algal hydrogen production. Philos Trans R Soc Lond B Biol Sci 357:1499–1511
Zhang L, Happe T, Melis A (2002) Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga). Planta 214:552–561
Acknowledgments
This study has been financed by Action de Recherche Concertée ARC07/12-04. F.F. is a Senior Research Associate of the Fonds de la Recherche Scientifique, F.R.S-FNRS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghysels, B., Franck, F. Hydrogen photo-evolution upon S deprivation stepwise: an illustration of microalgal photosynthetic and metabolic flexibility and a step stone for future biotechnological methods of renewable H2 production. Photosynth Res 106, 145–154 (2010). https://doi.org/10.1007/s11120-010-9582-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-010-9582-4